- 1DIMAMO Population Health Research Centre, University of Limpopo, Polokwane, South Africa
- 2Department of Public Health, University of Limpopo, Polokwane, South Africa
Background: Worldwide, it is estimated that 38 million people are HIV-positive and that over 36 million people have died from the virus. In South Africa, the prevalence of HIV was reported to be 20.6% with Limpopo Province having 17% HIV. Given the high rate of new HIV infection in Limpopo, there is therefore a need to assess factors promoting risky sexual behavior among people living with HIV in order to help design and develop behavioral interventions aimed at reducing risky behaviors among people living with HIV.
Methods: This was a quantitative cross-sectional prospective study, conducted in Mankweng Hospital. The study consisted of 116 participants of which 40 were males and 76 were females aged 18 years and above. The participants were selected using purposive sampling. The data was analyzed using Statistical Package for Social Sciences version 27. A comparison of proportions was performed using Chi-Square. The association between risky sexual practice and sociodemographic factors was analyzed using multivariate logistic regression.
Results: The proportion of risky sexual practices in the total population was 48.3%. Participants who were married, those aged 35–44, and those with tertiary qualifications were more likely to engage in risky sexual practices. Multivariate logistic regression showed widowed participants were less likely to practice risky sexual practices.
Conclusion: The present study reported a high prevalence of risky sexual practices of 48.3%. Risky sexual behavior was determined by age, marital status, and level of education. The proportion of married participants was higher in risky sexual behavior. Based on the findings of the present study, it is recommended that targeted interventions and educational programs should be implemented to reduce risky sexual behavior among married individuals, individuals aged 35–44, and individuals with tertiary qualifications.
1. Introduction
HIV/AIDS is one of the leading causes of death worldwide. In 2020, 1.5 million individuals acquired AIDS globally, and approximately 680,000 people died from AIDS-related causes (1). By the end of 2014, it was estimated that 25.8 million persons in sub-Saharan Africa were HIV and AIDS-positive (2, 3). Despite the 90–90–90 treatment goal introduced by UNAIDS/WHO in 2014, which aimed that by 2020, 90% of all individuals should know their HIV status, 90% of PLHIV should be on treatment, and, 90% of those should have viral suppression (4), the new cases of HIV infection in sub-Saharan Africa seems to be increasing particularly in the southern regions (5, 6).
Reports on the sexual behaviors of individuals on HIV treatment in Africa have indicated that individuals on treatment (ART) are more likely to have unprotected sex compared to those who are not on treatment (7, 8). Although ART is increasing globally, the impact of ART on sexual risk behavior and transmission of HIV in Africa is still unknown (9). Although ART may minimize the risk of HIV transmission by persistent viral suppression, therapy may also serve as an excuse for unsafe sexual activities (10). There are several factors, which were reported as contributing factors to the spread of HIV among adults, such as domestic violence, having multiple partners, the practice of unprotected sex, homosexuality, and commercial sex (8, 11, 12).
Very little is known about the sexual behavior of PLHIV on ART in Limpopo, South Africa (13, 14). The incidence of HIV in South Africa is driven primarily by sexual contact between PLHIV and those who are uninfected, a growing number of South African PLHIV receiving ART were reported to continue engaging in unprotected sex (15). Risky sexual behavior among people receiving ART is a major public health concern, not only because of the risk of HIV transmission, but also the potential risk of transmission of resistant strains (16–19). People who default on treatment develop resistance to antiretroviral drugs leading to incomplete viral suppression, which may limit both the magnitude and the duration of the response to treatment (20). People who develop resistance as a result of default, when recommitting to the treatment, may engage in risky sexual behavior with the impression that the viral load is suppressed thus contributing to the incidence of HIV (21).
The prevalence of HIV in South Africa was reported to be 20.6%, with Limpopo Province having 17% HIV prevalence (22, 23). Given the high rate of new HIV infection in Limpopo (23–25), there is a need to assess factors promoting risky sexual behavior among PLHIV to help design and develop behavioral interventions aimed at HIV-positive individuals. As a result, the study sought to identify the factors that contribute to risky sexual behavior among PLHIV.
2. Methods and materials
2.1. Study design, and study setting
In this study, a quantitative study approach was utilized. A cross-sectional study design was implemented at Mankweng Hospital situated in the Capricorn District of the Limpopo Province in South Africa. The hospital is a government-funded tertiary institution which is a type of hospital that provides highly specialized medical care that involves complex procedures and treatment situated in the Mankweng township. Mankweng Hospital has a 550-bed capacity and has a Phela o Mphedise (translation; live and make me live) POP clinic. The POP Clinic provides services to an average of 2000 HIV/AIDS patients within Mankweng and the surrounding rural areas of Polokwane Local Municipality. The study was conducted from 2017 to 2018.
2.2. Sampling
The participants were selected using the purposive sampling method. Based on statistics, the Mankweng Hospital POP ART Clinic provided services to a total of 2000 PLWH aged ≥18 years receiving ART at the time of data collection. The total sample calculated using (n = 2000) was 322 ART patients. The sample size was calculated using Yamane’s population proportion approach formula in which a 5% sampling error provision was used (26). This was rounded up to 350 participants to allow for spoilt responses. As this study was voluntary, only 151 respondents took part in the study by completing the questionnaire. Of the 151 respondents, 35 of the questionnaires were spoilt and could not be used in the analysis making the total number of responses 116 participants.
