
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 16 October 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1272562
 Ran Hu1
Ran Hu1 Yuanbao Liu1
Yuanbao Liu1 Lei Zhang1
Lei Zhang1 Guodong Kang1
Guodong Kang1 Borong Xu1
Borong Xu1 Mingma Li2
Mingma Li2 Jing Yu1
Jing Yu1 Yuanyuan Zhu1
Yuanyuan Zhu1 Hongxiong Guo1*
Hongxiong Guo1* Zhiguo Wang1*
Zhiguo Wang1*Background: This study is to evaluate the safety of two kinds of PCV13 carriers by monitoring the occurrence of adverse event following immunization (AEFI) after the launch of two kinds of PCV13 carriers in Jiangsu Province, China.
Methods: The AEFI Information System (CNAEFIS) of mainland China was used to monitor the incidence and classification of adverse reactions of the CRM197-carrier protein PCV13 and TT-carrier protein PCV13 vaccines.
Results: There was no statistical difference between the cumulative reported incidence of AEFI between the two vaccines from 2020 to 2022 (χ2 = 1.991, p < 0.158). 96.62% of the AEFIs were classified as common reactions; rare reactions and coincidental events only accounted for 2.99 and 0.39% of all the AEFI cases, respectively. Redness (2.6 cm–5 cm) is the commonest symptom at the injection site for both vaccines. More than 97% of AEFIs occurred between 30 min and 3 days after administration for both types of PCV13.
Conclusion: Both vaccines perform well in terms of safety. We did not identify any new/unexpected safety concern from the NAEFISS during a 4 years timespan.
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia, bacterial meningitis, bacteremia, and otitis media. As normal flora of human upper respiratory tract of human, it is often found in the nasopharynx of healthy people, and about 20 to 50% of people carry them (1, 2). When a human body lies under immunocompromising conditions, colonized pneumococcal is likely to develop into invasive disease (2). In recent years, pneumococcus has developed varying degrees of resistance to antibiotics, making the treatment of pneumonia more difficultly (3). In 2019, there were an estimated 13.7 million infection-related deaths globally of which more than 500,000 deaths were associated with pneumococcal infections (4, 5). Among the countries with the highest burden of pneumococcal diseases in children under 5 years of age, China ranks 4th,which accounts for about 3% of the total number of cases in the world (4, 6). Considering the infection rate, morbidity, mortality rate, the resistance of Streptococcus pneumonia, and cost-effectiveness of prevention and treatment of the disease, there is an urgent need to control pneumococcal disease using vaccines (3, 6–8).
The World Health Organization (WHO) recommends that all countries, especially those with a mortality rate of >50‰ for children under 5 years old, include the pneumococcal conjugate vaccine (PCV) in their National Immunization Programs (NIP) (9). As of 2023, China has not integrated PCVs into her NIP (10). At present, there are two kinds of products of the 13-valent pneumonia conjugate vaccine (PCV13) worldwide. One is PCV13 conjugated with cross-reactive material 197 (CRM197), and the other is PCV13 conjugated with tetanus toxoid (TT). The latter is China’s first PCV-13, which has been licensed by China National Medical Products Administration (NMPA) in December 2019 for all children aged 2 months to 5 years (4).
In view of the high number of serotypes and complex mechanism of PCV vaccines, manufacturers have been looking for suitable carrier proteins and combinations. Before the launch of TT-carrier protein PCV13, there were some doubts about the safety of the vaccine with TT as the carrier protein (11). According to the previous researches on TT, this carrier protein is the inactivated tetanus toxin manipulated with formaldehyde, which may suffers from formaldehyde residuals and the incomplete detoxification of tetanus toxin in vaccine products (11, 12). In other words, TT carrier protein is an inactivated tetanus toxin, whose safety depends on whether it is inactivated completely during production process. TT-based conjugate vaccine may show high rates of irritability, crying, and fever (11). Therefore, whether the post TT-carrier protein PCV13 is really affected by its carrier protein is still unclear. The study aimed to assess the safety of PCV13. Specifically, a comparison was conducted between the CRM197-carrier protein PCV13 and the TT-carrier protein PCV13. This study might help policy-makers in their decisions to continue adjusting its vaccination schedule or integrating it into NIP.
