- Department of Stomatology, North Sichuan Medical College, Nanchong, China
Objective: The aim of this meta-analysis is to evaluate the impact of light at night (LAN) exposure on the risk of breast cancer across varying factors.
Method: We conducted a systematic search of literature up to July 15, 2023, including PubMed, Cochrane Library, and Embase databases, using keywords related to breast cancer and LAN exposure. Cohort study and case–control study literature on night light exposure and breast cancer risk were included. Statistical analyses were performed using Stata software version 17.0. To address heterogeneity among different studies, we employed a random-effects model for analysis and assessed publication bias using funnel plots and Egger’s test.
Results: We included 13 case–control and 8 cohort studies with 734,372 participants worldwide. In the Newcastle-Ottawa Scale (NOS) assessments, the average score was 7.43 (ranging from 5 to 9). The overall meta-analysis demonstrated a significant association between exposure to LAN and risk of breast cancer (RR = 1.12; 95% CI: 1.06–1.17; I2 = 31.3%, p < 0.001). In the subgroup analysis, the results of the analysis for study types (case–control studies: RR = 1.16; 95% CI: 1.06–1.27; I2 = 40.4%, p = 0.001; cohort studies: RR = 1.08; 95% CI: 1.04–1.14; I2 = 0.0%, p < 0.001) and the results for light exposure types (outdoor LAN: RR = 1.07; 95% CI: 1.02–1.13; I2 = 30.9%, p = 0.004) are presented. In the analysis conducted for continents, the highest breast cancer risk was observed in the Asian population (Asian: RR = 1.24; 95% CI: 1.15–1.34; I2 = 0.0%, p < 0.001) and in the analysis of estrogen receptor status (ER+: RR = 1.10; 95% CI: 1.03–1.18; I2 = 17.0%, p = 0.005;). We also conducted an analysis on menopausal status and various lifestyles but did not find any statistically significant findings.
Conclusion: Our study demonstrates that LAN exposure is associated with an increased risk of breast cancer, particularly in the Asian population. Among the existing hypotheses, the idea that LAN exposure leads to a decrease in melatonin is widely accepted. However, until the mechanism of this effect is clearly elucidated, it is not recommended to take melatonin supplements for breast cancer prevention without medical advice. We hope to conduct more high-quality research, especially concerning the investigation of other environmental confounding factors, to further advance this field.
1 Introduction
Breast cancer is a prevalent form of cancer among women worldwide. The incidence and mortality of breast cancer have increased among women worldwide (1, 2). In 2018, the number of breast cancer cases in Italy increased by 12.5% compared to the prevalence in 2012 (3). Within North American countries, the incidence of breast cancer increased from 29% in 1987 to 70% in 2000 (4). Despite a decrease in the use of menopausal hormone therapy from 2000 to 2004 due to the influence of the Women’s Health Initiative (5, 6), the incidence of invasive breast cancer decreased (6, 7). However, since 2004, the incidence of invasive breast cancer has been slowly rising at a rate of 0.5% per year (8–10). Globally, the annual number of newly diagnosed breast cancer cases has exceeded 11.6% (11).
Breast cancer, as a highly heterogeneous and multifactorial disease (12), has been the subject of extensive research since the beginning of the 21st century (13). Efforts have been made to identify the associated risk factors and reduce the incidence of breast cancer in women. Current lifestyle risk factors for breast cancer primarily include factors such as ethnicity (14), geographical location (15), occupation (16), dietary choices (17), air quality levels (18), national economic development (19), smoking habits (20), alcohol consumption (21), and hormonal factors (22). Additionally, recent ecological research has identified an increase in body mass index (BMI) and a sustained decrease in birth rates as potential risk factors for an increased risk of breast cancer (23–25). In terms of environmental factors, an association has been observed between increased breast cancer risk and light pollution (26).
Light pollution is an emerging environmental issue that has intensified with urbanization and industrialization. Over the past few decades, light pollution has posed an increasing threat to human health (27). Between 2012 and 2016, artificial light at night (LAN) on Earth increased by 2.2% annually, with a total annual increase in radiance of 1.8% (28). According to the “New World Atlas of Artificial Sky Brightness,” more than 80% of the world’s population and over 99% of the population in the United States and Europe live under light-polluted skies (29). Outdoor LANs, such as urban artificial lighting, can infiltrate indoor environments, although the relationship between outdoor and indoor LAN exposure is not yet fully understood (30). Additionally, indoor LAN exposure has increased in recent decades, primarily due to household lights left on at night and new sources of exposure, such as screens and electronic devices such as smartphones, which have polluted the natural darkness of the night (31). In recent years, a substantial body of epidemiological evidence, including over 20 studies (32–52), has investigated the relationship between exposure to outdoor LAN (32, 39, 41, 45–48, 52), measured through satellite measurements in specific study areas, and breast cancer incidence. Self-reported indoor LAN exposure has also been studied (33, 35, 37, 43, 51). This evidence has identified LAN exposure as a risk factor for breast cancer.
Existing hypotheses suggest that exposure to LAN may inhibit the natural surge of melatonin during the night (53, 54), thereby diminishing the antitumor proliferative capacity or elevating circulating levels of estrogen and progesterone (55). Estrogen is a significant risk factor for breast cancer (56). Such an impact may lead to an increased susceptibility to breast cancer.
A series of epidemiological studies have examined the impact of LAN exposure on breast cancer risk, considering factors such as classification of this exposure, hormone receptor status, race, menopausal status, and others. In various studies examining different types of LAN exposure, previous research has reported a positive association between outdoor LAN exposure and breast cancer risk (46, 47). However, the results regarding self-reported indoor LAN exposure have been inconsistent. Specifically, in premenopausal women, some studies have suggested that LAN exposure increases the risk of breast cancer (46), while others have not observed any statistically significant associations (36). Furthermore, in analyses considering hormone receptor status, existing research has noted that LAN exposure has a significant promoting effect on estrogen receptor-positive breast cancer and in white populations. As this research area continues to receive increasing attention, new epidemiological studies are still ongoing to further investigate these relationships (33, 34, 41).
In the past 2 years, a recent meta-analysis included an analysis of BMI and reanalyzed the impact of breast cancer risk in different estrogen receptor status populations (57). Additionally, four new large-scale studies on nocturnal light exposure have drawn attention to research related to pregnancy hormone receptors, ethnicity, and population characteristics (37, 41, 45, 47). However, previous meta-analyses have overlooked the potential influence of nocturnal light and lifestyle factors, and they did not investigate the status of progesterone receptors, race, or other factors that are currently of interest in the field. Considering the potential impact of different races, regions, and progesterone receptor factors on breast cancer, we have included several recently published studies, updating the meta-analysis in this field. We have comprehensively re-evaluated the multifaceted associations between local area network exposure and breast cancer.
2 Methods
2.1 Study protocol
The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA; 58] guidelines for systematic reviews and meta-analysis. Our protocol has been registered on the PROSPERO platform under registration number CRD42023446724.
2.2 Search approach
We searched the PubMed, Cochrane Library, and Embase databases up to July 15, 2023. We employed Medical Subject Headings (MeSH) and relevant keywords. We drew upon the previous methodologies of meta-analysis. (57, 59–64). We included case–control and cohort studies without any language restrictions. The search terms included “Breast Neoplasms,” “Malignant Neoplasm of Breast,” “Light exposure,” and “Risk.” The complete search strategy for each database can be found in Supplementary Tables 1–3. Additionally, we scrutinized the reference lists of the included cohort studies and other published meta-analyses to identify relevant observational studies.
2.3 Study selection
The study selection process was carried out by two reviewers (LZN and LZL) in accordance with the predetermined inclusion and exclusion criteria. Both reviewers independently screened the literature and removed duplicate and irrelevant articles based on titles and abstracts. Subsequently, the full texts of potentially eligible articles were obtained and thoroughly reviewed to ensure the inclusion of all relevant studies. Any disagreements between the two reviewers were resolved through discussion until a consensus was reached.
2.4 Eligibility criteria
The inclusion criteria were as follows: (a) inclusion of an independent exposure variable pertaining to LAN exposure; (b) consideration of an outcome variable related to breast cancer; (c) incorporation of case–control or cohort study designs. The exclusion criteria were as follows: (a) reviews, conference proceedings, or commentaries; (b) duplicate literature (retaining the variant with the most comprehensive information).
2.5 Data extraction
The data extraction process was conducted independently by the first reviewer (LZN), and upon its completion, cross-verification and consultation was performed with the second reviewer (LZL). A predesigned data extraction form was utilized, and the following data were extracted: study type (case–control or cohort), first author, publication year, source of cases, source of controls, number of cases, number of controls, exposure definition, breast cancer definition, classification of exposure, information collection period and scope, baseline age, and values of outcome variables (odds ratio or hazard ratio) determined based on various confounding factors. Any potential discrepancies were addressed through discussions with LZL to achieve a consensus.
2.6 Quality assessment
The quality of our studies was evaluated using the Newcastle-Ottawa Scale (NOS) (65), which employs a star system ranging from 0 to 9 to evaluate participant selection and exposure measurement, with 2 stars allocated for result comparison and 3 stars designated for outcome assessment and follow-up adequacy. A higher score reflects superior study quality. Scores ranging from 0 to 3 indicate low quality, scores ranging from 4 to 6 indicate moderate quality, and scores ranging from 7 to 9 indicate high quality.
