
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 01 December 2023
Sec. Environmental Health and Exposome
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1287678
 Qu Cheng1*†
Qu Cheng1*† Qinlong Jing2†
Qinlong Jing2† Philip A. Collender3
Philip A. Collender3 Jennifer R. Head4
Jennifer R. Head4 Qi Li1
Qi Li1 Hailan Yu1
Hailan Yu1 Zhichao Li5
Zhichao Li5 Yang Ju6
Yang Ju6 Tianmu Chen7
Tianmu Chen7 Peng Wang1
Peng Wang1 Eimear Cleary8
Eimear Cleary8 Shengjie Lai8
Shengjie Lai8Introduction: Given the rapid geographic spread of dengue and the growing frequency and intensity of heavy rainfall events, it is imperative to understand the relationship between these phenomena in order to propose effective interventions. However, studies exploring the association between heavy rainfall and dengue infection risk have reached conflicting conclusions, potentially due to the neglect of prior water availability in mosquito breeding sites as an effect modifier.
Methods: In this study, we addressed this research gap by considering the impact of prior water availability for the first time. We measured prior water availability as the cumulative precipitation over the preceding 8 weeks and utilized a distributed lag non-linear model stratified by the level of prior water availability to examine the association between dengue infection risk and heavy rainfall in Guangzhou, a dengue transmission hotspot in southern China.
Results: Our findings suggest that the effects of heavy rainfall are likely to be modified by prior water availability. A 24–55 day lagged impact of heavy rainfall was associated with an increase in dengue risk when prior water availability was low, with the greatest incidence rate ratio (IRR) of 1.37 [95% credible interval (CI): 1.02–1.83] occurring at a lag of 27 days. In contrast, a heavy rainfall lag of 7–121 days decreased dengue risk when prior water availability was high, with the lowest IRR of 0.59 (95% CI: 0.43–0.79), occurring at a lag of 45 days.
Discussion: These findings may help to reconcile the inconsistent conclusions reached by previous studies and improve our understanding of the complex relationship between heavy rainfall and dengue infection risk.
Dengue is a viral infection caused by the dengue virus and transmitted by Aedes aegypti and Ae. Albopictus mosquitoes (1). The burden of disease attributed to dengue has increased drastically over the past two decades, with the number of cases reported to World Health Organization increasing from 0.5 million cases in 2000 to 5.2 million in 2019 (2). Moreover, the true dengue disease burden is likely to be substantially higher than the reported. Modeling and seroprevalence studies indicate that about 75% of dengue infections may be missed by the surveillance system once they do not present noticeable symptoms (3, 4).
Although a commercial vaccine is available, its application is limited to those who have had prior dengue infection, since the vaccine may increase the risk of severe dengue among those who have not been previously infected (5). As a result, vector control measures remain the primary means for prevention of dengue outbreaks. Therefore, having accurate knowledge of the environmental determinants of mosquito proliferation and dengue virus transmission is essential for reducing the burden of dengue via the design of appropriate vector control measures.
The vectors for dengue, mosquitoes of the genus Aedes, undergo four distinct life stages: the aquatic egg, larva and pupa stages, and the terrestrial adult stage (Figure 1). The rate of development between these stages and the mortality rate at each stage depend heavily on temperature and rainfall through complex pathways (8). Higher temperatures can accelerate mosquito development and reproduction, as well as viral replication within mosquitoes, potentially raising the risk of dengue infection (Figure 1). Higher temperatures can also increase mosquito mortality, potentially reducing the risk of transmission (8). Similarly, rainfall has been shown to increase dengue risk by providing additional water for mosquito breeding sites, thereby increasing the environmental capacity for the immature aquatic life stages of mosquitoes (9). On the other hand, it can flush out immature mosquitoes from their breeding sites, thereby impeding the transmission of dengue when the water level in breeding sites is already close to maximum capacity, as demonstrated by field and laboratory experiments (10).
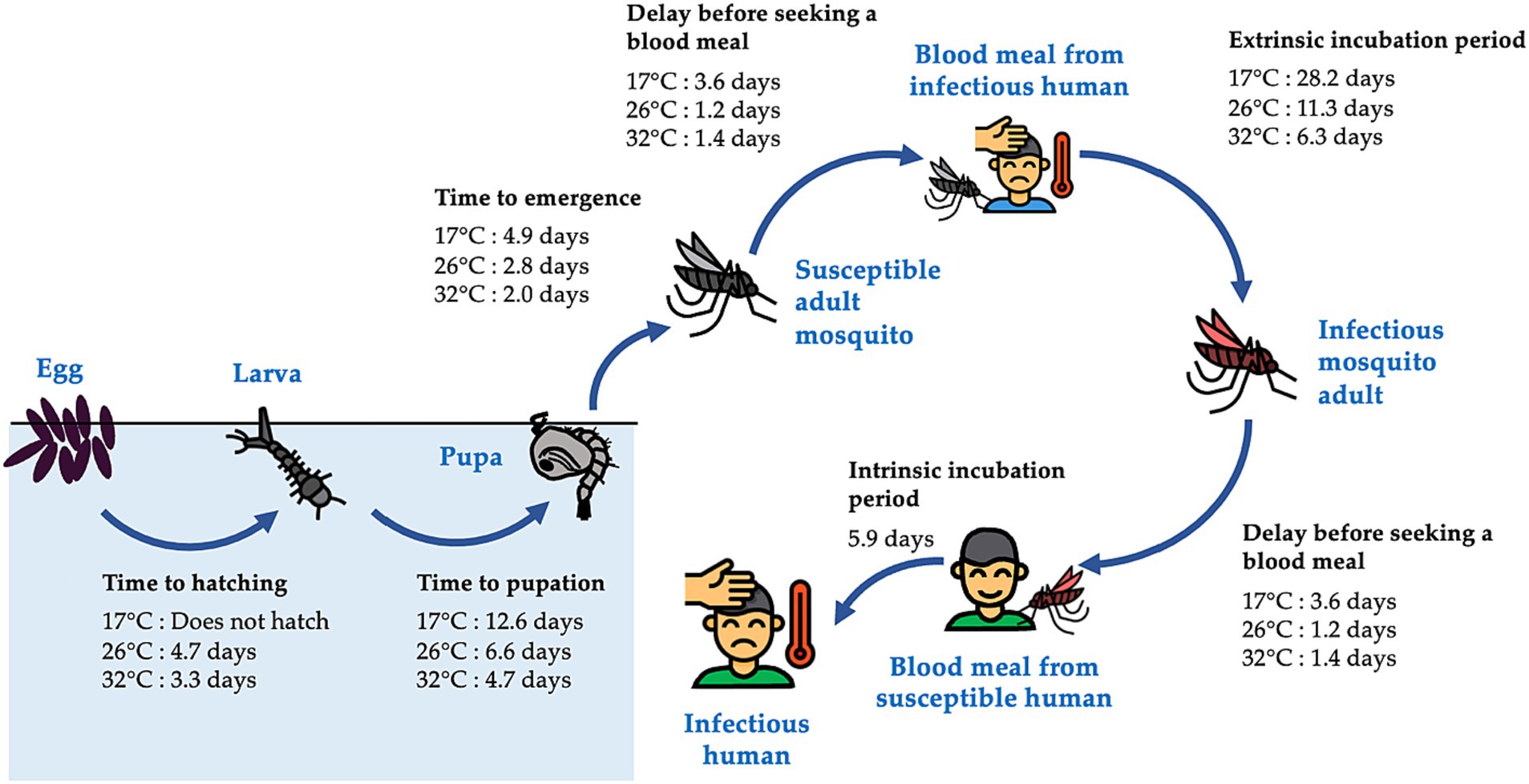
Figure 1. Life stages of the Aedes mosquitoes and the transmission of dengue virus. Aedes mosquitoes have four distinct life stages, including the aquatic egg, larva, and pupa stages, and the terrestrial adult stage. Upon emergence, female adults seek mates and blood meals before laying eggs. They can become infected with dengue virus by feeding on an infectious human, and, following an incubation period, can transmit the virus to other susceptible humans during subsequent blood meals. Times required for completion of each stage of the cycle at varying temperatures were estimated from enzyme kinetics models fitted to data collected from laboratory studies (6), except for the intrinsic incubation period, which was obtained directly from Chan and Johansson (7). The figure displays times required at 17, 26, and 32°C, which represent the minimum, average, and maximum of daily mean temperatures in Guangzhou, respectively, between August and October, the months during which Guangzhou experiences high dengue case counts (Figure 2C) and frequent heavy rainfall events (Supplementary Figure S2B).
