- 1School of Medicine and Health Sciences, Mulungushi University, Livingstone, Zambia
- 2International Vaccine Access Center, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 3Centers for Disease Control and Prevention, Lusaka, Zambia
- 4Provincial Medical Office, Ministry of Health, Choma, Zambia
- 5School of Public Health, University of Zambia, Lusaka, Zambia
Background: Globally, most countries have implemented a test-and-treat policy to reduce morbidity and mortality associated with HIV infection. However, the impact of this strategy has not been critically appraised in many settings, including Zambia. We evaluated the retention and clinical outcomes of adults enrolled in antiretroviral therapy (ART) and assessed the impact of the test-and-treat policy.
Methods: We conducted a retrospective cohort study among 6,640 individuals who initiated ART between January 1, 2014 and July 31, 2016 [before test-and-treat cohort (BTT), n = 2,991] and between August 1, 2016 and October 1, 2020 [after test-and-treat cohort (ATT), n = 3,649] in 12 districts of the Southern province. To assess factors associated with retention, we used logistic regression (xtlogit model).
Results: The median age [interquartile range (IQR)] was 34.8 years (28.0, 42.1), and 60.2% (n = 3,995) were women. The overall retention was 83.4% [95% confidence interval (CI) 82.6, 84.4], and it was significantly higher among the ATT cohort, 90.6 vs. 74.8%, p < 0.001. The reasons for attrition were higher in the BTT compared to the ATT cohorts: stopped treatment (0.3 vs. 0.1%), transferred out (9.3 vs. 3.2%), lost to follow-up (13.5 vs. 5.9%), and death (1.4 vs. 0.2%). Retention in care was significantly associated with the ATT cohort, increasing age and baseline body mass index (BMI), rural residence, and WHO stage 2, while non-retention was associated with never being married, divorced, and being in WHO stage 3.
Conclusion: The retention rate and attrition factors improved in the ATT compared to the BTT cohorts. Drivers of retention were test-and-treat policy, older age, high BMI, rural residence, marital status, and WHO stage 1. Therefore, there is need for interventions targeting young people, urban residents, non-married people, and those in the symptomatic WHO stages and with low BMI. Our findings highlight improved ART retention after the implementation of the test-and-treat policy.
1. Introduction
Increasing early access to antiretroviral therapy (ART) in resource-limited settings has improved the lives of people with HIV (PWH) in Africa (1, 2). Several strategies have been implemented to reduce morbidity and mortality among PWH. Among these strategies is the test-and-treat approach, where ART is initiated soon after diagnosis of HIV infection following the World Health Organization (WHO) recommendations of 2015 (3). Implementation of this universal test-and-treat policy was projected to prevent at least 21 million deaths and 28 million new infections by 2030 globally (4). In 2016, the Zambia Ministry of Health started implementing the universal HIV test-and-treat policy for all PWH, regardless of their CD4 count or WHO HIV clinical stage at the time of diagnosis. However, some scientists and program managers were concerned about the approaches to use to initiate ART for the newly diagnosed and supposedly healthy, to handle issues of HIV stigma, manpower, and infrastructure constraints, and to provide psychosocial counseling and clinical follow-up for large numbers of PWH (5–7).
However, the beneficial effects of early ART initiation are overwhelming in the prevention and treatment of HIV infection. Universal testing and treatment (UTT) has been linked to a reduction in HIV-associated morbidity and mortality (8–11). In the presence of optimal adherence, early initiation of ART leads to rapid attainment and sustained HIV viral suppression (8, 11–14). This is critical to the reduction of HIV transmission at the individual (15) and community (16, 17) levels and to achieving the UNAIDS 2030 target of 95-95-95 (18). The effect of an early ART start has also been observed in sub-Saharan Africa (SSA). Retention in care increased in Malawi (19) and Zambia (20), and reduced mortality was observed in the studies conducted in Uganda (21) and South Africa (22). Despite the well-documented benefit of UTT, other studies in Africa have shown an increase in loss to follow-up (LTFU) (23–25) and a decrease in ART retention in the first year (21, 26, 27). Some scholars have attributed the problems with retention and LTFU to a lack of realization of the beneficial effects of ART when individuals are initiated while enjoying a healthy state (23). Additionally, the lack of special support for individuals during UTT has been implicated in low retention rates (28).
For countries in SSA to achieve the sustainable development goal (SDG-3.3) of ending the AIDS epidemic by 2030 (29), retention in care and sustained viral suppression are critical. According to WHO, SSA has a substantial challenge with long-term retention in care (30). Persons who disengage from care and stop taking medication have a high probability of transmitting HIV infection, attaining AIDS status, and dying (30). The drivers of retention in care include female sex, older age, disclosure of HIV status, optimal ART adherence, viral suppression, a high CD4 count, and delayed ART initiation after HIV diagnosis (19, 26, 31, 32). Being divorced and in a relationship, acquiring HIV infection perinatally, and voluntary testing and counseling are some of the drivers of retention (33–35) in our setting. Therefore, there is an urgent need for locally tailored empirical evidence on the drivers of retention to attain HIV epidemic control in SSA.
In view of the existing literature, several studies in different settings have demonstrated the beneficial effect of the test-and-treat strategy (13, 14, 36) but with inconsistent findings on retention in care and other clinical outcomes such as death and LTFU. However, the impact of this strategy has not been adequately evaluated in a programmatic setting like Zambia, where the prevalence of HIV is high. We, therefore, conducted a multicenter retrospective cohort study to determine the effect of HIV test-and-treat on clinical outcomes of adults initiated on ART before and after implementation of the test-and-treat policy, with the hypothesis that the test-and-treat policy improved the HIV retention rate.
