
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 31 October 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1293710
 Jui-Hung Hsu1†
Jui-Hung Hsu1† Chien-Cheng Liu2,3,4
Chien-Cheng Liu2,3,4 I-Wen Chen1†
I-Wen Chen1† Jheng-Yan Wu5
Jheng-Yan Wu5 Po-Yu Huang6
Po-Yu Huang6 Ting-Hui Liu7
Ting-Hui Liu7 Kuo-Chuan Hung8,9*
Kuo-Chuan Hung8,9*Background: Mild cognitive impairment (MCI) is an intermediate stage between normal ageing and dementia. The early identification of MCI is important for timely intervention. The visual cognitive assessment test (VCAT) is a brief language-neutral screening tool for detecting MCI/mild dementia. This meta-analysis evaluated the diagnostic efficacy of the VCAT for MCI/mild dementia.
Methods: Medline, Embase, Google Scholar, and Cochrane Library were searched from their inception until August 2023 to identify studies using VCAT to diagnose MCI/mild dementia. The primary outcome was to assess the diagnostic accuracy of the VCAT for detecting MCI/mild dementia through area under the receiver operating characteristic curve (AU-ROC) analysis. The secondary outcome was to explore the correlation between VCAT scores and MCI/mild dementia presence by comparing scores among patients with and without MCI/mild dementia. Pooled sensitivity, specificity, and area under the curve (AUC) were calculated.
Results: Five studies with 1,446 older adults (mean age 64–68.3 years) were included. The percentage of participants with MCI/mild dementia versus controls ranged from 16.5% to 87% across studies. All studies were conducted in Asian populations, mostly Chinese, in Singapore and Malaysia. The pooled sensitivity was 80% [95% confidence interval (CI) 68%–88%] and the specificity was 75% (95% CI 68%–80%). The AU-ROCC was 0.77 (95% CI 0.73–0.81). Patients with MCI/mild dementia had lower VCAT scores than the controls (mean difference −6.85 points, p < 0.00001).
Conclusion: VCAT demonstrated acceptable diagnostic accuracy in distinguishing MCI/mild dementia in cognitively normal older adults. As a language-neutral and culturally unbiased tool, the VCAT shows promise in detecting MCI/mild dementia. Further studies in non-Asian populations are required.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42023453453.
Within the continuum of cognitive aging, mild cognitive impairment (MCI) represents the intermediate stage between normal aging and dementia (1, 2). A comprehensive review showed that 15.56% of community-dwelling adults aged ≥50 years have MCI (3), and this prevalence rate increases with age, reaching up to 25.2% among those aged 80–84 years (3, 4). MCI frequently manifests as objective cognitive impairments, including memory, thinking, and behavioral deterioration (5). Although MCI does not significantly impair older adults’ daily activities and independence (5), approximately 40% of individuals with MCI eventually develop dementia (6), highlighting that MCI is not a benign condition but rather often a transitional stage to more severe forms of cognitive impairment, including dementia. Compared with MCI, in which older adults’ daily activities and independence are not significantly impaired, mild dementia is characterized by more pronounced cognitive impairments that significantly interfere with daily functioning. Evidence suggests that both MCI and mild dementia are linked to higher mortality rates, suggesting that even minor cognitive deterioration reduces life expectancy (7, 8). A recent large-scale study examined Medicare claims records from 2015 to 2019, covering 38–41 million beneficiaries aged 65 years or older, to ascertain the real-world diagnosis rates for MCI and dementia (9). Findings from 2017 to 2019 showed that only 7.9% of anticipated MCI cases were actually diagnosed (9), implying that approximately 7.4 million cases went undetected. Consequently, considering the vast potential of the cognitively impaired population, there is an increasing need for early diagnosis of cognitive impairment and timely interventions.
Cognitive screening tools, including the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), are commonly used (10, 11). Although the MMSE and MoCA have good diagnostic utility, their clinical application may be limited in detecting MCI across diverse linguistic, cultural, and educational backgrounds (12, 13). The visual cognitive assessment test (VCAT) is a brief language-neutral cognitive screening tool designed to detect MCI/mild dementia. It consists of 10 subtests assessing memory, visuospatial function, and executive function using non-verbal, visually based items without complex verbal instructions (14). The test takes approximately 15–20 min to complete (14). The visual-based format of the VCAT makes it especially appropriate for identifying cognitive impairments in diverse, multicultural, and multilingual populations globally (12). Previous studies have affirmed the efficacy of the VCAT in identifying MCI/mild dementia (12, 15); however, the limited sample size in these studies underscores the need for a systematic evaluation of its effectiveness. This meta-analysis aimed to investigate the efficacy of the VCAT in the diagnosis of patients with MCI/mild dementia.
This review was designed and reported in accordance with PRISMA guidelines. Details of the protocol registration are available in PROSPERO (Number: CRD42023453453).
Articles were identified through a search of databases, including MEDLINE, Google Scholar, EMBASE, and the Cochrane Library, spanning from their inception to August 11, 2023. The search was performed employing the following keywords: (“cognitive impairment” or “cognitive decline” or “impaired cognition” or “cognitive dysfunction” or “cognitive disorder” or “cognitive disability” or “cognitive deficit”) and “visual cognitive assessment test.” Moreover, to augment the inclusiveness of the literature exploration, controlled vocabulary search terms, including MEDLINE (MeSH) and EMBASE (Emtree), were used. A manual review of the reference lists from the acquired studies was performed to identify additional studies that might be eligible for inclusion. There were no restrictions based on the language in which the studies were published. The search strategy used to scan the MEDLINE database is presented in Table 1.