2.3. Data collection
A 30-item questionnaire adapted from Purvis et al. (27) was given to patients, along with an introductory patient information form explaining the purpose of the research. The questionnaire covered 4 sections, namely (1) social-demographic data; (2) the sexual practices of the patients; (3) the health status of the patients; and (4) the use of alcohol and drugs. The participants who gave consent to participate in the research were offered a seat in a private room to complete the questionnaire, after signing a consent form. The data was collected from 1st January to the end of May 2018. Considering that Sepedi is the predominant language spoken among the study population, an option to choose between an English or Sepedi questionnaire was given to the participants. The participants’ information form and the consent form were read out to participants with no formal education to elicit their responses. Only those who gave consent to participate in the research were handed the questions and the options in the questionnaire were read out to them. The researchers recorded their responses on the survey form. To ensure the quality of data, data capturing and data cleaning were done by the researcher, which included removing outliers and participants with missing data.
2.4. Risky sexual behavior definition
In the present study risky sexual practice was defined as engaging in one of the following characteristics: unprotected oral sex, male-to-male sex, sex without the use of a condom or inconsistent use of a condom, and sexual intercourse under the influence of alcohol and drugs in the past 3 months (16, 18).
2.5. Data analysis
The data were analyzed using Statistical Package for Social Sciences (SPSS) version 27.0. Data were reported as frequencies and percentages. A comparison of proportions was performed using Chi-Square whilst a comparison of means was performed using an unpaired Student’s t-test. To determine the relationship between risky sexual behavior and associated factors we conducted a multivariate binary logistic regression. Risky sexual behavior was the exposure of interest. In the present study, the following variables were considered for the dependent variable, i.e., unprotected oral sex, male-to-male sex, sex without the use of a condom or inconsistent use of a condom, and sexual intercourse under the influence of alcohol and drugs. These variables were structured in a question format; the participants were asked if they have practiced any of the sexual practices listed in the following question; In the past 3 months, have you engaged in the following sexual practices, unprotected oral sex, male-to-male sex, sex without the use of a condom or inconsistent use of a condom, and sexual intercourse under the influence of alcohol and drugs in the past 3 months?. Risky sexual behavior was coded “1” if the participants answered yes to any of the sexual practices listed and coded “0” if they answered no to all the listed sexual practices. We adjusted for the main determinants of risky sexual practice which included sociodemographic (age, sex, educational status, and marital status). For all the inferential statistics, a p-value of less than 0.05 was considered statistically significant.
2.6. Ethical consideration
The study protocol was ethical approved by the Turfloop Research Ethics Committee (TREC) (TREC/320/2017:PG). The permission to conduct this study was granted by the Provincial Department of Health and the management of the Mankweng Hospital.
2.7. Rights of participants
The rights of the participants were not violated during this study and the principles of non-maleficence, human dignity, confidentiality, beneficence, and justice were maintained at all times.
2.8. Non-maleficence
During this research, none of the participants were harmed physically, verbally, or emotionally. A social worker and psychologist were made available, on referral, to counsel any participant who may have become distressed as a result of responding to the study questionnaire.
2.9. Human dignity
The researchers went through the informed consent form with the participants, making sure that they were aware of the study’s objectives and what was expected of them. The participants were made aware that they could opt out of participating in the research at any point, even after signing the consent form.
2.10. Confidentiality and privacy
No names of the participants were used in this research, instead, unique identifier codes were used to maintain the confidentiality of all questionnaires. The same level of confidentiality was applied when storing the data. All the data collected was kept on a laptop that was only accessed by the researcher. During data collection, participants were allowed to complete the questionnaires in a private space to maintain the privacy of all the participants.
2.11. Justice
All the participants in this study were treated equally and fairly and with a high degree of respect by the researcher. The researcher mentioned to the participants that participating in this research was voluntary and that there would be no material gain.
2.12. Beneficence
The results of this research will be presented to district health in Limpopo and to the national Department of Health. The outcome of the research will assist in collecting information that will help in the development of policies aimed at improving HIV and AIDS awareness.
