- 1Department of Emergency Medicine, University of Washington, Seattle, WA, United States
- 2Department of Global Health, University of Washington, Seattle, WA, United States
- 3Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, CT, United States
- 4Department of Family Medicine, University of Washington, Seattle, WA, United States
- 5Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, United States
- 6Post-COVID Rehabilitation and Recovery Clinic, University of Washington, Seattle, WA, United States
- 7Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT, United States
- 8Department of Biostatistics, University of Washington, Seattle, WA, United States
- 9Department of Health Systems and Population Health, University of Washington, Seattle, WA, United States
- 10Department of Epidemiology, Yale School of Public Health, New Haven, CT, United States
- 11Yale Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, CT, United States
- 12Department of Emergency Medicine, University of California San Francisco, San Francisco, CA, United States
- 13Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
- 14Divisions of Infectious Diseases, Department of Internal Medicine, Rush University Medical Center, Chicago, IL, United States
- 15Department of Medicine, Cook County Hospital, Chicago, IL, United States
- 16National Center for Immunizations and Respiratory Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, GA, United States
- 17Department of Emergency Medicine, Rush University Medical Center, Chicago, IL, United States
- 18UTHealth Houston McGovern Medical School Department of Emergency Medicine, Houston, TX, United States
- 19Department of Emergency Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA, United States
- 20Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 21Clinical Informatics Center, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 22Center for Connected Care, Thomas Jefferson University, Philadelphia, PA, United States
- 23Department of Emergency Medicine, Yale School of Medicine, New Haven, CT, United States
- 24Biomedical Informatics and Medical Education, University of Washington, Seattle, WA, United States
Introduction: Data on ethnic and racial differences in symptoms and health-related impacts following SARS-CoV-2 infection are limited. We aimed to estimate the ethnic and racial differences in symptoms and health-related impacts 3 and 6 months after the first SARS-CoV-2 infection.
Methods: Participants included adults with SARS-CoV-2 infection enrolled in a prospective multicenter US study between 12/11/2020 and 7/4/2022 as the primary cohort of interest, as well as a SARS-CoV-2-negative cohort to account for non-SARS-CoV-2-infection impacts, who completed enrollment and 3-month surveys (N = 3,161; 2,402 SARS-CoV-2-positive, 759 SARS-CoV-2-negative). Marginal odds ratios were estimated using GEE logistic regression for individual symptoms, health status, activity level, and missed work 3 and 6 months after COVID-19 illness, comparing each ethnicity or race to the referent group (non-Hispanic or white), adjusting for demographic factors, social determinants of health, substance use, pre-existing health conditions, SARS-CoV-2 infection status, COVID-19 vaccination status, and survey time point, with interactions between ethnicity or race and time point, ethnicity or race and SARS-CoV-2 infection status, and SARS-CoV-2 infection status and time point.
Results: Following SARS-CoV-2 infection, the majority of symptoms were similar over time between ethnic and racial groups. At 3 months, Hispanic participants were more likely than non-Hispanic participants to report fair/poor health (OR: 1.94; 95%CI: 1.36–2.78) and reduced activity (somewhat less, OR: 1.47; 95%CI: 1.06–2.02; much less, OR: 2.23; 95%CI: 1.38–3.61). At 6 months, differences by ethnicity were not present. At 3 months, Other/Multiple race participants were more likely than white participants to report fair/poor health (OR: 1.90; 95% CI: 1.25–2.88), reduced activity (somewhat less, OR: 1.72; 95%CI: 1.21–2.46; much less, OR: 2.08; 95%CI: 1.18–3.65). At 6 months, Asian participants were more likely than white participants to report fair/poor health (OR: 1.88; 95%CI: 1.13–3.12); Black participants reported more missed work (OR, 2.83; 95%CI: 1.60–5.00); and Other/Multiple race participants reported more fair/poor health (OR: 1.83; 95%CI: 1.10–3.05), reduced activity (somewhat less, OR: 1.60; 95%CI: 1.02–2.51; much less, OR: 2.49; 95%CI: 1.40–4.44), and more missed work (OR: 2.25; 95%CI: 1.27–3.98).
Discussion: Awareness of ethnic and racial differences in outcomes following SARS-CoV-2 infection may inform clinical and public health efforts to advance health equity in long-term outcomes.
Introduction
The COVID-19 pandemic accentuated health disparities. Early in the pandemic, ethnic and racial minoritized (1, 2) populations were reported to be at greater risk of SARS-CoV-2 infection in association with their overrepresentation in the essential workforce (3, 4), fewer opportunities to work from home (4–7), and less ability to practice social distancing (4, 5, 7, 8). Ethnic or racial minoritized individuals with acute SARS-CoV-2 infection had greater barriers to care, including underinsurance (9), lack of primary care (10), and greater economic consequences from missed work (11, 12). Disparities in health outcomes following acute SARS-CoV-2 infection included higher rates of hospitalization and SARS-CoV-2-related mortality among Black and Hispanic populations (13–20). Converging systems of oppression for ethnic and racial minoritized populations magnified health disparities (21, 22).
Disparities in recovery after SARS-CoV-2 infection remain largely under-explored (23–25). Limitations in the few studies that have reported on ethnic and racial differences in recovery from SARS-CoV-2 include heterogeneity in follow-up duration and definition of post-COVID conditions (26–28), inconsistency of findings (29–32), limited focus on symptom presence (as opposed to impact) (29–31), and lack of adjustment for potential confounding by social health determinants (29, 30). We sought to evaluate symptoms and health-related impacts following SARS-CoV-2 infection by ethnicity and race to inform effective and equitable health interventions.
Methods
Study design and participant recruitment
This is a secondary analysis of data from the Innovative Support for Patients with SARS-CoV-2 Infections Registry (INSPIRE), a multicenter, longitudinal cohort study of the sequelae of SARS-CoV-2 in the United States. Adult participants were followed prospectively with patient-reported outcomes collected every 3 months via survey and linked to digital health data. Additional methods of the parent study were described previously (33). In this study, we focus on reporting results among SARS-CoV-2-positive participants to address our primary objective, namely to assess for differences in symptoms and health-related impacts by ethnicity and race following a first SARS-CoV-2 infection. The SARS-CoV-2-negative cohort was included in the analysis to account for non-SARS-CoV-2 impacts.
Study sample
Participants with self-reported symptoms suggestive of acute SARS-CoV-2 infection who tested SARS-CoV-2-positive or SARS-CoV-2-negative within the past 42 days were eligible for enrollment in INSPIRE. Participants who completed enrollment and the 3-month post-enrollment surveys were included in this analysis; among those included in the analysis, 6-month post-enrollment survey data were included as available. Participants with a prior SARS-CoV-2-positive test (>42 days before enrollment) were excluded.
Exposures
Ethnicity and race were self-reported at enrollment. Ethnicity was reported as Hispanic or non-Hispanic. Race was reported as American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, white, and/or ‘some other race’. This approach was in accordance with the U.S. Office of Management and Budget (OMB) standards (34). Ethnicity and race data were collected separately, first asking participants to indicate whether they were of Hispanic, Latin, or Spanish origin and then asking them to select their race category to permit the most granular presentation of findings and because this is the preferred method for data collection (35). For the analysis, due to the small sample size for some subcategories, race data were collapsed into four categories: Asian, Black, Other/Multiple (participants who identified as American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, ‘some other race’, and who selected two or more races), and white.