The criteria for article inclusion were as follows: first, studies that employed the VCAT for diagnosing MCI/mild dementia irrespective of ethnicity were considered. Second, studies that provided data on the sensitivity, specificity, and number of patients afflicted with MCI/mild dementia were considered. A diverse range of study designs (e.g., cohort, case-control, and randomized controlled studies) were deemed eligible for inclusion in this meta-analysis.
Studies falling into any of the following four categories were excluded from the analysis: (1) non-peer-reviewed reports; (2) abstracts, letters, review articles, and case series; (3) studies devoid of information pertaining to MCI/mild dementia or VCAT; and (4) reports for which full-text versions could not be accessed.
Two researchers independently collected the following details: study characteristics (e.g., sample size), demographics of the enrolled patients, criteria for the definition of MCI/mild dementia, ethnicity, language, education level, sensitivity and specificity values, area under the curve (AUC) value, and the time required for VCAT. Any disagreements were resolved by a third investigator. Efforts were made to contact the authors of the studies to acquire missing or incomplete data.
The main objective was to evaluate the diagnostic precision of the VCAT in diagnosing MCI/mild dementia by calculating the area under the receiver operating characteristic curve (AU-ROC). The criteria for defining MCI/mild dementia were derived from those used in individual studies. The secondary objective focused on the relationship between VCAT scores and the presence of MCI/mild dementia by calculating the score differences between patients with and without MCI/mild dementia.
To evaluate the quality and potential bias of studies assessing the accuracy of diagnostic tests, the Quality Assessment for Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used (16). The QUADAS-2 tool has four domains: patient selection, index test, reference standard, and flow/timing. Independent reviewers assign judgments of “low,” “high,” or “unclear” risk of bias to each signaling question within the domains. The authors engaged in thorough discussions to reach consensus in instances where disagreements or discrepancies emerged. If discrepancies remained unresolved, a third team member was brought in to facilitate the resolution.
In this meta-analysis, ROC curve analysis was employed to determine the area under the curve (AUC), offering a comprehensive gauge of diagnostic accuracy. Furthermore, Fagan’s nomogram was applied to estimate the post-test probability of the disease by integrating the pre-test probability with the test likelihood ratios (17). These ratios provide insights into how much a positive or negative test result alters the likelihood of the disease: values between two and five marginally increase probability, five to ten moderately increase probability, and above ten substantially elevate the post-test likelihood compared to the pre-test probability (18). Deek’s funnel plot was used for publication bias assessment. It analyzes the correlation between study size and effect size. To identify whether the findings were statistically significant, a significance level of 0.05 was set. Statistical analyses were performed using RevMan software or MIDAS command in Stata 15 (StataCorp LLC., College Station, TX, United States).
The selection process for the eligible studies is shown in Figure 1. The PRISMA flow diagram presented in Figure 1 was constructed based on the recommendations of a previous study (19). During the search process, we did not limit the publication year. Given that VCAT is a new tool, only a limited number of records were identified. Overall, a comprehensive literature search yielded only 37 records. Of these, 27 were subsequently excluded owing to duplication (n = 12) and title- and abstract-based exclusion (n = 15). After a thorough full-text reading and assessment of the 10 reports, we decided to exclude 5 of them from our review for various reasons. One study was excluded because it did not provide the necessary outcomes relevant to our review. Two reports were conference abstracts and did not provide comprehensive data for our analysis. Another report was a review, and as our aim was to include the original research, we decided not to incorporate it. Finally, one of the reports was not a peer-reviewed article, and given the importance of peer review in ensuring the quality and validity of research, we deemed it inappropriate for inclusion in our review. Finally, five studies published between 2016 and 2023 were determined eligible for inclusion (12, 15, 20–22). Further search of the reference lists within these selected articles did not reveal any additional studies aligned with the inclusion criteria.
An overview of the study characteristics, collectively comprising 1,446 patients, is provided in Table 2. Notably, all studies encompassed individuals aged >60 years, ranging from 64 to 68.3 years. Regarding sex distribution, a female preponderance was observed, with male proportions varying between 39.5% and 51.4%. The sample sizes varied across the studies, ranging from 184 to 471 participants. Of these, four included patients with MCI or mild dementia of the Alzheimer’s disease (AD) type (12, 15, 20, 21), whereas one exclusively focused on individuals with MCI (22). The percentage of participants with MCI/mild dementia versus controls (i.e., case: control ratio) across these studies ranged from 16.5% to 87%. All the studies included in the analysis were conducted within the geographical confines of Asian countries, with four of them conducted in Singapore (12, 15, 20, 22) and the remaining one in Malaysia (21). The ethnic composition predominantly consists of Chinese individuals, varying between 52.5% and 92.5%. The participants demonstrated an educational background of over 10 years, encompassing durations ranging from 10.5 to 13.59 years. Four studies specified the language employed in assessments (12, 15, 21, 22), while one omitted this detail (20). Three studies reported the time required for VCAT completion (12, 15, 21), indicating that the test did not exceed 20 min.
An overview of the risk of bias and applicability issues in the included studies is shown in Figure 2. One area of concern relates to the selection or recruitment of patients, which could introduce sample bias; consequently, the risk of bias for patient selection remains unclear in all studies (12, 15, 20–22). Regarding the index test, three studies did not pre-specify a threshold for what would constitute a positive test result on the VCAT, leaving the risk of bias in this area also unclear (20–22). All other risks of bias or concerns regarding applicability were deemed low across all the included studies.
The definitions of MCI/mild dementia are summarized in Table 3. For MCI diagnosis, one study adopted the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria (20), another study employed Petersen’s criteria (22), and three studies concurrently utilized both criteria for diagnosis (12, 15, 21). For mild dementia, four studies relied upon the NIA-AA criteria (12, 15, 20, 21), whereas one study also referenced the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria (21).
