
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 07 December 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1244482
This article is part of the Research TopicWorld Antimicrobial Awareness WeekView all 25 articles
 Jens Thomsen1,2
Jens Thomsen1,2 Najiba M. Abdulrazzaq3
Najiba M. Abdulrazzaq3 the UAE AMR Surveillance Consortium‡
the UAE AMR Surveillance Consortium‡ Dean B. Everett2,4,5†
Dean B. Everett2,4,5† Godfred Antony Menezes6†
Godfred Antony Menezes6† Abiola Senok7,8†
Abiola Senok7,8† Carole Ayoub Moubareck9*†
Carole Ayoub Moubareck9*†Background: Carbapenem-resistant Enterobacterales (CRE) are spreading in the United Arab Emirates (UAE) where their dissemination is facilitated by international travel, trade, and tourism. The objective of this study is to describe the longitudinal changes of CRE as reported by the national AMR surveillance system of the UAE.
Methods: In this study, we retrospectively describe CRE isolated from 317 surveillance sites, including 87 hospitals and 230 centers/clinics from 2010 to 2021. The associated clinical, demographic, and microbiological characteristics are presented by relying on the UAE national AMR surveillance program. Data was analyzed using WHONET microbiology laboratory database software (http://www.whonet.org).
Results: A total of 14,593 carbapenem resistant Enterobacterales were analyzed, of which 48.1% were carbapenem resistant Klebsiella pneumoniae (CRKp), 25.1% carbapenem resistant Escherichia coli (CREc), and 26.8% represented 72 other carbapenem resistant species. Carbapenem resistant strains were mostly associated with adults and isolated from urine samples (36.9% of CRKp and 66.6% of CREc) followed by respiratory samples (26.95% for CRKp) and soft tissue samples (19.5% for CRKp). Over the studied period carbapenem resistance rates remained high, especially in K. pneumoniae, and in 2021 were equivalent to 67.6% for imipenem, 76.2% for meropenem, and 91.6% for ertapenem. Nevertheless, there was a statistically significant decreasing trend for imipenem and meropenem resistance in Klebsiella species (p < 0.01) while the decrease in ertapenem resistance was non-significant. Concerning E. coli, there was a statistically significant decreasing trend for meropenem and imipenem resistance over the 12 years, while ertapenem resistance increased significantly with 83.8% of E. coli exhibiting ertapenem resistance in 2021. Resistance rates to ceftazidime and cefotaxime remained higher than 90% (in 2021) for CRKp and cefotaxime rates increased to 90.5% in 2021 for CREc. Starting 2014, resistance to colistin and tigecycline was observed in carbapenem resistant Enterobacterales. CRE were associated with a higher mortality (RR: 6.3), admission to ICU (RR 3.9), and increased length of stay (LOS; 10 excess inpatient days per CRE case).
Conclusion: This study supports the need to monitor CRE in the UAE and draws attention to the significant increase of ertapenem resistance in E. coli. Future surveillance analysis should include a genetic description of carbapenem resistance to provide new strategies.
Nowadays, carbapenem-resistant Enterobacterales (CRE) represent a serious health concern worldwide, causing a distressing burden on morbidity, mortality and healthcare costs, and contributing to the socio-economic and public health consequences of antimicrobial resistance (1, 2). Gram-negative, rod-shaped, facultatively anaerobic bacteria inhabiting the gastrointestinal tract, Enterobacterales (formerly Enterobacteriaceae) represent the largest group of bacterial pathogens in humans (3, 4). They are associated with a wide range of severe infections including septicemia, urinary tract infections (UTIs), intra-abdominal infections, and pneumonia, which can be community-acquired, hospital-acquired, or ventilator-associated (5–9). The widespread, empiric use of carbapenems as the most reliable antibiotics for the treatment of infections caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacterales has driven the emergence of CRE, whose infections are more challenging to treat (10). According to the Centers for Disease Control and Prevention (CDC), CRE are defined as Enterobacterales strains that test resistant to at least one of the carbapenem antibiotics (ertapenem, meropenem, doripenem, or imipenem) or produce a carbapenemase (11). CRE acquire resistance to carbapenems via efflux pump overactivity, loss or mutation of outer membrane proteins, and/or carbapenemase production, the latter being the most prevalent mechanism (12, 13). With increasing incidence of infections caused by CRE and the lack of new, approved treatment modalities, such infections are associated with worse outcomes, lengthier hospitalizations, and increased costs compared to their susceptible counterparts (14). CRE continue to be labeled as critical priority pathogens by the World Health Organization (WHO), and the necessity for discovery, research, and development of new antibiotics targeting these pathogens remains an urgent need (15).
The global spread of CRE and changes in their epidemiology continue to evolve, inevitably complicating therapy and hampering effective antimicrobial stewardship and infection prevention and control programs (1, 16). In general, longitudinal studies of antibiotic susceptibility in a specific region over time allow identification of trends of resistance and emerging pathogens at national levels. Such routine surveillance is key for generating and establishing approaches to control antimicrobial resistance and guide informed therapy decisions (16), and appears critical as far as CRE are concerned (17). The trends obtained will detect either a rise in CRE prevalence (18, 19), thus revisiting and improving the current infection control strategies, or its decline (20), thus reinforcing the possible beneficial factors. The United Arab Emirates (UAE), a thriving hub for international travel, trade, tourism and medical services, has been susceptible to CRE spread, like many other countries in the Arabian Peninsula (21). Currently, the country hosts a population of nearly 10 million people of which approximately 1 million are Emirati citizens, and the rest are expatriates from various nationalities. The majority of this population resides in Abu Dhabi and Dubai, the two biggest Emirates of the seven that form the UAE (22).
Previous data have described the epidemiology and resistance patterns of CRE from the UAE, the latest of which being the study by Pál et al. (17), which compared CRE collected between 2009 and 2015 to those collected between 2018 and 2019 in the Emirate of Abu Dhabi. The study revealed that highly resistant Klebsiella pneumoniae clones started dominating the area since 2009, severely impacting the overall antibiotic resistance patterns, including those of colistin and tigecycline. Moreover, a recent surveillance of CRE carried out over 9 months in 15 Emirati hospitals showed around 100% non-susceptibility to ertapenem and 80% non-susceptibility to each of imipenem and meropenem (23). Likewise, resistance rates of 100% to ertapenem, 21% to imipenem, and 17% meropenem were observed in a collection of Enterobacterales in an epidemiological investigation from Dubai (24). Smaller scale investigations of CRE in the UAE also reported clusters of NDM-1-producing Enterobacterales (25), and more recently of K. pneumoniae with OXA-181/NDM-5 carbapenemases (26). The accumulation of such body of evidence supports the notion that timely, focused, and systematic, surveillance could offer a possible guidance to health authorities to mitigate the countrywide progress of the CRE epidemic. As such, it is imperative to address the current gap in literature regarding the spread of CRE infections and their resistance trends over the years, especially given the multicultural, heterogeneous, and diverse nature of the UAE population.
The objective of the current study is to describe the characteristics and longitudinal changes in CRE resistance levels and trends as reported by the national AMR surveillance system spanning all the seven emirates of the UAE, in order to assess the nationwide status of the CRE epidemic. It represents the first documentation of changes in CRE isolated from UAE medical centers over a period of 12 years, from 2010 to 2021.
A multi-institutional retrospective observational study was conducted between 2010 and 2021 in the UAE using data extracted from the WHONET microbiology laboratory database software (www.whonet.org) supported by the Global AMR Surveillance System protocol (GLASS, World Health Organization). Data was generated, collected, cleaned, and analyzed through the UAE national AMR Surveillance programs as described by Thomsen et al. (27).
Starting 2010, UAE healthcare institutions were enrolled as AMR surveillance sites into the UAE national AMR surveillance program based on epidemiological needs assessment, readiness, and willingness of facilities to participate, availability of high-quality electronic AMR data, lab accreditation status, and qualification of staff. Hospitals, centers, and clinics representing all seven emirates of the UAE joined the AMR surveillance network gradually over the years.
All Enterobacterales isolated from clinical samples by medical professionals in the national AMR surveillance sites were part of this surveillance analysis from January 2010 to December 2021. Repeat isolates were marked and only the first isolate was included for each patient per year.
The associated patient demographic information, clinical data, and microbiologic laboratory results were extracted from the national WHONET laboratory database software. The demographic variables included age, sex, nationality, clinical variables revealed the type of facility reporting the isolate (hospital/center/clinic), patient location, location type, specimen collection date, types of infection/specimen source, and microbiology variables revealed types of organism and antibiotic susceptibility testing results. The infection was considered to originate outside the center for outpatients or those presenting with the infection at the emergency department.
Bacterial identification was performed at the national AMR surveillance sites by medical professionals. The participating centers used at least one commercial, automated system for identification of bacteria, including VITEK® (BioMérieux SA, Craponne, France), BD Phoenix™ (Becton Dickinson, New Jersey, USA) and MicroScan (Beckman Coulter, Brea, CA, USA). Only one lab relied on manual systems like API® (Analytical Profile Index. BioMérieux SA, Craponne, France) solely for identification.