3. Results
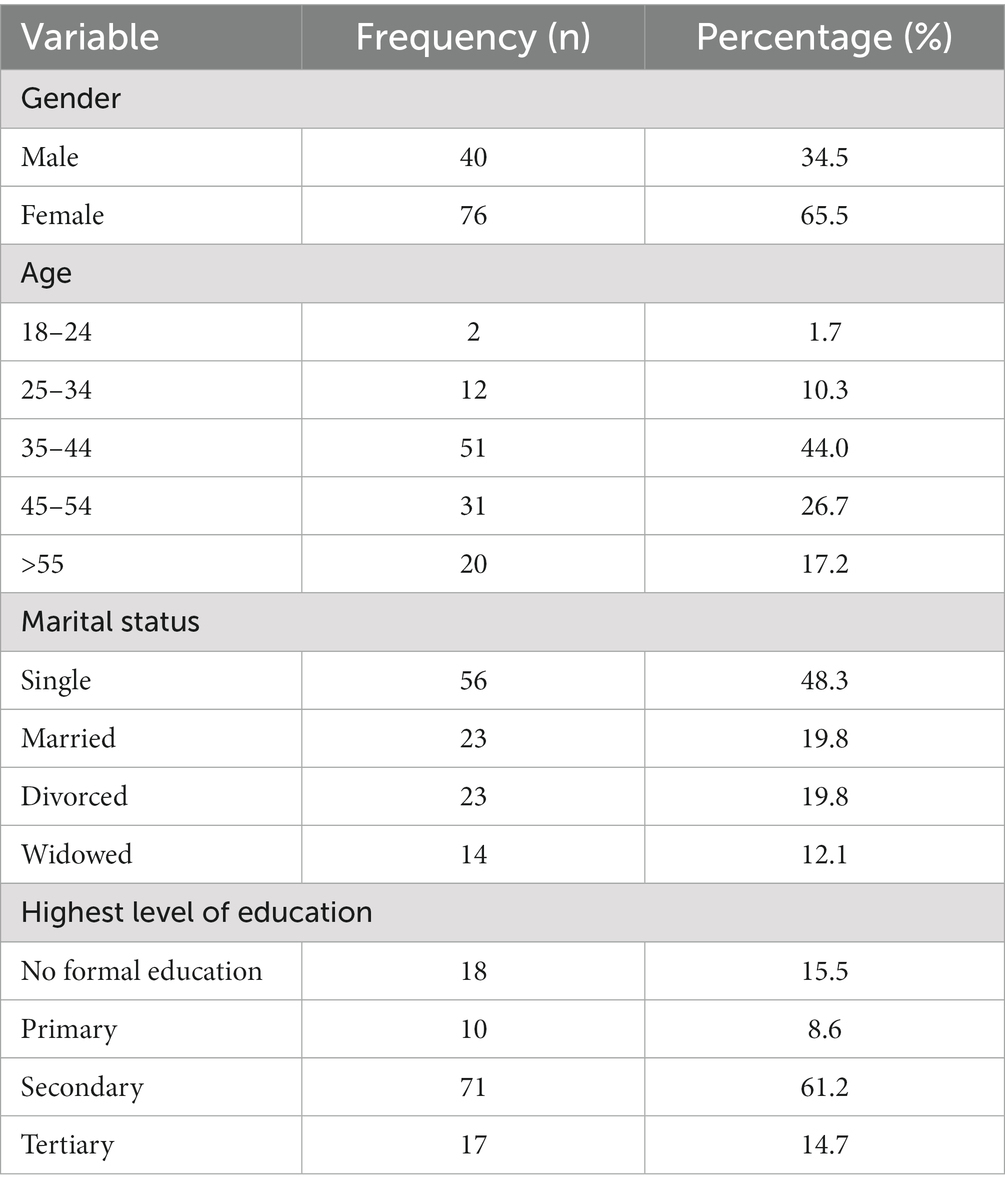
All the participants who took part in the study were HIV positive. The majority of the participants were females. Most participants were of the age category of 35–44. In addition, the majority of the participants were single and had secondary school as their highest level of education (Figure 1 and Table 1).
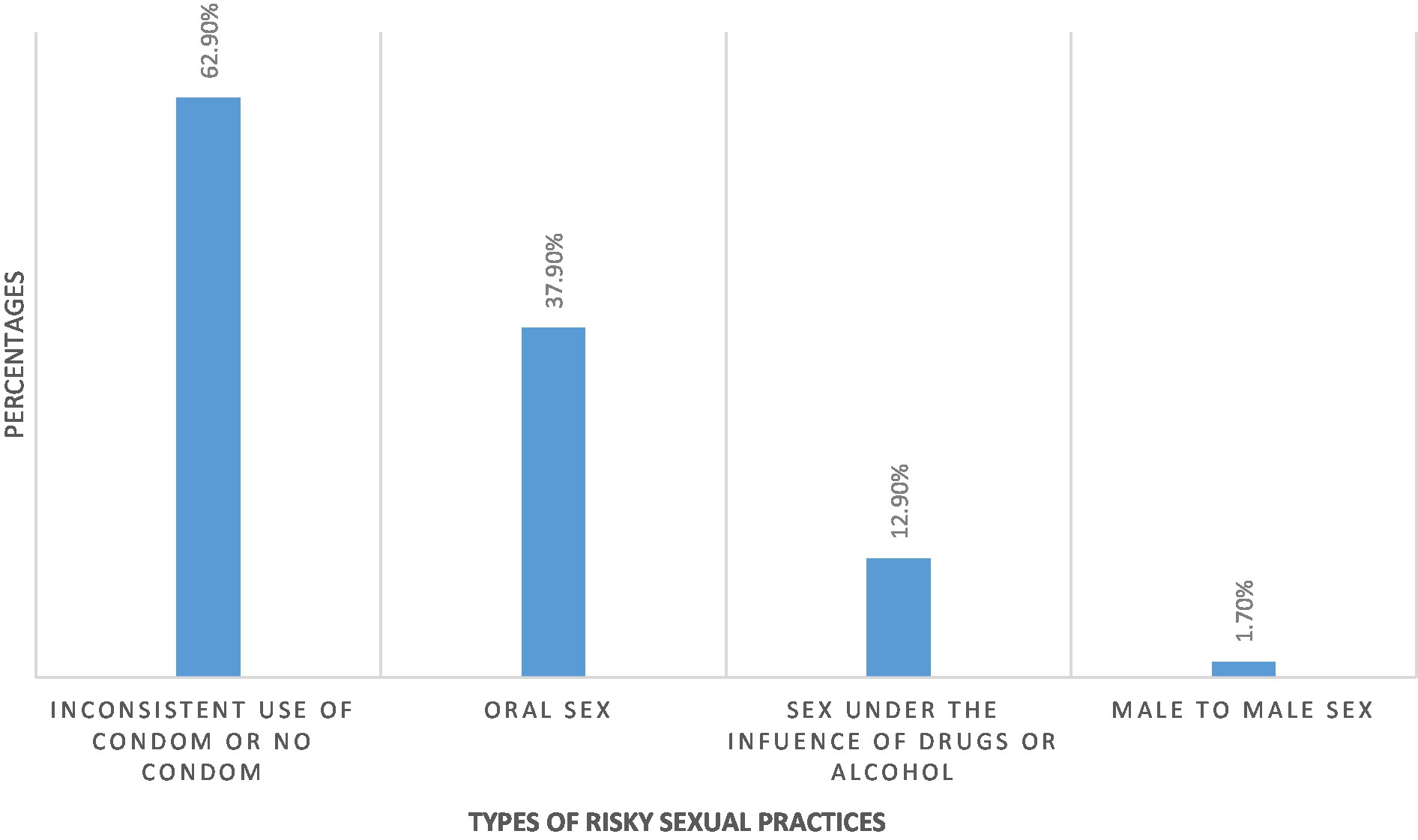
The prevalence of risky sexual practice in the present study was 48.3%. Amongst all participants who practiced risky behavior, 62.9% had sexual intercourse without a condom, 37.9% had oral sex, 12.9% had sexual intercourse under the influence of drugs and 1.7% had male-to-male sex.
There was no significant difference in gender (both males and females) between risky sexual behavior and non-risky sexual behavior. The proportion of participants aged 35–44 was significantly higher in participants with risky sexual behavior (56.8% vs. 36.1%, p = 0.048) as compared to those without risky sexual behavior. The proportion of participants aged 45–54 and