- 1Department of Endocrinology and Metabolism, Affiliated Hospital of Weifang Medical University, School of Clinical Medicine, Weifang Medical University, Weifang, China
- 2Clinical Research Center, Affiliated Hospital of Weifang Medical University, Weifang, China
- 3Department of Pathophysiology, School of Basic Medical Sciences, Weifang Medical University, Weifang, China
- 4Department of Pathology, Affiliated Hospital of Weifang Medical University, Weifang, China
Aging is a progressive and irreversible pathophysiological process that manifests as the decline in tissue and cellular functions, along with a significant increase in the risk of various aging-related diseases, including metabolic diseases. While advances in modern medicine have significantly promoted human health and extended human lifespan, metabolic diseases such as obesity and type 2 diabetes among the older adults pose a major challenge to global public health as societies age. Therefore, understanding the complex interaction between risk factors and metabolic diseases is crucial for promoting well-being and healthy aging. This review article explores the environmental and behavioral risk factors associated with metabolic diseases and their impact on healthy aging. The environment, including an obesogenic environment and exposure to environmental toxins, is strongly correlated with the rising prevalence of obesity and its comorbidities. Behavioral factors, such as diet, physical activity, smoking, alcohol consumption, and sleep patterns, significantly influence the risk of metabolic diseases throughout aging. Public health interventions targeting modifiable risk factors can effectively promote healthier lifestyles and prevent metabolic diseases. Collaboration between government agencies, healthcare providers and community organizations is essential for implementing these interventions and creating supportive environments that foster healthy aging.
1. Introduction
Aging is an ongoing and irreversible physiological process that leads to the gradual deterioration of tissue and cellular functions, increasing the susceptibility to age-related diseases, including metabolic disorders (1). While remarkable advancements in modern medicine have improved human health and extended lifespan, metabolic conditions like obesity and type 2 diabetes (T2D) continue to pose significant global public health challenges as populations age (1–5). Understanding the complex relationship between metabolic diseases and healthy aging is crucial for promoting well-being and preventing disease burden. While genetics play a role in metabolic disease development, environmental and behavioral factors also contribute significantly (6–10). An obesogenic environment, characterized by air pollution, pesticides and exposure to environmental toxins, correlates strongly with the rising prevalence of obesity and its associated comorbidities (8, 10–14). Additionally, research suggests that behavioral factors, such as dietary choices, physical activity levels, and sleep patterns, significantly influence the risk of metabolic diseases throughout aging (15–18).
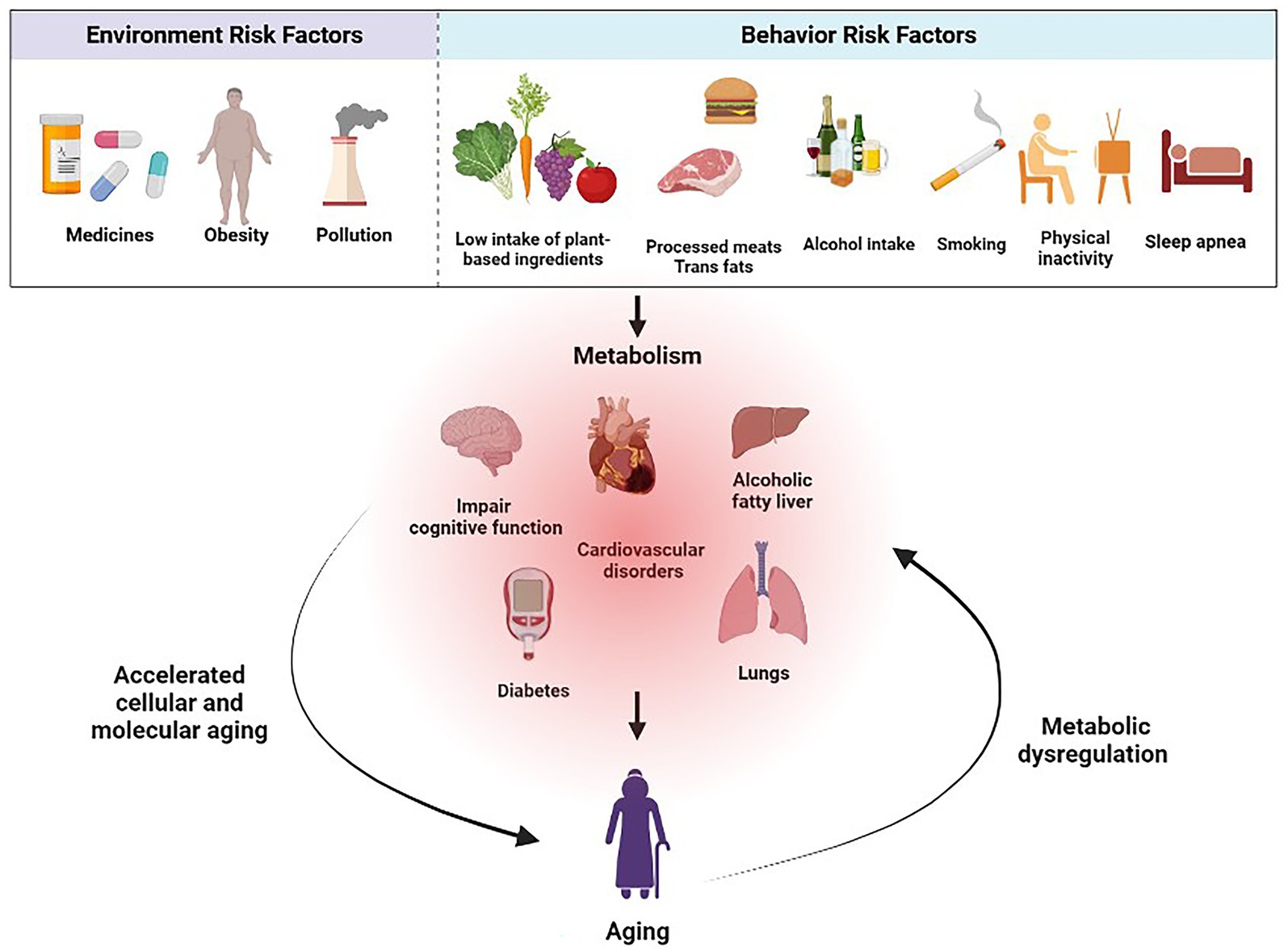
Effective strategies must be implemented to promote healthy aging and prevent metabolic diseases. This involves understanding the underlying risk factors and their impact on public health, which can then inform the development of targeted interventions and policies that encourage healthier lifestyles. Public health initiatives can address these risks to promote healthier choices among the population by identifying modifiable environmental and behavioral factors (Figure 1).
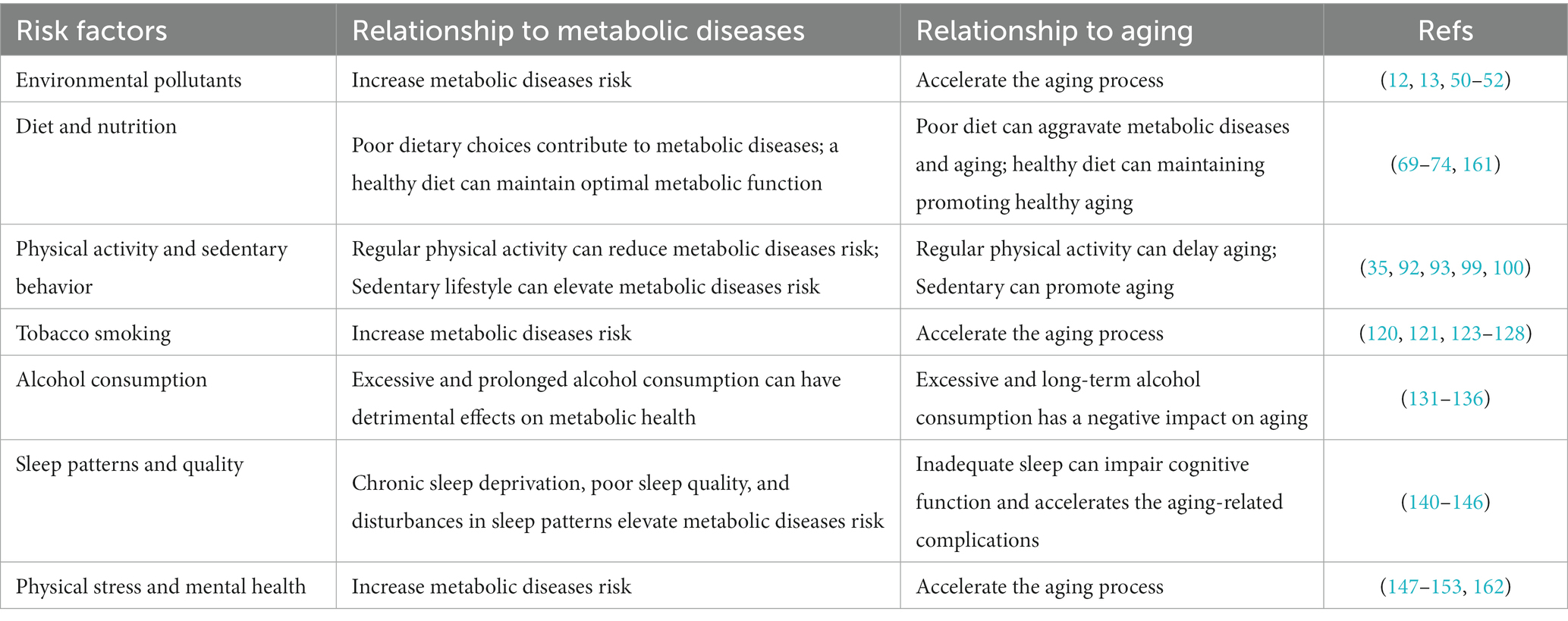
This review article aims to explore the intricate relationship between metabolic diseases and healthy aging by examining the environmental and behavioral risk factors contributing to their onset and progression. By analyzing current research and existing literature, we will delve into the multifaceted nature of these diseases, considering factors such as diet, physical activity, stress, sleep patterns, and socioeconomic determinants. Furthermore, we will explore the potential of public health interventions to mitigate the impact of metabolic diseases and promote healthy aging (Table 1).