Antimicrobial susceptibility testing was performed at the National AMR surveillance sites using at least one commercial, automated system for routine antimicrobial susceptibility testing. Only two laboratories used manual testing methods (disc diffusion/Kirby Bauer). All labs followed CLSI guidelines for antimicrobial susceptibility testing of bacteria (CLSI-M100) (28). The EUCAST guidelines were used for interpretation of tigecycline results (29). Unusual antibiotic susceptibility testing results were confirmed locally. To assess the multidrug-resistant (MDR) phenotype of the isolates, a slightly modified version of the standard definition by Magiorakos et al. (30) was used.
Strains of CRE were defined as Enterobacterales species such as K. pneumoniae, E. coli, Klebsiella oxytoca, Enterobacter cloacae, and Enterobacter aerogenes and others that are resistant to at least one carbapenem antibiotic or produce a carbapenemase enzyme. Proteus spp., Morganella spp., and Providencia spp. that have intrinsic elevated minimum inhibitory concentrations to imipenem but are susceptible to ertapenem and/or meropenem were not counted. Repeat isolates were marked and only the first ones expressing any distinct carbapenem resistance mechanisms were included for each patient during the surveillance period (2010–2021).
Statistical significance of temporal trends for antimicrobial resistance percentages was calculated if data from at least 5 years was available. If fewer than 30 isolates per year were reported, or data was not available for all years within the considered period, trend analysis was not conducted. Statistical significance of trends is expressed as a p-value, calculated by a Chi-square for trend test (extended Mantel-Haenszel), using SPSS or Epi Info™. For testing the statistical significance of the difference for mortality and ICU admission a Chi2-test was used. For testing the statistical significance of the difference for length of stay (LOS), the weighted log-rank survival analysis was used. This was done to take care of differences in sample size between the groups. A p < 0.05 was considered statistically significant.
The UAE national AMR surveillance program was in 2010 in the Abu Dhabi Emirate with six hospitals and 16 centers/clinics enrolled as AMR surveillance sites. Additional sites were recruited over the years, starting with 22 participating sites located only in the Emirate of Abu Dhabi in 2010, which is the first year during which the study was initiated, and reaching in 2021 a total of 317 surveillance sites, including 87 hospitals and 230 centers/clinics and representing all seven emirates of the country. Figure 1 represents the distribution of reporting sites for National AMR Surveillance from 2010 to 2021.
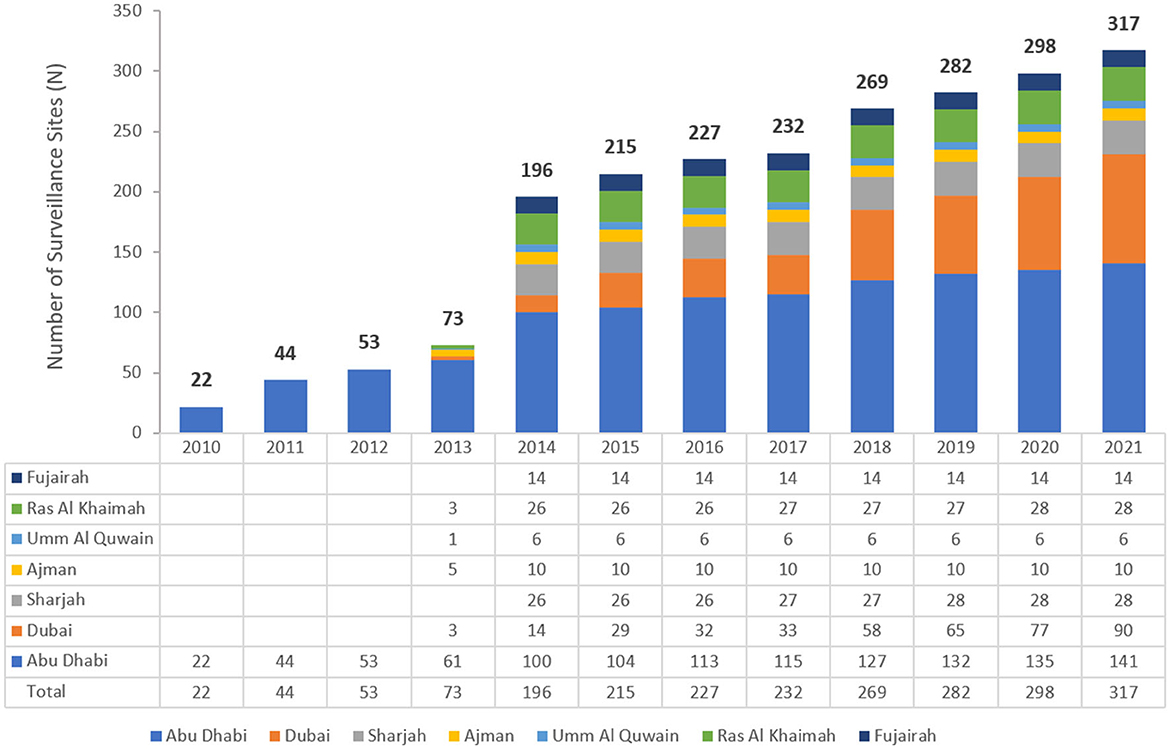
Figure 1. Number of AMR Surveillance sites by Emirate over the years of the surveillance period (2010–2021) in the UAE.
From 2010 to 2021, a total of 381,535 non-repetitive Enterobacterales were included in the analysis of which 14,593 (3.8%) were carbapenem resistant (CRE), representing 74 different species. Figure 2 represents the percentage of CRE and non-CRE isolates per year.
Figure 3 represents the prevalence of CRE calculated per year during the 12 years of the study. A gradual rise in this prevalence was seen from 2010 (0.2%) and for 4 consecutive years until 2014 (3.7%). Starting 2014, CRE prevalence was oscillating between 3.4 and 4.2%, with a steady decrease between 2016 and 2020, then showing a tendency to increase noted in 2021. The overall prevalence of CRE over the 12 years of surveillance averages at 3.8%.
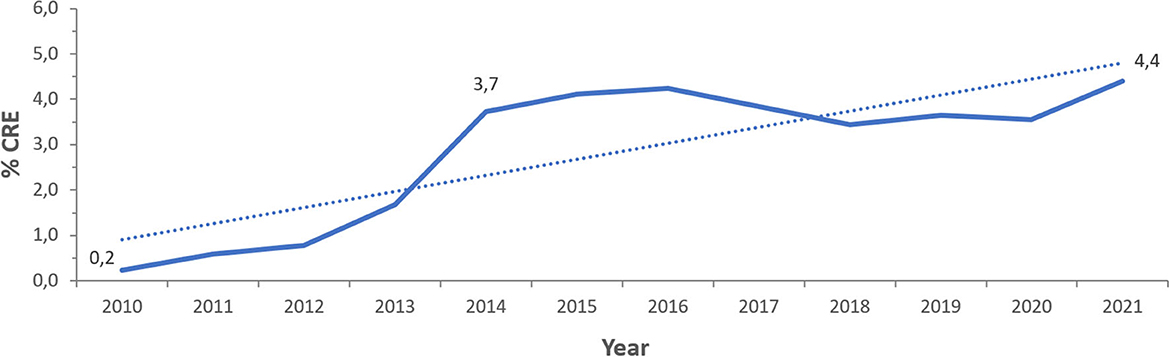
Figure 3. Time trends of CRE prevalence (% CRE) in the UAE over the surveillance period (2010–2021).
Among the 14,593 carbapenem resistant Enterobacterales isolates analyzed, 7,023 (48.1%) were carbapenem resistant K. pneumoniae (CRKp), 3,668 (25.1%) were carbapenem resistant Escherichia coli (CREc), and the remaining 3,902 (26.8%) isolates represented 72 other species. The CRE species distribution over the surveillance period is shown for species with at least 10 isolates in Figure 4.
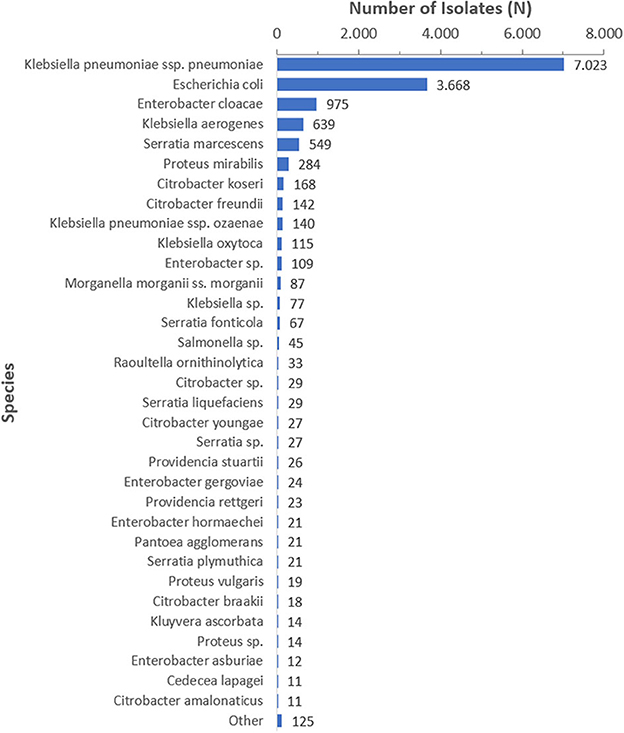
Figure 4. CRE species distribution over the surveillance period (2010–2021), by species (N = 14,593), species is shown for n ≥ 10.