2.7 Statistical analysis
The adjusted relative ratios (RRs) and 95% confidence intervals (CIs) for each observational study were used to assess the association between breast cancer incidence and LAN exposure. Heterogeneity was evaluated using I2 values (66) and Cochran’s Q heterogeneity test (67). Furthermore, we utilized Z-tests to assess whether the effect size (RRs) is equal among different subgroups (67). Based on the precedent of previous meta-analyses (33–35), we used a random effects model in each study to address confounding factors and minimize study errors. Sensitivity analysis was conducted by predefining the exclusion of studies that did not pass the sensitivity analysis to ensure the robustness of the overall effect. A funnel plot was examined for symmetry to detect publication bias, and Egger’s regression test was used for statistical assessment (68). The primary data analysis was conducted using Stata statistical software version 17.0 (Stata Corp, College Station, Texas), while the Z-tests were performed using R language (version 4.2.2).
3 Results
3.1 Literature search
A total of 2,964 observational studies were initially identified after excluding duplicates. After conducting title and abstract screening, 2,871 articles were subsequently excluded. An in-depth review was conducted on 93 articles, and after the further exclusion of 72 articles, 21 studies were eligible for the meta-analysis (Figure 1). The primary reasons for exclusion were irrelevant research topics, inappropriate study types, missing data, inappropriate study subjects, availability of only abstracts, studies sharing the same data, and unclear definitions within the LAN. Among the 21 included studies, 8 were cohort studies (36, 39, 41, 45–47, 50, 52) and 13 were case–control studies (32–35, 37, 38, 40, 42–44, 48, 49, 51).
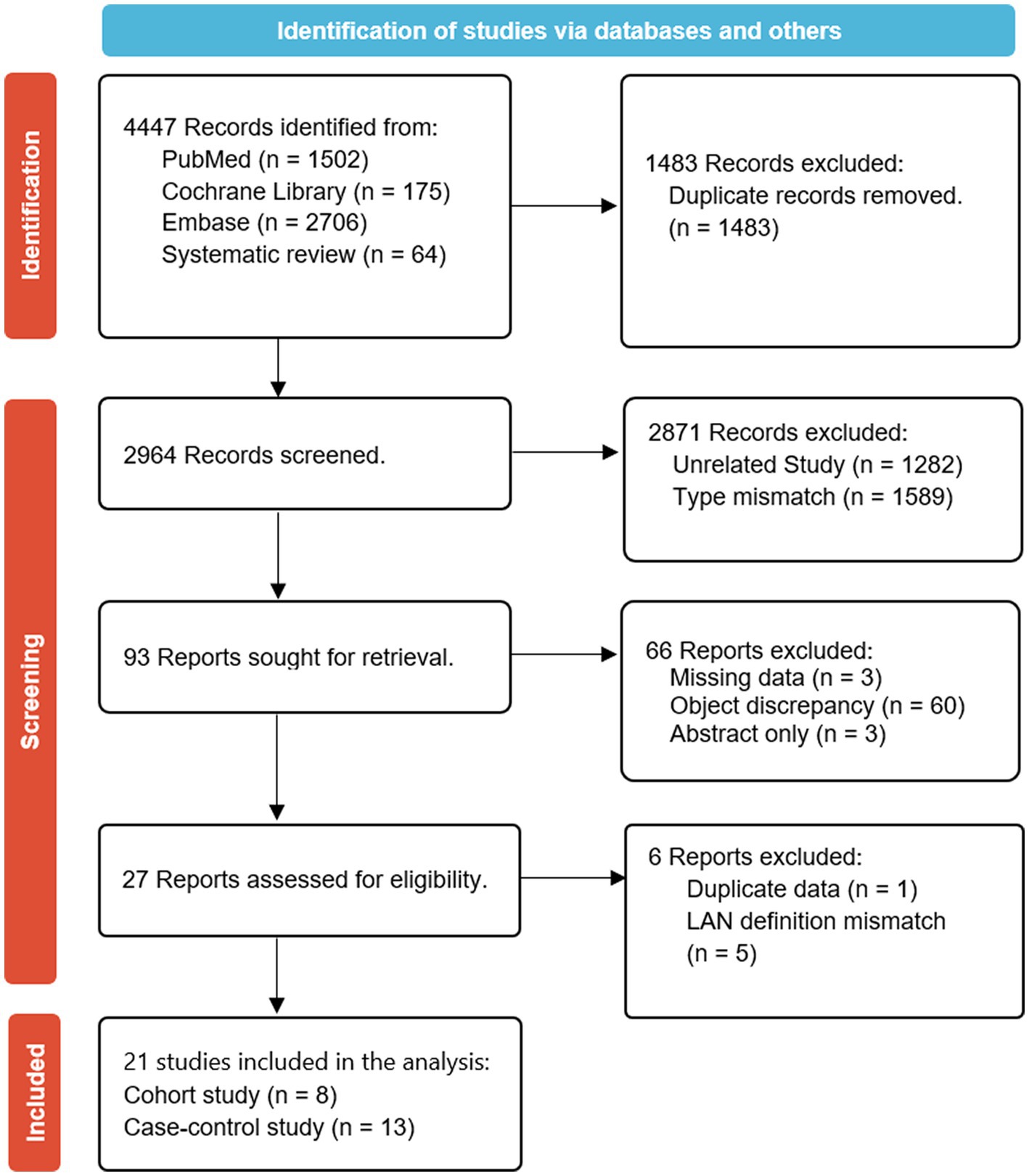
Figure 1. Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram summarizing the search strategy and study selection for the meta-analysis of the association between LAN exposure and risk of breast cancer.
3.2 Study characteristics
The 21 studies included in this study were published between 2001 and 2023 and involved a total of 734,372 participants. Of these studies, 11 originated from North America (32, 39–41, 43, 46–50, 52), 3 from Europe (36, 37, 45), 5 from Asia (33–35, 38, 42), and an additional 2 from Australia (44, 51). The average follow-up time for these studies was 12.8 years.
Among the various confounding factors examined in these studies, the proportion of studies focused on estrogen receptor status was 42.9% (n = 9) (36, 37, 41, 42, 45–47, 50, 52), while studies on menopausal status accounted for 47.6% (n = 10) (36, 39–42, 45–48, 51). Additionally, studies investigating indoor LAN accounted for 66.7% (n = 14) (33–44, 50, 51), and studies on outdoor LAN accounted for 66.7% (n = 14) (32–35, 37, 39–41, 45–48, 50, 52).
Regarding lifestyle habits, the proportions were as follows: 19.0% (n = 4) of studies examined the habit of watching TV while sleeping (35, 40, 41, 50), 23.8% (n = 5) looked at the use of bedroom shutters (33–35, 38, 40), and 14.3% (n = 3) explored the habit of waking up and turning on lights (36, 43, 49).
Each study provided clear definitions for both LAN exposure and breast cancer. Adjusted estimates were available for analysis, despite the presence of varying confounding factors across the studies. The characteristics of the cohort (Table 1) and case–control studies (Table 2) are presented.
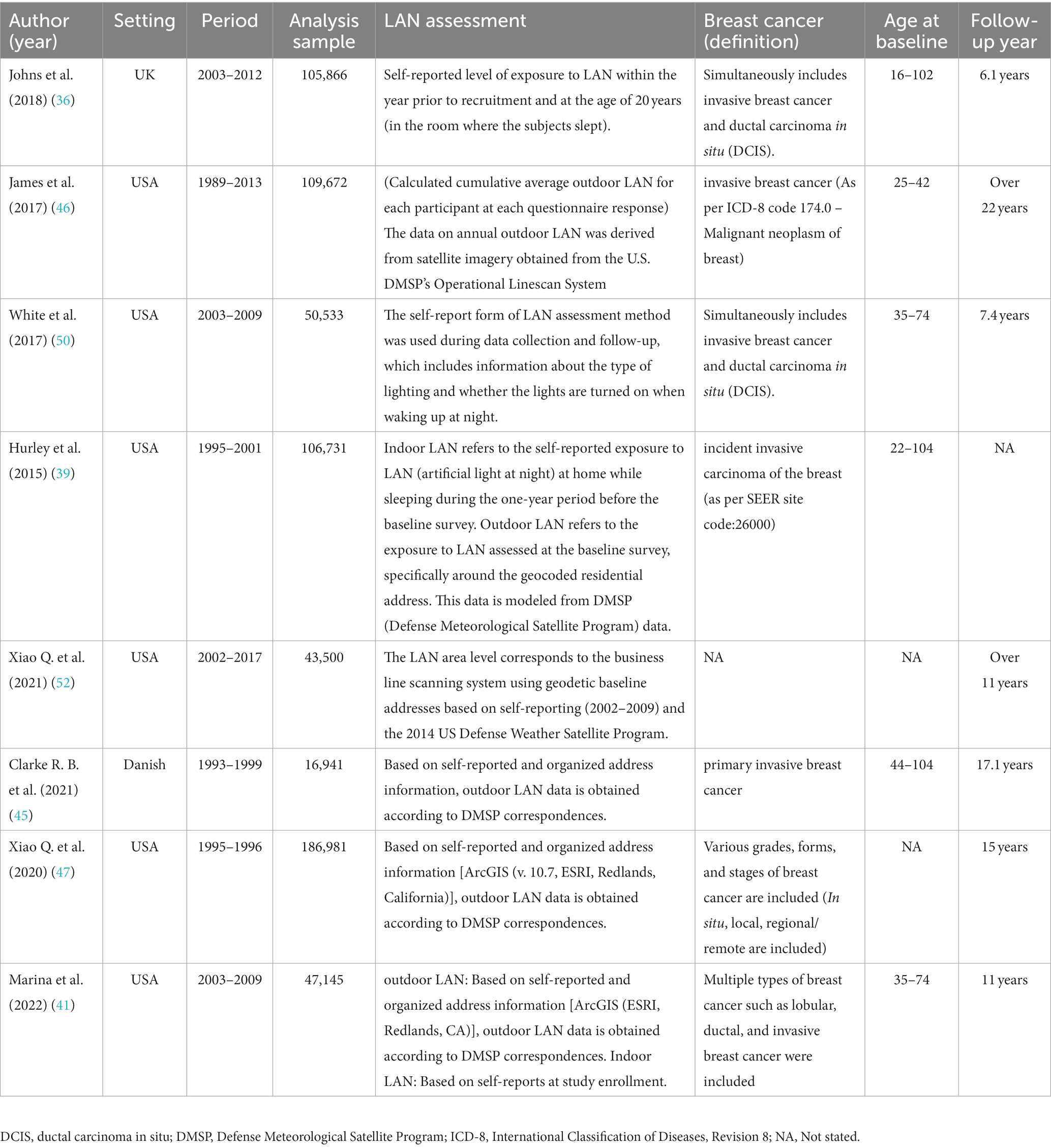
Table 1. Summary of main characteristics of the eight cohort studies examining association between exposure to LAN and risk of breast cancer.