Other variables
Information on demographics, social determinants of health, and substance use was collected at enrollment. Social determinants of health were assessed using a validated tool and included health insurance status, housing insecurity, food insecurity, utility access, transportation access, and employment (36). Substance use in the past 12 months was assessed for tobacco, alcohol, prescription drugs, marijuana, and illicit drugs. Pre-existing health conditions were collected in the 3-month survey and included asthma, hypertension, diabetes, obesity, emphysema or chronic obstructive pulmonary disease, heart conditions, smoking/tobacco consumption, and kidney and liver disease. Acute SARS-CoV-2 infection status was reported at enrollment and confirmed using the electronic health record (EHR) and/or test result image. In the 3- and 6-month surveys, participants were asked about new SARS-CoV-2 infections. Those who were SARS-CoV-2-negative at enrollment and who subsequently reported testing SARS-CoV-2-positive in their 3- or 6-month survey were censored at that time. COVID-19 vaccination status (receiving any COVID-19 vaccine) was assessed through self-report and EHR data.
Outcomes
The CDC’s Person Under Investigation for SARS-CoV-2 symptom list was used to assess 21 COVID-19-like symptoms and/or “other symptoms” at enrollment, 3 months, and 6 months (37). Health status (5-point scale, excellent to poor), activity level compared to before SARS-CoV-2-like symptoms (same, somewhat less, and much less), and missed work due to health reasons in the past 3 months (0–5 workdays, 6–10 workdays, 10–20 workdays, up to 4 weeks, and do not work; overlap of intervals reflects the answer options presented in the survey) were assessed at 3 and 6 months.
Statistical methods
We described socio-demographic and clinical characteristics across ethnicity and race groups and displayed frequency counts by initial SARS-CoV-2 test results. Outcomes were described at enrollment, 3 months, and 6 months after SARS-CoV-2 infection for each ethnic and racial group.
We used generalized estimating equations (GEE) logistic regression to model the association between ethnicity and race (separately) and study outcomes (symptoms, health status, activity level, and missed work) at 3 and 6 months. We leveraged data from the full dataset (including those with and without acute SARS-CoV-2) to fit GEE models with robust standard errors. Independent variables include (1) demographic characteristics (age, gender, education, and family income), social determinants of health, tobacco use, substance use, pre-existing health conditions, and COVID-19 vaccination status (vaccinated or not vaccinated before the index SARS-CoV-2 test); and (2) interactions between ethnicity or race and time point, ethnicity or race and SARS-CoV-2 infection status, and SARS-CoV-2 infection status and time point. Time point was modeled as a categorical variable to account for a non-linear trajectory. Social determinants of health were considered a binary variable coded as “any problem” (vs. no problem) if housing, food security, access to utilities, or access to transportation were unstable (Supplementary Material 1). Substance use was included as present if there was a ‘moderate to severe problem’ with at least one substance. Individual comorbidities were included as indicator variables. Where appropriate, variable subgroups were collapsed to create larger subgroups.
Using the GEE models, we calculated marginal odds ratios, for brevity referred to as odds ratios hereafter, of individual symptoms (yes, no) by comparing each ethnicity and racial group to the corresponding referent group (non-Hispanic for ethnicity and white for race). For the outcome ‘health status’, we estimated the odds ratio of being in ‘very good’ or ‘excellent’ health and being in ‘fair’ or ‘poor’ health compared to ‘good’ health. For activity level, the odds ratio of being ‘somewhat less’ and being ‘much less’ able to do activities was estimated compared to being the ‘same as before’. For missed work, the odds ratio of missing >5 workdays in the prior 3 months due to health reasons was compared to ‘0 to 5 days’.
We did not adjust for multiple comparisons, given the exploratory nature of this study. All tests were two-sided with an alpha criterion of 0.05. Statistical analyses were performed using the GENMOD procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). Additional information about our GEE methods is available (Supplementary Material 2).
Human subjects approval
This study was approved by the Institutional Review Boards of all eight study sites (33).
Results
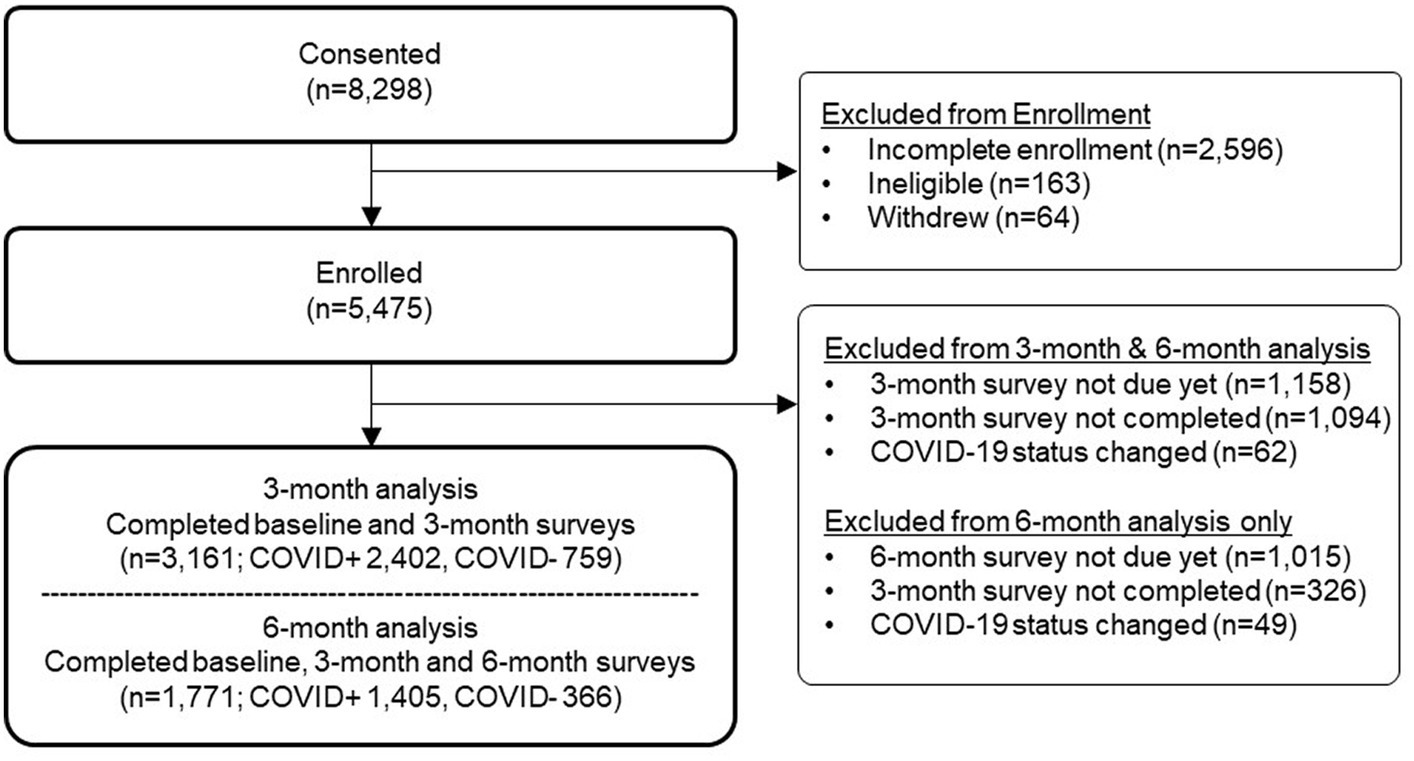
The participant flow diagram is shown in Figure 1. Three-month data were available for all participants (N = 3,161), and 6-month data were available for 1,771 participants. Of the 3,161 total participants, ethnicity was reported by 3,155 participants, and race was reported by 3,133 participants. There were differences by ethnicity and race in the proportion of participants completing the follow-up surveys, with lower completion among Hispanic compared to non-Hispanic participants and among Black participants compared to other race groups (Supplementary Material 3).
