
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 04 January 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1295165
This article is part of the Research TopicChallenges of Non-AIDS-Defining Diseases in People Living with HIVView all 7 articles
Background and Aim: Europe faces an elevated risk of nonalcoholic fatty liver disease (NAFLD) among people living with HIV (PLWH), contributing to the region’s highest global burden of NAFLD. However, the prevalence of NAFLD across various European countries and regions remains unclear. This study aims to investigate the prevalence and risk factors associated with NAFLD among PLWH across European countries.
Methods: A systematic search was conducted across four databases: PubMed, Embase, Web of Science, and Cochrane Library. Data on the prevalence of NAFLD, nonalcoholic steatohepatitis (NASH), and fibrosis, as well as the associated risk factors, were collected among PLWH in Europe.
Results: Thirty-six studies from 13 European nations were included. The prevalence of NAFLD, NASH, and fibrosis were 42% (95%CI 37–48), 35% (95%CI 21–50) and 13% (95%CI 10–15), respectively. Male gender, BMI, waist circumference, Diabetes, hypertension, metabolic syndrome, dyslipidemia, triglycerides, HDL, LDL, ALT, AST, and years on antiretroviral therapy (ART) were found to be risk factors for NAFLD. High BMI and triglycerides were associated with NASH. Patients with high BMI and triglycerides are at increased risk of significant liver fibrosis.
Conclusion: The high prevalence of NAFLD, NASH, and fibrosis among PLWH in Europe highlights the need for early screening, intervention, and increased research focus on adolescents living with HIV. Furthermore, the significant variations observed between countries and regions underscore the influence of related risk factors.
Approximately 40 million people globally were carrying HIV, with around five thousand new cases of HIV infection occurring each day (1). However, the distribution of HIV across the world is uneven. The earliest reported cases were among men who have sex with men in Western Europe and the United States (2). But now, Eastern Europe is one of the regions with the highest HIV infection rates globally (1, 3). Additionally, with the advent of Direct-Acting Antiviral (DAA) treatment against HCV and significant improvement in the clearance rate of HBV and HCV treatment in recent years, the impact of non-infectious NAFLD on the liver has become more prominent (4, 5). On the other hand, over the past 20 years, the global coverage of antiretroviral therapy services has expanded from less than one million HIV-infected individuals in 2000 to 28.7 million in 2021 (6). With the massive expansion of HIV treatment and prevention, as well as the emergency of newer, more effective antiretroviral therapies, the global mortality rate from HIV infection has significantly decreased, greatly reducing the disparity in life expectancy with the general population (7). Under such circumstances, non-infectious chronic diseases, such as NAFLD, have a more widespread impact on the health of the PLWH population (8, 9). Recent research has found that people living with HIV (PLWH) have a higher risk of non-alcoholic fatty liver disease (NAFLD) compared to the general population (10–12), and this is believed to be related to HIV-specific factors (13).
NAFLD refers to the abnormal accumulation of triglycerides in liver cells (≥5%), with the absence of excessive alcohol consumption (men30 g/d, women20 g/d), and the exclusion of other secondary causes of fatty liver, such as genetic diseases, parenteral nutrition, viral hepatitis, etc. (14–16). NAFLD is, in fact, an umbrella term that encompasses everything from early-stage hepatic fat deposition to more severe conditions like non-alcoholic steatohepatitis (NASH), liver fibrosis, and cirrhosis (17). Since its first report in 1980, the prevalence of NAFLD has been on the rise (18). In the past two decades, NAFLD has become the most common cause of chronic liver in adults and children in developed countries (19, 20). In PLWH, chronic liver disease has also become the second leading cause of non-HIV-related mortality (21). The latest guidelines from the European AIDS Clinical Society identify PLWH as a high-risk population for NAFLD and liver fibrosis (22).
According to the recent analysis by Kalligeros et al. (23, 24), the prevalence of NAFLD in PLWH is 33.96%, significantly higher than the general population’s rate of 25.24%. On a worldwide level, Europe (42.79%) exhibits the highest prevalence of NAFLD among PLWH, greatly exceeding South America’s second-ranking rate of 35.85% (23). While Europe carries the heaviest burden of liver diseases worldwide and also has the highest NAFLD prevalence among PLWH globally, previous analyses of NAFLD prevalence in PLWH did not provide a stratified assessment of NAFLD prevalence among PLWH in different European countries and regions (25). It is widely recognized that the occurrence of HIV and NAFLD varies significantly across diverse countries, regions, populations, and ethnic groups worldwide (18, 26). However, the prevalence of NAFLD among People Living with HIV (PLWH) in varying European countries and regions remains uncertain. The primary objective of this meta-analysis is to conduct a comprehensive assessment of the prevalence and risk factors associated with NAFLD, NASH, and fibrosis in diverse European countries and regions.
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The protocol for this meta-analysis has been successfully registered with PROSPERO (CRD42023462228) and available in full on the http://www.crd.york.ac.uk/prospero/display.record.php?ID=CRD42023462228 (27).
We searched all relevant literature in four databases, namely PubMed, Web of Science, Embase, and Cochrane Library, from their inception up to August 20, 2023. There were no language restrictions, Table 1 provides an example of the search strategy used in PubMed.
Two reviewers independently screened and evaluated studies that potentially met the inclusion criteria. In case of a disagreement, a third reviewer made the final decision. The inclusion criteria were as follows: the article must report the prevalence of NAFLD and/or NASH and/or liver fibrosis in people living with HIV who are mono-infected in European countries, and it should describe the diagnostic or assessment techniques used (e.g., liver biopsy, ultrasonography, or CT scan). Exclusion criteria: studies conducted in non-European populations, studies that did not exclude excessive alcohol consumption (men≥30 g/d, women≥20 g/d), concurrent infections with HBV or HCV infections, or liver abnormalities caused by other secondary factors, animal experiments, review articles, conference abstracts, or case reports.
For each included study, we extracted the following information: author(s), publication year, nation, total number of patients (TN), study period, study design, population (adult/adolescent), diagnostic method (DxM) for NAFLD/NASH, DxM for fibrosis.
For the assessment of study quality, we employed the JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies for both cross-sectional studies and longitudinal prospective observational studies. For the longitudinal studies, only baseline measurements were used. Each study could score a maximum of eight points. Studies with a score of five or higher were included. This assessment was independently conducted by two researchers, and any discrepancies were settled through group discussion.
The primary outcomes of interest of this study are the prevalence of NAFLD, NASH, and related liver fibrosis in European PLWH who are mono-infected, with subgroup analysis based on the patients’ countries and different regions. Additionally, the study aims to analyze the differences between adolescents and adults with HIV mono-infection in Europe. Secondary outcomes of interest include identifying potential risk factors for NAFLD, NASH, and liver fibrosis, such as age, male gender, BMI, waist circumference, hypertension, diabetes, metabolic syndrome, dyslipidemia, triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine transaminase (ALT), aspartate transaminase (AST), CD4 count, CD4 nadir, years of HIV infection, years on antiretroviral therapy, and undetectable HIV viral load.
The Cochran’s Q statistic and the inconsistency index I-squared (I2) statistic were employed to evaluate the presence of statistical heterogeneity among studies within each model (28). In the event that the value of I2 surpasses 50%, we employed the constrained maximum likelihood estimator to implement the inverse variance weighted random-effects model. If the value of I2 did not surpass 50%, a fixed-effects model with inverse variance weighting was utilized. We utilized the Freeman-Tukey double arcsine transformation approach in order to address the issue of variance stabilization for the prevalence measure (29). In order to assess the impact of different diagnostic methods, countries, and regions on the results, we conducted subgroup analyses and meta-regression, respectively. To evaluate the presence of publication bias, we employed funnel plots and Begg’s test. Further, sensitivity analyses were performed on all studies encompassed in the investigation pertaining to NAFLD, NASH, and liver fibrosis. These analyses aimed to assess the potential influence of excluding any one study on the overall conclusions drawn from the investigation. In the meta-analysis conducted on risk factors, dichotomous variables were presented as discrete data, while continuous variables were represented as mean values accompanied by their standard deviations. This approach was employed to calculate odds ratios (OR) for dichotomous variables and mean differences for continuous variables. The 95% confidence interval (CI) was determined and provided to estimate the range within which the true prevalence in a new study is likely to fall. p-value was computed in order to assess their compatibility with the null hypothesis of no effect. The predetermined level of statistical significance was established at 0.05. Significance was attributed only to p-values that were less than or equal to 0.05. A p-value exceeding 0.05 was deemed to indicate a lack of statistical significance. All statistical analyses for prevalence and risk factors were conducted using STAT 15.1 software (STATA CORPORATION, College Station, TX).
We initially retrieved a total of 1,549 articles, and after removing duplicates, we were left with 553 articles. After screening the titles and abstracts, 309 articles were excluded, leaving us with 244 articles. The excluded articles included irrelevant articles, reviews, abstracts, animal experiments, and case reports. After reviewing the full texts, a total of 36 studies met the inclusion criteria and were included in the final analysis (11, 30–64) (Figure 1). These studies were all from European countries, including the United Kingdom (42, 46), France (31, 35, 38–40, 64), Belgium (39, 40), Greece (30, 41), Italy (11, 34, 44, 48, 53, 62), Spain (33, 45, 50, 51, 55, 56), Denmark (36), Finland (37, 57), Germany (32, 39, 40, 43, 49, 58, 63), Switzerland (54), Serbia (47, 61), Austria (59), and Turkey (52, 60) (Supplementary Table S1). Most of the studies were cross-sectional in design, with a few prospective cohort studies.
Only one study focused on adolescents (64), while the rest were conducted on adults. Based on the geographical distribution of the study population across different European countries, they were categorized into Central Europe (CE) (32, 43, 49, 54, 58, 59, 63), Western Europe (WE) (31, 35, 38, 42, 46, 64), Eastern Europe (EE) (47, 52, 60, 61), Southern Europe (SE) (11, 30, 33, 34, 41, 44, 45, 48, 50, 51, 53, 55, 56, 62), and Northern Europe (NE) (36, 37, 57). Two multicenter studies (39, 40) included patients from Belgium, France, and Germany. In principle, these two studies were analyzed separately and were not included in the national and regional subgroup analyses. An exception was made for the subgroup analysis of NASH prevalence, as it only included one multicenter study.