The number of previous studies that have investigated the impact of heavy rainfall events on dengue risk have been limited and have reached inconsistent conclusions. Using data from 35 provinces in Southeast Asia, Wang et al. found that extreme rainfall was associated with a 25 percent reduction in dengue risk (RR: 0.75, 95% CI: 0.62–0.90) compared to no rainfall (11). Conversely, studies in Asia observed a positive association between heavy rainfall and dengue risk (12–15). However, none of the studies mentioned above stratified the analyses by prior water availability, and many were constrained by their reliance on data with low temporal resolution (i.e., weekly or monthly, instead of daily), as well as their limited consideration of the effects of heavy rainfall across multiple time lags.
In this study, we examined the relationship between heavy rainfall and dengue risk using a distributed lag non-linear modeling approach taking into consideration interactions between heavy rainfall events and prior water availability. Using data on the daily number of dengue cases in Guangzhou collected by a passive surveillance system from 2006 to 2018, we aimed to test the hypothesis that prior water availability modified the relationship between heavy rainfall and dengue risk.
Guangzhou, a subtropical prefecture located in southern China, has the fifth largest population (18.7 million) and the fourth highest gross domestic product across all prefectures in the country (16). The burden of dengue in this region is high: between 1990 and 2015, dengue cases in Guangzhou accounted for over 65 percent of dengue cases reported in China (6, 17). Guangzhou is located around the Tropic of Cancer and has a typical subtropical climate with hot and humid summers and mild and dry winters (Figures 2A,B), which is favorable for mosquito growth. Heavy rainfall is common in summer months (Figure 3C; Supplementary Figure S2B). Notably, unlike most other dengue transmission areas, where the primary vector is Ae. aegypti, the sole vector in Guangzhou is Ae. albopictus, which tolerates cold during the winter months better through egg diapause (18). Dengue transmission in Guangzhou follows a clearly defined seasonal pattern. No local transmission is reported during the winter months, when the climate is cooler, and over 99 percent of cases are reported between July and December, when the climate is more favorable by the vectors (Figure 2C). Each year, the initiation of local transmission requires the arrival of imported cases (17, 19). The starting time of local transmissions play a crucial role in determining the outbreak size of that year, with earlier local transmission associated with larger outbreaks (6). As a result, the number of cases varies significantly from year to year. For example, 37,338 cases were recorded in 2014 (89 percent of the 41,939 cases recorded during the 2006–2018 study period) while only three cases were reported during 2008 and 2009 (Figure 2D).
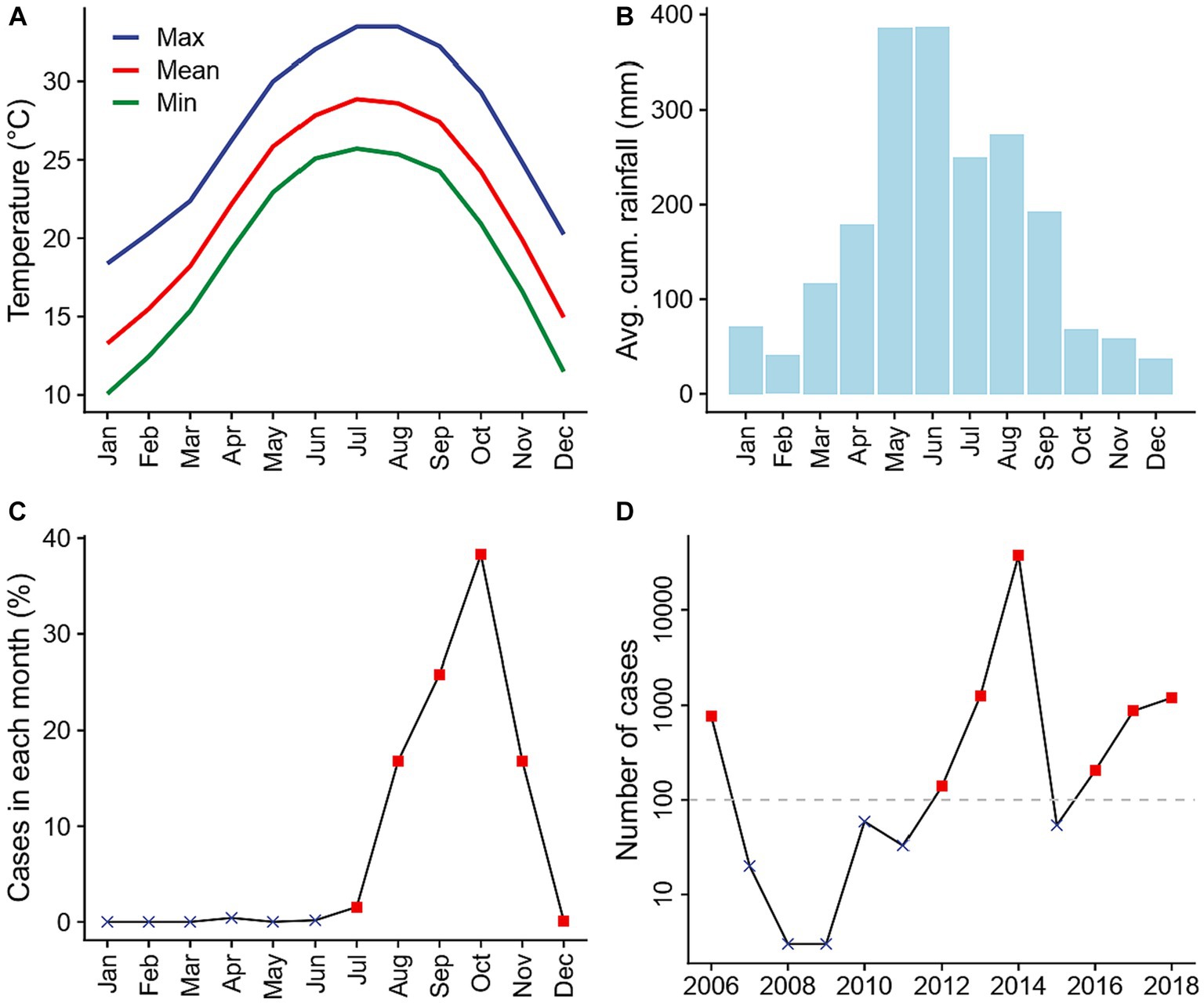
Figure 2. Climate conditions and dengue transmission in Guangzhou prefecture, China between 2006 and 2018. (A) Monthly average daily maximum (blue), mean (red), and minimum (green) temperatures. (B) Monthly average cumulative rainfall. (C) Average percentage of annual dengue cases reported in each month. Red squares represent transmission season months included in the statistical analyses, while black crosses represent those excluded. (D) Number of dengue cases reported in each year. Only years with more than 100 reported cases (red squares) were included in the statistical analyses. Climate data was obtained from China Meteorological Data Service Centre, while dengue case count data was obtained from Guangzhou Center for Disease Control and Prevention.