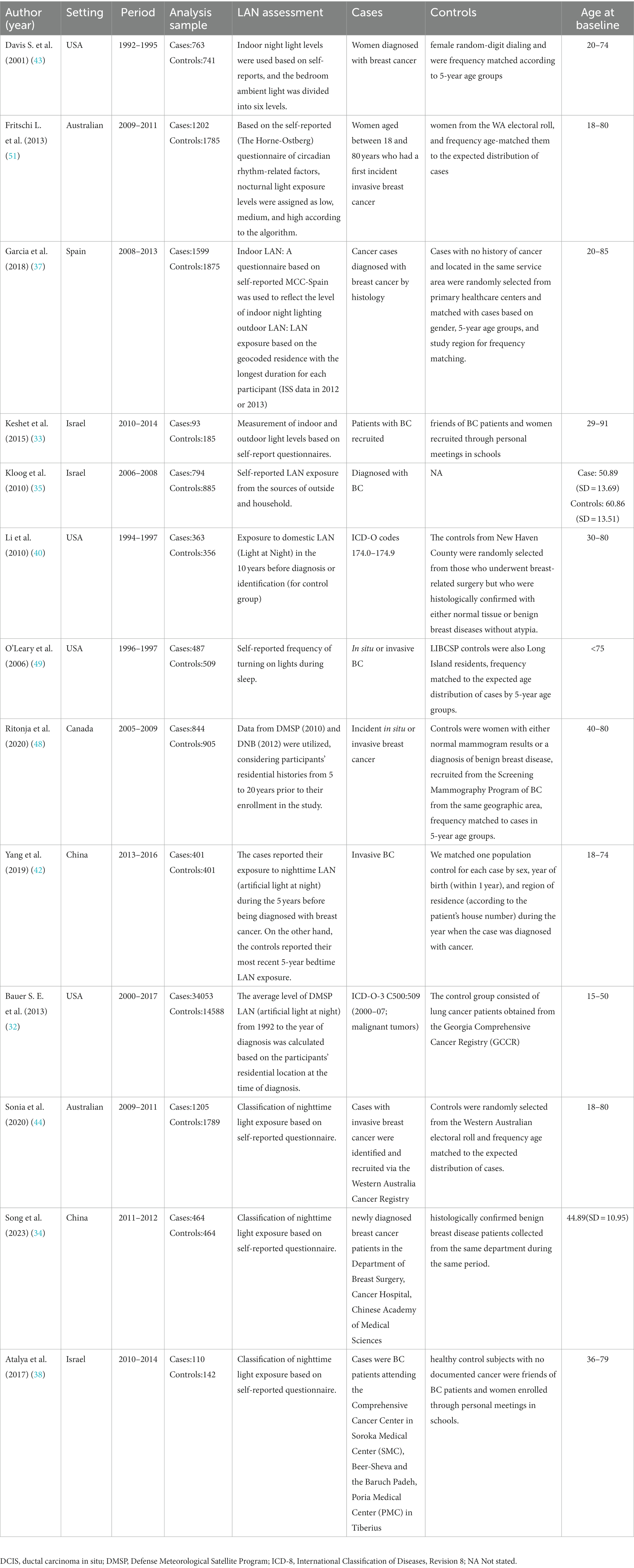
Table 2. Summary of main characteristics of the 13 case–control/case-referent studies examining association between exposure to LAN and risk of breast cancer.
3.3 Quality assessment
In the Newcastle-Ottawa Scale (NOS) assessments conducted on the various studies, the average score for the included research was 7.43 (ranging from 5 to 9). A score of 7 is considered as the cut-off point for high-quality research. Studies with a score of 7 or above are regarded as of higher quality. The scores of the included studies are illustrated in Figure 2.
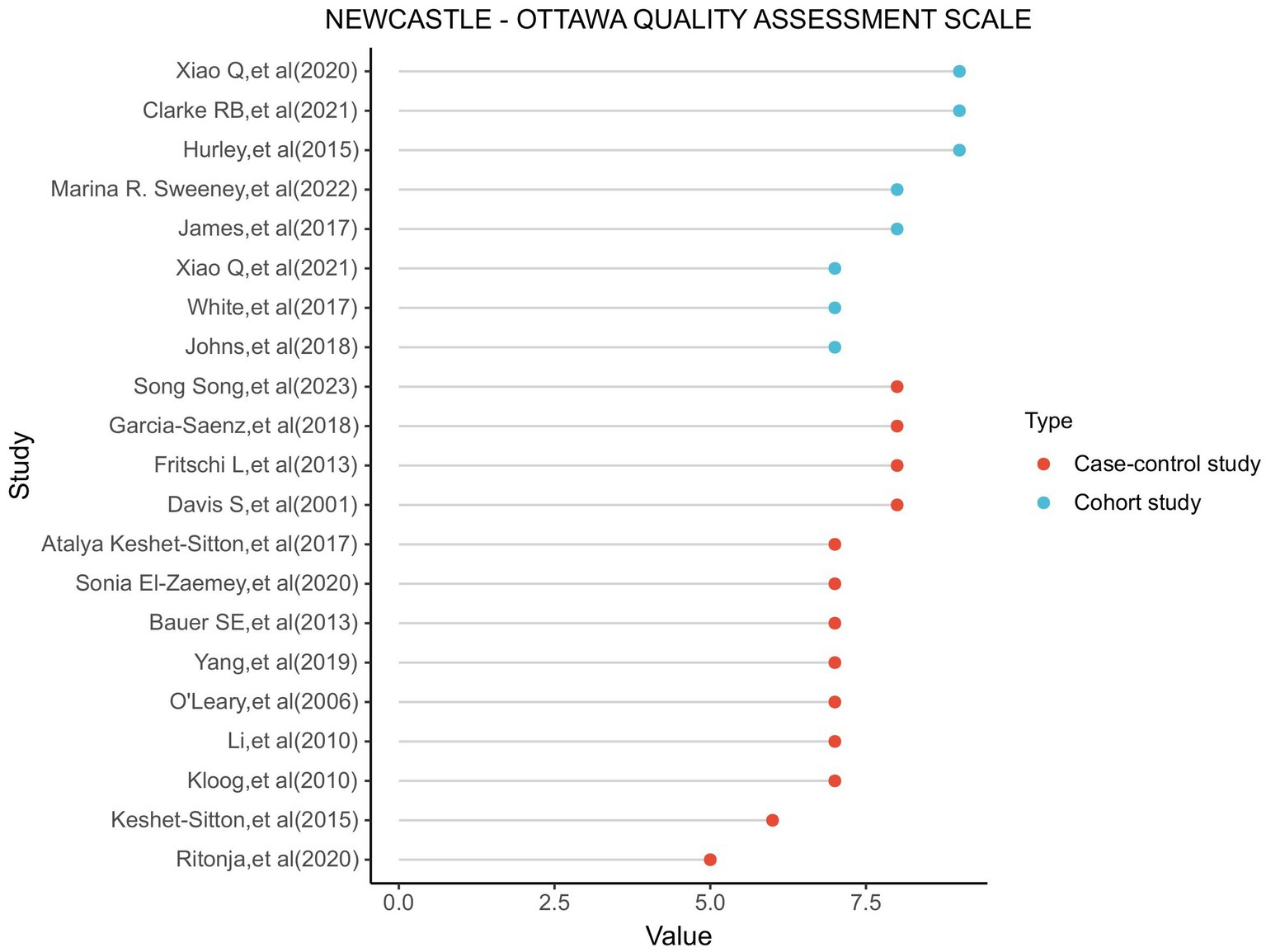
Figure 2. Utilizing the NOS (Newcastle-Ottawa scale) to assess the outcomes of each incorporated study, we evaluate the graphical representation.