2. Methods
2.1. Study design
We conducted a retrospective cohort study of PWH enrolled in HIV care in 12 President’s Emergency Plan for AIDS Relief (PEPFAR)-supported districts in one province of Zambia.
2.2. Study population
Clinical data from patient records were extracted for PWH aged 15 years and older who were initiated on ART between January 1, 2014 and October 1, 2020, regardless of their clinical outcome at the time of data abstraction. PWH initiated on ART before the launch of the test-and-treat policy (January 1, 2014 to July 31, 2016) formed the pre-test-and-treat cohort, and those initiated in the period following the implementation of the test-and-treat policy (August 1, 2016 to October 1, 2020) formed the post-test-and-treat cohort.
All PWHs that enrolled in care but were not initiated on ART by the time of data abstraction were excluded from the analysis. However, the details of those who were not on ART were provided to the ART health providers for further follow-up and counseling with the aim of commencing ART. PWH with either a missing date of birth or age or a missing ART initiation date were excluded from the study.
2.3. Data collection and study procedures
Clinical data for PWH were abstracted either from HIV paper-based or electronic medical records known as SmartCare in 45 health facilities located in 12 districts of Southern Province onto the Electronic Data Capture (REDCap) software. Patient outcomes were recorded around the follow-up period of 3, 6, 12, and 24 months ±30 days and the last date of contact with the health care provider. In addition to clinical data (ART regimen, WHO stage, BMI, duration on ART, retention, stopped treatment, death, transferred out, and LTFU), laboratory (CD4 count and HIV viral load), and pharmacy-related data, including medicine refill dates, were collected from laboratory and pharmacy registers, respectively, in facilities where SmartCare was not fully functional. Where possible, the data were triangulated from both SmartCare and paper registers.
A line list of PWH was created from the ART clinic enrollment register per national guidelines. If the site had fewer than 100 patients on ART, data were abstracted from all the patient SmartCare medical records. For sites with more than 100 patient records, a systematic sampling scheme was used. At each site, we determined a sampling interval (X) by dividing the total number of client records at the site by the sample size and taking the whole number only (dropping the decimals); this process was done for the allocated sample sizes in the before and after test-and-treat strategies. The first record for review was selected (N) by randomly selecting a number between 1 and X (sampling interval). Data abstractors selected the Nth record and proceeded with selecting record N + X as the first consecutive record for review, which continued with adding X until the quota for that site had been reached. All the selected records were entered into the REDCap application and screened for inclusion and exclusion criteria. If any patient record from the selected list was excluded from the study, the next eligible record fulfilling the inclusion criteria was selected to replace the excluded record.
2.4. Data quality control
Data entries from 10% of the selected patient records were re-abstracted by the team facilitator immediately after the needed records were abstracted by the data extraction team using a systematic random sampling method. Once the files had been re-abstracted, any discrepancies between the data initially abstracted and the data that were re-abstracted were discussed, and the option that was unanimously agreed to be correct was selected. We have used guidelines for strengthening the reporting of observational studies in epidemiology (see Supplementary File 1, STROBE checklist).
2.5. Outcomes
The primary outcome was retention in HIV care, defined as regular attendance at appointments or engagement with the ART clinic at 3, 6, 12, 24, and ≥ 24 months after ART initiation. Secondary outcomes were LTFU, transferred out, stopped treatment, and death. LTFU was defined as a PWH whose medical records showed no evidence of any form of contact with a health facility for 30 or more days after the scheduled visit day. Transferred out is when a PWH moves to another ART clinic to continue receiving ART services. Stopped treatment was when a PWH stopped HIV treatment. We defined HIV viral suppression as having a viral load of less than 1,000 copies/mL after being on ART for ≥6 months as per national guidelines (37).
2.6. Sample size estimation
PS software was used to estimate the sample size (38). We hypothesized that the proportion of retention would be slightly higher after a test-and-treat period. An uncorrected chi-squared test was used to test the difference in proportions of retention between the two groups (before and after test-and-treat implementation), and the sample size was estimated accordingly with reference to a study conducted in Malawi (19). The proportion of retention BTT was 76.2%, and we expected a 3.7% increase in ATT (79.9%). We estimated requiring 6,688 records to achieve at least a 90% chance of detecting a difference (one-sided α = 0.05, allowing 5% type I error, conservative design effect of 2, power = 0.9).
2.7. Facility and participant sampling methods
We selected 45 health facilities [21 rural health centers (RHC), 16 urban health centers (UHC), and eight hospitals] across the 12 districts from 274 ART sites. In the initial step, the 274 ART sites were stratified into rural and urban sites, and then, a probability based on the number of PWH on ART at each health facility was used to select the required number of sites from each stratum.
2.8. Data analysis
The data were entered in REDCap and thereafter exported to STATA version 15 and R for standard data analysis. Data were described using frequency and percentages for categorical variables and medians (interquartile ranges) for continuous variables. To test for normality, the Shapiro–Wilk test was used. The Wilcoxon rank-sum test was used to ascertain the statistical difference between the two medians. A relationship between two categorical variables was determined using a chi-squared test. Multivariable logistic regression was used to examine the factors associated with retention using generalized estimating equations (GEE) via the logit link function to account for correlation structure within observations. The variables [cohort (ATT), age, sex, facility location, marital status, duration to ART initiation after HIV diagnosis, WHO stage, ART regimen, and BMI] in the adjusted analysis were selected based on previous literature (19, 26, 31, 32) and knowledge from HIV treatment and management experts. To ascertain statistical significance, a p value of <0.05 was used.
2.9. Ethics
Ethical approval was obtained from the Macha Research Trust Institution Review Board (IRB) and the Zambia National Health Research Authority (ZNHRA). This project was also reviewed in accordance with the Centers for Disease Control and Prevention’s (CDC) human research protection guidelines. In this study, we analyzed de-identified data from healthcare facilities.