An overview of the diagnostic efficacy of the VCAT within each individual study is presented in Table 4. Notably, four studies exhibited an AU-ROC exceeding 0.8, spanning from 0.837 to 0.933, whereas a single study reported an AU-ROC of 0.794. The sensitivity values ranged from 0.754 to 0.921, whereas the specificity values were within the range of 0.711–0.872. Supplementary Table S1 summarizes the potential limitations enumerated for each study included in the meta-analysis.
Five studies reported VCAT values in patients with (n = 857) or without (n = 589) MCI/mild dementia. Patients with MCI/mild dementia had lower VCAT values than those without MCI/mild dementia (mean difference, −6.85; 95% CI: −9.18 to −4.51; p < 0.00001; I2 = 96%) (Figure 3) (12, 15, 20–22).
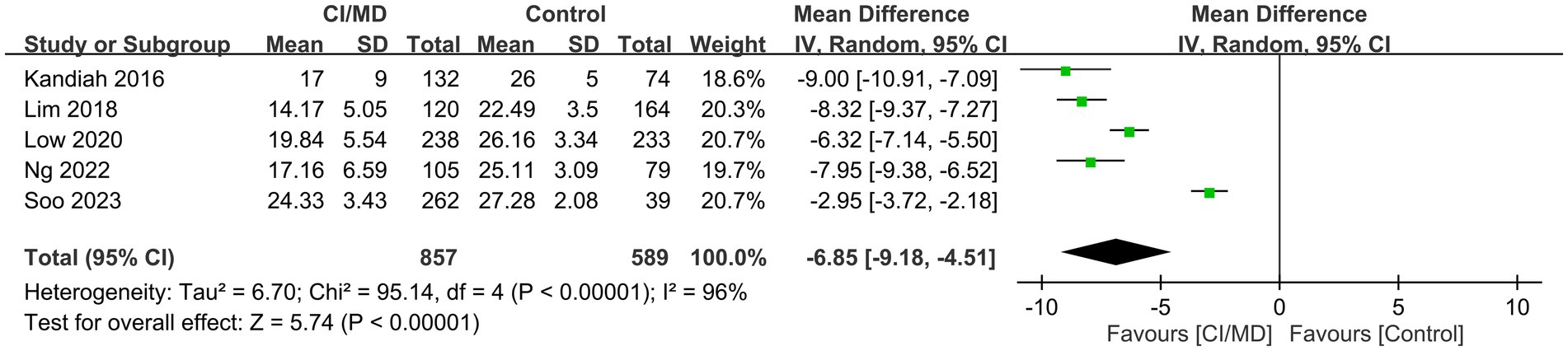
Figure 3. Forest plot showing low visual cognitive assessment test (VCAT) values in patients with mild cognitive impairment (MCI)/mild dementia compared with those without MCI/mild dementia.