Carbapenem resistant strains were mostly associated to infections in adults (89.6% of patients) with an average of 93.7 and 85.5% of infections caused, respectively, by CRKp and CREc in that population group. The number of CRE isolates recovered from patients below 19 years increased from two isolates in the first year of the study to 265 for CRKp and 373 for CREc in the last year.
The most commonly isolated species of CRE being CRKp, looking into features of these isolates revealed they were mostly recovered from male patients (57%). Patients were of unknown nationality (47.9%), although 16.3% of the patients were Emirati citizens. The most frequent Emirate for isolation of CRKp was Abu Dhabi (31.8%). Most patients developing infections due to CRKp were detected in clinical settings (83.9%) and were enrolled in general medical wards (48.1%) followed by ICUs (30.1%) and critical care units (1.2%). A proportion of 20.5% of studied isolates originated in outpatient basis, being recovered either in the community or in emergency departments.
Following CRKp, the second most frequent CRE species was CREc, commonly isolated from females (65.2%), of unknown nationality (44.4%), and in the Emirate of Abu Dhabi (43.4%). Most patients developing infections due to CREc were detected in clinical settings (50%) and were enrolled in general medical wards (31.8%) followed by ICUs (11%) and critical care units (0.2%). A proportion of 57% of studied isolates originated in outpatient basis, being recovered either in the community or in emergency departments.
A subgroup analysis including the nine clinical institutions that reported mortality was performed. In these institutions, a total of 101,762 patients were associated with non-CRE of whom 3,717 patients died (mortality rate: 3.65%), while a total of 1,824 were associated with CRE, of whom 389 patients died (mortality rate: 21.33%). The difference in mortality between CRE patients and non-CRE patients is statistically significant (RR 6.31, 95% C.I. 5.74, 6.93, p < 0.01).
A total of 249,844 patients were associated with non-CRE of whom 13,567 patients were admitted to ICU (ICU admission rate is 5.43%), while a total of 10,011 patients were associated with CRE, of whom 2,142 patients were admitted to ICU (ICU admission rate: 21.40%). The difference in ICU admission rate is statistically significant (RR 3.94, 95% C.I. 3.78, 4.11, p < 0.01).
A subgroup analysis including those patients for whom the date of admission as well as the date of discharge was known was performed (N = 34,195). For those patients who were associated with non-CRE (n = 33,462) the median length of stay was 7.0 days, while for those patients who were associated with CRE (n = 733) the median length of stay was 17.0 days, equivalent to 7,330 excess days of hospitalization. The difference in length of stay (LOS) was equal to 10 days and was statistically highly significant (p < 0.001).
After applying the above-mentioned difference in the LOS on the total number of patients associated with CRE (n = 14,593) during the whole observation period (2010–2021), a total of 145,930 excess days of hospitalization is estimated attributable to CRE. For the year 2021 only (n = 3,448 CRE cases), a total of 34,480 excess hospitalization days is estimated attributable to CRE.
Carbapenem resistant strains were mostly isolated from urine samples (36.85% of CRKp and 66.55% of CREc) followed by sputum samples (26.95% of CRKp and 6.35% of CREc) and soft tissue samples (19.52% of CRKp and 13.79 % of CREc) as described in Tables 1, 2.
Resistance rates to cefotaxime increased from 87.2% (n = 179) in 2014 to 95.8% (n = 612) in 2021 for CRKp and from 76.3% (n = 59) in 2014 to 90.5% (n = 243) in 2021 for CREc. The trends of resistance in CRKp and CREc to different antibiotics for a selected time frame of the surveillance period are shown in Figures 5–8. It is noticed that the resistance to the antibiotics tested did not change much for CRKp over these years, while a more fluctuating pattern was seen for CREc, especially for cefotaxime and piperacillin/tazobactam. Regarding colistin, sensitivity fluctuated over the years but notably increased in CRKp after 2017, and it remained above 80% toward the end of the data collection period. Fosfomycin resistance levels were the lowest, with maximum upper limit of 8.6% in 2019 for CREc, while resistance levels for CRKp persisted close to zero with isolates remaining highly sensitive to this antibiotic all over the years.
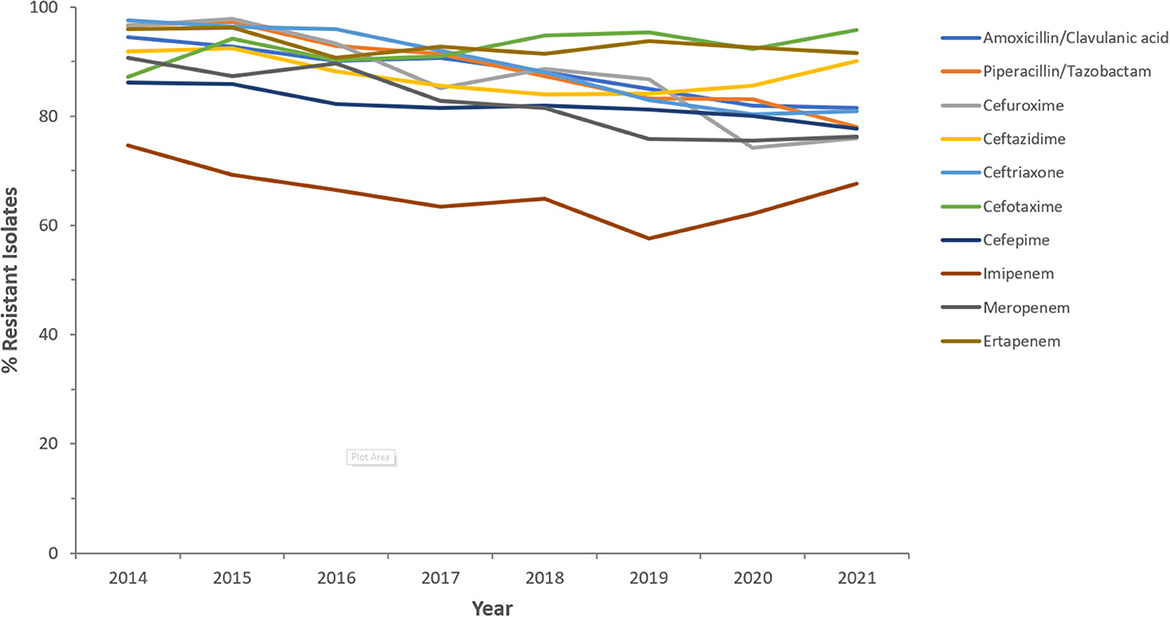
Figure 5. Resistance patterns (%R) to antibiotics of the β-lactam group among CRKp for the period between 2014 and 2021. The graph shows a selected period due to small number of participating centers prior to 2014.
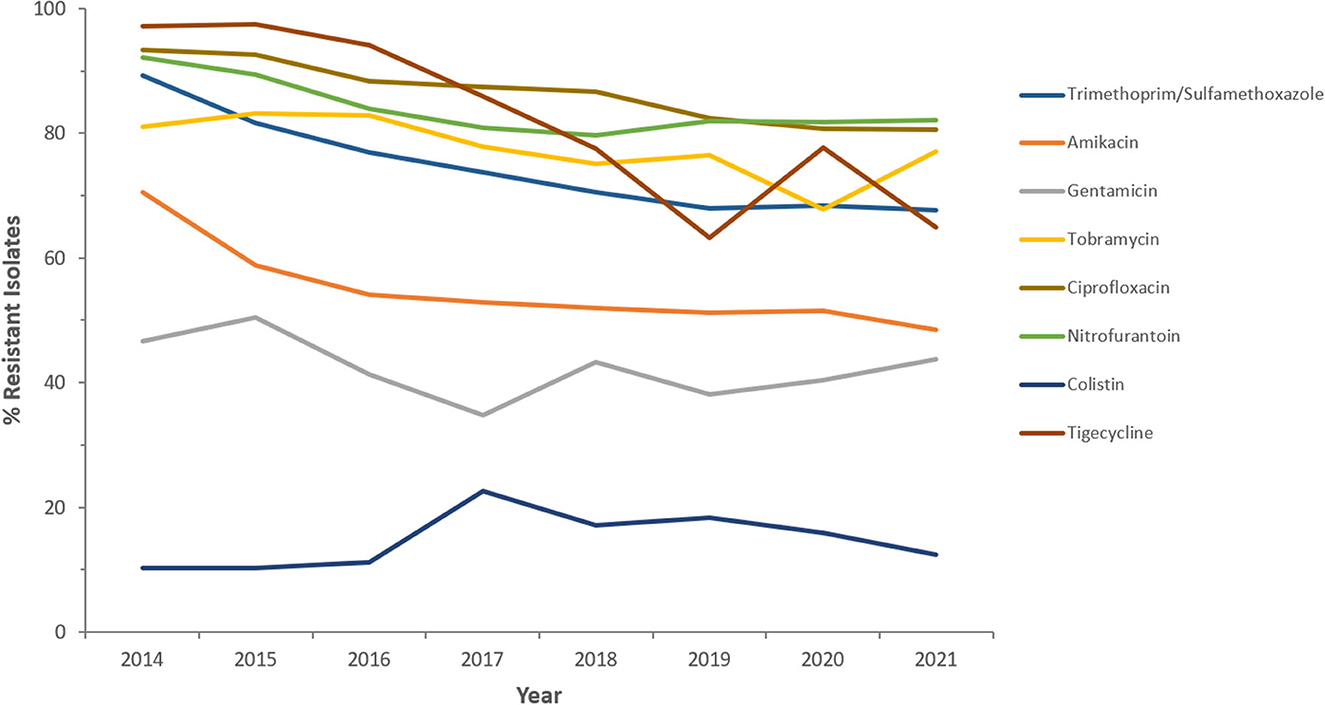
Figure 6. Resistance patterns (%R) to non β-lactam antibiotics among CRKp for the period between 2014 and 2021. The graph shows a selected period due to small number of participating centers prior to 2014.