those aged >55 was higher in participants without risky sexual behavior and compared to those with risky sexual behavior, respectively, (33% vs. 15.9%, p = 0.048), (19.4% vs. 13.6%, p = 0.048). There was no significant difference between risky sexual behavior and those without risky sexual behavior in single and divorced participants. The proportion of married participants was higher in risky sexual behavior (25.0% vs. 15.0%, p = 0.054) as compared to those without risky sexual behavior. The proportion of widowed participants was higher in non-risky sexual behavior (18.3% vs. 5.4%, p = 0.016) as compared to risky sexual behavior. There was no significant difference in sexual behavior between participants who had no formal education, primary and secondary school. However, risky sexual behavior (25.0% vs. 8.30%, p = 0.028) was significant in participants who had tertiary education (Table 2).
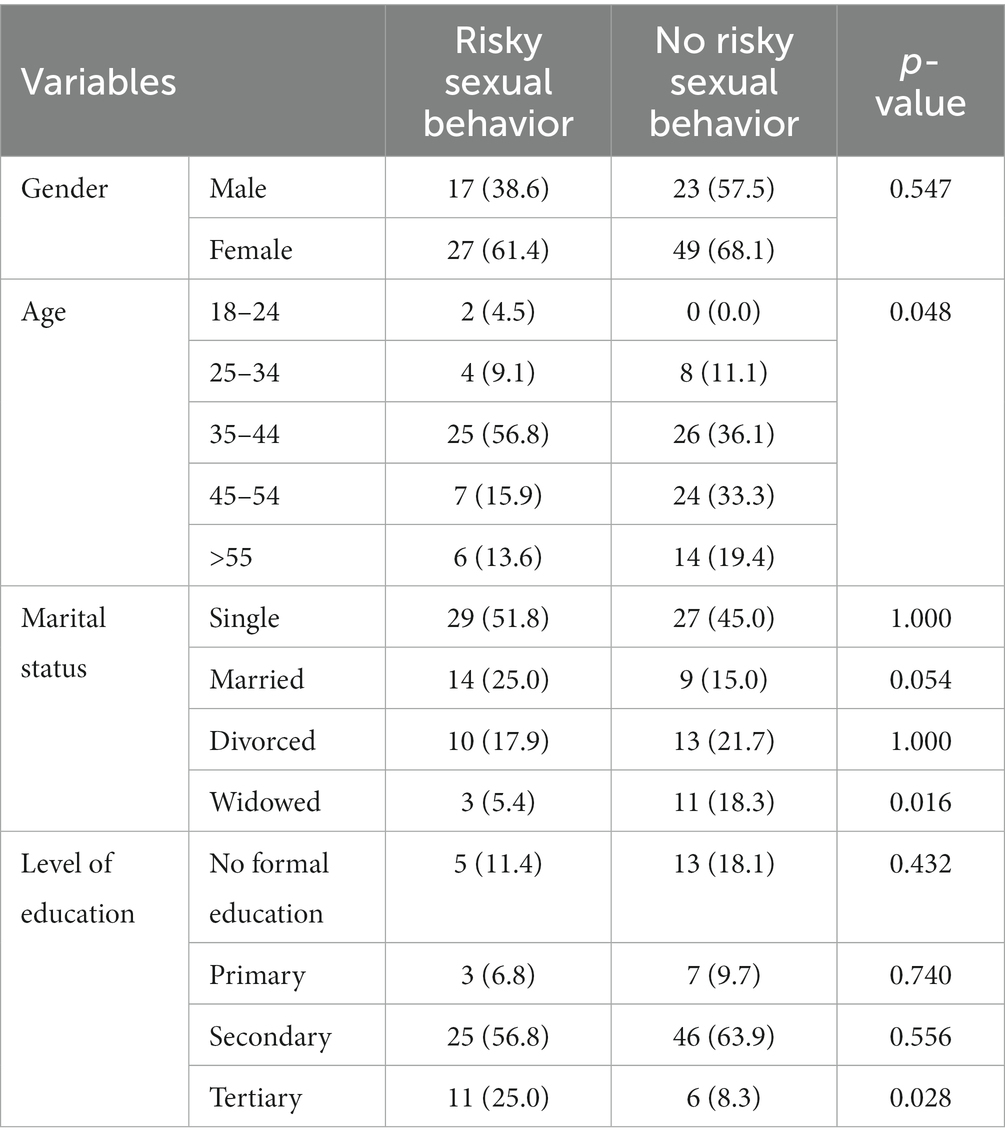
Table 2. Comparison of sociodemographic profile between risky sexual behavior and non-risky sexual behavior.