The combined sensitivity was 80% (68%–88%), whereas the pooled specificity was 75% (68%–80%) (Figure 4). The combined AUC was 0.77 (95% CI, 0.73–0.81) (Figure 5). The pooled positive likelihood ratio was 3 and the negative likelihood ratio was 0.27. If the pre-test probability was set at 50%, the post-test probability increased to 76% for a positive test and decreased to 21% for a negative test according to Fagan’s nomogram (Figure 6). The risk of publication bias was low (p = 0.65) (Figure 7). These results suggest that VACT is useful in diagnosing MCI/mild dementia.
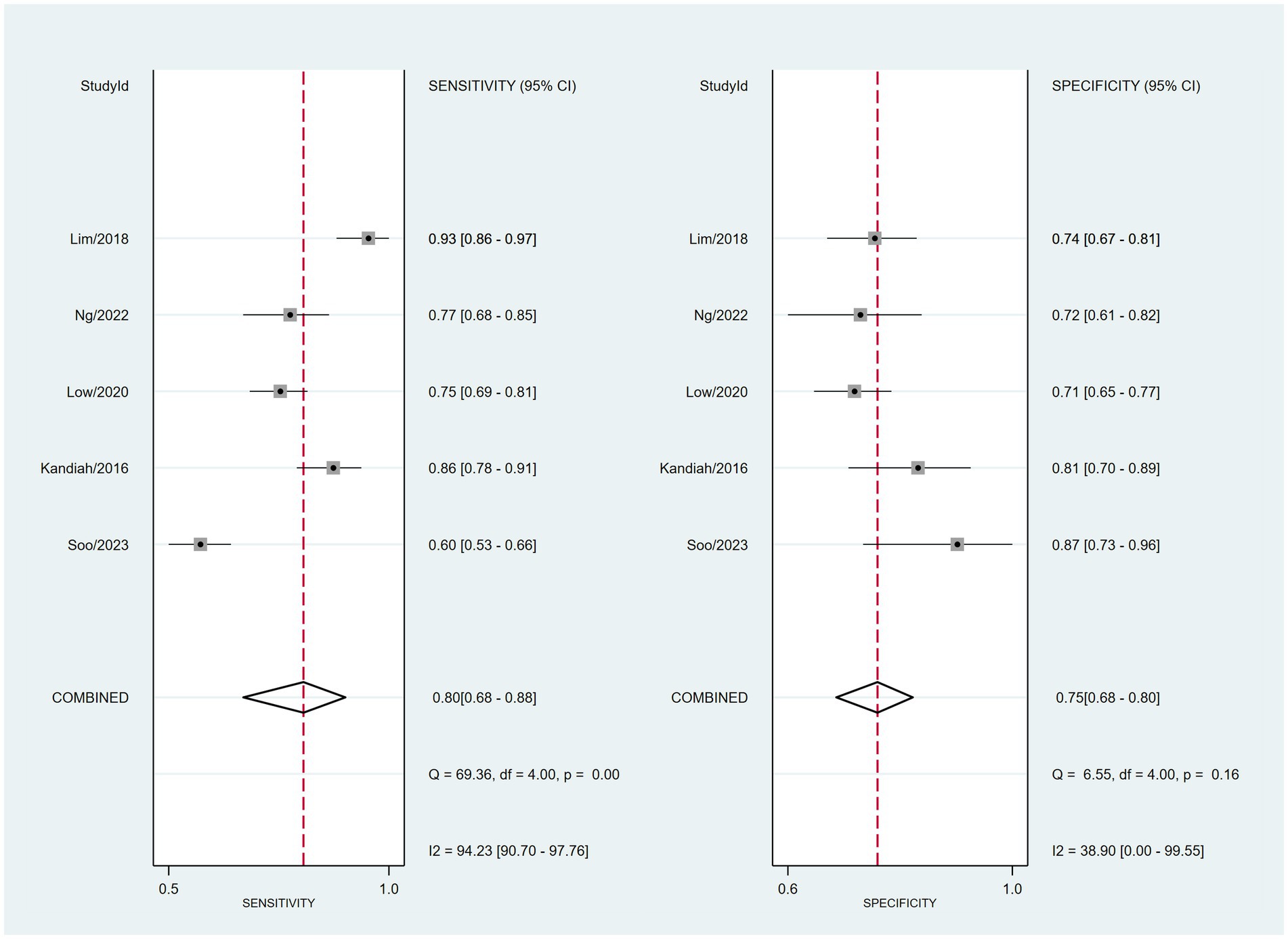
Figure 4. Forest plot depicting the combined sensitivity and specificity of 80% (68%–88%) and 75% (68%–80%), respectively, of the VCAT for mild cognitive impairment (MCI)/mild dementia.
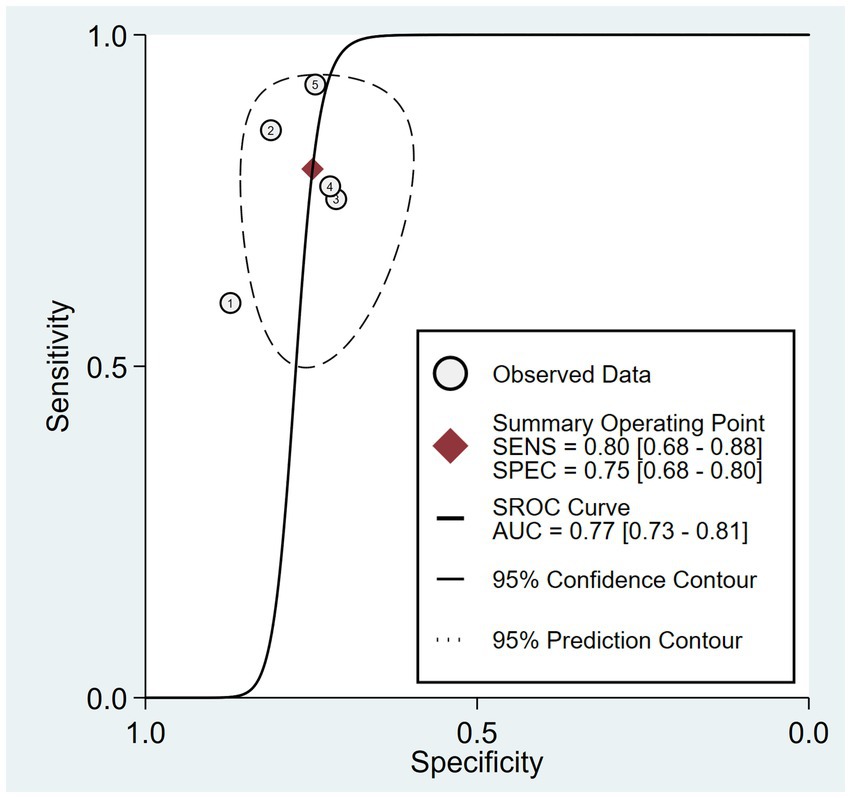
Figure 5. Hierarchical summary receiver operating characteristic (HSROC) curves illustrating an area under the curve (AUC) of 0.77.