According to the JBI Critical Appraisal Checklist, all 36 studies scored five or higher (Supplementary Table S2), and therefore, no studies were excluded due to quality issues. Except for age analysis in the risk factor studies of NAFLD, the majority of studies had Begg’s test p-values>0.05 (Supplementary Table S3). Thus, there was no significant publication bias. The funnel plot is roughly symmetrical on both sides, indicating no obvious publication bias (Supplementary Figures S1–S5). No literature was found through sensitivity analysis to have a significant influence on the analysis’s overall findings (Figure 2). Therefore, the results can be considered robust and reliable.
A total of 32 studies were analyzed, including 1 study (64) on adolescents and 31 studies (11, 31–37, 39–52, 54–58, 60–63) on adults. It involved 9,962 adults and 23 adolescents. The analysis showed that the overall prevalence of NAFLD in the people living with HIV mono-infection in Europe was 42% (95%CI, 37–48) (Figure 3A). Subgroup analysis for different populations showed that the incidence of NAFLD in adults (43, 95%CI: 37–49) was significantly higher than in adolescents (17, 95%CI: 2–33) (Supplementary Table S4). Subgroup analysis based on national levels (Figures 3B, 4) showed that Denmark (9, 95%CI: 6–11) and Turkey (27, 95%CI: 14–40) had significantly lower prevalence compared to other European countries, followed by Finland (33, 95%CI: 23–43), Germany (35, 95%CI: 29–41), and Italy (39, 95%CI: 35–44) at a relatively lower level. However, these three countries did not differ significantly from the overall level (Figures 3B, 4). Among PLWH, France had the highest prevalence of NAFLD (61, 95%CI: 47–76), followed by Greece (55, 95%CI, 46–64) and Switzerland (51, 95%CI, 46–56) (Figures 3B, 4). There were no differences in prevalence between other countries and the overall level. Comparing different regions in Europe, the lowest prevalence was found in Northern Europe (24, 95%CI, 4–44), and the highest was in Western Europe (55, 95%CI, 30–80) but the differences between regions in Europe were not statistically significant (Figures 3C, 4). Two multicenter studies from Belgium, France, and Germany showed a high level of prevalence (68, 95%CI, 57–79) for NAFLD (Figures 3D, 4).

Figure 3. Forest plot for the prevalence of NAFLD in PLWH in Europe (A), and subgrouped by nation (B), region (C), multicenter (D).
It is important to note that, consistent with the findings of Kalligeros, et al.’s meta-analysis, the incidence of NAFLD may be influenced by the choice of diagnostic methods and patient selection. For example, in Lemoine, et al.’s study, if only individuals with persistently elevated liver transaminases were included, the prevalence of NAFLD may be overestimated (39). Another study by Lemone, et al. involving 39 individuals and utilizing high-sensitivity MRI-PDFF for diagnosis, may yield a higher prevalence compared to studies employing other diagnostic methods (40). Therefore, while our overall prevalence results align with Kalligeros, et al.’s meta-analysis, it is crucial approach these results objectively and prudently. To investigate the sources of heterogeneity, we conducted subgroup analyses based on diagnostic methods (Supplementary Figure S6) and meta-regression analyses (Table 2). The subgroup based on diagnostic methods yielded the following prevalence rates for NAFLD: transient elastography (TE) with continuous attenuation parameters (CAP), 46% (95%CI 38–55); ultrasound (U/S), 54% (95%CI 38–70); computed tomography (CT), 26% (95%CI 5–47); hepatic steatosis index (HSI) 60% (95%CI 2–118); magnetic resonance imaging-proton density fat fraction (MRI-PDFF), 42% (95%CI −2–0.86); 1H-magnetic resonance spectroscopy (1H-MRS), 34% (95%CI 22–44); OWLiver Test, 34% (95%CI 17–52); liver biopsy, 63% (95%CI 31–96). From these results, it is evident that liver biopsy has the highest prevalence, which may be attribute to the fact that patients willing to undergo invasive diagnostic methods, such as liver biopsy, are often those with more severe conditions. Meta-regression analyses (Table 2) further revealed that no matter whether the selected independent variable was diagnostic methods (p = 0.000), nations (p = 0.003), or regions (p = 0.001), the resulting p-values were all less than 0.05. This also confirmed that both diagnostic methods and different populations contribute as influencing factors to the variable in prevalence.
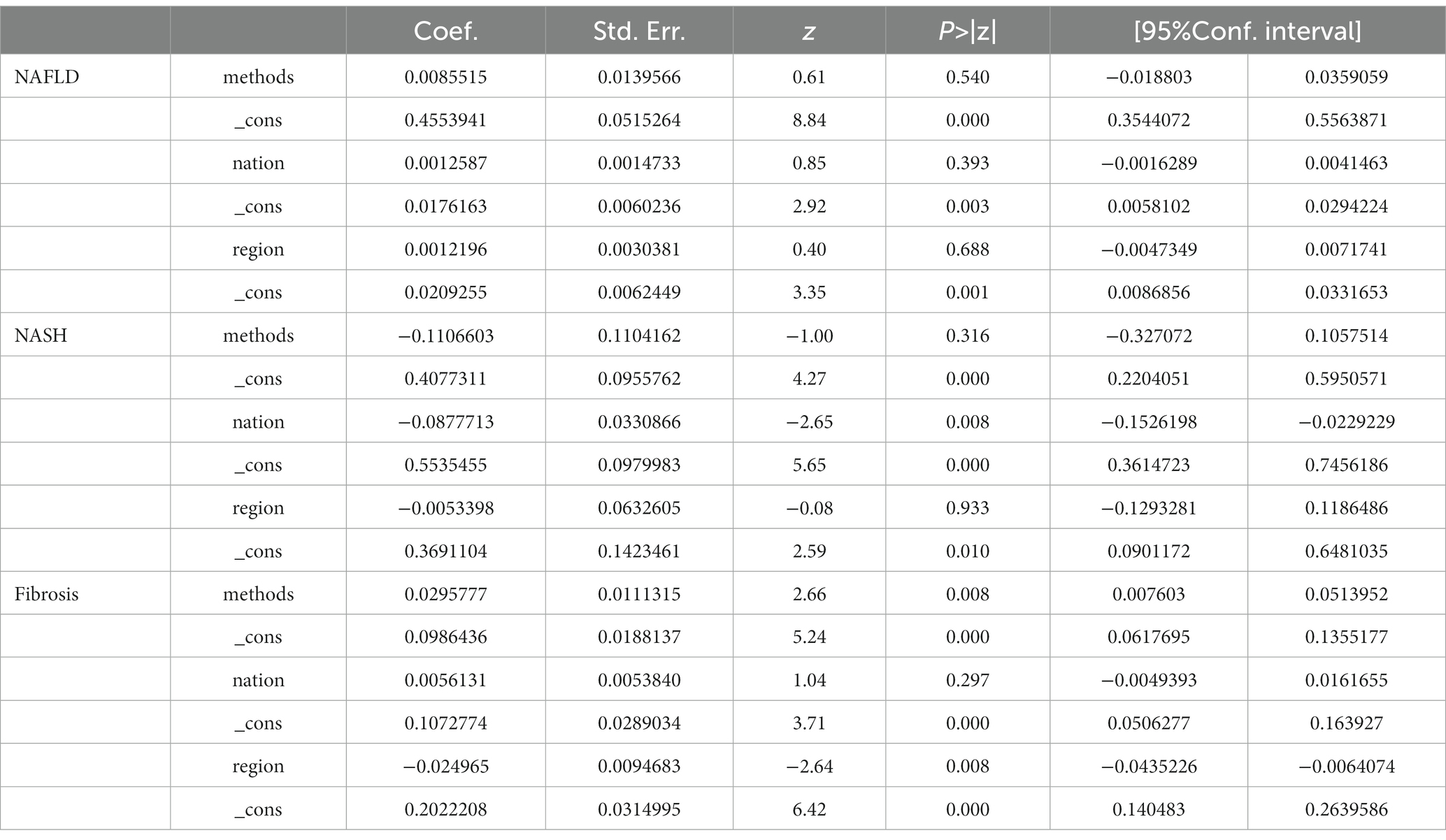
Table 2. Meta-regression for the prevalence of NAFLD, NASH, and liver fibrosis with diagnostic methods, nations, and regions.
The analysis comprised 579 patients from seven studies (31, 33, 35, 39, 43, 46, 52). In the European PLWH, the overall prevalence of NASH was found to be 35% (95%CI: 21–50) (Figure 5). Subgroup analysis based on countries revealed that the lowest NASH prevalence was observed in the United Kingdom (8, 95%CI: 3–14) and Germany (12, 95%CI: 8–16), while the highest rates were observed in France (55, 95%CI: 40–69) and Spain (55, 95%CI: 43–67), with significant differences between them and overall prevalence (Table 3). When comparing different regions in Europe, Central Europe had the lowest prevalence (12, 95%CI: 8–16), showing significant differences compared to other regions. Southern Europe had the highest prevalence (55, 95%CI: 44–64), significantly higher than other regions (Table 3). The prevalence of NASH in the multicenter study (47, 95%CI: 33–61) was not significantly higher than the overall level (35, 95%CI: 21–50) (Figure 5).