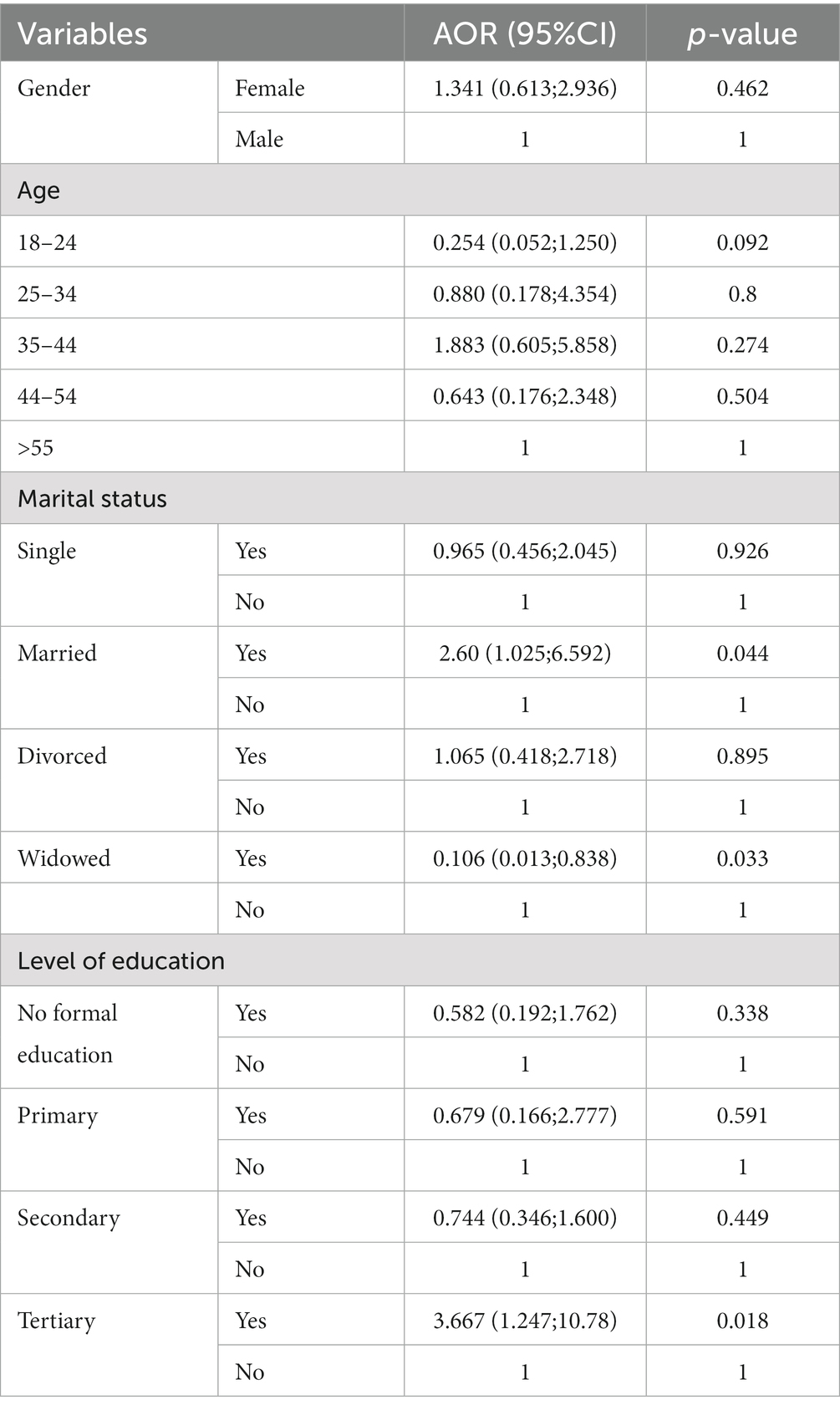
In multivariate regression, there was no relationship between risky sexual behavior with gender and age. Married participants were 2.60 (aOR = 2.60; 95%CI: 1.025–6.592) times more likely to practice risky sexual behavior (p = 0.044). Widowed participants were 0.106 (aOR = 0.106; 95%CI: 0.013–0.838) times less likely to practice risky sexual behavior (p = 0.033). There was no relationship between risky sexual behavior and level of education (i.e., No formal education, primary, and secondary). Participants with tertiary education were 3.669 (aOR = 3.669; 95%CI: 1.247–10.78) times more likely to practice risky sexual behavior (p = 0.018) (Table 3).