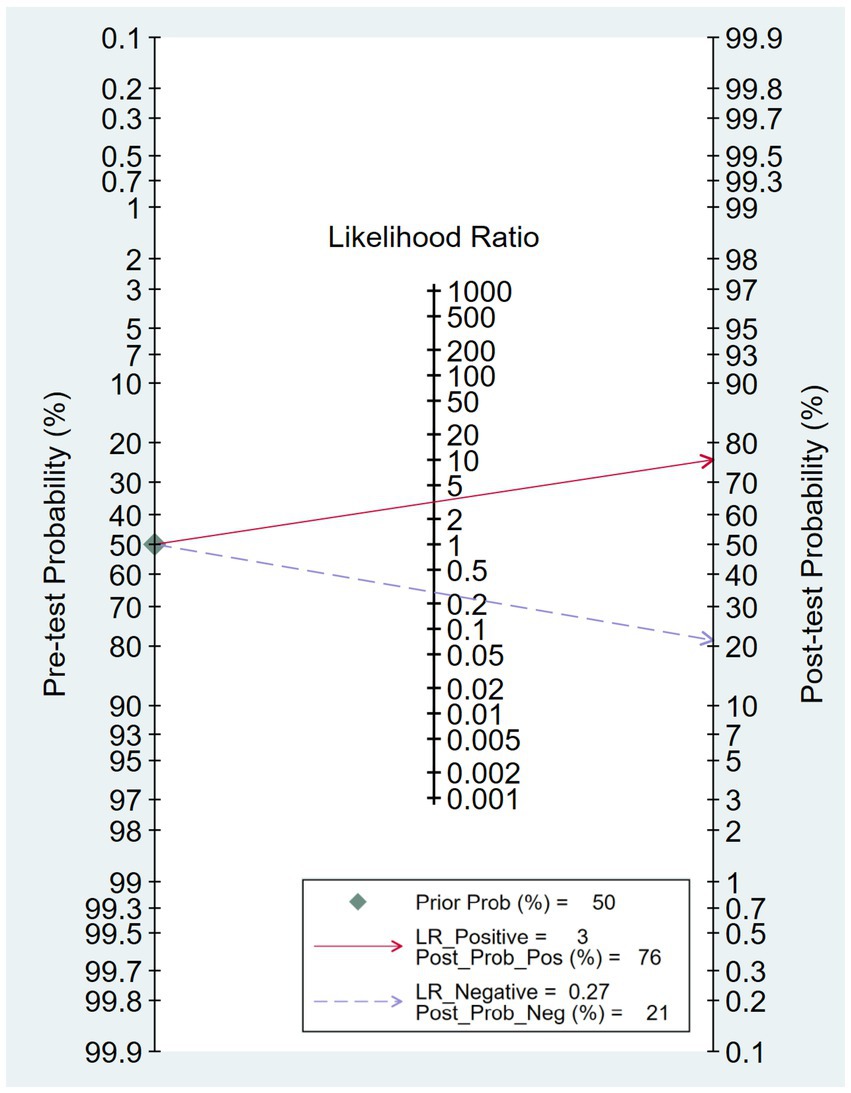
Figure 6. Fagan’s nomogram revealing the post-test probability of mild cognitive impairment (MCI)/mild dementia.
The increased use of brief cognitive assessments in primary care could improve diagnosis rates and provide early and appropriate treatment to patients. Considering the increasing attention toward cognitive impairment screening tools, to the best of our knowledge, this is the first meta-analysis designed to assess the diagnostic efficacy of the VCAT in identifying cognitive impairment, specifically including both MCI and mild dementia. Based on a meta-analysis of 1,446 patients from studies conducted in Asian countries (four in Singapore and one in Malaysia), patients with MCI/mild dementia had lower VCAT scores than those without MCI/mild dementia, with a mean difference of −6.85. VCAT showed a combined sensitivity and specificity of 80% and 75%, respectively, with an overall AU-ROC of 0.77.
In the current meta-analysis, VCAT showed a combined sensitivity and specificity of 80% and 75%, respectively, with an overall AU-ROC of 0.77. This finding indicates an acceptable diagnostic performance of the VCAT in distinguishing MCI/mild dementia from the healthy control group. In a previous systematic review, the AU-ROC for the MoCA and MMSE was 0.883 and 0.78, respectively (23). While these findings suggest that the MoCA and MMSE exhibit higher diagnostic efficacy than the VCAT, this comparison was not conducted head-to-head owing to the absence of primary data. Several studies have reported similar diagnostic efficacies for VCAT and MoCA (15, 21). Lim et al. (15) reported that the AU-ROC for detecting patients with MCI/mild dementia was 0.905 and 0.916 for the VCAT and MoCA, respectively, suggesting that both tests are highly effective in identifying MCI/mild dementia. Similarly, Ng et al. (21) demonstrated no statistically significant difference in diagnostic efficacy between MoCA and VCAT. Recent evidence from 13 studies has shown that the effectiveness of the MoCA in identifying MCI varies, with sensitivity and specificity ranging from 73.5% to 83.8% and from 70.8% to 91.3%, respectively, depending on the selected cutoff score (24). Considering that the VCAT demonstrated a combined sensitivity and specificity of 80% and 75%, respectively, both tools appeared to be effective in detecting MCI/mild dementia.
Traditional cognitive tests, including the MMSE and MoCA, have demonstrated limitations when applied to diverse populations, primarily owing to sociodemographic factors and linguistic dependency-related biases (24–28). In contrast, VCAT presents a more universally accessible alternative. For instance, a multicenter study across four Asian countries demonstrated that the language used to administer VCAT did not significantly affect its performance (15). Another study showed that even when adjusting VCAT scores for factors including race and education, the tool remained effective in differentiating between cognitively normal individuals and those with MCI from those with mild dementia (20). Furthermore, racial analysis of VCAT scores showed no significant disparities, emphasizing cross-cultural fairness (15). Contrary to language-dependent tests such as the MMSE and MoCA, which necessitate translation and cultural adaptation (29–31), the VCAT relies on visual cognition and nonverbal responses, thereby eliminating the need for such modifications. This feature makes it adaptable across diverse linguistic and cultural contexts. Moreover, traditional screening tools (e.g., MoCA) are known to exhibit a ceiling effect, wherein a large number of cognitively normal individuals score near the test’s maximum, thereby reducing the tool’s sensitivity in detecting MCIs (31, 32). Conversely, the VCAT shows a more normally distributed scoring pattern among cognitively normal adults without excessive clustering at the upper end. This absence of a ceiling effect more effectively enhances the ability of the VCAT to distinguish between normal cognitive function and MCI/mild dementia than its MMSE and MoCA counterparts (12, 33).
The emphasis of the VCAT on evaluating episodic memory and executive function, with half of the test specifically geared toward assessing episodic memory, makes it a potential screening tool for early AD detection and monitoring. A previous study indicated that deficits in episodic memory can signal the future development of Alzheimer’s dementia in otherwise healthy adults (34). Furthermore, another study established a correlation between VCAT scores and the presence of amyloid beta, a biomarker of Alzheimer’s pathology (35). Based on imaging findings, another study reported a possible link between VCAT scores and medial temporal lobe atrophy, a sign of neurodegeneration (22). Collectively, these findings suggest that VCAT is sensitive to cognitive domains and biological markers associated with the early stages of Alzheimer’s pathology. This makes the VCAT a promising clinical instrument for identifying early neurodegenerative alterations in individuals at the preclinical stage of AD.