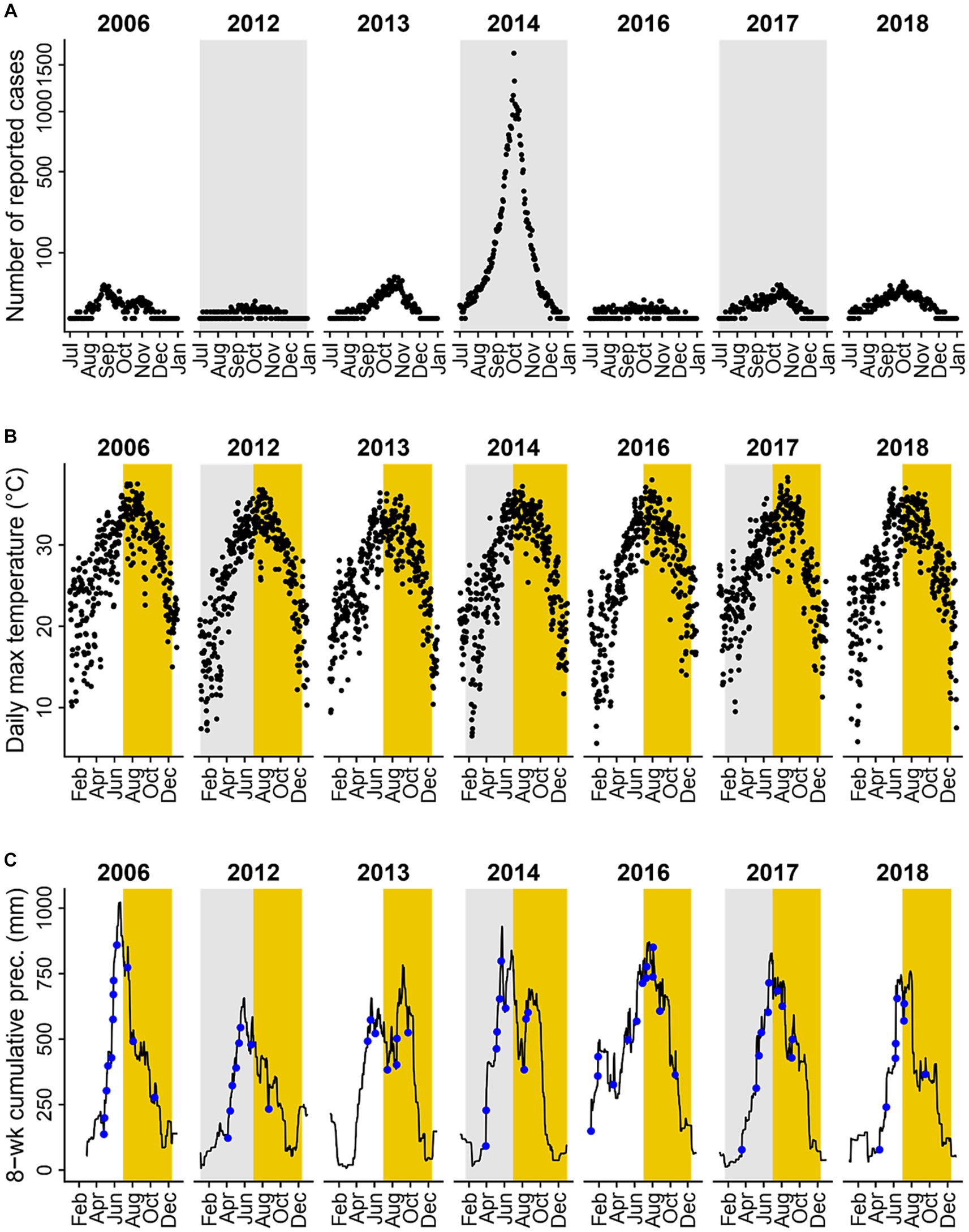
Figure 3. Dengue incidence and climate variables in years where over 100 dengue cases were reported. (A) Daily incidence of locally acquired dengue cases reported during transmission seasons (black dots). (B) Daily maximum temperature (°C); yellow shaded areas represent the transmission season of July to December. (C) Daily prior water availability (defined as 8-week cumulative precipitation; black line) and heavy rainfall events (defined as daily precipitation levels exceeding the 95th percentile value of rainy days in the study period, 51 mm, blue dots); yellow shaded areas represent the transmission seasons of July to December. Dengue incidence and climate variables for all years and months between 2006 and 2018 are shown in Supplementary Figure S1.
Dengue is a notifiable disease in China. Cases are diagnosed according to the National Diagnostic Criteria for Dengue Fever (WS216-2008) (20). Once diagnosed, cases are required to be reported within 24 h via the web-based National Infectious Disease Reporting System that covers almost all healthcare facilities in China (20, 21). Travel history is a mandatory reporting field, and cases where the patient did not report travel to dengue endemic areas in the 15 days prior to the onset of illness are considered to be locally-acquired (21). We obtained daily counts of locally acquired incidence cases, including both clinically-diagnosed and laboratory-confirmed infections, in Guangzhou throughout the period between January 1st, 2006 to December 31st, 2018 from the Guangzhou Center for Disease Control and Prevention. Year-end population data for Guangzhou from 2005 to 2018 were collected from the Guangdong Statistical Yearbooks (22) and used to interpolate the population size on each day linearly.
