- Department of Blood Transfusion, Ningbo Medical Center Lihuili Hospital, Ningbo University, Ningbo, China
Background: Coronavirus disease (COVID-19) has posed a significant threat to the lives and health of people worldwide since its onset in 2019. However, the relationship between the number of vaccination shots and the severity of SARS-CoV-2 infection in Chinese patients remains unclear.
Methods: We retrospectively collected information from 829 patients infected with SARS-CoV-2 in Ningbo Medical Center Lihuili Hospital from December 05, 2022 to March 31, 2023, then divided them into four groups based on the severity of pneumonia. Last, we compared the difference in the number of shots of COVID-19 vaccine between the four groups, considering potential confounding factors using univariate and multivariate logistic regression.
Results: Vaccination with two and three doses was positively associated with low prevalence of pneumonia and severe pneumonia both in crude and optimal models, while only three doses of the vaccine was correlated with low prevalence of death in SARS-CoV-2-infected patients. In optimal models, male SARS-CoV-2-infected individuals with advanced age were positively associated with high prevalence of pneumonia, severe pneumonia, and death; comorbidity with hypertension (OR = 2.532, p < 0.001) was positively associated with high prevalence of pneumonia (OR = 2.532, p < 0.001); and comorbidity with diabetes was positively associated with high prevalence of death (OR = 1.856, p = 0.011). However, this is a cross-sectional study and the causal relationships need to be further studied.
Conclusion: One dose of vaccine may not have a protective effect against pneumonia, severe pneumonia, and death; more than one dose of vaccine is an independent protective factor for pneumonia and severe pneumonia; and three doses of vaccine is an independent protective factor for death.
Introduction
Coronavirus disease (COVID-19) was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since COVID-19 swept the globe in 2019, it has presented a huge challenge to human life and health globally, greatly thwarting human economic and social development, and healthcare systems around the world are under unprecedented pressure (1). In particular, with the mutation of the SARS-CoV-2 virus, the Omicron variant became globally prevalent for a time (2), putting people at higher risk of reinfection (3), which presented yet another challenge.
Coronaviruses (CoVs) are a highly diverse family of envelope-positive single-stranded RNA viruses characterized by high rates of genetic recombination and mutation leading to their ecological diversity (4). On the basis of genome sequence and serologic response, they can be classified into alphacoronavirus, betacoronavirus, gammacoronavirus, and deltacoronavirus. SARS-CoV-2 belongs to betacoronavirus. SARS-CoV-2 infection involves multiple cell surface receptors and multiple pathways (5–7), and it is mainly transmitted through the respiratory tract, and the clinical manifestations of SARS-CoV-2 infections vary widely in clinical manifestations, ranging from asymptomatic or mild infections to pneumonia, and can also develop into life-threatening respiratory diseases in severe cases (8, 9). Currently, the treatment of COVID-19 has a corresponding approach according to different clinical syndromes (10). At the same time, many anti-COVID-19 drugs are being developed (11). Nevertheless, Long COVID occurs in at least 10% of SARS-CoV-2 infections and is an often debilitating illness (12). Therefore, the prevention of COVID-19 is still the most effective means of safeguarding the lives and health of the public against this disease.
Scientists around the world have been working on vaccine research and development for as long as SARS-CoV-2 has been recognized in humans (13). The process of developing and producing a vaccine for COVID-19 was further accelerated with the introduction of the COVID-19 Vaccines Global Access (COVAX). Thanks to this program, as of August 2023, 70.48% of the world population has received at least one dose of a COVID-19 vaccine, and China reached its first peak for the SARS-CoV-2 vaccine in June 2020 (14). Nonetheless, there is still a portion of the population that is not up-to-date or not fully vaccinated for various reasons, such as uneven distribution of vaccine resources, vaccine reactions, and a lack of knowledge about vaccines (15–17). However, strict adherence to the vaccination regimen is essential for the vaccine to produce optimal protection (18). Booster immunization involves the administration of an additional dose of vaccine after the initial vaccination to induce immune memory and improve protection against the corresponding viral infection. Several studies have also emphasized the importance of booster shots (19–22). Nowadays, although the pandemic trend of COVID-19 has passed, the number of new infections still fluctuates within a certain range globally (14), so we still cannot completely let down our guard.
The importance of the number of COVID-19 vaccinations, especially complete vaccination courses, has been reported in a few studies (8, 23–25). There have also been several studies conducted in the Chinese population. A study based in Yunan, China, reported that the vaccine reduced the risk of pneumonia and severe pneumonia after SARS-CoV-2 infection (26). A study based in the Hong Kong Special Administrative Region, China, reported that the COVID-19 vaccine reduced the risk of death (27). However, there is a lack of broader population-based studies on the number of shots of COVID-19 vaccine and the risk of pneumonia, severe pneumonia, and death in patients with new SARS-CoV-2 virus infections.
Method
Study design and population
In this study, we retrospectively collected data from December 05, 2022 to March 31, 2023. A total of 837 COVID-19 cases were admitted to Ningbo Medical Center Lihuili Hospital during the period, totaling 829 cases after the exclusion of 8 cases of underage patients, including 456 male and 373 female patients. Patients attending COVID-19 were categorized into non-pneumonia versus pneumonia and non-severe pneumonia versus severe pneumonia groups according to clinical diagnosis. Patients were also categorized into non-death and death groups. This study was approved by the ethics committee of Ningbo Medical Center Lihuili Hospital (Approval No: Li Huili Hospital Ethics Review 2023 Research No. 082).
Data collection and definitions of covariates
The diagnosis of COVID-19 and basic patient information, including gender, age, BMI, diabetes mellitus, hypertension, and malignant tumors, were collected from electronic medical records (EMR) (KINGT Software, Ningbo Jintang Software Co., Ltd., China). Vaccination information, including the number of vaccination shots and types, was collected from the SaaS Yunjinmiao vaccination system of Zhejiang Province (Shensu Science & Technology (Suzhou) Co., Ltd., China). Age and BMI were categorized according to the median, which was 71 for age and 23.07 for BMI.
Measurement of outcomes
The diagnosis of COVID-19 and the definition of the severity of pneumonia were based on examination by doctors according to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 9) (28).