The CRM197-carrier protein PCV13 was administered to children aged from 6 weeks to 15 months old, whereas the TT-carrier protein PCV13 was administered to children aged from 6 weeks to 5 years old. Both kinds of PCV13 vaccines were administered via the intramuscular route using a four-dose schedule. But TT-carrier protein PCV13 immunization schedule is more flexible. Children aged 7–11 months can be vaccinated with 2 doses and reinforced immunization with 1 dose after 12 months. Children aged 12–23 months can be vaccinated with 2 doses, with an interval of at least 2 months. Children aged 2–5 years can be vaccinated with 1 dose.
In mainland China, a passive adverse event following immunization(AEFI) Information System (CNAEFIS) to be used for AEFI monitoring was established in 2008, and updated twice in 2015 and 2018, respectively. Through this system, doctors of VCs (vaccination clinics) with authority report and preliminary classify AEFI. This system has been described in our previous research (13). The definition of adverse event following immunization in Mainland China is similar to the requirements of WHO National Regulatory Authority (NRA) assessment. AEFI refers to the reaction that may cause the damage of organs and functions of the person affected in the course of preventive inoculation or after inoculation, and suspected to be related to preventive inoculation.
AEFIs occurring after the administration of the PCV13 in Jiangsu Province from January 1st 2019 to December 31st 2022 were reviewed. AEFI data that were extracted from the CNAEFIS during the above period were included in this study for cases in which any type or dose of PCV13 was administered. We obtained the unique identification code, gender, age, inoculation time, reaction time, preliminary AEFI classification, final clinical diagnosis, final AEFI classification and other information of the recipients with AEFI after PCV13 inoculation in the selected time period from CNAEFIS.
The Chinese individual-level Electronic Immunization Registries System (EIRS) has already been introduced in our team’s previous research. Jiangsu has developed a new system (Jiangsu Province Vaccination Integrated Service Management Information System, SMIS) on the basis of that system. The name, age, sex, inoculation record, place of residence and other information of the recipient can be queried in detail and summarized statistically in SMIS. With this new system, the number of PCV13 doses was obtained and selected as a denominator to calculate the IR of the AEFI.
The differences of AEFI occurrence between two kinds of vaccines on gender, doses, year of AEFI reporting were analyzed by chi-square test with R package. Poisson test was performed on the distribution of various AEFI proportion of both PCV13 vaccines. In this study, the Northern Jiangsu includes Xuzhou, Huai’an, Lianyungang, Suqian, Yancheng; the Central Jiangsu includes Nantong, Yangzhou, Taizhou; the Southern Jiangsu includes Nanjing, Zhenjiang, Changzhou, Wuxi, Suzhou. The incidence rate of an AEFI (per 100,000 doses) is the reported number of AEFI per 100,000 doses administrated.
During the period from January 1, 2019 to December 31, 2022, a total of 1,915,958 doses of PCV13 were registered in Jiangsu Province, China, of which 1,439,808 doses of CRM197-carrier protein vaccine were inoculated, accounting for 75.1%; 476,150 doses of TT-carrier protein vaccine were administered, accounting for 24.9%. 2,726 AEFI cases linked to CRM197-carrier protein PCV13 and 853 linked to TT- carrier protein PCV13 were reported in CNAEFIS, respectively.
