- 1Department of Pediatric Dentistry and Orthodontics, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 2Laser Biospectroscopy Laboratory, Prokhorov General Physics Institute of the Russian Academy of Sciences, Moscow, Russia
- 3Department of Laser Micro-, Nano- and Biotechnologies, Institute for Physics and Engineering in Biomedicine, National Research Nuclear University MEPhI (Moscow Engineering Physics Institute), Moscow, Russia
- 4Laboratory of Laser Biospectroscopy, Prokhorov General Physics Institute of the Russian Academy of Sciences, Moscow, Russia
- 5Department of Maxillofacial Surgery, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 6Department of Maxillofacial Surgery, The Peoples' Friendship University of Russia, Moscow, Russia
Dental diseases occur in children with cerebral palsy three times higher than in healthy children. Low values of the unstimulated salivation rate (<0.3 ml per minute), pH and buffer capacity, changes in enzyme activity and sialic acid concentration, as well as increased saliva osmolarity and total protein concentration, which indicates impaired hydration, are the factors in the development of a gingiva disease in case of cerebral palsy. This leads to increased bacterial agglutination and the formation of acquired pellicle and biofilm, leading to the formation of dental plaque. There is a tendency toward an increase in the concentration of hemoglobin and a decrease in the degree of hemoglobin oxygenation, as well as an increase in the generation of reactive oxygen and nitrogen species. Photodynamic therapy (PDT) with the use of photosensitizer methylene blue improves blood circulation and the degree of oxygenation in periodontal tissues, as well as eliminates a bacterial biofilm. Analysis of back diffuse reflection spectra makes it possible to conduct non-invasive monitoring determine tissue areas with a low level of hemoglobin oxygenation for precision photodynamic exposure.
Aim: To improve the effectiveness of phototheranostics methods using, namely PDT with simultaneous optical-spectral control, for the treatment of gingivitis in children with complex dental and somatic status (cerebral palsy).
Methods: The study involved 15 children (6-18 y.o.) with various forms of cerebral palsy, in particular, spastic diplegia and atonic-astatic form and with gingivitis. The degree of hemoglobin oxygenation was measured in tissues before PDT and on the 12th day. PDT was performed using laser radiation (λ = 660 nm) with a power density of 150 mW/cm2 with a five-minute application of 0.01% MB. The total light dose was 45 ± 15 J/cm2. For statistical evaluation of the results, a paired Student's t-test was used.
Results: The paper presents the results of phototheranostics using methylene blue in children with cerebral palsy. An increase in the level of hemoglobin oxygenation from 50 to 67% (p < 0.001) and a decrease in blood volume in the microcirculatory bed of periodontal tissues were shown.
Conclusion: Photodynamic therapy methods with application of methylene blue make it possible to assess the state of the gingival mucosa tissue diseases objectively in real time, and to provide effective targeted therapy for gingivitis in children with cerebral palsy. There is a prospect that they can become widely used clinical methods.
Introduction
Cerebral palsy (CP) is a common (2.0–2.5 cases per 1,000) chronic movement disorder, often accompanied by impaired coordination, cognitive functions, communication and seizure disorders (1–3). These disorders, as well as medications used by children with CP (4), have a direct impact on the development of various diseases, but one of the most common pathologies in case of cerebral palsy, affecting the child's quality of life, are dental or oral diseases (5, 6). All research groups that studied the prevalence of diseases of periodontal and dental hard tissues in children found that children with CP had the prevalence of dental diseases in three times higher than in the control group (7). The more severe the degree of neurological stroke was and, as a result, cognitive and motor deficits, the higher the risk of developing dental diseases (8–11). Inability to maintain oral hygiene, difficulty in chewing and swallowing, excessive drooling (sialorrhea), recurrent regurgitation and vomiting, insufficient calcium intake, vitamin D deficiency, use of antiepileptic medications are only a part of the factors leading to the onset and development of dental pathologies in children with CP (12–16). Along with caries, gingivitis is observed in the vast majority of children with CP (17–19). Low indices of the unstimulated salivation rate, pH and buffer capacity, changes in enzyme activity and sialic acid concentration, as well as increased saliva osmolarity and total protein concentration, which indicates impaired hydration, are the factors in the development of gingiva diseases in case of cerebral palsy (20, 21). This leads to increased bacterial agglutination and the formation of acquired pellicle and biofilm, leading to the formation of dental plaque (22).
The inflammatory response of the gingivas after the onset of the plaque formation is characterized by a change in vascular morphology that precedes clinical changes. As the inflammation of the gingivas increases, the expansion and proliferation of the gingiva vessels is observed, as well as their course changes and the number of functioning units increases. However, as shown in the study (23) of the degree of oxygenation of the inflamed periodontal tissues, the increase in blood supply is insufficient to meet the oxygen demand of the inflamed gingivas, in which the Hb index (tissue blood filling) was significantly higher than in clinically healthy gingivas. A lower level of tissue SO2 was observed in the inflamed area. Hypoxia and inflammation are usually closely related (24). Hypoxia regulates vascular tone and is a powerful stimulus for angiogenesis (25), which ensures the supply of oxygen and nutrients to tissues, and restores homeostasis during wound healing (26). Endothelial cells are located at the boundary between blood and tissues and their function directly depends on changes in oxygen tension (27). Endothelial cells respond to hypoxia through the expression of regulatory genes mediated by various oxygen-dependent signaling cascades (28, 29). Hypoxia-inducible factor (HIF) is a key regulatory protein for hypoxia-mediated events (30). It regulates the expression of many genes involved in adaptation to oxygen deficiency, including cell proliferation, apoptosis, metabolism, immune responses, genomic instability and vascularization, the expression of VEGF, nitric oxide synthase (NOS), and the release of cytokines that regulate angiogenesis (31).
