
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 08 January 2024
Sec. Public Mental Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1281689
This article is part of the Research Topic The Association Between Oral Health and Mental Health View all 6 articles
 Zhi-qiang Zhang1
Zhi-qiang Zhang1 Jing-yang Li2
Jing-yang Li2 Si-tong Ge1
Si-tong Ge1 Tian-yi Ma1
Tian-yi Ma1 Fu-yao Li1
Fu-yao Li1 Jun-liang Lu3
Jun-liang Lu3 Shu-rui Si1
Shu-rui Si1 Zhe-zhu Cui1
Zhe-zhu Cui1 Yu-lian Jin4,5,6
Yu-lian Jin4,5,6 Xiang-hua Jin1*
Xiang-hua Jin1*Background: Recently, the prevalence of sensorineural hearing loss (SNL) has been increasing, and several studies have suggested that depression, anxiety, and SNL may be associated with each other, however, individual findings still have discrepancies. To the best of our knowledge, no scholars have systematically elucidated the bidirectional associations between SNL, depression, and anxiety disorders from the perspective of meta-analysis. In this study, we aimed to systematically evaluate the bidirectional associations between SHL and depressive and anxiety symptoms, and to provide evidence-based medical evidence for reducing SNL, depression, and anxiety disorders.
Methods: We performed systematic review based on priori protocol that was registered with PROSPERO (No. CRD42022365963). Systematic search of PubMed, Embase, and Web of Science databases identified articles published as of June 1, 2023, on the relationship between SNL and depression and anxiety. Meta-analysis was performed to calculate the odds ratios (OR) and 95% confidence intervals (CIs) for the outcome metrics, and the results were combined to assess bivariate associations between the disorders with fixed or random effects. Sensitivity and subgroup analyzes were conducted to analyze sources of heterogeneity, and Egger’s and Begg’s tests combined with funnel plots were applied to assess publication bias.
Results: Summary analysis of the results of 20 studies covering 675,291 individuals showed that the bidirectional association between SNL and depression and anxiety disorders. The incidence (OR = 0.17, 95% CI: 0.09–0.28) and risk (OR = 1.43, 95% CI: 1.32–1.55) of depression and morbidity were higher in SNL patients than the general population. Elevated prevalence (OR = 0.46, 95% CI: 0.28–0.65) and risk (OR = 1.30, 95% CI: 1.11–1.48) of SNL were also observed in depressed patients. The prevalence of anxiety disorders among SNL patients was about 40% (OR = 0.40, 95% CI: 0.24%-0.57), which was associated with higher risk (OR = 1.83, 95% CI: 1.42–2.24) of development than the general population. Incidence of SNL in patients with anxiety disorders was approximately 31% (OR = 0.31, 95% CI: 0.29–0.33). Additionally, subgroup analyzes showed that the bidirectional associations between SNL, depression, and anxiety disorders was influenced by age, region, and mode of diagnosis of the disorders (SNL, depression, anxiety).
Conclusion: There are bidirectional associations between SNL and depression and anxiety disorders, which was influenced by age and region and the method the disorders (SNL, depression, anxiety) were diagnosed.
Recently, the incidence of sensorineural hearing loss (SHL) has been increasing year by year, with more than 1.5 billion people around the world suffering from varying degrees of SHL, including at least 430 million people suffering from moderate SHL or worse (1). Notably, the prevalence of SHL may increase and become worse with age, studies have shown that the frequency of SHL is approximately four times greater in older adults aged 90 and over than in 60-year-olds, which is accompanied by more severe SHL (2).
Over the past two decades, depression and anxiety disorders have become one of the major public health concerns globally and are considered to be the most common mental disorders, affecting more than 264 million people worldwide and potentially leading to severe mental stress and dysfunction, and even suicide, especially in low- and middle-income countries (3). Furthermore, depression and anxiety increased in prevalence with age (4), with approximately 15% of older adults experiencing clinically significant depressive symptoms and 1–5% suffering from major anxiety disorders (5).
Various studies (6–11) have shown that SHL may contribute to the more frequent occurrence of depression and anxiety disorders. Interestingly, depression and anxiety could also be responsible for the development and progression of SHL (9). Associations have been reported between SHL and depression and anxiety disorders in recent years (7, 10, 12–14), nevertheless, the majority of studies have focused merely on the effect of SHL on the risk of depression and anxiety disorders (6–8, 10, 15, 16), while evidence of an inverse association between SHL and events of depression and anxiety disorders is limited, and the results of the various studies have been inconsistent and the conclusions are still somewhat controversial. To the best of our knowledge, currently nobody has systematically elaborated the bidirectional associations between SHL and depression and anxiety disorders from the perspective of evidence-based medicine, therefore the systematic evaluation and meta-analysis of the existing evidence is necessitated.
The primary objective of this meta-analysis was to succinctly summarize the existing evidence on the prevalence of depression and anxiety disorders in SHL. Additionally, it aimed to evaluate the bidirectional associations related to the risk of developing depression and anxiety disorders in individuals with SHL. The ultimate goal is to provide valuable insights for clinical practitioners.
This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (17). We performed a systematic review based on a priori protocol that was registered with PROSPERO (No. CRD42022365963), which was to ensure the originality of our selected topic.
Inclusion criteria: (1) patients with SHL whose exposure was confirmed by pure tone audiometry (PTA), questionnaires, or self-reported hearing loss; (2) patients with or at risk for outcome-confirmed diagnosis or self-reported depression or anxiety via the Depression or Anxiety Scale; (3) age of the patient needs to be ≥18 years old for either the exposure or the outcome; and (4) type of study: observational, which can be a case–control study, cohort study, or cross-sectional study.
Exclusion criteria included: Literature with no access to full text and missing raw data; Literature with illogical study design protocols; Literature that did not report on the ethical review process; and meeting abstracts, reviews and Letters were also excluded.
The computerized search of PubMed, Embase, and Web of Science databases was conducted to assemble case–control studies, cohort studies, or cross-sectional studies on the relationship between SHL and depression and anxiety. All search timeframes were from library construction to June 1, 2023. The English search terms included Hypoacusis, Hearing Loss, Hypoacusis, Hearing Impairment, Deafness, Depression, Depressive Symptom, Emotional Depression, Melancholia, Anxiety, Angst, Hypervigilance, Nervousness, Anxiousness, etc. Detailed literature search formula and search details are shown in Supplementary Table S1.