Participant characteristics
Among 2,354 SARS-CoV-2-positive participants with ethnicity data, 330 (14.0%) were Hispanic and 2,024 (86.0%) were non-Hispanic. Among 801 SARS-CoV-2-negative participants with ethnicity data, 132 (16.5%) were Hispanic and 669 (83.5%) were non-Hispanic. Assessing both SARS-CoV-2-positive and SARS-CoV-2-negative participants, compared with non-Hispanic participants, Hispanic participants were younger, less educated, more likely never married, and had lower family income (Table 1). Hispanic participants were more likely to lack health insurance, experience housing and/or food insecurity, have limited access to utilities and transportation, and be non-health essential workers working outside the home throughout the pandemic. Hispanic participants were more likely to report prescription abuse and pre-existing obesity and less likely to report hypertension and having no pre-existing health conditions. The proportion of SARS-CoV-2-positive participants was similar among Hispanic and non-Hispanic participants (75.2% vs. 71.4%, respectively, value of p = 0.089). Hispanic participants were less likely to be vaccinated against COVID-19, and the two groups utilized different COVID-19 testing sites (Table 1).

Table 1. Characteristics by ethnicity of adult INSPIRE participants stratified by SARS-CoV-2 infection status (N = 3,155).
Among 2,341 SARS-CoV-2-positive participants with race data, 258 (11.0%) were Asian, 186 (7.9%) were Black, 232 (9.9%) were Other/Multiple races, and 1,665 (71.1%) were white (Table 2). Among 792 SARS-CoV-2-negative participants with race data, 117 (14.8%) were Asian, 104 (13.1%) were Black, 64 (8.1%) were Other/Multiple races, and 507 (64.0%) were white. Assessing both SARS-CoV-2-positive and SARS-CoV-2-negative participants, Black participants had the highest prevalence of low family income, lack of health insurance, housing insecurity, and food insecurity, limited utility access, and limited transportation access, followed by Other/Multiple race participants in most instances. Black participants also had the highest prevalence of pre-existing health conditions. Employment in essential services was highest among Black and Other/Multiple race participants, and healthcare setting employment was highest among Asian and Other/Multiple race participants. Black participants were most likely to be tested for COVID-19 in a hospital setting, and COVID-19 vaccination was lowest among Black and Other/Multiple race participants.

Table 2. Characteristics by race of adult INSPIRE participants stratified by SARS-CoV-2 status (N = 3,133).
Symptoms, health status, activity level, and work among SARS-CoV-2-positive participants
At each time point, the reported prevalence of symptoms, health status, activity level, and missed work among SARS-CoV-2-positive participants varied by ethnicity and race (Table 3; results for the SARS-CoV-2-negative cohort included in Supplementary Material 4).

Table 3. Symptoms, health status, activity level, and missed work over time among adult INSPIRE SARS-CoV-2-positive participants by ethnicity and race.
Association between ethnicity and race and study outcomes among SARS-CoV-2-positive participants
Figure 2 presents adjusted odds ratios for having 21 COVID-like symptoms or “other symptoms” (22 symptoms queried total) comparing Hispanic to non-Hispanic participants and comparing different races to white participants who tested positive for SARS-CoV-2 at 3 and 6 months (entire cohort results are included in Supplementary Materials 5.1–5.3 GEE adjusted odds ratio output).

Figure 2. Forest plots to demonstrate study outcomes by ethnicity and race using adjusted analyses among adult SARS-CoV-2-positive INSPIRE participants.
Compared to non-Hispanic participants, at 3 and 6 months, Hispanic participants reported headaches more frequently (3 months, OR: 1.70, 95%CI: 1.20–2.42; 6 months, OR: 1.97, 95%CI: 1.25–3.11), and at 6 months, they reported more nausea or vomiting (OR: 2.20, 95%CI: 1.01–4.77) and ‘other symptoms’ (OR: 2.32, 95%CI: 1.14–4.72) (Figure 2; Supplementary Material 5.1).
By race, compared to white participants, at 3 months, Asian participants reported more sore throat (OR: 1.72, 95%CI: 1.09–2.72) and less shortness of breath (OR: 0.32, 95%CI: 0.14–0.74); Black participants reported less loss of smell (OR: 0.45, 95%CI: 0.25–0.82), wheezing (OR: 0.29, 95%CI: 0.09–0.98) and ‘other symptoms’ (OR: 0.35, 95%CI: 0.13–0.95); and Other/Multiple race participants reported more wheezing (OR: 2.22, 95%CI: 1.04–4.75). At 6 months, Other/Multiple race participants had less loss of smell than white participants (OR: 0.23, 95%CI: 0.05–0.95) (Figure 2; Supplementary Materials 5.2, 5.3).
Evaluating health status, activity level, and missed work at 3 and 6 months after SARS-CoV-2 infection, some minoritized groups had worse health status, less physical activity, and more missed days of work. At 3 months, Hispanic compared to non-Hispanic participants were more likely to report fair/poor health (OR: 1.94, 95%CI: 1.36–2.78) and less activity (somewhat less, OR: 1.47, 95%CI: 1.06–2.02; much less, OR: 2.23, 95%CI: 1.38–3.61) (Figure 2, estimates reported in Supplementary Material 5.1). These differences in health and activity level by ethnicity were not found at 6 months (Figure 2, estimates reported in Supplementary Material 5.1). There were no significant differences in missed work at 3 or 6 months by ethnicity.
By race, there were differences in health status and activity level at 3 months and in health status, activity level, and work missed at 6 months. At 3 months, Other/Multiple race compared to white participants fared worse in terms of health (fair/poor health, OR: 1.90, 95%CI: 1.25–2.88) and activity level (somewhat less, OR: 1.72, 95%CI: 1.21–2.46; much less, OR: 2.08, 95%CI: 1.18–3.65). At 6 months, these differences persisted for Other/Multiple race participants in terms of health (fair/poor health, OR: 1.83, 95%CI: 1.10–3.05) and activity level (somewhat less, OR: 1.60, 95%CI: 1.02–2.51; much less, OR: 2.49, 95%CI: 1.40–4.44), and higher odds of missed work was found (OR: 2.25, 95%CI: 1.27–3.98). There were no differences at 3 months between Asian and Black participants compared to white participants. At 6 months, Asian compared to white participants reported more fair/poor health (OR: 1.88, 95%CI: 1.13–3.12). At 6 months, Black compared to white participants reported more missed work (OR: 2.83, 95%CI: 1.60–5.00) (Figure 2, estimates reported in Supplementary Materials 5.2, 5.3).
The relative importance of ethnicity and race in driving study outcomes compared to other covariates
The adjusted GEE models (fit using the complete dataset of SARS-CoV-2-positive and negative participants) were examined to explore associations between included covariates and study outcomes to gauge the relative importance of ethnicity and race, respectively, in driving study outcomes (Supplementary Materials 6.1–6.3 summary plots of GEE model parameter estimates). Adjusted GEE parameter estimates demonstrate a broad positive association between SARS-CoV-2-positive status, older age, female gender, any problem in social determinants of health, asthma, and lack of COVID vaccination and the 22 symptoms assessed (Supplementary Materials 6.1, 6.2 summary plots of GEE model parameter estimates). By contrast, infrequent associations between ethnicity or race and symptoms were found when adjusting for other covariates.
Examining associations of covariates that were adjusted for in the model with health status, activity level, and missed work, prominent associations were found between identifying as female or transgender, any problem in social determinants of health, asthma, and obesity and worse study outcomes (Supplementary Material 6.3 summary plot of GEE model parameter estimates).