The bacterium F. nucleatum plays a special role in the progression of periodontitis and gingivitis (32). It stimulates pro-inflammatory changes in endothelial cells (33, 34) and induces hypoxia by itself, reducing the oxygen content in the environment (35, 36). As a result, endothelial cells demonstrate decreased expression of CD31 and increased CD34 (37), which leads to endothelial dysfunction, increased inflammation, and increased activation of T-cells (38). Another inflammatory factor during hypoxia is an increase in the immunoreactivity of NF-kB, HIF-1 and VEGF, as well as an increase in the expression of IL-1β and MMP-1 in accordance with the progression of diseases (39), which induces vascular permeability and stimuli of invasion of immune cells, such as as monocytes/macrophages and neutrophils, which contribute to the destruction of periodontal tissue.
In modern clinical practice, the diagnosis of periodontal disease is reduced to asking about complaints, writing a case history, instrumental examination, radiography. The evaluation is carried out taking into account the condition of soft tissues, the integrity of the epithelial attachment, the presence and depth of periodontal pockets, the degree of tooth mobility. The Green Vermillion Hygiene Index (OHI-S), the Muehlemann Bleeding Index (SBI), the Schiller-Pisarev test, the papillary-marginal-alveolar index (PMA), and others are commonly used. The general drawback of these methods lies in the fact that the degree of the disease is determined visually, therefore, it is difficult to get rid of the problem of conventionality of digital values determined by a doctor. These methods do not give an opportunity to evaluate the dynamic processes occurring in the tissues and do not allow us to determine the degree of hemoglobin oxygenation in the microvasculature and the level of blood flow in the tissue. Since today microbial factors (recognized by WHO) are responsible for inflammatory-destructive periodontal lesions, the choice of etiotropic therapy of inflammatory periodontal diseases is carried out on the basis of bacteriological examination of the contents of gingival pockets, giving an opportunity to eliminate or weaken its influence by the subsequent use of an etiotropic complex (40). This complex usually provides the direct removal of a biofilm and hard dental plaque; the use of agents that suppress both the maturation of the biofilm and the degree of its pathogenic effect on tissues—antiseptics, and, if necessary, antibiotics. However, more and more antibiotic-resistant bacteria species are emerging, which complicate the possibility of drug treatment, and standard therapies are no longer effective. The use of photodynamic therapy (PDT) with photosensitizers (PS) (41, 42) may help to overcome these difficulties in the treatment of periodontal diseases.
PDT is a method used as a therapy for oral diseases of various origins, such as oncology, precancerous diseases, caries, periodontitis, and gingivitis (43). The advantages of this method are multifactorial effects, antibacterial, anti-inflammatory and wound healing (44). Methylene blue (45), phthalocyanines (46), chlorins (47), porphyrins, etc. are usually used for antimicrobial PDT.
The effect of MB on the regulation of inflammatory processes has been studied (48). It has been shown that MB attenuates the activation of inflammasomes that induce maturation of IL-1β ℵ IL-18, and also a protein such as caspase-1, which mediates cell death known as apoptosis, is part of the inflammasome. In addition, MB inhibits the initial signal of activation of the inflammatory process, phagocytosis, and expression of genes of inflammatory components by inhibiting NF-kB signaling.
The use of PDT with MB in severe chronic periodontitis showed that clinical variables showed significant improvement in IL-1α, IL-1β, IL-8, IL-1ra, IFN-γ, IL-10 and VEGF (49).
The explanation of the mechanism of anti-inflammatory photodynamic action in gingivitis is a non-trivial task (Figure 1). Biofilm products secreted by bacteria, such as endotoxins, trypsin-like enzymes, acid and alkaline phosphatases, etc. (51) induce functional disorders and death of gingival epithelial cells. As a result, a cascade inflammatory response is induced, triggered by resident macrophages, neutrophils, and T-cells (52). Inflammation in periodontal pockets is characterized by low oxygen levels and an acidic medium, and mechanical stress caused by chewing, rubbing, or orthodontic treatment can provoke deformation of the blood vessels, contributing to ischemia and hypoxia (53).