3.4 Light at night exposure and breast cancer risk
A comprehensive analysis of 21 studies (32–52) revealed a noteworthy correlation between exposure to LAN and a higher susceptibility to breast cancer (RR = 1.12; 95% CI: 1.06–1.17; I2 = 31.3%, p < 0.001; Figure 3). Heterogeneity analysis indicated a slight level of heterogeneity in our study, whereas sensitivity analysis did not uncover any individual study findings that overturned the overall results, thus providing evidence for the reliability of our analytical outcomes (Supplementary Figure A).
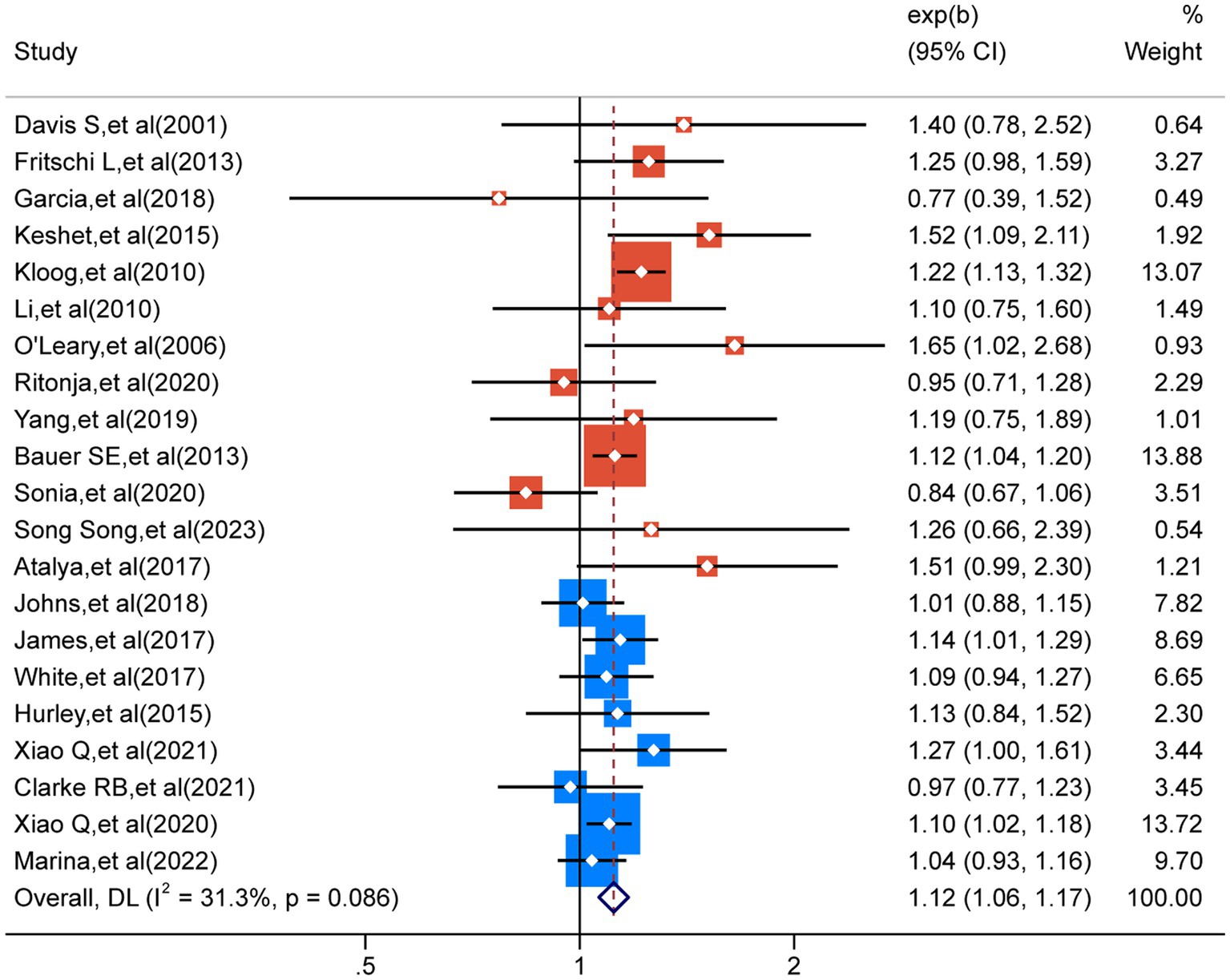
Figure 3. The heterogeneity analysis results for the 21 outcomes concerning nocturnal light exposure.
3.5 Subgroup analysis
We conducted subgroup analyses on the 21 included studies to explore the impact of various factors on the incidence of breast cancer (32–52). Based on existing evidence, we categorized these factors into confounding factors (factors that are associated with LAN exposure and breast cancer incidence but are not mediators of the relationship) and effect-modifying factors (those that modify the relationship under different LAN conditions, breast cancer subtypes, or study types). Specifically, confounding factors included geography, menopausal status, BMI, and ethnicity. Effect-modifying factors encompassed study type, LAN (light at night) type, estrogen and progesterone receptor status, and lifestyle. When analyzed as independent confounding factors, no heterogeneity (I2 = 0%) was observed for continents, white race, and postmenopausal individuals, which was considered the main factor contributing to the slight heterogeneity observed in the overall analysis.
Regarding confounding factors, in our subgroup analyses that focused on different continents, we observed a clear disparity in breast cancer incidence rates associated with exposure to the LAN. Specifically, the breast cancer incidence rate in Asia (RR = 1.24; 95% CI: 1.15–1.34; I2 = 0.0%, p < 0.001) was significantly higher than that in North America (RR = 1.11; 95% CI: 1.06–1.15; I2 = 0.0%, p < 0.001). However, the breast cancer incidence rate in Europe (RR = 0.99; 95% CI: 0.89–1.11; I2 = 0.0%, p = 0.898), despite being analyzed, did not exhibit any statistically significant difference. When considering racial differences, the incidence of breast cancer was higher in the white population (RR = 1.12; 95% CI: 1.05–1.20; I2 = 0.0%, p < 0.001), with no statistically significant difference observed in the Black population (RR = 1.15; 95% CI: 0.98–1.35; I2 = 4.8%, p = 0.080). Additionally, subgroup analysis for menopausal status did not yield statistically significant differences between the premenopausal (RR = 1.09; 95% CI: 0.99–1.19; I2 = 17.6%, p = 0.083) and postmenopausal groups (RR = 1.03; 95% CI: 0.97–1.09; I2 = 0.0%, p = 0.352).
In terms of effect-modifying factors, similar to the meta-analysis conducted in 2021 (57), we conducted separate analyses to assess the effects of LAN exposure on breast cancer risk based on estrogen receptor status, types of study, and types of LAN exposure. The analysis of different types of LAN exposure showed that outdoor LAN exposure (RR = 1.07; 95% CI: 1.02–1.13; I2 = 30.9%, p = 0.004) had a slightly higher promoting effect on breast cancer incidence than indoor LAN exposure (RR = 1.02; 95% CI: 0.92–1.13; I2 = 65.6%, p = 0.675). In the analysis of estrogen receptor status, LAN exposure exhibited a slightly stronger impact on breast cancer risk for cases with estrogen receptor positivity (RR = 1.10; 95% CI: 1.03–1.18; I2 = 17.0%, p = 0.005) compared to cases with estrogen receptor negativity (RR = 1.07; 95% CI: 0.94–1.21; I2 = 0.0%, p = 0.316). Subgroup analyses for different study types revealed that LAN exposure had a higher promoting effect on individual breast cancer incidence in case–control studies (RR = 1.16; 95% CI: 1.06–1.27; I2 = 40.4%, p = 0.001) compared to cohort studies (RR = 1.08; 95% CI: 1.04–1.14; I2 = 0.0%, p < 0.001).We also conducted analyses on various lifestyle factors summarized in the literature. When considering TV on while sleeping as an effect-modifying factor, the impact of LAN exposure on breast cancer did not show statistical significance (RR = 1.06; 95% CI: 0.97–1.16; I2 = 0.0%, p = 0.168). This observation holds true for “Turn on the light when you wake up” (RR = 1.09; 95% CI: 0.87–1.37; I2 = 47.5%, p = 0.449) and “Bedroom shutters (open)” (RR = 1.07; 95% CI: 0.84–1.37; I2 = 64.4%, p = 0.589). The complete dataset can be found in Table 3. The results of the Z-tests and the forest plot for the subgroups can be viewed in Supplementary Figure C.