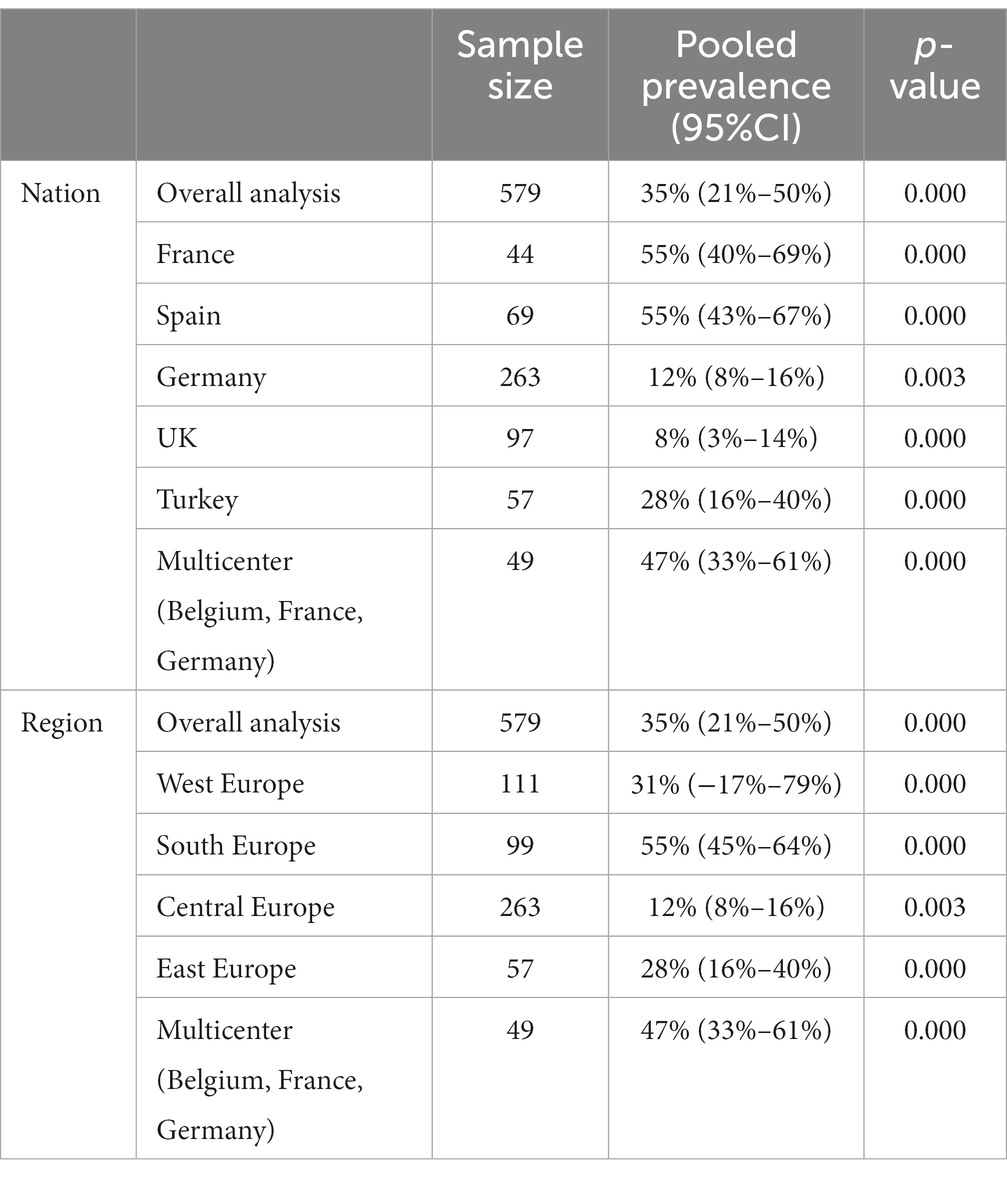
Table 3. Prevalence of NASH in PLWH mono-infection in Europe subgroup analysis by nation and region.
Furthermore, subgroup analysis based on different diagnostic methods yielded the following prevalence for NASH: biopsy, 43% (95%CI 18–69); fibroscan-AST score (FAST score), 12% (95%CI 8–16); magnetic resonance elastography (MRE), 28% (95%CI 16–40) (Supplementary Figure S7). Similar to NAFLD, the biopsy group had the highest prevalence. Meta-regression analyses revealed that different diagnostic methods (p = 0.001), different nations (p = 0.000), and different regions (p = 0.010) all contributed as influencing factors to the variable in NASH prevalence (Table 2).
This analysis encompassed 916 patients from twenty studies (30, 31, 33, 35, 37–46, 48, 49, 52–54, 59). The overall prevalence of fibrosis in the population was found to be 13% (95%CI: 10–15) (Figure 6A). Subgroup analysis based on countries revealed that Switzerland (3, 95%CI: 2–5) and Germany (8, 95%CI: 4–11) had significantly lower prevalence compared to the overall prevalence. On the other hand, the United Kingdom (33, 95%CI: 24–42), France (17, 95%CI: 14–20), and Austria (16, 95%CI: 14–18) had a significantly higher prevalence than the overall prevalence (Figure 6B). When comparing different regions in Europe, Western Europe (24, 95%CI: 14–34) had a significantly higher prevalence compared to the overall level, while there was no significant difference in the prevalence between other regions in Europe (Figure 6C), including the multicenter studies and the overall level (Figure 6D).
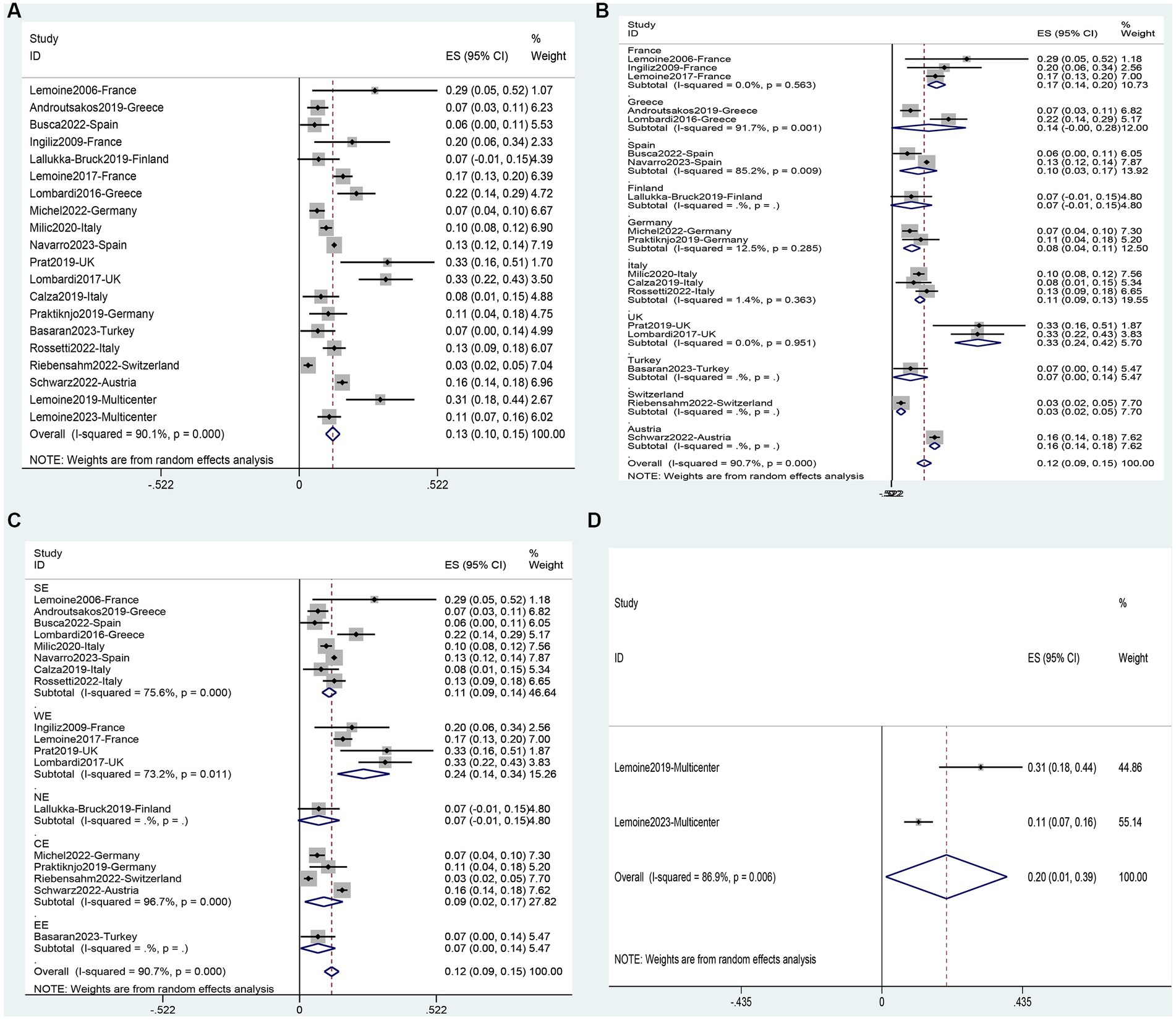
Figure 6. Prevalence of Fibrosis in PLWH in Europe: (A), and subgrouped by nation (B), region (C), and multicenter (D).
The prevalence of liver fibrosis obtained through subgroup analysis using different diagnostic methods were as follows: fibroscan 10% (95%CI 17–13); NFS, 13% (95%CI 9–16); APRI, 24% (95%CI 8–40), MRE, 7% (95%CI 2–12); and biopsy, 28% (95%CI 20–36) (Supplementary Figure S8). These results showed similarities with the outcome of NAFLD and NASH, where the biopsy group had the highest prevalence. Meta-regression analyses (Table 2) revealed that different diagnostic methods (p = 0.000), nations (p = 0.000), and regions (p = 0.000) were all significant influencing factors for the prevalence of liver fibrosis.
Data on risk factors for NAFLD in HIV mono-infected patients was obtained from fourteen studies (11, 36, 40–43, 45–47, 49, 52, 60, 62, 63) involving 7,444 individuals. Using these data, a meta-analysis was conducted on 18 variables (Figures 7–9). Male gender (OR 2.32, 95%CI 1.29–4.19, p = 0.005) (Figure 7B), BMI (SMD 1.08, 95%CI 0.46–1.70, p = 0.001) (Figure 7C), waist circumference (SMD 1.54, 95%CI 0.95–2.14, p = 0.000) (Figure 7D), Diabetes (OR 3.19, 95%CI 2.02–5.05, p = 0.000) (Figure 7E), Hypertension (OR 1.95, 95%CI 1.65–2.32, p = 0.000) (Figure 7F), metabolic syndrome (OR 3.19, 95%CI 2.39–4.26, p = 0.000) (Figure 8A), dyslipidemia (OR 2.40, 95%CI 2.11–2.72, p = 0.000) (Figure 8B), triglycerides (SMD 0.58, 95%CI 0.27–0.89, p = 0.000) (Figure 8C), HDL (SMD -0.44, 95%CI -0.74—0.13, p = 0.005) (Figure 8D), LDL (SMD 0.13, 95%CI 0.03–0.23, p = 0.008) (Figure 8E), ALT (SMD 0.44, 95%CI 0.21–0.67, p = 0.000) (Figure 8F), AST (SMD 0.19, 95%CI 0.03–0.36, p = 0.019) (Figure 9A), and years on antiretroviral therapy (SMD 0.64, 95%CI 0.11–1.16, p = 0.017) (Figure 9E) were found to be associated with an increased risk of NAFLD (Supplementary Table S5). However, age (SMD 0.30, 95%CI −0.21–0.81, p = 0.253) (Figure 7A), CD4 count (SMD 0.20, 95%CI −0.00–0.39, p = 0.052) (Figure 9B), CD4 nadir (SMD −0.03, 95%CI −0.21–0.15, p = 0.733) (Figure 9C), years of HIV infection (SMD 0.22, 95%CI −0.09–0.53, p = 0.155) (Figure 9D), undetectable HIV viral load (OR 1.38, 95%CI 0.91–2.09, p = 0.125) (Figure 9F) were not significantly associated with the prevalence of NAFLD (Supplementary Table S5).