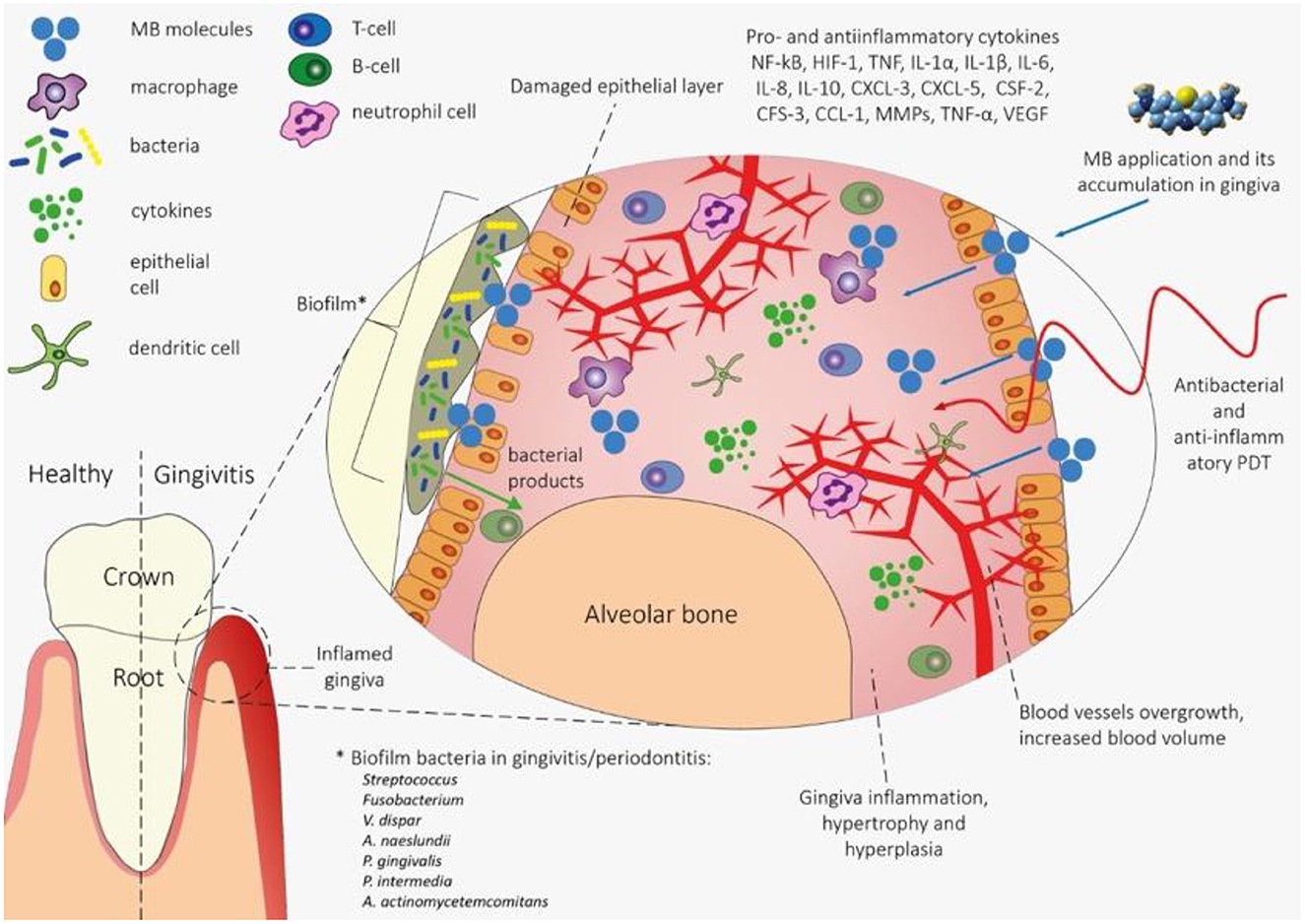
Figure 1. The mechanism of PDT effects on gingiva tissue in gingivitis. The waste products of biofilm bacteria lead to functional disruption and death of epithelial cells, which contributes to the onset of the inflammatory process triggered by immunocompetent cells (macrophages, neutrophils and T cells). There is increased blood flow, decreased hemoglobin oxygenation and gingiva hyperplasia. When MS is applied to gingival tissue, bacteria and gingival tissue cells are sensitized with a photosensitizer (primarily phagocytes). With the help of photodynamic action, bacteria are eliminated in the biofilm and pro-inflammatory macrophages are deactivated, which leads to a decrease in inflammation and normalization of blood flow and the degree of hemoglobin oxygenation. Methylene blue (MB) is of greatest interest as a PS for anti-inflammatory PDT in order to increase the degree of hemoglobin oxygenation in the microvasculature of periodontal tissues. Initially, methylene blue was the first stain used in medicine as an antiseptic and the first photosensitizer tested and approved for use for antimicrobial photodynamic therapy (50).
This leads to an increase in the immunoreactivity of NF-kB, HIF-1, VEGF, IL-1β and MMP-1 and promotes the invasion of immune cells such as monocytes and/or macrophages and neutrophils. Immune cells, in turn, express IL-8, TNF, IL-6, IL-1β, and IL-10, and epithelial cells show elevated regulation of IL-1α, CXCL-3, CXCL-5, CSF-2, CFS- 3, CCL-1 (54).
The use of PDT with MB can deactivate inflammation and completely eliminate bacteria in the biofilm (55), which will contribute to the functional normalization of the gingiva tissue and the restoration of oxygenation in the microvasculature (56, 57).
Thus, to control PDT of gingivitis, it is necessary to monitor the degree of hemoglobin oxygenation and the level of hemoglobin oxygenation in the microvasculature of periodontal tissues. Optical methods are best suited for these purposes, since they are non-invasive, fast and relatively cheap. In modern clinical dentistry, hemodynamic parameters can be determined using laser Doppler flowmetry (LDF) (58–60) laser speckle contrast imaging (LSCI) (61), Doppler OCT and using backsward diffuse reflection spectroscopy (BDR) (23, 62). Compared to single-point laser Doppler flowmetry (LDF), the LSCI is capable of showing multiple examined regions rather than one. Another unique feature of LSCI is its fast imaging, which reduces movement artifacts and shortens the time of each measurement session. The drawback of LDF is its high sensitivity to the position of the sensor relative to the measured area, which complicates the processing of the results. The measurement results also depend not only on the blood flow in the measured area, but also on the scattering properties of the surrounding tissues. Another limitation of the LDF method is the lack of absolute zero measurement, even if the flow of red blood cells is reduced to zero experimentally or surgically. This is explained by the Brownian motion of macromolecules in the interstitial compartment (63). As a result, changes in gingival blood flow cannot be compared between patients, and values of blood flow at different sites cannot be compared even in the same patient. The main shortcoming of the LSCI methods is the low probing depth (up to 300 microns) due to a loss of light coherence during dispersion in biological tissue. With the help of OCT one can only determine the structure of blood vessels without dynamic parameters. Doppler OCT can only measure blood flow velocity in large vessels where pulsating blood flow is observed. Thus, diffuse backscatter spectroscopy, the probing depth of which reaches several millimeters, and also allows one to determine the degree of hemoglobin oxygenation and the level of blood circulation in the microvasculature of tissues, is the most preferred method for assessing the effectiveness of photodynamic effects in case of gingivitis. BDR spectroscopy is also well combined with fluorescence spectroscopy, which makes it possible to determine the PS concentration in tissues before and after PDT (64).