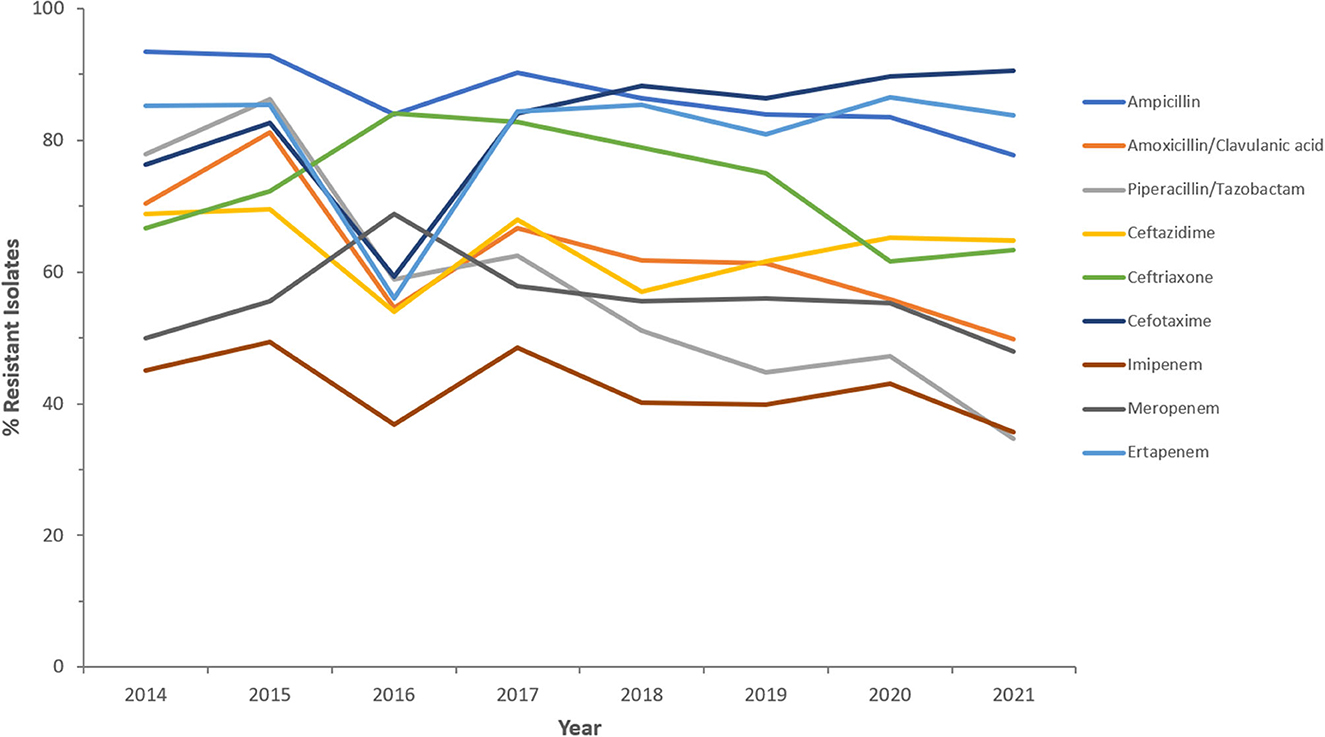
Figure 7. Resistance patterns (%R) to antibiotics of the β-lactam group among CREc for the period between 2014 and 2021. The graph shows a selected period due to small number of participating centers prior to 2014.
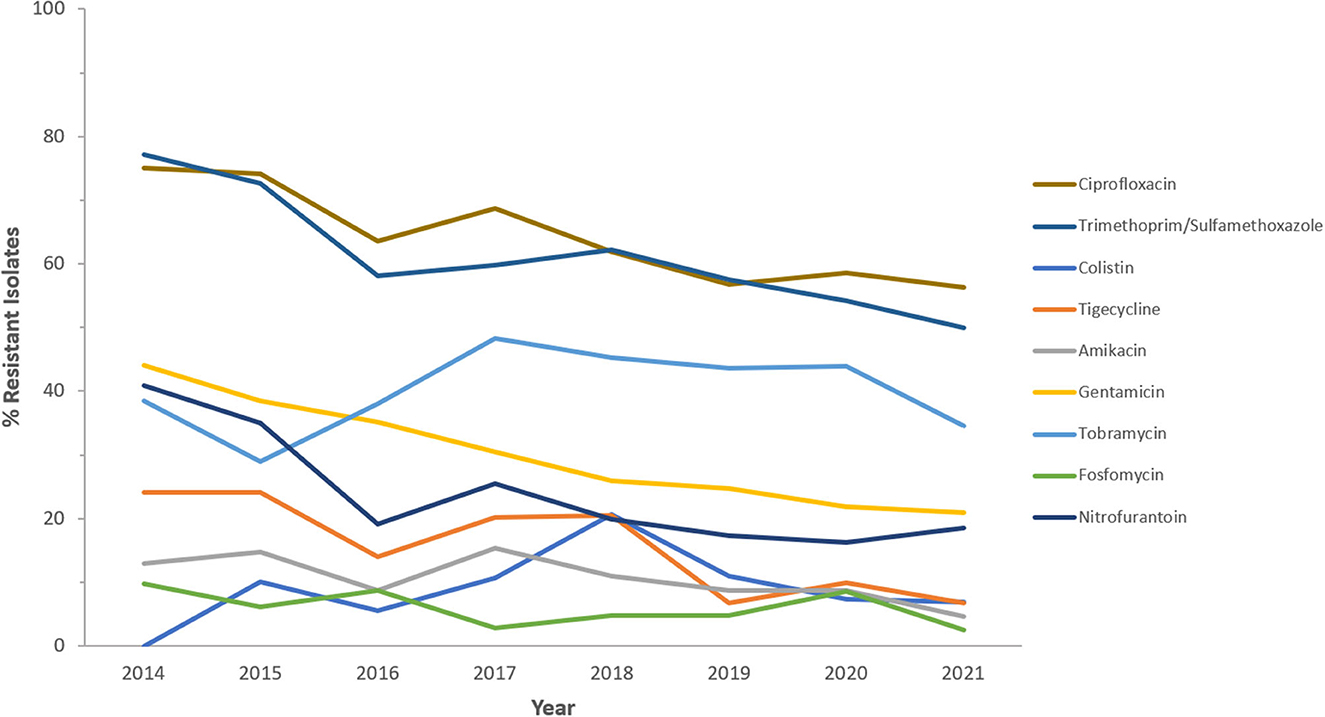
Figure 8. Resistance patterns (%R) to non β-lactam antibiotics among CREc for the period between 2014 and 2021. The graph shows a selected period due to small number of participating centers prior to 2014.
Over the surveillance period, the resistance rates to individual carbapenems remained high, especially in CRKp, and in 2021 were equivalent to 67.6% for imipenem, 76.2% for meropenem, and 91.6% for ertapenem. Concerning CREc, resistance rates to meropenem and ertapenem were oscillating around 58 and 83% respectively over the 12 years, while for imipenem a progressive decrease was noted from 45.1% (n = 91) in 2014 to 35.6% (n = 932) in 2021.
Statistical analysis revealed a significant decreasing trend for imipenem and meropenem resistance in Klebsiella species (p < 0.01) while the decrease in ertapenem resistance was non-significant. Concerning E. coli, there was a statistically significant decreasing trend for meropenem and imipenem resistance over the 12 years while ertapenem resistance was associated to a statistically significant increasing trend with 83.8% of E. coli exhibiting ertapenem resistance in 2021.
A total of 1,002 CRKp and 982 CREc was tested for ceftolozane/tazobactam susceptibility and results revealed, respectively 65.6 and 57.9% resistance to this β-lactam/β-lactamase inhibitor combination. A total of 913 CRKp and 146 CREc was tested for ceftazidime/avibactam susceptibility and results revealed, respectively, 62.7 and 65.8% resistance to that second combination.
This study was carried out to assess the contemporary trends of carbapenem resistance among Enterobacterales of medical relevance in the United Arab Emirates (UAE) over a 12-year period. The follow-up of CRE, characterized by mobile, easily transmissible resistance determinants, as well as easy spread facilitated by international travel and medical tourism, is imperative for infection surveillance and control in a country with huge cross-cultural exchange like the UAE. The participation of healthcare sites, both hospitals and clinics, in contribution to Enterobacterales data increased over the years from only 22 centers in the first year of reporting to more than 300 sites toward the end of the study period, representing the seven Emirates. This reflects not only the increasing coverage and representation of the surveillance database, but probably also the increased alertness across the country to the importance of antimicrobial resistance surveillance and mitigation.