Literature was screened, extracted and cross-checked independently by 2 researchers, consulting a third party for assistance in any disagreements, and contacting the corresponding authors to supplement any missing information wherever possible. During literature screening, the title and abstract were read initially, and obviously irrelevant literature was excluded, and then the full text was read further to determine final inclusion. The extracted data mainly included: basic information of the included studies, including the first author, investigation area, publication time, etc.; sample size of the study population, patients’ (average) age, gender and disease diagnosis criteria; outcome indicators and result measures (prevalence rate, ratio, risk ratio, etc.) of the studies; specific details of the interventions, disease status after the interventions, etc.; and the key elements of the evaluation of the risk of bias. Two investigators independently evaluated the risk of bias of the included studies, and any disagreement was resolved through discussion and negotiation. Risk of bias was assessed using the New Castle-Ottawa scale (NOS) for case–control and cohort studies, and the Agency for Healthcare Research and Quality (AHRQ) risk of bias criteria for cross-sectional studies, and ≥ 5 were classified as high quality.
Stata 16.0 software were used to perform this meta-analysis. Measurement data utilized the odds ratio (OR) and the combined percentage as effect indicators, each of which was provided with points estimates and 95% confidence intervals (CIs). Heterogeneity between the results of the included studies was analyzed using the X2 test (the test level was α = 0.1), and the magnitude of heterogeneity was quantitatively determined by combining with I2. Fixed effects model was used to combine the effects if I2 was ≤50%, and random effects model to combine the effects if I2 was >50%, followed by sensitivity analysis or subgroup analysis to explore the source of heterogeneity. Funnel plots were drawn for outcome metrics for ≥6 articles included in the literature and combined with Begg’s and Egger’s tests to assess publication bias.
Five thousand, eight hundred eighty-six articles were initially generated from database and manual searches. Critically reviewed based on title and abstract by two independent reviewers after removing duplicate studies. 236 papers were selected for evaluation in full text and finally, according to the previously established inclusion criteria, 20 articles were incorporated into this study for the meta-analysis. The detailed literature screening process is presented in Figure 1. Ultimately, 20 studies containing 675,291 subjects participated in this meta-analysis, and the major characteristics of the included studies are shown in Table 1. Among all eligible studies included, 7 are cross-sectional, while 6 are case–control and 7 are cohort studies.
Cross-sectional studies were evaluated using AHRQ criteria and included studies which explicitly stated the research question, specified the target study population and utilized valid and reliably administered exposure and outcomes measures. The results of the quality assessment indicated that no high-risk studies were included through detailed review, which are shown in Table 2.
Assessment of all the included cohort and case–control studies using the NOS scores indicated that 6 studies were of high quality, 7 studies were of moderate quality and no low-quality studies were included in this meta-analysis (Table 3).
The outcome variables were plotted in funnel plots for the number of included literatures ≥5, and combined with Egger’s and Begg’s tests (Table 4), which showed no publication bias was observed for our outcomes (Supplementary Figures S1–S4).

Table 4. Results of meta-analysis of the bidirectional relationship between sensorineural hearing loss and depression-anxiety disorder.
A total of 14 studies (6–8, 12–16, 18–23) were presented on the prevalence of depression among SHL patients, and the results of the meta-analysis showed (Figure 2; Table 4) that the overall prevalence of SHL patients suffering from comorbid depression was 17% (Rate = 0.17, 95% CI: 0.09–0.28; I2 = 99.86%, p < 0.001), furthermore the meta-analysis of the 13 studies (6–10, 15, 16, 20–22, 24–26) indicated that SNL patients had higher risk of incidence of depression (Figure 3; Table 4) compared to the general population (OR = 1.43, 95% CI: 1.32–1.55; I2 = 69.1%, p = 0.000). Considering the relatively high heterogeneity of both findings, we conducted the sensitivity analysis (Supplementary Figures S5–S6) to explore whether the heterogeneity was sourced by excluding tests one-by-one, which showed that excluding any one piece of literature has no significant influence on the finding.
The current study included 7 articles [6-,8,15-16,20–21] assessing the prevalence of SNL in depressed patients, which suggest that the overall prevalence of SNL occurring in depressed patients was 46% (Rate = 0.46, 95% CI: 0.28–0.65; I2 = 99.61%, p < 0.001; Figure 4; Table 4). This heterogeneity could not be explained through sample size (large or small) or study design (prospective or retrospective), and sensitivity analyzes showed that none of the studies contributed significantly to the summary findings (Supplementary Figure S7). Combined 5-study (7, 9, 10, 16, 21) findings revealed that the risk of SNL prevalence is increased in patients with a history of depression (OR = 1.30, 95% CI: 1.11–1.48; I2 = 68.2%, p < 0.001; Figure 5; Table 4). The results of sensitivity analysis showed (Supplementary Figure S8) that the conclusion of Liu et al. (21) significantly deviated from the midline, and the heterogeneity was significantly reduced by excluding the findings of that study, and the recombined results using a fixed-effects model were (OR = 1.20, 95% CI: 1.09–1.32; I2 = 0%, p = 0.476).
We performed meta-analysis of the findings of the 4 studies (7, 11, 12, 18) indicating that the prevalence of anxiety disorders among SNL patients is approximately 40% (Rate = 0.40, 95% CI: 0.24–0.57; I2 = 98.82%, p < 0.001; Figure 6; Table 4), which is considerably high compared to the general population. Meanwhile, analyzing the results of study (7, 8, 11) showed that the risk of anxiety disorders in SNL patients is 1.83 times (OR = 1.83, 95% CI: 1.42–2.24; I2 = 86.1%, p < 0.001; Figure 7; Table 4) higher than that of the general population. Additionally, the prevalence of SNL in patients with anxiety disorders was 31% (OR = 0.31, 95% CI: 0.29–0.33; I2 = 0%, p < 0.001; Supplementary Figure S9; Table 4) (7, 11), suggesting perhaps the bidirectional association between the two disorders and the sensitivity analysis failed to find that none of the any one of included studies had influence on the conclusions (Supplementary Figures S10–S12).