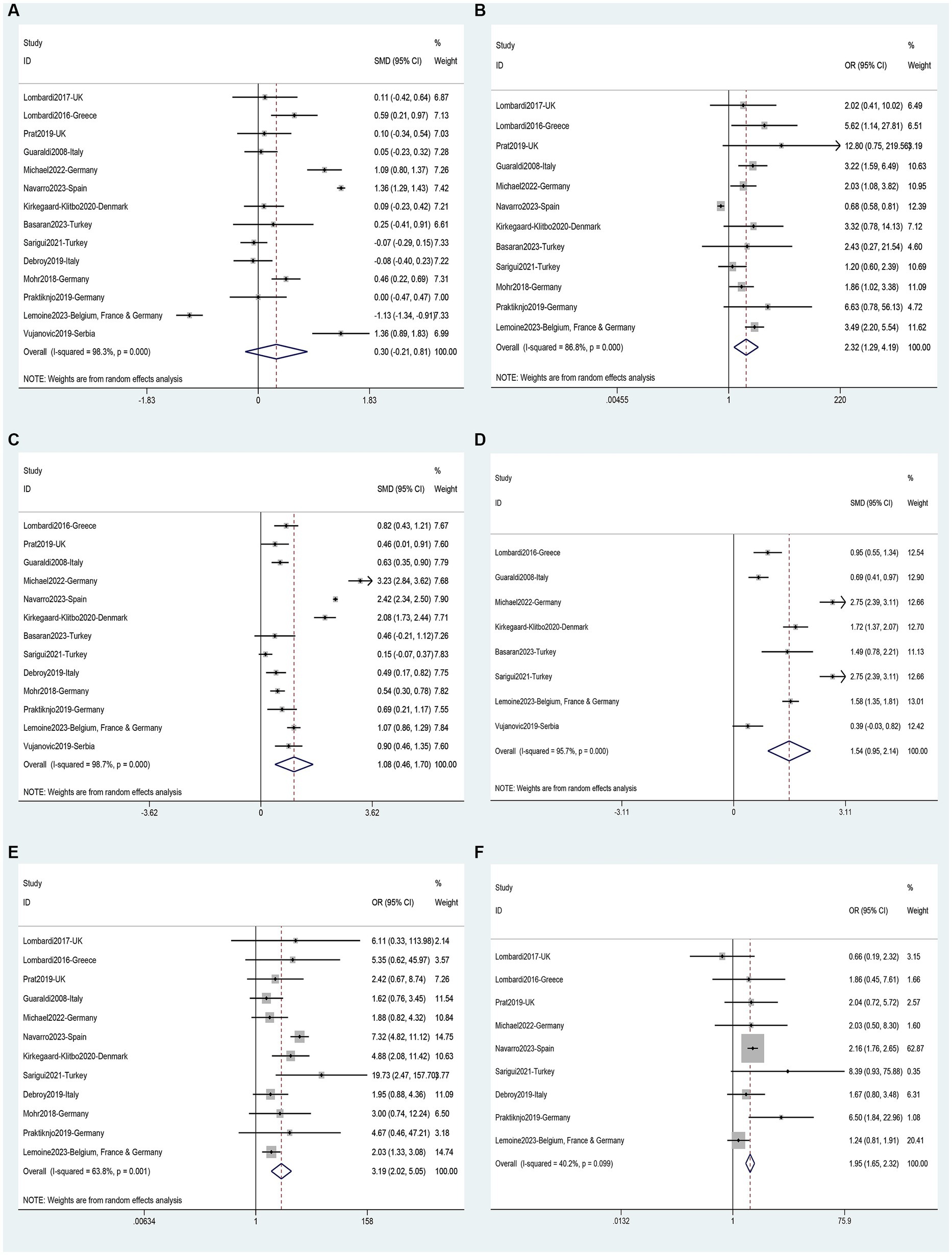
Figure 7. Forest plot for NAFLD risk factors: (A) Age, (B) Male gender, (C) BMI, (D) Waist circumference, (E) Diabetes, (F) Hypertension.

Figure 8. Forest plot for NAFLD risk factors: (A) Metabolic syndrome, (B) Dyslipidemia, (C) Triglycerides, (D) HDL, (E) LDL, (F) ALT.
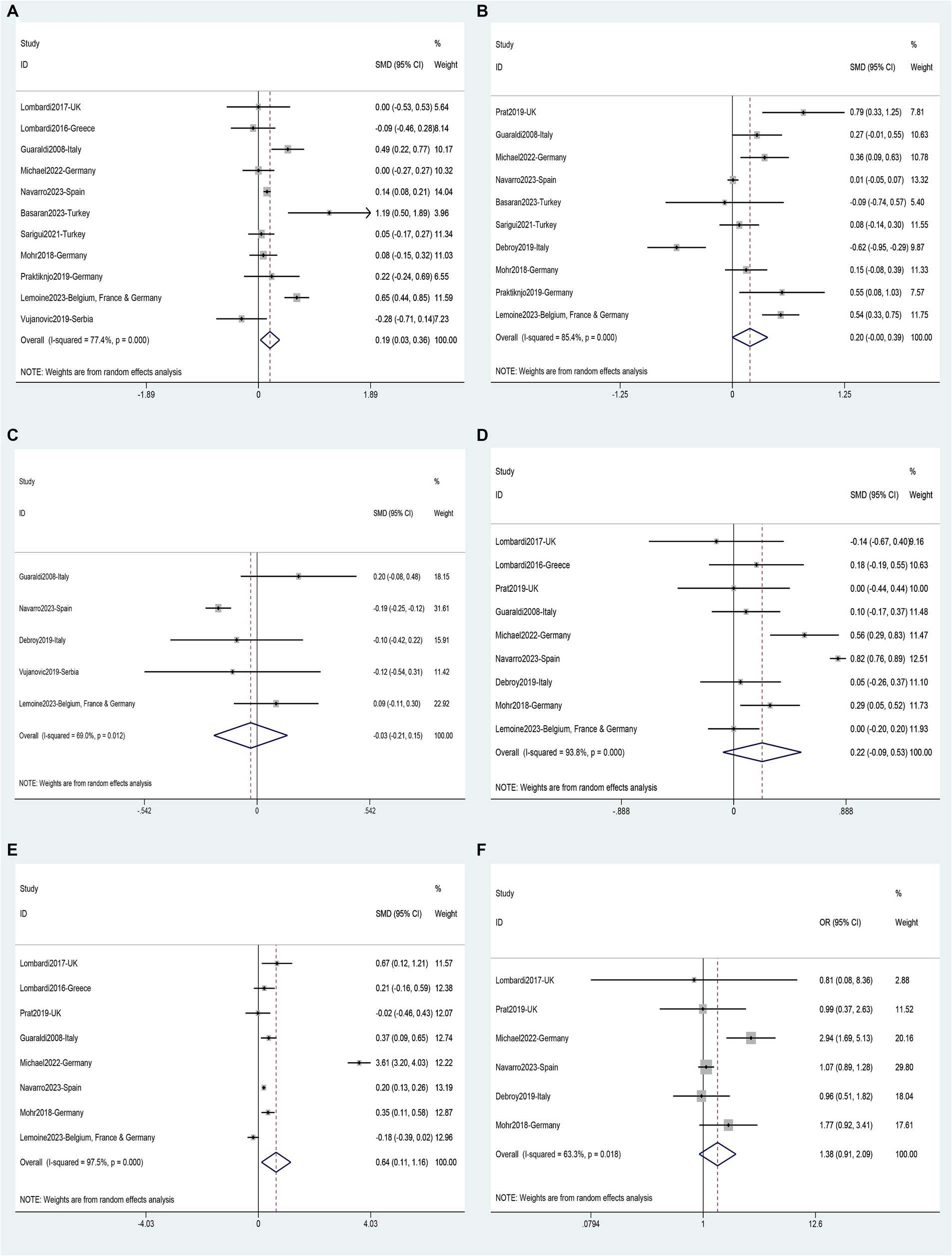
Figure 9. Forest plot for NAFLD risk factors: (A) AST, (B) CD4 count, (C) CD4 nadir, (D) Years of HIV infection, (E) Years on antiretroviral therapy, (F) Undetectable HIV viral load.
Two studies (35, 43) involving a total of 293 patients provided data for the analysis of seven possible risk factors for NASH. Body Mass Index (BMI) (SMD 0.68, 95%CI 0.35–1.02, p = 0.000) (Figure 10B) and triglycerides (SMD 0.45, 95%CI 0.12–0.78, p = 0.008) (Figure 10E) were identified as risk factors for NASH, while age (SMD 0.05, 95%CI −0.28–0.38, p = 0.765) (Figure 10A), ALT (SMD 1.09, 95%CI −0.90–3.09, p = 0.283) (Figure 10C), AST (SMD 1.13, 95%CI −1.17–3.43, p = 0.336) (Figure 10D), total cholesterol (SMD −0.29, 95%CI −0.62–0.04, p = 0.085) (Figure 10F) and CD4 count (SMD 0.43, 95%CI −0.29–1.16, p = 0.239) (Figure 10G) were not supported as risk factors for NASH (Supplementary Table S5).