2. The interactions between aging and metabolic diseases
2.1. Aging as a risk factor for metabolic diseases
Aging is a significant risk factor for developing and progressing metabolic diseases in older adults due to various physiological changes that occur with age (19, 20). These changes affect metabolic regulation and contribute to the increased risk of metabolic disorders. One notable change associated with aging is the decline in metabolic rate. If calorie intake remains constant or increases, this decline in metabolic rate can lead to weight gain, obesity and insulin resistance (IR) (21). IR refers to repaired responses to insulin stimulation in specific tissues, primarily the liver, muscles, and adipose tissues. This impairment leads to ineffective glucose utilization, prompting a compensatory increase in β-cell insulin production and ultimately resulting in hyperinsulinemia (22, 23). This age-related decline in insulin sensitivity becomes more prominent as people grow older. Moreover, aging affects body composition, leading to increased adiposity (fat accumulation) and decreased lean muscle mass (24). This shift in body composition, known as sarcopenic obesity, contributes to metabolic dysregulation and raises the risk of metabolic diseases (24, 25). Besides these factors, aging-related hormonal changes play a role in metabolic dysregulation. Aging is accompanied by a natural decline in growth hormone and insulin-like growth factor-1 (IGF-1) levels (26, 27). This reduction increases adiposity, particularly abdominal fat, while decreasing muscle mass. Lower hormone levels lead to IR, glucose intolerance (the body’s struggle to control blood glucose levels, resulting in elevated levels not yet classified as diabetes), and the potential development of diabetes (28, 29). In women, menopause triggers a substantial decrease in estrogen levels, potentially resulting in IR and an increase in abdominal fat, since estrogen renders tissues more sensitive to insulin (30, 31). Similarly, men experience a gradual decline in testosterone levels with aging, resulting in higher body fat levels, loss of muscle mass, and IR (32). These hormonal changes influence fat metabolism, insulin sensitivity, and energy expenditure, thereby increasing the risk of metabolic diseases in older adults (33, 34).
Furthermore, aging is associated with a higher prevalence of additional risk factors for metabolic diseases. Reduced physical activity, sedentary behavior, underlying genetic predispositions, and cumulative exposure to environmental factors over a lifetime increase risk in older adults (17, 35). When combined with age-related changes in metabolic regulation, these factors contribute to the bidirectional relationship between aging and metabolic diseases (6, 8, 10).
2.2. Impact of metabolic diseases on the aging process
Metabolic diseases, particularly diabetes and hypertension, significantly impact aging by accelerating age-related decline in health and functionality (19, 20). Both conditions foster systemic inflammation and oxidative stress, contributing to cellular and molecular aging (36–39). Chronic inflammation from these diseases elevates reactive oxygen species levels, inflicting cellular damage and disrupting normal functions. It also induces IR, which inhibits glucose uptake by cells and raises blood glucose levels, thereby impairing glucose metabolism and leading to complications like renal disease and cognitive decline (40–43). Diabetes causes microvascular and macrovascular damage, increasing the risk of complications such as diabetic retinopathy, peripheral neuropathy, and cardiovascular issues. These conditions impede sensory and motor functions, limit mobility, and negatively affect overall well-being (44–47). Hypertension, often related to metabolic disorders like obesity and IR, imposes strain on the cardiovascular system, accelerating aging and promoting heart-related complications (38, 39). It also exacerbates organ damage, leading to chronic conditions like kidney disease, dementia, and heart failure, thereby further speeding up the aging process when coupled with metabolic syndrome (42, 43, 48, 49).
3. Environment risk factors
Environmental pollutants, including air pollutants, pesticides, heavy metals, and endocrine-disrupting compounds (EDCs), pose substantial risks to metabolic health and can accelerate aging (12, 50, 51). Air pollution, especially fine particulate matter (PM2.5) and nitrogen dioxide has been linked to IR, inflammation, and an increased risk of metabolic disorders such as obesity, T2D, and cardiovascular disease (CVD) (13, 52). Similarly, pesticides can disrupt metabolic processes and hormonal regulation, increasing the risk of diseases like IR and obesity (12, 14). Heavy metals, such as lead, cadmium, and mercury, can also induce metabolic dysfunction and oxidative stress (50).
EDCs are present in various consumer products and can interfere with the production, transport, or metabolism of hormones. It is estimated that there are more than one thousand different EDCs. EDCs disrupt hormonal action, while metabolic disruptors affect metabolism. It is crucial to clarify that not all metabolic disruptors are EDCs. EDCs specifically pertain to substances that interfere with the hormonal aspects of metabolism (51, 53–56). Therefore, mitigating exposure to these pollutants can help reduce the risk of metabolic diseases and age-related complications.
The term “exposome” encompasses both external and internal environmental factors that impact human health. It includes the ecto-exposome, which pertains to external environmental influences, and the endo-exposome, which relates to the immediate adjacent extracellular environment. Both the ecto-exposome and the endo-exposome are conditions that support cell survival and organ/system function. According to reports, persistent organic pollutants, also known as ‘EDCs,’ interfere with the endocrine system and are found in the environment, causing lifelong exposure in certain populations (57, 58). Occupational exposure to toxins, including heavy metals, solvents, pesticides, and industrial chemicals, is a significant risk factor for metabolic diseases and healthy aging (14, 59–61). The heavy use of these toxins in industries such as manufacturing, agriculture, and mining can lead to adverse health effects like IR, dyslipidemia, impaired glucose metabolism, hormonal disruption, and altered lipid metabolism (62–64). Toxic exposure can also influence aging by promoting inflammation, DNA damage, mitochondrial dysfunction, and impairing natural defense mechanisms against oxidation, thereby accelerating cellular aging and increasing the risk of age-related diseases (12, 65–68). However, little is known about how these exposures actually affect humans or potentially affect the onset of metabolic diseases. It is critical to further explore the impact of environmental exposure on health under the concept of exposure and enforce strict safety regulations and promote adequate workplace protection to reduce these risks. Training and regular health screenings can also contribute to early detection and prevention of occupational health issues.
4. Behavior risk factors
4.1. Role of diet in metabolic diseases and healthy aging
Diet plays a significant role in the onset and progression of metabolic diseases, with varying dietary habits yielding distinct impacts on these conditions. A well-balanced diet, rich in fruits, vegetables, whole grains, lean meats, and healthy fats, contributes to weight maintenance, blood sugar regulation, lowered cholesterol levels, and reduced risk of chronic diseases (69–71). Specific dietary factors, such as the overconsumption of sugary beverages and processed foods, elevate the risk of obesity and T2D. Conversely, diets high in fiber, antioxidants and omega-3 fatty acids, along with adequate intake of micronutrients and phytochemicals, can protect against these diseases and age-related decline (72–74). Interestingly, studies in invertebrates and rodents have found that calorie or diet restriction can slow down age-related diseases and prolong life expectancy (75). Simultaneously, Longo et al. (76) demonstrated that dietary interventions, such as intermittent fasting and protein restriction, can attenuate aging and extend a healthy lifespan (73). Furthermore, the Mediterranean diet has been proven to reduce mortality from cardiovascular disease (77, 78).