Purpose
To improve the effectiveness of the gingivitis treatment in children with complicated dental and somatic status (cerebral palsy), based on the use of photodynamic therapy with the simultaneous use of optical diagnostic methods.
Materials and methods
The patients
We analyzed the results of therapeutic treatment for catarrhal gingivitis in 15 patients with various forms of cerebral palsy, in particular, spastic diplegia and atonic-astatic form. The patients were in the 6-18 age range with a male predominance (10:5).
Optical spectral assessment of tissue oxygenation
To quantify the degree of oxygenation and the relative amount of hemoglobin in the tissue, the spectroscopic method was used, which was described in detail in the works of researchers (65, 66) (see Figure 2). The principle of this method consists in analyzing the logarithm of the diffuse reflectance spectrum in the spectral range of 500–600 nm, where the shapes of the absorption spectra of hemoglobin in oxygenated and reduced forms have the greatest differences.
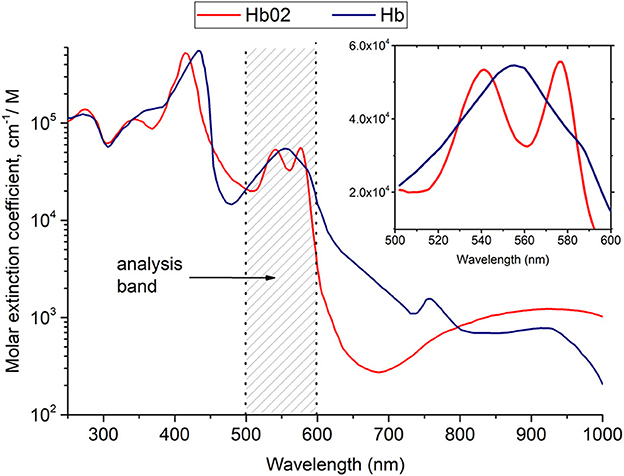
Figure 2. Results of hemoglobin oxygenation measurement in the microcirculatory supply of periodontal tissues.
To measure the absorption spectra of periodontal tissues, we used an LESA-01-BioSpec fiber-optic spectrometer with a spectral resolution of 8 nm and an entrance slit of 200 μm. A halogen lamp with a fiber optic output was used as a source of broadband radiation for recording absorption spectra. A fiber-optic probe with a central illumination fiber was used to provide delivery and reception of radiation, which supplied either laser radiation exciting fluorescence or broadband radiation to the tissue, and six peripheral fibers collecting radiation. The measurement of spectra is carried out in gentle contact (without pressure) with the biological tissue to exclude the Fresnel reflection of light coming from the surface of the biological tissue from getting into the receiving fibers and to avoid the displacement of blood from the examined area. For each patient, an area with clinically pronounced gingivitis was selected, as well as an area of conditionally healthy tissue. From 5 to 10 measurements of back diffuse reflection spectra were measured in each area, then the results were averaged for each area of each patient. For statistical evaluation of the results, a paired Student's t-test was used.
The obtained spectra were processed using the UnoMomento software (BioSpec LLC).
Photodynamic therapy using MB
The MB solution was 0.01% MB (absorption maximum λ = 662 nm). PS was applied for 5 min. An LFT-02-Biospec semiconductor laser with a wavelength λmax = 660 nm was used as a radiation source for photodynamic exposure. Laser radiation was delivered to the PDT area using a fiber-optic bundle with a microlens, which provided distinct boundaries of the exposure spot. The power density of the exposure for each selected area of tissue was from 100 to 200 mW/cm2, the exposure time was 5 minutes, the light dose was 45 ± 15 J/cm2.
Results
These results testify to a positive anti-inflammatory effect of MB and high-quality treatment using PDT and MB. Figure 2 shows the results of hemoglobin oxygenation in the microvasculature of tissues (see Figure 3).
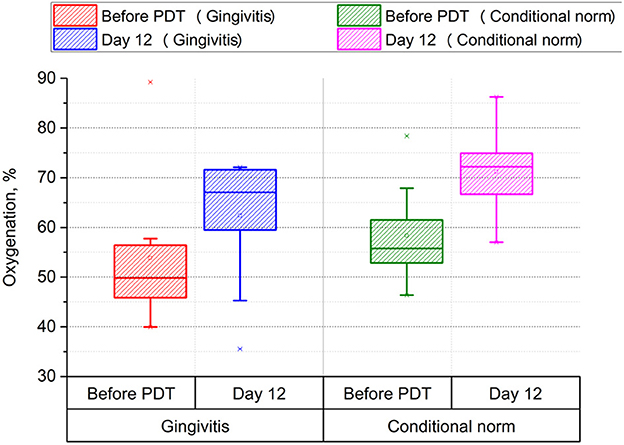
Figure 3. Assessment of the level of gingival tissue oxygenation before and after photodynamic therapy (p < 0.001, power > 0.99).