3. Results
3.1. Characteristics of the study participants
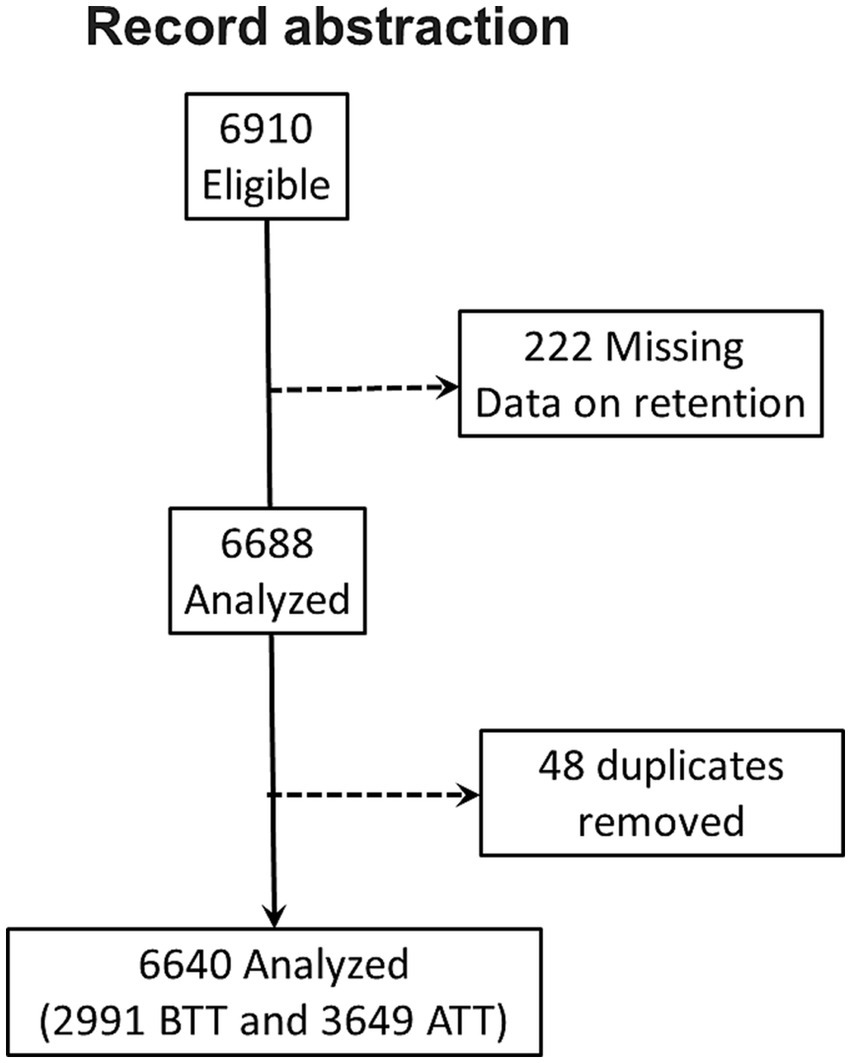
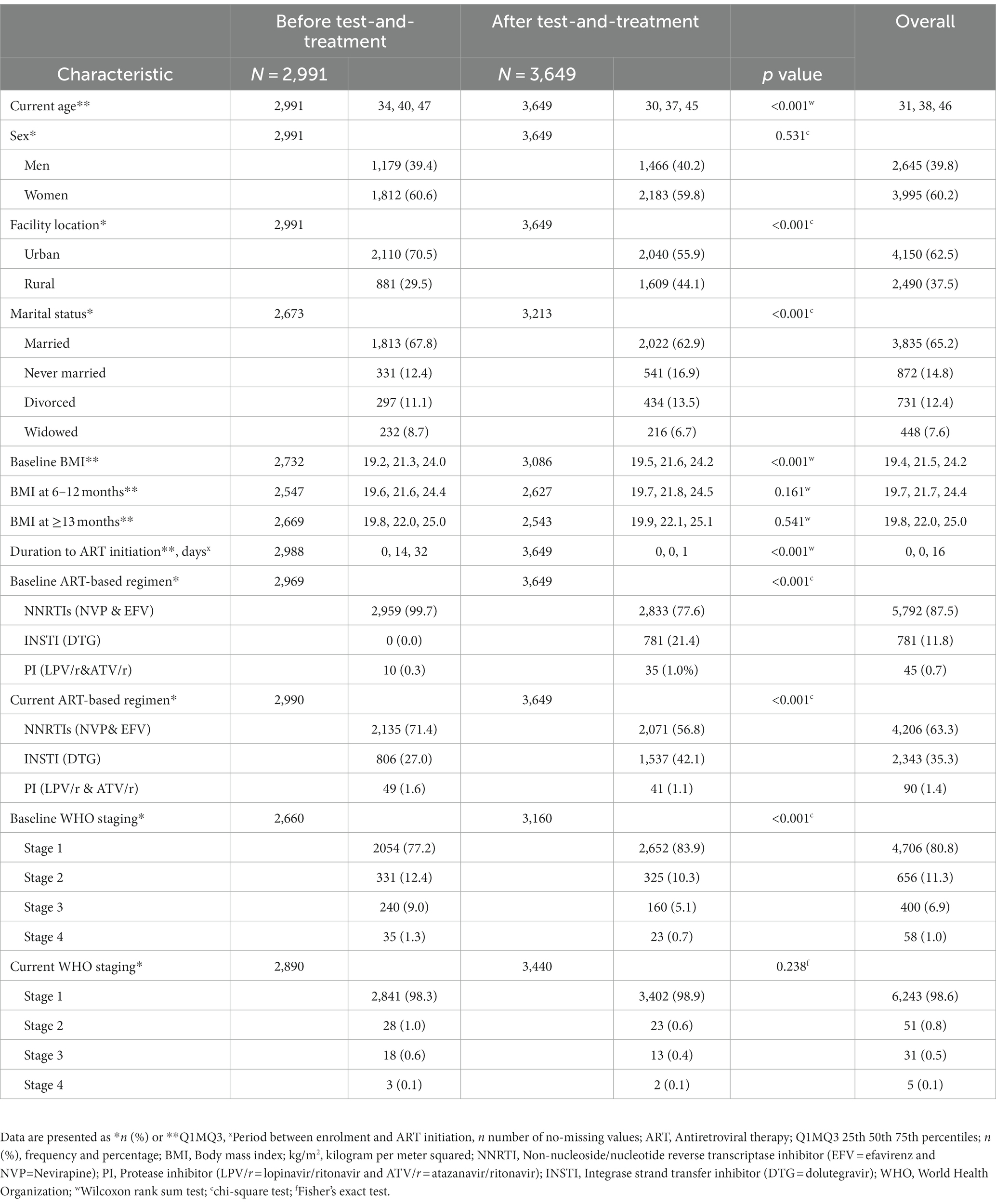
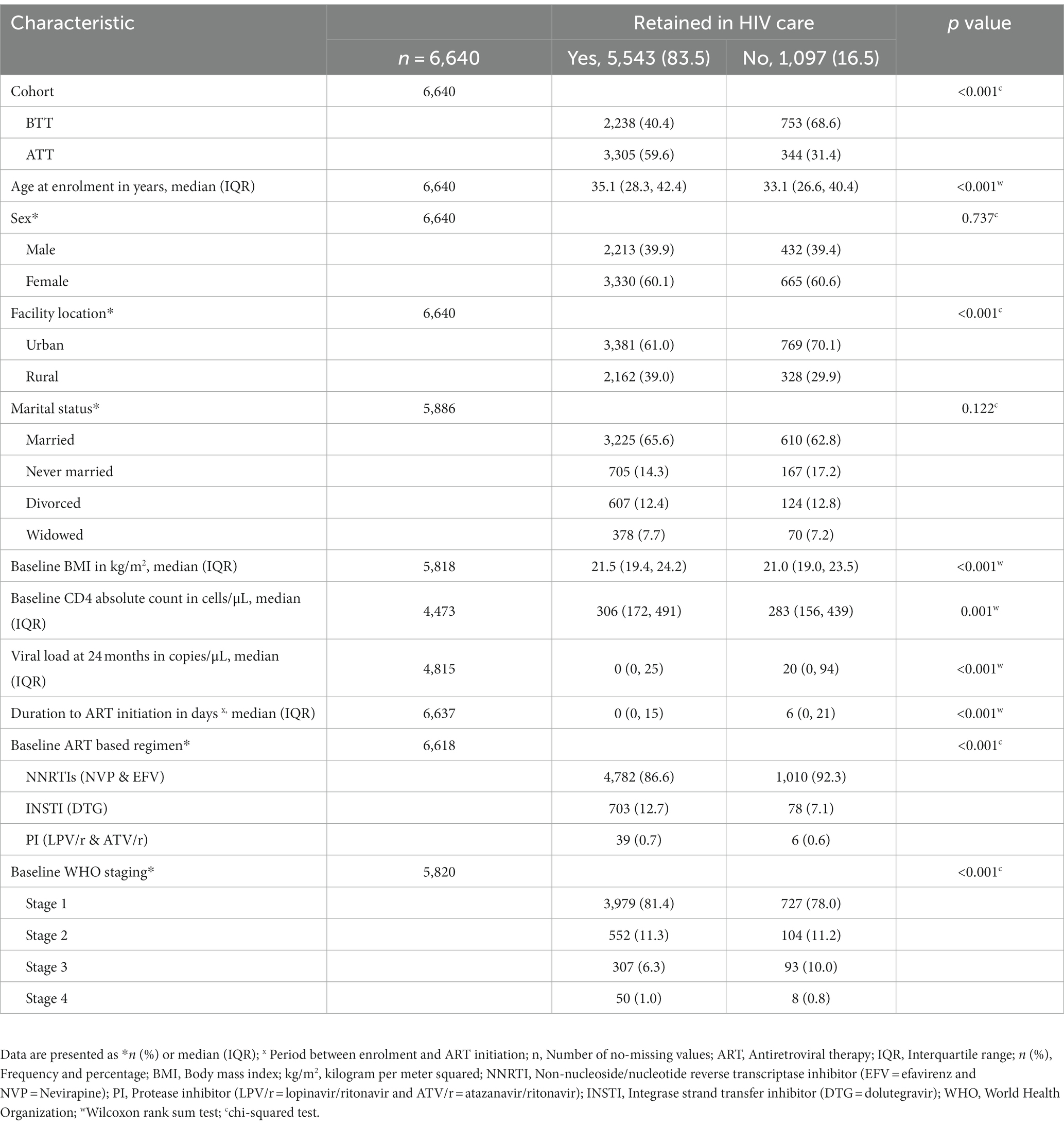
Owing to the anticipated potential presence of duplicates and missing information, we abstracted 6,910 records. After removing duplicates and records with missing information on the outcome variable, only 6,640 records were included in the analysis (Figure 1). We identified 2,991 (45.1%) records for PWH initiated on ART before test-and-treat (BTT) and 3,649 (54.9%) after test-and-treat (ATT) policies (Figure 1). The median age was 38 years [interquartile range (IQR), 31.0, 46.0], and participants in the BTT policy were significantly older, 40 vs. 37 years, p < 0.001. Women comprised 60.2% (n = 3,995) of PWH in the study sample. The majority of the participants, BTT (70.5%, n = 2,110) and ATT (55.9%, n = 2040), were from urban areas. There were more married individuals than those who were never married, divorced, or widowed in both the BTT (67.8%, n = 1813) and ATT (62.9%, n = 2022) cohorts. The baseline body mass index (BMI) was significantly higher among participants in the ATT than the BTT (21.6 vs. 21.3 kg/m2, p < 0.001). BTT participants took 14 days (IQR: 0, 32) to be initiated on ART from the time of HIV diagnosis compared with the ATT cohort that took 0 days (IQR: 0, 1), p = 0.001. A high proportion of the participants were taking non-nucleotide reverse transcriptase inhibitors (NNRTIs) at baseline (99.7% BTT and 77.6% ATT) and currently (71.4% BTT and 56.8% ATT). At baseline, the majority of patients had WHO stage I HIV, with a higher proportion of patients in the ATT cohort with Stage I (83.9%) vs. those in the BTT cohort (77.2%; Table 1).