The overall prevalence of CRE over the 12 years of surveillance averages at 3.8%. This result should be interpreted by comparison with figures of resistance obtained from similar follow-up studies that monitored carbapenem resistance for longitudinal periods, especially those from the region, due to patient and cultural exchange that connects these countries with the UAE. For example, in a surveillance from Africa and Middle East, a rate of 5.7% of resistance among Enterobacterales to carbapenems was reported (31). A report of antimicrobial resistance trends in Lebanon over 10 years, from 2000 till 2010, showed carbapenem resistance rates among E. coli and K. pneumoniae that did not exceed 2% (32). In a more recent analysis in 2022, the rate in Lebanon was 2.8% (33), while in Jordan, the rate was 1.6% in 2015 according to the Study for Monitoring Antimicrobial Resistance Trends (SMART) (34), and 1% in another study of 5 hospitals in 2018 (35). A version of the SMART study in Asia-Pacific region from 2002 to 2010 showed an overall carbapenem resistance rate of 10% among Enterobacterales (36). A recent surveillance report from Saudi Arabia, a neighboring country, showed resistance rates of about 5% to carbapenems among these bacteria (37). Another 5-year surveillance study from the Kingdom of Bahrain showed CRE average incidence of approximately 23/10,000 hospital admissions, with a decrease noted in the last two study years due to development and implementation of new CRE policy based on initial CRE screening for high risk patients, reinforcement of contact precautions, strengthened communication about CRE across hospital units, and staff education (38). As such, UAE, like other countries in the region, is facing the challenge of an important number of reported cases of CRE. Hence, update and follow-up on the prevalence, epidemiology and microbiological characteristics of CRE is mandatory for adequate public health and infection control practices.
Between 2013 and 2014, CRE prevalence increased from 1.7 to 3.7%, whereas from 2016 to 2020, CRE resistance prevalence shows a slightly decreasing pattern, which triggers the exploration of what factors resulted in such changes? In June 2013, the Health Authority of Abu Dhabi issued a circular on CRE, which has alerted healthcare facilities in the Emirate of Abu Dhabi and may have led to an increased detection of carbapenem-resistant pathogens, hence the increased prevalence of CRE in 2014. The number of surveillance sites in 2018 increased by 37 compared to 2017, but CRE prevalence in 2018 declined by 0.4 and 0.8% compared to 2017 and 2016, respectively. This decline cannot be directly explained from our results but warrants investigation of any national policies that may have produced such effect in these 2 years. In December 2017, the Department of Health of Abu Dhabi issued a standard and a guideline for antimicrobial stewardship (ASP), which may have contributed to improved prevention and control of multi-drug resistant organisms, including CRE, in the Emirate of Abu Dhabi. In 2019 and 2020, almost a steady pattern is observed, which tends to raise again in 2021, warranting to explore the effect of the COVID-19 pandemic on such changes. Previous data from other countries have reported a decline in CRE in the wake of the global pandemic (39, 40), and factors that may explain such decline like improved hygiene, social distancing, reduced travel, constricted transfer of critically ill patients, and others, have been described (41), although precise data in this regard remain conflicting (42). Moreover, the mortality rate, according to our observations, was about six-fold higher in patients associated with CRE compared to those associated with non-CRE Enterobacterales. Patients associated with CRE were four-fold more likely to be admitted to ICU, and their median length of stay was increased by 10 days, as compared to patients associated with non-CRE. This is consistent with other findings that indicated high mortality rate and poor outcomes in patients with CRE (43, 44), and highlights need for surveillance and control for better health outcomes.
When looking into the age of the population affected by CRE, it was found that over the study period, almost 90% of the patients with CRE samples were adults aged above 19 years. It is worth mentioning that the number of CRE isolates recovered from patients below 19 years increased from two isolates in the first year of the study to over 250 for CRKp and over 350 for CREc in the last year. Whether such an increase is due to resistance spread in the pediatric patients or merely due to increased inclusiveness of our samples by more centers getting involved, cannot be accurately determined, but indeed, warrants attention to monitor CRE in pediatrics. Although studies exist on CRE infections in pediatric patients (45–47), the true prevalence and proportionality to adult infections remains to be identified. One study reported that the frequency of carbapenem resistance among Enterobacterales in children in the United States raised from 0% in 1999–2000 to 0.47% in 2010–2011 (48). While the therapeutic paradigms for CRE have evolved with the introduction of novel β-lactam/β-lactamase inhibitor combinations like ceftazidime/avibactam, meropenem/vaborbactam, and imipenem/cilastatin-relebactam, optimal treatment of CRE infections in children remains challenging given limited pediatric-specific clinical data and experience (49). With the complexity of CRE treatment in children, and the need for expert consultation and individualized approach, our results call for a more meticulous surveillance of these infections in children while they are still limited, in a way to benefit from time until treatment paradigms evolve and new agents in the antibiotic pipeline become available and well-studied in pediatrics.
The majority of CRE identified throughout the study period were recovered from urine samples followed by sputum then blood. These data are somehow in alignment with other studies (50) but are unlike results of some large-scale multicenter studies from China and Taiwan reporting the highest number of CRE infections to originate in the lower respiratory tract (51–53). Urine samples may have outnumbered other samples in the UAE since most of the participating centers were public or private clinics rather than tertiary care centers, and these clinics may have urine as the easiest and most convenient sample. This highlights the possible community spread of these strains, that has been already reported elsewhere (6, 54), and warrants close monitoring in the UAE.
Throughout the study, CRKp remained the most prevalent CRE isolated from the studied samples, with an increase in its numbers consistently shown across the years. Pathogenic strains of K. pneumoniae cause widely diverse infectious diseases, including urinary tract, respiratory tract and blood infections, and are known as key menace to public health, being a common agent of nosocomial and community acquired infections (55). The results obtained regarding the demographic features of patients from whom CRKp isolates were recovered, together with antimicrobial susceptibility profiles, add to previous longitudinal data on CRKp in other countries over several years, like those from China (56–58), Singapore (59), Italy (60), and Germany (61). As a first time-trend study in the UAE, it will be beneficial to capitalize on these data for further surveillance of CRKp, and to try to associate its infections with particular risk factors. Our results did not reveal molecular epidemiology of the strains, a highly demanding task given the large number of samples and the long study period, but such properties, indeed, are tempting to analyse. So far, carbapenemase production, especially the Ambler class A K. pneumoniae carbapenemase (KPC) and the Ambler class B metallo-β-lactamases (MBL) like IMP, VIM, and NDM constitute the basic molecular mechanisms of CRKp emergence (12). According to recent evidence, knowledge of the exact mechanism of CRKp emergence is crucial to select an appropriate antimicrobial agent among choices such as plazomicin, eravacycline, temocillin, cefiderocol, ceftolozane/tazobactam, imipenem/cilastatin/relebactam, meropenem/vaborbactam, ceftazidime/avibactam, or aztreonam/avibactam (62). For instance, meropenem/vaborbactam combination is known for its effectiveness against KPC producers, ceftazidime/avibactam against both KPC and OXA-48 producers, and cefiderocol against MBL producers (63). It is anticipated that if resistance mechanism data support the phenotypic and demographic characteristics of CRKp, a better guide into antimicrobial therapy selection for these strains in the UAE can be established.
Regarding CREc and its isolation mostly from urine samples of outpatients, especially adult females, these are trends consistent with previously reported data about this organism (64–66). They may relate to its association with urinary tract infections (67, 68), which are among the most common infections worldwide, with substantial morbidity, mortality, and economic burden (69). Due to the physiological and structural factors, women are more vulnerable to urinary tract infections and almost half of them will experience at least one episode during their lifetime (70). In addition, the prevalence of the infection increases with age, weak immune system, and low estrogen levels (71). The high empiric use of antibiotics for the treatment of urinary tract infections has driven antibacterial resistance in E. coli (72), and this is not an exception in UAE, with its mixed and fluctuating population. Perhaps a more thorough investigation of carbapenem resistance in this organism by molecular and genomic methods will add to the available data from this 12-year long follow-up to better understand and mitigate carbapenem resistance in this organism.
Looking into the carbapenem resistance rates for specific CRE, it was noticed that in 2021, K. pneumoniae were to 67.6, 66.2, and 91.6% resistant to imipenem, meropenem, and ertapenem, respectively. There is a need to activate and reinforce stewardship programs and infection control to reduce further raise in carbapenem resistance in K. pneumoniae in the UAE. For imipenem, a progressive decrease in resistance among CREc was noted, reaching 35.6% in 2021, and is similar to some other studies describing trends in other areas in the region like Iraq and Jordan (73). This observation is interesting and emphasizes the effectiveness of infection control programs and the importance of targeted antimicrobial stewardship programs in reducing resistance rates.