Statistical analysis
Statistical analyses were performed with SPSS 22.0 and R.4.2.0. Continuous variables are described as mean and standard deviation and categorical variables are described as frequency and percentage. First, univariate logistic regression was performed to calculate the odds ratio (OR) of the number of vaccination shots, and the differences between the groups categorized by pneumonia, severe pneumonia, and death after SARS-CoV-2 infection were compared. Participants without pneumonia were treated as the reference group. The indicators with statistical significance (p < 0.05) in univariate logistic regression were adopted as the potential confounding factors in the multivariate logistic regression model to further examine the association between the number of vaccination shots and the severity of pneumonia after SARS-CoV-2 Infection. Last, we removed the variables with p > 0.05 in multivariate logistic models and re-conducted the multivariate logistic regression to fit the optimal model. All reported probabilities (p-values) were two-sided, with p < 0.05 considered statistically significant.
Result
Basic characters of participants
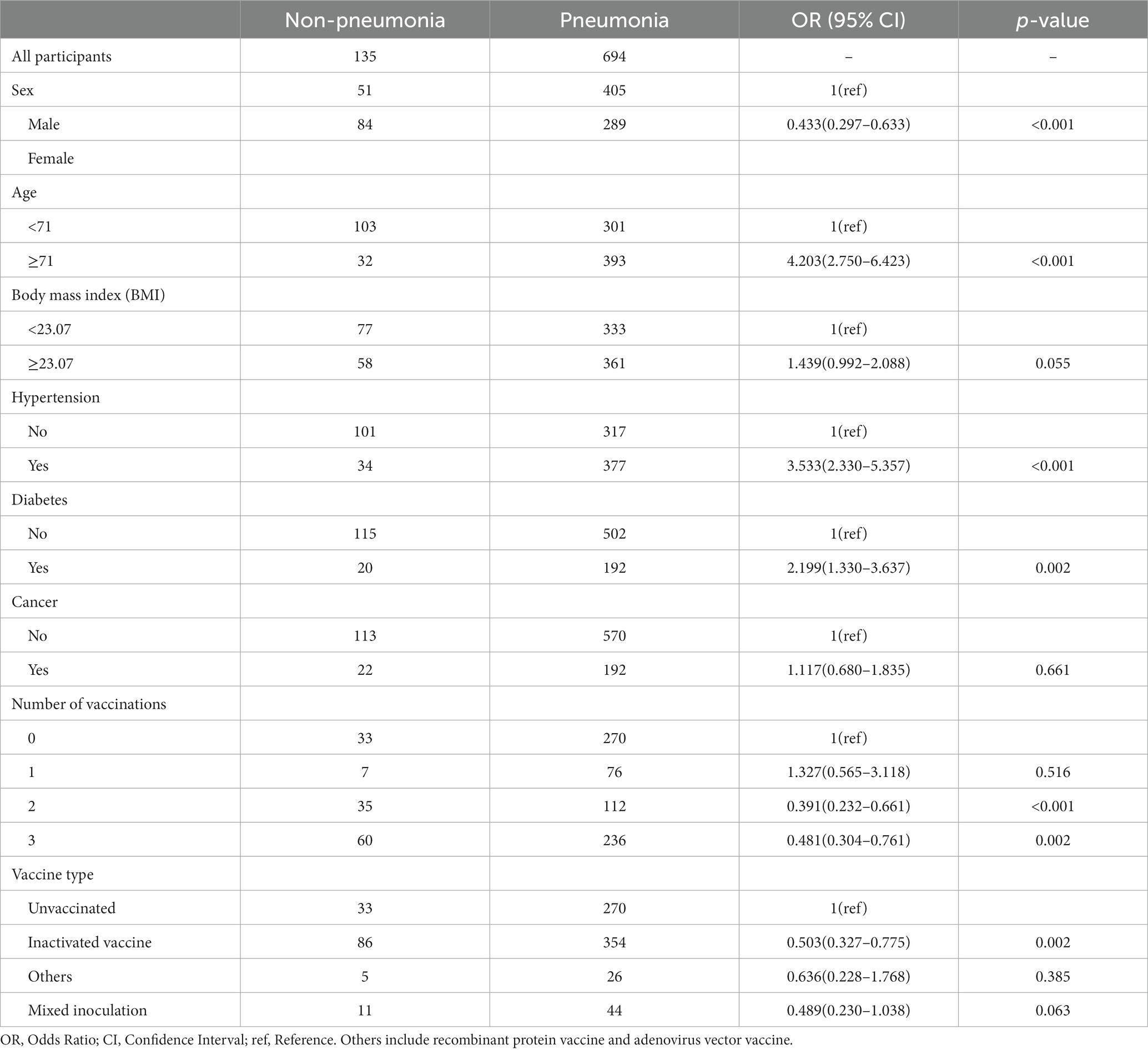
The general characteristics of the study population are summarized in Table 1. We finally included 829 participants in the analysis, of which 456(55.00%) were male and 373(45.00%) were female. Of these, 404 participants were aged greater than or equal to 71 years, representing 51.27% of the study population. BMI greater than or equal to 23.07 was 410(49.46%), 411(49.58%) were hypertensive, 212(25.57%) were diabetic, and 146(17.61%) had malignancy. The number of people vaccinated with 0 to 3 COVID-19 vaccine doses were 303 (36.55%), 83 (10.01%), 147 (17.73%), and 296 (35.71%), respectively. As for the type of vaccine, the number of people vaccinated with inactivated vaccine for all injections was 440, which is 53.08% of the total number of people in the study and 83.65% of the number of people vaccinated. The number of people vaccinated with adenovirus vector vaccine or recombinant protein vaccine for all injections and the number of people vaccinated with a mixture of vaccines of different attributes were 31 and 55, respectively, accounting for 3.74% and 6.63% of the total number of people in the study, respectively.
Relationship between number of COVID-19 vaccination shots and pneumonia
We observed that two and three vaccination shots were positively correlated with low prevalence of pneumonia in SARS-CoV-2-infected patients in a univariable logistic model (Two shots: OR = 0.391, p < 0.001; Three shots: OR = 0.481, p = 0.002), while one shot of vaccination was not significantly associated with pneumonia (Table 2). In addition, other factors, namely, gender, age, and comorbidity, showed significant differences between the non-pneumonia and pneumonia groups (Table 2). After performing multivariable logistic regression and retaining nominally significant variables, two and three vaccination shots remained significant, and their OR were 0.445 and 0.558 in the optimal model (Two shots: p = 0.004; Three shots: p = 0.017) (Figure 1). Moreover, male patients (OR = 1.343, p < 0.001), patients with age ≥ 71 (OR = 2.850, p < 0.001) or those that were comorbid with hypertension (OR = 2.532, p < 0.001) were positively correlated with high prevalence of pneumonia.