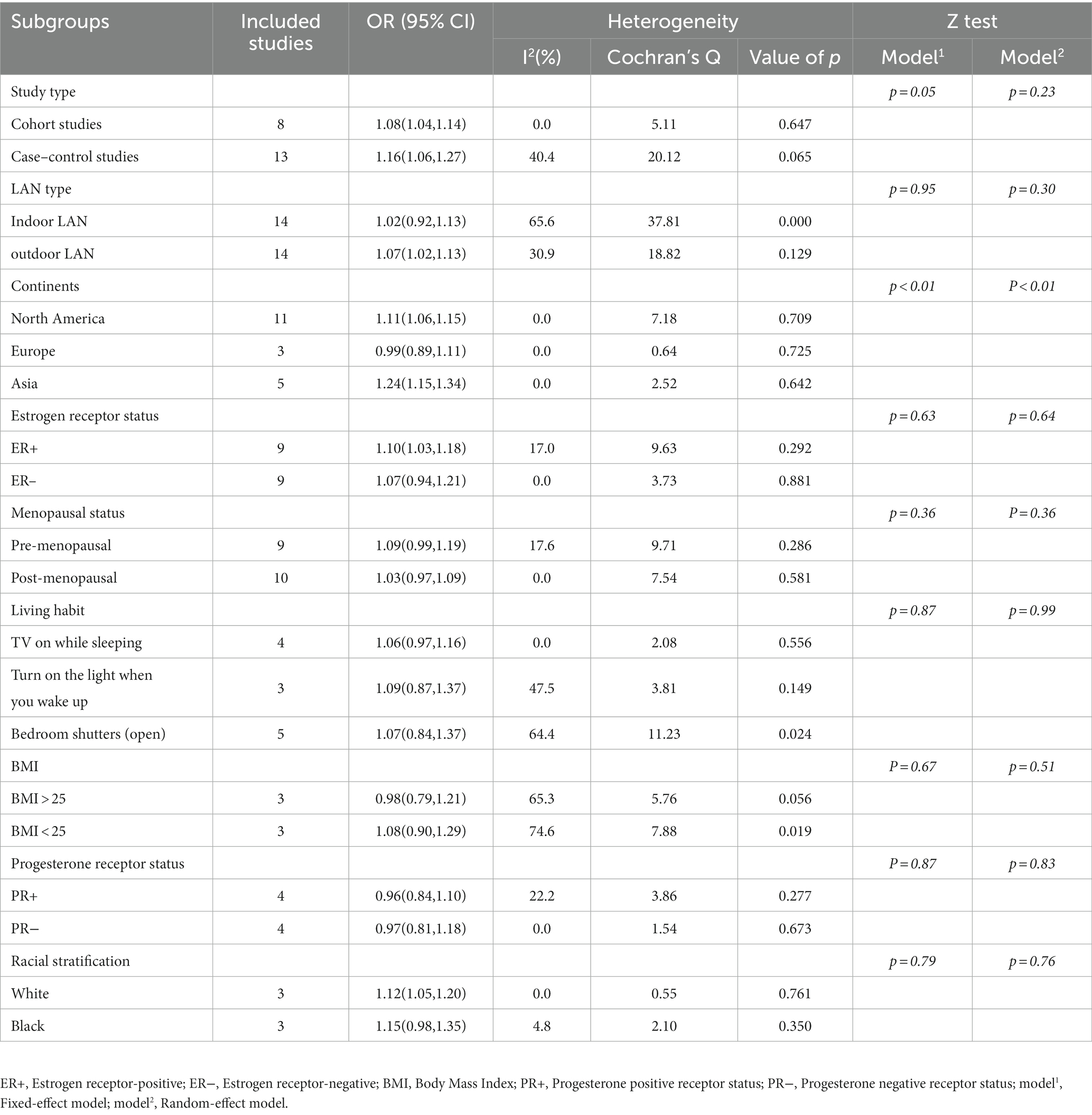
Table 3. Subgroup analysis of the risk of breast cancer in patients with exposure to LAN (light at night).
3.6 Publication bias
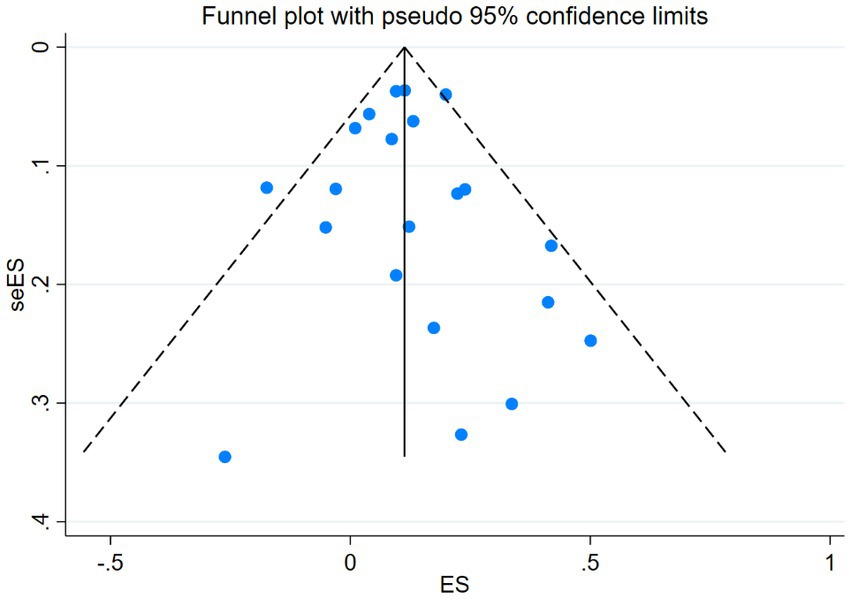
Visual inspection of the funnel plot did not reveal any significant publication bias in the occurrence of breast cancer. This observation holds true for the overall analysis funnel plot (Figure 4), which is consistent with the results of our Egger’s regression test (p = 0.746). Similar findings of bias were observed across various subgroups (Supplementary Figure B).
4 Discussion
4.1 Main findings
Our meta-analysis included a total of 21 observational studies. The collective data analysis unveiled a significant correlation between exposure to LAN and an escalated susceptibility to breast cancer. Regarding the confounding factors, Jack Sariego first proposed the relationship between geographic correlation and breast cancer (69). This relationship is typically associated with fertility rates and taxation (70). High-quality lifestyles or habits in developed regions are effective in curtailing the incidence of breast cancer (71). We observed that LAN exposure in Asian countries showed the highest increase in breast cancer risk, followed by North America, while in Europe, the breast cancer risk outcome did not reach statistical significance. At the level of menopausal status, many studies have observed a protective effect in premenopausal women in terms of obesity (72), night shift work (73), and physical activity (74). We found that breast cancer risk outcomes did not reach statistical significance, whether in premenopausal or postmenopausal states. We considered additional factors that are associated with LAN exposure and breast cancer incidence but are not mediators of the relationship, such as BMI and race, which could potentially introduce significant bias to the risk outcomes. Multicenter studies have indicated a clear association between higher BMI and breast cancer in women (75), while in terms of race, the annual statistical graphs from the SEER Cancer Statistics Review show consistently higher breast cancer incidence rates in the white population than in the Black population (76). Subgroup analysis for LAN exposure revealed that white individuals have a higher breast cancer risk. Existing evidence supports the notion that cultural dynamics, as well as differences in sociodemographic and behavioral characteristics among different population subgroups, modulate the expression of biological diseases, possibly contributing to the higher breast cancer risk in the white population (14). In terms of modifying factors, previous observational studies have consistently shown a significant increase in breast cancer risk associated with outdoor LAN exposure, particularly among estrogen receptor-positive cases. Our findings align with two prior meta-analyses (57, 60). In a previous meta-analysis, Ka Yan Lai et al. highlighted the impact of indoor LAN exposure in their discussion (60). Lifestyle factors such as window orientation, the use of blinds, and television watching may all contribute to an elevated risk of breast cancer (77). Due to the limited number of studies available, they did not conduct a subgroup analysis in this regard. We have addressed this gap in the literature. Although the five studies included in our analysis did not demonstrate a statistically significant difference, the influence of confounding factors within the lifestyle domain is substantial. Therefore, future, more rigorous research focusing on LAN exposure should be conducted.
4.2 Comparison with previous studies
To date, a total of 8 related meta-analyses have been identified (57, 59–62, 64, 78). Regarding the types of included studies, the predominant ones include: four studies assessing the association between breast cancer risk and indoor and outdoor LAN (57.1%) (59–61, 78), four studies investigating the relationship between breast cancer risk and menopausal status (57.1%) (60, 61, 64, 78), and two studies examining the association between breast cancer risk and estrogen receptor status (28.6%) (60, 78).
Ka Yan Lai et al. (60) published a meta-analysis on LAN exposure in 2021. Their results showed a significant association between ER+ and outdoor LAN exposure and cancer risk. They also observed some correlation in premenopausal women. However, they did not consider the potential influence of the patients’ life habits on the experimental results. Furthermore, although their study included participants from seven countries, they did not explore differences in the effects of LAN exposure on breast cancer between countries, although there is solid evidence that cancer risks vary due to economic differences between countries (79). In our analysis, we supplemented subgroups with continental differences and lifestyle habits. We did not observe significant differences in LAN exposure and breast cancer risk by different menopausal states. Therefore, our study provides more subgroup analysis and new evidence in this field, which has certain reference value.