The AEFI IR sex ratio of both PCV13 were 1:0.92, while the difference was not statistically significant (χ2 = 2.35, p = 0.125). There was no difference in the incidence of AEFI among different vaccines after gender stratification was taken into account (CMHχ2 = 1.938, p = 0.164). The IR of AEFIs in 2020 was higher than that in other years (χ2 = 31.282, p < 0.001). There was no significant difference in the incidence of AEFI among different carrier protein vaccines after considering the influence of year stratification factors (CMHχ2 = 0.028, p = 0.868). There were significant differences in the incidence of AEFI among various regions in Jiangsu Province. The incidence of AEFI in northern Jiangsu Province was significantly higher than that in central and southern areas (χ2 = 4780.58, p < 0.001). Among children received the fourth dose of vaccine, the incidence of AEFIs is higher than in those only received the first three doses (χ2 = 120.81, p < 0.001). We compared the occurrence of AEFI among different recipient groups, and found that there was significant difference between the first dose and the third dose (χ2 = 5.50, p = 0.019) while no significant difference between the second dose and the third dose (χ2 = 0.20, p = 0.653). There was no statistical difference between the cumulative reported incidence of AEFI between the two vaccines from 2020 to 2022 (χ2 = 1.991, p < 0.158) (shown in Table 1).
96.62% of the AEFIs belongs to common reactions; rare reactions and coincidental events only accounted for 2.99 and 0.39% of all the AEFI cases, respectively (Table 2). No vaccine quality events, program errors, psychogenic reactions, or deaths were reported. Among the individuals with AEFI in all years, there was a statistical difference in the proportion of various types of AEFIs caused by these two vaccines (χ2 = 17.32, p = 0.0002).
A total of 2,726 AEFIs linked to the CRM197-carrier protein PCV13 were reported, in which 1,390 were injection site reactions. The IRs of different symptoms ranged from 0.07 to 70.15 (per 100,000 doses). The top three systemic symptoms were fever, irritability and loss of appetite (Table 3).
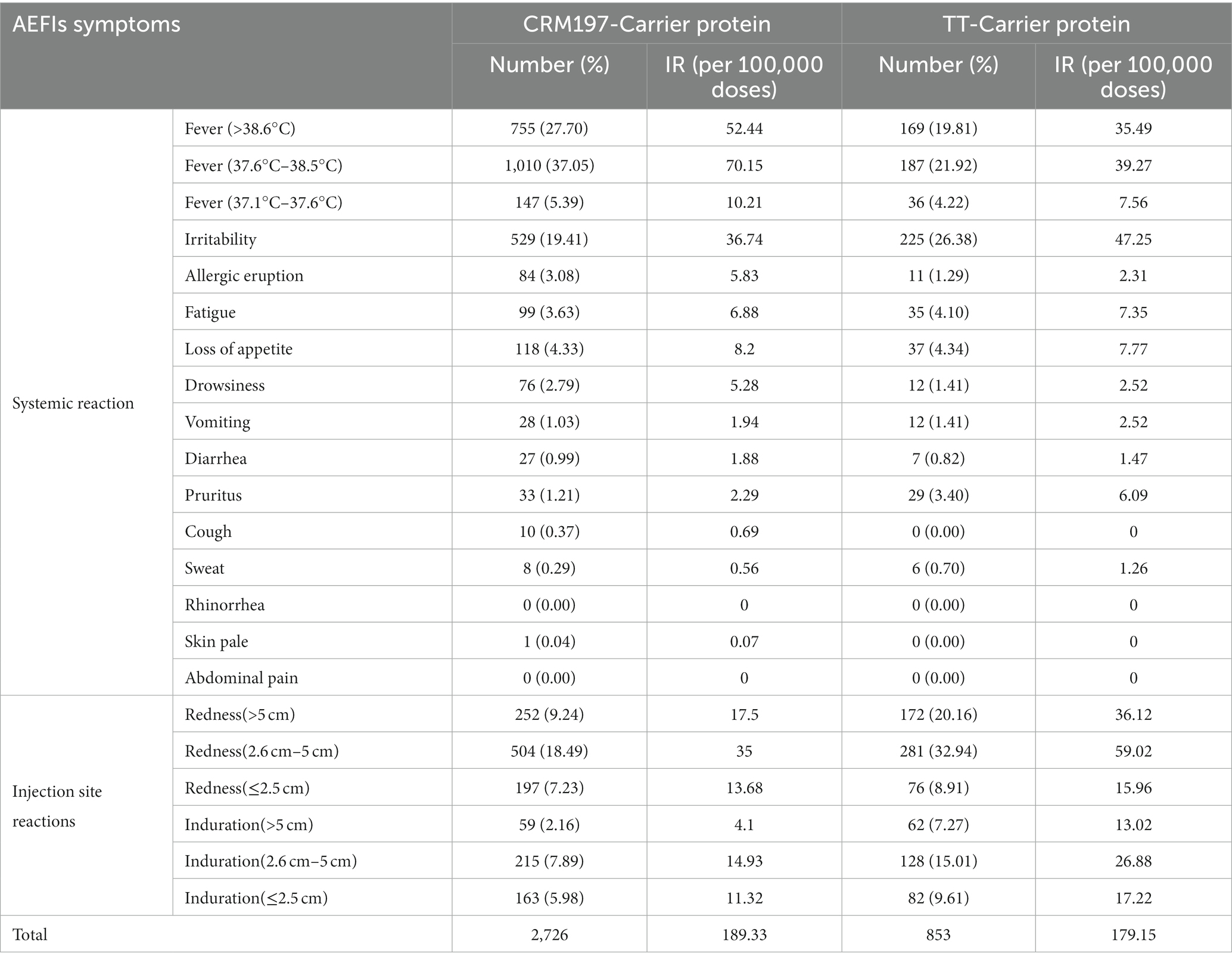
Table 3. CRM197-Carrier protein and TT-Carrier protein systemic reaction and injection site reactions.