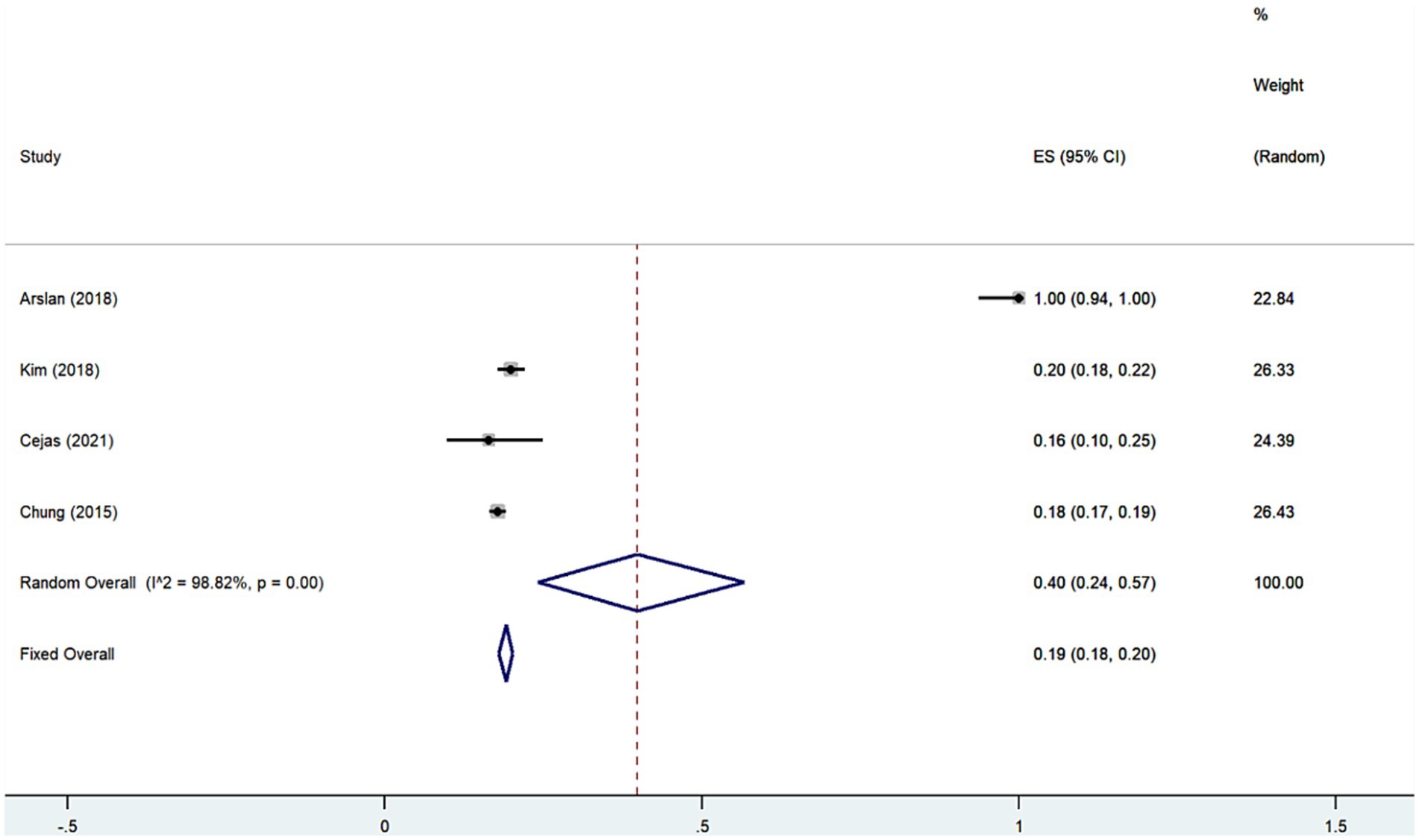
Figure 6. Forest plot of prevalence of anxiety disorders among patients with sensorineural hearing loss.
Fourteen studies discussed the percentage of SNL patients who developed depression and the results showed strong heterogeneity (I2 = 99.92%, p < 0.001), subgroup analysis was conducted according to age in the youth group (6–8, 12, 13, 15, 16, 18, 19) versus the older adults group (14, 20–23). The results of the combined Meta-analysis using the random-effects model showed that in the older adults group (Rate = 0.26, 95% CI: 0.11–0.44; I2 = 99.75%, p < 0.001) The percentage of SNL patients who developed depression was higher than that of the young group (Rate = 0.13, 95% CI (0.06–0.23); I2 = 99.81%, p < 0.001). Furthermore, it was essentially equal between the risk of depression in SNL patients in the younger (6–8, 10, 15, 16) (OR = 1.53, 95% CI: 1.35–1.71; I2 = 62.3%, p = 0.021) and older adults groups (20, 21, 24–26) (OR = 1.40, 95%CI: 1.26–1.53; I2 = 69.5%, p = 0.011), which was shown in Table 5. Seven studies discussed the percentage of depressed patients with hearing loss, which were categorized into young (6–8, 15, 16) and older adults (20, 21) groups according to age to carry out subgroup analyzes, with a significant decrease in heterogeneity in the old group (I2 = 0%, p < 0.001) and no significant change in the young group (I2 = 96.79%, p < 0.001). The results of the subgroup analyzes showed that a higher prevalence of SNL in the older adults group (OR = 0.70, 95% CI: 0.68–0.71; Table 5) depressed patients than in the youth group (OR = 0.35, 95% CI: 0.26–0.44; Table 5).
We conducted subgroup analyzes based on geography which showed approximately equal percentages of depression in SNL patients between the Asian (6–8, 13, 15, 16, 20–22) and American (12, 14, 19, 23) groups (Table 6). Interestingly, the included European study (18) (Rate = 0.55) showed higher prevalence of depression in SNL patients compared to Asia (Rate = 0.15, 95% CI: 0.06–0.27; I2 = 99.89%, p < 0.001) and the Americas (Rate = 0.17; 95% CI: 0.08–0.28; I2 = 99.13%, p < 0.001), the difference that may be due to sample size. Furthermore, the risk of depression in the Asian group (7–10, 15, 16, 20, 21, 26) (OR = 1.43, 95%CI: 1.31–1.56; I2 = 70.7%, p = 0.001) of SNL patients was considerable compared with that of the Oceania group (25) (OR = 1.47, 95% CI: 1.31–1.65) and slightly higher than in the study of the Americas group (24) (OR = 1.26, 95% CI: 1.11–1.44).