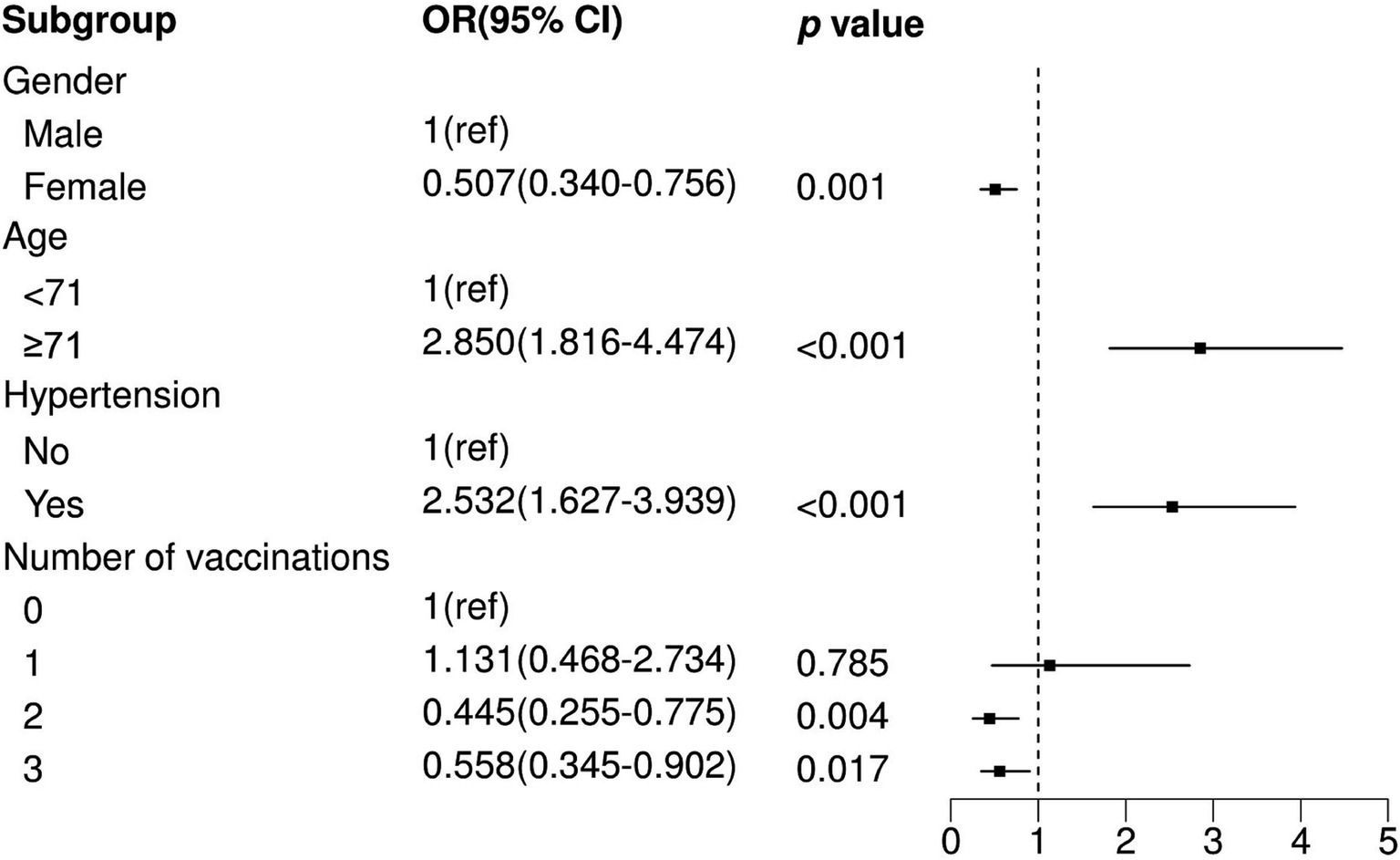
Figure 1. Multivariate logistic regression analysis between non-pneumonia and pneumonia. OR, Odds Ratio; CI, Confidence Interval; ref., Reference.