Discussion
In this prospective longitudinal cohort of individuals with acute SARS-CoV-2-like symptoms, there were few differences in adjusted odds of symptoms by ethnicity or race at 3 and 6 months among SARS-CoV-2-positive participants. However, there were differences in health status, activity level, and missed work. By ethnicity, at 3 months, Hispanic compared to non-Hispanic participants had worse health and lower activity levels; these differences were not present at 6 months. By race, at 3 months, Other/Multiple races compared to white participants had worse health and lower activity levels; at 6 months, these differences persisted. Additionally, at 6 months, higher odds of worse health were found for Asian participants and of missed work for Black and Other/Multiple race participants compared to white participants. The definition currently used to identify post-COVID conditions (i.e., Long COVID) is limited in scope to continuing or developing “signs, symptoms, and conditions” following acute SARS-CoV-2 infection. Notably, the differential impacts on participants’ lives by ethnicity and race identified in this study would not be captured within the current definition of post-COVID conditions. A broadening of our understanding of post-COVID conditions may be necessary to fully capture the health-related consequences of SARS-CoV-2. The strength of the association between health status, activity level, and missed work following acute SARS-CoV-2 illness and ethnicity or race is eclipsed by the strength of the association between these impacts and other determinants that we adjusted for in our model, including pre-existing health conditions and social determinants of health.
Few prior studies have evaluated longer-term sequelae of COVID-19 through the lens of ethnicity and race. Several studies used a threshold of ≥28 days to define Long COVID symptoms (28–30). We assessed the presence of SARS-CoV-2-like symptoms at least 3 months after initial infection in accordance with the current World Health Organization definition of Long COVID (38). We accounted for known ethnic and racial disparities in social determinants of health, adjusted for demographic characteristics and pre-existing health conditions, controlled for non-SARS-CoV-2 impacts through the inclusion of participants testing negative for SARS-CoV-2, and considered not only differences in persisting symptoms but other overall health measures as well. Associations between SARS-CoV-2-like symptoms and SARS-CoV-2 infection status, time point from the onset of acute symptoms, pre-existing health conditions, and lack of vaccination found in our GEE models are consistent with what is known in the literature, supporting the validity of our results.
Others have reported inconsistent associations between ethnicity and race and Long COVID symptoms. The Arizona CoVHORT study and a study of American SARS-CoV-2-positive adults who tested positive during the Omicron surge reported no significant differences by race in self-reported Long COVID symptoms (29, 30). Conversely, a U.S. Veterans Affairs EHR-based cohort study found differences between Black and white participants at 6 months, though not to the detriment of one particular group (31). The COVID States Project, a 6-weekly internet survey conducted in all 50 states and the District of Columbia between February 2021 and July 2022 (N > 16,000), found that Hispanic, Other, and white participants testing positive for SARS-CoV-2 were more likely than Asian participants to report Long COVID symptoms (32). Multiple prior studies found ethnic and racial minoritized populations to have a higher risk of Long COVID (28, 39–41). Some investigations have indicated that differences by ethnicity and race might be partially accounted for by other factors. In the RECOVER Program, a retrospective study using EHR data of participants with and without COVID-19, Black and Hispanic participants experienced higher symptom burden and a different distribution of symptoms/conditions 31–180 days after testing positive for SARS-CoV-2 than white participants (27); however, adjusting for neighborhood-level socioeconomic status attenuated several differences. In the University of California Los Angeles COVID Ambulatory Monitoring Program, a prospective cohort study of adults with SARS-CoV-2, no significant difference in symptoms 60 days after acute illness was found by ethnicity or race after adjusting for other factors, including demographic/clinical characteristics, insurance type, social vulnerability index, and baseline function (26).
Differences that we observed among ethnic and racial minoritized populations in health-related outcomes 3 and 6 months following acute SARS-CoV-2 illness might be explained by additional factors, beyond those adjusted for in this study. Several potential factors are described in the literature on health disparities. Socioeconomic deprivation has independently been associated with a higher risk of Long COVID and may mediate disparities in SARS-CoV-2 impact for ethnic and racial minoritized populations (42). Higher loss of work days may be driven by the overrepresentation of minoritized populations in physically demanding frontline industries without the option to work from home (6, 43, 44). Poor health outcomes may result from barriers to care, including inadequate health insurance and medical mistrust (6, 43–46). Mistrust and fear have been shown to deter ethnic and racial minoritized individuals with persistent SARS-CoV-2 symptoms from seeking care, compounding structural and systemic barriers (47). Ethnic and racial inequities in access to care have been illustrated in an administrative claims study, which showed that for Asian, Black, and Hispanic patients, a significantly longer time elapsed between initial infection and Long COVID diagnosis than for non-Hispanic white patients (48). Activity levels among ethnic and racial minoritized groups have been associated with differences in the built environments where they live (49–55). Finally, sequelae of COVID-19 among ethnic and racial minoritized populations may be driven by institutional, cultural, and structural racism. Experiences of discrimination adversely impact mental health and physical health through inflammation, telomere shortening, cortisol dysregulation, and increased allostatic load (56–58). Experiences of discrimination have been shown to negatively affect the quality of care and form a barrier to seeking help (59, 60). Further research is needed to understand the remaining variation in SARS-CoV-2’s impact on health status, activity level, and missed work by ethnicity and race.
Our study has several limitations. First, various ethnic and racial subgroups had small sample sizes, which reduced precision in identifying differences within these subgroups. Second, sparse data precluded adjustment for insurance and frontline worker status in GEE analysis. Third, individuals who agreed to participate in this study may not have been representative of their larger ethnic and racial subgroups. Fourth, representativeness across survey time points may have been further impacted by non-response bias. Given the variation in response rates by ethnicity and race, these limitations likely differentially impact the conclusions for specific ethnic and racial groups. Fifth, we did not evaluate important neurological and mental health sequelae of SARS-CoV-2, including cognitive impairment, difficulty concentrating, and anxiety (26, 31). Sixth, participants were recruited at different stages of the pandemic, and the ethnic and racial composition of newly recruited participants fluctuated over time (61). Heterogeneity in symptom profile and SARS-CoV-2 impact by ethnicity and race may be influenced by differences in the dominant SARS-CoV-2 variant at the time of enrollment. Finally, we did not adjust for multiple comparisons, and the generated hypotheses should be tested in confirmatory studies.
Conclusion
Despite similar symptom prevalence, ethnic minoritized populations compared to non-Hispanic populations and racial minoritized populations compared to white populations experience more negative impacts following SARS-CoV-2 infection in terms of health status, activity level, and missed work. Increased focus on understanding drivers of ethnic and racial differences in health impacts may inform approaches to advance health equity after SARS-CoV-2 infection.
Data availability statement
The datasets presented in this article are not readily available because the data are not approved for outside use. Requests to access the datasets should be directed to KNO, a29sYXVnaEB1dy5lZHU=.