Daily minimum, mean, and maximum temperature and cumulative precipitation between 2006 and 2018 from a weather station in Guangzhou were collected from the China Meteorological Data Service Centre.1 We defined a heavy rainfall event as a day when total precipitation exceeded the 95th percentile of all non-zero daily precipitation values in the study period (13, 14). The resulting cutoff of 51 mm corresponds well with the definition used by the China Meteorological Administration, which considers a heavy rainfall event as cumulative rainfall exceeding 50 mm within 24 h (23). We used the cumulative precipitation during the preceding 8 weeks as a proxy measure of water availability in mosquito breeding sites (hereafter referred to as prior water availability) following previous literature (24, 25).
We constructed a distributed lag non-linear model (DLNM) to examine the effects of temperature, heavy rainfall, prior water availability, and the interaction between heavy rainfall and prior water availability on dengue incidence at multiple lags. We used the distributed lag model to account for the delayed effects of climatic and hydrological factors on dengue infection risk. We assumed the outcome followed a negative binomial distribution to account for potential overdispersion of daily case counts. We specified the DLNM as:
where represents the negative binomial distribution; and represent the number of observed and expected locally-acquired dengue cases whose symptoms onset on day t, respectively; k is the dispersion parameter of the negative binomial distribution; is the model intercept; is a natural spline smooth function of prospective time with 7 degrees of freedom per year, included to control for long-term and seasonal trends of any unmeasured confounders as in a majority of the existing environmental epidemiology studies (26–28); and DOW (day-of-week) is a categorical variable for day of the week, included to control for the day of week effects in reporting and incidence. The symbol denotes the cross-basis function in DLNM, which models the smooth effect surface in both lag and variable value dimensions. In the lag dimension, we modeled the delayed effects of temperature ( ), heavy rainfall events ( ), prior water availability ( ) and the interaction between heavy rainfall events and prior water availability ( ) with natural cubic splines with two internal knots equally placed in the log range of lags (29, 30). We used a maximum lag of 140 days (i.e., 20 weeks) based on the results of previous studies about the lagged effects of climatic factors on dengue transmission (12, 31). In the variable value dimension, we modeled the effects of temperature with a natural cubic spline with two internal knots equally placed along the range of observed temperatures (29, 30), the effect of a heavy rainfall using binary indicators for occurrence of heavy rainfall events, and effects of prior water availability and their modification by heavy rainfall as linear functions (24). To account for shifts over time in the population at risk, a population offset term was included. Daily maximum temperature, rather than the mean or minimum temperature, was used in the model since it exhibited the lowest collinearity with the other variables. We used instead of to control for long-term and seasonal trends of any unmeasured confounders, since the seasonal pattern of dengue incidence rates in Guangzhou varied from year to year, and the model with flexible seasonal terms had a remarkably lower deviance than the model with a fixed seasonal term (4749.0 vs. 5807.3). To obtain the effects of heavy rainfall events under the low, medium, and high prior water level scenarios, we centered at its 5th, 50th, and 95th percentile values between 2006 and 2018 (25.0, 260.6, and 786.9 mm) in three separate model fits. In doing so, the cross basis on heavy rainfall events can be interpreted as the delayed effects of a heavy rainfall event when prior water availability is at the centered value (either high, medium, or low), as the interaction term in Equation ((1) will equal zero. We restricted our analysis to local transmission months (i.e., July–December) in years with over 100 reported cases (2006, 2012–14, and 2016–18, Figure 2D) to examine effects of heavy rainfall events and prior water availability on dengue only among time periods with continuous local transmission. Cases during these periods contributed over 99 percent of all cases between 2006 and 2018.
To test the robustness of our findings, we conducted sensitivity analyses that explored the results with alternative centering values for prior water availability (such as using the 15th, 25th, …, 85th percentile values); definitions of heavy rainfall events (daily precipitation levels above the 90th percentile value on rainy days within the study period, which is 34.6 mm); number of preceding weeks over which to aggregate measures of prior water availability (7 or 9 weeks); degrees of freedom used for controlling long-term and seasonal trends of potential unmeasured confounders (8 or 9 degrees of freedom per year); and numbers of knots for the lag-response relationship of heavy rainfall events (3 or 4 knots). We also examined the results when excluding data in 2014 from the analyses due to its notably high number of reported cases (Figure 2D).
All analyses were conducted in R 4.2.1 (32) with packages tidyverse 1.3.2 (33), data.table 1.14.2 (34), and lubridate 1.8.0 (35) for data processing, tsModel 0.6–1 (36), splines 4.2.1 (37), dlnm 2.4.7 (38) and INLA 22.07.23 (39) for statistical modeling, and ggplot2 3.3.6. (40), ggsci 2.9 (41) and cowplot 1.1.1 (42) for visualization. All data and code are available from https://github.com/qu-cheng/dengue_heavyrain.
From 2006 to 2018, a total of 41,939 locally acquired and clinically-diagnosed or laboratory-confirmed dengue cases were reported in Guangzhou. The number of newly-onset cases reported varied across years, with 37,338 cases reported in 2014, and only 3 cases reported in both 2008 and 2009. Seven years (2006, 2012–2014, and 2016–2018) met our inclusion criteria of having more than 100 cases reported. Figure 3A illustrates the number of dengue cases reported per day between July and December for included years.
In years with more than 100 reported dengue cases, daily maximum temperature ranged from 5.6 to 38.3°C, with an average of 26.8°C and a median of 27.9°C (Figure 3B). The number of heavy rainfall events varied year-to-year (Figure 3C; Supplementary Figure S2A), with 2016 having the most (13 events) and 2013 having the least (7 events). Heavy rainfall events were most frequent between April and September, and least frequent between November to February (Supplementary Figure S2B), with two seasonal peaks of event frequency in May and August. Average prior water availability, calculated as the cumulative precipitation during the previous 8 weeks, was at its highest in 2016 and its lowest in 2012 among years included in the statistical analyses (Figure 3C; Supplementary Figure S3A). Across months, prior water availability was greatest from May to October and lowest from November to March (Supplementary Figure S3B). Mean 8-week cumulative rainfall exhibited a single seasonal peak in June.