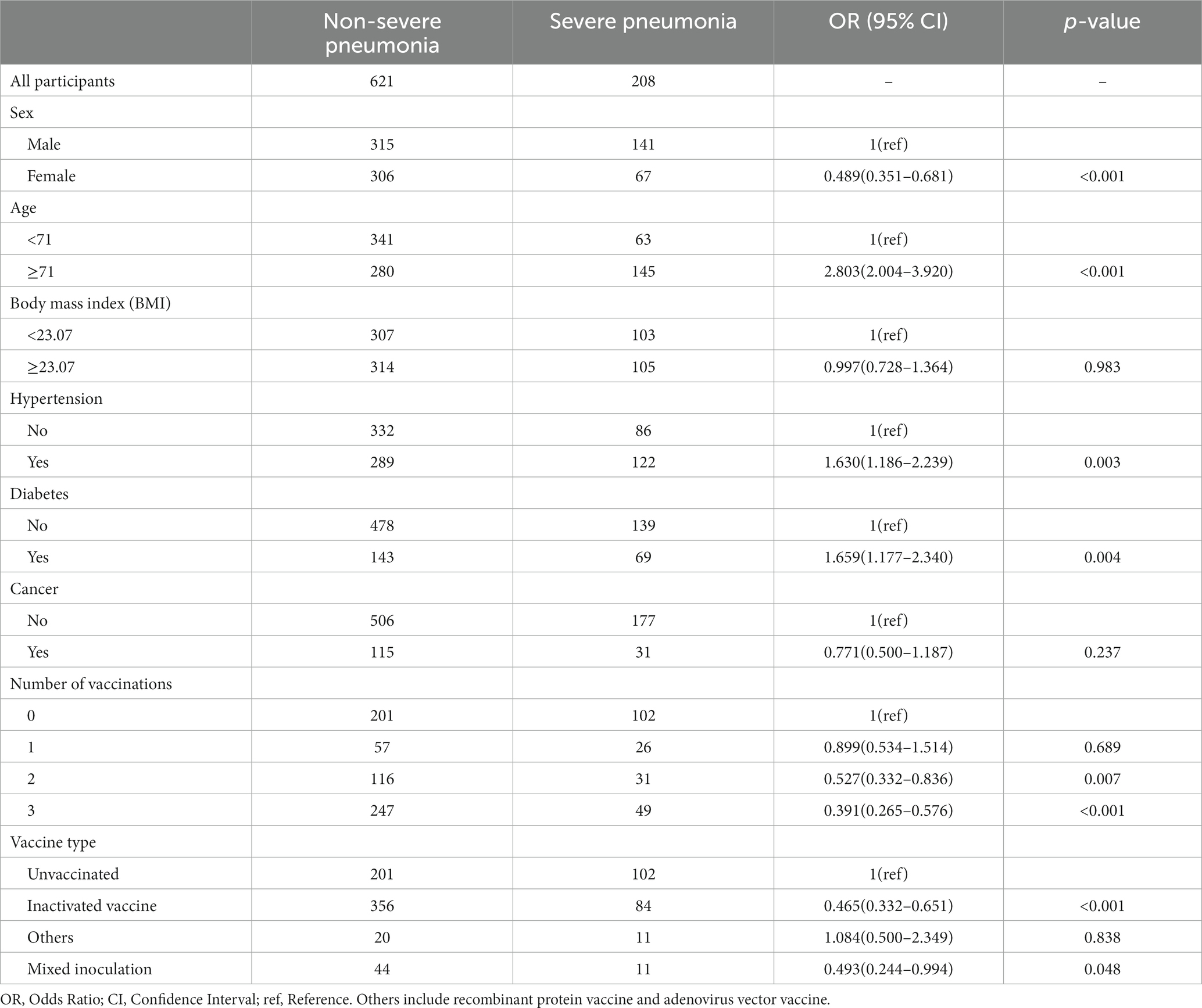
Relationship between number of vaccination shots and severe pneumonia
We observed that two and three vaccination shots were positively correlated with low prevalence of severe pneumonia in SARS-CoV-2-infected patients (Two shots: OR = 0.527, p = 0.007; Three shots: OR = 0.391, p < 0.001) in univariable logistic analysis (Table 3). In addition, other factors, namely, gender, age, hypertension, and diabetes, showed significant differences between the severe pneumonia and pneumonia groups (Table 3). After performing multivariable logistic regression and retaining nominally significant variables, two and three vaccination shots were still significant in the optimal model (Figure 2; Two shots: OR = 0.554, p = 0.015; Three shots: OR = 0.408, p < 0.001). Moreover, male patients (OR = 1.332, p < 0.001), patients with age ≥ 71 (OR = 2.550, p < 0.001) were also positively correlated with high prevalence of severe pneumonia.
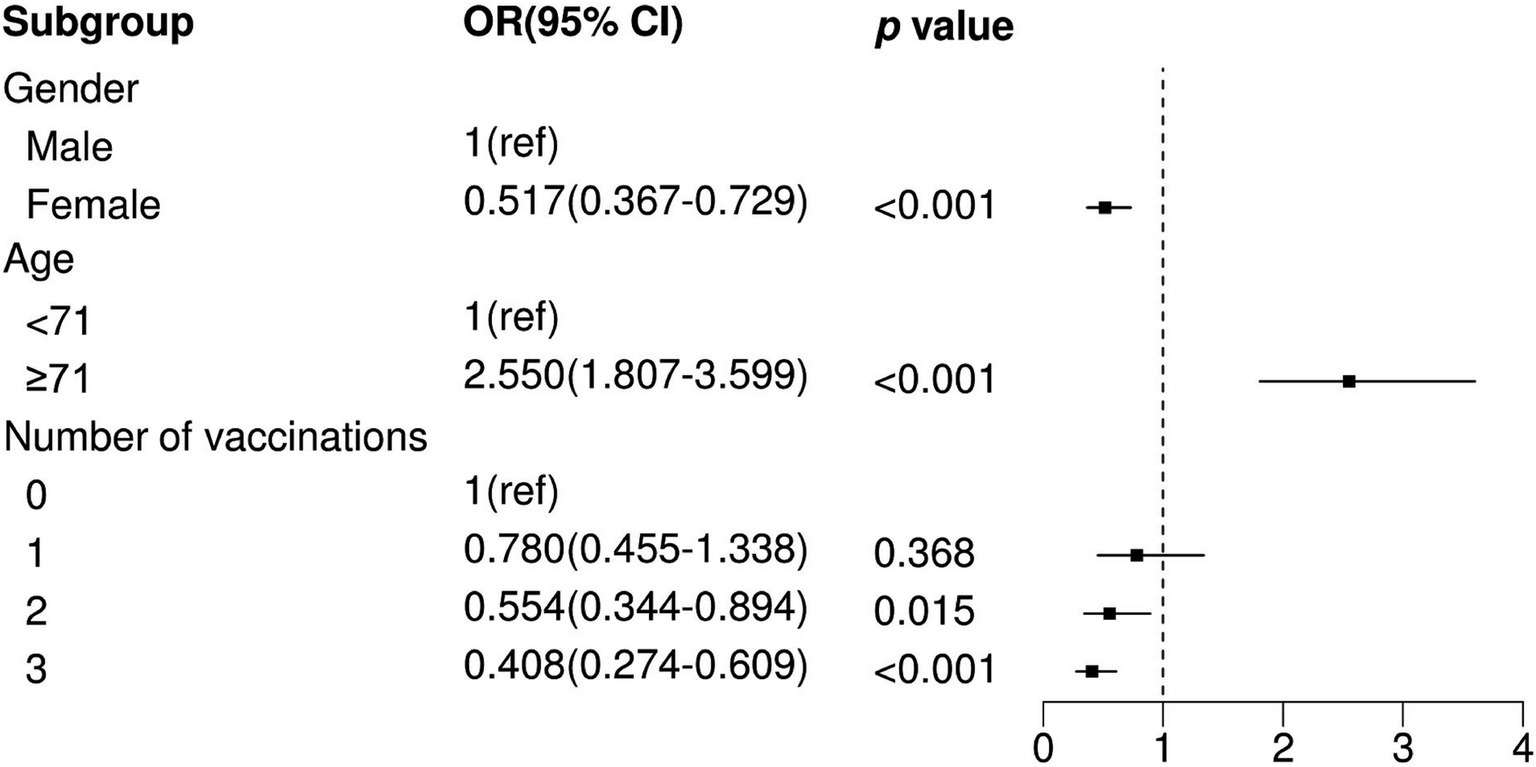
Figure 2. Multivariate logistic regression analysis between non-severe pneumonia and pneumonia. OR, Odds Ratio; CI, Confidence Interval; ref., Reference.