Ethics statement
Ethics approval of this protocol has been obtained at each individual site including Rush University (protocol number: 20030902, approved 3/14/2020), Yale University (2000027976, approved 4/30/2020), the University of Washington (UW Human Subjects Division, STUDY00009920, approved 4/2/2020), Thomas Jefferson University (20p.1150, approved 1/21/2021), the University of Texas Southwestern Medical Center (STU 2020-1352, approved 2/3/2021), the University of Texas, Houston (HSC-MS-20-0981, approved 9/10/2020), the University of California, San Francisco (20-32222, approved 1/25/2021) and the University of California, Los Angeles (20-001683, approved 12/18/2020). The Yale University ethics approval includes the role as the analytic lead. Additionally, the Rush University ethics approval includes INSPIRE data storage on the Hugo platform and transfer of data to Rush for secure storage. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KNO: Conceptualization, Methodology, Writing – original draft, Investigation. REK: Conceptualization, Methodology, Writing – original draft. IEM: Data curation, Formal analysis, Writing – review & editing. NLG: Conceptualization, Methodology, Writing – review & editing, Investigation. REG: Conceptualization, Methodology, Writing – review & editing, Investigation. ZZ: Conceptualization, Methodology, Writing – original draft. HY: Data curation, Formal analysis, Methodology, Writing – review & editing. S-XL: Data curation, Formal analysis, Visualization, Writing – review & editing. KCGC: Conceptualization, Methodology, Writing – review & editing. ESS: Investigation, Writing – review & editing. RCW: Investigation, Writing – review & editing. ML: Investigation, Writing – review & editing. RAW: Funding acquisition, Investigation, Writing – review & editing. IDP: Writing – review & editing. MG: Investigation, Writing – review & editing. RMH: Investigation, Writing – review & editing. MH: Writing – review & editing. JGE: Investigation, Writing – review & editing. MJH: Investigation, Writing – review & editing. MK: Investigation, Writing – review & editing. SM: Investigation, Writing – review & editing. KLR: Investigation, Writing – review & editing. RMR: Investigation, Writing – review & editing. AV: Investigation, Methodology, Writing – review & editing. AHI: Investigation, Writing – review & editing. MS: Investigation, Writing – review & editing. KK: Investigation, Writing – review & editing. SS: Conceptualization, Methodology, Writing - review & editing. GN: Conceptualization, Methodology, Writing – original draft. KAS: Conceptualization, Methodology, Writing – original draft.
INSPIRE group
Karen Adams, BA, Regulatory Specialist, University of Washington, Clinical Core & Enrolling Site; Zohaib Ahmed, BS, Research Assistant, Rush University, Administrative Core & Enrolling Site; Grace Amadio, MD, CCRP, Research Assistant, Thomas Jefferson University, Enrolling Site; Jill Anderson, BSN, RN, Clinical Core Program Manager, University of Washington, Clinical Core & Enrolling Site; Mireya Arreguin, Research Coordinator, University of California, San Francisco, Enrolling Site; Phouthavang (Jimmie) Boliboun, BS, Research Assistant, Rush University, Administrative Core & Enrolling Site; Melissa Briggs-Hagen, MD, MPH, Centers for Disease Control and Prevention (CDC); Virginia Chan, MPH, Research Coordinator, University of California, San Francisco, Enrolling Site; Chris Chandler, BA, Research Assistant, University of California, Los Angeles, Enrolling Site; Gary Chang, PhD, Senior Biostatistician, University of Washington, Clinical Core & Enrolling Site; Alex Charlton, BS, Research Coordinator, Thomas Jefferson University, Enrolling Site; Cecilia Lara Chavez, Research Coordinator, University of California, San Francisco, Enrolling Site; David Cheng, BS, Research Coordinator, Thomas Jefferson University, Enrolling Site; Michael Choi, MBS, BA, Research Assistant, Rush University, Administrative Core & Enrolling Site; Antonia Derden, BA, Administrative Assistant, Rush University, Administrative Core & Enrolling Site; Kate Diaz Roldan, MPH, Research Assistant, University of California, Los Angeles, Enrolling Site; Jocelyn Dorney, MPH, Research Coordinator, Yale University, Analytic Core & Enrolling Site; Megan Eguchi, MPH, Data Analyst, University of California, Los Angeles, Enrolling Site; Joann G. Elmore, MD, MPH, Site Principal Investigator, University of California, Los Angeles, Enrolling Site; David Gallegos, Research Coordinator, University of Texas Southwestern Medical Center, Enrolling Site; Kristyn Gatling, MA, Research Coordinator, Rush University, Administrative Core & Enrolling Site; Caitlin A. Gaylord, Research Assistant, Rush University, Administrative Core & Enrolling Site; Nicole Gentile, MD, PhD, Co-Investigator, University of Washington, Clinical Core & Enrolling Site; Rachel E. Geyer, MPH, Research Coordinator, University of Washington, Clinical Core & Enrolling Site; Chloe Gomez, Research Assistant, Rush University, Administrative Core & Enrolling Site; Michael Gottlieb, MD, Principal Investigator, Rush University, Administrative Core & Enrolling Site; Dylan Grau, BS, Research Coordinator, Thomas Jefferson University, Enrolling Site; Diego Guzman, BS, Research Assistant, Rush University, Administrative Core & Enrolling Site; Aron J. Hall, DVM, MSPH, Centers for Disease Control and Prevention (CDC); Paavali Hannikainen, BS, Research Assistant, Thomas Jefferson University, Enrolling Site; Minna Hassaballa, BA, Research Assistant, Rush University, Administrative Core & Enrolling Site; Mandy Hill, DrPH, MPH, Site Principal Investigator, University of Texas Health Science Center at Houston, Enrolling Site; Ryan Huebinger, MD, Site Principal Investigator, University of Texas Health Science Center at Houston, Enrolling Site; Ahamed H. Idris, MD, Site Principal Investigator, University of Texas Southwestern Medical Center, Enrolling Site; Ryan Jerger, Research Assistant, Rush University, Administrative Core & Enrolling Site; Amro (Marshall) Kaadan, BS, Research Assistant, Rush University, Administrative Core & Enrolling Site; Arun Kane, BA, Research Coordinator, University of Texas Health Science Center at Houston, Enrolling Site; Efrat Kean, MD, Co-Investigator, Thomas Jefferson University, Enrolling Site; Morgan Kelly, BS, Research Coordinator, Thomas Jefferson University, Enrolling Site; Robin Kemball, MPH, Program Manager, University of California, San Francisco, Enrolling Site; Avinash Kesari, Research Assistant, Rush University, Administrative Core & Enrolling Site; Jeremiah Kinsman, MPH, NREMT, Research Manager, Yale University, Analytic Core & Enrolling Site; Robin E. Klabbers, MSc in Medicine, MSc in Global Health, Research Assistant, University of Washington, Clinical Core & Enrolling Site; Katherine Koo, MS-HSM, Program Manager, Rush University, Administrative Core & Enrolling Site; Michelle L’Hommedieu, PhD, Site Program Director, University of California, Los Angeles, Enrolling Site; Shu-Xia Li, PhD, Core Statistician, Yale University, Analytic Core & Enrolling Site; Zhenqiu Lin, PhD, Core Statistician, Yale University, Analytic Core & Enrolling Site; Mengni Liu, MS, Core Statistician, Yale University, Analytic Core & Enrolling Site; Elizabeth Lomas, Research Assistant, Rush University, Administrative Core & Enrolling Site; Victoria Lyon, MPH, Project Manager, University of Washington, Clinical Core & Enrolling Site; Zenoura Maat, Research Assistant, University of Washington, Clinical Core & Enrolling Site; Caitlin Malicki, MPH, Senior Research Manager, Yale University, Analytic Core & Enrolling Site; Kerry Malone, BA, Research Assistant, University of Washington, Clinical Core & Enrolling Site; Imtiaz Ebna Mannan, MS, Core Statistician, Yale University, Analytic Core & Enrolling Site; Riley Martin, Research Assistant, University of Texas Southwestern Medical Center, Enrolling Site; Samuel McDonald, MD, Co-Investigator, University of Texas Southwestern Medical Center, Enrolling Site; Jessica Miao, BA, Research Assistant, Thomas Jefferson University, Enrolling Site; Juan Carlos Montoy, MD, PhD, Site