A total of 853 AEFIs linked to the TT-carrier protein PCV13 were reported, in which 801 were injection site reactions. The IRs of different symptoms ranged from 0 to 59.05 (per 100,000 doses) for the TT-carrier protein PCV13. The top three systemic symptoms were fever, irritability and loss of appetite. Redness (2.6 cm–5 cm) is the commonest symptom at the injection site for both vaccines (Table 3).
More than 97% of AEFIs occurred between 30 min and 3 days after administration for both kinds of PCV13 (Table 4). The main symptoms with a time interval of less than 30 min were allergy related symptoms, mainly allergic rash. The longest interval was 17 days. The clinical symptom of this case was thrombocytopenic purpura after vaccination. The recipient’s guardian believed that it was caused by vaccine. However, the AEFI was classified as a coincidental event after the investigation of the AEFI expert panel.
Widespread use of 23-valent pneumococcal polysaccharide vaccine (PPV23) and PCV13 has significantly decreased invasive pneumococcal disease (IPD) in children (14, 15). The significant reduction in the burden of pneumococcal disease in children worldwide over the last decade underscores the public health value of infant immunization with pneumonia vaccine (16–18). Although the 13-valent pneumonia vaccine is not included in China’s National Expanded Programme on Immunization, Chinese parents are highly motivated to vaccinate their children against pneumonia.
Previous study shows that no significant differences on AEFIs were observed between PHiD-CV + MenC-TT and PHiD-CV + MenC-CRM, which is accordance with our results on PCV13-CRM197 and PCV13-TT (19). In addition, our results showed that the reported incidence of AEFI associated with these two kinds of vaccines was higher than that of many other vaccines reported in the national AEFI monitoring system (20). As one of the most expensive category II vaccines for children to be vaccinated at present, which is mainly administrated among children whose parents with good economic status and educational level (4, 21, 22). These parents often pay more attention to the situation of their children after vaccination than others, accompanying with the increasing active reporting rate and sensitivity of AEFI.