The effects of heavy rainfall on the risk of dengue infection were modified by prior water availability. When the cumulative rainfall during the preceding 8 weeks was at its 5th percentile value (indicating low prior water availability), the occurrence of heavy rainfall events was associated with increased risk of dengue infection at a lag of 24–55 days. The strongest increase in dengue incidence occurred 27 days after a heavy rainfall event (incidence rate ratio, IRR: 1.37, 95% CI: 1.02–1.83, Figure 4A). By contrast, when the cumulative rainfall during the preceding 8 weeks was at its 95th percentile value (indicating high prior water availability), the occurrence of heavy rainfall events was associated with reduced dengue risk at a lag of 7–121 days, with the strongest negative effect occurring 45 days after the heavy rainfall events (IRR: 0.59, 95% CI: 0.43–0.79, Figure 4C). The shape of the lag-response curve of heavy rainfall events under medium prior water availability scenario was very similar to that of the low prior water availability scenario. However, the delayed effects of heavy rainfall events observed under medium prior water availability on dengue incidence were closer to the null (Figure 4B).
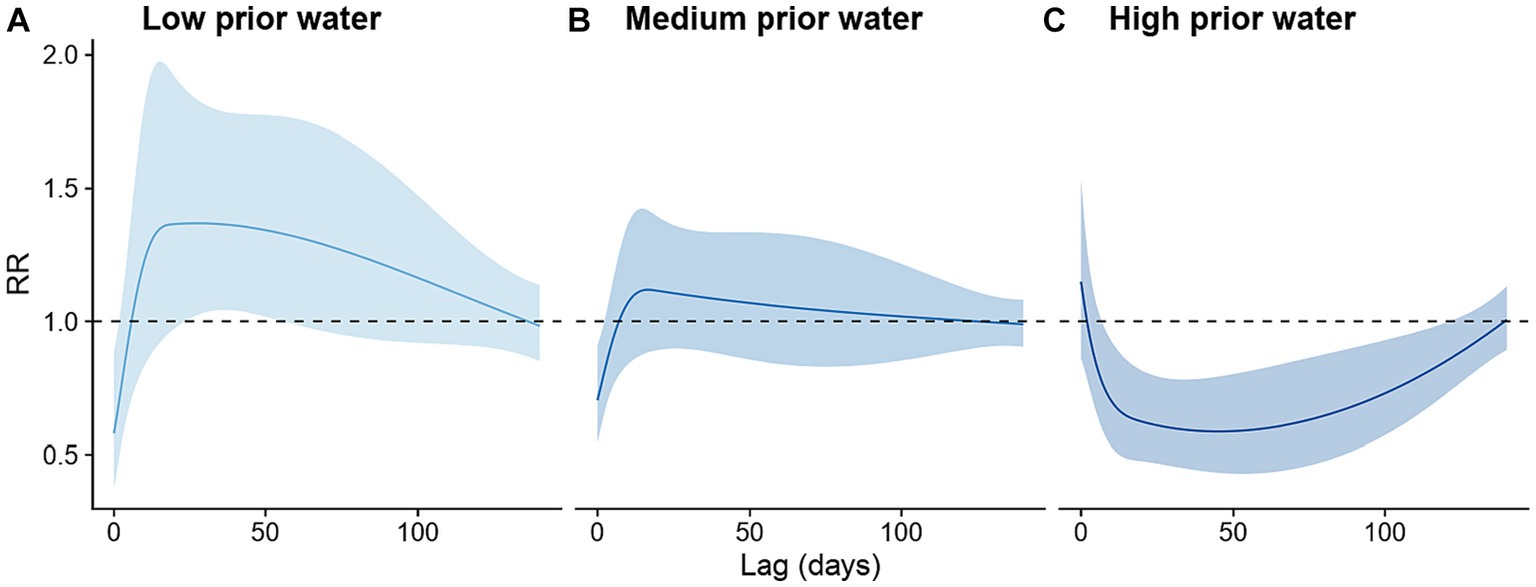
Figure 4. Lagged effects of heavy rainfall events on dengue infection risk stratified by prior water availability scenarios. (A) Low prior water availability scenario, defined as cumulative rainfall during the preceding 8 weeks at its 5th percentile value; (B) Medium prior water availability scenario, defined as cumulative rainfall during the preceding 8 weeks at its 50th percentile value; and (C) High prior water availability scenario, defined as cumulative rainfall during the preceding 8 weeks at its 95th percentile value.
To identify the level of prior water availability at which the effect of heavy rainfall events switches from increasing to decreasing dengue incidence rates, we examined other centering values for the cumulative rainfall in the proceeding 8 weeks (i.e., the 15th, 25th, …, 85th percentile values, Supplementary Figure S5). Using the RR at a 35-day lag, we found that the association between heavy rainfall events was positive when prior rainfall availability was below the 15th percentile, null when prior rainfall availability was between the 25th and 75th percentile, and negative when prior rainfall availability was above the 85th percentile.
We examined the lag-response relationship for different temperatures compared to 28°C, the median daily maximum temperature observed between 2006 and 2018. Our findings revealed that lower temperatures were associated with a reduced risk of dengue infection, while higher temperatures were associated with an increased risk (Figure 5). When compared with the reference temperature, a daily maximum temperature at 18°C, the 10th percentile of daily maximum temperature observed between 2006 and 2018, was associated with reduced dengue infection risk at lags of 1–32 days. The strongest negative association occurred at a lag of 10 days (IRR: 0.89, 95% CI: 0.85–0.94, Figure 5B). In comparison, a daily temperature of 35°C, the 90th percentile of daily maximum temperature observed between 2006 and 2018, was associated with increased risk of dengue infection at lags of 7–140 days, with the strongest positive association occurring at a lag of 63 days (IRR: 1.07, 95% CI: 1.03–1.11) (Figure 5B).