The use of PDT in treatment leads to a significant increase in oxygenation both in the area affected with gingivitis (from 50 to 67%, p < 0.001) and in the conditionally healthy area of periodontal tissue (from 57 to 72%, p < 0.001), which indicates a positive effect of PDT on energy and metabolic processes in periodontal tissues.
Discussion
The difficulty of performing most dental procedures in children with CP is beyond doubt. Almost all dental procedures in these patients involve sedation and anesthesia. Children with CP may be difficult to handle and uncooperative during dental examination and treatment. Therefore, in this case, non-invasive methods of exposure, such as phototheranostics, are of particular relevance. Unfortunately, at the moment in scientific practice there are practically no studies on the use of PDT and optical-spectral diagnostics in patients with cerebral palsy. First of all, this is due to the difficulties of obtaining all permits for conducting these studies.
Although in the world practice extensive research is being carried out on the possibility of using photodynamic therapy for diseases of the oral cavity (67), our scientific group for the first time applied phototheranostics methods in children with cerebral palsy.
The choice of this particular treatment method is due to the multifactorial nature of the photodynamic effect: antibacterial (68), anti-inflammatory (69) and wound healing (70), and the fact that phototherasnostics is a non-invasive and conservative method with few side effects (71). Another advantage of phototheranostics is its minimally invasive action, which can be especially sought after by children, youth and adults who are afraid of dental procedures. Considering that many children and patients with special needs find it difficult to cooperate during long-term clinical dental treatment, the use of PDT in the field of pediatric dentistry and in patients with special needs is very promising. Because it is a painless treatment with no unpleasant taste, it allows the clinician to have regular consultations (72).
Phototheranostics methods for the treatment and prevention of periodontal isease can be non-invasive, and therefore well suited for use in such patients. The widespread introduction of phototheranostics methods is especially important for patients who are unable to independently monitor the health of the oral cavity and tolerate standard treatment methods due to the limited choice of specific dental tools and diagnostic and treatment methods that ensure rapidity, non-invasiveness and effectivenessThe results of our study showed a significant increase in the oxygenation of biological tissues (from 50 to 67%) as a result of photodynamic exposure with a five-minute application of 0.01% MB solution. This results provide prerequisites for the development of new clinical methods that allow non-invasive and painless, real-time assessment of the periodontal diseases state, as well as effective therapy for gingivitis in children with cerebral palsy.
Our future research will focus on obtaining data on biochemical parameters and indicators of inflammation obtained as a result of the work that will allow a deeper understanding of the mechanisms of photodynamic effects on tissues, which can allow the development of algorithms for the clinical application of photodynamic therapy using the photosensitizer methylene blue not only for periodontal diseases, but also for other inflammatory diseases.
Conclusion
At present, the clinical experience that has been accumulated in the world is not sufficient to formulate the optimal tactics for treating children with cerebral palsy. It is necessary to select an individual approach to such patients, and, as a rule, making emphasis on more comfortable treatment.
Children with cerebral palsy are at increased risk of developing dental problems compared to healthy children, which can lead to their increased incidence and prevalence and significantly affect children's well-being and quality of life.
The conducted study showed that the use of photodynamic therapy with methylene blue gives reliable results in the treatment of children with cerebral palsy. There was a trend toward an increase in tissue saturation on the 12th day after photodynamic exposure (from 50 to 67%).
The indisputable advantage of the methylene blue is that this drug has already been approved for use in pediatric dentistry, which means that the possibility of a wide practical implementation of the results seems very likely. Methods of phototheranostics using methylene blue make it possible to objectively assess in real time the state of the gingival mucosa tissues diseases, and to provide effective targeted therapy for gingivitis in children with cerebral palsy and can become promising clinical methods.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Local Ethics Committee FSAEI HE I. M. Sechenov First Moscow State University of the Ministry of Health of the Russian Federation (Sechenov University). The patients' guardian provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The research was supported by the Ministry of Education and Science of the Russian Federation, grant for the creation and development of world-class research centers No. 075-15-2022-315—Photonics Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grzić R, Bakarcić D, Prpić I, Jokić NI, Sasso A, Kovac Z, et al. Dental health and dental care in children with cerebral palsy. Coll Antropol. (2011) 35:761–4.
2. Sehrawat N, Marwaha M, Bansal K, Chopra R. Cerebral palsy: a dental update. Int J Clin Ped Dent. (2014) 7:109–18. doi: 10.5005/jp-journals-10005-1247
3. Jan BM, Jan MM. Dental health of children with cerebral palsy. Neurosciences (Riyadh, Saudi Arabia). (2016) 21:314–8. doi: 10.17712/nsj.2016.4.20150729
4. Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat. (2020) 16:1505–18. doi: 10.2147/NDT.S235165
5. Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. (2016) 22:609–19. doi: 10.1111/odi.12428
6. de Carvalho RB, Mendes RF, Prado RR Jr, Moita Neto JM. Oral health and oral motor function in children with cerebral palsy. Spec Care Dentist. (2011) 31:58–62. doi: 10.1111/j.1754-4505.2011.00180.x
7. Botti RS, Masiero MT, Novo D, Simionato MRL. Oral conditions in children with cerebral palsy. J Dent Child. (2003) 70:40–6.