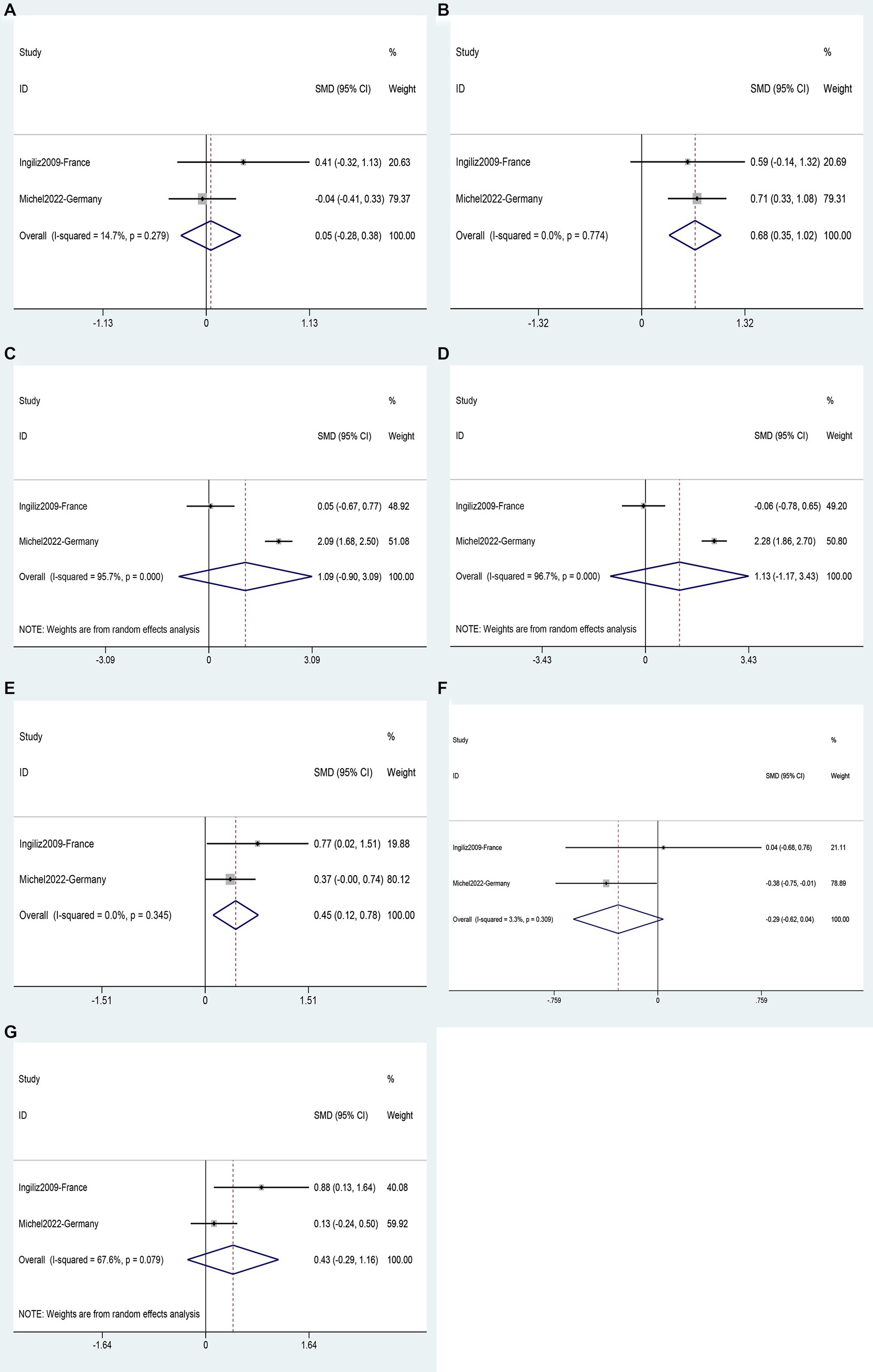
Figure 10. Forest plot for NASH risk factors: (A) Age, (B) BMI, (C) ALT, (D) AST, (E) Triglycerides, (F) Total cholesterol, (G) CD4 count.
The analysis of risk factors for liver fibrosis was based on data from 6 studies (40–44, 59) involving 2,734 patients. Fibrosis was found to be associated with age (SMD 0.35, 95%CI 0.12–0.58, p = 0.003) (Figure 11A); BMI (SMD 0.74, 95%CI 0.44–1.04, p = 0.016) (Figure 11C), waist circumference (SMD 0.56, 95%CI 0.38–0.74, p = 0.000) (Figure 11D); diabetes (OR 3.72, 95%CI 1.90–7.27, p = 0.111) (Figure 11E); ALT (SMD 0.66, 95%CI 0.06–1.27, p = 0.000) (Figure 11F), and AST (SMD 0.75, 95%CI: 0.32–1.18, p = 0.000) (Figure 11G). Male gender (OR 1.32, 95%CI 1.00–1.73, p = 0.111) (Figure 11B), and years of HIV infection (SMD 0.13, 95%CI −0.33–0.59, p = 0.640) (Figure 11H) were not observed to be associated with Fibrosis (Supplementary Table S5).
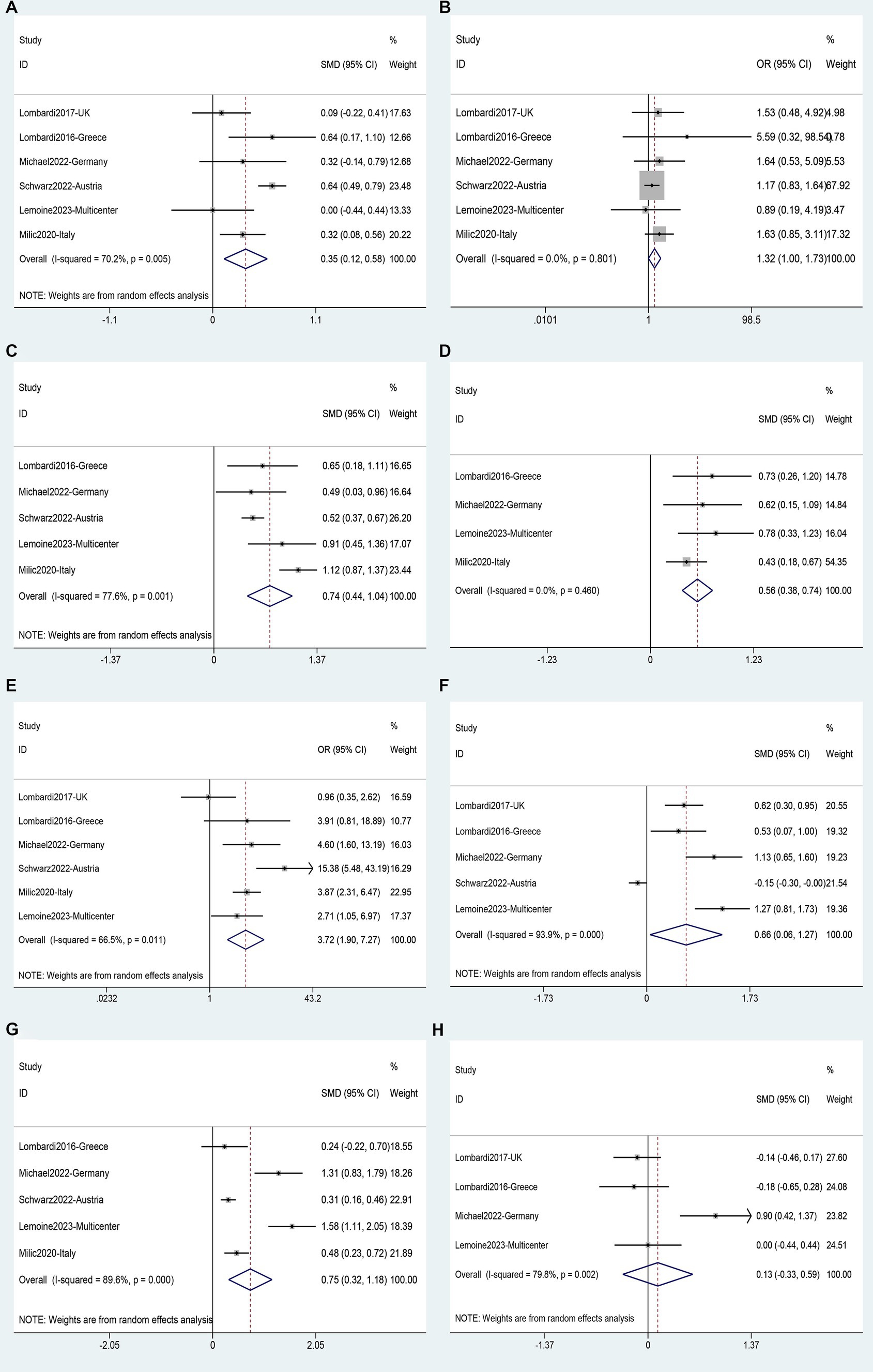
Figure 11. Forest plot for Liver fibrosis risk factors: (A) Age, (B) Male gender, (C) BMI, (D) Waist circumference, (E) Diabetes, (F) ALT, (G) AST, (H) Years of HIV infection.
This study represents the first meta-analysis of the prevalence of NAFLD, NASH, and fibrosis, along with associated risk factors, in PLWH across European countries. We meticulously excluded studies that involved concurrent infections with hepatitis viruses such as HCV and HBV, as well as those with high alcohol intake. In total, we included thirty-six studies in our analysis. It is important to note that different diagnostic methods can have some impact on the positivity rate for NAFLD, but this variability is inevitable (23, 24). To mitigate heterogeneity, we adopted a methodology similar to previous studies, prioritizing diagnostic results obtained through ultrasound examinations or other imaging techniques (23). Moreover, for studies with excessive heterogeneity, we rigorously applied a random-effects model in our analysis (23).
Based on the research data from thirty-six studies, encompassing 7,893 patients, our analysis revealed that the overall prevalence of NAFLD among European individuals with HIV mono-infection stands at a substantial 42%. In the adult population, this figure reaches 43%. Notably, this finding aligns perfectly with the 42.79% reported by Kalligeros et al. in their recent study (23). While they included eighteen studies from Europe, only half the number included in our analysis, the consistency in research outcomes is remarkable. This further underscores the reliability and stability of the study results (23).
The reasons behind the high prevalence of NAFLD in European PLWH are not yet clear. However, it is speculated that this may be related to variations in the distribution of NAFLD susceptibility genes among different ethnic groups. For instance, variations in the PNPLA3 gene, which encodes a lipid-editing enzyme, have been shown to significantly impact liver fat content and susceptibility to NAFLD. This variation is more common in European Caucasians and Hispanics than in African populations (65, 66). Recently, the TM6SF2 gene has also been found to play a significant role in the development and progression of NAFLD, with the non-synonymous E167K mutation within it considered one of the strongest genetic risk factors for NAFLD development (67). Additionally, Mancia et al. discovered that the MBOAT7 gene variant rs641738 may be associated with the occurrence and progression of NAFLD in Europeans. However, further research is necessary to investigate the distribution of these genetic variations across different populations. A recent report has highlighted the spread of a highly virulent strain of HIV in Europe (68). Nevertheless, given that undetectable HIV RNA viral load were not linked to a decreased likelihood of NAFLD, NASH and liver fibrosis, there is no enough evidence to suggest that viral virulence is a risk factor.