4. Discussion
The present study aimed to determine the prevalence of risky sexual behavior and its associated factors in PLHIV on ART, at Mankweng Hospital in Limpopo Province. In the present study, the prevalence of risky sexual behavior was 48.3%. These findings are congruent with the results of other studies conducted in Africa where the prevalence of risky sexual behavior was reported in Addis Ababa, Ethiopia, Johannesburg, South Africa, and Northwest Ethiopia at Gondar University Referral Hospital at 39.1, 30.4, and 38%, respectively (18, 28, 29). However, a study conducted in Zambia explicit a contrary results to this study in that it has reported a higher prevalence of risky sexual practice of 72.2% amongst participants aged 18–25 (30). The difference between the present study and the study conducted by Yang et al. could be the difference in age groups. In addition to oral sex, sex without the use of a condom, and sexual intercourse under the influence of alcohol, which were used to define risky sexual behavior in the present study, Yang et al. broadened the definition by including multiple sexual partners and casual sex with strangers thus likely contributing to the difference in prevalence.
In the present study, the proportion of the married category was significantly higher in risky sexual behavior as compared to non-risky sexual behavior. In addition, married participants were more likely to practice risky sexual behavior as compared to widowed. These findings concur with the results of a study conducted by Molla and Gelagay, which posits that married participants are more likely to engage in risky sexual behavior as compared to unmarried participants (29). Multivariate logistic regression showed that married participants were 2.60 times more likely to practice risky sexual behavior, a finding which is in agreement with research done by Tolmay et al. (31), who reported that married participants had the highest odds of inconsistence use of condoms as compared to those who are not married. The study found that low sexual decision-making capacity, relationship instability worry, and infidelity accusation fear were the main causes of inconsistent condom use in married women (31). Furthermore, Madiba and Ngwenya, cited that most men have indicated that they cannot pay lobola and still use a condom (32). In patriarchal societies, refusing unprotected sex or suggesting condom use is viewed as a threat to a husband’s authority or an indication of unfaithfulness, and may be confronted with physical abuse by the husband (31). In addition, a study by Tadesse and Gelagay (18), attributed the high prevalence of risky sexual behavior in married women to the need to have children, while some participants see no use of condoms since they are already infected with HIV.
The widowed category had a significantly higher proportion of non-risky sexual behavior as compared to risky sexual behavior. In multivariate binary logistic regression, widowed participants were 0.106 times less likely to practice risky sexual behavior. In agreement with the present study, reported widowed participants to be 0.30 times less likely to practice risky sexual behaviors study (33). The reason for widowed participants to practice less risky sexual behavior could be that, they are stigmatized in some areas which limits their social interactions (34).
There was no relationship between sexual behavior in participants with no formal education, primary, and secondary school. However, the proportion of risky sexual behavior was high in participants with tertiary education. In multivariate binary logistic regression, participants with tertiary education were 3.669 times more likely to practice risky sexual behavior. In agreement with the present study, Akinyi et al. reported a high proportion of inconsistent use of condoms in tertiary participants (35). There is still no clear explanation for why people with tertiary qualifications and living with HIV engage in risky sexual behaviors in rural South African populations. However, Ifeanyi Brian et al. (36) reported that 63.2% of tertiary students were engaging in risky sexual behavior for sexual experimentation and pleasure, oftentimes ignorant of the associated negative consequences.
4.1. Limitations of study
The study was conducted in rural Mankweng, which falls under the Capricorn District. The results, however, cannot be generalized to other rural communities as cultural and socio-demographic differences, depending on the area where research is conducted, and may influence sexual behaviors, sample size, the definition of risky sexual behavior, purposive sampling, and cross-sectional.
4.2. Recommendations
Based on the findings of the present study, it is recommended that targeted interventions and educational programs be implemented to address risky sexual behavior among married individuals, individuals aged 35–44, and individuals with tertiary qualifications. These interventions should focus on promoting safer sexual practices and increasing awareness about the potential risks associated with risky sexual behavior. Additionally, efforts should be made to improve sexual and reproductive health education, particularly among married individuals, to ensure they have the necessary knowledge and skills to make informed decisions about their sexual health.
5. Conclusion
The present study reported a high prevalence of risky sexual practice of 42.8%. Risky sexual behavior was determined by age, marital status, and the highest level of education. Married participants were more likely to practice risky sexual behavior. The present study found no significant difference in sexual behavior between participants who had no formal education, primary and secondary school. However, risky sexual behavior was found significant in participants who had tertiary education.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Turfloop Research Ethics Committee (TREC) (TREC/320/2017:PG). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CBN made substantial contributions to the conception, and design including analysis, and interpretation of data. RGM, KPS, TN, and EM made substantial contributions in methodology, interpretation of data, and reviewing and editing the manuscript. CBN, RGM, KPS, TN, and EM extensively reviewed the manuscript. All authors have read and agreed to the published version of the manuscript, read and approved the published version of the manuscript, collaboratively wrote the manuscript, did the design of the study including the data management and writing of the article, and involved in the study.