In the studies included in the current meta-analysis, Low et al. (20) examined the VCAT scores derived from healthy comparisons and MCI/mild dementia groups, discovering significant differences in the results of memory-related tasks between these two groups. This observation was further supported by Soo et al. (22), who found that only memory-related domains exhibited pronounced differences between healthy comparisons and MCI/mild dementia groups (22). Pearson’s correlation analysis further revealed a high correlation between VCAT scores and memory domain neuropsychological assessments (22). Studies by Ng et al. (21) and Lim et al. (15), also yielded similar results, showing significant differences in VCAT scores across various domains between healthy comparisons and MCI/mild dementia groups, with memory exhibiting the most pronounced decline. These findings not only reflect the substantial weighting of memory tasks within the VCAT, but also underscores the effectiveness of the test in identifying MCI/mild dementia. A systematic review recommended the MoCA over the MMSE as the preferred tool for screening MCI/mild dementia in primary care settings (36). The finding that Ng et al. (21) revealed a high correlation (i.e., r = 0.819) between the MoCA and VCAT, further highlighting the robustness of the VCAT as a cognitive assessment tool.
Several studies have highlighted the feasibility of integrating the VCAT into clinical settings, reporting its ease of completion as a significant advantage (22, 37). The average time needed to complete the VCAT was comparable to that of the MoCA for both healthy controls (10.0 vs. 10.4 min) and those with cognitive impairment (15.4 vs. 15.4 min), indicating that participants typically perceived its instructions as easy or easier to understand than those of the MoCA (37). In a study by Soo et al. (22), 94.3% of the participants believed that VCAT instructions were either equivalent to or simpler than those of the MoCA. However, VCAT is designed for patients with normal visual capabilities, making it less suitable for those with visual impairments. To optimize MCI/mild dementia detection using the VCAT, we recommend its use in conjunction with a brief clinical interview. This should include checks for subjective memory complaints using tools such as the subjective memory complaints questionnaire (38) and an assessment for functional decline using measures such as the instrumental activities of daily living scale (39).
The use of a visual-based format in the VCAT not only minimizes potential variables that could influence the results but also facilitates digital implementation (14). This clinical application is particularly significant for individuals with undiagnosed dementia who do not actively seek medical care or consultation (40). When cognitive decline becomes a concern, digital technology makes it easier to conduct initial assessments at home by shifting away from old-fashioned paper-and-pencil methods to more efficient digital platforms (41). Moreover, growing acceptance of technology among older adults has spurred the development of remote digital cognitive assessments capable of detecting subtle cognitive shifts (42). Previous visual-based tests, such as the phototest for dementia, were largely developed using Western populations (43). However, the VCAT was conceived within Eastern cohorts, providing that our study added significance in confirming its diagnostic effectiveness, particularly within the Asian context.
The notable heterogeneity in our meta-analysis can be partially attributed to the study by Soo et al. (22), which reported lower sensitivity and AUC values than other studies. This discrepancy could arise from the unique composition of the study population, which consisted solely of patients with MCI (22). In contrast, other studies have included patients with both MCI and mild dementia. There is a distinction between MCI and mild dementia. While individuals with MCI show subtle but observable cognitive issues, those with mild dementia have more severe cognitive impairments that affect daily activities. The notable heterogeneity suggests that the VCAT might have reduced sensitivity in detecting MCI compared to mild dementia, which could explain the diminished sensitivity observed in Soo’s et al. (22) study. While analyzing data from patients with MCI or mild dementia can potentially confound the discriminative value of the tool, our results offer clinicians initial insight into the strengths and weaknesses of the test. We believe that future research may build upon our findings and further delineate the diagnostic value of VACT in specific patient populations.
The current meta-analysis has several limitations. First, all included studies were conducted in Asian populations, primarily Chinese participants in Singapore and Malaysia. This limits the generalizability of the findings to non-Asian and diverse populations. Further studies on other ethnicities and cultural groups are required. Second, only five studies were included in the meta-analysis. A greater number of high-quality studies would allow for a more robust quantitative synthesis and subgroup analyses to explore potential sources of heterogeneity. Third, the criteria used for defining MCI/mild dementia varied somewhat across studies, which could have introduced inconsistencies. Standardized diagnostic criteria improve the comparability. Fourth, data on key demographic variables, including gender, education, and language, have been inconsistently reported across studies. The impact of these factors on the VCAT accuracy could not be fully evaluated. Fifth, no data have been reported regarding the reliability, learning effects, or responsiveness of the VCAT to repeat administration. This further demonstrates its utility in monitoring cognitive changes over time. Finally, given the significant heterogeneity observed in the pooled sensitivity (94.23%), a systematic review might be better suited than a meta-analysis to present the existing literature on the use of VCAT in diagnosing cognitive decline. While pooling data in a meta-analysis can amplify the risk of misinterpretation owing to such pronounced heterogeneity, our findings underscore the limitations of the included studies and highlight potential directions for future research.