3.2. Immunovirological outcomes by cohort
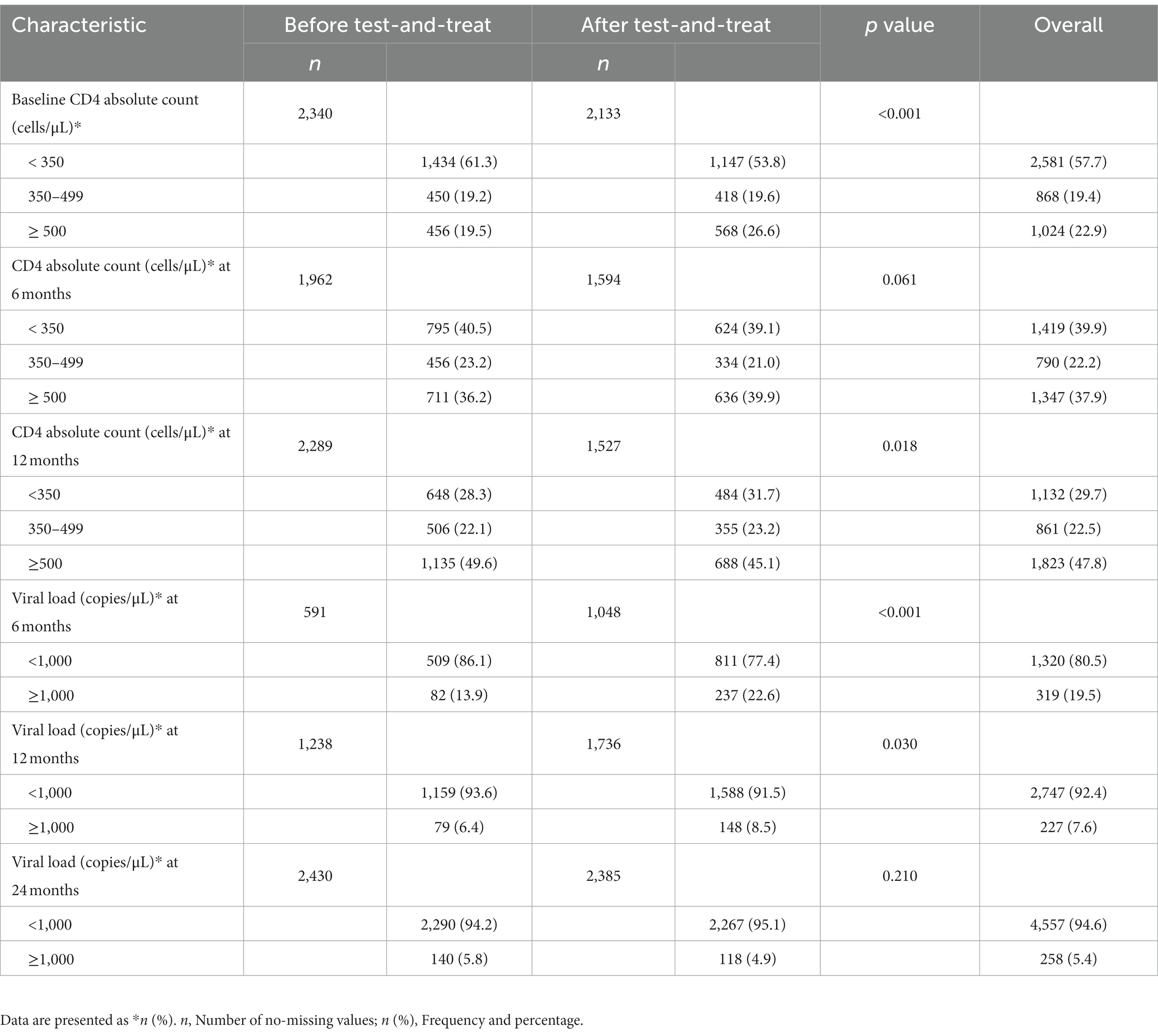
The overall prevalence of CD4 ≥ 500 cells/μL was 22.9, 37.9, and 47.8% at baseline, 6 months, and 12 months, respectively. Similarly, an increase in the proportion of patients with viral suppression (VL <1,000 copies/μL) was observed at different time points: 6 months (80.5%), 12 months (92.4%), and 24 months (94.6%).
At baseline, a significantly higher proportion of participants in the ATT cohort had CD4 ≥ 500 cells/μL than BTT (26.6 vs. 19.5%, p < 0.001). However, at 12 months, a higher proportion of participants in BTT than ATT had CD4 ≥ 500 cells/μL, 49.6 vs. 45.1%, p = 0.018. A higher proportion of viral suppression was observed among the participants in the BTT cohort at 6 months (86.1 vs. 77.4%, p < 0.001) and 12 months (93.6 vs. 91.5%, p = 0.030). At 24 months, the viral load categories were comparable (Table 2).