Another meta-analysis, conducted by Teresa Urbano et al. (57), encompassed 17 studies. Subgroup analysis was performed based on study type, menopausal status, and estrogen receptor (ER) status. Their findings revealed a noteworthy elevation in the risk of breast cancer attributed to exposure to LAN in cohort studies, premenopausal women, and ER-positive women. In contrast, our study employed a larger sample size (n = 21). Notably, our findings demonstrated a more substantial risk association among the case–control study population, contradicting the results reported by Urbano et al. (57).
4.3 Interpretation of findings
The existing three hypotheses attempt to explain the connection between LAN exposure and the risk of various cancers: the direct inhibition of melatonin secretion (80), sleep deprivation affecting cell proliferation and cytokine production (81), and the effect of chrono disruption (82, 83). The function of melatonin was described in detail in 2018 (84). Melatonin is considered a pleiotropic and multitasking molecule (85). In addition to regulating circadian rhythms, it plays a significant role in anticancer effects. It inhibits tumor cell proliferation and invasion, suppresses DNA damage, and modulates the immune system to aid in the elimination of tumor cells. All these factors support its inhibitory effect on breast cancer (55, 81). Additionally, some observational studies have confirmed the suppression of melatonin secretion due to indoor LAN exposure (86). In addition, we should also acknowledge the potential impacts of sleep deprivation and circadian disruption. The sleep–wake cycle is a natural component of human life (87), including waking up during the day and sleeping overnight. The sleep pattern is controlled by static sleep pressure and the circadian rhythm (88), so as the day progresses, the combined effect of these two factors makes it easier for us to fall asleep, while signals from the circadian rhythm can prevent us from falling asleep. Experimental evidence has shown that sleep deprivation can significantly impair learning, memory, judgment, and concentration (89). In terms of health-related risk factors, according to the Nurse’s Health Study, shift work nurses, especially those who work at least three times a month for 15 years or more, have a significantly increased risk of colorectal cancer (90). The risk of breast cancer in nurses who have worked in shift rotations for over 20 years is 1.79 times higher (91). The detrimental effects of LAN exposure on sleep have been confirmed in the experiments conducted by Yu-Xiang Xu et al. (92).
4.4 Implications and limitations
Nonetheless, our meta-analysis has limitations. First, as is typical with observational studies, uncontrolled confounding factors can affect our evaluations. When analyzing lifestyle factors, we did not consider potential influences from factors such as “turn on the light when you wake up” due to insufficient descriptions or sample sizes in observational studies. To control for confounding factors within acceptable limits, we did not assess the potential impact of LAN exposure during night shifts or rotating shift work on breast cancer. The impacts of insufficient sleep duration and sleep quality were not explored, despite prior research showing their significance (93).Other established risk factors for breast cancer, such as noise, air quality, smoking, and alcohol intake, were not adequately examined due to small sample sizes. For instance, observational studies on alcohol accounted for only 4.8% (n = 1) (47), and subgroup studies on smoking were similarly limited at 9.5% (n = 2) (46, 47). While the influence of BMI on breast cancer has been established (94), we could not analyze its association with LAN exposure due to the limited data available. The use of personal electronic devices, lightning, and magnetic fields represent potential sources of exposure that should be considered. Second, due to inconsistent indicators of outdoor LAN exposure data of various materials, DMSP (47) or DNP (48) was used for LAN dose detection in existing experiments, and no updated outdoor LAN exposure dose data were available after 2021. Therefore, we did not conduct a dose analysis on the correlation between LAN exposure and breast cancer. Additionally, it is inevitable that we included retrospective cohort studies and case–control studies, where patients’ subjective descriptions may introduce potential biases and ultimately lead to recall bias. Therefore, the interpretation of the results should be approached with caution. The last factor is the subjective design error. In many indoor LAN exposure studies based on self-reporting (35–39, 43, 50, 51), statistical analysis is conducted to compare the population with the highest LAN exposure or presence of LAN exposure to those without LAN exposure or with the lowest dose. However, due to different questionnaire designs and varying definitions of high and low doses (for example, Davis et al. (43) categorized indoor LAN exposure into six levels, while Johns et al. (36) used only three levels), the subjectivity present in each study cannot be avoided in our selection. Undeniably, our study still has many strengths. (1) Following the suggestion of Ka Yan Lai et al. (60), we have, for the first time, considered continents and lifestyle habits in the meta-analysis of LAN exposure and breast cancer and obtained favorable results. (2) Our study supports the claim that there is no significant difference in breast cancer risk between pre- and postmenopausal women exposed to the LAN, as we did not observe any statistical significance in our meta-analysis, providing new evidence for this factor. (3) Our study found that individuals with high exposure to the LAN, especially among Asian populations, are more susceptible to breast cancer. Therefore, it may be of great importance for relevant public health agencies to implement necessary protective measures and preventive strategies for specific groups. Therefore, future research should incorporate relevant influencing factors, particularly those related to unhealthy lifestyle choices. Additionally, designing standardized indoor LAN exposure assessments will aid in evaluating factors associated with breast cancer risk.
5 Conclusion
Our meta-analysis provides evidence to support the hypothesis that exposure to LAN is associated with an elevated risk of breast cancer incidence, thereby adding valuable insights to the existing body of research. Although we did not observe a statistically significant correlation between lifestyle factors and the association between LAN and breast cancer incidence, our findings indicate a stronger relationship in certain subgroups, such as study populations located in Asia or investigations focusing on outdoor LAN exposure. These results contribute to the identification of important avenues for future research and offer meaningful recommendations.
Author contributions
ZIL: Conceptualization, Data curation, Formal analysis, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. ZHL: Conceptualization, Funding–acquisition, Writing – review & editing. HC: Data curation, Investigation, Supervision, Writing – review & editing. YL: Writing – review & editing-Methodology/Investigation. NT: Investigation, Supervision, Writing – review & editing. HL: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Science and Technology Strategic Cooperation Project of Nanchong Government & University (18SXHZ0482). Student College Computer Design Competition of Sichuan (20230410).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1276290/full#supplementary-material
References
1. Ferlay, J, Shin, HR, Bray, F, Forman, D, Mathers, C, and Parkin, DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. (2010) 127:2893–917. doi: 10.1002/ijc.25516
2. Jemal, A, Bray, F, Center, MM, Ferlay, J, Ward, E, and Forman, D. Global cancer statistics. CA Cancer J Clin. (2011) 61:69–90. doi: 10.3322/caac.20107
3. AIRTUM. The numbers of cancer in Italy-2020. (2020). Available at: https://www.registritumori.it/cms/sites/default/fles/pubblicazioni/new_NDC2020-operatori-web.pdf. 2020.
4. Giaquinto, AN, Sung, H, Miller, KD, Kramer, JL, Newman, LA, Minihan, A, et al. Breast Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:524–41. doi: 10.3322/caac.21754
5. Coombs, NJ, Cronin, KA, Taylor, RJ, Freedman, AN, and Boyages, J. The impact of changes in hormone therapy on breast cancer incidence in the US population. Cancer Causes Control. (2010) 21:83–90. doi: 10.1007/s10552-009-9437-5
6. Ravdin, PM, Cronin, KA, Howlader, N, Berg, CD, Chlebowski, RT, Feuer, EJ, et al. The decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. (2007) 356:1670–4. doi: 10.1056/NEJMsr070105
7. DeSantis, C, Howlader, N, Cronin, KA, and Jemal, A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. (2011) 20:733–9. doi: 10.1158/1055-9965.EPI-11-0061
8. Islami, F, Ward, EM, Sung, H, Cronin, KA, Tangka, FKL, Sherman, RL, et al. Annual report to the nation on the status of Cancer, part 1: national cancer statistics. J Natl Cancer Inst. (2021) 113:1648–69. doi: 10.1093/jnci/djab131
9. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*stat database: Incidence—SEER research data with delay-adjustment, 8 registries, malignant only, November 2021 submission (1975–2019)—Linked to county attributes—Time dependent (1990–2019) income/rurality, 1969–2020 counties. Surveillance research program, division of Cancer control and population sciences, National Cancer Institute. (2022). Accessed July 1, 2022. seer.cancer.gov.
10. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*stat database: Incidence—SEER research limited-field data with DelayAdjustment, 22 registries, malignant only, November 2021 submission (2000–2019)—Linked to county attributes—Time dependent (1990–2019) income/rurality, 1969–2020 counties. Surveillance research program, division of Cancer control and population sciences, National Cancer Institute; (2022). Accessed July 1, 2022. seer.cancer.gov.
11. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
12. Anastasiadi, Z, Lianos, GD, Ignatiadou, E, Harissis, HV, and Mitsis, M. Breast cancer in young women: an overview. Updates Surg. (2017) 69:313–7. doi: 10.1007/s13304-017-0424-1
13. Cheng, H, Lin, L, Liu, T, Wang, S, Zhang, Y, and Tian, L. Financial toxicity of breast cancer over the last 30 years: a bibliometrics study and visualization analysis via CiteSpace. Medicine. (2023) 102:e33239. doi: 10.1097/MD.0000000000033239
14. Hunter, CP. Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer. (2000) 88:1193–202. doi: 10.1002/(SICI)1097-0142(20000301)88:5+<1193::AID-CNCR3>3.0.CO;2-D
15. Williams, F, Jeanetta, S, and James, AS. Geographical location and stage of breast Cancer diagnosis: a systematic review of the literature. J Health Care Poor Underserved. (2016) 27:1357–83. doi: 10.1353/hpu.2016.0102
16. Brito-Marcelino, A, Duarte-Tavares, RJ, Marcelino, KB, and Silva-Neto, JA. Breast cancer and occupational exposures: an integrative review of literature. Revista Brasileira de Medicina do Trabalho. (2020) 18:488–96. doi: 10.47626/1679-4435-2020-595
17. Lu, S, Huang, X, Yu, H, Yang, J, Han, R, Su, J, et al. Dietary patterns and risk of breast cancer in Chinese women: a population-based case-control study. Lancet. (2016) 388:S61. doi: 10.1016/S0140-6736(16)31988-2
18. Andersen, ZJ, Stafoggia, M, Weinmayr, G, Pedersen, M, Galassi, C, Jørgensen, JT, et al. Long-term exposure to ambient air pollution and incidence of postmenopausal breast Cancer in 15 European cohorts within the ESCAPE project. Environ Health Perspect. (2017) 125:107005. doi: 10.1289/EHP1742
19. Hastert, TA, Beresford, SA, Sheppard, L, and White, E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. (2014) 69:168–76. doi: 10.1136/jech-2014-204417
20. Gaudet, MM, Gapstur, SM, Sun, J, Diver, WR, Hannan, LM, and Thun, MJ. Active smoking and breast Cancer risk: original cohort data and Meta-analysis. JNCI: J Nat Cancer Institute. (2013) 105:515–25. doi: 10.1093/jnci/djt023
21. Zhang, SM, Lee, IM, Manson, JE, Cook, NR, Willett, WC, and Buring, JE. Alcohol consumption and breast Cancer risk in the Women's health study. Am J Epidemiol. (2007) 165:667–76. doi: 10.1093/aje/kwk054
22. Cotterchio, M, Kreiger, N, Theis, B, Sloan, M, and Bahl, S. Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev. (2003) 12:1053–60. doi: 10.1002/ijc.27788
23. Davis Lynn, BC, Chernyavskiy, P, Gierach, GL, and Rosenberg, PS. Decreasing incidence of estrogen receptor-negative breast cancer in the United States: trends by race and region. J Natl Cancer Inst. (2022) 114:263–70. doi: 10.1093/jnci/djab186
24. Pfeiffer, RM, Webb-Vargas, Y, Wheeler, W, and Gail, MH. Proportion of U.S. trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol Biomarkers Prev. (2018) 27:1214–22. doi: 10.1158/1055-9965.EPI-18-0098
25. Rosenberg, PS, Barker, KA, and Anderson, WF. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst. (2015) 107:djv159. doi: 10.1093/jnci/djv159
26. Walker, WH, Bumgarner, JR, Walton, JC, Liu, JA, Meléndez-Fernández, OH, Nelson, RJ, et al. Light pollution and Cancer. Int J Mol Sci. (2020) 21:9360. doi: 10.3390/ijms21249360
27. Davies, TW, and Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob Chang Biol. (2017) 24:872–82. doi: 10.1111/gcb.13927
28. Kyba, CCM, and Spitschan, M. Comment on ‘domestic light at night and breast cancer risk: a prospective analysis of 105000 UK women in the generations study’. Br J Cancer. (2018) 120:276–7. doi: 10.1038/s41416-018-0203-x
29. Falchi, F, Cinzano, P, Duriscoe, D, Kyba, CC, Elvidge, CD, Baugh, K, et al. The new world atlas of artificial night sky brightness. Sci Adv. (2016) 2:e1600377. doi: 10.1126/sciadv.1600377
30. Rea, MS, Brons, JA, and Figueiro, MG. Measurements of light at night (LAN) for a sample of female school teachers. Chronobiol Int. (2011) 28:673–80.
31. Chang, A-M, Aeschbach, D, Duffy, JF, and Czeisler, CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. PANAS. (2015) 112:1232–7. doi: 10.1073/pnas.1418490112
32. Bauer, SE, Wagner, SE, Burch, J, Bayakly, R, and Vena, JE. A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr. (2013) 12:23. doi: 10.1186/1476-072X-12-23
33. Keshet-Sitton, A, Or-Chen, K, Yitzhak, S, Tzabary, I, and Haim, A. Can avoiding light at night reduce the risk of breast Cancer? Integr Cancer Ther. (2016) 15:145–52. doi: 10.1177/1534735415618787
34. Song, S, Lei, L, Zhang, R, Liu, H, du, J, Li, N, et al. Circadian disruption and breast Cancer risk: evidence from a case-control study in China. Cancers (Basel). (2023) 15:419. doi: 10.3390/cancers15020419
35. Kloog, I, Portnov, BA, Rennert, HS, and Haim, A. Does the modern urbanized sleeping habitat pose a breast Cancer risk? Chronobiol Int. (2011) 28:76–80. doi: 10.3109/07420528.2010.531490
36. Johns, LE, Jones, ME, Schoemaker, MJ, McFadden, E, Ashworth, A, and Swerdlow, AJ. Domestic light at night and breast cancer risk: a prospective analysis of 105 000 UK women in the generations study. Br J Cancer. (2018) 118:600–6. doi: 10.1038/bjc.2017.359
37. Garcia-Saenz, A, Sánchez de Miguel, A, Espinosa, A, Valentin, A, Aragonés, N, Llorca, J, et al. Evaluating the association between artificial light-at-night exposure and breast and prostate Cancer risk in Spain (MCC-Spain study). Environ Health Perspect. (2018) 126:047011. doi: 10.1289/EHP1837
38. Keshet-Sitton, A, Or-Chen, K, Yitzhak, S, Tzabary, I, and Haim, A. Light and the City: breast Cancer risk factors differ between urban and rural women in Israel. Integr Cancer Ther. (2017) 16:176–87. doi: 10.1177/1534735416660194
39. Hurley, S, Goldberg, D, Nelson, D, Hertz, A, Horn-Ross, PL, Bernstein, L, et al. Light at night and breast cancer risk among California teachers. Epidemiology. (2014) 25:697–706. doi: 10.1097/EDE.0000000000000137
40. Li, Q, Zheng, T, Holford, TR, Boyle, P, Zhang, Y, and Dai, M. Light at night and breast cancer risk: results from a population-based case-control study in Connecticut, USA. Cancer Causes Control. (2010) 21:2281–5. doi: 10.1007/s10552-010-9653-z
41. Sweeney, MR, Nichols, HB, Jones, RR, Olshan, AF, Keil, AP, Engel, LS, et al. Light at night and the risk of breast cancer: findings from the sister study. Environ Int. (2022) 169:107495. doi: 10.1016/j.envint.2022.107495
42. Yang, W, Shi, Y, Ke, X, Sun, H, Guo, J, and Wang, X. Long-term sleep habits and the risk of breast cancer among Chinese women: a case–control study. Eur J Cancer Prev. (2019) 28:323–9. doi: 10.1097/CEJ.0000000000000458
43. Davis, S, Mirick, DK, Stevens, RG, and Work, NS. Light at night, and risk of breast Cancer. JNCI: J Nat Cancer Institute. (2001) 93:1557–62. doi: 10.1093/jnci/93.20.1557
44. el-Zaemey, S, Fritschi, L, Heyworth, J, Boyle, T, Saunders, C, Wylie, E, et al. No association between night shiftwork and mammographic density. Occup Environ Med. (2020) 77:564–7. doi: 10.1136/oemed-2019-106315
45. Clarke, RB, Amini, H, James, P, von Euler-Chelpin, M, Jørgensen, JT, Mehta, A, et al. Outdoor light at night and breast cancer incidence in the Danish nurse cohort. Environ Res. (2021) 194:110631. doi: 10.1016/j.envres.2020.110631
46. James, P, Bertrand, KA, Hart, JE, Schernhammer, ES, Tamimi, RM, and Laden, F. Outdoor light at night and breast Cancer incidence in the Nurses' health study II. Environ Health Perspect. (2017) 125:087010. doi: 10.1289/EHP935
47. Xiao, Q, James, P, Breheny, P, Jia, P, Park, Y, Zhang, D, et al. Outdoor light at night and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int J Cancer. (2020) 147:2363–72. doi: 10.1002/ijc.33016
48. Ritonja, J, McIsaac, MA, Sanders, E, Kyba, CCM, Grundy, A, Cordina-Duverger, E, et al. Outdoor light at night at residences and breast cancer risk in Canada. Eur J Epidemiol. (2020) 35:579–89. doi: 10.1007/s10654-020-00610-x
49. O'Leary, ES, Schoenfeld, ER, Stevens, RG, Kabat, GC, Henderson, K, Grimson, R, et al. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol. (2006) 164:358–66. doi: 10.1093/aje/kwj211
50. White, AJ, Weinberg, CR, Park, YM, D'Aloisio, AA, Vogtmann, E, Nichols, HB, et al. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int J Cancer. (2017) 141:2204–14. doi: 10.1002/ijc.30920