We observed no significant difference in reported AEFI incidence between boys and girls, which is generally consistent with previous reports (23, 24). We found that the reported incidence of AEFI in 2020 was highest from 2019 to 2022. Since February 2021, population-wide immunization of COVID-19 vaccine programme was carried out, more efforts and manpower were put in to strengthen AEFI surveillance linked to COVID-19 vaccination in many provinces. It could cause indirectly that the reported incidence of AEFIs except COVID-19 vaccine declined, and this phenomenon lasted from 2021 to 2022. Nonetheless, the reduction in reported AEFIs was mainly focused on general response induced by vaccine. Our results indicated that the reported incidence of AEFI after inoculating the fourth dose of PCV13 is highest whatever for CRM197 or TT conjugated vaccine. Which is distinct from some previous studies which showed that the reported incidence of AEFI after inoculating the first dose is highest (25, 26). Their argument is the recipients and their guardians pay more attention to the reaction after the first dose of vaccine (25, 26). However, there are some studies that support our conclusions (24, 27). The view they take is the subsequent dose was more likely to cause allergic reactions due to the body sensitization by the early dose. No significant difference on the reported incidence of AEFIs was observed among three geographical regions in Jiangsu Province, which was in accordance with our previous studies (13, 25).
AEFI in PCV13 occurs mainly within 2 days after vaccination and generally lasts about 72 h in previous studies (23–25). This is basically consistent with the conclusion of our results. The main clinical symptoms of AEFI caused by PCV13 are general reactions such as fever, redness or scleroma, which usually occur in 2–5 days after vaccination. Guardians’ awareness of reporting AEFI often wanes over time. Even though some minor adverse reactions occur after 5 days, they are often neglected by guardians because they think it is only mild presentation. Similar phenomena have been observed in post-marketing studies of other vaccines. This is actually one of the drawbacks of passive monitoring (21).
There are three limitations to this study. (1) Recipients have different tolerance for adverse events from domestic vaccine (developed and produced in China) and imported one (developed and produced outside China). Some recipients may subconsciously believe that imported vaccines have fewer adverse effects. Even if there is an adverse reaction, they get used to attribute it to personal physique. However, we do not think it will have a significant impact on the conclusions given the nearly 2 million doses. (2) Reporting bias and information bias cannot be well controlled, for the passive monitoring system. This may affect the accuracy of the results to some extent. Active surveillance study in the future may be a way to solve this problem. (3) The AEFI data associated with TT-carrier protein PCV13 in 2019 was unavailable. So we compare reported AEFI incidence between various years, only the data collected during 2020–2022 were used to conduct statistical analysis.
In conclusion, our findings demonstrated that most AEFIs caused by both PCV13 vaccines were mild and belonged common reactions. No significant differences in rare reactions were observed between TT-carrier protein PCV13 vaccine and CRM197-carrier PCV13. Which was broadly consistent with the safety data from the previous pre- or post-licensure trials. Our results is helpful to reduce the concern from parents for the safety of TT-carrier protein PCV13 vaccine and increase the coverage of PCV13 vaccine in Chinese children.