Acknowledgments
The authors acknowledge the participants for agreeing to participate in the study, the Mankweng Hospital, and the Department of Health for granting permission for the study to take place at Mankweng Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kaushal, A, Kaushal, D, Shegokar, R, and Pathak, Y. Viral diseases: cellular understanding of diseases In: R Shegokar and Y Pathak, editors. Viral drug delivery systems: advances in treatment of infectious diseases. Cham: Springer (2023). 27–63.
2. Awofala, AA, and Ogundele, OE. HIV epidemiology in Nigeria. Saudi J Biol Sci. (2018) 25:697–703. doi: 10.1016/j.sjbs.2016.03.006
3. Gubernick, SI, Félix, N, Lee, D, Xu, JJ, and Hamad, B. The HIV therapy market. Nat Rev Drug Discov. (2016) 15:451–2. doi: 10.1038/nrd.2016.69
5. Kharsany, AB, and Karim, QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. (2016) 10:34–48. doi: 10.2174/1874613601610010034
6. Nsanzimana, S, and Mills, EJ. Estimating HIV incidence in sub-Saharan Africa. Lancet HIV. (2023) 10:e146–8. doi: 10.1016/S2352-3018(22)00366-6
7. Anore, DL, Mengistie, B, and Geltore, TE. Unprotected sexual practices and associated factors among adult people living with HIV on antiretroviral therapy in public hospitals of Kembata Tembaro Zone, Southern Ethiopia. Pan Afr Med J. (2021) 38:176. doi: 10.11604/pamj.2021.38.176.26105
8. Nkhoma, K, Ahmed, A, Alli, Z, Sherr, L, and Harding, R. Does sexual behaviour of people with HIV reflect antiretroviral therapy as a preventive strategy? A cross-sectional study among outpatients in Kenya. BMC Public Health. (2019) 19:1–7. doi: 10.1186/s12889-019-7581-8
9. Schaefer, R, Gregson, S, and Benedikt, C. Widespread changes in sexual behaviour in eastern and southern Africa: challenges to achieving global HIV targets? Longitudinal analyses of nationally representative surveys. J Int AIDS Soc. (2019) 22:e25329. doi: 10.1002/jia2.25329
10. Katz, MH, Schwarcz, SK, Kellogg, TA, Klausner, JD, Dilley, JW, Gibson, S, et al. Impact of Highly Active Antiretroviral Treatment on HIV Seroincidence Among Men Who Have Sex With Men: Ed. Am J Public Health. San Francisco (2002) 92:388–394. doi: 10.2105/AJPH.92.3.388
12. Basten, M, den Daas, C, Heijne, JC, Boyd, A, Davidovich, U, Rozhnova, G, et al. The rhythm of risk: sexual behaviour, PreP use and HIV risk perception between 1999 and 2018 among men who have sex with men in Amsterdam, the Netherlands. AIDS Behav. (2021) 25:1800–09.
13. Shuper, PA, Pillay, S, MacDonald, S, Christie, S, Cornman, DH, Fisher, WA, et al. One in four HIV-positive South Africans awaiting ART initiation report condomless sex with a serodiscordant partner. J Acquir Immune Defic Syndr. (2016) 72:e77–9. doi: 10.1097/QAI.0000000000001016
14. Wekesa, E . A new lease of life: sexual and reproductive behaviour among PLWHA in the ART era in Nairobi slums. London: London School of Economics and Political Science (2012).
15. Earnshaw, VA, Smith, LR, Shuper, PA, Fisher, WA, Cornman, DH, and Fisher, JD. HIV stigma and unprotected sex among PLWH in KwaZulu-Natal, South Africa: a longitudinal exploration of mediating mechanisms. AIDS Care. (2014) 26:1506–13. doi: 10.1080/09540121.2014.938015
16. Dessie, Y, Gerbaba, M, Bedru, A, and Davey, G. Risky sexual practices and related factors among ART attendees in Addis Ababa public hospitals, Ethiopia: a cross-sectional study. BMC Public Health. (2011) 11:1–10. doi: 10.1186/1471-2458-11-422
17. Diura-Vere, O, Mokgatle, MM, and Oguntibeju, OO. Sexual behaviour and attitudes towards safe sex of youth receiving antiretroviral care at public health facilities in Palapye District, Botswana. Int J Environ Res Public Health. (2023) 20:3790. doi: 10.3390/ijerph20053790
18. Tadesse, WB, and Gelagay, AA. Risky sexual practice and associated factors among HIV positive adults visiting ART clinics in public hospitals in Addis Ababa city, Ethiopia: a cross sectional study. BMC Public Health. (2019) 19:113–8. doi: 10.1186/s12889-019-6438-5
19. Wondemagegn, F, and Berkessa, T. High level risky sexual behavior among persons living with HIV in the urban setting of the highest HIV prevalent areas in Ethiopia: implications for interventions. PLoS One. (2020) 15:e0242701. doi: 10.1371/journal.pone.0242701
20. Volberding, PA, and Deeks, SG. Antiretroviral therapy and management of HIV infection. Lancet. (2010) 376:49–62. doi: 10.1016/S0140-6736(10)60676-9
21. Mattson, CL, Freedman, M, Fagan, JL, Frazier, EL, Beer, L, Huang, P, et al. Sexual risk behaviour and viral suppression among HIV-infected adults receiving medical care in the United States. AIDS. (2014) 28:1203–11. doi: 10.1097/QAD.0000000000000273
22. Conan, N, Simons, E, Chihana, ML, Ohler, L, FordKamara, E, Mbatha, M, et al. Increase in HIV viral suppression in KwaZulu-Natal, South Africa: community-based cross sectional surveys 2018 and 2013. What remains to be done? PLoS One. (2022) 17:e0265488. doi: 10.1371/journal.pone.0265488
23. Moshidi, ML, Malema, RN, Muthelo, L, and Mothiba, TM. Provision of care to the people with HIV: voices of professional nurses in the public hospitals of Limpopo Province, South Africa. Int J Environ Res Public Health. (2021) 18:3112. doi: 10.3390/ijerph18063112
24. Ramathuba, DU, Ramashala, RM, and Mashau, NS. Contributory factors that lead to increase of mother-to-child transmission of HIV in Capricorn district of Limpopo Province, South Africa. HIV AIDS Rev. (2021) 20:39–45. doi: 10.5114/hivar.2021.105111
25. Sibeko, Z.R., Molato, B.J., and Moloko-Phiri, S. (2023). Perceptions of people living with human immunodeficiency virus regarding the use of dolutegravir based regimen, Limpopo Province.