Apart from carbapenems, and for other antibiotic/antibiotic combinations tested throughout the study, resistance was high especially in CRKp, and showed a heterogeneous pattern for CREc. Nevertheless, both pathogens remained sensitive to fosfomycin, known for effectiveness in urinary tract infections caused by resistant E. coli and K. pneumoniae strains (74–76). However, in light of the recent observations of acquired fosfomycin resistance in these pathogens (77–79), practitioners in the UAE should remain vigilant about the use of this antibiotic to preserve its effectiveness. Moreover, CREc remained, throughout the study, sensitive to tigecycline, which persists among the last resort options for CRE (80, 81). Likewise, emerging reports of increased resistance in E. coli to this antibiotic (82, 83), as well as of hypervirulent K. pneumoniae which is tigecycline non-susceptible (84, 85) highlight the urgent need to enhance clinical awareness regarding this issue, the responsible use of tigecycline, and continuous epidemiologic surveillance to prevent compromising the usefulness of this antibiotic. Also, and with spread of mobilized colistin resistance genes (mcr) among Gram-negative pathogens (86) and reports from surrounding regions (87) as well as national observations (88), care must be taken to advance the knowledge about colistin resistance while supporting the efforts toward better stewardship to maintain clinical utility of this antibiotic. Moreover, the increase in MDR phenotype recovery in CRKp over the study years, being resistant to at least three antibiotic classes, indicates the need for follow-up, and both species need to be monitored in this regard, given the paucity of treatment options with multi-resistance (Figure 9).
In summary, this manuscript shows the trend over time of carbapenem resistance rates in the UAE among Enterobacterales and points out important findings for research and follow-up. It also shows that CRE infections are associated with higher mortality, increased ICU admission rates, and a longer hospitalization, thus poorer clinical outcome and higher associated costs. The phenotypic and demographic resistance profiles of CRE remain dynamic, and should be continuously updated, as well as supported by molecular epidemiology and genomic data, to help diminish the spread of these isolates across the UAE.
The datasets presented in this article are not readily available because the national AMR Surveillance database managed by the UAE Ministry of Health and Prevention (MOHAP) contains confidential health information. Requests to access the datasets should be directed to the UAE Ministry of Health and Prevention (https://mohap.gov.ae/).
Ethical approval for this study was provided by the Ministry of Health and Prevention Research Ethics Committee (MOHAP/DXB-REC/D.D.D/No.131/2021 and MOHAP/DXB-REC/J.J.J./No. 86/2023), Dubai Scientific Research Ethics Committee (DSREC-GL17-2023), and Abu Dhabi Health Research and Technology Ethics Committee (DOH/ZHCD/2023/1316).
Conceptualization and data interpretation: CA, JT, AS, NA, GM, and DE. Data collection and manuscript review and editing: JT, CA, AS, NA, GM, DE, and The UAE AMR surveillance consortium. Formal analysis and manuscript preparation: JT and CA. All authors have read and agreed to the published version of the manuscript.
The APC was funded by Zayed University, UAE.
The authors wish to thank the UAE Ministry of Health and Prevention (MOHAP), Regional Health Authorities (DHA, DoH/ADPHC), AMR Focal points at participating sites, and all healthcare professionals and other experts involved in national AMR surveillance in the UAE for their continuous collaboration and support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Coppola N, Maraolo AE, Onorato L, Scotto R, Calò F, Atripaldi L, et al. Epidemiology, mechanisms of resistance and treatment algorithm for infections due to carbapenem-resistant gram-negative bacteria: an expert panel opinion. Antibiotics. (2022) 11:1263. doi: 10.3390/antibiotics11091263
2. Rabaan AA, Eljaaly K, Alhumaid S, Albayat H, Al-Adsani W, Sabour AA, et al. An Overview on phenotypic and genotypic characterisation of carbapenem-resistant enterobacterales. Medicina. (2022) 58:1675. doi: 10.3390/medicina58111675
3. Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. (2021) 40:2053–68. doi: 10.1007/s10096-021-04296-1
4. Ordonez AA, Wintaco LM, Mota F, Restrepo AF, Ruiz-Bedoya CA, Reyes CF, et al. Imaging Enterobacterales infections in patients using pathogen-specific positron emission tomography. Sci Transl Med. (2021) 13:eabe9805. doi: 10.1126/scitranslmed.abe9805
5. Molina J, Montero-Mateos E, Praena-Segovia J, León-Jiménez E, Natera C, López-Cortés LE, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect. (2022) 28:550–7. doi: 10.1016/j.cmi.2021.12.008
6. Shrestha R, Luterbach CL, Dai W, Komarow L, Earley M, Weston G, et al. Characteristics of community-acquired carbapenem-resistant Enterobacterales. J Antimicrob Chemother. (2022) 77:2763–71. doi: 10.1093/jac/dkac239
7. Dunne MW, Puttagunta S, Aronin SI, Brossette S, Murray J, Gupta V. Impact of empirical antibiotic therapy on outcomes of outpatient urinary tract infection due to nonsusceptible Enterobacterales. Microbiol Spectr. (2022) 10:e0235921. doi: 10.1128/spectrum.02359-21
8. Chen YC, Chen WY, Hsu WY, Tang HJ, Chou Y, Chang YH, et al. Distribution of β-lactamases and emergence of carbapenemases co-occurring Enterobacterales isolates with high-level antibiotic resistance identified from patients with intra-abdominal infection in the Asia-Pacific region, 2015-2018. J Microbiol Immunol Infect. (2022) 55(6 Pt 2):1263–72. doi: 10.1016/j.jmii.2021.07.007
9. Gao B, Li X, Yang F, Chen W, Zhao Y, Bai G, et al. Molecular epidemiology and risk factors of ventilator-associated pneumonia infection caused by carbapenem-resistant Enterobacteriaceae. Front Pharmacol. (2019) 10:262. doi: 10.3389/fphar.2019.00262
10. Sheu CC, Lin SY, Chang YT, Lee CY, Chen YH, Hsueh PR. Management of infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae: current evidence and future prospects. Expert Rev Anti Infect Ther. (2018) 16:205–18. doi: 10.1080/14787210.2018.1436966
11. CRE Technical Information. CRE | HAI | CDC [Internet]. (2021). Available online at: https://www.cdc.gov/hai/organisms/cre/technical-info.html (cited March 30, 2023).
12. Hammoudi Halat D, Ayoub Moubareck C. The current burden of Carbapenemases: review of significant properties and dissemination among gram-negative bacteria. Antibiotics. (2020) 9:E186. doi: 10.3390/antibiotics9040186
13. Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. (2014) 44:51–6. doi: 10.1016/j.medmal.2013.11.007
14. Pérez-Galera S, Bravo-Ferrer JM, Paniagua M, Kostyanev T, Kraker MEA de, Feifel J, et al. Risk factors for infections caused by carbapenem-resistant Enterobacterales: an international matched case-control-control study (EURECA). eClinicalMedicine. (2023) 57:101871. doi: 10.1016/j.eclinm.2023.101871
15. Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. (2018) 18:318–27. doi: 10.1016/S1473-3099(17)30753-3
16. Brink AJ. Epidemiology of carbapenem-resistant gram-negative infections globally. Curr Opin Infect Dis. (2019) 32:609–16. doi: 10.1097/QCO.0000000000000608
17. Pál T, Butt AB, Ghazawi A, Thomsen J, Rizvi TA, Sonnevend Á. Early years of carbapenem-resistant Enterobacterales epidemic in Abu Dhabi. Antibiotics. (2022) 11:1435. doi: 10.3390/antibiotics11101435
18. Tadese BK, Darkoh C, DeSantis SM, Mgbere O, Fujimoto K. Clinical epidemiology of carbapenem-resistant Enterobacterales in the Greater Houston region of Texas: a 6-year trend and surveillance analysis. J Glob Antimicrob Resist. (2022) 30:222–7. doi: 10.1016/j.jgar.2022.06.019
19. Kotb S, Lyman M, Ismail G, Abd El Fattah M, Girgis SA, Etman A, et al. Epidemiology of Carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare-associated Infections Surveillance Data, 2011-2017. Antimicrob Resist Infect Control. (2020) 9:2. doi: 10.1186/s13756-019-0639-7
20. Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Ghitan M, et al. Rise and fall of KPC-producing Klebsiella pneumoniae in New York City. J Antimicrob Chemother. (2016) 71:2945–8. doi: 10.1093/jac/dkw242
21. Al-Baloushi AE, Pál T, Ghazawi A, Sonnevend A. Genetic support of carbapenemases in double carbapenemase producer Klebsiella pneumoniae isolated in the Arabian Peninsula. Acta Microbiol Immunol Hung. (2018) 65:135–50. doi: 10.1556/030.65.2018.005
22. Population Demographic Mix - The Official Portal of the UAE Government [Internet]. Available online at: https://u.ae/en/information-and-services/social-affairs/preserving-the-emirati-national-identity/population-and-demographic-mix (cited March 27, 2023).