Generally, the SNL diagnostic modalities of the studies we included were categorized into PTA group, Hearing Impairment Scale (HIS) group, and self-report (SR) group (Table 7). The results of subgroup analysis showed that the incidence of depression was highest in the SR group (13, 20, 21, 23) (Rate = 0.27, 95% CI: 0.10–0.48; I2 = 99.73%, p < 0.001) followed by the PTA group (6, 7, 12, 14, 15, 18, 19) (Rate = 0.16, 95% CI: 0.09–0.24; I2 = 99.36%, p < 0.001) and the HIS group (8, 16, 22) (Rate = 0.09, 95% CI: 0.02–0.20; I2 = 99.68%, p < 0.001) among SNL patients. Interestingly, patients diagnosed with SNL by HIS (8, 16) (OR = 1.66, 95% CI: 1.44–1.87; I2 = 15.10%, p = 0.278) had a slightly higher OR for depression than the PTA (6, 10, 15, 24, 25) (OR = 1.37, 95% CI: 1.28–1.45; I2 = 45.9%, p = 0.116) and SR (9, 20, 21, 26) (OR = 1.37, 95% CI: 1.18–1.55; I2 = 78.30%, p = 0.003). Additionally, the risk of developing SNL in depressed patients may be associated with the diagnostic modality of SNL, and the results of subgroup analyzes showed that the HIS group (16) (OR = 1.42, 95% CI: 1.02–1.98) and the SR (9, 21) (OR = 1.41, 95% CI: 1.22–1.60; I2 = 56.60%, p = 0.129) group had a roughly similar and higher SNL risk was broadly similar and higher than in the PTA group (7, 10) (OR = 1.15, 95% CI: 1.01–1.29; I2 = 0.00%, p = 0.491). The incidence of SNL among depressed patients also demonstrated differences among the subgroups, and the results showed that the SR (20, 21) group (Rate = 0.70, 95% CI: 0.68–0.71; I2 = 0.00%, p = 0.926) exhibited the highest prevalence of SNL followed by the HIS (8, 16) (Rate = 0.40, 95% CI: 0.37–0.43; I2 = 0.00%, p = 0.999) and PTA (6, 7, 15) (Rate = 0.27, 95% CI: 0.23–0.30; I2 = 69.57%, p = 0.04) groups. Risk of anxiety in patients with SNL disorders was lower in the PTA group (7, 11) (OR = 1.63, 95% CI: 1.33–1.92; I2 = 75.20%, p = 0.44) than in the HIS group (8) (OR = 2.38, 95% CI: 1.95–2.89).
Subgroup analysis (Table 8) was conducted by dividing the study into medical diagnosis (MD) group, depression scale (DS) group, and self-report (SR) group based on the mode of depression diagnosis, and the results showed that the risk of developing depression was higher in the SR (22) (OR = 4.90, 95% CI: 2.63–9.14) group than the risk of developing depression in the MD (6, 7, 10, 15, 16, 25) (OR = 1.45, 95% CI: 1.36–1.54; I2 = 44.6%, p = 0.108) group and DS (9, 20, 21, 24) (OR = 1.30, 95% CI: 1.15–1.46; I2 = 64.9%, p = 0.036) group among SNL patients. Nevertheless, the incidence rate perspective analysis of the outcome showed that the percentage of SNL occurrence was lower in the MD (6, 7, 15, 16) (OR = 0.06, 95% CI: 0.01–0.15; I2 = 99.80%, p < 0.001) group than in the DS (8, 13, 14, 18, 20, 21) (Rate = 0.23, 95% CI: 0.09–0.40; I2 = 99.79%, p = 0.00) group and in the SR (12, 19, 22, 23) (Rate = 0.24, 95% CI: 021–0.28; I2 = 81.79%; p = 0.00) groups. The results of the subgroup analysis confirmed that the OR of having SNL among depressed patients was slightly higher in the MD (7, 10, 16) group (OR = 1.17, 95% CI: 1.03–1.30; I2 = 0.0%, p = 0.436) than in the DS (9, 21) group (OR = 1.41, 95% CI: 1.22–1.60; I2 = 56.6%, p = 0.129). The prevalence of SNL among depressed patients was different depending on the diagnostic modality of depression, with a high prevalence in the DS group (8, 20, 21) (Rate = 0.60, 95% CI: 0.35–0.83; I2 = 99.61%, p = 0.00), followed by the MD group (6, 7, 15) (OR = 0.27, 95% CI: 0.23–0.30%; I2 = 69.57%, p = 0.04). Moreover, the diagnostic modality of anxiety disorders in SNL patients also influenced the OR of patients developing anxiety disorders, with the diagnostic group relying on the Anxiety Scale (8) (OR = 2.38, 95% CI: 1.95–2.89) relative to the MD group (7, 11) (OR = 1.63, 95% CI: 1.33–1.92; I2 = 75.2%, p = 0.044) the OR was higher. Regarding the prevalence of anxiety disorders in SNL patients, relationships between the Anxiety Scale diagnostic group (12, 18) (Rate = 0.52, 95% CI: 0.44–0.59; I2 = 0.0%, p = 0.997) and the MD (7, 11) (Rate = 0.18, 95% CI: 0.17–0.19; I2 = 0.0%, p = 0.996) group relations showed the similar trend.
In recent years, the association between SNL and depression and anxiety disorders has emerged as a major focus of scholarly study. There is growing evidence of association between SNL and psychiatric disorders including depression, anxiety disorders, and cognitive impairment which begins earlier than previously recognized (subclinical stage of normal hearing) (28–30).
Nevertheless, some controversy may exist regarding the conclusions, and therefore the integration of the studies’ conclusions to provide reference for clinical diagnosis and treatment could be necessary. To the best of our knowledge, no meta-analysis systematically addressed the bi-directional association of SHL with depression and anxiety disorders in any of the studies up to now, especially the risk and incidence of the development of comorbid SNL in patients with depression or anxiety disorders. Therefore, we performed a meta-analysis based on the bidirectional association of SHL with depression and anxiety disorders in expectation of providing further evidence-based medical evidence for clinical practitioners.