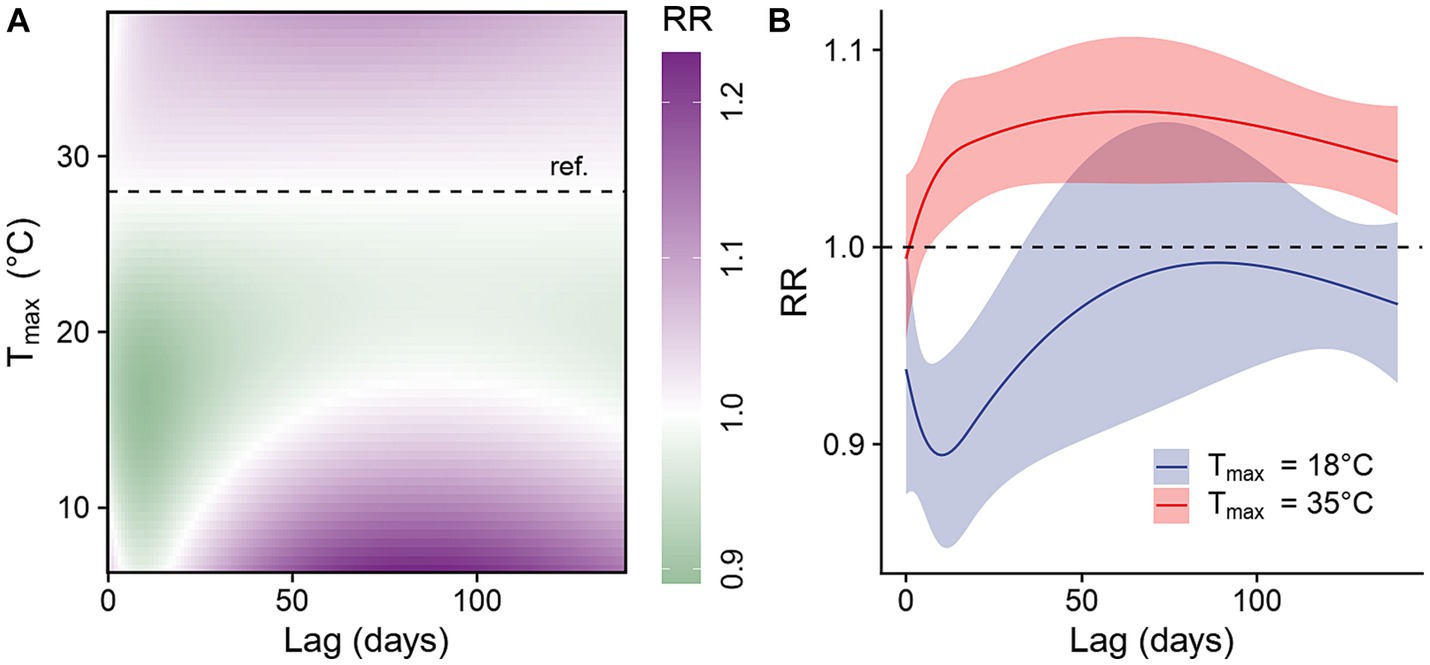
Figure 5. Lagged effects of daily maximum temperature on dengue infection risk. (A) Contour plot of the association between daily maximum temperature and the risk of dengue infection at various lag times, relative to the study period’s median daily maximum temperature of 28°C (horizontal dashed line). (B) Lag-response association for low (18°C, the 10th percentile of daily maximum temperature observed between 2006 and 2018) and high (35°C, the 90th percentile of daily maximum temperature observed between 2006 and 2018) daily maximum temperature relative to median daily maximum temperature (28°C).
Varying thresholds used to define heavy rainfall (Supplementary Figure S6), weeks over which prior precipitation was aggregated to estimate water availability (Supplementary Figure S7), degrees of freedom in the temporal trend term (Supplementary Figure S8), knots used to model the lag-response of temperature, heavy rainfall events, and prior water availability (Supplementary Figure S9), and excluding data from 2014 (Supplementary Figure S10) did not qualitatively change the conclusion of our analyses. Across these scenarios, we consistently observed a positive association between heavy rainfall events and dengue infection risk when prior water availability was low, and a negative association when prior water availability was high, although the strength of the associations varied. Comparable to the baseline scenario, the occurrence of a heavy rainfall event was generally associated with a significant change in dengue risk at a lag of 25–45 days. The exact lag days with significant associations, the lag day with the strongest association (including both positive and negative association), and the relative risk and its 95% CI on the lag day with the strongest association are shown in Supplementary Table S1.
As the climate changes, heavy rainfall events are projected to increase in frequency and intensity globally (43). Understanding the effects of heavy rainfall events on dengue transmission is critical for developing targeted public health interventions to reduce the risk of dengue transmission following extreme weather events. Heavy rainfall has long been hypothesized to increase dengue risk when water availability is low by creating more mosquito habitats. However, risk may decrease when water availability is high as immature aquatic stages of mosquitoes are flushed out of existing habitats (44, 45). To the best of our knowledge, this hypothesis had not been formally tested with observational data up to this point. Using daily weather and dengue case data from Guangzhou, China, we developed a distributed lag non-linear model and found evidence supporting this theory. Our results suggest that heavy rainfall events were likely to increase dengue risk at a lag of 25–45 days (3–7 weeks) when water availability before the heavy rainfall was medium to low, but decrease dengue risk when prior water availability was high.
This lag period corresponds well with the time from development of immature aquatic stages to the onset of human dengue cases at 26°C, the average daily mean temperature in Guangzhou between August and October. These months observe high dengue case counts and frequent heavy rainfall events in our study area. According to a previous study using enzyme kinetic models fit to data collected from laboratory studies (6), at 26°C, the times required for the development of eggs into larvae, larvae into pupae, and pupae into adults are 5, 7, and 3 days, respectively; the average time required for Ae. albopictus to find a host for a blood meal is 1 day, meaning at least 2 days would be required for a mosquito to acquire the virus from a human and transmit it to another; and the average length of the extrinsic and intrinsic incubation periods are 11 and 6 days (7), respectively (Figure 1). Together these processes take 22 days when counting from the last aquatic stage (pupa), or 34 days when counting from the first aquatic stage (egg). The concordance between these time periods and the lag times identified by our study (25–45 days) suggest that heavy rainfall events can influence various stages of the entire process.
Although some research has shed light on the effects of heavy rainfall on dengue infection risk, results have been inconsistent and heterogenous between study locations. For instance, a study conducted across all 21 prefectures in Guangdong Province, China, found that heavy rainfall events were significantly associated with increased dengue risk in seven prefectures (including Guangzhou) yet reduced dengue risk in four prefectures (13). Another study across 35 provinces in Southeast Asia observed a negative association between heavy rainfall and dengue risk in Sri Lanka, with no significant associations in Malaysia, Thailand, and Singapore (11). Stratifying data by prior water availability could potentially result in more consistent conclusions.
Our findings suggest that warmer daily maximum temperatures are linked to a higher risk of dengue infection, with the delayed effects of warmer temperatures likely to persist for several months. These results align with earlier studies conducted in regions with a subtropical climate. For example, a study in Guangzhou observed the highest dengue risk between 20 and 45 days after a daily maximum temperature of 30 to 35°C, with the lagged effects lasting up to 141 days (46). Similarly, a study in Barbados identified a positive association between monthly minimum temperature and dengue infection risk, with peaks occurring after 1–2 months (29). Our results also make sense in the context of the mosquito and dengue lifecycle, as temperatures in the upper range observed in our study region are hypothesized to enhance nearly all stages, ranging from egg development to virus replication (Figure 1). In contrast, research from tropical regions has suggested an inverted U-shaped relationship between temperature and dengue risk, where relative risk increases first before decreasing (11, 47, 48). While these results suggest that extreme heat may hinder parts of the mosquito or viral lifecycle, the optimal temperature for mosquito development and virus transmission may vary depending on the location (11, 47, 48).