8. Sankar C, Mundkur N. Cerebral palsy definition, classification, etiology and early diagnosis. Indian J Ped. (2015) 72:865–8. doi: 10.1007/BF02731117
9. Cardoso AM, Gomes LN, Silva CR, Soares R, Abreu MH, Padilha WW, et al. Dental caries and periodontal disease in Brazilian children and adolescents with cerebral palsy. Int J Environ Res Public Health. (2014) 12:335–53. doi: 10.3390/ijerph120100335
10. Dourado MR, Andrade PM, Ramos-Jorge ML, Moreira RN, Oliveira-Ferreira F. Association between executive/attentional functions and caries in children with cerebral palsy. Res Dev Disabil. (2013) 34:2493–9. doi: 10.1016/j.ridd.2013.05.003
11. Abanto J, Ortega AO, Raggio DP, Bönecker M, Mendes FM, Ciamponi AL. Impact of oral diseases and disorders on oral-health-related quality of life of children with cerebral palsy. Spec Car Dent. (2014) 34:56–63. doi: 10.1111/scd.12028
12. Russman BS, Ashwal S. Evaluation of the child with cerebral palsy. Semin Ped Neurol. (2004) 11:47–57. doi: 10.1016/j.spen.2004.01.007
13. Eltumi M, Sullivan PB. Nutritional management of the disabled child: the role of percutaneous endoscopic gastrostomy. Dev Med Child Neurol. (1997) 39:66–8. doi: 10.1111/j.1469-8749.1997.tb08207.x
14. Ferreira ACFM, Eveloff RJ, Freire M, Santos MTBR. The Impact of Oral-Gut Inflammation in Cerebral Palsy. Front Immunol. (2021) 12:619262. doi: 10.3389/fimmu.2021.619262
15. Santos MT, Ferreira MC, Guaré RO, Diniz MB, Rösing CK, Rodrigues JA, et al. Gingivitis and salivary osmolality in children with cerebral palsy. Int J Paediatr Dent. (2016) 26:463–70. doi: 10.1111/ipd.12220
16. Subasi F, Mumcu G, Koksal L, Cimilli H, Bitlis D. Factors affecting oral health habits among children with cerebral palsy: pilot study. Pediatr Int. (2007) 49:853–7. doi: 10.1111/j.1442-200X.2007.02445.x
17. Chunyang Z, Xiaoming X, Minqi X, Chun F, Michael GS, Kan-Zhi L, et al. Assessment of tissue oxygenation of periodontal inflammation in patients with coronary artery diseases using optical spectroscopy. BMC Oral Health. (2014) 14:25. doi: 10.1186/1472-6831-14-25
18. Guare RDO, Ciampioni AL. Prevalence of periodontal disease in the primary dentition of children with cerebral palsy. J Dent Child. (2004) 71:27–32.
19. Hashmi A, Mawlood H, Kowash AH, Al HM. Oral health status among children with cerebral palsy in Dubai, United Arab Emirates. J Int Soc Prev Com Dent. (2017) 7:149–54. doi: 10.4103/jispcd.JISPCD_295_17
20. Ferreira ACFM, Mayer MPA, Kawamoto D, Santos MTBR. Constipation, antiepileptic drugs, and gingivitis in children and adolescents with cerebral palsy. Int J Paediatr Dent. (2019) 29:635–41. doi: 10.1111/ipd.12488
21. Santos MT, Ferreira MC, Mendes FM, Oliveira GR. Assessing salivary osmolality as a caries risk indicator in cerebral palsy children. Int J Paed Dent. (2014) 24:84–9. doi: 10.1111/ipd.12030
22. Santos MT, Guare RO, Celiberti P, Siqueira WL. Caries experience in individuals with cerebral palsy in relation to oromotor dysfunction and dietary consistency. Spec Care Dent. (2009) 29:198–203. doi: 10.1111/j.1754-4505.2009.00092.x
23. Hanioka T, Shizukuishi S, Tsunemitsu A. Hemoglobin concentration and oxygen saturation of clinically healthy and inflamed gingiva in human subjects. J Per Res. (1990) 25:93–8. doi: 10.1111/j.1600-0765.1990.tb00898.x
24. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. (2004) 95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5
25. Bartruff JB, Yukna RA, Layman DL. Outer membrane vesicles from Porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol. (2005) 76:972–9. doi: 10.1902/jop.2005.76.6.972
26. Cochran DL. Inflammation and bone loss in periodontal disease. J Per. (2008) 79:1569–76. doi: 10.1902/jop.2008.080233
27. Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. (2015) 27:27. doi: 10.1186/s13073-015-0153-3
28. Faller DV. Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol. (1999) 26:74–84. doi: 10.1046/j.1440-1681.1999.02992.x
29. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. (2003) 9:669–76. doi: 10.1038/nm0603-669
30. Folkman J, Shing Y. Angiogenesis. J Biol Chem. (1992) 267:10931–4. doi: 10.1016/S0021-9258(19)49853-0
31. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. (2008) 79:1585–91. doi: 10.1902/jop.2008.080183
32. Lin MI, Sessa WC. Vascular endothelial growth factor signaling to endothelial nitric oxide synthase: more than a FLeeTing moment. Circ Res. (2006) 99:666–8. doi: 10.1161/01.RES.0000245430.24075.a4
33. Mendes RT, Nguyen D, Stephens D, Pamuk F, Fernandes D, Van Dyke TE, Kantarci A. Endothelial Cell Response to Fusobacterium nucleatum. Infect Immun. (2016) 84:2141–48. doi: 10.1128/IAI.01305-15
34. Liu L, Shi GP. CD31: beyond a marker for endothelial cells. Card Res. (2012) 94:3–5. doi: 10.1093/cvr/cvs108
35. Mendes RT, Nguyen D, Stephens D, Pamuk F, Fernandes D, Hasturk H. Hypoxia-induced endothelial cell responses–possible roles during periodontal disease. Clin Exp Dent Res. (2018) 4:241–8. doi: 10.1002/cre2.135
36. Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Per. (2011) 38:28–35. doi: 10.1111/j.1600-051X.2010.01673.x