This meta-analysis of five studies involving 1,446 older adults indicated that the VCAT, as a nonverbal and visual-based cognitive screening tool, has moderate diagnostic accuracy in distinguishing individuals with MCI/mild dementia from cognitively normal controls. The pooled sensitivity and specificity were 80% and 75%, respectively, with an AU-ROC of 0.77. Further rigorous studies are required to confirm its applicability and validity across different populations and settings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
J-HH: Data curation, Investigation, Writing – original draft, Writing – review & editing. C-CL: Investigation, Writing – original draft, Writing – review & editing. I-WC: Investigation, Visualization, Writing – original draft, Writing – review & editing. J-YW: Methodology, Validation, Writing – original draft, Writing – review & editing. P-YH: Data curation, Resources, Writing – original draft, Writing – review & editing. T-HL: Data curation, Methodology, Writing – original draft, Writing – review & editing. K-CH: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Chi Mei Medical Center, Tainan, Taiwan (Grant number: CMOR11203).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1293710/full#supplementary-material
1. Petersen, RC. Mild cognitive impairment. Continuum. (2016) 22:404. doi: 10.1212/CON.0000000000000313
2. Knopman, DS, Beiser, A, Machulda, MM, Fields, J, Roberts, RO, Pankratz, VS, et al. Spectrum of cognition short of dementia: Framingham heart study and Mayo clinic study of aging. Neurology. (2015) 85:1712–21. doi: 10.1212/WNL.0000000000002100
3. Bai, W, Chen, P, Cai, H, Zhang, Q, Su, Z, Cheung, T, et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing. (2022) 51:afac173. doi: 10.1093/ageing/afac173
4. Petersen, RC, Lopez, O, Armstrong, MJ, Getchius, TS, Ganguli, M, Gloss, D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
5. Langa, KM, and Levine, DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. (2014) 312:2551–61. doi: 10.1001/jama.2014.13806
6. Mitchell, AJ, and Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. (2009) 119:252–65. doi: 10.1111/j.1600-0447.2008.01326.x
7. Zhang, Y, Natale, G, and Clouston, S. Incidence of mild cognitive impairment, conversion to probable dementia, and mortality. Am J Alzheimers Dis Other Dement. (2021) 36:153331752110122. doi: 10.1177/15333175211012235
8. Wilson, RS, Segawa, E, Hizel, LP, Boyle, PA, and Bennett, DA. Terminal dedifferentiation of cognitive abilities. Neurology. (2012) 78:1116–22. doi: 10.1212/WNL.0b013e31824f7ff2
9. Mattke, S, Jun, H, Chen, E, Liu, Y, Becker, A, and Wallick, C. Expected and diagnosed rates of mild cognitive impairment and dementia in the US Medicare population: observational analysis. Alzheimers Res Ther. (2023) 15:1–8. doi: 10.1186/s13195-023-01272-z
10. Ciesielska, N, Sokołowski, R, Mazur, E, Podhorecka, M, Polak-Szabela, A, and Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State xamination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. (2016) 50:1039–52. doi: 10.12740/PP/45368
11. Fage, BA, Chan, CC, Gill, SS, Noel-Storr, AH, Herrmann, N, Smailagic, N, et al. Mini-Cog for the detection of dementia within a community setting. Cochrane Database Syst Rev. (2021) 2021:Cd010860. doi: 10.1002/14651858.CD010860.pub3
12. Kandiah, N, Zhang, A, Bautista, DC, Silva, E, Ting, SKS, Ng, A, et al. Early detection of dementia in multilingual populations: visual cognitive assessment test (VCAT). J Neurol Neurosurg Psychiatry. (2016) 87:156–60. doi: 10.1136/jnnp-2014-309647
13. Ng, KP, Chiew, HJ, Lim, L, Rosa-Neto, P, Kandiah, N, and Gauthier, S. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Rev Neurother. (2018) 18:859–69. doi: 10.1080/14737175.2018.1532792
14. Gauthier, S, Rosa-Neto, P, Morais, JA, and Webster, C. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Dis Int. (2021) 2022:30.
15. Lim, L, Ng, TP, Ong, AP, Tan, MP, Cenina, AR, Gao, Q, et al. A novel language-neutral visual cognitive assessment test (VCAT): validation in four southeast Asian countries. Alzheimers Res Ther. (2018) 10:1–9. doi: 10.1186/s13195-017-0333-z
16. Whiting, PF, Rutjes, AW, Westwood, ME, Mallett, S, Deeks, JJ, Reitsma, JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
17. Tsai, WW, Hung, KC, Huang, YT, Yu, CH, Lin, CH, Chen, IW, et al. Diagnostic efficacy of sonographic measurement of laryngeal air column width difference for predicting the risk of post-extubation stridor: a meta-analysis of observational studies. Front Med. (2023) 10:1109681. doi: 10.3389/fmed.2023.1109681
18. Grimes, DA, and Schulz, KF. Refining clinical diagnosis with likelihood ratios. Lancet. (2005) 365:1500–5. doi: 10.1016/S0140-6736(05)66422-7
19. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Low, A, Lim, L, Lim, L, Wong, B, Silva, E, Ng, KP, et al. Construct validity of the visual cognitive assessment test (VCAT)—a cross-cultural language-neutral cognitive screening tool. Int Psychogeriatr. (2020) 32:141–9. doi: 10.1017/S1041610219000504
21. Ng, LY, Chin, CJ, Danial, M, Albart, SA, Suppiah, PD, Ganasegeran, K, et al. Validation of the visual cognitive assessment test (VCAT) for the early diagnosis of cognitive impairment in multilingual population in Malaysia. Psych. (2022) 4:38–48. doi: 10.3390/psych4010003