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RH: Conceptualization, Data curation, Formal analysis, Writing – original draft, Investigation, Supervision. YL: Investigation, Methodology, Data curation, Software, Visualization, Validation, Writing – review & editing. LZ: Data curation, Methodology, Resources, Writing – review & editing. GK: Investigation, Project administration, Validation, Data curation, Formal analysis, Writing – review & editing. BX: Investigation, Methodology, Validation, Visualization, Writing – review & editing. ML: Data curation, Methodology, Formal analysis, Investigation, Writing – review & editing. JY: Data curation, Methodology, Investigation, Visualization, Writing – review & editing. YZ: Investigation, Data curation, Validation, Visualization, Writing – review & editing. HG: Data curation, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. ZW: Data curation, Conceptualization, Methodology, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported partly by the project funded by the Scientific Research Project of the Jiangsu Provincial Health Commission (M2021030) &Yangtze River Delta Immunization Program Leading Talent Research Project (CSJP001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Xiaochuan, W, Jikui, D, Kunling, S, et al. Chinese experts’ consensus statement on diagnosis, treatment, and prevention of pneumococcal diseases in children. Chin J Appl Clin Pediatr. (2020) 7:35. doi: 10.3760/cma.j.cn112338-20201111-01322
2. Syrogiannopoulos, GA, Grivea, IN, Moriondo, M, Nieddu, F, Michoula, AN, Calabrese, MR, et al. Molecular surveillance of pneumococcal carriage following completion of immunization with the 13-valent pneumococcal conjugate vaccine administered in a 3+1 schedule. Sci Rep. (2021) 11:24534. doi: 10.1038/s41598-021-03720-y
3. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
4. Wang, C, Su, L, Mu, Q, Gu, X, Guo, X, and Wang, X. Cost-effectiveness analysis of domestic 13-valent pneumococcal conjugate vaccine for children under 5 years of age in mainland China. Hum Vaccin Immunother. (2021) 17:2241–8. doi: 10.1080/21645515.2020.1870396
5. GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2022) 400:2221–48. doi: 10.1016/S0140-6736(22)02185-7
6. Wahl, B, O’rien, KL, Greenbaum, A, Majumder, A, Liu, L, Chu, Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–2015. Lancet Glob Health. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
7. Lewnard, JA, Lo, NC, Arinaminpathy, N, Frost, I, and Laxminarayan, R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. (2020) 581:94–9. doi: 10.1038/s41586-020-2238-4
8. Vaccine and Immunization Branch, Chinese Preventive Medicine AssociationHuaqing, W, et al. Expert consensus on influenza vaccination and pneumococcal vaccination for patients with certain chronic diseases (2020). Chin J Vacc Immun. (2021) 21:1. doi: 10.19914/j.CJVI.2021001
9. WHO. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019
10. World Health Organization. Global Immunization coverage: (2022), (Accessed September 1, 2023). Available at: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
11. Yu, R, Xu, J, Hu, T, and Chen, W. The pneumococcal polysaccharide-tetanus toxin native C-fragment conjugate vaccine: the carrier effect and immunogenicity. Mediat Inflamm. (2020) 2020:9596129. doi: 10.1155/2020/9596129
12. Martinón-Torres, F, Gimenez-Sanchez, F, Gurtman, A, Bernaola, E, Diez-Domingo, J, Carmona, A, et al. 3007 study group. 13-valent pneumococcal conjugate vaccine given with meningococcal C-tetanus toxoid conjugate and other routine pediatric vaccinations: immunogenicity and safety. Pediatr Infect Dis J. (2012) 31:392–9. doi: 10.1097/INF.0b013e31824b972b.22301472
13. Ran, H, Peng, S, Liu, Y, Tang, F, Wang, Z, Zhang, L, et al. The characteristics and trend of adverse events following immunization reported by information system in Jiangsu province, China, 2015–2018. BMC Public Health. (2021) 21:1338. doi: 10.1186/s12889-021-11387-3
14. Ben-Shimol, S, Regev-Yochay, G, Givon-Lavi, N, van der Beek, BA, Brosh-Nissimov, T, Peretz, A, et al. Dagan R; Israeli pediatric bacteremia and meningitis group (IPBMG); Israeli adult invasive pneumococcal disease (IAIPD) group. Dynamics of invasive pneumococcal disease in Israel in children and adults in the 13-valent pneumococcal conjugate vaccine (PCV13) era: a Nationwide prospective surveillance. Clin Infect Dis. (2022) 74:1639–49. doi: 10.1093/cid/ciab645