Moreover, diet significantly influences gut microbiota homeostasis, impacting energy metabolism and fat storage. Imbalances in gut flora, or dysbiosis, can lead to increased energy extraction and fat storage, contributing to obesity and diabetes (79, 80). Specifically, an increase in Firmicutes and a decrease in Bacteroidetes are commonly observed in these conditions (81, 82). In addition, certain potentially harmful bacterial groups may become overrepresented. For instance, the family Enterobacteriaceae, which includes potentially harmful species such as Escherichia coli, is often found to increase in metabolic diseases (83). These bacteria can lead to inflammation and further disrupt the balance in the gut microbiota. Conversely, beneficial bacteria may decrease in metabolic diseases. Examples include Akkermansia muciniphila, known to help maintain the health of the gut lining and Bifidobacterium, a genus often used in probiotics known for its health-promoting effects (84). These bacteria produce short-chain fatty acids that help regulate metabolism and inflammation, and their reduction can exacerbate metabolic issues. Positive dietary interventions, such as increasing the intake of prebiotic fibers and probiotic-rich foods, can help maintain healthy gut microbiota and promote healthy aging (85, 86). Additionally, age-related dysbiosis is linked to inflammation, promoting metabolic disorders and frailty, accelerating aging (87, 88). Understanding the intricate relationship between diet, gut flora, and overall health is crucial for mitigating metabolic diseases and fostering healthier aging outcomes.
4.2. Influence of physical activity and sedentary behavior on metabolic health
Declining activity and unhealthful changes in body composition are linked to aging. Physical activity and sedentary behavior have significant impacts on metabolic health.
Exercise prevents many chronic diseases and mitigates certain undesirable physiological changes brought on by aging. Interestingly, the impact of physical training on the reward system is a topic of growing interest. Engaging in regular physical activity reduces the motivation to seek unhealthy rewards like high-fat, sugary foods, and drugs. Additionally, it changes attitudes toward overeating, making individuals more mindful of their food choices. This transformation promotes healthier lifestyle preferences (89, 90).
Regular exercise also promotes glucose metabolism by increasing cells’ sensitivity to insulin, reducing the risk of developing T2D characterized by IR (91–93). Engaging in aerobic exercise training, such as brisk walking, jogging, swimming, wheel running, or cycling, increases energy expenditure, promotes cardiovascular health, and aids in weight management (94–96). Studies in the natural aging mouse model (C57BL/J6) have shown that early lifelong aerobic exercise training is crucial in preventing age-related issues, including muscle loss, decreased motility, and testicular atrophy, along with overall organ pathology (97). Testicular atrophy, the shrinking of testicles, can impact reproductive health. The findings highlight that aerobic exercise, when initiated early in life, can effectively mitigate these age-related challenges. Moreover, early-onset, lifelong running can inhibit inflammation, prevent multiple types of cancer, and prolong the healthy lifespan of naturally aging mice (97). The wheel running exercise can improve insulin sensitivity and treat IR in aging rats by restoring the role of hepatic insulin sensitizing substance. This substance is also known as hepatalin, a liver-produced hormone that enhances insulin sensitivity, particularly in skeletal muscle, promoting glucose storage as glycogen and contributing to the post-meal glucose disposal effect of insulin (53, 98). Resistance training, including weightlifting, improves metabolic health by enhancing muscle strength and mass, leading to an increased resting metabolic rate and insulin sensitivity (99, 100).
The World Health Organization recommends that adults engage in at least 150 min of moderate-intensity aerobic exercise or 75 min of vigorous exercise per week, or a combination of both (101, 102). It is well known that the level and intensity of physical activity decline as humans age, and during long-term aging, exercise affects changes in body weight and body composition in a gender-dependent manner (103). In women, estrogen plays a crucial role in regulating various aspects of metabolism. Estrogens regulate fat development by inhibiting preadipocyte differentiation, reducing lipolysis, favoring subcutaneous fat storage, and influencing energy expenditure. They boost the basal metabolic rate and can help maintain a healthy weight (104, 105). However, during menopause, declining estrogen levels can lead to increased abdominal fat accumulation. Studies in humans and rodents have shown that reduced estrogen production leads to IR and inflammation, and exercise relieves glucose intolerance and IR (106, 107). Moreover, estrogen deficiency in postmenopausal women and ovariectomized rodents results in increased respiratory exchange rate levels, decreased lipid oxidation, and increased carbohydrate oxidation during rest and exercise, accelerated cell senescence, and impaired bone formation (108, 109). Similarly, changes in male muscle mass, physical activity level, body weight, and fat percentage are closely related to testosterone. A decrease in testosterone levels will lead to an increase in body fat and a decrease in physical activity level (110, 111).
Conversely, a sedentary lifestyle characterized by prolonged sitting or inactivity is associated with an elevated risk of metabolic diseases, including obesity, T2D, and cardiovascular problems (112–114). Sedentary behavior leads to weight gain, reduced muscle mass, and impaired glucose and lipid metabolism (115, 116). It is important to note that even individuals who engage in regular exercise may still be at risk if they spend prolonged periods sitting or being inactive throughout the day (35). To promote metabolic health, it is crucial to incorporate regular physical activity into daily routines. This can include structured exercise sessions, such as workouts or sports, as well as simple lifestyle modifications like choosing to take the stairs instead of the elevator, walking or cycling for transportation, incorporating movement breaks during extended periods of sitting (117, 118). Elderly people are encouraged to walk to shopping when supermarkets are less than a kilometer away, as it is often the preferred mode of transportation over driving a car for their shopping needs (119).
4.3. Smoking and its association with metabolic diseases and aging
Tobacco smoking significantly elevates the risk for metabolic diseases and negatively affects the aging process. Cigarette smoke contains harmful compounds such as nicotine, carbon monoxide, and carcinogens that damage tissues and organs throughout the body. Smoking is linked to metabolic diseases like T2D, IR, obesity, and CVD, primarily due to nicotine’s contribution to IR and impaired glucose metabolism (120–123). In addition, smoking induces inflammation, oxidative stress, and endothelial dysfunction, leading to blood vessel damage and an increased risk of CVD (121, 124, 125). It also accelerates aging, causing premature skin aging, impaired wound healing, and elevating the risk of age-related conditions like osteoporosis and cognitive decline (126–128). Quitting smoking is crucial for reducing metabolic disease risk and promoting healthy aging. Immediate benefits post-cessation includes improved lung function, cardiovascular health, and overall well-being (129, 130).
4.4. Alcohol consumption and its impact on metabolic health in aging populations
Alcohol consumption’s impact on metabolic health hinges on individual factors and the quantity consumed. While moderate consumption might confer some cardiovascular benefits, excessive and chronic alcohol intake poses severe risks to metabolic health, particularly in aging populations (131). Heavy alcohol use promotes weight gain and obesity due to its caloric density and effects on appetite regulation. It also disrupts glucose metabolism, escalating the risk of IR and T2D (131–133). Toxic byproducts from alcohol metabolism can harm the liver, resulting in fatty liver disease, alcoholic hepatitis, and cirrhosis. Such chronic abuse hampers liver function, negatively impacting nutrient and medication metabolism and clearance, exacerbating metabolic health concerns (134, 135). Furthermore, alcohol can impede the absorption and utilization of essential nutrients, including vitamins and minerals, that are critical for maintaining metabolic health (136). Thus, for maintaining metabolic health in aging populations, it is recommended to moderate alcohol intake (131). Nonetheless, personal health conditions, medications, and other variables may necessitate adjustments to these guidelines, warranting consultation with a healthcare professional.