Some limitations should be acknowledged for this study. First, our findings rely on data acquired via passive surveillance, which may be influenced by local healthcare-seeking behavior, disease reporting practices, and the clinical severity of disease. However, these factors seem unlikely to change with the exposure status, meaning that any resulting bias would be expected to pull effect estimates toward the null. Second, because of data availability, our study was limited to a single subtropical prefecture in southern China, which has different seasonal patterns in rainfall compared to other dengue transmission hotspots in tropical regions. While we expect that effect modification by prior water availability of the association between heavy rainfall events and dengue incidence may work similarly in other locations, further research from diverse settings is needed to confirm this and to estimate location-specific effects.
Our study has shown that the impact of heavy rainfall events on dengue risk relies on prior water availability. To gain a deeper understanding of the modification effect of prior water availability, it is essential to conduct future studies that go beyond the scope of our research. These studies include epidemiological investigations conducted in regions beyond southern China, allowing for a broader assessment of our hypothesis. Additionally, direct observational studies on mosquito density would provide valuable insights into the association between prior water availability and mosquito populations. These findings may help to reconcile the inconsistent conclusions reached by previous studies and improve our understanding of the complex relationship between heavy rainfall and dengue risk. Moreover, they can provide support for the development of more precise prevention strategies during extreme events under future climate conditions.
All data and code used is publicly available via https://github.com/qu-cheng/dengue_heavyrain. Further inquiries should be directed to the corresponding author(s).
The studies involving humans were approved by Ethics Committee of the Guangzhou Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Collecting routine infectious disease surveillance data from hospitals.
QC: Conceptualization, Formal analysis, Funding acquisition, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. QJ: Conceptualization, Data curation, Resources, Writing – review & editing. PC: Methodology, Visualization, Writing – review & editing. JH: Methodology, Visualization, Writing – review & editing. QL: Writing – review & editing. HY: Writing – review & editing. ZL: Writing – review & editing. YJ: Writing – review & editing. TC: Writing – review & editing. PW: Methodology, Writing – review & editing. EC: Methodology, Writing – review & editing. SL: Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. QC was supported by National Natural Science Foundation of China (82204110). SL was supported by the EU Horizon (MOOD 874850), the Bill & Melinda Gates Foundation (INV-024911), and the National Institute for Health (MIDAS Mobility R01AI160780).
The computation is completed in the HPC Platform of Huazhong University of Science and Technology. Figure 1 has been designed using images from Flaticon.com made by surang and itim2101.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1287678/full#supplementary-material
2. World Health Organization. Dengue and severe dengue. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
3. Wang, T, Wang, M, Shu, B, Chen, X-q, Luo, L, Wang, J-y, et al. Evaluation of inapparent dengue infections during an outbreak in southern China. PLoS Negl Trop Dis. (2015) 9:e0003677. doi: 10.1371/journal.pntd.0003677
4. Bhatt, S, Gething, PW, Brady, OJ, Messina, JP, Farlow, AW, Moyes, CL, et al. The global distribution and burden of dengue. Nature. (2013) 496:504–7. doi: 10.1038/nature12060
5. World Health Organization. Dengue vaccine: WHO position paper, September 2018-recommendations. Vaccine. (2019) 37:4848–9. doi: 10.1016/j.vaccine.2018.09.063
6. Cheng, Q, Jing, Q, Spear, RC, Marshall, JM, Yang, Z, and Gong, P. Climate and the timing of imported cases as determinants of the dengue outbreak in Guangzhou, 2014: evidence from a mathematical model. PLoS Negl Trop Dis. (2016) 10:e0004417. doi: 10.1371/journal.pntd.0004417
7. Chan, M, and Johansson, MA. The incubation periods of dengue viruses. PLoS One. (2012) 7:e50972. doi: 10.1371/journal.pone.0050972
8. Morin, CW, Comrie, AC, and Ernst, K. Climate and dengue transmission: evidence and implications. Environ Health Perspect. (2013) 121:1264–72. doi: 10.1289/ehp.1306556
9. Karl, S, Halder, N, Kelso, JK, Ritchie, SA, and Milne, GJ. A spatial simulation model for dengue virus infection in urban areas. BMC Infect Dis. (2014) 14:1–17. doi: 10.1186/1471-2334-14-447
10. Dieng, H, Rahman, G, Abu Hassan, A, Che Salmah, M, Satho, T, Miake, F, et al. The effects of simulated rainfall on immature population dynamics of Aedes albopictus and female oviposition. Int J Biometeorol. (2012) 56:113–20. doi: 10.1007/s00484-011-0402-0
11. Wang, Y, Wei, Y, Li, K, Jiang, X, Li, C, Yue, Q, et al. Impact of extreme weather on dengue fever infection in four Asian countries: a modelling analysis. Environ Int. (2022) 169:107518. doi: 10.1016/j.envint.2022.107518
12. Chien, L-C, and Yu, H-L. Impact of meteorological factors on the spatiotemporal patterns of dengue fever incidence. Environ Int. (2014) 73:46–56. doi: 10.1016/j.envint.2014.06.018
13. Cheng, J, Bambrick, H, Yakob, L, Devine, G, Frentiu, FD, Williams, G, et al. Extreme weather conditions and dengue outbreak in Guangdong, China: spatial heterogeneity based on climate variability. Environ Res. (2021) 196:110900. doi: 10.1016/j.envres.2021.110900
14. Cheng, J, Bambrick, H, Frentiu, FD, Devine, G, Yakob, L, Xu, Z, et al. Extreme weather events and dengue outbreaks in Guangzhou, China: a time-series quasi-binomial distributed lag non-linear model. Int J Biometeorol. (2021) 65:1033–42. doi: 10.1007/s00484-021-02085-1
15. Meng, H, Xiao, J, Liu, T, Zhu, Z, Gong, D, Kang, M, et al. The impacts of precipitation patterns on dengue epidemics in Guangzhou city. Int J Biometeorol. (2021) 65:1929–37. doi: 10.1007/s00484-021-02149-2
16. National Bureau of Statistics of China. China statistical yearbook 2021. Beijing: China Statistics Press (2021).
17. Lai, S, Huang, Z, Zhou, H, Anders, KL, Perkins, TA, Yin, W, et al. The changing epidemiology of dengue in China, 1990-2014: a descriptive analysis of 25 years of nationwide surveillance data. BMC Med. (2015) 13:1–12. doi: 10.1186/s12916-015-0336-1
18. Jin, L, and Li, D. A recent survey of mosquito fauna in Guandong Province, southern China, with a review of past records. Med Vet Entomol. (2008) 22:359–63. doi: 10.1111/j.1365-2915.2008.00758.x
19. Sang, S, Chen, B, Wu, H, Yang, Z, di, B, Wang, L, et al. Dengue is still an imported disease in China: a case study in Guangzhou. Infect Genet Evol. (2015) 32:178–90. doi: 10.1016/j.meegid.2015.03.005
20. Ministry of Health of the People's Republic of China. Diagnostic criteria for dengue fever (WS 216–2008). Beijing: People's Medical Publishing House (2008).