15. Igarashi, A, Ueyama, M, Idehara, K, and Nomoto, M. Burden of illness associated with pneumococcal infections in Japan – a targeted literature review. J Mark Access Health Policy. (2021) 10:2010956. doi: 10.1080/20016689.2021.2010956
16. Du, Y, Wang, Y, Zhang, T, Li, J, Song, H, Wang, Y, et al. Economic evaluations of 13-valent pneumococcal conjugate vaccine: a systematic review. Expert Rev Vaccines. (2023) 22:193–206. doi: 10.1080/14760584.2023.2173176.PMID: 36719062
17. de Miguel, S, Domenech, M, González-Camacho, F, Sempere, J, Vicioso, D, Sanz, JC, et al. Nationwide trends of invasive pneumococcal disease in Spain from 2009 through 2019 in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. (2021) 73:e3778–87. doi: 10.1093/cid/ciaa1483
18. Sanchez Picot, V, Keovichith, I, Paboriboune, P, Flaissier, B, Saadatian-Elahi, M, and Rudge, JW. Epidemiology and serotype distribution of Streptococcus pneumoniae carriage among influenza-like illness cases in metropolitan Vientiane, Lao PDR: a community-based cohort study. Front Public Health. (2023) 11:1124016. doi: 10.3389/fpubh.2023.1124016
19. Chevallier, B, Vesikari, T, Brzostek, J, Knuf, M, Bermal, N, Aristegui, J, et al. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis J. (2009) 28:S109–18. doi: 10.1097/INF.0b013e318199f62d
20. Li, K, Wu, W, Ye, J, Xu, D, and Liu, D. Adverse events following immunization: 6th international conference, GSES 2018, Handan, China, September 25–26, 2018, revised selected papers (2019). doi: 10.1007/978-981-13-2438-3_4,
21. Che, X, Chen, Q, Liu, Y, Gu, L, Lu, Z, Gu, W, et al. Analysis of 13-valent pneumococcal polysaccharide conjugate vaccine among children born in Hangzhou from 2017 to 2021. Front Public Health. (2023) 11:1184059. doi: 10.3389/fpubh.2023.1184059
22. Lizhi, L, Lixia, Y, Yi, C, Ting, F, Liang, Z, Hongjun, D, et al. Vaccination coverage of 13-valent pneumococcal conjugate vaccine among children born in 2017–2018 in Ningbo City, Zhejiang Province, China. Clin JBiol. (2020) 33:50–4.
23. Kang, G, Tang, F, Wang, Z, Hu, R, Yu, J, and Gao, J. Surveillance of adverse events following the introduction of inactivated poliovirus vaccine made from Sabin strains (sIPV) to the Chinese EPI and a comparison with adverse events following inactivated poliovirus vaccine made from wild strains (wIPV) in Jiangsu. China Hum Vaccin Immunother. (2021) 17:2568–74. doi: 10.1080/21645515.2021.1898306
24. Pan, X, Lv, H, Liang, H, Wang, Y, Shen, L, Chen, F, et al. Surveillance on the adverse events following immunization with the pentavalent vaccine in Zhejiang, China. Hum Vaccin Immunother. (2022) 18:2021711. doi: 10.1080/21645515.2021.2021711
25. Gao, J, Tang, F, Wang, Z, Yu, J, Hu, R, Liu, L, et al. Post-marketing safety surveillance for inactivated enterovirus 71 vaccines in Jiangsu, China from 2017 to 2019. Vaccine. (2021) 39:1415–9. doi: 10.1016/j.vaccine.2021.01.048
26. Luo, S, Wu, F, Ye, X, Fu, T, Tao, J, Luo, W, et al. Safety comparison of two enterovirus 71 (EV71) inactivated vaccines in Yiwu (65, 545, 2019). J Trop Pediatr. (2020) 65:547–51. doi: 10.1093/tropej/fmz004
Keywords: vaccine, pneumococcal polysaccharide conjugate vaccine, adverse reaction, carrier protein, safety, post-marketing surveillance
Citation: Hu R, Liu Y, Zhang L, Kang G, Xu B, Li M, Yu J, Zhu Y, Guo H and Wang Z (2023) Post-marketing safety surveillance for both CRM197 and TT carrier proteins PCV13 in Jiangsu, China. Front. Public Health. 11:1272562. doi: 10.3389/fpubh.2023.1272562
Received: 04 August 2023; Accepted: 28 September 2023;
Published: 16 October 2023.
Edited by:
Faris Lami, University of Baghdad, IraqReviewed by:
Kamal A. Kadhim, Ministry of Health (Iraq), IraqCopyright © 2023 Hu, Liu, Zhang, Kang, Xu, Li, Yu, Zhu, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Wang, MTIwNzE0OTkxQHFxLmNvbQ==; Hongxiong Guo, Z3VvaG9uZ3hpb25nQGpzY2RjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.