4.5. Sleep patterns and their relationship with metabolic diseases and aging
Sleep quality and patterns profoundly affect metabolic health and the aging process. Chronic sleep deprivation and disturbances are linked to increased metabolic disease risk and accelerated aging-related complications (137–139). Insufficient sleep and poor sleep quality can lead to obesity, T2D, CVD, and other metabolic disorders, primarily through disturbances in hormonal regulation of appetite and glucose metabolism (140–142). Sleep disruptions can also disrupt the body’s circadian rhythm, which regulates metabolic processes. These disturbances, such as shift work or irregular sleep schedules, contribute to metabolic dysregulation, hormonal imbalances, and increased inflammation (143, 144). Moreover, inadequate sleep impairs cognitive function and memory consolidation, which are essential for healthy aging (145, 146). Thus, promoting good sleep hygiene, including maintaining a consistent sleep schedule, creating a comfortable sleep environment, and using relaxation techniques, is crucial. Ensuring sufficient sleep duration, typically 7–9 h for adults, and seeking professional help for sleep disorders can support metabolic health and healthy aging.
4.6. The role of psychological stress and mental health in metabolic diseases and aging
Psychological stress and mental health significantly influence metabolic diseases and aging. Stress, depression, anxiety, and other mental health conditions are linked with metabolic diseases and can hasten the aging process (147–150). Stress and negative emotions can incite unhealthy behaviors such as overeating and inactivity, leading to obesity and related metabolic disorders (151, 152). Moreover, chronic stress disrupts hormonal balance, impairing the body’s ability to regulate blood sugar and manage inflammation (153). Recognizing the interplay between mental and physical health, public health initiatives should equally emphasize mental well-being. Promoting stress management, access to mental health services, and lifestyle changes that foster overall health could enhance interventions targeting healthy aging and metabolic disease prevention.
5. Promoting public health for healthy aging
Healthy aging is the focus of the World Health Organization’s aging policy by 2030 (154). The development of clear and targeted public health interventions is essential for mitigating the rising prevalence of metabolic diseases and promoting healthy aging.
These interventions should tackle determinants of metabolic diseases such as unhealthy dietary patterns, sedentary lifestyles, tobacco use, and environmental factors to effectively reduce their incidence and complications (7, 10, 35, 121, 124). Long-term healthy aging approaches require prevention throughout the life course. Specific public health strategies include: building strong social support systems, promoting health literacy promotion and lifestyle awareness and health education for older adult that can empower individuals to prevent metabolic diseases (155). Policies facilitating access to affordable, nutritious food, such as nutrition education, labeling requirements, and restrictions on marketing unhealthy foods have shown effectiveness in preventing metabolic diseases (156, 157).
Promoting physical activity or community volunteer work through community initiatives, workplace wellness programs, and accessible recreational facilities can improve metabolic health by maintaining healthy weight and enhancing insulin sensitivity. Infrastructure that prioritizes active transportation, green spaces, and safe recreational areas can further promote activity and reduce sedentary behavior (158, 159). Additionally, lifelong health promotion and disease prevention activities can help prevent or delay the onset and progression of metabolic diseases. The government should enhance and improve the healthcare system and primary healthcare services. Implementing regular routine screenings for middle-aged and elderly individuals can lead to the early detection and timely intervention of metabolic diseases, thereby minimizing their consequences. Furthermore, providing long-term, effective treatment, nursing care, and psychological support for patients with advanced diseases can enhance their sense of social well-being (160). Health regulations are also critical for promoting healthy aging and metabolic disease prevention. Therefore, a combination of multi-sectoral initiatives can create an environment that supports healthy behavior and promotes public health and healthy aging.
This article has certain limitations. It offers a comprehensive overview of metabolic diseases, but it fails to carry out in-depth analysis of the molecular processes associated with healthy aging and account for individual differences and subtle nuances, such as genetic factors and socio-economic circumstances. These elements play a significant role in the risk and progression of these diseases. Moreover, due to the complexity of the system and confounding variables, determining the causality between environmental/behavioral factors and metabolic diseases presents a challenge. For a complete understanding of metabolic diseases and healthy aging, further research and personalized assessments are critically essential.
6. Conclusion
In summary, this review article emphasizes the important contribution of environmental and behavioral risk factors to the emergence of metabolic diseases and healthy aging. Key risk factors, including diet, physical activity, smoking, alcohol consumption, environmental pollutants, and sleep patterns, have been identified and linked to various metabolic disorders and age-related complications. The findings underscore the importance of public health interventions in addressing metabolic diseases and promoting healthy aging. Further research is needed to understand risk factor interactions, mechanisms, and intervention effects. Addressing metabolic diseases is vital for healthy aging, reducing chronic conditions, and improving quality of life. A holistic approach combining behavior changes, supportive environments, and public health interventions can lead to a healthier future with lower metabolic disease rates and improved well-being in aging populations.
Author contributions
KZ: Conceptualization, Data curation, Methodology, Writing – original draft. YuM: Conceptualization, Data curation, Methodology, Writing – original draft. YL: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. YS: Conceptualization, Data curation, Investigation, Writing – review & editing. GX: Data curation, Investigation, Writing – review & editing. YaM: Data curation, Investigation, Conceptualization, Writing – review & editing. XS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. CK: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
This work was supported by the Taishan Scholars Project of Shandong Province (tsqn202211365) and funding for the Key Disciplines of Medicine and Health in Shandong Province (Endocrinology and Metabolism).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo, J, Huang, X, Dou, L, Yan, M, Shen, T, Tang, W, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. (2022) 7:391. doi: 10.1038/s41392-022-01251-0
2. Paneni, F, Diaz Cañestro, C, Libby, P, Lüscher, TF, and Camici, GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. (2017) 69:1952–67. doi: 10.1016/j.jacc.2017.01.064
3. Batsis, JA, and Villareal, DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
4. Bellary, S, Kyrou, I, Brown, JE, and Bailey, CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. (2021) 17:534–48. doi: 10.1038/s41574-021-00512-2
5. Santos, AL, and Sinha, S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res Rev. (2021) 67:101268. doi: 10.1016/j.arr.2021.101268