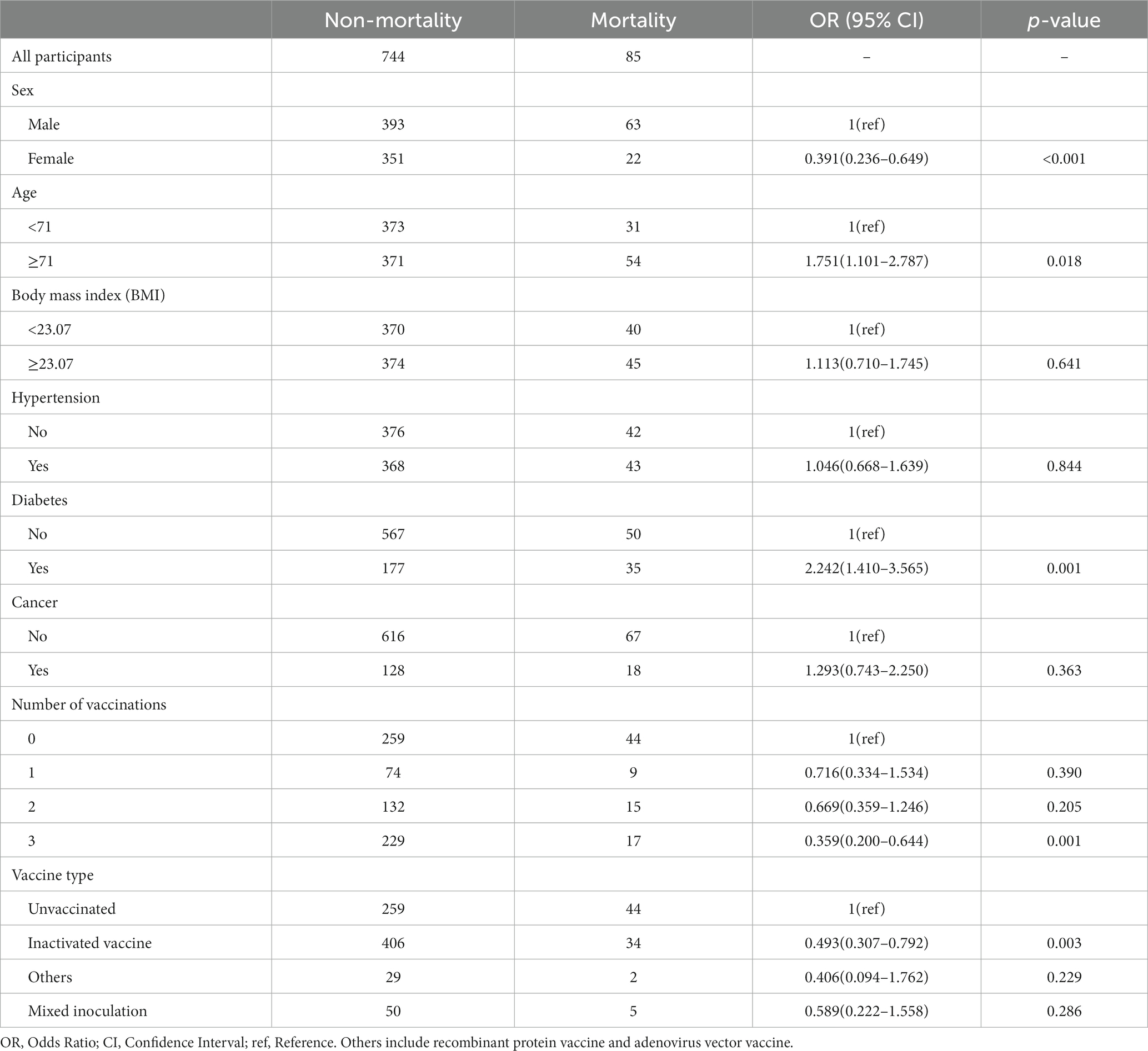
Relationship between death and number of vaccination shots
To further explore the relationship between the number of vaccination shots and death in SARS-CoV-2-infected patients, we adopted the same analytical approach as previously mentioned. The results showed that only three vaccination shots were positively correlated with low prevalence of death in SARS-CoV-2-infected patients (Three shots: OR = 0.359, p = 0.001) in a univariable logistic model (Table 4) and were still significant in the optimal model (Figure 3; Three shots: OR = 0.393, p = 0.002). Moreover, individuals who were comorbid with diabetes (OR = 1.856, p = 0.011) and male (OR = 1.468, p = 0.001) were also positively correlated with high prevalence of death in SARS-CoV-2-infected patients.