According to subgroup analyses conducted at the national and regional levels, the trends in the prevalence of NAFLD, NASH, and liver fibrosis among European PLWH are not entirely consistent. For instance, the prevalence of NAFLD in Switzerland is significantly higher than the overall level, but the prevalence of fibrosis is notably lower than the overall rate. This inconsistency in trends may be related to specific genetic, and environmental factors, and medical practices in different countries and regions. In Northern Europe, countries like Denmark and Finland have relatively low rates of NAFLD and fibrosis among PLWH compared to the European average. This might reflect successful practices in the prevention and treatment of NAFLD and fibrosis in the Northern European region or the protective role of specific genetic and environmental factors in this area. Conversely, in Western Europe, France stands out with significantly higher rates of NAFLD, NASH, and fibrosis compared to the overall average. However, it is worth noting that the prevalence in France were obtained using the biopsy diagnostic method, which is invasive and usually accepted by patients with more severe conditions. Therefore, the results obtained may be biased towards higher prevalence. This is further supported by the results of the subgroup analyses (Supplementary Figures S6–S8) and meta-regression analysis (Table 2). As a result, these results should be interpreted with caution. However, France boasts the highest HIV treatment coverage in Europe. Antiretroviral therapy (ART) may contribute to a likelihood of NAFLD-related metabolic abnormalities among patients (69, 70). While genetic and environmental factors may play a role, the impact of ART treatment cannot be overlooked as a potential contributing factor.
This study has uncovered that the duration of ART is the only variable related to HIV in the PLWH population that poses a risk factor for NAFLD. All other risk factors align with those found in the general population (71–73). As for NASH and liver fibrosis, no risk factors different from the general population have been found. This emphasizes the need to follow largely similar preventive strategies for promoting overall health in PLWH individuals, as is recommended for the general population. However, the impact of ART on NAFLD should not be overlooked. Early protease inhibitors, such as Lopinavir and Atazanavir, are known to cause moderate to severe liver toxicity (74). Nevertheless, Darunavir is currently the only commonly used protease inhibitor for HIV treatment, which is utilized as a booster in combination with Ritonavir or Cobicistat. Notably, it has significantly reduced liver toxicity compared to the first-generation protease inhibitors (75, 76). Pires et al. have suggested that NAFLD induced by antiretroviral therapy may primarily be associated with D-analogs (e.g., Didanosine/ddI, Stavudine/d4T, and Zalcitabine/ddC) (70). Nevertheless, these medications are not commonly used in contemporary HIV treatment in Europe as well. A recent five-year study from Germany found that the duration of treatment with second generation nucleoside reverse transcriptase inhibitors, Tenofovir Alafenamide (TAF)/Emtricitabine and second generation integrase inhibitors, Dolutegravir (DTG), was associated with increased risk of NAFLD in HIV patients (32). However, the third generation of Integrase inhibitors, such as Lenacapvir, have minimal impact on cytochrome P450 enzymes, leading to very rare instances of liver toxicity (77). It remains unclear whether second-generation NRTIs and Integrase inhibitors are the primary ARTs responsible for causing NAFLD in PLWH in Europe. Furthermore, since most HIV patients are typically exposed to multiple types of ART drugs, further research is required to investigate this matter in the future.
NASH represents a more severe progression of NAFLD, involving liver cell damage during the inflammatory process and carrying an increased risk of further developing liver fibrosis, cirrhosis, and liver cancer (78, 79). It is estimated that the average incidence of NASH in the general population is 3.5–5% (80). However, PLWH who have NAFLD are more prone to developing NASH than NAFLD patients in the general population (81). Our analysis based on seven studies indicated that the prevalence of NASH in the European PLWH is 35%. It should be emphasized that, although liver biopsy is the most accurate method for diagnosing NASH, only patients with more severe conditions would undergo invasive liver biopsy examinations. Therefore, the prevalence of NASH obtained from liver biopsies could be overestimated, as it represents a group that is enriched due to the inherent selection bias associated with an intrusive diagnostic procedure. Nevertheless, the results obtained are comparable to those of previously published meta-analyses with the same inclusion criteria, thus rendering them significant. For instance, compared with the results of a meta-analysis on NASH prevalence among liver biopsy patients completed by Maurice et al. (24), the prevalence of NASH in European PLWH (35%) is lower than the global prevalence (42%) reported by Maurice et al. This regional difference may be associated with specific genetic factors within the European population (82) or may be attributed to early screening, diagnosis, and treatment of NAFLD. A Finnish study conducted genome-wide analysis on 8,434 European ancestry NAFLD patients and identified five susceptibility genes, indicating that NAFLD could be a kind of disease caused by multiple factors related to genetic variation (82). However, there is currently a lack of information about the distribution of susceptibility genes among different population worldwide, thus requiring future studies to be more comprehensive and in-depth. In April 2016, the European Association for the Study of the Liver released NAFLD clinical practice guidelines, which provided thirty-eight recommendations for the screening, diagnosis, treatment, and follow-up of NAFLD in European countries (83). Effective early intervention and management strategies can help prevent the progression of NAFLD to NASH. Furthermore, our meta-analysis of risk factors for NASH in the PLWH for the first time reveals that BMI and hypertriglyceridemia are associated with the prevalence of NASH in the European PLWH. This suggests that factors related to NASH in the European PLWH may be linked to weight and lipid abnormalities.
Previous studies have suggested that 36.1% of NAFLD patients may experience progressive liver fibrosis (73). Consistent with this, our study showed the prevalence of liver fibrosis in the European PLWH is 13%, which is almost exactly one-third of the NALFD prevalence (42%) in the European PLWH and nearly identical to the prevalence (12%) of fibrosis in the global PLWH as reported by Kallilgeros et al. (23). Similar to NASH, this finding may reflect some positive measures in liver health management and interventions in Europe, as well as other influencing factors that may exist among PLWH in different regions, such as regional genetic differences, more frequent liver function tests, regular health education, easier access to treatment, and support. These factors may contribute to preventing NAFLD from progressing to liver fibrosis, thereby reducing the incidence of liver fibrosis.
Through the analysis of liver fibrosis risk factors in the European PLWH, we found a significantly increased incidence of liver fibrosis associated with age, BMI, waist circumference, diabetes, ALT, AST, and years of HIV infection. This suggests that metabolic abnormalities, especially obesity and diabetes, play a significant role in the development of liver fibrosis. This conclusion is consistent with previous findings on risk factors for liver fibrosis (24). Furthermore, our study did not find a correlation between the duration of HIV infection and liver fibrosis, which supports the findings of Maurice’s research (24).
The limitations of this study primarily include the following aspects: (1) Only one study on European adolescents living with HIV was included, and this study only provided data on the prevalence of NAFLD, making comprehensive comparison and analysis difficult. Nonetheless, given the considerably lower prevalence of NAFLD among adolescents compared to adults, the focus of research remains on adults. (2) Most of the included studies were cross-sectional studies, which limited the ability of this study to infer causal relationships. (3) From the analysis results of this study, it can be observed that the duration of receiving ART treatment in HIV patients is also one of the risk factors for NAFLD. If data on the impact of different types of HAART treatment drugs on NAFLD prevalence were available, it might reveal valuable information. However, due to the lack of relevant data, such an analysis cannot be conducted at this time. (4) There are relatively few studies from Central and Eastern Europe, thereby limiting their representativeness. This will be addressed with updates as more relevant research becomes available in the future.
In summary, this study conducted a stratified assessment of the prevalence of NAFLD, NASH, and fibrosis, as well as potential risk factors among PLWH in different countries and regions in Europe. The analysis guided the future prevention and treatment of NAFLD and related diseases in PLWH in Europe. It is advisable to implement early and proactive screening and interventions based on the risk factors that may influence the occurrence, progression, and prognosis of NAFLD, as well as the prevalence rates in different European countries and regions. This approach can lead to more targeted public health policies and medical measures, ultimately contributing to a fundamental improvement in the health of PLWH in Europe.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
DJ: Writing – original draft, Writing – review & editing, Formal analysis, Methodology, and Project administration. TZ: Conceptualization, Investigation, Writing – review & editing. SJ: Data curation, Investigation, Writing – review & editing. ZC: Data curation, Investigation, Writing – review & editing. BG: Methodology, Software, Writing – review & editing. GL: Project administration, Validation, Writing – review & editing. CZ: Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the He’nan Province Medical Science and Technology Research Projects (Province and Ministry joint Project) (Grant No. LHGJ20191102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1295165/full#supplementary-material
1. Challacombe, SJ. Global inequalities in HIV infection. Oral Dis. (2020) 26:16–21. doi: 10.1111/odi.13386
2. De Cock, KM, Jaffe, HW, and Curran, JW. The evolving epidemiology of HIV/AIDS. AIDS (London, England). (2012) 26:1205–13. doi: 10.1097/QAD.0b013e328354622a
3. Wise, J. New cases of HIV in Europe reach highest level since 1980s. BMJ. (2015) 351:h6419. doi: 10.1136/bmj.h6419
4. Martinello, M, Solomon, SS, Terrault, NA, and Dore, GJ. Hepatitis C. Lancet. (2023) 402:1085–96. doi: 10.1016/S0140-6736(23)01320-X
5. Song, A, Lin, X, and Chen, X. Functional cure for chronic hepatitis B: accessibility, durability, and prognosis. Virol J. (2021) 18:114. doi: 10.1186/s12985-021-01589-x
6. Nachega, JB, Musoke, P, Kilmarx, PH, Gandhi, M, Grinsztejn, B, Pozniak, A, et al. Global HIV control: is the glass half empty or half full? Lancet HIV. (2023) 10:e617–22. doi: 10.1016/S2352-3018(23)00150-9
7. Kalra, DK, Vorla, M, Michos, ED, Agarwala, A, Virani, S, Duell, PB, et al. Dyslipidemia in human immunodeficiency virus disease: JACC review topic of the week. J Am Coll Cardiol. (2023) 82:171–81. doi: 10.1016/j.jacc.2023.04.050
8. Davis, K, Perez-Guzman, P, Hoyer, A, Brinks, R, Gregg, E, Althoff, KN, et al. Association between HIV infection and hypertension: a global systematic review and meta-analysis of cross-sectional studies. BMC Med. (2021) 19:105. doi: 10.1186/s12916-021-01978-7
9. Monroe, AK, Glesby, MJ, and Brown, TT. Diagnosing and managing diabetes in HIV-infected patients: current concepts. Clin Infect Dis. (2015) 60:453–62. doi: 10.1093/cid/ciu779
10. Crum-Cianflone, N, Dilay, A, Collins, G, Asher, D, Campin, R, Medina, S, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. (2009) 50:464–73. doi: 10.1097/QAI.0b013e318198a88a
11. Guaraldi, G, Squillace, N, Stentarelli, C, Orlando, G, D’Amico, R, Ligabue, G, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. (2008) 47:250–7. doi: 10.1086/589294
12. Guaraldi, G, Stentarelli, C, Orlando, G, Zona, S, Carli, F, Ballestri, S, et al. Nonalcoholic fatty liver disease in HIV-infected persons: epidemiology and the role of nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. (2010) 53:278. doi: 10.1097/QAI.0b013e3181c990ed
13. Bulteel, N, and Leen, C. Editorial: NAFLD in HIV infection – call for action. Aliment Pharmacol Ther. (2015) 41:590. doi: 10.1111/apt.13077
14. Than, NN, and Newsome, PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. (2015) 239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001
15. Tomic, D, Kemp, WW, and Roberts, SK. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. Eur J Gastroenterol Hepatol. (2018) 30:1103–15. doi: 10.1097/MEG.0000000000001235
16. Francque, S, Lanthier, N, Verbeke, L, Reynaert, H, Van Steenkiste, C, Vonghia, L, et al. The Belgian Association for Study of the liver guidance document on the Management of Adult and Paediatric non-Alcoholic Fatty Liver Disease. Acta Gastro-Enterol Belg. (2018) 81:55–81.