37. Sluimer JC, Gasc JM, van Wanroji JL, et al. Hypoxia, hypoxia- inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. (2008) 51:1258–65. doi: 10.1016/j.jacc.2007.12.025
38. Tandle AT, Calvani M, Uranchimeg B, Zahavi D, Melillo G, Libutti SK. Endothelial monocyte activating polypeptide-II modulates endothelial cell responses by degrading hypoxia-inducible factor- 1alpha trough interaction with PSMA7, a component of the proteasome. Exp Cel Res. (2009) 315:1850–9. doi: 10.1016/j.yexcr.2009.03.021
39. Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, et al. Hypoxia and P. gingivalis synergistically induce HIF-1 and NF-κB activation in PDL cells and periodontal diseases. Mediators Inflamm. (2015) 2015:438085. doi: 10.1155/2015/438085
40. Sanz I, Alonso B, Carasol M, Herrera D, Sanz M. Nonsurgical Treatment of Periodontitis. J Evid Bas Dent Pract. (2012) 12:76–86. doi: 10.1016/S1532-3382(12)70019-2
41. Meimandi M. The effect of photodynamic therapy in the treatment of chronic periodontitis: a review of literature. J Las Med Scien. (2017) 8:S7–S11. doi: 10.15171/jlms.2017.s2
42. Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. (2007) 86:694–707. doi: 10.1177/154405910708600803
43. Gursoy H. Photodynamic therapy in dentistry: a literature review. Clin Or Inv. (2013) 17:1113–25. doi: 10.1007/s00784-012-0845-7
44. Sperandio FF, Simões A, Aranha A. C, Corrêa, L, Orsini, Machado de Sousa SC. Photodynamic therapy mediated by methylene blue dye in wound healing. Photomed Laser Surg. (2010) 28:581–7. doi: 10.1089/pho.2009.2601
45. Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. (2002) 93:155–60. doi: 10.1067/moe.2002.120051
46. Allen CM, Sharman WM, Van LJE. Current status of phthalocyanines in the photodynamic therapy of cancer. J Porphyr Phthalocyanines. (2001) 5:161–9. doi: 10.1002/jpp.324
47. Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobio Bio. (2004) 73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002
48. Ahn H, Kang SG, Yoon S, Ko HJ, Kim PH, Hong EJu, An BS, Lee E, Lee GSh. Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci Rep. (2017) 7:12409. doi: 10.1038/s41598-017-12635-6
49. Boltes Cecatto R, Siqueira de Magalhães L, Fernanda Setúbal Destro Rodrigues M, Pavani C, Lino-Dos-Santos-Franco A, Teixeira Gomes M, et al. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagnosis Photodyn Ther. (2020) 31:101828. doi: 10.1016/j.pdpdt.2020.101828
50. Klepac-Ceraj V, Patel N, Song X, Holewa C, Patel C, Kent R, et al. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Las Surg Med. (2011) 43:600–6. doi: 10.1002/lsm.21069
51. Khurshid Z, Mali M, Naseem M, Najeeb S, Zafar MS. Human gingival crevicular fluids (GCF) proteomics: an overview. Dent J (Basel). (2017) 5:12. doi: 10.3390/dj5010012
52. Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Muc Imm. (2016) 9:1163–72. doi: 10.1038/mi.2015.136
53. Geng X, Horst WJ, Golz JF, Lee JE, Ding Z, Yang ZB. LEUNIG_HOMOLOG transcriptional co-repressor mediates aluminium sensitivity through PECTIN METHYLESTERASE46-modulated root cell wall pectin methylesterification in Arabidopsis. Plant J. (2017) 90:491–504. doi: 10.1111/tpj.13506
54. Brown JL, Johnston W, Delaney C, Rajendran R, Butcher J, Khan S, et al. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci Rep. (2019) 9:1–14. doi: 10.1038/s41598-019-52115-7
55. Oliveira RR, Schwartz-Filho HO, Novaes AB Jr, Garlet GP, Freitas de Souza R, Taba Jr M, Ribeiro FJ. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Cytokine profile in gingival crevicular fluid, preliminary results. J Periodont. (2009) 80:98–105. doi: 10.1902/jop.2009.070465
56. Vieira DL, Leite AF, Figueiredo PTDS, Vianna LM, Moreira-Mesquita CR, Melo NS. A conservative approach for localized spongiotic gingivitis hyperplasia using photodynamic therapy: a case report and review of the literature. Photobiomod Photomed Las Surg. (2019) 37:57–61. doi: 10.1089/photob.2018.4454
57. Gómez C, Abellán R, Palma JC. Efficacy of photodynamic therapy vs ultrasonic scaler for preventing gingival inflammation and white spot lesions during orthodontic treatment. Photo Photodyn Ther. (2018) 24:377–83. doi: 10.1016/j.pdpdt.2018.11.001
58. Mavropoulos A, Brodin P, Rösing CK, Aass AM, Aars H. Gingival blood flow in periodontitis patients before and after periodontal surgery assessed in smokers and non-smokers. J Perio. (2007) 78:1774–82. doi: 10.1902/jop.2007.060472
59. Donos N, D'Aiuto F, Retzepi M, Tonetti M. Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study J Perio Res. (2005) 40:129–37. doi: 10.1111/j.1600-0765.2005.00777.x
60. Baab DA, ÖBerg PA, Holloway GA. Gingival blood flow measured with a laser Doppler flowmeter. J Perio Res. (1986) 21:73–85. doi: 10.1111/j.1600-0765.1986.tb01440.x
61. Molnár E, Molnár B, Lohinai Z, Tóth Z, Benyó Z, Hricisák L, et al. Evaluation of laser speckle contrast imaging for the assessment of oral mucosal blood flow following periodontal plastic surgery: an exploratory study. BioMed Res Inter. (2017) 2017:4042902. doi: 10.1155/2017/4042902
62. Loschenov VB, Konov VI, Prokhorov AM. Photodynamic therapy and fluorescence diagnostics. LAS Physics-lawr. (2000) 10:1188–207.