23. Sonnevend Á, Abdulrazzaq N, Ghazawi A, Thomsen J, Bharathan G, Makszin L, et al. The first nationwide surveillance of carbapenem-resistant Enterobacterales in the United Arab Emirates - increased association of Klebsiella pneumoniae CC14 clone with Emirati patients. Int J Infect Dis. (2022) 120:103–12. doi: 10.1016/j.ijid.2022.04.034
24. Moubareck CA, Mouftah SF, Pál T, Ghazawi A, Halat DH, Nabi A, et al. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int J Antimicrob Agents. (2018) 52:90–5. doi: 10.1016/j.ijantimicag.2018.03.003
25. Sonnevend A, Al Baloushi A, Ghazawi A, Hashmey R, Girgis S, Hamadeh MB, et al. Emergence and spread of NDM-1 producer Enterobacteriaceae with contribution of IncX3 plasmids in the United Arab Emirates. J Med Microbiol. (2013) 62(Pt 7):1044–50. doi: 10.1099/jmm.0.059014-0
26. Sonnevend Á, Ghazawi A, Hashmey R, Haidermota A, Girgis S, Alfaresi M, et al. Multihospital occurrence of pan-resistant Klebsiella pneumoniae sequence type 147 with an ISEcp1-directed blaOXA-181 insertion in the mgrB gene in the United Arab Emirates. Antimicrob Agents Chemother. (2017) 61:e00418–17. doi: 10.1128/AAC.00418-17
27. Thomsen J, Abdulrazzaq NM, AlRand H, The UAE AMR Surveillance Consortium. Surveillance of antimicrobial resistance in the United Emirates: the early implementation phase. Front Public Health. (2023) 11:1247627. doi: 10.3389/fpubh.2023.1247627
28. Clinical Laboratory and Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th Edn. (2020). Wayne, PA: Clinical Laboratory and Standards Institute (CLSI).
29. eucast: Breakpoint Tables Dosages v 12.0. (2022). Available online at: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=464&cHash=ea8540c0fbdaa71b3bbcb3bf765239de (cited April 29, 2023).
30. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
31. Karlowsky JA, Bouchillon SK, El Mahdy Kotb R, Mohamed N, Stone GG, Sahm DF. Carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa causing infection in Africa and the Middle East: a surveillance study from the ATLAS programme (2018-20). JAC Antimicrob Resist. (2022) 4:dlac060. doi: 10.1093/jacamr/dlac060
32. Araj GF, Avedissian AZ, Ayyash NS, Bey HA, El Asmar RG, Hammoud RZ, et al. A reflection on bacterial resistance to antimicrobial agents at a major tertiary care center in Lebanon over a decade. J Med Liban. (2012) 60:125–35.
33. Rima M, Oueslati S, Dabos L, Daaboul D, Mallat H, Bou Raad E, et al. Prevalence and molecular mechanisms of Carbapenem resistance among gram-negative bacilli in three hospitals of Northern Lebanon. Antibiotics. (2022) 11:1295. doi: 10.3390/antibiotics11101295
34. Hayajneh WA, Hajj A, Hulliel F, Sarkis DK, Irani-Hakimeh N, Kazan L, et al. Susceptibility trends and molecular characterization of Gram-negative bacilli associated with urinary tract and intra-abdominal infections in Jordan and Lebanon: SMART 2011-2013. Int J Infect Dis. (2015) 35:56–61. doi: 10.1016/j.ijid.2015.04.011
35. Aqel AA, Findlay J, Al-Maayteh M, Al-Kaabneh A, Hopkins KL, Alzoubi H, et al. Characterization of Carbapenemase-producing Enterobacteriaceae from patients in Amman, Jordan. Microb Drug Resist. (2018) 24:1121–7. doi: 10.1089/mdr.2017.0238
36. Huang CC, Chen YS, Toh HS, Lee YL, Liu YM, Ho CM, et al. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002-2010. Int J Antimicrob Agents. (2012) 40(Suppl.):S4–10. doi: 10.1016/S0924-8579(12)70003-1
37. Bazaid AS, Saeed A, Alrashidi A, Alrashidi A, Alshaghdali K, A Hammam S, et al. Antimicrobial surveillance for bacterial uropathogens in Ha'il, Saudi Arabia: a five-year multicenter retrospective study. Infect Drug Resist. (2021) 14:1455–65. doi: 10.2147/IDR.S299846
38. Saeed NK, Alkhawaja S, Azam NFAEM, Alaradi K, Al-Biltagi M. Epidemiology of carbapenem-resistant Enterobacteriaceae in a tertiary care center in the kingdom of Bahrain. J Lab Phys. (2019) 11:111–7. doi: 10.4103/JLP.JLP_101_18
39. Polly M, Almeida BL de, Lennon RP, Cortês MF, Costa SF, Guimarães T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. (2021) 50:32–8. doi: 10.1016/j.ajic.2021.09.018
40. Abubakar U, Al-Anazi M, Alanazi Z, Rodríguez-Baño J. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram negative pathogens: a systematic review. J Infect Public Health. (2023) 16:320–31. doi: 10.1016/j.jiph.2022.12.022
41. Rusic D, Vilovic M, Bukic J, Leskur D, Seselja Perisin A, Kumric M, et al. Implications of COVID-19 pandemic on the emergence of antimicrobial resistance: adjusting the response to future outbreaks. Life. (2021) 11:220. doi: 10.3390/life11030220
42. Ayoub Moubareck C, Hammoudi Halat D. The collateral effects of COVID-19 pandemic on the status of Carbapenemase-producing pathogens. Front Cell Infect Microbiol. (2022) 12:823626. doi: 10.3389/fcimb.2022.823626
43. Chen HY, Jean SS, Lee YL, Lu MC, Ko WC, Liu PY, et al. Carbapenem-resistant Enterobacterales in long-term care facilities: a global and narrative review. Front Cell Infect Microbiol. (2021) 11:601968. doi: 10.3389/fcimb.2021.601968
44. Yu H, Hernández González A, Estévez Torres G, González Molina MK, Hart Casares M, Han X, et al. A retrospective study of risk factors, mortality, and treatment outcomes for infections with Carbapenemase-producing Enterobacterales in a Tertiary Hospital in Havana, Cuba. Antibiotics. (2022) 11:942. doi: 10.3390/antibiotics11070942
45. Zhang X, Xue J, Shen MJ, An WH, Chen ZQ, Wu KF. Molecular typing and drug resistance analysis of carbapenem-resistant Klebsiella pneumoniae from paediatric patients in China. J Infect Dev Ctries. (2022) 16:1726–31. doi: 10.3855/jidc.17003
46. Tian D, Pan F, Wang C, Sun Y, Zhang H. Resistance phenotype and clinical molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae among pediatric patients in Shanghai. Infect Drug Resist. (2018) 11:1935–43. doi: 10.2147/IDR.S175584
47. Sekar R, Mythreyee M, Srivani S, Sivakumaran D, Lallitha S, Saranya S. Carbapenem-resistant Enterobacteriaceae in pediatric bloodstream infections in rural Southern India. Indian Pediatr. (2017) 54:1021–4. doi: 10.1007/s13312-017-1204-1
48. Logan LK, Renschler JP, Gandra S, Weinstein RA, Laxminarayan R, Centers for Disease Control, et al. Carbapenem-resistant Enterobacteriaceae in children, United States, 1999-2012. Emerg Infect Dis. (2015) 21:2014–21. doi: 10.3201/eid2111.150548
49. Chiotos K, Hayes M, Gerber JS, Tamma PD. Treatment of Carbapenem-resistant Enterobacteriaceae infections in children. J Pediatric Infect Dis Soc. (2019) 9:56–66. doi: 10.1093/jpids/piz085
50. Huang YS, Tsai WC, Li JJ, Chen PY, Wang JT, Chen YT, et al. Increasing New Delhi metallo-β-lactamase-positive Escherichia coli among carbapenem non-susceptible Enterobacteriaceae in Taiwan during 2016 to 2018. Sci Rep. (2021) 11:2609. doi: 10.1038/s41598-021-82166-8
51. Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of Carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. (2018) 62:e01882-17. doi: 10.1128/AAC.01882-17
52. Chang YY, Chuang YC, Siu LK, Wu TL, Lin JC, Lu PL, et al. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. (2015) 48:219–25. doi: 10.1016/j.jmii.2014.05.010
53. Wang Q, Wang X, Wang J, Ouyang P, Jin C, Wang R, et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. (2018) 67(Suppl. 2):S196–205. doi: 10.1093/cid/ciy660
54. Moghnia OH, Rotimi VO, Al-Sweih NA. Preponderance of bla KPC-carrying carbapenem-resistant Enterobacterales among fecal isolates from community food handlers in Kuwait. Front Microbiol. (2021) 12:737828. doi: 10.3389/fmicb.2021.737828
55. Candan ED, Aksöz N. Klebsiella pneumoniae: characteristics of carbapenem resistance and virulence factors. Acta Biochim Pol. (2015) 62:867–74. doi: 10.18388/abp.2015_1148
56. Hu Y, Liu C, Shen Z, Zhou H, Cao J, Chen S, et al. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008-2018. Emerg Microbes Infect. (2020) 9:1771–9. doi: 10.1080/22221751.2020.1799721
57. Huang N, Jia H, Zhou B, Zhou C, Cao J, Liao W, et al. Hypervirulent carbapenem-resistant Klebsiella pneumoniae causing highly fatal meningitis in southeastern China. Front Public Health. (2022) 10:991306. doi: 10.3389/fpubh.2022.991306