17. Benedict, M, and Zhang, X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. (2017) 9:715–32. doi: 10.4254/wjh.v9.i16.715
18. Jennings, J, Faselis, C, and Yao, MD. NAFLD-NASH: an under-recognized epidemic. Curr Vasc Pharmacol. (2018) 16:209–13. doi: 10.2174/1570161115666170622074007
19. Bush, H, Golabi, P, and Younossi, ZM. Pediatric non-alcoholic fatty liver disease. Children (Basel). (2017) 4:48. doi: 10.3390/children4060048
20. Bhandari, P, Sapra, A, Ajmeri, MS, Albers, CE, and Sapra, D. Nonalcoholic fatty liver disease: could it be the next medical tsunami? Cureus. (2022) 14:e23806. doi: 10.7759/cureus.23806
21. Lake, JE, Overton, T, Naggie, S, Sulkowski, M, Loomba, R, Kleiner, DE, et al. Expert panel review on nonalcoholic fatty liver disease in persons with human immunodeficiency virus. Clinical Gastroenterol Hepatol. (2022) 20:256–68. doi: 10.1016/j.cgh.2020.10.018
22. Ryom, L, De Miguel, R, Cotter, AG, Podlekareva, D, Beguelin, C, Waalewijn, H, et al. Major revision version 11.0 of the European AIDS clinical society guidelines 2021. HIV Med. (2022) 23:849–58. doi: 10.1111/hiv.13268
23. Kalligeros, M, Vassilopoulos, A, Shehadeh, F, Vassilopoulos, S, Lazaridou, I, Mylonakis, E, et al. Prevalence and characteristics of nonalcoholic fatty liver disease and fibrosis in people living with HIV Monoinfection: a systematic review and meta-analysis. Clinical Gastroenterol Hepatol. (2023) 21:1708–22. doi: 10.1016/j.cgh.2023.01.001
24. Maurice, JB, Patel, A, Scott, AJ, Patel, K, Thursz, M, and Lemoine, M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS (London, England). (2017) 31:1621–32. doi: 10.1097/QAD.0000000000001504
25. Pimpin, L, Cortez-Pinto, H, Negro, F, Corbould, E, Lazarus, JV, Webber, L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. (2018) 69:718–35. doi: 10.1016/j.jhep.2018.05.011
26. Riazi, K, Swain, MG, Congly, SE, Kaplan, GG, and Shaheen, AA. Race and ethnicity in non-alcoholic fatty liver disease (NAFLD): a narrative review. Nutrients. (2022) 14:4556. doi: 10.3390/nu14214556
27. Jin, DC, Zhou, T, Jin, SQ, Cui, ZF, Guo, BQ, and Li, GM. Prevalence and risk factors of non-alcoholic fatty liver disease in people living with HIV across European countries: a protocol for meta-analysis. PROSPERO. (2023). Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023462228
28. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
29. Freeman, MF, and Tukey, JW. Transformation related to the angular and the square root. Ann Math Stat. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
30. Androutsakos, T, Schina, M, Pouliakis, A, Kontos, A, Sipsas, N, and Hatzis, G. Liver fibrosis assessment in a cohort of Greek HIV mono-infected patients by non-invasive biomarkers. Curr HIV Res. (2019) 17:173–82. doi: 10.2174/1570162X17666190809153245
31. Lemoine, M, Barbu, V, Girard, PM, Kim, M, Bastard, JP, Wendum, D, et al. Altered hepatic expression of SREBP-1 and PPARγ is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS (London, England). (2006) 20:387–95. doi: 10.1097/01.aids.0000206503.01536.11
32. Bischoff, J, Gu, W, Schwarze-Zander, C, Boesecke, C, Wasmuth, JC, van Bremen, K, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine. (2021) 40:101116. doi: 10.1016/j.eclinm.2021.101116
33. Busca, C, Sánchez-Conde, M, Rico, M, Rosas, M, Valencia, E, Moreno, A, et al. Assessment of noninvasive markers of steatosis and liver fibrosis in human immunodeficiency virus-monoinfected patients on stable antiretroviral regimens. Open Forum Infect Dis. (2022) 9:ofac279. doi: 10.1093/ofid/ofac279
34. Guaraldi, G, Lonardo, A, Ballestri, S, Zona, S, Stentarelli, C, Orlando, G, et al. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res. (2011) 42:690–7. doi: 10.1016/j.arcmed.2011.12.009
35. Ingiliz, P, Valantin, MA, Duvivier, C, Medja, F, Dominguez, S, Charlotte, F, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. (2009) 49:436–42. doi: 10.1002/hep.22665
36. Kirkegaard-Klitbo, DM, Fuchs, A, Stender, S, Sigvardsen, PE, Kühl, JT, Kofoed, KF, et al. Prevalence and risk factors of moderate-to-severe hepatic steatosis in human immunodeficiency virus infection: the Copenhagen co-morbidity liver study. J Infect Dis. (2020) 222:1353–62. doi: 10.1093/infdis/jiaa246
37. Lallukka-Brück, S, Isokuortti, E, Luukkonen, PK, Hakkarainen, A, Lundbom, N, Sutinen, J, et al. Natural course of nonalcoholic fatty liver disease and type 2 diabetes in patients with human immunodeficiency virus with and without combination antiretroviral therapy-associated Lipodystrophy: a 16-year follow-up study. Clin Infect Dis. (2019) 70:1708–16. doi: 10.1093/cid/ciz435
38. Lemoine, M, Lacombe, K, Bastard, JP, Sébire, M, Fonquernie, L, Valin, N, et al. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients. AIDS (London, England). (2017) 31:1955–64. doi: 10.1097/QAD.0000000000001587
39. Lemoine, M, Assoumou, L, De Wit, S, Girard, PM, Valantin, MA, Katlama, C, et al. Diagnostic accuracy of noninvasive markers of steatosis, NASH, and liver fibrosis in HIV-Monoinfected individuals at risk of nonalcoholic fatty liver disease (NAFLD): results from the ECHAM study. J Acquir Immune Defic Syndr. (2019) 80:E86–94. doi: 10.1097/QAI.0000000000001936
40. Lemoine, M, Assoumou, L, Girard, PM, Valantin, MA, Katlama, C, de Wit, S, et al. Screening HIV patients at risk for NAFLD using MRI-PDFF and transient elastography: a European multicenter prospective study. Clinical Gastroenterol Hepatol. (2023) 21:713–722.e3. doi: 10.1016/j.cgh.2022.03.048
41. Lombardi, R, Sambatakou, H, Mariolis, I, Cokkinos, D, Papatheodoridis, GV, and Tsochatzis, EA. Prevalence and predictors of liver steatosis and fibrosis in unselected patients with HIV mono-infection. Dig Liver Dis. (2016) 48:1471–7. doi: 10.1016/j.dld.2016.08.117
42. Lombardi, R, Lever, R, Smith, C, Marshall, N, Rodger, A, Bhagani, S, et al. Liver test abnormalities in patients with HIV mono-infection: assessment with simple noninvasive fibrosis markers. Ann Gastroenterol. (2017) 30:349–56. doi: 10.20524/aog.2017.0141
43. Michel, M, Labenz, C, Wahl, A, Anders, M, Armandi, A, Huber, Y, et al. Prevalence and risk factors of nonalcoholic steatohepatitis with significant fibrosis in people with HIV. AIDS (London, England). (2022) 36:1665–74. doi: 10.1097/QAD.0000000000003312
44. Milic, J, Menozzi, V, Schepis, F, Malagoli, A, Besutti, G, Franconi, I, et al. Liver steatosis and nonalcoholic fatty liver disease with fibrosis are predictors of frailty in people living with HIV. AIDS (London, England). (2020) 34:1915–21. doi: 10.1097/QAD.0000000000002650
45. Navarro, J, Curran, A, Raventós, B, García, J, Suanzes, P, Descalzo, V, et al. Prevalence of non-alcoholic fatty liver disease in a multicentre cohort of people living with HIV in Spain. Eur J Intern Med. (2023) 110:54–61. doi: 10.1016/j.ejim.2023.01.028
46. Prat, LI, Roccarina, D, Lever, R, Lombardi, R, Rodger, A, Hall, A, et al. Etiology and severity of liver disease in HIV-positive patients with suspected NAFLD: lessons from a cohort with available liver biopsies. J Acquir Immune Defic Syndr. (2019) 80:474–80. doi: 10.1097/QAI.0000000000001942
47. Vujanović, M, Brkić-Jovanović, N, Ilić, D, Drvendžija, Z, Srdić-Galić, B, Turkulov, V, et al. Associations of visceral fat thickness and anthropometric measurements with non-alcoholic fatty liver disease development in male patients mono-infected with human immunodeficiency virus. South Afr J HIV Med. (2019) 20:968. doi: 10.4102/sajhivmed.v20i1.968
48. Calza, L, Colangeli, V, Borderi, M, Coladonato, S, Tazza, B, Fornaro, G, et al. Improvement in liver steatosis after the switch from a ritonavir-boosted protease inhibitor to raltegravir in HIV-infected patients with non-alcoholic fatty liver disease. Infect Dis (Lond). (2019) 51:593–601. doi: 10.1080/23744235.2019.1629008
49. Praktiknjo, M, Djayadi, N, Mohr, R, Schierwagen, R, Bischoff, J, Dold, L, et al. Fibroblast growth factor 21 is independently associated with severe hepatic steatosis in non-obese HIV-infected patients. Liver Int. (2019) 39:1514–20. doi: 10.1111/liv.14107