3.3. Overall retention in HIV care
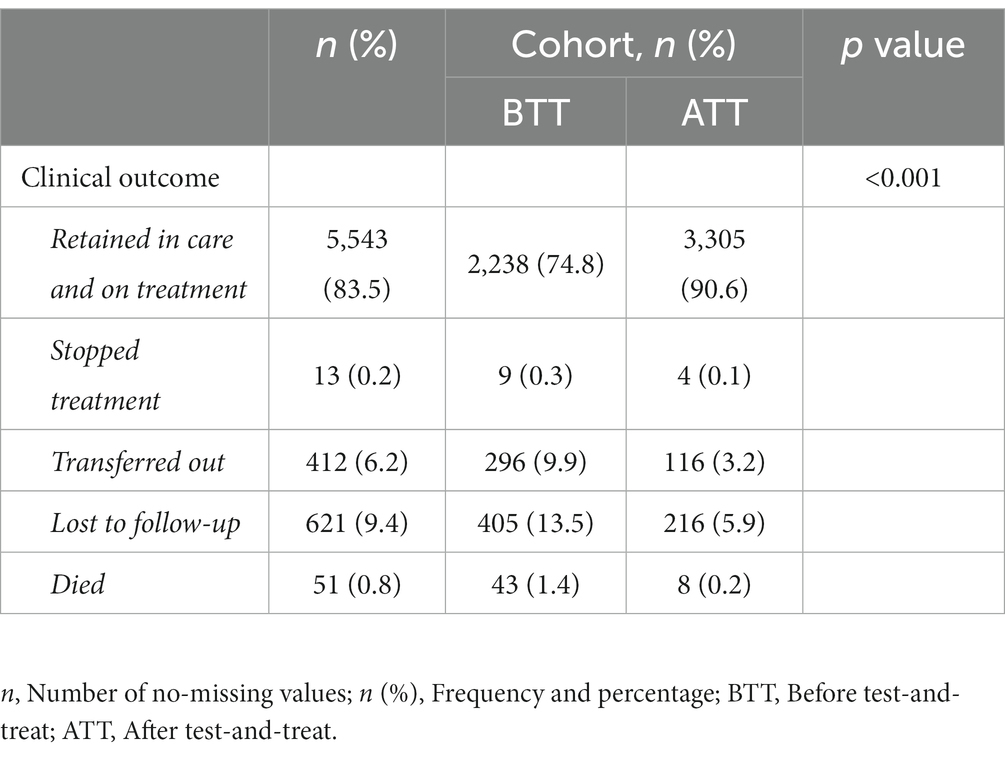
At the time of data abstraction, 5,543 (83.4%) were actively on ART, 13 (0.2%) had stopped ART, 412 (6.2%) were transferred out, 621 (9.4%) were LTFU, and 51 (0.8%) were dead (Table 3). There was a statistically higher proportion of retention on ART for the ATT cohort as compared to the BTT cohort (90.5 vs. 74.8%, p < 0.001), while attrition was generally high in the BTT cohort as compared to the ATT cohort: death (1.4 vs. 0.2%, p < 0.001), stopped treatment (0.3 vs. 0.1%, p < 0.001), transferred out (9.9 vs. 3.2%, p < 0.001), and LTFU (13.5 vs. 5.9%, p < 0.001).
3.4. Retention at different time points by cohort
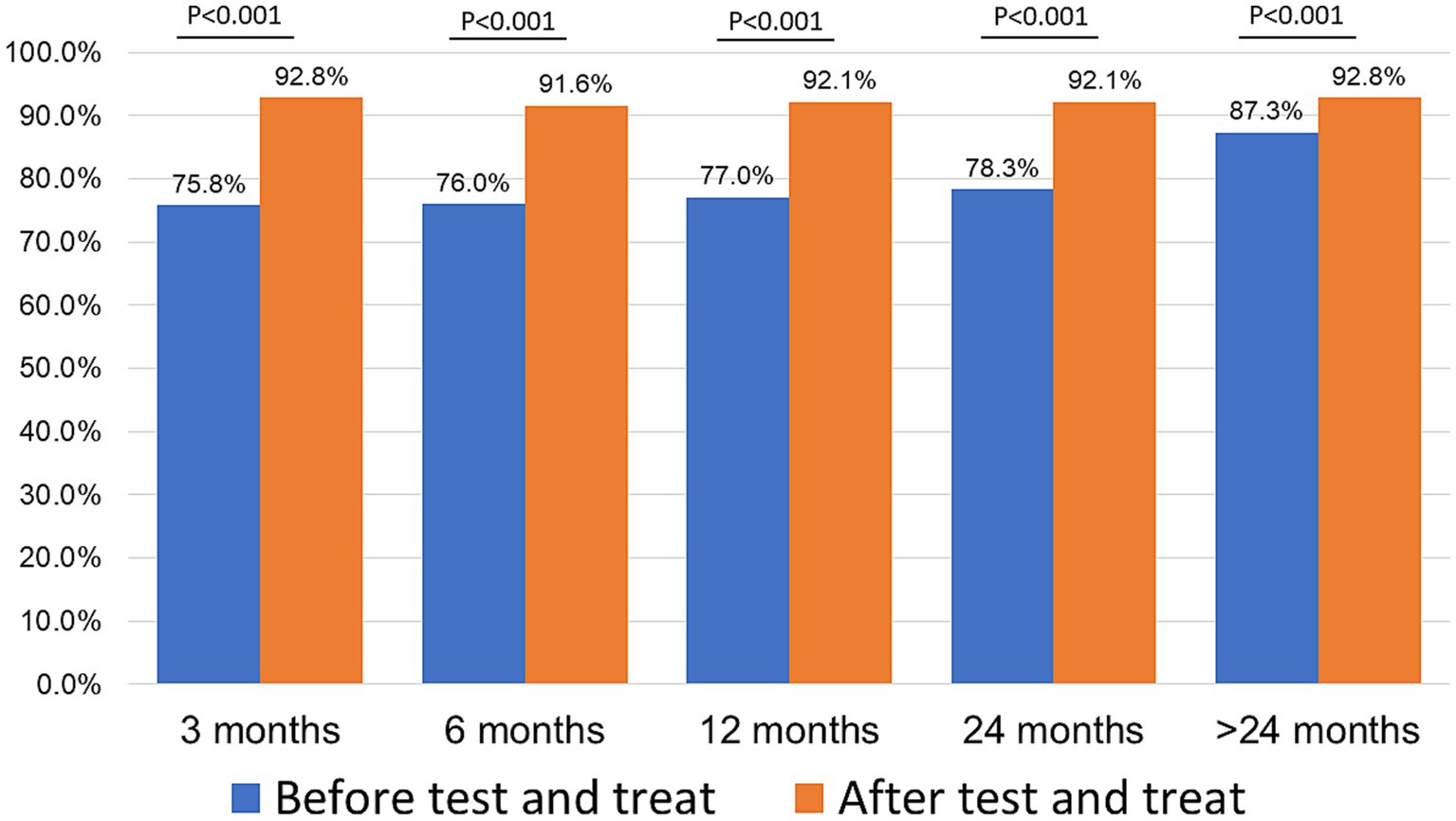
Retention was higher in the ATT cohort as compared to the BTT cohort (Figure 2) at 3 months (92.8 vs. 75.8%, p < 0.001), 6 months (91.6 vs. 76.0%, p < 0.001,), 12 months (92.1 vs. 77.0%, p < 0.001), 24 months (92.1 vs. 78.3%, p < 0.001), and > 24 months (92.8 vs. 87.3%, p < 0.001), that is, all time points.
3.5. Retention in HIV care at ≥24 months sorted by demographic and clinical characteristics
3.5.1. Combined analysis (BTT and ATT)
Over half of the patients (59.6%, n = 3,305) who were retained in care were from the ATT cohort, whereas over two-thirds of the patients (40.4%, n = 2,238) were not retained in care were from the BTT cohort. The individuals who were retained in HIV care were significantly older than the ones who were not retained (35.1 vs. 33.1 years, p < 0.001). There was a higher proportion of urban participants who were not retained (70.1%) compared to those retained in HIV care. Conversely, there was a higher proportion of rural participants who were retained in HIV care (39.0%) vs. not retained in care (29.9%). Baseline BMI (21.5 vs. 21.0 kg/m2) and CD4 count (306 vs. 283 cells/μL) were significantly higher among the participants who were retained in HIV care in comparison to non-retained ones, with a p value of <0.05. The duration from HIV diagnosis to initiation of treatment was significantly lower among the retained participants as compared with those who were not retained (0 vs. 6 days, p < 0.001). Equally, the viral load at 24 months among the retained individuals in HIV care was lower (0 vs. 20 copies/μL, p < 0.001). A higher proportion of the participants whose dolutegravir (DTG) was their baseline ART regimen were retained in HIV care compared to those who were not retained (12.7 vs. 7.1%, p < 0.001). Among those in the NNRTI group, the preponderance of them were not retained in care (92.3 vs. 86.6%). A significantly higher percentage of the baseline participants with WHO stage 1 were retained in HIV care compared to those who were not retained (81.4 vs. 78.0%, p < 0.001; Table 4).