Principal Investigator, University of California, San Francisco, Enrolling Site; Raul Moreno, BA, Administrative Analyst, University of California, Los Angeles, Enrolling Site; Dana Morse, RN, BSN, Research Coordinator, University of Washington, Clinical Core & Enrolling Site; Graham Nichol, MD, Principal Investigator, University of Washington, Clinical Core & Enrolling Site; Peter Nikonowicz, BA, Research Coordinator, University of Texas Health Science Center at Houston, Enrolling Site; Kelli N. O’Laughlin, MD, MPH, Site Principal Investigator, University of Washington, Clinical Core & Enrolling Site; Jasmine Park, Research Assistant, University of Washington, Clinical Core & Enrolling Site; Krisna Patel, BA, Research Assistant, Rush University, Administrative Core & Enrolling Site; Ariana Pavlopoulos, Research Assistant, Rush University, Administrative Core & Enrolling Site; Senyte Pierce, Research Assistant, Yale University, Analytic Core & Enrolling Site; Ian D. Plumb, MBBS, MSc, Centers for Disease Control and Prevention (CDC); Xavier Puente, Research Assistant, Yale University, Analytic Core & Enrolling Site; Nicole Renzi, RN, Nurse Coordinator, Thomas Jefferson University, Enrolling Site; Kristin Rising, MD, MS, Site Principal Investigator, Thomas Jefferson University, Enrolling Site; Robert Rodriguez, MD, Site Principal Investigator, University of California, San Francisco, Enrolling Site; Luis Ruiz, BA, Research Assistant, University of Washington, Clinical Core & Enrolling Site; Wafa Salah, Research Assistant, Yale University, Analytic Core & Enrolling Site; Michelle Santangelo, MS, Research Manager, Rush University, Administrative Core & Enrolling Site; Sarah Sapp, MPH, Research Coordinator, University of Texas Health Science Center at Houston, Enrolling Site; Sharon Saydah, PhD, Centers for Disease Control and Prevention (CDC); Kevin Schaeffer, BS, Research Coordinator, Thomas Jefferson University, Enrolling Site; Mary Schiffgens, Grant & Finance Manager, University of Washington, Clinical Core & Enrolling Site; Hailey Shughart, BA, CCRP, Research Assistant, Thomas Jefferson University, Enrolling Site; Lindsey Shughart, BS, Research Assistant, Thomas Jefferson University, Enrolling Site; Carly Shutty, BSN, Research Coordinator, Thomas Jefferson University, Enrolling Site; Erica S. Spatz, MD, MHS, Principal Investigator, Yale University, Analytic Core & Enrolling Site; Kari A. Stephens, PhD, MS, Principal Investigator, University of Washington, Clinical Core & Enrolling Site; Tracy Stober, BA, MA, Patient Representative, University of Washington, Clinical Core & Enrolling Site; Andrew Ulrich, MD, Co-Investigator, Yale University, Analytic Core & Enrolling Site; Arjun Venkatesh, MD, MBA, MHS, Principal Investigator, Yale University, Analytic Core & Enrolling Site; Ralph C. Wang, MD, MAS, Site Principal Investigator, University of California, San Francisco, Enrolling Site; Phillip Watts, BA, MM, CCRP, Research Coordinator, Thomas Jefferson University, Enrolling Site; Robert A. Weinstein, MD, Principal Investigator, Rush University, Administrative Core & Enrolling Site; Michael Willis, AS, BSHS, Research Coordinator, University of Washington, Clinical Core & Enrolling Site; Lauren E. Wisk, PhD, Co-Investigator, University of California, Los Angeles, Enrolling Site; Angela Wong, BA, Research Coordinator, University of California, San Francisco, Enrolling Site; Geoffrey Yang, BA, Research Assistant, Rush University, Administrative Core & Enrolling Site; Zimo Yang, MS, Core Statistician, Yale University, Analytic Core & Enrolling Site; Huihui Yu, PhD, Core Statistician, Yale University, Analytic Core & Enrolling Site; Zihan Zhang, Analyst, University of Washington, Clinical Core & Enrolling Site.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE) is funded by the Centers for Disease Control and Prevention (CDC, www.cdc.gov) and the National Center of Immunization and Respiratory Diseases (NCIRD) (contract number: 75D30120C08008; PI, RAW).
Acknowledgments
We would like to thank Public Health Seattle King County, the California Department of Public Health, the CTSI COVID Clinical Research Steering Committee and the CTSI Office of Clinical Research Patient Navigation Team and Bioinformatics Program for their assistance with participant recruitment for this study.
Conflict of interest
JGE is Editor in Chief of Adult Primary Care topics for UpToDate. MG reports grant funding from the Rush Center for Emerging Infectious Diseases Research Grant, Biomedical Advanced Research and Development Authority Research Grant, Emergency Medicine Foundation/Council of Residency Directors in Emergency Medicine Education Research Grant, Emergency Medicine: Reviews and Perspectives Medical Education Research Grant, University of Ottawa Department of Medicine Education Grant; and Society of Directors of Research in Medical Education Grant. KLR reports research grant funding from Abbott Diagnostics, DermTech, MeMed, Prenosis, and Siemens Healthcare Diagnostics. RMR reports research funding for PROCOVAXED funded by NIAID R01AI166967-01 (PI: Rodriquez). KK reports HECAP funded by RWJF (contract number: 79308 PI: Ansell); Chicago Department of Public Health Order 2020–4 COVID-19 Data Sharing for Patient Safety and Capacity Management funded by CDC (contract number: 6NU50CK000556-01-04 PI: Saldanha). GN reports funding through National Institutes of Health. PROCOVAXED Trial, Site PI. Centers for Disease Control and Prevention. Clinical Core, INSPIRE Registry, PI. Patient-Centered Outcomes Research Institute, Washington, DC. University of Washington PCORNet Expansion Award, Joint PI. Abiomed Inc., Danvers, MA. Emergency Care Core for Trial of Impella in Patients with STEMI and Cardiogenic Shock (RECOVER IV), PI. ZOLL Medical Corp., Chelmsford, MA, Multidimensional Study of Oxygenation in Early Post-Resuscitation (MOSER), PI. Vapotherm Inc., Exeter, NH. Vapotherm Device for Rapid Cooling Study (VOS), Co-PI. ZOLL Circulation Inc., San Jose, CA. Better Resuscitation with Supersaturated Oxygen (BASSO) Study, Co-PI. Powerful Medical Inc., Bratislava, Slovakia, US Validation Study of AI-Enhanced Diagnosis of Occluding Myocardial Infarction, PI. CPR Therapeutics Inc., Putney, VT. Consultant. Heartbeam Inc., Santa Clara, CA. Consultant. Invero Health LLC, Montville, NJ. Consultant. Kestra Medical Technologies Inc., Kirkland, WA. Consultant. Orixha Inc., Saint Cyr Au Mont d’Or, France. Consultant. BrainCool AB, Lund, Sweden. Consultant Patent for measurement of blood flow during CPR; non-provisional patent pending for blood flow measurement during CPR using signal gating; non-provisional patent pending for reperfusion-injury modifying device; all assigned to University of Washington. KNO reports research grant funding for PROCOVOXED funded by NIAID R01 AI166967 (PI: Rodriguez).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1324636/full#supplementary-material
References
1. Wingrove-Haugland, E, and McLeod, J. Not minority but minoritized. Teach Ethics. (2021) 21:1–11. doi: 10.5840/tej20221799
2. Black, C, Cerdeña, JP, and Spearman-McCarthy, EV. I am not your minority. Lancet Reg Health Am. (2023) 19:100464. doi: 10.1016/j.lana.2023.100464
3. Stringer, SM. New York City’s frontline workers. Report Bureau of Policy and Research. (2020) Available at: https://comptroller.nyc.gov/reports/new-york-citys-frontline-workers/
4. Dubay, L, Aarons, J, Brown, KS, and Kenney, GM: How risk of exposure to the coronavirus at work varies by race and ethnicity and how to protect the health and well-being of workers and their families. (2020). Available at: https://www.urban.org/sites/default/files/publication/103278/how-risk-of-exposure-to-the-coronavirus-at-work-varies_1.pdf
5. Khanijahani, A, Iezadi, S, Gholipour, K, Azami-Aghdash, S, and Naghibi, D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. (2021) 20:248. doi: 10.1186/s12939-021-01582-4
6. Gould, E, and Wilson, V: Black workers face two of the most lethal preexisting conditions for coronavirus-- racism and economic inequality. (2020). Available at: https://www.epi.org/publication/black-workers-covid/ (Accessed January 3, 2024).