22. Soo, SA, Kumar, D, Leow, YJ, Koh, CL, Saffari, SE, and Kandiah, N. Usefulness of the visual cognitive assessment test in detecting mild cognitive impairment in the community. J Alzheimers Dis. (2023) 93:755–63. doi: 10.3233/JAD-221301
23. Pinto, TCC, Machado, L, Bulgacov, TM, Rodrigues-Júnior, AL, Costa, MLG, Ximenes, RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) in the elderly? Int Psychogeriatr. (2019) 31:491–504. doi: 10.1017/S1041610218001370
24. Islam, N, Hashem, R, Gad, M, Brown, A, Levis, B, Renoux, C, et al. Accuracy of the Montreal Cognitive Assessment tool for detecting mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Dement. (2023) 19:3235–43. doi: 10.1002/alz.13040
25. Gibbons, LE, van Belle, G, Yang, M, Gill, C, Brayne, C, Huppert, FA, et al. Cross-cultural comparison of the Mini-Mental State Examination in United Kingdom and United States participants with Alzheimer’s disease. Int J Geriatr Psychiatry. (2002) 17:723–8. doi: 10.1002/gps.683
26. Dong, Y, Sharma, VK, Chan, BP-L, Venketasubramanian, N, Teoh, HL, Seet, RCS, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. (2010) 299:15–8. doi: 10.1016/j.jns.2010.08.051
27. Xu, Y, Mirelman, A, Saunders-Pullman, R, Mejia-Santana, H, Caccappolo, E, Raymond, D, et al. Differences in performance on English and Hebrew versions of the MoCA in Parkinson’s patients. Clin Park Relat Disord. (2020) 3:100042. doi: 10.1016/j.prdoa.2020.100042
28. O’Driscoll, C, and Shaikh, M. Cross-cultural applicability of the Montreal Cognitive Assessment (MoCA): a systematic review. J Alzheimers Dis. (2017) 58:789–801. doi: 10.3233/JAD-161042
29. O’Bryant, SE, Humphreys, JD, Smith, GE, Ivnik, RJ, Graff-Radford, NR, Petersen, RC, et al. Detecting dementia with the Mini-Mental State Examination in highly educated individuals. Arch Neurol. (2008) 65:963–7. doi: 10.1001/archneur.65.7.963
30. Ng, TP, Feng, L, Lim, WS, Chong, MS, Lee, TS, Yap, KB, et al. Montreal Cognitive Assessment for screening mild cognitive impairment: variations in test performance and scores by education in Singapore. Dement Geriatr Cogn Disord. (2015) 39:176–85. doi: 10.1159/000368827
31. Trzepacz, PT, Hochstetler, H, Wang, S, Walker, B, Saykin, AJ, Alzheimer’s Disease Neuroimaging Initiative, et al. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. (2015) 15:107. doi: 10.1186/s12877-015-0103-3
32. Hoops, S, Nazem, S, Siderowf, A, Duda, J, Xie, S, Stern, M, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. (2009) 73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47
33. Sarah, IK. A visual based cognitive assessment tool (VCAT) for diagnosis of dementia in a multilingual Asian population/Sarah Iyang Kiyu University of Malaya (2017).
34. Rabin, LA, Wang, C, Katz, MJ, Derby, CA, Buschke, H, and Lipton, RB. Predicting Alzheimer’s disease: neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc. (2012) 60:1128–34. doi: 10.1111/j.1532-5415.2012.03956.x
35. Tolar, M, Hey, J, Power, A, and Abushakra, S. Neurotoxic soluble amyloid oligomers drive Alzheimer’s pathogenesis and represent a clinically validated target for slowing disease progression. Int J Mol Sci. (2021) 22:6355. doi: 10.3390/ijms22126355
36. Abd Razak, MA, Ahmad, NA, Chan, YY, Mohamad Kasim, N, Yusof, M, Abdul Ghani, MKA, et al. Validity of screening tools for dementia and mild cognitive impairment among the elderly in primary health care: a systematic review. Public Health. (2019) 169:84–92. doi: 10.1016/j.puhe.2019.01.001
37. Song, C-S, Lee, H-S, and Chun, B-Y. Comparison of Montreal Cognitive Assessment in Korean version for predicting mild cognitive assessment in 65-year and over individuals. Occup Ther Int. (2022) 2022:1–6. doi: 10.1155/2022/4108434
38. Youn, JC, Kim, KW, Lee, DY, Jhoo, JH, Lee, SB, Park, JH, et al. Development of the subjective memory complaints questionnaire. Dement Geriatr Cogn Disord. (2009) 27:310–7. doi: 10.1159/000205512
39. Mathuranath, PS, George, A, Cherian, PJ, Mathew, R, and Sarma, PS. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. (2005) 17:461–74. doi: 10.1017/S1041610205001547
40. Savva, GM, and Arthur, A. Who has undiagnosed dementia? A cross-sectional analysis of participants of the aging, demographics and memory study. Age Ageing. (2015) 44:642–7. doi: 10.1093/ageing/afv020
41. Staffaroni, AM, Tsoy, E, Taylor, J, Boxer, AL, and Possin, KL. Digital cognitive assessments for dementia: digital assessments may enhance the efficiency of evaluations in neurology and other clinics. Pract Neurol. (2020) 2020:24–45.
42. Öhman, F, Hassenstab, J, Berron, D, Schöll, M, and Papp, KV. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimers Dement. (2021) 13:e12217. doi: 10.1002/dad2.12217
Keywords: mild cognitive impairment, visual cognitive assessment test, sensitivity, specificity, mild dementia
Citation: Hsu J-H, Liu C-C, Chen I-W, Wu J-Y, Huang P-Y, Liu T-H and Hung K-C (2023) Efficacy of the visual cognitive assessment test for mild cognitive impairment/mild dementia diagnosis: a meta-analysis. Front. Public Health. 11:1293710. doi: 10.3389/fpubh.2023.1293710
Received: 13 September 2023; Accepted: 17 October 2023;
Published: 31 October 2023.
Edited by:
Yan Press, Ben-Gurion University of the Negev, IsraelReviewed by:
Ryuichi Morishita, Osaka University, JapanCopyright © 2023 Hsu, Liu, Chen, Wu, Huang, Liu and Hung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuo-Chuan Hung, ZWQxMDI2MDVAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.