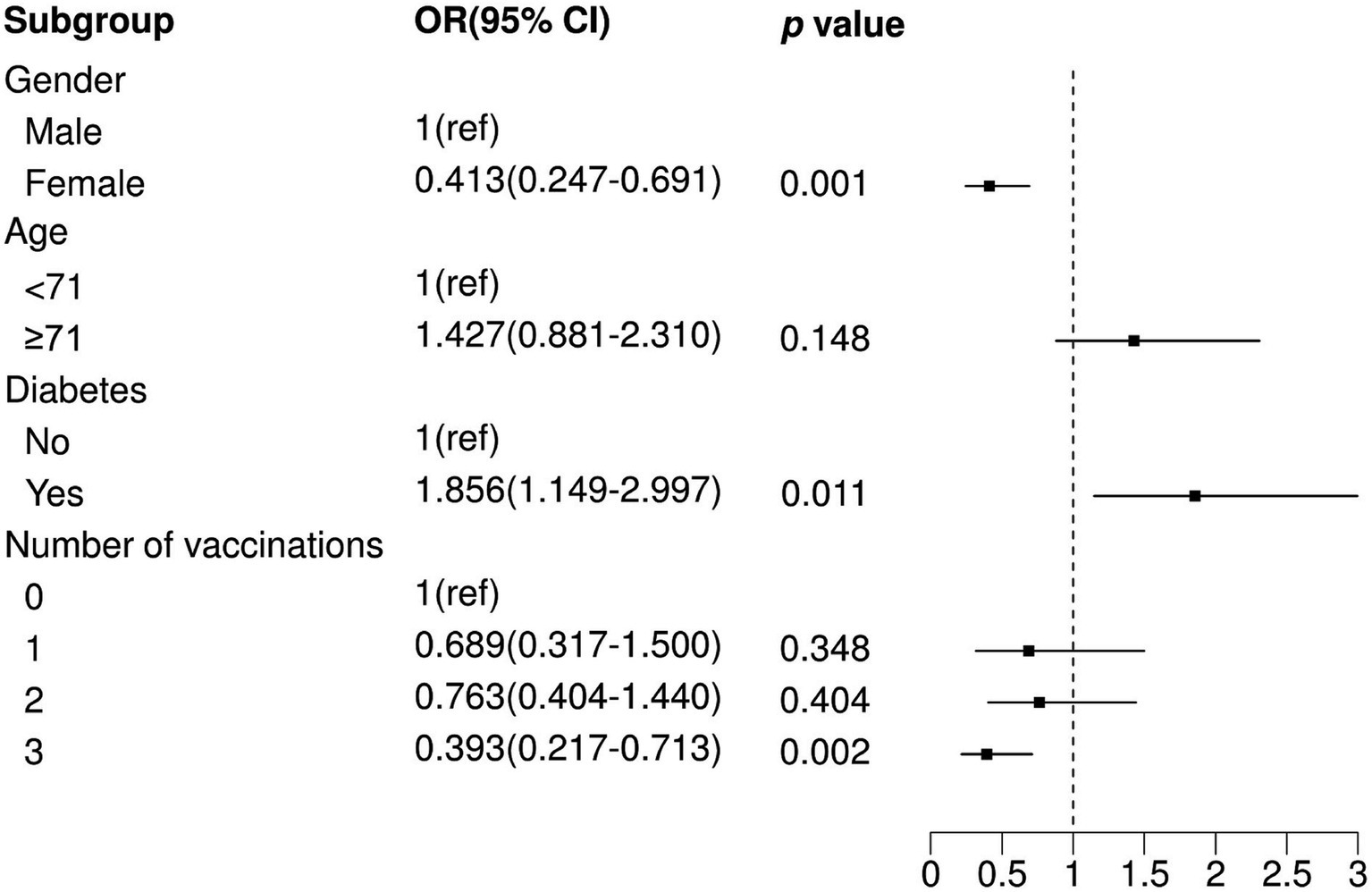
Figure 3. Multivariate logistic regression analysis between non-mortality and mortality. OR, Odds Ratio; CI, Confidence Interval; ref., Reference.
Discussion
In this study, we retrospectively collected information about SARS-CoV-2-infected patients to investigate the relationships between the number of vaccination shots and the severity of pneumonia. Our main finding suggested that two or three vaccination shots may decrease the risk of pneumonia and severe pneumonia in SARS-CoV-2-infected patients, while only three vaccination shots may reduce the risk of death after COVID-19 infection, which highlights the importance of multiple vaccinations. Our findings also illustrate that one dose of COVID-19 vaccine is not significantly protective in SARS-CoV-2-infected patients, whereas more than one dose of vaccine may be effective in reducing the incidence of pneumonia and severe pneumonia in SARS-CoV-2-infected patients. However, three doses are necessary for the vaccine to be protective against death. This may be closely linked to the production of sufficient amounts of protective antibodies (29).
There are three main types of COVID-19 vaccines in China. The first is inactivated vaccines, including three inactivated vaccines produced by Sinopharm Zhongsheng (Beijing), Sinopharm Zhongsheng (Wuhan), and Beijing Kexing Zhongwei (Beijing); these are the vaccines administered to the majority of the population in China. The second is adenovirus vector vaccine type 5, produced by Tianjin Kangxinuo Company. Third is the recombinant protein vaccine (mRNA), a recombinant new coronavirus vaccine (CHO cells) produced by Anhui Zhifei. Three injections are required for the inactivated vaccine and the recombinant protein vaccine, and the adenovirus vector vaccine requires one injection. The different types of vaccines mentioned here did not differ in effect in our study. It can be seen that the production process of the vaccine does not make a difference in the level of protection of SARS-CoV-2-infected patients.
Our study found that men are more likely than women to have adverse outcomes after SARS-CoV-2 infection, which is also consistent with previous studies (30–33). These differences may be strongly related to factors such as gender-specific behaviors and biological pathways related to sex hormones and SARS-CoV-2 infection (31, 34). Several studies have also shown that women have stronger immune responses to vaccines than men, which may be one of the reasons why women have stronger immune efficacy when infected with SARS-CoV-2 (35, 36). In addition, there are different degrees of age-related declines in immune responses in older adult patients due to the onset of immune senescence (37, 38), which greatly elevates the risk of patients developing severe COVID-19 (39), which also corroborates the conclusions we obtained.
The presence of comorbidities is considered to be an important risk factor for exacerbating adverse outcomes in SARS-CoV-2-infected patients (40), and in a study by Guangtong Deng et al., hypertension, diabetes mellitus, and cancer were all reported to be risk factors for mortality in COVID-19 patients (41). It has been reported that genetically predicted high IGF-1 levels are associated with reduced COVID-19 susceptibility and risk of hospitalization (42), and diabetic patients tend to have reduced blood levels of IGF-1, which may be an important reason why diabetes is a risk factor for death in COVID-19 patients. Interestingly, however, our study only identified hypertension and diabetes mellitus as risk factors for pneumonia and death from infection, respectively, and there was no effect of cancer on the occurrence of pneumonia, severe pneumonia, and death after SARS-CoV-2 infection. This finding is not entirely consistent with the above report, and we speculate that the possible reason for this is that, in addition to the small number of cases included in the analysis compared to other studies, 83 of the 146 SARS-CoV-2-infected cases with cancer were also vaccinated with COVID-19 vaccine, and the vaccine still has some protective effect in these patients. This conjecture is supported by the fact that the majority of cancer patients found in the Luigi Cavanna et al. meta-analysis had an immunogenic response to COVID-19 vaccination (43).