The results of our study showed an increased prevalence and risk of depression in subjects with SNL compared to normal hearing subjects. Several population-based studies have shown that people with SHL have higher risk of depression than those with normal hearing (6–8, 10), which is similar to the conclusions of our study. Furthermore, our analysis of the combined results of the included studies confirmed that the prevalence of SNL and the risk of developing SNL were also increased in depressed patients, suggesting that perhaps the bidirectional association exists between SNL and depression. Individuals with hearing loss may experience communication difficulties (31), social and emotional isolation (32), and affective disorders (7), all of which are independently associated with the development of depressive symptoms (7, 31, 32). A clinical trial demonstrated that depression symptoms are controlled in SNL patients who provide interventions to treat (26). Moreover, recent studies have shown that audiological rehabilitation, including the use of sound amplification devices (31), and the availability of hearing aids and sound amplification devices (33), which probably would have slowed down the incidence of depression in SNL patients. Besides, SHL patients commonly suffer from varying degrees of social isolation, which may increase the development of depressive symptoms (34–36). The existence of a link between social isolation/loneliness has been found to decrease regional brain volume perception in areas that support emotional processing and socialization (37). Similarly, patients with depression and anxiety disorders are usually under stress, and increased sensitivity or anxiety in stressed individuals may sensitize them to the perception of hearing loss (38). Liu et al. showed that the risk of SNL in depressed patients was 1.49 folds that in normal individuals, which is similar to our findings (21). On the other hand, social isolation has been associated with abnormalities in ventral striatal function, with worse connectivity in response to social information and dysfunctional frontal limbic emotion processing, which may be a contributing factor to the development of depressive symptoms in SNL patients (39).
Meanwhile, the risk and prevalence of anxiety disorders were found to be elevated in patients with SNL, and the prevalence of SNL was also identified to be increased in patients with anxiety disorders, which may demonstrate that there is a bilateral link between SNL and anxiety disorders. Kevin et al.’s findings suggest that the association between SNL and anxiety disorders is perhaps mediated via other mental health factors (e.g., social isolation and sensory deprivation) (40). Fatih et al. indicated that patients with SNL had significantly elevated anxiety scores relative to the general population, which is consistent with the findings of our study (18). Additionally, amygdala is a critical structure involved in several emotional functions and has been associated with multiple mood disorders, including anxiety and depression (41). The results of several studies suggest (42, 43) that SNL may cause abnormal neural responses in the amygdala. The amygdala’s decreased response to emotional stimulation in patients with SHL (43, 44) suggests that long-term hearing loss could diminish the transmission of auditory and emotional valence information by affecting the pattern of connectivity between the auditory cortex and the amygdala. Tang et al. (45) showed that SHL impairs temporal synchronization between the amygdala and the striatum. Such abnormalities ultimately can result in abnormal responses to emotionally significant stimuli or even emotional deficits in patients with SHL, which could lead to the development of anxiety disorders.
Age has been recognized as a marker of negative prognosis in a variety of disorders, and our findings suggest that older patients with depression or/and SNL experience greater risk and incidence of SNL or/and depression than the younger population. Hearing impairment is commonly recognized as a natural part of the aging process and the older adults tend to be more vulnerable to hearing loss than the younger population (46). Suzanne et al. showed that declines in social communication and activities of daily living in older patients with SNL were identified as a significant factor contributing to poorer mental health outcomes (47). Several studies have confirmed that the prevalence of depression in older adults SNL patients rises dramatically over time (48, 49). Similarly, depression-induced social communication deficits and social withdrawal may further amplify the role of social isolation in SNL development. The treatment of SNL may reverse or reduce symptoms of depression, especially in the older adults population. Mulrow et al. reported significant reductions in depressive symptoms in older adults patients at 6 weeks and 4 months after wearing hearing aids, while quality of life and cognitive functioning increased significantly. The treatment of SNL has been shown to be effective in the treatment of depression in the older adults, particularly in the older adults population (50, 51).
While our study suggests bidirectional relationship between SNL and depression. Interestingly, differences may exist between different geographic regions. Despite the fact that the majority of the studies we included were conducted based on Asian populations, nevertheless, subgroup analyzes showed that the incidence of depression was roughly the same among SNL patients in Asia and the Americas. Patients with SNL in Asia, however, experienced a higher risk of depression than in the Americas. This difference is perhaps due in part to differences in racial composition. A genome-wide association study based on depression suggests that depression-related genetic variant sites differ between individuals of East Asian and European ancestry (52). On the other hand, since the onset of depression is partially correlated with an individual’s social background, economic level, and level of medical care, the greater number of developing countries in Asia may partially explain the higher risk of depression in SNL patients (53). Further studies are necessary to continue to explore the influence of region on the bidirectional association between SNL and depression.
In our study, we discovered that SNL and diagnostic methods for depression and anxiety disorders perhaps influenced the results of the meta-analysis. As we described SNL diagnosed based on self-report and the Hearing Impairment Scale had a modest increased risk of depression onset relative to the PTA diagnostic group. Prevalence differences in depression between different SNL diagnostic groups could be attributed to the fact that we included studies of younger populations, who typically perceive their hearing impairment more accurately than older adults populations. A population-based cross-sectional study showed that the sensitivity of self-reported hearing loss in older adults was 41–65% (54). However, a prospective study that included younger subjects noted sensitivity of 81% for PTA as a diagnostic criterion for hearing loss (55). In addition, ethnicity, socioeconomic status, and educational attainment may perhaps have partially influenced the results, attributable to the fact that most of the studies we included were conducted in Asian countries. Higher levels of education have been associated with concordance between self-reported hearing loss and audiometric PTA results in several studies that have shown that age and education are associated with the correct perception of hearing impairment (47, 56, 57). The prevalence of self-reported hearing loss and audiometric hearing loss is higher in low-education and/or low-income populations (58). The greater accessibility of healthcare content and services in higher education groups may influence the concordance between self-reported hearing loss and audiometric hearing (58). The results of our subgroup analyzes showed that the prevalence of SNL was found to be higher in the population with self-reported diagnosis of depression and/or anxiety disorders compared to the scale-diagnosed group as compared to the medical-diagnosed group. Kim et al. (59) demonstrated that anxiety and depression showed significant correlation with overestimation of hearing loss. On the other hand, hearing impairment has adverse influence on depression and cognition, especially in the older adults (60, 61). Moreover, depression and anxiety have been reported to be strongly correlated with self-reported hearing loss (61, 62).