6. van Dijk, SJ, Tellam, RL, Morrison, JL, Muhlhausler, BS, and Molloy, PL. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin Epigenetics. (2015) 7:66. doi: 10.1186/s13148-015-0101-5
7. Mattson, MP, Longo, VD, and Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. (2017) 39:46–58. doi: 10.1016/j.arr.2016.10.005
8. Münzel, T, Sørensen, M, Gori, T, Schmidt, FP, Rao, X, Brook, J, et al. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. (2017) 38:550–6. doi: 10.1093/eurheartj/ehw269
9. Wei, M, Brandhorst, S, Shelehchi, M, Mirzaei, H, Cheng, CW, Budniak, J, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. (2017):9. doi: 10.1126/scitranslmed.aai8700
10. Howell, NA, and Booth, GL. The weight of place: built environment correlates of obesity and diabetes. Endocr Rev. (2022) 43:966–83. doi: 10.1210/endrev/bnac005
11. Park, SY, Byun, EJ, Lee, JD, Kim, S, and Kim, HS. Air pollution, autophagy, and skin aging: impact of particulate matter PM(10) on human dermal fibroblasts. Int J Mol Sci. (2018):19. doi: 10.3390/ijms19092727
12. He, B, Ni, Y, Jin, Y, and Fu, Z. Pesticides-induced energy metabolic disorders. Sci Total Environ. (2020) 729:139033. doi: 10.1016/j.scitotenv.2020.139033
13. Yang, BY, Fan, S, Thiering, E, Seissler, J, Nowak, D, Dong, GH, et al. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res. (2020) 180:108817. doi: 10.1016/j.envres.2019.108817
14. Zhang, Y, Dong, T, Hu, W, Wang, X, Xu, B, Lin, Z, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int. (2019) 123:325–36. doi: 10.1016/j.envint.2018.11.076
15. Poggiogalle, E, Jamshed, H, and Peterson, CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. (2018) 84:11–27. doi: 10.1016/j.metabol.2017.11.017
16. Geidl, W, Schlesinger, S, Mino, E, Miranda, L, and Pfeifer, K. Dose-response relationship between physical activity and mortality in adults with noncommunicable diseases: a systematic review and meta-analysis of prospective observational studies. Int J Behav Nutr Phys Act. (2020) 17:109. doi: 10.1186/s12966-020-01007-5
17. Oppert, JM, Bellicha, A, and Ciangura, C. Physical activity in management of persons with obesity. Eur J Intern Med. (2021) 93:8–12. doi: 10.1016/j.ejim.2021.04.028
18. Manoogian, E, Chow, LS, Taub, PR, Laferrère, B, and Panda, S. Time-restricted eating for the prevention and Management of Metabolic Diseases. Endocr Rev. (2022) 43:405–36. doi: 10.1210/endrev/bnab027
19. Anisimova, AS, Alexandrov, AI, Makarova, NE, Gladyshev, VN, and Dmitriev, SE. Protein synthesis and quality control in aging. Aging (Albany NY). (2018) 10:4269–88. doi: 10.18632/aging.101721
20. Fang, P, She, Y, Yu, M, Min, W, Shang, W, and Zhang, Z. Adipose-muscle crosstalk in age-related metabolic disorders: the emerging roles of adipo-myokines. Ageing Res Rev. (2023) 84:101829. doi: 10.1016/j.arr.2022.101829
21. Arner, P, and Rydén, M. Human white adipose tissue: a highly dynamic metabolic organ. J Intern Med. (2022) 291:611–21. doi: 10.1111/joim.13435
22. Petersen, MC, and Shulman, GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
23. Sampath Kumar, A, Maiya, AG, Shastry, BA, Vaishali, K, Ravishankar, N, Hazari, A, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 62:98–103. doi: 10.1016/j.rehab.2018.11.001
24. Li, CW, Yu, K, Shyh-Chang, N, Jiang, Z, Liu, T, Ma, S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. (2022) 13:781–94. doi: 10.1002/jcsm.12901
25. Dowling, L, Duseja, A, Vilaca, T, Walsh, JS, and Goljanek-Whysall, K. MicroRNAs in obesity, sarcopenia, and commonalities for sarcopenic obesity: a systematic review. J Cachexia Sarcopenia Muscle. (2022) 13:68–85. doi: 10.1002/jcsm.12878
26. Bartke, A. Growth hormone and aging. Rev Endocr Metab Disord. (2021) 22:71–80. doi: 10.1007/s11154-020-09593-2
27. Frater, J, Lie, D, Bartlett, P, and McGrath, JJ. Insulin-like growth factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: a review. Ageing Res Rev. (2018) 42:14–27. doi: 10.1016/j.arr.2017.12.002
28. Wang, W, Tesfay, EB, van Klinken, JB, Willems van Dijk, K, Bartke, A, van Heemst, D, et al. Clustered Mendelian randomization analyses identify distinct and opposing pathways in the association between genetically influenced insulin-like growth factor-1 and type 2 diabetes mellitus. Int J Epidemiol. (2022) 51:1874–85. doi: 10.1093/ije/dyac119
29. Janssen, J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and Cancer. Int J Mol Sci. (2021) 22:22. doi: 10.3390/ijms22157797
30. De Paoli, M, Zakharia, A, and Werstuck, GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am J Pathol. (2021) 191:1490–8. doi: 10.1016/j.ajpath.2021.05.011
31. Julien, B, Pinteur, C, Vega, N, Vidal, H, Naville, D, and Le Magueresse-Battistoni, B. Estrogen withdrawal and replacement differentially target liver and adipose tissues in female mice fed a high-fat high-sucrose diet: impact of a chronic exposure to a low-dose pollutant mixture(☆). J Nutr Biochem. (2019) 72:108211. doi: 10.1016/j.jnutbio.2019.07.002
32. Kelly, DM, and Jones, TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. (2013) 217:R25–45. doi: 10.1530/JOE-12-0455
33. van den Beld, AW, Kaufman, JM, Zillikens, MC, Lamberts, S, Egan, JM, and van der Lely, AJ. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. (2018) 6:647–58. doi: 10.1016/S2213-8587(18)30026-3
34. Donato, J Jr, Wasinski, F, Furigo, IC, Metzger, M, and Frazão, R. Central regulation of metabolism by growth hormone. Cells. (2021):10. doi: 10.3390/cells10010129
35. Lavie, CJ, Ozemek, C, Carbone, S, Katzmarzyk, PT, and Blair, SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. (2019) 124:799–815. doi: 10.1161/CIRCRESAHA.118.312669
36. Palmer, AK, Gustafson, B, Kirkland, JL, and Smith, U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. (2019) 62:1835–41. doi: 10.1007/s00125-019-4934-x
37. Laiteerapong, N, Ham, SA, Gao, Y, Moffet, HH, Liu, JY, Huang, ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care. (2019) 42:416–26. doi: 10.2337/dc17-1144
38. Cicalese, SM, da Silva, JF, Priviero, F, Webb, RC, Eguchi, S, and Tostes, RC. Vascular stress signaling in hypertension. Circ Res. (2021) 128:969–92. doi: 10.1161/CIRCRESAHA.121.318053
39. Forte, M, Stanzione, R, Cotugno, M, Bianchi, F, Marchitti, S, and Rubattu, S. Vascular ageing in hypertension: focus on mitochondria. Mech Ageing Dev. (2020) 189:111267. doi: 10.1016/j.mad.2020.111267
40. Glass, CK, and Olefsky, JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. (2012) 15:635–45. doi: 10.1016/j.cmet.2012.04.001
41. Grandl, G, and Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. (2018) 40:215–24. doi: 10.1007/s00281-017-0666-5
42. Franceschi, C, Garagnani, P, Parini, P, Giuliani, C, and Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
43. Gonzalez, FJ, Xie, C, and Jiang, C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. (2018) 15:21–32. doi: 10.1038/s41574-018-0096-z
44. Knapp, M, Tu, X, and Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. (2019) 40:1–8. doi: 10.1038/s41401-018-0042-6
45. Ali, MK, Pearson-Stuttard, J, Selvin, E, and Gregg, EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. (2022) 65:3–13. doi: 10.1007/s00125-021-05585-2
46. Zampino, M, AlGhatrif, M, Kuo, PL, Simonsick, EM, and Ferrucci, L. Longitudinal changes in resting metabolic rates with aging are accelerated by diseases. Nutrients. (2020):12. doi: 10.3390/nu12103061
47. Silveira Rossi, JL, Barbalho, SM, Reverete de Araujo, R, Bechara, MD, Sloan, KP, and Sloan, LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38:e3502. doi: 10.1002/dmrr.3502
48. Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension. (2015) 65:252–6. doi: 10.1161/HYPERTENSIONAHA.114.03617
49. Wan, Z, Song, L, Hu, L, Lei, X, Huang, Y, Lv, Y, et al. The role of systemic inflammation in the association between serum 25-hydroxyvitamin D and type 2 diabetes mellitus. Clin Nutr. (2021) 40:3661–7. doi: 10.1016/j.clnu.2021.04.029