21. Sang, S, Yin, W, Bi, P, Zhang, H, Wang, C, Liu, X, et al. Predicting local dengue transmission in Guangzhou, China, through the influence of imported cases, mosquito density and climate variability. PLoS One. (2014) 9:e102755. doi: 10.1371/journal.pone.0102755
22. Statistics Bureau of Guangdong Province. Guangdong statistical yearbook, 2005–2022. Beijing: China Statistics Press (2022).
23. China Meteorological Administration. Definitions of heavy rain Beijing. (2018). Available at: https://www.cma.gov.cn/2011xzt/2018zt/20100728/2010072804/202111/t20211103_4140377.html (Accessed July 6, 2018).
24. Carlton, EJ, Eisenberg, JN, Goldstick, J, Cevallos, W, Trostle, J, and Levy, K. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol. (2014) 179:344–52. doi: 10.1093/aje/kwt279
25. Collender, PA, Morris, C, Glenn-Finer, R, Acevedo, A, Chang, HH, Trostle, JA, et al. Mass gatherings and diarrheal disease transmission among rural communities in coastal Ecuador. Am J Epidemiol. (2019) 188:1475–83. doi: 10.1093/aje/kwz102
26. Bhaskaran, K, Gasparrini, A, Hajat, S, Smeeth, L, and Armstrong, B. Time series regression studies in environmental epidemiology. Int J Epidemiol. (2013) 42:1187–95. doi: 10.1093/ije/dyt092
27. Huang, W, Li, S, Vogt, T, Xu, R, Tong, S, Molina, T, et al. Global short-term mortality risk and burden associated with tropical cyclones from 1980 to 2019: a multi-country time-series study. Lancet Planet Health. (2023) 7:e694–705. doi: 10.1016/S2542-5196(23)00143-2
28. O’Brien, E, Masselot, P, Sera, F, Roye, D, Breitner, S, Ng, CFS, et al. Short-term association between sulfur dioxide and mortality: a multicountry analysis in 399 cities. Environ Health Perspect. (2023) 131:037002. doi: 10.1289/EHP11112
29. Lowe, R, Gasparrini, A, van Meerbeeck, CJ, Lippi, CA, Mahon, R, Trotman, AR, et al. Nonlinear and delayed impacts of climate on dengue risk in Barbados: a modelling study. PLoS Med. (2018) 15:e1002613. doi: 10.1371/journal.pmed.1002613
30. Lowe, R, Lee, SA, O'Reilly, KM, Brady, OJ, Bastos, L, Carrasco-Escobar, G, et al. Combined effects of hydrometeorological hazards and urbanisation on dengue risk in Brazil: a spatiotemporal modelling study. Lancet Planet Health. (2021) 5:e209–19. doi: 10.1016/S2542-5196(20)30292-8
31. Chen, S-C, Liao, C-M, Chio, C-P, Chou, H-H, You, S-H, and Cheng, Y-H. Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: insights from a statistical analysis. Sci Total Environ. (2010) 408:4069–75. doi: 10.1016/j.scitotenv.2010.05.021
32. R Core Team. R: A language and environment for statistical computing Vienna. Austria: R Foundation for Statistical Computing (2022) Available at: https://www.R-project.org/.
33. Wickham, H, Averick, M, Bryan, J, Chang, W, McGowan, LDA, François, R, et al. Welcome to the Tidyverse. J Open Source Softw. (2019) 4:1686. doi: 10.21105/joss.01686
34. Dowle, M, Srinivasan, A, Gorecki, J, Chirico, M, Stetsenko, P, Short, T, et al. Package ‘data. Table’. Extension of ‘data frame’. (2019):596.
35. Grolemund, G, and Wickham, H. Dates and times made easy with lubridate. J Stat Softw. (2011) 40:1–25. doi: 10.18637/jss.v040.i03
36. Peng, RD, and Dominici, F. Statistical methods for environmental epidemiology with R. A Case Study in Air Pollution and Health. New York, NY: Springer (2008).
38. Gasparrini, A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. (2011) 43:1–20. doi: 10.18637/jss.v043.i08
39. Blangiardo, M, Cameletti, M, Baio, G, and Rue, H. Spatial and spatio-temporal models with R-INLA. Spatial Spatio-temporal Epidemiol. (2013) 4:33–49. doi: 10.1016/j.sste.2012.12.001
40. Wickham, H. ggplot2: Elegant graphics for data analysis. Springer (2016). p. 189–201. Available at: https://link.springer.com/book/10.1007/978-3-319-24277-4
41. Xiao, N. Ggsci: scientific journal and sci-fi themed color palettes for ‘ggplot2’. R package version 29. (2018)
42. Wilke, CO. Cowplot: streamlined plot theme and plot annotations for ‘ggplot2’. R package version 111. (2022):4.
43. Intergovernmental Panel on Climate Change. AR6 synthesis report: Climate change 2023. (2023). Available at: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (Accessed March 19, 2023).
44. Benedum, CM, Seidahmed, OM, Eltahir, EA, and Markuzon, N. Statistical modeling of the effect of rainfall flushing on dengue transmission in Singapore. PLoS Negl Trop Dis. (2018) 12:e0006935. doi: 10.1371/journal.pntd.0006935
45. Koenraadt, C, and Harrington, L. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J Med Entomol. (2008) 45:28–35. doi: 10.1093/jmedent/45.1.28
46. Xiang, J, Hansen, A, Liu, Q, Liu, X, Tong, MX, Sun, Y, et al. Association between dengue fever incidence and meteorological factors in Guangzhou, China, 2005–2014. Environ Res. (2017) 153:17–26. doi: 10.1016/j.envres.2016.11.009
47. Seah, A, Aik, J, Ng, L-C, and Tam, CC. The effects of maximum ambient temperature and heatwaves on dengue infections in the tropical city-state of Singapore–a time series analysis. Sci Total Environ. (2021) 775:145117. doi: 10.1016/j.scitotenv.2021.145117
Keywords: dengue, heavy rainfall events, prior water availability, DLNM, Guangzhou
Citation: Cheng Q, Jing Q, Collender PA, Head JR, Li Q, Yu H, Li Z, Ju Y, Chen T, Wang P, Cleary E and Lai S (2023) Prior water availability modifies the effect of heavy rainfall on dengue transmission: a time series analysis of passive surveillance data from southern China. Front. Public Health. 11:1287678. doi: 10.3389/fpubh.2023.1287678
Received: 02 September 2023; Accepted: 31 October 2023;
Published: 01 December 2023.
Edited by:
Mo Salman, Colorado State University, United StatesReviewed by:
Kamil Demirberk Ünlü, Atılım University, TürkiyeCopyright © 2023 Cheng, Jing, Collender, Head, Li, Yu, Li, Ju, Chen, Wang, Cleary and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qu Cheng, Y2hlbmdxdUBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.