3.6. Retention in HIV care at ≥24 months sorted by BTT and ATT
In the cohort BTT, the factors significantly associated with retention in HIV care were older age, facility location, marital status, higher baseline BMI and CD4 count, and being in baseline WHO stage 1. While ATT participants, older age, lower viral load at 24 months, and being initiated on ART on the same day of HIV diagnosis were the factors significantly associated with retention in HIV care (Supplementary Table 1).
3.7. Predictors of overall retention in HIV care
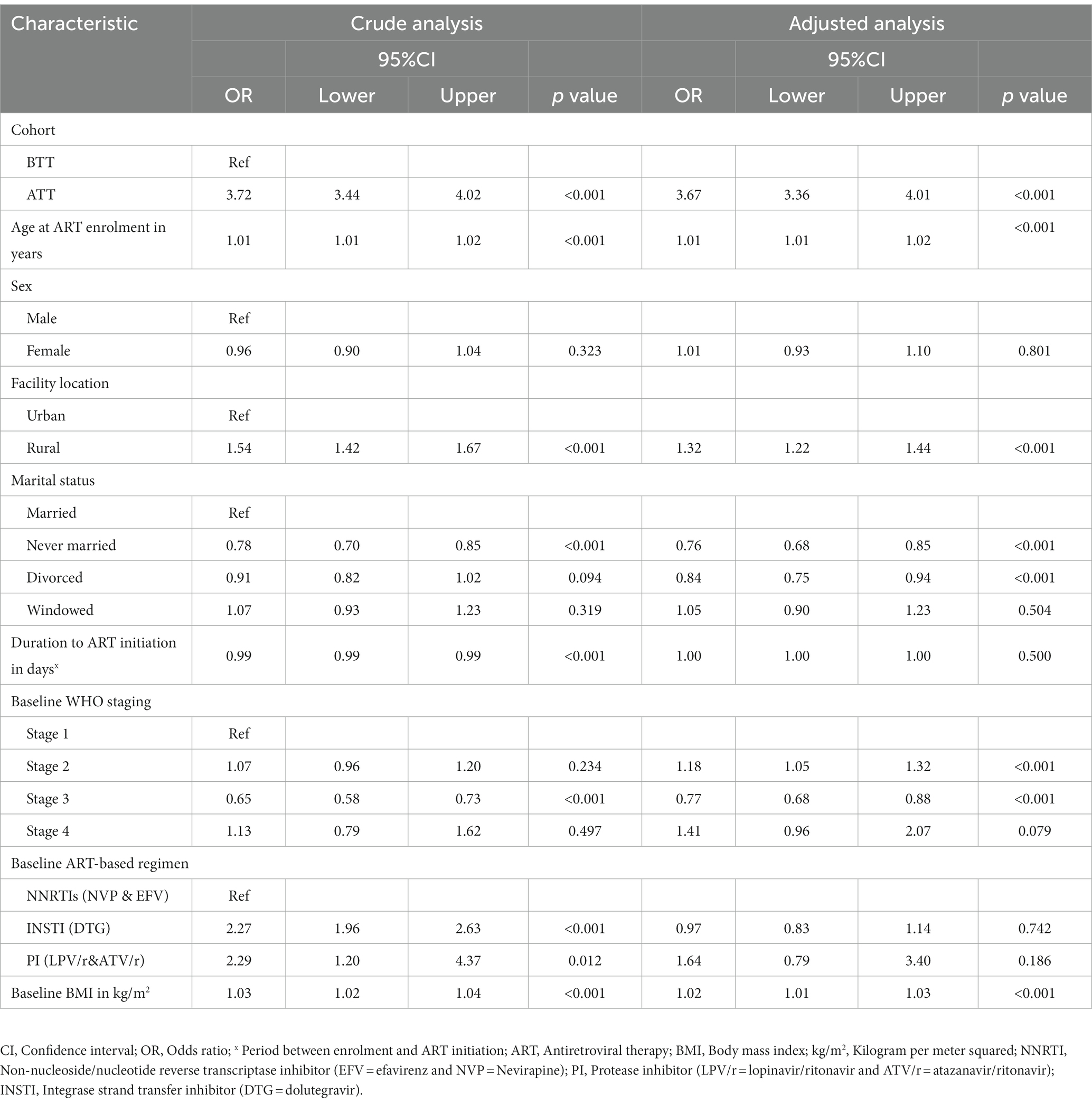
In the univariate analysis, there was a statistically significant association between retention and the following determinants: Participants in the ATT cohort were 3.72 times more likely to be retained in HIV care compared to individuals in BTT (OR: 3.72, 95%CI 3.44, 4.02). A year increase in age was significantly associated with 1% increased odds of being retained in HIV care [odds ratio (OR) 1.01; 95%CI 1.01, 1.02]. Participants from rural areas had a significantly higher probability (54%) of being retained in HIV care compared to those from urban areas (OR 1.54; 95%CI 1.42, 1.67). Individuals who had never been married had 22% reduced odds of being retained in HIV care compared to those who were married (OR 0.78; 95%CI 0.70, 0.85). An increase in the number of days from HIV diagnosis to ART initiation was significantly associated with a 1% reduced chance of being retained in HIV care. Participants who were in WHO stage 3 at baseline had 35% reduced odds of being retained in HIV care compared to the ones in stage 1 (OR 0.65; 95%CI 0.58, 0.73). Clients on a DTG and protease inhibitor (PI)-based regimen were 2.27 and 2.29 times more likely to be retained in HIV care relative to those on NNRTIs, respectively. A unit increase in baseline BMI was significantly associated with 3% increased odds of being retained in HIV care (OR 1.03; 95%CI 1.02, 1.04).