58. Zhou Y, Zhao Z, Zeng L, Peng J, Zhou S, Min L, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China revealed the dynamics of carbapenemase and the prevalence of ST2735 K. pneumoniae. J Med Microbiol. (2022) 71. doi: 10.1099/jmm.0.001482
59. Teo JQM, Tang CY, Tan SH, Chang HY, Ong SM, Lee SJY, et al. Genomic surveillance of carbapenem-resistant Klebsiella pneumoniae from a major public health hospital in Singapore. Microbiol Spectr. (2022) 10:e0095722. doi: 10.1128/spectrum.00957-22
60. Ripabelli G, Tamburro M, Guerrizio G, Fanelli I, Flocco R, Scutellà M, et al. Tracking multidrug-resistant Klebsiella pneumoniae from an Italian Hospital: molecular epidemiology and surveillance by PFGE, RAPD and PCR-based resistance genes prevalence. Curr Microbiol. (2018) 75:977–87. doi: 10.1007/s00284-018-1475-3
61. Koppe U, von Laer A, Kroll LE, Noll I, Feig M, Schneider M, et al. Carbapenem non-susceptibility of Klebsiella pneumoniae isolates in hospitals from 2011 to 2016, data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob Resist Infect Control. (2018) 7:71. doi: 10.1186/s13756-018-0362-9
62. Karampatakis T, Tsergouli K, Behzadi P. Carbapenem-resistant Klebsiella pneumoniae: virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics. (2023) 12:234. doi: 10.3390/antibiotics12020234
63. Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol. (2019) 10:80. doi: 10.3389/fmicb.2019.00080
64. Milano A, Sulejmani A, Intra J, Sala MR, Leoni V, Carcione D. Antimicrobial resistance trends of Escherichia coli isolates from outpatient and inpatient urinary infections over a 20-year period. Microb Drug Resist. (2022) 28:63–72. doi: 10.1089/mdr.2021.0010
65. Adegoke AA, Ikott WE, Okoh AI. Carbapenem resistance associated with coliuria among outpatient and hospitalised urology patients. New Microbes New Infect. (2022) 48:101019. doi: 10.1016/j.nmni.2022.101019
66. Gondal AJ, Choudhry N, Bukhari H, Rizvi Z, Yasmin N. Characterization of genomic diversity among Carbapenem-resistant Escherichia coli clinical isolates and antibacterial efficacy of silver nanoparticles from Pakistan. Microorganisms. (2022) 10:2283. doi: 10.3390/microorganisms10112283
67. Huang L, Huang C, Yan Y, Sun L, Li H. Urinary tract infection etiological profiles and antibiotic resistance patterns varied among different age categories: a retrospective study from a tertiary general hospital during a 12-year period. Front Microbiol. (2021) 12:813145. doi: 10.3389/fmicb.2021.813145
68. Li J, Jiang F, Xie A, Jiang Y. Analysis of the distribution and drug resistance of pathogens in patients with urinary tract infection in the Eastern Chongming area of Shanghai from 2018 to 2020. Infect Drug Resist. (2022) 15:6413–22. doi: 10.2147/IDR.S384515
69. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. (2015) 13:269–84. doi: 10.1038/nrmicro3432
70. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. (2003) 49:53–70. doi: 10.1067/mda.2003.7
71. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. (2012) 366:1028–37. doi: 10.1056/NEJMcp1104429
72. Mazzariol A, Bazaj A, Cornaglia G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. J Chemother. (2017) 29(Supp1.):2–9. doi: 10.1080/1120009X.2017.1380395
73. Moghnieh RA, Moussa JA, Aziz MA, Matar GM. Phenotypic and genotypic characterisation of cephalosporin-, carbapenem- and colistin-resistant Gram-negative bacterial pathogens in Lebanon, Jordan and Iraq. J Glob Antimicrob Resist. (2021) 27:175–99. doi: 10.1016/j.jgar.2021.08.005
74. Sojo-Dorado J, López-Hernández I, Rosso-Fernandez C, Morales IM, Palacios-Baena ZR, Hernández-Torres A, et al. Effectiveness of fosfomycin for the treatment of multidrug-resistant Escherichia coli bacteremic urinary tract infections: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2137277. doi: 10.1001/jamanetworkopen.2021.37277
75. Ruiz Ramos J, Salavert Lletí M. Fosfomycin in infections caused by multidrug-resistant Gram-negative pathogens. Rev Esp Quimioter. (2019) 32(Suppl 1):45–54.
76. Mikhail S, Singh NB, Kebriaei R, Rice SA, Stamper KC, Castanheira M, et al. Evaluation of the synergy of ceftazidime-avibactam in combination with meropenem, amikacin, aztreonam, colistin, or fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. (2019) 63:e00779–19. doi: 10.1128/AAC.00779-19
77. Ortiz-Padilla M, Portillo-Calderón I, de Gregorio-Iaria B, Blázquez J, Rodríguez-Baño J, Pascual A, et al. Interplay among different fosfomycin resistance mechanisms in Klebsiella pneumoniae. Antimicrob Agents Chemother. (2021) 65:e01911-20. doi: 10.1128/AAC.01911-20
78. Walflor HSM, Lucena ARC, Tuon FF, Medeiros LCS, Faoro H. Resensitization of fosfomycin-resistant Escherichia coli using the CRISPR system. Int J Mol Sci. (2022) 23:9175. doi: 10.3390/ijms23169175
79. Falagas ME, Athanasaki F, Voulgaris GL, Triarides NA, Vardakas KZ. Resistance to fosfomycin: mechanisms, frequency and clinical consequences. Int J Antimicrob Agents. (2019) 53:22–8. doi: 10.1016/j.ijantimicag.2018.09.013
80. Tian Y, Zhang Q, Wen L, Chen J. Combined effect of polymyxin B and tigecycline to overcome heteroresistance in carbapenem-resistant Klebsiella pneumoniae. Microbiol Spectr. (2021) 9:e0015221. doi: 10.1128/Spectrum.00152-21
81. Zhang J, Yu L, Fu Y, Zhao Y, Wang Y, Zhao J, et al. Tigecycline in combination with other antibiotics against clinical isolates of carbapenem-resistant Klebsiella pneumoniae in vitro. Ann Palliat Med. (2019) 8:622–31. doi: 10.21037/apm.2019.09.11
82. Sun J, Chen C, Cui CY, Zhang Y, Liu X, Cui ZH, et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat Microbiol. (2019) 4:1457–64. doi: 10.1038/s41564-019-0496-4
83. Bai L, Du P, Du Y, Sun H, Zhang P, Wan Y, et al. Detection of plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong Provinces, China, February 2019. Euro Surveill. (2019) 24:1900340. doi: 10.2807/1560-7917.ES.2019.24.25.1900340
84. Cheng YH, Huang TW, Juan CH, Chou SH, Tseng YY, Chen TW, et al. Tigecycline-non-susceptible hypervirulent Klebsiella pneumoniae strains in Taiwan. J Antimicrob Chemother. (2020) 75:309–17. doi: 10.1093/jac/dkz450
85. Zhang Y, Wang X, Wang Q, Chen H, Li H, Wang S, et al. Emergence of tigecycline nonsusceptible and IMP-4 carbapenemase-producing K2-ST65 hypervirulent Klebsiella pneumoniae in China. Microbiol Spectr. (2021) 9:e0130521. doi: 10.1128/Spectrum.01305-21
86. Hussein NH, Al-Kadmy IMS, Taha BM, Hussein JD. Mobilized colistin resistance (mcr) genes from 1 to 10: a comprehensive review. Mol Biol Rep. (2021) 48:2897–907. doi: 10.1007/s11033-021-06307-y
87. Aris P, Robatjazi S, Nikkhahi F, Amin Marashi SM. Molecular mechanisms and prevalence of colistin resistance of Klebsiella pneumoniae in the Middle East region: a review over the last 5 years. J Glob Antimicrob Resist. (2020) 22:625–30. doi: 10.1016/j.jgar.2020.06.009
88. Habib I, Elbediwi M, Ghazawi A, Mohamed MYI, Lakshmi GB, Khan M. First report from supermarket chicken meat and genomic characterization of colistin resistance mediated by mcr-1.1 in ESBL-producing, multidrug-resistant Salmonella Minnesota. Int J Food Microbiol. (2022) 379:109835. doi: 10.1016/j.ijfoodmicro.2022.109835
Keywords: carbapenem-resistant Enterobacterales, surveillance, Enterobacterales, healthcare associated infections, antibiotics, antimicrobial resistance, UAE
Citation: Thomsen J, Abdulrazzaq NM, the UAE AMR Surveillance Consortium, Everett DB, Menezes GA, Senok A and Ayoub Moubareck C (2023) Carbapenem resistant Enterobacterales in the United Arab Emirates: a retrospective analysis from 2010 to 2021. Front. Public Health 11:1244482. doi: 10.3389/fpubh.2023.1244482
Received: 22 June 2023; Accepted: 24 October 2023;
Published: 07 December 2023.
Edited by:
Dalal Hammoudi Halat, Qatar University, QatarReviewed by:
Yancheng Yao, University of Giessen, GermanyCopyright © 2023 Thomsen, Abdulrazzaq, the UAE AMR Surveillance Consortium, Everett, Menezes, Senok and Ayoub Moubareck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carole Ayoub Moubareck, Y2Fyb2xlQG1vdWJhcmVjay5jb20=
†These authors have contributed equally to this work and share last authorship
‡The UAE AMR Surveillance Consortium group members are listed in Appendix
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.