50. Wang, X, Mukherjee, B, and Park, SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003-2014. Environ Int. (2018) 121:683–94. doi: 10.1016/j.envint.2018.09.035
51. Lind, PM, and Lind, L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. (2018) 61:1495–502. doi: 10.1007/s00125-018-4621-3
52. Zhao, L, Fang, J, Tang, S, Deng, F, Liu, X, Shen, Y, et al. PM2.5 and serum metabolome and insulin resistance, potential mediation by the gut microbiome: a population-based panel study of older adults in China. Environ Health Perspect. (2022) 130:27007. doi: 10.1289/EHP9688
53. Le Magueresse-Battistoni, B, Vidal, H, and Naville, D. Environmental pollutants and metabolic disorders: the multi-exposure scenario of life. Front Endocrinol. (2018) 9:582. doi: 10.3389/fendo.2018.00582
54. Lauretta, R, Sansone, A, Sansone, M, Romanelli, F, and Appetecchia, M. Endocrine disrupting chemicals: effects on endocrine glands. Front Endocrinol. (2019) 10:178. doi: 10.3389/fendo.2019.00178
55. Le Magueresse-Battistoni, B. Endocrine disrupting chemicals and metabolic disorders in the liver: what if we also looked at the female side. Chemosphere. (2021) 268:129212. doi: 10.1016/j.chemosphere.2020.129212
56. Le Magueresse-Battistoni, B. Adipose tissue and endocrine-disrupting chemicals: does sex matter. Int J Environ Res Public Health. (2020) 17:9403. doi: 10.3390/ijerph17249403
57. Beulens, J, Pinho, M, Abreu, TC, den Braver, NR, Lam, TM, Huss, A, et al. Environmental risk factors of type 2 diabetes-an exposome approach. Diabetologia. (2022) 65:263–74. doi: 10.1007/s00125-021-05618-w
58. Sun, J, Fang, R, Wang, H, Xu, DX, Yang, J, Huang, X, et al. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: where exposomics meets metabolomics. Environ Int. (2022) 158:106941. doi: 10.1016/j.envint.2021.106941
59. Park, SK, Schwartz, J, Weisskopf, M, Sparrow, D, Vokonas, PS, Wright, RO, et al. Low-level lead exposure, metabolic syndrome, and heart rate variability: the VA normative aging study. Environ Health Perspect. (2006) 114:1718–24. doi: 10.1289/ehp.8992
60. Valcke, M, Ouellet, N, Dubé, M, Laouan Sidi, EA, LeBlanc, A, Normandin, L, et al. Biomarkers of cadmium, lead and mercury exposure in relation with early biomarkers of renal dysfunction and diabetes: results from a pilot study among aging Canadians. Toxicol Lett. (2019) 312:148–56. doi: 10.1016/j.toxlet.2019.05.014
61. Vasto, S, Baldassano, D, Sabatino, L, Caldarella, R, Di Rosa, L, and Baldassano, S. The role of consumption of molybdenum biofortified crops in bone homeostasis and healthy aging. Nutrients. (2023):15. doi: 10.3390/nu15041022
62. Li, B, Zhang, Q, Chang, X, Shen, Y, Liu, T, Liang, X, et al. Association of urinary metal levels with metabolic syndrome in coal workers. Environ Sci Pollut Res Int. (2023) 30:62892–904. doi: 10.1007/s11356-023-26452-0
63. Liang, J, Tian, SS, Qiao, N, Wang, C, Huang, JJ, Sun, CM, et al. Relationship between physical activity patterns and metabolic syndrome among male coal miners of Shanxi Province in China. Int J Sport Nutr Exerc Metab. (2017) 27:50–8. doi: 10.1123/ijsnem.2015-0320
64. Cotrim, HP, Carvalho, F, Siqueira, AC, Lordelo, M, Rocha, R, and De Freitas, LA. Nonalcoholic fatty liver and insulin resistance among petrochemical workers. JAMA. (2005) 294:1618–20. doi: 10.1001/jama.294.13.1618-b
65. Pearson, BL, and Ehninger, D. Environmental chemicals and aging. Curr Environ Health Rep. (2017) 4:38–43. doi: 10.1007/s40572-017-0131-6
66. Ko, E, Choi, M, and Shin, S. Bottom-line mechanism of organochlorine pesticides on mitochondria dysfunction linked with type 2 diabetes. J Hazard Mater. (2020) 393:122400. doi: 10.1016/j.jhazmat.2020.122400
67. Xu, X, Liao, W, Lin, Y, Dai, Y, Shi, Z, and Huo, X. Blood concentrations of lead, cadmium, mercury and their association with biomarkers of DNA oxidative damage in preschool children living in an e-waste recycling area. Environ Geochem Health. (2018) 40:1481–94. doi: 10.1007/s10653-017-9997-3
68. Sanmarco, LM, Chao, CC, Wang, YC, Kenison, JE, Li, Z, Rone, JM, et al. Identification of environmental factors that promote intestinal inflammation. Nature. (2022) 611:801–9. doi: 10.1038/s41586-022-05308-6
69. Ludwig, DS, and Ebbeling, CB. The carbohydrate-insulin model of obesity: beyond "calories in, calories out". JAMA Intern Med. (2018) 178:1098–103. doi: 10.1001/jamainternmed.2018.2933
70. Craig, WJ, Mangels, AR, Fresán, U, Marsh, K, Miles, FL, Saunders, AV, et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients. (2021):13. doi: 10.3390/nu13114144
71. Li, Y, Schoufour, J, Wang, DD, Dhana, K, Pan, A, Liu, X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. (2020) 368:l6669. doi: 10.1136/bmj.l6669
72. Heianza, Y, Zhou, T, Sun, D, Hu, FB, and Qi, L. Healthful plant-based dietary patterns, genetic risk of obesity, and cardiovascular risk in the UK biobank study. Clin Nutr. (2021) 40:4694–701. doi: 10.1016/j.clnu.2021.06.018
73. Hu, Y, Ding, M, Sampson, L, Willett, WC, Manson, JE, Wang, M, et al. Intake of whole grain foods and risk of type 2 diabetes: results from three prospective cohort studies. BMJ. (2020) 370:m2206. doi: 10.1136/bmj.m2206
74. Tardy, AL, Pouteau, E, Marquez, D, Yilmaz, C, and Scholey, A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. (2020):12. doi: 10.3390/nu12010228
75. Longo, VD, Antebi, A, Bartke, A, Barzilai, N, Brown-Borg, HM, Caruso, C, et al. Interventions to slow aging in humans: are we ready. Aging Cell. (2015) 14:497–510. doi: 10.1111/acel.12338
76. Dato, S, Bellizzi, D, Rose, G, and Passarino, G. The impact of nutrients on the aging rate: a complex interaction of demographic, environmental and genetic factors. Mech Ageing Dev. (2016) 154:49–61. doi: 10.1016/j.mad.2016.02.005
77. de Lorgeril, M, and Salen, P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. (2006) 9:118–23. doi: 10.1079/PHN2005933
78. Battino, M, Forbes-Hernández, TY, Gasparrini, M, Afrin, S, Cianciosi, D, Zhang, J, et al. Relevance of functional foods in the Mediterranean diet: the role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit Rev Food Sci Nutr. (2019) 59:893–920. doi: 10.1080/10408398.2018.1526165
79. Makki, K, Deehan, EC, Walter, J, and Bäckhed, F. The impact of dietary Fiber on gut microbiota in host health and disease. Cell Host Microbe. (2018) 23:705–15. doi: 10.1016/j.chom.2018.05.012
80. Myhrstad, M, Tunsjø, H, Charnock, C, and Telle-Hansen, VH. Dietary Fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients. (2020):12. doi: 10.3390/nu12030859
81. Magne, F, Gotteland, M, Gauthier, L, Zazueta, A, Pesoa, S, Navarrete, P, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut Dysbiosis in obese patients. Nutrients. (2020):12. doi: 10.3390/nu12051474
82. Shi, J, Qiu, H, Xu, Q, Ma, Y, Ye, T, Kuang, Z, et al. Integrated multi-omics analyses reveal effects of empagliflozin on intestinal homeostasis in high-fat-diet mice. iScience. (2023) 26:105816. doi: 10.1016/j.isci.2022.105816
83. Shuwen, H, and Kefeng, D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes. (2022) 14:2113717. doi: 10.1080/19490976.2022.2113717
84. Stenman, LK, Burcelin, R, and Lahtinen, S. Establishing a causal link between gut microbes, body weight gain and glucose metabolism in humans - towards treatment with probiotics. Benef Microbes. (2016) 7:11–22. doi: 10.3920/BM2015.0069
85. Valdes, AM, Walter, J, Segal, E, and Spector, TD. Role of the gut microbiota in nutrition and health. BMJ. (2018) 361:k2179. doi: 10.1136/bmj.k2179
86. Dalile, B, Van Oudenhove, L, Vervliet, B, and Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
87. Haran, JP, and McCormick, BA. Aging, frailty, and the microbiome-how Dysbiosis influences human aging and disease. Gastroenterology. (2021) 160:507–23. doi: 10.1053/j.gastro.2020.09.060
88. Thevaranjan, N, Puchta, A, Schulz, C, Naidoo, A, Szamosi, JC, Verschoor, CP, et al. Age-associated microbial Dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. (2017) 21:455–66.e4. doi: 10.1016/j.chom.2017.03.002