7. Dickinson, KL, Roberts, JD, Banacos, N, Neuberger, L, Koebele, E, Blanch-Hartigan, D, et al. Structural racism and the COVID-19 experience in the United States. Health Secur. (2021) 19:S-14–26. doi: 10.1089/hs.2021.0031
8. Chang, S, Pierson, E, Koh, PW, Gerardin, J, Redbird, B, Grusky, D, et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. (2021) 589:82–7. doi: 10.1038/s41586-020-2923-3
9. Kaiser Family Foundation Uninsured rates for the nonelderly by race/ethnicity. (2019). Available at: https://www.kff.org/uninsured/state-indicator/nonelderly-uninsured-rate-by-raceethnicity/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D (Accessed January 3, 2024).
10. Arnett, MJ, Thorpe, RJ Jr, Gaskin, DJ, Bowie, JV, and LaVeist, TA. Race, medical mistrust, and segregation in primary care as usual source of care: findings from the exploring health disparities in integrated communities study. J Urban Health. (2016) 93:456–67. doi: 10.1007/s11524-016-0054-9
11. Creamer, J: Poverty rates for blacks and hispanics reached historic lows in 2019. Available at: https://www.census.gov/library/stories/2020/09/poverty-rates-for-blacks-and-hispanics-reached-historic-lows-in-2019.html (2020)
12. Klerman, JA, Daley, K, and Pozniak, A. Family and medical leave in 2012: technical report. Cambridge, MA: Abt Associates Inc. (2012).
13. Romano, SD, Blackstock, AJ, Taylor, EV, El Burai, FS, Adjei, S, Singleton, CM, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region – United States, March–December 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:560–5. doi: 10.15585/mmwr.mm7015e2
14. Garg, S, Kim, L, Whitaker, M, O’Halloran, A, Cummings, C, Holstein, R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. Morb Mortal Wkly Rep. (2020) 69:458–64. doi: 10.15585/mmwr.mm6915e3
15. Karaca-Mandic, P, Georgiou, A, and Sen, S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. (2021) 181:131–4. doi: 10.1001/jamainternmed.2020.3857
16. Mackey, K, Ayers, CK, Kondo, KK, Saha, S, Advani, SM, Young, S, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. (2021) 174:362–73. doi: 10.7326/M20-6306
17. Rossen, LM, Branum, AM, Ahmad, FB, Sutton, P, and Anderson, RN. Excess deaths associated with COVID-19, by age and race and ethnicity – United States, January 26-October 3, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1522–7. doi: 10.15585/mmwr.mm6942e2
18. Webb Hooper, M, Nápoles, AM, and Pérez-Stable, EJ. COVID-19 and racial/ethnic disparities. JAMA. (2020) 323:2466–7. doi: 10.1001/jama.2020.8598
19. Hawkins, RB, Charles, EJ, and Mehaffey, JH. Socio-economic status and COVID-19-related cases and fatalities. Public Health. (2020) 189:129–34. doi: 10.1016/j.puhe.2020.09.016
20. Magesh, S, John, D, Li, WT, Li, Y, Mattingly-app, A, Jain, S, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and Meta-analysis. JAMA Netw Open. (2021) 4:e2134147. doi: 10.1001/jamanetworkopen.2021.34147
21. Crenshaw, K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev. (1991) 43:1241–99. doi: 10.2307/1229039
22. Collins, PH. Intersectionality’s definitional dilemmas. Annu Rev Sociol. (2015) 41:1–20. doi: 10.1146/annurev-soc-073014-112142
23. Centers for Disease Control and Prevention Long COVID or post-COVID conditions. (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/ (Accessed January 3, 2024).
24. Herrera, JE, Niehaus, WN, Whiteson, J, Azola, A, Baratta, JM, Fleming, TK, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PM R. (2021) 13:1027–43. doi: 10.1002/pmrj.12684
25. Chen, C, Haupert, SR, Zimmermann, L, Shi, X, Fritsche, LG, and Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/infdis/jiac136
26. Yoo, SM, Liu, TC, Motwani, Y, Sim, MS, Viswanathan, N, Samras, N, et al. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med. (2022) 37:1988–95. doi: 10.1007/s11606-022-07523-3
27. Khullar, D, Zhang, Y, Zang, C, Xu, Z, Wang, F, Weiner, MG, et al. Racial/ethnic disparities in post-acute sequelae of SARS-CoV-2 infection in New York: an EHR-based cohort study from the RECOVER program. J Gen Intern Med. (2023) 38:1127–36. doi: 10.1007/s11606-022-07997-1
28. Durstenfeld, MS, Peluso, MJ, Peyser, ND, Lin, F, Knight, SJ, Djibo, A, et al. Factors associated with long COVID symptoms in an online cohort study. Open Forum Infect Dis. (2023) 10:ofad047. doi: 10.1093/ofid/ofad047
29. Jacobs, ET, Catalfamo, CJ, Colombo, PM, Khan, SM, Austhof, E, Cordova-Marks, F, et al. Pre-existing conditions associated with post-acute sequelae of COVID-19. J Autoimmun. (2023) 135:102991. doi: 10.1016/j.jaut.2022.102991
30. Qasmieh, SA, Robertson, MM, Teasdale, CA, Kulkarni, SG, Jones, HE, McNairy, M, et al. The prevalence of SARS-CoV-2 infection and long COVID in U.S. adults during the BA.4/BA.5 surge, June-July 2022. Prev Med. (2023) 169:107461. doi: 10.1016/j.ypmed.2023.107461
31. Xie, Y, Bowe, B, and Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. (2021) 12:1–12. doi: 10.1038/s41467-021-26513-3
32. Perlis, RH, Santillana, M, Ognyanova, K, Safarpour, A, Lunz Trujillo, K, Simonson, MD, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open. (2022) 5:e2238804. doi: 10.1001/jamanetworkopen.2022.38804
33. O’Laughlin, KN, Thompson, M, Hota, B, Gottlieb, M, Plumb, ID, Chang, AM, et al. Study protocol for the innovative support for patients with SARS-COV-2 infections registry (INSPIRE): a longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection. PLoS One. (2022) 17:e0264260. doi: 10.1371/journal.pone.0264260
34. United States Census Bureau 2020 census frequently asked questions about race and ethnicity. (2021). Available at: https://www.census.gov/programs-surveys/decennial-census/decade/2020/planning-management/release/faqs-race-ethnicity.html (Accessed January 3, 2024).
35. U.S. Department of the Interior Office of diversity inclusion and civil rights: standards for maintaining, collecting, and presenting federal data on race and ethnicity. Appendix A (1997) (Accessed September 18, 2023).
36. Billioux, A, Verlander, K, Anthony, S, and Alley, D. Standardized screening for health-related social needs in clinical settings: the accountable health communities screening tool. NAM Perspect. (2017) 7. doi: 10.31478/201705b
37. Centers for Disease Control and Prevention Information for health departments on reporting cases of COVID-19. (2019). Available at: https://www.cdc.gov/coronavirus/2019-ncov/php/reporting-pui.html (Accessed January 3, 2024).