To the best of our knowledge, this meta-analysis constitutes the first attempt to argue for the bidirectional association between SNL and depression and anxiety disorders in the context of prevalence and risk of incidence. Our meta-analysis was based on comprehensive search that included 3 databases, and extensive manual searches were conducted for more comprehensive literature screening. Furthermore, we performed numerous analyzes and conducted subgroup studies seeking to demonstrate bi-directional associations between SNL and depression and anxiety disorders previously from multiple perspectives. Another strength of this study is the large sample size included, we had included altogether 675,291 patients including from 4 continents, which makes our results more credible and generalizable. The majority of the studies we included were characterized by high quality, most of which were adjusted for multiple confounders, possessed higher levels of evidence, and demonstrated efficacy in increasing the potential association of disease. However, several limitations should be recognized as well in our study, which considered with criticality. Although we included 20 studies for analysis, nevertheless the findings were relatively scattered, especially in targeting the association of anxiety disorders with SNL. Furthermore, despite the considerable amount of sensitivity analyzes and subgroup analyzes we performed, partially unexplained heterogeneity still existed, which may be attributed to differences in study design methods and selection of populations. The studies in the meta-analysis partly assessed depression and anxiety by using self-report questionnaires, however self-report questionnaires lacked interpretability and had lower specificity in identifying depression and anxiety. Meanwhile, older adults depressed individuals typically present more frequently with somatic symptoms, which may not be identified by general screening tools, and reliance on self-reported data on hearing ability may introduce bias. Secondly, some studies have utilized cross-sectional designs, which perhaps leads to an inability to pinpoint disease causation and accurately assess individuals when symptoms are present. Perhaps more importantly for the typing aspect of SNL, we attempted to analyze SNL in subgroups of age-related deafness and sudden deafness, regrettably some studies we included were unable to accurately characterize the type of SNL the patients suffered from. We continue to expect further studies to elaborate our findings from the perspective of SNL typing (age-related deafness and sudden deafness) in the future. Due to the large number of findings, the available evidence for subgroup analyzes had a relatively small number of subgroups and significant heterogeneity in the literature for each subgroup design. Further research is needed to understand the mechanisms underlying the relationship, especially research that demonstrates the link between dose–response relationships. Even so, regardless of these limitations, our analysis is extremely significant. This meta-analysis has revealed, for the first time, the bidirectional association between SNL and depressives and anxiety disorders in terms of prevalence and risk of onset, which can contribute to the identification of SNL and susceptible populations of depressives and anxiety, and provide new strategies for prevention and early intervention in the development of the disorders.
The current study found the bidirectional relationship between SNL and depression-anxiety disorders. Nevertheless, published reports are still relatively underdeveloped. Further studies are required to understand the mechanisms underlying the relationship and to conduct detailed subgroup analyzes for typing between disorders, especially to demonstrate the influence of dose–response on the relationship between disorders.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Z-qZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. J-yL: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. S-tG: Conceptualization, Investigation, Methodology, Methodology, Writing – review & editing. T-yM: Conceptualization, Formal analysis, Investigation, Project administration, Writing – review & editing. F-yL: Conceptualization, Conceptualization, Methodology, Writing – review & editing. J-lL: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. S-rS: Investigation, Methodology, Supervision, Validation, Writing – review & editing. Z-zC: Investigation, Methodology, Software, Writing – review & editing. Y-lJ: Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing. X-hJ: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81860189, 2019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1281689/full#supplementary-material
1. Chadha, S, Kamenov, K, and Cieza, A. The world report on hearing, 2021. Bull World Health Organ. (2021) 99:242-A. doi: 10.2471/BLT.21.285643
2. Clark, JL, and Swanepoel, W. The world report on hearing - a new era for global hearing care. Int J Audiol. (2021) 60:161. doi: 10.1080/14992027.2021.1881318
3. Disease, GBD, Injury, I, and Prevalence, C. Global, regional, and National Incidence, Prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of Disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. Freeman, AT, Santini, ZI, Tyrovolas, S, Rummel-Kluge, C, Haro, JM, and Koyanagi, A. Negative perceptions of ageing predict the onset and persistence of depression and anxiety: findings from a prospective analysis of the Irish longitudinal study on ageing (Tilda). J Affect Disord. (2016) 199:132–8. doi: 10.1016/j.jad.2016.03.042
5. Fiske, A, Wetherell, JL, and Gatz, M. Depression in older adults. Annu Rev Clin Psychol. (2009) 5:363–89. doi: 10.1146/annurev.clinpsy.032408.153621.1
6. Tseng, CC, Hu, LY, Liu, ME, Yang, AC, Shen, CC, and Tsai, SJ. Risk of depressive disorders following sudden sensorineural hearing loss: a Nationwide population-based retrospective cohort study. J Affect Disord. (2016) 197:94–9. doi: 10.1016/j.jad.2016.03.020
7. Kim, JY, Lee, JW, Kim, M, Kim, MJ, and Kim, DK. Association of Idiopathic Sudden Sensorineural Hearing Loss with affective disorders. JAMA Otolaryngol Head Neck Surg. (2018) 144:614–21. doi: 10.1001/jamaoto.2018.0658