89. Zhou, Y, Stubbs, RJ, and Finlayson, G. A neurocognitive perspective on the relationship between exercise and reward: implications for weight management and drug addiction. Appetite. (2023) 182:106446. doi: 10.1016/j.appet.2022.106446
90. Guo, X, Gong, S, Chen, Y, Hou, X, Sun, T, Wen, J, et al. Lifestyle behaviors and stress are risk factors for overweight and obesity in healthcare workers: a cross-sectional survey. BMC Public Health. (2023) 23:1791. doi: 10.1186/s12889-023-16673-w
91. Amanat, S, Ghahri, S, Dianatinasab, A, Fararouei, M, and Dianatinasab, M. Exercise and type 2 diabetes. Adv Exp Med Biol. (2020) 1228:91–105. doi: 10.1007/978-981-15-1792-1_6
92. Savikj, M, Stocks, B, Sato, S, Caidahl, K, Krook, A, Deshmukh, AS, et al. Exercise timing influences multi-tissue metabolome and skeletal muscle proteome profiles in type 2 diabetic patients - a randomized crossover trial. Metabolism. (2022) 135:155268. doi: 10.1016/j.metabol.2022.155268
93. Ibeas, K, Herrero, L, Mera, P, and Serra, D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochem Pharmacol. (2021) 190:114640. doi: 10.1016/j.bcp.2021.114640
94. Diaz, KM, and Shimbo, D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. (2013) 15:659–68. doi: 10.1007/s11906-013-0386-8
95. Palatini, P. Blood pressure behaviour during physical activity. Sports Med. (1988) 5:353–74. doi: 10.2165/00007256-198805060-00002
96. Lin, WY. A large-scale observational study linking various kinds of physical exercise to lipoprotein-lipid profile. J Int Soc Sports Nutr. (2021) 18:35. doi: 10.1186/s12970-021-00436-2
97. Nilsson, MI, Bourgeois, JM, Nederveen, JP, Leite, MR, Hettinga, BP, Bujak, AL, et al. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS One. (2019) 14:e0210863. doi: 10.1371/journal.pone.0210863
98. Chowdhury, KK, Legare, DJ, and Lautt, WW. Insulin sensitization by voluntary exercise in aging rats is mediated through hepatic insulin sensitizing substance (HISS). Exp Gerontol. (2011) 46:73–80. doi: 10.1016/j.exger.2010.10.006
99. Pan, B, Ge, L, Xun, YQ, Chen, YJ, Gao, CY, Han, X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. (2018) 15:72. doi: 10.1186/s12966-018-0703-3
100. Oppert, JM, Bellicha, A, van Baak, MA, Battista, F, Beaulieu, K, Blundell, JE, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of obesity physical activity working group. Obes Rev. (2021) 22:e13273. doi: 10.1111/obr.13273
101. Ramos, JS, Dalleck, LC, Tjonna, AE, Beetham, KS, and Coombes, JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. (2015) 45:679–92. doi: 10.1007/s40279-015-0321-z
102. Haganes, KL, Silva, CP, Eyjólfsdóttir, SK, Steen, S, Grindberg, M, Lydersen, S, et al. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: a randomized controlled trial. Cell Metab. (2022) 34:1457–71.e4. doi: 10.1016/j.cmet.2022.09.003
103. McMullan, RC, Kelly, SA, Hua, K, Buckley, BK, Faber, JE, Pardo-Manuel de Villena, F, et al. Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging. Physiol Rep. (2016) 4:e13011. doi: 10.14814/phy2.13011
104. Zhu, J, Zhou, Y, Jin, B, and Shu, J. Role of estrogen in the regulation of central and peripheral energy homeostasis: from a menopausal perspective. Ther Adv Endocrinol Metab. (2023) 14:20420188231199359. doi: 10.1177/20420188231199359
105. Kuryłowicz, A. Estrogens in adipose tissue physiology and obesity-related dysfunction. Biomedicine. (2023) 11:690. doi: 10.3390/biomedicines11030690
106. Ko, SH, and Kim, HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. (2020) 12:202. doi: 10.3390/nu12010202
107. Ko, SH, and Jung, Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. (2021) 13:4556. doi: 10.3390/nu13124556
108. Ali, D, Figeac, F, Caci, A, Ditzel, N, Schmal, C, Kerckhofs, G, et al. High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: evidence from ovariectomized mice. Aging Cell. (2022) 21:e13726. doi: 10.1111/acel.13726
109. Klinge, CM. Estrogenic control of mitochondrial function. Redox Biol. (2020) 31:101435. doi: 10.1016/j.redox.2020.101435
110. Bhasin, S, Woodhouse, L, Casaburi, R, Singh, AB, Bhasin, D, Berman, N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. (2001) 281:E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172
111. Mudali, S, and Dobs, AS. Effects of testosterone on body composition of the aging male. Mech Ageing Dev. (2004) 125:297–304. doi: 10.1016/j.mad.2004.01.004
112. Blümel, JE, Fica, J, Chedraui, P, Mezones-Holguín, E, Zuñiga, MC, Witis, S, et al. Sedentary lifestyle in middle-aged women is associated with severe menopausal symptoms and obesity. Menopause. (2016) 23:488–93. doi: 10.1097/GME.0000000000000575
113. Kotseva, K, De Backer, G, De Bacquer, D, Rydén, L, Hoes, A, Grobbee, D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. (2019) 26:824–35. doi: 10.1177/2047487318825350
114. Carter, S, Hartman, Y, Holder, S, Thijssen, DH, and Hopkins, ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev. (2017) 45:80–6. doi: 10.1249/JES.0000000000000106
115. Ekelund, U, Brage, S, Besson, H, Sharp, S, and Wareham, NJ. Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality. Am J Clin Nutr. (2008) 88:612–7. doi: 10.1093/ajcn/88.3.612
116. Hamburg, NM, McMackin, CJ, Huang, AL, Shenouda, SM, Widlansky, ME, Schulz, E, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. (2007) 27:2650–6. doi: 10.1161/ATVBAHA.107.153288
117. Swift, DL, McGee, JE, Earnest, CP, Carlisle, E, Nygard, M, and Johannsen, NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. (2018) 61:206–13. doi: 10.1016/j.pcad.2018.07.014
118. Rigamonti, AE, De Col, A, Tamini, S, Cicolini, S, Caroli, D, De Micheli, R, et al. Multidisciplinary integrated metabolic rehabilitation in elderly obese patients: effects on cardiovascular risk factors, fatigue and muscle performance. Nutrients. (2019):11. doi: 10.3390/nu11061240
119. Ishikawa, M, Yokoyama, T, Nakaya, T, Fukuda, Y, Takemi, Y, Kusama, K, et al. Food accessibility and perceptions of shopping difficulty among elderly people living alone in Japan. J Nutr Health Aging. (2016) 20:904–11. doi: 10.1007/s12603-015-0694-6
120. Durlach, V, Vergès, B, Al-Salameh, A, Bahougne, T, Benzerouk, F, Berlin, I, et al. Smoking and diabetes interplay: a comprehensive review and joint statement. Diabetes Metab. (2022) 48:101370. doi: 10.1016/j.diabet.2022.101370
121. Tsai, PC, Glastonbury, CA, Eliot, MN, Bollepalli, S, Yet, I, Castillo-Fernandez, JE, et al. Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin Epigenetics. (2018) 10:126. doi: 10.1186/s13148-018-0558-0
122. Dikalov, S, Itani, H, Richmond, B, Vergeade, A, Rahman, S, Boutaud, O, et al. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am J Physiol Heart Circ Physiol. (2019) 316:H639–639H646. doi: 10.1152/ajpheart.00595.2018
123. Seoane-Collazo, P, Diéguez, C, Nogueiras, R, Rahmouni, K, Fernández-Real, JM, and López, M. Nicotine' actions on energy balance: friend or foe. Pharmacol Ther. (2021) 219:107693. doi: 10.1016/j.pharmthera.2020.107693
124. Rezk-Hanna, M, and Benowitz, NL. Cardiovascular effects of hookah smoking: potential implications for cardiovascular risk. Nicotine Tob Res. (2019) 21:1151–61. doi: 10.1093/ntr/nty065
125. Yang, Y, Peng, N, Chen, G, Wan, Q, Yan, L, Wang, G, et al. Interaction between smoking and diabetes in relation to subsequent risk of cardiovascular events. Cardiovasc Diabetol. (2022) 21:14. doi: 10.1186/s12933-022-01447-2
126. Klopack, ET, Carroll, JE, Cole, SW, Seeman, TE, and Crimmins, EM. Lifetime exposure to smoking, epigenetic aging, and morbidity and mortality in older adults. Clin Epigenetics. (2022) 14:72. doi: 10.1186/s13148-022-01286-8
127. Tarantino, U, Cariati, I, Greggi, C, Gasbarra, E, Belluati, A, Ciolli, L, et al. Skeletal system biology and smoke damage: from basic science to medical clinic. Int J Mol Sci. (2021):22. doi: 10.3390/ijms22126629