26. Louangrath, P . Sample size determination for non-finite population. SSRN Electr J. (2014) 3:141–52. doi: 10.2139/ssrn.2710455
27. Purvis, CP, Barnett, RV, and Forthun, L. Parental involvement during adolescence and contraceptive use in college. Chattanooga: University of Tennessee at Chattanooga (2014).
28. Madiba, S, and Letsoalo, B. Disclosure, multiple sex partners, and consistent condom use among HIV positive adults on antiretroviral therapy in Johannesburg, South Africa. World J AIDS. (2014) 4:62–73. doi: 10.4236/wja.2014.41008
29. Molla, AA, and Gelagay, AA. Risky sexual practice and associated factors among HIV positive adults attending anti-retroviral treatment clinic at Gondar University referral hospital, Northwest Ethiopia. PLoS One. (2017) 12:e0174267. doi: 10.1371/journal.pone.0174267
30. Yang, X, Yuan, S, Zhang, R, Yu, J, Nzala, SH, Wang, P, et al. Risky sexual behaviors and associated factors among college students in Lusaka, Zambia. Arch Sex Behav. (2019) 48:2117–23. doi: 10.1007/s10508-019-1442-5
31. Tolmay, J, Knight, L, Muvhango, L, Polzer-Ngwato, T, Stöckl, H, and Ranganathan, M. Women’s economic contribution, relationship status and risky sexual behaviours: a cross-sectional analysis from a microfinance-plus programme in rural South Africa. AIDS Behav. (2022) 26:2349–62. doi: 10.1007/s10461-021-03566-5
32. Madiba, S, and Ngwenya, N. Cultural practices, gender inequality and inconsistent condom use increase vulnerability to HIV infection: narratives from married and cohabiting women in rural communities in Mpumalanga province, South Africa. Glob Health Action. (2017) 10:1341597. doi: 10.1080/16549716.2017.1341597
33. Pappin, M, Wouters, E, and Booysen, FL. Anxiety and depression amongst patients enrolled in a public sector antiretroviral treatment programme in South Africa: a cross-sectional study. BMC Public Health. (2012) 12:244. doi: 10.1186/1471-2458-12-244
34. Asfaw, S, Demissie, K, Kiros, G, and Abebe, L. Sexual behaviors and associated factors among antiretroviral treatment attendees in Ethiopia. HIVAIDS. (2015) 7:183–90. doi: 10.2147/HIV.S78300
35. Akinyi, B, Odhiambo, C, Otieno, F, Inzaule, S, Oswago, S, Kerubo, E, et al. Prevalence, incidence and correlates of HSV-2 infection in an HIV incidence adolescent and adult cohort study in western Kenya. PLoS One. (2017) 12:e0178907. doi: 10.1371/journal.pone.0178907
Keywords: PLHIV, risky sexual behaviors, married, education, South Africa
Citation: Ntimana CB, Mashaba RG, Seakamela KP, Netshapapame T and Maimela E (2023) Risky sexual behaviors and associated factors among adult patients on antiretroviral treatment at Mankweng Hospital in Limpopo Province, South Africa. Front. Public Health. 11:1245178. doi: 10.3389/fpubh.2023.1245178
Edited by:
Aida Bianco, University Magna Graecia of Catanzaro, ItalyReviewed by:
Alexandra M. Bitty-Anderson, African Research Center in Epidemiology and Public Health (CARESP), TogoMagarsa Lami, Haramaya University, Ethiopia
Copyright © 2023 Ntimana, Mashaba, Seakamela, Netshapapame and Maimela E. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cairo B. Ntimana, Y2Fpcm8ubnRpbWFuZUB1bC5hYy56YQ==
 Cairo B. Ntimana
Cairo B. Ntimana Reneilwe G. Mashaba
Reneilwe G. Mashaba Kagiso P. Seakamela
Kagiso P. Seakamela Tshifhiwa Netshapapame
Tshifhiwa Netshapapame Eric Maimela
Eric Maimela