8. Hsu, WT, Hsu, CC, Wen, MH, Lin, HC, Tsai, HT, Su, P, et al. Increased risk of depression in patients with acquired sensory hearing loss: a 12-year follow-up study. Medicine (Baltimore). (2016) 95:e5312. doi: 10.1097/MD.0000000000005312
9. Wu, C. Bidirectional association between depression and hearing loss: evidence from the China health and retirement longitudinal study. J Appl Gerontol. (2022) 41:971–81. doi: 10.1177/07334648211042370
10. Kim, SY, Min, C, Lee, CH, Park, B, and Choi, HG. Bidirectional relation between depression and sudden sensorineural hearing loss: two longitudinal follow-up studies using a National Sample Cohort. Sci Rep. (2020) 10:1482. doi: 10.1038/s41598-020-58547-w
11. Chung, SD, Hung, SH, Lin, HC, and Sheu, JJ. Association between sudden sensorineural hearing loss and anxiety disorder: a population-based study. Eur Arch Otorhinolaryngol. (2015) 272:2673–8. doi: 10.1007/s00405-014-3235-8
12. Cejas, I, Coto, J, Sanchez, C, Holcomb, M, and Lorenzo, NE. Prevalence of depression and anxiety in adolescents with hearing loss. Otol Neurotol. (2021) 42:e470–5. doi: 10.1097/MAO.0000000000003006
13. Yang, Y, Xiao, Y, Liu, Y, Li, Q, Shan, C, Chang, S, et al. Mental health and psychological impact on students with or without hearing loss during the recurrence of the Covid-19 pandemic in China. Int J Environ Res Public Health. (2021) 18:1421. doi: 10.3390/ijerph18041421
14. Powell, DS, Brenowitz, WD, Yaffe, K, Armstrong, NM, Reed, NS, Lin, FR, et al. Examining the combined estimated effects of hearing loss and depressive symptoms on risk of cognitive decline and incident dementia. J Gerontol B Psychol Sci Soc Sci. (2022) 77:839–49. doi: 10.1093/geronb/gbab194
15. Kim, SY, Kim, HJ, Park, EK, Joe, J, Sim, S, and Choi, HG. Severe hearing impairment and risk of depression: a National Cohort Study. PLoS One. (2017) 12:e0179973. doi: 10.1371/journal.pone.0179973
16. Lin, CS, Lin, YS, Liu, CF, Weng, SF, Lin, C, and Lin, BS. Increased risk of sudden sensorineural hearing loss in patients with depressive disorders: population-based cohort study. J Laryngol Otol. (2016) 130:42–9. doi: 10.1017/S0022215115002960
17. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and Meta-analysis protocols (Prisma-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
18. Arslan, F, Aydemir, E, Kaya, YS, Arslan, H, and Durmaz, A. Anxiety and depression in patients with sudden one-sided hearing loss. Ear Nose Throat J. (2018) 97:E7–9. doi: 10.1177/0145561318097010-1101
19. Crowson, MG, Franck, KH, Rosella, LC, and Chan, TCY. Predicting depression from hearing loss using machine learning. Ear Hear. (2021) 42:982–9. doi: 10.1097/AUD.0000000000000993
20. Huang, H, Wang, J, Jiang, CQ, Zhu, F, Jin, YL, Zhu, T, et al. Hearing loss and depressive symptoms in older Chinese: whether social isolation plays a role. BMC Geriatr. (2022) 22:620. doi: 10.1186/s12877-022-03311-0
21. Liu, W, Yang, C, Liu, L, Kong, G, and Zhang, L. Bidirectional associations of vision loss, hearing loss, and dual sensory loss with depressive symptoms among the middle-aged and older adults in China. J Affect Disord. (2022) 301:225–32. doi: 10.1016/j.jad.2022.01.066
22. Marmamula, S, Kumbham, TR, Modepalli, SB, Barrenkala, NR, Yellapragada, R, and Shidhaye, R. Depression, combined visual and hearing impairment (dual sensory impairment): a hidden multi-morbidity among the elderly in residential Care in India. Sci Rep. (2021) 11:16189. doi: 10.1038/s41598-021-95576-5
23. Paiva, KM, Samelli, AG, Oliveira, PL, Hillesheim, D, Haas, P, Medeiros, PA, et al. Negative self-perception of hearing and depression in older adults: a population-based study. Rev Saude Publica. (2023) 57:15. doi: 10.11606/s1518-8787.2023057004675
24. Golub, JS, Brewster, KK, Brickman, AM, Ciarleglio, AJ, Kim, AH, Luchsinger, JA, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. (2020) 28:545–56. doi: 10.1016/j.jagp.2019.12.008
25. Lawrence, BJ, Jayakody, DMP, Bennett, RJ, Eikelboom, RH, Gasson, N, and Friedland, PL. Hearing loss and depression in older adults: a systematic review and Meta-analysis. Gerontologist. (2020) 60:e137–54. doi: 10.1093/geront/gnz009
26. Guan, L, Liu, Q, Chen, D, Chen, C, and Wang, Z. Hearing loss, depression, and medical service utilization among older adults: evidence from China. Public Health. (2022) 205:122–9. doi: 10.1016/j.puhe.2022.01.025
27. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. J European journal of epidemiology. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Deal, JA, Betz, J, Yaffe, K, Harris, T, Purchase-Helzner, E, Satterfield, S, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health Abc study. J Gerontol A Biol Sci Med Sci. (2017) 72:703–9. doi: 10.1093/gerona/glw069
29. Golub, JS, Brickman, AM, Ciarleglio, AJ, Schupf, N, and Luchsinger, JA. Association of Subclinical Hearing Loss with cognitive performance. JAMA Otolaryngol Head Neck Surg. (2020) 146:57–67. doi: 10.1001/jamaoto.2019.3375
30. Golub, JS, Brewster, KK, Brickman, AM, Ciarleglio, AJ, Kim, AH, Luchsinger, JA, et al. Association of Audiometric age-Related Hearing Loss with depressive symptoms among Hispanic individuals. JAMA Otolaryngol Head Neck Surg. (2019) 145:132–9. doi: 10.1001/jamaoto.2018.3270
31. Zivkovic Marinkov, EM, Rancic, NK, Milisavljevic, DR, Stankovic, MD, Milosevic, VD, Malobabic, MM, et al. Impact of sensorineural hearing loss during the pandemic of Covid-19 on the appearance of depressive symptoms, anxiety and stress. Medicina (Kaunas). (2022) 58:233. doi: 10.3390/medicina58020233
32. Fu, X, Eikelboom, RH, Liu, B, Wang, S, and Jayakody, DMP. The impact of untreated hearing loss on depression, anxiety, stress, and loneliness in tonal language-speaking older adults in China. Front Psychol. (2022) 13:917276. doi: 10.3389/fpsyg.2022.917276
33. Barbosa, MG, Oliveira, D, Martinelli, MC, Keinert, AAM, Lima-Costa, MF, Suemoto, CK, et al. The Association of Hearing Loss with depressive symptoms and cognitive function among older people: results from the Brazilian longitudinal study of aging. Int J Geriatr Psychiatry. (2023) 38:e5904. doi: 10.1002/gps.5904
34. Mick, P, Kawachi, I, and Lin, FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. (2014) 150:378–84. doi: 10.1177/0194599813518021
35. Pichora-Fuller, MK, Mick, P, and Reed, M. Hearing, cognition, and healthy aging: social and public health implications of the links between age-related declines in hearing and cognition. Semin Hear. (2015) 36:122–39. doi: 10.1055/s-0035-1555116