128. Ratajczak, AE, Szymczak-Tomczak, A, Rychter, AM, Zawada, A, Dobrowolska, A, and Krela-Kaźmierczak, I. Impact of cigarette smoking on the risk of osteoporosis in inflammatory bowel diseases. J Clin Med. (2021):10. doi: 10.3390/jcm10071515
129. Russo, C, Walicka, M, Caponnetto, P, Cibella, F, Maglia, M, Alamo, A, et al. Efficacy and safety of Varenicline for smoking cessation in patients with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2217709. doi: 10.1001/jamanetworkopen.2022.17709
130. Hu, Y, Zong, G, Liu, G, Wang, M, Rosner, B, Pan, A, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. (2018) 379:623–32. doi: 10.1056/NEJMoa1803626
131. Hendriks, H. Alcohol and human health: what is the evidence. Annu Rev Food Sci Technol. (2020) 11:1–21. doi: 10.1146/annurev-food-032519-051827
132. Minzer, S, Losno, RA, and Casas, R. The effect of alcohol on cardiovascular risk factors: is there new information. Nutrients. (2020):12. doi: 10.3390/nu12040912
133. Millwood, IY, Walters, RG, Mei, XW, Guo, Y, Yang, L, Bian, Z, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. (2019) 393:1831–42. doi: 10.1016/S0140-6736(18)31772-0
134. Seitz, HK, Bataller, R, Cortez-Pinto, H, Gao, B, Gual, A, Lackner, C, et al. Alcoholic liver disease. Nat Rev Dis Primers. (2018) 4:16. doi: 10.1038/s41572-018-0014-7
135. Åberg, F, Byrne, CD, Pirola, CJ, Männistö, V, and Sookoian, S. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol. (2023) 78:191–206. doi: 10.1016/j.jhep.2022.08.030
136. Butts, M, Sundaram, VL, Murughiyan, U, Borthakur, A, and Singh, S. The influence of alcohol consumption on intestinal nutrient absorption: a comprehensive review. Nutrients. (2023):15. doi: 10.3390/nu15071571
137. Li, SB, Damonte, VM, Chen, C, Wang, GX, Kebschull, JM, Yamaguchi, H, et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science. (2022) 375:eabh3021. doi: 10.1126/science.abh3021
138. Marjot, T, Ray, DW, Williams, FR, Tomlinson, JW, and Armstrong, MJ. Sleep and liver disease: a bidirectional relationship. Lancet Gastroenterol Hepatol. (2021) 6:850–63. doi: 10.1016/S2468-1253(21)00169-2
139. Adam, TC, Drummen, M, Macdonald, I, Jalo, E, Siig-Vestentoft, P, Martinez, JA, et al. Association of Psychobehavioral Variables with HOMA-IR and BMI differs for men and women with prediabetes in the PREVIEW lifestyle intervention. Diabetes Care. (2021) 44:1491–8. doi: 10.2337/dc21-0059
140. Antza, C, Kostopoulos, G, Mostafa, S, Nirantharakumar, K, and Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. (2021) 252:125–41. doi: 10.1530/JOE-21-0155
141. Besedovsky, L, Lange, T, and Haack, M. The sleep-immune crosstalk in health and disease. Physiol Rev. (2019) 99:1325–80. doi: 10.1152/physrev.00010.2018
142. Morrison, M, Halson, SL, Weakley, J, and Hawley, JA. Sleep, circadian biology and skeletal muscle interactions: implications for metabolic health. Sleep Med Rev. (2022) 66:101700. doi: 10.1016/j.smrv.2022.101700
143. Liu, H, and Chen, A. Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. (2019) 219:231–7. doi: 10.1016/j.lfs.2019.01.006
144. Papatriantafyllou, E, Efthymiou, D, Zoumbaneas, E, Popescu, CA, and Vassilopoulou, E. Sleep deprivation: effects on weight loss and weight loss maintenance. Nutrients. (2022):14. doi: 10.3390/nu14081549
145. Agorastos, A, and Olff, M. Sleep, circadian system and traumatic stress. Eur J Psychotraumatol. (2021) 12:1956746. doi: 10.1080/20008198.2021.1956746
146. Nassan, M, and Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. (2022) 18:7–24. doi: 10.1038/s41582-021-00577-7
147. Winkley, K, Upsher, R, Stahl, D, Pollard, D, Kasera, A, Brennan, A, et al. Psychological interventions to improve self-management of type 1 and type 2 diabetes: a systematic review. Health Technol Assess. (2020) 24:1–232. doi: 10.3310/hta24280
148. Brugnera, A, Compare, A, Omboni, S, Greco, A, Carrara, S, Tasca, GA, et al. Psychological covariates of blood pressure among patients with hypertension and metabolic syndrome. Health Psychol. (2022) 41:946–54. doi: 10.1037/hea0001205
149. Verhoeven, JE, Révész, D, van Oppen, P, Epel, ES, Wolkowitz, OM, and Penninx, BW. Anxiety disorders and accelerated cellular ageing. Br J Psychiatry. (2015) 206:371–8. doi: 10.1192/bjp.bp.114.151027
150. Verhoeven, JE, Révész, D, Epel, ES, Lin, J, Wolkowitz, OM, and Penninx, BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. (2014) 19:895–901. doi: 10.1038/mp.2013.151
151. Konttinen, H. Emotional eating and obesity in adults: the role of depression, sleep and genes. Proc Nutr Soc. (2020) 79:283–9. doi: 10.1017/S0029665120000166
152. Charansonney, OL, and Després, JP. Disease prevention--should we target obesity or sedentary lifestyle. Nat Rev Cardiol. (2010) 7:468–72. doi: 10.1038/nrcardio.2010.68
153. Kymissis, P, Shatin, L, and Brown, W. Relationship between high fasting blood sugar and depression in a mental hygiene clinic population. Can J Psychiatr. (1979) 24:133–8. doi: 10.1177/070674377902400204
154. Willans, B, Desai, M, Owen, L, and Leng, G. NICE public health guidance update. J Public Health (Oxf). (2019) 41:642–4. doi: 10.1093/pubmed/fdz087
155. Zhang, K, Kan, C, Luo, Y, Song, H, Tian, Z, Ding, W, et al. The promotion of active aging through older adult education in the context of population aging. Front Public Health. (2022) 10:998710. doi: 10.3389/fpubh.2022.998710
156. Reséndiz Lara, T, Muñoz Torres, AV, Mendoza Salmerón, G, Zendejas Vela, DD, Medina Bravo, P, Roy García, I, et al. Education with a multimedia web platform improves knowledge and HbA1c of Mexican patients with type 2 diabetes. Open clinical trial. Endocrinol Diabetes Nutr. (2020) 67:530–9. doi: 10.1016/j.endinu.2019.07.011
157. Grummon, AH, Reimold, AE, and Hall, MG. Influence of the San Francisco, CA, sugar-sweetened beverage health warning on consumer reactions: implications for equity from a randomized experiment. J Acad Nutr Diet. (2022) 122:363–70.e6. doi: 10.1016/j.jand.2021.07.008
158. Pollack Porter, KM, Prochnow, T, Mahoney, P, Delgado, H, Bridges Hamilton, CN, Wilkins, E, et al. Transforming City streets to promote physical activity and health equity. Health Aff. (2019) 38:1475–83. doi: 10.1377/hlthaff.2019.00454
159. Juul, V, and Nordbø, E. Examining activity-friendly neighborhoods in the Norwegian context: green space and walkability in relation to physical activity and the moderating role of perceived safety. BMC Public Health. (2023) 23:259. doi: 10.1186/s12889-023-15170-4
160. Longevity, TLH. Primary care workers vital for healthy longevity. Lancet Healthy Longev. (2023) 4:e357. doi: 10.1016/S2666-7568(23)00142-3
161. Blaner, WS. Vitamin a signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. (2019) 197:153–78. doi: 10.1016/j.pharmthera.2019.01.006
Keywords: metabolic diseases, healthy aging, risk factors, public health, environment
Citation: Zhang K, Ma Y, Luo Y, Song Y, Xiong G, Ma Y, Sun X and Kan C (2023) Metabolic diseases and healthy aging: identifying environmental and behavioral risk factors and promoting public health. Front. Public Health. 11:1253506. doi: 10.3389/fpubh.2023.1253506
Edited by:
Emiliana Giacomello, University of Trieste, ItalyReviewed by:
Brigitte Le Magueresse-Battistoni, INSERM U1060 Laboratoire de Recherche en Cardiovasculaire, Métabolisme, diabétologie et Nutrition, FranceGiuseppe Sirago, Université de Lausanne, Switzerland
Copyright © 2023 Zhang, Ma, Luo, Song, Xiong, Ma, Sun and Kan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Sun, eGlhb2Rvbmcuc3VuQHdmbWMuZWR1LmNu; Chengxia Kan, ZnlrYW5jaGVuZ3hpYUB3Zm1jLmVkdS5jbg==
‡ORCID: Chengxia Kan, https://orcid.org/0000-0002-4593-0303
†These authors share first authorship
 Kexin Zhang
Kexin Zhang Yujie Ma
Yujie Ma Youhong Luo
Youhong Luo Yixin Song
Yixin Song Guoji Xiong
Guoji Xiong Yanhui Ma
Yanhui Ma Xiaodong Sun
Xiaodong Sun Chengxia Kan
Chengxia Kan