63. Choi C, Bennet R. Laser Dopplers to determine cutaneous blood flow. Derm Surg. (2003) 29:272–80. doi: 10.1046/j.1524-4725.2003.29042.x
64. Stratonnikov AA, Ermishova NYVE, Loshchenov VB. Diagnostics of a laser-induced response of capillary vessels in tissues. Quan Electron. (2002) 32:917–92. doi: 10.1070/QE2002v032n10ABEH002317
65. Séguier S, Souza SL, Sverzut AC, Simioni AR, Primo FL, Bodineau A, et al. Impact of photodynamic therapy on inflammatory cells during human chronic periodontitis. J Photochem photobio B: Biol. (2010) 101:348–54. doi: 10.1016/j.jphotobiol.2010.08.007
66. Stratonnikov AA, Meerovich GA, Ryabova AV, Savel'eva TA, Loshchenov VB. Application of backward diffuse reflection spectroscopy for monitoring the state of tissues in photodynamic therapy. Quant Electron. (2006) 36:1103–10. doi: 10.1070/QE2006v036n12ABEH013331
67. Chambrone L, Wang HL, Romanos GE. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol. (2018) 89:783–803.
68. Nemezio MA, de Souza Farias SS, Borsatto MC, Aires CP, Corona SAM. Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagnosis Photodyn Ther. (2017) 20:234–7. doi: 10.1016/j.pdpdt.2017.10.025
69. Phutim-Mangkhalthon A, Teerakapong A, Tippayawat P, Morales NP, Morkmued S, Puasiri S, et al. Anti-inflammatory effect of photodynamic therapy using guaiazulene and red lasers on peripheral blood mononuclear cells. Photodiagnosis Photodyn Ther. (2020) 31:101747. doi: 10.1016/j.pdpdt.2020.101747
70. Shivakumar V, Shanmugam M, Sudhir G, Priyadarshoni SP. Scope of photodynamic therapy in periodontics and other fields of dentistry. J Interdiscip Dentist. (2012) 2:78. doi: 10.4103/2229-5194.100598
71. Alves LVGL, Curylofo-Zotti FA, Borsatto MC, Salvador SLS, Valério RA, Souza-Gabriel AE, et al. Influence of antimicrobial photodynamic therapy in carious lesion. Randomized split-mouth clinical trial in primary molars. Photodiag Photodyn Ther. (2019) 26:124–30. doi: 10.1016/j.pdpdt.2019.02.018
Keywords: optical spectral diagnostics, photodynamic therapy, phototheranostic, periodontal disease, gingivitis, children with cerebral palsy, methylene blue
Citation: Morozova NS, Kozlitina IA, Makarov VI, Loschenov VB, Grinin VM, Ivanov SY and Kashtanova MS (2023) Optical spectral diagnostics of the oxygenation level in periodontal tissues and photodynamic therapy using methylene blue in children with cerebral palsy. Front. Public Health 11:961066. doi: 10.3389/fpubh.2023.961066
Received: 21 October 2022; Accepted: 13 January 2023;
Published: 30 January 2023.
Edited by:
Guliz N. Guncu, Hacettepe University, TürkiyeReviewed by:
Ali Cekici, Istanbul University, TürkiyeDeepti Shrivastava, Al Jouf University, Saudi Arabia
Copyright © 2023 Morozova, Kozlitina, Makarov, Loschenov, Grinin, Ivanov and Kashtanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalia S. Morozova,  bmF0YWxpYXMubW9yb3pvdmFAeWFuZGV4LmNvbQ==;
bmF0YWxpYXMubW9yb3pvdmFAeWFuZGV4LmNvbQ==;  S25zNzRAYmsucnU=
S25zNzRAYmsucnU=
†ORCID: Natalia S. Morozova orcid.org/0000-0002-6453-1615
Iuliia A. Kozlitina orcid.org/0000-0002-4964-7441
Vladimir I. Makarov orcid.org/0000-0001-9622-1550
Victor B. Loschenov orcid.org/0000-0002-0507-2367
Vasiliy M. Grinin orcid.org/0000-0002-2280-8559
Sergey Yu. Ivanov orcid.org/0000-0001-5458-0192
Maria S. Kashtanova orcid.org/0000-0002-5478-0837
 Natalia S. Morozova
Natalia S. Morozova Iuliia A. Kozlitina
Iuliia A. Kozlitina Vladimir I. Makarov
Vladimir I. Makarov Victor B. Loschenov3,4†
Victor B. Loschenov3,4†