38. World Health Organization Post COVID-19 condition (long COVID). (2022). Available at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=It%20is%20defined%20as%20the,months%20with%20no%20other%20explanation (Accessed January 3, 2024).
39. Jacobs, MM, Evans, E, and Ellis, C. Racial, ethnic, and sex disparities in the incidence and cognitive symptomology of long COVID-19. J Natl Med Assoc. (2023) 115:233–43. doi: 10.1016/j.jnma.2023.01.016
40. Abramoff, BA, Dillingham, TR, Brown, LA, Caldera, F, Caldwell, KM, McLarney, M, et al. Psychological and cognitive functioning among patients receiving outpatient rehabilitation for post-COVID sequelae: an observational study. Arch Phys Med Rehabil. (2023) 104:11–7. doi: 10.1016/j.apmr.2022.09.013
41. United States Census Bureau: Household pulse survey shows 31.1% reported symptoms three months or longer after they had COVID-19. Available at: https://www.census.gov/library/stories/2023/05/long-covid-19-symptoms-reported.html (2023) (Accessed January 3, 2024).
42. Shabnam, S, Razieh, C, Dambha-Miller, H, Yates, T, Gillies, C, Chudasama, YV, et al. Socioeconomic inequalities of long COVID: a retrospective population-based cohort study in the United Kingdom. J R Soc Med. (2023) 116:263–73. doi: 10.1177/01410768231168377
43. Rho, HJ, Brown, H, and Fremstad, S. A basic demographic profile of workers in frontline industries. Washington, DC: Center for Economic and Policy Research (2020).
44. Saffer, H, Dave, D, Grossman, M, and Ann, LL. Racial, ethnic, and gender differences in physical activity. J Hum Cap. (2013) 7:378–410. doi: 10.1086/671200
45. Bogart, LM, Ojikutu, BO, Tyagi, K, Klein, DJ, Mutchler, MG, Dong, L, et al. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J Acquir Immune Defic Syndr. (1999) 86:200–7. doi: 10.1097/QAI.0000000000002570
46. LaVeist, TA, Isaac, LA, and Williams, KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. (2009) 44:2093–105. doi: 10.1111/j.1475-6773.2009.01017.x
47. Baz, SA, Fang, C, Carpentieri, JD, and Sheard, L. I don't know what to do or where to go'. Experiences of accessing healthcare support from the perspectives of people living with long COVID and healthcare professionals: a qualitative study in Bradford, UK. Health Expect. (2023) 26:542–54. doi: 10.1111/hex.13687
48. Song, Z, and Giuriato, M. Demographic and clinical factors associated with long COVID. Health Aff. (2023) 42:433–42. doi: 10.1377/hlthaff.2022.00991
49. Williams, DR, and Collins, C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. (2016). doi: 10.1093/phr/116.5.404
50. Moore, LV, Roux, AVD, Evenson, KR, McGinn, AP, and Brines, SJ. Availability of recreational resources in minority and low socioeconomic status areas. Am J Prev Med. (2008) 34:16–22. doi: 10.1016/j.amepre.2007.09.021
51. Auchincloss, AH, Roux, AVD, Mujahid, MS, Shen, M, Bertoni, AG, and Carnethon, MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis. Arch Intern Med. (2009) 169:1698–704. doi: 10.1001/archinternmed.2009.302
52. Powell, LM, Chaloupka, FJ, and Bao, Y. The availability of fast-food and full-service restaurants in the United States: associations with neighborhood characteristics. Am J Prev Med. (2007) 33:S240–5. doi: 10.1016/j.amepre.2007.07.005
53. French Simone, A, Story, M, and Jeffery, RW. Environmental influences on eating and physical activities. Annu Rev Public Health. (2001) 22:309–35. doi: 10.1146/annurev.publhealth.22.1.309
54. Morland, K, Roux, AVD, and Wing, S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prev Med. (2006) 30:333–9. doi: 10.1016/j.amepre.2005.11.003
55. Morello-Frosch, R, and Lopez, R. The riskscape and the color line: examining the role of segregation in environmental health disparities. Environ Res. (2006) 102:181–96. doi: 10.1016/j.envres.2006.05.007
56. Gibbons, FX, and Stock, ML. Perceived racial discrimination and health behavior: mediation and moderation In: B Major, JF Dovidio, and BG Link, editors. The Oxford handbook of stigma, discrimination, and health. Oxford: Oxford University Press (2018). 355–77.
57. Brosschot, JF, Gerin, W, and Thayer, JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. (2006) 60:113–24. doi: 10.1016/j.jpsychores.2005.06.074
58. Lewis, TT, Cogburn, CD, and Williams, DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. (2015) 11:407–40. doi: 10.1146/annurev-clinpsy-032814-112728
59. Bergmans, RS, Chambers-Peeple, K, Aboul-Hassan, D, Dell’Imperio, S, Martin, A, Wegryn-Jones, R, et al. Opportunities to improve long COVID care: implications from semi-structured interviews with Black patients. Patient. (2022) 15:715–28. doi: 10.1007/s40271-022-00594-8
60. Ioannidis, JPA, Powe, NR, and Yancy, C. Recalibrating the use of race in medical research. JAMA. (2021) 325:623–4. doi: 10.1001/jama.2021.0003
61. Gottlieb, M, Wang, RC, Yu, H, Spatz, ES, Montoy, JCC, Rodriguez, RM, et al. Severe fatigue and persistent symptoms at 3 months following severe acute respiratory syndrome coronavirus 2 infections during the pre-delta, delta, and omicron time periods: a multicenter prospective cohort study. Clin Infect Dis. (2023) 76:1930–41. doi: 10.1093/cid/ciad045
Keywords: COVID-19, disparities, cohort, race, ethnicity, SARS-CoV-2, survey
Citation: O’Laughlin KN, Klabbers RE, Ebna Mannan I, Gentile NL, Geyer RE, Zheng Z, Yu H, Li S-X, Chan KCG, Spatz ES, Wang RC, L’Hommedieu M, Weinstein RA, Plumb ID, Gottlieb M, Huebinger RM, Hagen M, Elmore JG, Hill MJ, Kelly M, McDonald S, Rising KL, Rodriguez RM, Venkatesh A, Idris AH, Santangelo M, Koo K, Saydah S, Nichol G and Stephens KA the INSPIRE Group (2024) Ethnic and racial differences in self-reported symptoms, health status, activity level, and missed work at 3 and 6 months following SARS-CoV-2 infection. Front. Public Health. 11:1324636. doi: 10.3389/fpubh.2023.1324636
Edited by:
Myer Glickman, Office for National Statistics, United KingdomReviewed by:
Ana Izabel Passarella Teixeira, Federal University of Mato Grosso do Sul, BrazilCopyright © 2024 O’Laughlin, Klabbers, Ebna Mannan, Gentile, Geyer, Zheng, Yu, Li, Chan, Spatz, Wang, L’Hommedieu, Weinstein, Plumb, Gottlieb, Huebinger, Hagen, Elmore, Hill, Kelly, McDonald, Rising, Rodriguez, Venkatesh, Idris, Santangelo, Koo, Saydah, Nichol, Stephens and the INSPIRE Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelli N. O’Laughlin, a29sYXVnaEB1dy5lZHU=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Kelli N. O’Laughlin
Kelli N. O’Laughlin Robin E. Klabbers
Robin E. Klabbers Imtiaz Ebna Mannan3
Imtiaz Ebna Mannan3 Rachel E. Geyer
Rachel E. Geyer Ian D. Plumb
Ian D. Plumb Joann G. Elmore
Joann G. Elmore Michelle Santangelo
Michelle Santangelo Katherine Koo
Katherine Koo the INSPIRE Group
the INSPIRE Group