Nowadays, although we have safely passed the most menacing stage of COVID-19, the protective effect of the SARS-CoV-2 vaccine is weakening over time (44), and there are still many individuals experiencing multiple infections due to SARS-CoV-2. In our study, as well as in previous studies, older adults with the presence of underlying diseases remain the most prominent victims of COVID-19 (40, 45, 46). Therefore, now, half a year after the peak of COVID-19, we still call on the public to strengthen awareness of prevention and to receive timely vaccination or catch-up vaccination when the situation permits in order to minimize the damage to health caused by COVID-19.
However, our study did have some limitations. First, as this is a cross-sectional study, the causal effect of the number of vaccination shots on the severity of pneumonia in SARS-CoV-2-infected patients could not be inferred. Second, although some potential confounding factors were considered in our study, other unmeasured confounders could affect the relationship between vaccination shots and the outcomes. Last, our study is not a multicenter study, which may limit the representativeness of the population.
Conclusion
Our findings show that one dose of vaccine may not have a protective effect against pneumonia, while two and three vaccination shots were an independent protective factor for pneumonia and severe pneumonia, and three doses of vaccine was an independent protective factor for death.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Ningbo Medical Center Lihuili Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SX: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. WC: Investigation, Writing – review & editing. QY: Investigation, Writing – review & editing. LG: Investigation, Writing – review & editing. GL: Data curation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El-Shabasy, RM, Nayel, MA, Taher, MM, Abdelmonem, R, Shoueir, KR, and Kenawy, ER. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol. (2022) 204:161–8. doi: 10.1016/j.ijbiomac.2022.01.118
2. Araf, Y, Akter, F, Tang, YD, Fatemi, R, Parvez, MSA, Zheng, C, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. (2022) 94:1825–32. doi: 10.1002/jmv.27588
3. Xia, S, Wang, L, Zhu, Y, Lu, L, and Jiang, S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 omicron sublineages. Signal Transduct Target Ther. (2022) 7:241. doi: 10.1038/s41392-022-01105-9
4. Cui, J, Li, F, and Shi, ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. (2019) 17:181–92. doi: 10.1038/s41579-018-0118-9
5. V'Kovski, P, Kratzel, A, Steiner, S, Stalder, H, and Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. (2021) 19:155–70. doi: 10.1038/s41579-020-00468-6
6. Jackson, CB, Farzan, M, Chen, B, and Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. (2022) 23:3–20. doi: 10.1038/s41580-021-00418-x
7. Malone, B, Urakova, N, Snijder, EJ, and Campbell, EA. Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat Rev Mol Cell Biol. (2022) 23:21–39. doi: 10.1038/s41580-021-00432-z
8. Zhang, J, Chen, N, Zhao, D, Zhang, J, Hu, Z, and Tao, Z. Clinical characteristics of COVID-19 patients infected by the omicron variant of SARS-CoV-2. Front Med (Lausanne). (2022) 9:912367. doi: 10.3389/fmed.2022.912367
9. He, X, Cheng, X, Feng, X, Wan, H, Chen, S, and Xiong, M. Clinical symptom differences between mild and severe COVID-19 patients in China: a meta-analysis. Front Public Health. (2020) 8:561264. doi: 10.3389/fpubh.2020.561264
10. Peng, Y, Tao, H, Satyanarayanan, SK, Jin, K, and Su, H. A comprehensive summary of the knowledge on COVID-19 treatment. Aging Dis. (2021) 12:155–91. doi: 10.14336/ad.2020.1124
11. Ji, X, Meng, X, Zhu, X, He, Q, and Cui, Y. Research and development of Chinese anti-COVID-19 drugs. Acta Pharm Sin B. (2022) 12:4271–86. doi: 10.1016/j.apsb.2022.09.002
12. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
13. Le, TT, Cramer, JP, Chen, R, and Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. (2020) 19:667–8. doi: 10.1038/d41573-020-00151-8
14. Our World in Data. (2023). Available at: https://ourworldindata.org/.
15. Watson, OJ, Barnsley, G, Toor, J, Hogan, AB, Winskill, P, and Ghani, AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. (2022) 22:1293–302. doi: 10.1016/S1473-3099(22)00320-6
16. Zimmerman, T, Shiroma, K, Fleischmann, KR, Xie, B, Jia, C, Verma, N, et al. Misinformation and COVID-19 vaccine hesitancy. Vaccine. (2023) 41:136–44. doi: 10.1016/j.vaccine.2022.11.014
17. Chen, Y, Xu, Z, Wang, P, Li, XM, Shuai, ZW, Ye, DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. (2022) 165:386–401. doi: 10.1111/imm.13443
18. Zhao, H, Zhou, X, and Zhou, YH. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. (2020) 16:1533–44. doi: 10.1080/21645515.2020.1732166
19. Yao, J, Ren, J, Shen, L, Chen, Y, Liang, X, Cui, F, et al. The effects of booster vaccination of hepatitis B vaccine on anti-HBV surface antigen negative children 11-15 years after primary vaccination. Hum Vaccin. (2011) 7:1055–9. doi: 10.4161/hv.7.10.15990
20. Poorolajal, J, and Hooshmand, E. Booster dose vaccination for preventing hepatitis B. Cochrane Database Syst Rev. (2016) 2016:CD008256. doi: 10.1002/14651858.CD008256.pub3
21. Chenchula, S, Karunakaran, P, Sharma, S, and Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review. J Med Virol. (2022) 94:2969–76. doi: 10.1002/jmv.27697
22. Menni, C, May, A, Polidori, L, Louca, P, Wolf, J, Capdevila, J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID study. Lancet Infect Dis. (2022) 22:1002–10. doi: 10.1016/S1473-3099(22)00146-3