36. Wang, HX, Karp, A, Winblad, B, and Fratiglioni, L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. (2002) 155:1081–7. doi: 10.1093/aje/155.12.1081
37. Duzel, S, Drewelies, J, Gerstorf, D, Demuth, I, Steinhagen-Thiessen, E, Lindenberger, U, et al. Structural brain correlates of loneliness among older adults. Sci Rep. (2019) 9:13569. doi: 10.1038/s41598-019-49888-2
38. Inagaki, TK, Muscatell, KA, Moieni, M, Dutcher, JM, Jevtic, I, Irwin, MR, et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc Cogn Affect Neurosci. (2016) 11:1096–101. doi: 10.1093/scan/nsv076
39. Cacioppo, JT, Norris, CJ, Decety, J, Monteleone, G, and Nusbaum, H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J Cogn Neurosci. (2009) 21:83–92. doi: 10.1162/jocn.2009.21007
40. Contrera, KJ, Betz, J, Deal, J, Choi, JS, Ayonayon, HN, Harris, T, et al. Association of Hearing Impairment and Anxiety in older adults. J Aging Health. (2017) 29:172–84. doi: 10.1177/0898264316634571
41. Janak, PH, and Tye, KM. From circuits to behaviour in the amygdala. Nature. (2015) 517:284–92. doi: 10.1038/nature14188
42. Chen, GD, Sheppard, A, and Salvi, R. Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience. (2016) 315:228–45. doi: 10.1016/j.neuroscience.2015.12.005
43. Husain, FT, Carpenter-Thompson, JR, and Schmidt, SA. The effect of mild-to-moderate hearing loss on auditory and emotion processing networks. Front Syst Neurosci. (2014) 8:10. doi: 10.3389/fnsys.2014.00010
44. Hein, TC, Mattson, WI, Dotterer, HL, Mitchell, C, Lopez-Duran, N, Thomason, ME, et al. Amygdala habituation and Uncinate fasciculus connectivity in adolescence: a multi-modal approach. NeuroImage. (2018) 183:617–26. doi: 10.1016/j.neuroimage.2018.08.058
45. Tang, TY, Luan, Y, Jiao, Y, Zhang, J, Ju, SH, and Teng, GJ. Disrupted amygdala connectivity is associated with elevated anxiety in sensorineural hearing loss. Front Neurosci. (2020) 14:616348. doi: 10.3389/fnins.2020.616348
46. Kamil, RJ, Genther, DJ, and Lin, FR. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear. (2015) 36:164–7. doi: 10.1097/AUD.0000000000000075
47. Cosh, S, Helmer, C, Delcourt, C, Robins, TG, and Tully, PJ. Depression in elderly patients with hearing loss: current perspectives. Clin Interv Aging. (2019) 14:1471–80. doi: 10.2147/CIA.S195824
48. Yu, A, and Liljas, AEM. The relationship between self-reported sensory impairments and psychosocial health in older adults: a 4-year follow-up study using the English longitudinal study of ageing. Public Health. (2019) 169:140–8. doi: 10.1016/j.puhe.2019.01.018
49. Kiely, KM, Anstey, KJ, and Luszcz, MA. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci. (2013) 7:837. doi: 10.3389/fnhum.2013.00837
50. Mondelli, MF, and Souza, PJ. Quality of life in elderly adults before and after hearing aid fitting. Braz J Otorhinolaryngol. (2012) 78:49–56. doi: 10.1590/S1808-86942012000300010
51. Mulrow, CD, Aguilar, C, Endicott, JE, Tuley, MR, Velez, R, Charlip, WS, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. (1990) 113:188–94. doi: 10.7326/0003-4819-113-3-188
52. Giannakopoulou, O, Lin, K, Meng, X, Su, MH, Kuo, PH, Peterson, RE, et al. The genetic architecture of depression in individuals of east Asian ancestry: a genome-wide association study. JAMA Psychiatry. (2021) 78:1258–69. doi: 10.1001/jamapsychiatry.2021.2099
53. Tibubos, AN, Beutel, ME, Schulz, A, Klein, EM, Brahler, E, Michal, M, et al. Is assessment of depression equivalent for migrants of different cultural backgrounds? Results from the German population-based Gutenberg health study (Ghs). Depress Anxiety. (2018) 35:1178–89. doi: 10.1002/da.22831
54. Agrawal, Y, Platz, EA, and Niparko, JK. Prevalence of hearing loss and differences by demographic characteristics among us adults: data from the National Health and nutrition examination survey, 1999-2004. Arch Intern Med. (2008) 168:1522–30. doi: 10.1001/archinte.168.14.1522
55. Ranganathan, B, Counter, P, and Johnson, I. Validation of self-reported hearing loss using television volume. J Laryngol Otol. (2011) 125:18–21. doi: 10.1017/S0022215110001210
56. Kiely, KM, Gopinath, B, Mitchell, P, Browning, CJ, and Anstey, KJ. Evaluating a dichotomized measure of self-reported hearing loss against gold standard audiometry: Prevalence estimates and age Bias in a pooled National Data set. J Aging Health. (2012) 24:439–58. doi: 10.1177/0898264311425088
57. Zekveld, AA, George, EL, Houtgast, T, and Kramer, SE. Cognitive abilities relate to self-reported hearing disability. J Speech Lang Hear Res. (2013) 56:1364–72. doi: 10.1044/1092-4388(2013/12-0268)
58. Feder, K, Michaud, D, Ramage-Morin, P, McNamee, J, and Beauregard, Y. Prevalence of hearing loss among Canadians aged 20 to 79: audiometric results from the 2012/2013 Canadian health measures survey. Health Rep. (2015) 26:18–25.
59. Kim, SY, Kim, HJ, Kim, MS, Park, B, Kim, JH, and Choi, HG. Discrepancy between self-assessed hearing status and measured audiometric evaluation. PLoS One. (2017) 12:e0182718. doi: 10.1371/journal.pone.0182718
60. Castiglione, A, Benatti, A, Velardita, C, Favaro, D, Padoan, E, Severi, D, et al. Aging, cognitive decline and hearing loss: effects of auditory rehabilitation and training with hearing aids and Cochlear implants on cognitive function and depression among older adults. Audiol Neurootol. (2016) 21:21–8. doi: 10.1159/000448350
61. Grewal, M, and Golub, J. Association between hearing loss and multiple negative emotional states in the us Hispanic population. Otolaryngol Head Neck Surg. (2023) 168:1047–53. doi: 10.1002/ohn.208
Keywords: sensorineural hearing loss, anxiety, depression, meta-analysis, mental disorder
Citation: Zhang Z-q, Li J-y, Ge S-t, Ma T-y, Li F-y, Lu J-l, Si S-r, Cui Z-z, Jin Y-l and Jin X-h (2024) Bidirectional associations between sensorineural hearing loss and depression and anxiety: a meta-analysis. Front. Public Health. 11:1281689. doi: 10.3389/fpubh.2023.1281689
Received: 22 August 2023; Accepted: 12 December 2023;
Published: 08 January 2024.
Edited by:
Jianhua Wu, Queen Mary University of London, United KingdomReviewed by:
Yao Wang, Tianjin Polytechnic University, ChinaCopyright © 2024 Zhang, Li, Ge, Ma, Li, Lu, Si, Cui, Jin and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-hua Jin, Y3VpenoyMDA4QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.