51. Fritschi, L, Erren, TC, Glass, DC, Girschik, J, Thomson, AK, Saunders, C, et al. The association between different night shiftwork factors and breast cancer: a case-control study. Br J Cancer. (2013) 109:2472–80. doi: 10.1038/bjc.2013.544
52. Xiao, Q, Gierach, GL, Bauer, C, Blot, WJ, James, P, and Jones, RR. The association between outdoor artificial light at night and breast Cancer risk in black and White women in the southern community cohort study. Environ Health Perspect. (2021) 129:87701. doi: 10.1289/EHP9381
53. Stevens, RG, and Rea, MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. (2001) 12:279–87. doi: 10.1023/A:1011237000609
54. Stevens, RG. Electric power use and breast CANCER: a hypothesis. Am J Epidemiol. (1987) 125:556–61. doi: 10.1093/oxfordjournals.aje.a114569
55. Cohen, M, Lippman, M, and Chabner, B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet. (1978) 2:814–6. doi: 10.1016/S0140-6736(78)92591-6
56. Yager, JD, and Davidson, NE. Estrogen carcinogenesis in breast Cancer. N Engl J Med. (2006) 354:270–82. doi: 10.1056/NEJMra050776
57. Urbano, T, Vinceti, M, Wise, LA, and Filippini, T. Light at night and risk of breast cancer: a systematic review and dose-response meta-analysis. Int J Health Geogr. (2021) 20:44. doi: 10.1186/s12942-021-00297-7
58. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
59. He, C, Anand, ST, Ebell, MH, Vena, JE, and Robb, SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health. (2015) 88:533–47. doi: 10.1007/s00420-014-0986-x
60. Lai, KY, Sarkar, C, Ni, MY, Cheung, LWT, Gallacher, J, and Webster, C. Exposure to light at night (LAN) and risk of breast cancer: a systematic review and meta-analysis. Sci Total Environ. (2021) 762:143159. doi: 10.1016/j.scitotenv.2020.143159
61. Wu, Y, Gui, SY, Fang, Y, Zhang, M, and Hu, CY. Exposure to outdoor light at night and risk of breast cancer: a systematic review and meta-analysis of observational studies. Environ Pollut. (2021) 269:116114. doi: 10.1016/j.envpol.2020.116114
62. Yang, WS, Deng, Q, Fan, WY, Wang, WY, and Wang, X. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. European J Cancer Preven Official J European Cancer Preven Organ. (2014) 23:269–76. doi: 10.1097/CEJ.0000000000000030
63. Stevens, RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. (2009) 38:963–70. doi: 10.1093/ije/dyp178
64. Megdal, SP, Kroenke, CH, Laden, F, Pukkala, E, and Schernhammer, ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. (2005) 41:2023–32. doi: 10.1016/j.ejca.2005.05.010
65. Wells, GA. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2014). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
66. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
67. Borenstein, M, Hedges, LV, Higgins, JPT, and Rothstein, HR. Introduction to Meta-analysis. US: Wiley (2009).
68. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34.
69. Sariego, J. Patterns of breast Cancer presentation in the United States: does geography matter? Am Surg. (2009) 75:545–50. doi: 10.1177/000313480907500703
70. Hakama, M, Soini, I, Kuosma, E, Lehtonen, M, and Aromaa, A. Breast Cancer incidence: geographical correlations in Finland. Int J Epidemiol. (1979) 8:33–40. doi: 10.1093/ije/8.1.33
71. Walker, CL, and Nies, MA. Health promoting lifestyles: healthy women and women with breast Cancer. Nurse Educ. (1998) 23:6. doi: 10.1097/00006223-199805000-00001
72. Rose, DP, and Vona-Davis, L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. (2010) 66:33–8. doi: 10.1016/j.maturitas.2010.01.019
73. Hansen, J. Night shift Work and risk of breast Cancer. Current Environ Heal Reports. (2017) 4:325–39. doi: 10.1007/s40572-017-0155-y
74. Friedenreich, CM. Physical activity and breast Cancer risk: the effect of menopausal status. Exerc Sport Sci Rev. (2004) 32:180–4. doi: 10.1097/00003677-200410000-00010
75. Noureen, A, Javed, A, Siddiqui, RH, and Shakir, FTZ. Correlation between stages of breast cancer and BMI in females: a multi-Centre study. J Pak Med Assoc. (2023) 73:467–70. doi: 10.47391/JPMA.5443
76. LAG, R, Kosary, CL, Hankey, BF, Miller, BA, and Edwards, BK. SEER Cancer statistics review, 1973–1996. National Cancer Institute: Bethesda, MD (1998).
77. Harvie, M, Howell, A, and Evans, DG. Can diet and lifestyle prevent breast Cancer: what is the evidence? Am Soc Clin Oncol Educ Book. (2015) 35:e66–73. doi: 10.14694/EdBook_AM.2015.35.e66
78. Urbano, T., Vinceti, M., and Filippini, T. Is artificial light-at-night associated with increased breast cancer risk? A systematic review and dose-response meta-analysis. J Prev Med Hyg. (2021) 2:E16–E17.
79. Forouzanfar, MH, Foreman, KJ, Delossantos, AM, Lozano, R, Lopez, AD, Murray, CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. (2011) 378:1461–84. doi: 10.1016/S0140-6736(11)61351-2
80. Costa, G, Haus, E, and Stevens, R. Shift work and cancer – considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health. (2010) 36:163–79. doi: 10.5271/sjweh.2899
81. Haus, EL, and Smolensky, MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. (2013) 17:273–84. doi: 10.1016/j.smrv.2012.08.003
82. Touitou, Y, Reinberg, A, and Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. (2017) 173:94–106. doi: 10.1016/j.lfs.2017.02.008
83. Erren, TC, Reiter, RJ, and Piekarski, C. Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften. (2003) 90:485–94. doi: 10.1007/s00114-003-0468-6
84. Giudice, A, Crispo, A, Grimaldi, M, Polo, A, Bimonte, S, Capunzo, M, et al. The effect of light exposure at night (LAN) on carcinogenesis via decreased nocturnal melatonin synthesis. Molecules. (2018) 23:1308. doi: 10.3390/molecules23061308
85. Bhattacharya, S, Patel, KK, Dehari, D, Agrawal, AK, and Singh, S. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem. (2019) 462:133–55. doi: 10.1007/s11010-019-03617-5
86. Gooley, JJ, Chamberlain, K, Smith, KA, Khalsa, SBS, Rajaratnam, SMW, van Reen, E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metabol. (2011) 96:E463–72. doi: 10.1210/jc.2010-2098
87. Bjorvatn, B, Dale, S, Hogstad-Erikstein, R, Fiske, E, Pallesen, S, and Waage, S. Self-reported sleep and health among Norwegian hospital nurses in intensive care units. Nurs Crit Care. (2012) 17:180–8. doi: 10.1111/j.1478-5153.2012.00504.x
88. Sugden, C, Athanasiou, T, and Darzi, A. What are the effects of sleep deprivation and fatigue in surgical practice? Semin Thorac Cardiovasc Surg. (2012) 24:166–75. doi: 10.1053/j.semtcvs.2012.06.005
89. Eanes, L. The potential effects of sleep loss on a nurse’s health. Am J Nurs. (2015) 115:34–40. doi: 10.1097/01.NAJ.0000463025.42388.10
90. Nojkov, B, Rubenstein, JH, Chey, WD, and Hoogerwerf, WA. The impact of rotating shift Work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. (2010) 105:842–7. doi: 10.1038/ajg.2010.48
91. Culpepper, L. The social and economic burden of shift-work disorder. J Fam Pract. (2010) 59:S3–S11.
92. Xu, Y, Zhang, JH, Tao, FB, and Sun, Y. Association between exposure to light at night (LAN) and sleep problems: a systematic review and meta-analysis of observational studies. Sci Total Environ. (2023) 857:159303. doi: 10.1016/j.scitotenv.2022.159303
93. Qin, Y, Zhou, Y, Zhang, X, Wei, X, and He, J. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer. (2013) 134:1166–73. doi: 10.1002/ijc.28452
Keywords: breast cancer, light at night, lighting, menopausal status, meta-analysis
Citation: Luo Z, Liu Z, Chen H, Liu Y, Tang N and Li H (2023) Light at night exposure and risk of breast cancer: a meta-analysis of observational studies. Front. Public Health. 11:1276290. doi: 10.3389/fpubh.2023.1276290
Edited by:
Michela Baccini, University of Florence, ItalyReviewed by:
Francesco Sera, University of Florence, ItalyPaolo Vineis, Imperial College London, United Kingdom
Copyright © 2023 Luo, Liu, Chen, Liu, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenglong Liu, MTUyNjU4M0BxcS5jb20=: Ying Liu, eWluZ2xpdTAwMDY2NkBuc21jLmVkdS5jbg==
†Present addresses: Zhenglong Liu, School of Basic Medical Sciences and Forensic Medicine, North Sichuan Medical College, Nanchong, China
Ying Liu, Department of Stomatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Nenghuan Tang and Haoran Li, Department of Clinical Medicine, North Sichuan Medical College, Nanchong, China
 Zining Luo
Zining Luo Zhenglong Liu
Zhenglong Liu Hongjie Chen
Hongjie Chen Ying Liu*†
Ying Liu*†