23. Ali, AM, Tofiq, AM, Rostam, HM, Ali, KM, and Tawfeeq, HM. Disease severity and efficacy of homologous vaccination among patients infected with SARS-CoV-2 Delta or omicron VOCs, compared to unvaccinated using main biomarkers. J Med Virol. (2022) 94:5867–76. doi: 10.1002/jmv.28098
24. Accorsi, EK, Britton, A, Fleming-Dutra, KE, Smith, ZR, Shang, N, Derado, G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and Delta variants. JAMA. (2022) 327:639–51. doi: 10.1001/jama.2022.0470
25. Kulper-Schiek, W, Piechotta, V, Pilic, A, Batke, M, Dreveton, LS, Geurts, B, et al. Facing the omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review. Front Immunol. (2022) 13:940562. doi: 10.3389/fimmu.2022.940562
26. Ma, C, Sun, W, Tang, T, Jia, M, Liu, Y, Wan, Y, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: evidence from an outbreak in Yunnan, China, 2021. Vaccine. (2022) 40:2869–74. doi: 10.1016/j.vaccine.2022.03.067
27. Smith, DJ, Hakim, AJ, Leung, GM, Xu, W, Schluter, WW, Novak, RT, et al. COVID-19 mortality and vaccine coverage – Hong Kong special administrative region, China, January 6, 2022-march 21, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:545–8. doi: 10.15585/mmwr.mm7115e1
28. Commission RbNH. National Administration of traditional Chinese medicine on March 15. Diagnosis and treatment protocol for COVID-19 patients (trial version 9). Health care. Science. (2022) 1:14–28. doi: 10.1002/hcs2.1
29. Walmsley, SL, Szadkowski, L, Wouters, B, Clarke, R, Colwill, K, Rochon, P, et al. COVID-19 vaccine antibody responses in community-dwelling adults to 48 weeks post primary vaccine series. iScience. (2023) 26:106506. doi: 10.1016/j.isci.2023.106506
30. Alwani, M, Yassin, A, Al-Zoubi, RM, Aboumarzouk, OM, Nettleship, J, Kelly, D, et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol. (2021) 31:e2223. doi: 10.1002/rmv.2223
31. Haitao, T, Vermunt, JV, Abeykoon, J, Ghamrawi, R, Gunaratne, M, Jayachandran, M, et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. (2020) 95:2189–203. doi: 10.1016/j.mayocp.2020.07.024
32. Pivonello, R, Auriemma, RS, Pivonello, C, Isidori, AM, Corona, G, Colao, A, et al. Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology. (2021) 111:1066–85. doi: 10.1159/000513346
33. Li, AJ, and Li, X. Sex-dependent immune response and lethality of COVID-19. Stem Cell Res. (2020) 50:102116. doi: 10.1016/j.scr.2020.102116
34. Cai, Z, Zhong, J, Jiang, Y, and Zhang, J. Associations between COVID-19 infection and sex steroid hormones. Front Endocrinol (Lausanne). (2022) 13:940675. doi: 10.3389/fendo.2022.940675
35. Fink, AL, and Klein, SL. The evolution of greater humoral immunity in females than males: implications for vaccine efficacy. Curr Opin Physio. (2018) 6:16–20. doi: 10.1016/j.cophys.2018.03.010
36. Fischinger, S, Boudreau, CM, Butler, AL, Streeck, H, and Alter, G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. (2019) 41:239–49. doi: 10.1007/s00281-018-0726-5
37. Effros, RB. Roy Walford and the immunologic theory of aging. Immun Ageing. (2005) 2:7. doi: 10.1186/1742-4933-2-7
38. Kadambari, S, Klenerman, P, and Pollard, AJ. Why the elderly appear to be more severely affected by COVID-19: the potential role of immunosenescence and CMV. Rev Med Virol. (2020) 30:e2144. doi: 10.1002/rmv.2144
39. Tizazu, AM, Mengist, HM, and Demeke, G. Aging, inflammaging and immunosenescence as risk factors of severe COVID-19. Immun Ageing. (2022) 19:53. doi: 10.1186/s12979-022-00309-5
40. Peterfi, A, Meszaros, A, Szarvas, Z, Penzes, M, Fekete, M, Feher, A, et al. Comorbidities and increased mortality of COVID-19 among the elderly: a systematic review. Physiol Int. (2022) 109:163–76. doi: 10.1556/2060.2022.00206
41. Deng, G, Yin, M, Chen, X, and Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care. (2020) 24:179. doi: 10.1186/s13054-020-02902-w
42. Li, X, Zhou, Y, Yuan, S, Zhou, X, Wang, L, Sun, J, et al. Genetically predicted high IGF-1 levels showed protective effects on COVID-19 susceptibility and hospitalization: a Mendelian randomisation study with data from 60 studies across 25 countries. elife. (2022) 11:e79720. doi: 10.7554/eLife.79720
43. Cavanna, L, Citterio, C, and Toscani, I. COVID-19 vaccines in cancer patients. seropositivity and safety. Systematic review and meta-analysis. Vaccines (Basel). (2021) 9:1048. doi: 10.3390/vaccines9091048
44. Hall, V, Foulkes, S, Insalata, F, Kirwan, P, Saei, A, Atti, A, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. (2022) 386:1207–20. doi: 10.1056/NEJMoa2118691
45. Gado, K, Kovacs, AK, Domjan, G, Nagy, ZZ, and Bednarik, GD. COVID-19 and the elderly. Physiol Int. (2022) 109:177–85. doi: 10.1556/2060.2022.00203
Keywords: COVID-19, vaccination, pneumonia, severe pneumonia, death
Citation: Xin S, Chen W, Yu Q, Gao L and Lu G (2024) Effect of the number of coronavirus disease 2019 (COVID-19) vaccination shots on the occurrence of pneumonia, severe pneumonia, and death in SARS-CoV-2-infected patients. Front. Public Health. 11:1330106. doi: 10.3389/fpubh.2023.1330106
Edited by:
Faris Lami, University of Baghdad, IraqReviewed by:
Sayed Himatt, The Eastern Mediterranean Public Health Network (EMPHNET), JordanEduardo Vasconez, University of the Americas, Ecuador
Copyright © 2024 Xin, Chen, Yu, Gao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genjie Lu, bHVnZW5qaWVAMTYzLmNvbQ==
 Shijun Xin
Shijun Xin Genjie Lu
Genjie Lu