50. Macías, J, Real, LM, Rivero‐Juarez, A, Merchante, N, Camacho, A, Neukam, K, et al. Changes in liver steatosis evaluated by transient elastography with the controlled attenuation parameter in HIV-infected patients. HIV Med. (2016) 17:766–73. doi: 10.1111/hiv.12384
51. Macías, J, Mancebo, M, Merino, D, Téllez, F, Montes-Ramírez, ML, Pulido, F, et al. Changes in liver steatosis after switching from Efavirenz to Raltegravir among human immunodeficiency virus-infected patients with nonalcoholic fatty liver disease. Clin Infect Dis. (2017) 65:1012–9. doi: 10.1093/cid/cix467
52. Başaran, NÇ, İdilman, İ, Tokuçoğlu, HA, Onur, MR, Sönmezer, MÇ, Özışık, L, et al. Detection of non-alcoholic fatty liver disease with non-invasive tools in Turkish people living with HIV and with apparently normal liver function. Curr HIV Res. (2023) 21:192–201. doi: 10.2174/1570162X21666230714122716
53. Rossetti, B, Borgo, V, Emiliozzi, A, Colaneri, M, Zanelli, G, d’Alessandro, M, et al. Discordant liver fibrosis predictors in virologically suppressed people living with HIV without hepatitis virus infection. Diagnostics. (2022) 12:14. doi: 10.3390/diagnostics12010014
54. Riebensahm, C, Berzigotti, A, Surial, B, Günthard, HF, Tarr, PE, Furrer, H, et al. Factors associated with liver steatosis in people with human immunodeficiency virus on contemporary antiretroviral therapy. Open Forum Infect Dis. (2022) 9:ofac538. doi: 10.1093/ofid/ofac538
55. Moreno‐Perez, O, Reyes‐Garcia, R, Muñoz‐Torres, M, Merino, E, Boix, V, Reus, S, et al. High Irisin levels in nondiabetic HIV-infected males are associated with insulin resistance, nonalcoholic fatty liver disease, and subclinical atherosclerosis. Clin Endocrinol. (2018) 89:414–23. doi: 10.1111/cen.13800
56. Curran, A, Rull, A, Navarro, J, Vidal-González, J, Martin-Castillo, M, Burgos, J, et al. Lipidomics reveals reduced inflammatory lipid species and storage lipids after switching from EFV/FTC/TDF TO RPV/FTC/TDF: a randomized open-label trial. J Clin Med. (2020) 9:1246. doi: 10.3390/jcm9051246
57. Hanttu, A, Vuoti, S, Kivelä, P, Arkkila, P, Lundbom, N, Hakkarainen, A, et al. Liver fat, adipose tissue, and body composition changes after switching from a protease inhibitor or Efavirenz to Raltegravir. AIDS Patient Care STDs. (2021) 35:335–41. doi: 10.1089/apc.2021.0106
58. Austermann, C, Schierwagen, R, Mohr, R, Anadol, E, Klein, S, Pohlmann, A, et al. microRNA-200a: a stage-dependent biomarker and predictor of steatosis and liver cell injury in human immunodeficiency virus patients. Hepatol Commun. (2017) 1:36–45. doi: 10.1002/hep4.1017
59. Schwarz, C, Chromy, D, Bauer, D, Duong, N, Schmidbauer, VU, Schwarz, M, et al. Prevalence and dynamics of NAFLD-associated fibrosis in people living with HIV in Vienna from first presentation to last follow-up. Wien Klin Wochenschr. (2022) 135:420–8. doi: 10.1007/s00508-022-02133-9
60. Sarigul, F, and Deniz, M. Prevalence and related risk factors of non-alcoholic fatty liver disease in HIV/AIDS patients. Klimik J. (2021) 34:50–5. doi: 10.36519/kd.2021.09
61. Marić, D, Brkić, S, Ignjatović, VB, Nikolaševic, Ž, Ilić, D, Vujanović, M, et al. Relation of steatosis to neurocognitive function in people living with HIV. Curr HIV Res. (2020) 18:172–80. doi: 10.2174/1570162X18666200227114310
62. Debroy, P, Lake, JE, Malagoli, A, and Guaraldi, G. Relationship between grip strength and nonalcoholic fatty liver disease in men living with HIV referred to a metabolic clinic. J Frailty Aging. (2019) 8:150–3. doi: 10.14283/jfa.2018.37
63. Mohr, R, Boesecke, C, Dold, L, Schierwagen, R, Schwarze-Zander, C, Wasmuth, JC, et al. Return-to-health effect of modern combined antiretroviral therapy potentially predisposes HIV patients to hepatic steatosis. Medicine (United States). (2018) 97:e0462. doi: 10.1097/MD.0000000000010462
64. Rubio, A, Monpoux, F, Huguon, E, Truchi, R, Triolo, V, Rosenthal-Allieri, MA, et al. Noninvasive procedures to evaluate liver involvement in HIV-1 vertically infected children. J Pediatr Gastroenterol Nutr. (2009) 49:599–606. doi: 10.1097/MPG.0b013e3181a15b72
65. Liu, YL, Patman, GL, Leathart, JBS, Piguet, AC, Burt, AD, Dufour, JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. (2014) 61:75–81. doi: 10.1016/j.jhep.2014.02.030
66. Romeo, S, Kozlitina, J, Xing, C, Pertsemlidis, A, Cox, D, Pennacchio, LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. (2008) 40:1461–5. doi: 10.1038/ng.257
67. Luo, F, Oldoni, F, and Das, A. TM6SF2: a novel genetic player in nonalcoholic fatty liver and cardiovascular disease. Hepatol Commun. (2022) 6:448–60. doi: 10.1002/hep4.1822
68. Wymant, C, Bezemer, D, Blanquart, F, Ferretti, L, Gall, A, Hall, M, et al. A highly virulent variant of HIV-1 circulating in the Netherlands. Science (New York, NY). (2022) 375:540–5. doi: 10.1126/science.abk1688
70. Pires, LB, Rocha, R, Vargas, D, Daltro, C, and Cotrim, HP. Non-alcoholic fatty liver disease in patients infected with human immunodeficiency virus: a systematic review. Rev Assoc Med Bras (1992). (2020) 66:81–6. doi: 10.1590/1806-9282.66.1.81
71. Gerges, SH, Wahdan, SA, Elsherbiny, DA, and El-Demerdash, E. Non-alcoholic fatty liver disease: an overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. (2021) 271:119220. doi: 10.1016/j.lfs.2021.119220
72. Lim, HW, and Bernstein, DE. Risk factors for the development of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including genetics. Clin Liver Dis. (2018) 22:39–57. doi: 10.1016/j.cld.2017.08.008
73. Huh, Y, Cho, YJ, and Nam, GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. (2022) 31:17–27. doi: 10.7570/jomes22021
74. Núñez, MHepatotoxicity of antiretrovirals: incidence, mechanisms and management. Journal of Hepatology. (2006). 44 Suppl:S139–9.
75. Clotet, B, Bellos, N, Molina, JM, Cooper, D, Goffard, JC, Lazzarin, A, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. (2007) 369:1169–78. doi: 10.1016/S0140-6736(07)60497-8
76. Eron, JJ, Orkin, C, Gallant, J, Molina, JM, Negredo, E, Antinori, A, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS (London, England). (2018) 32:1431–42. doi: 10.1097/QAD.0000000000001817
77. Pelchen-Matthews, A, Larsen, JF, Shepherd, L, Begovac, J, Pedersen, K, de Wit, S, et al. Hypersensitivity reactions, hepatotoxicity, and other discontinuations in persons receiving integrase strand transfer inhibitors: results from the EuroSIDA study. HIV Res Clin Pract. (2021) 22:160–8.
78. Dominguez, C. NASH syndrome: the coming epidemic. Gastroenterol Nurs. (2018) 41:316–20. doi: 10.1097/SGA.0000000000000334
79. Mehta, R, and Younossi, ZM. Natural history of nonalcoholic fatty liver disease. Clin Liver Dis. (2012) 1:112–3. doi: 10.1002/cld.27
80. Akter, S. Non-alcoholic fatty liver disease and steatohepatitis: risk factors and pathophysiology. Middle East J Dig Dis. (2022) 14:167–81. doi: 10.34172/mejdd.2022.270
81. Vodkin, I, Valasek, MA, Bettencourt, R, Cachay, E, and Loomba, R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. (2015) 41:368–78. doi: 10.1111/apt.13052
82. Ghodsian, N, Abner, E, Emdin, CA, Gobeil, É, Taba, N, Haas, ME, et al. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease. Cell Rep Med. (2021) 2:100437. doi: 10.1016/j.xcrm.2021.100437
Keywords: NAFLD, people living with HIV, prevalence, risk factor, Europe, meta-analysis
Citation: Jin D, Jin S, Zhou T, Cui Z, Guo B, Li G and Zhang C (2024) Regional variation in NAFLD prevalence and risk factors among people living with HIV in Europe: a meta-analysis. Front. Public Health. 11:1295165. doi: 10.3389/fpubh.2023.1295165
Received: 15 September 2023; Accepted: 08 December 2023;
Published: 04 January 2024.
Edited by:
Jiaye Liu, Shenzhen University, ChinaReviewed by:
Dinesh Yadav, University of Alabama at Birmingham, United StatesCopyright © 2024 Jin, Jin, Zhou, Cui, Guo, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dachuan Jin, ZGFjaHVhbmppbkBnbWFpbC5jb20=; Guangming Li, bGdtMTc3Z3JvdXBAMTYzLmNvbQ==; Chunming Zhang, emhhbmdjaHVubWluZzIwMjJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.