In the adjusted analysis, individuals from the ATT cohort were 3.67 times more likely to be retained in care. A unit increase in age was associated with a 1% increase in the odds of being retained in HIV care. Participants from rural areas had 32% higher odds of being retained in care than those from urban areas. Never married and divorced participants had 24 and 16% reduced odds of being retained in HIV care, respectively, compared to married ones. Baseline participants in WHO stage 2 had 18% increased odds of being retained in HIV care (OR 1.18; 95%CI 1.05, 1.32). However, baseline participants with WHO stage 3 had 23% reduced odds of being retained in HIV care. A unit increase in BMI increased the chance of being retained in care by 2% (Table 5).
3.8. Predictors of retention in HIV care among individuals (BTT and ATT)
In the BTT participant group, factors significantly associated with retention upon adjusted analysis included receiving care at a rural health facility (OR 1.54; 95%CI 1.21, 1.94) and being never married (OR 0.70; 95%CI 0.52, 0.95). In contrast, for the ATT cohort, factors linked to ART retention were increasing age (OR 1.02; 95%CI 1.01, 1.04) and being in WHO stage 2 (OR 2.32; 95%CI 1.20, 4.52). See Supplementary Table 2.
4. Discussion
This study aimed to determine the differences in retention rates and clinical outcomes for adult PWH enrolled on ART, BTT, and ATT policies. Participants who initiated ART and were enrolled in care after the test-and-treat policy had better retention in HIV care and health outcomes than those enrolled before the test-and-treat program. Our finding showed a higher prevalence of retention under ATT than what was previously reported in SSA (14) and Haiti (36); the variations could result from the fact that our study was conducted recently, and many other interventions have been aimed at improving retention in HIV care. Moreover, the majority of the participants in our project were on an INSTI-based regimen, which has shown higher tolerability than the other ART regimens (39). Retention was significantly associated with cohort (ATT), increasing baseline age, facility location (rural participants), marital status (never married and divorced), increasing baseline body mass index (BMI), and baseline WHO staging (2 and 3).
The evaluation results suggest that the universal and rapid test-and-treat implementation policy effectively retained clients in care. However, the clinical and immunological outcomes were better than BTT based on the studies conducted in Nigeria (40) and Zambia (20). Moreover, our follow-up period was longer than that in studies conducted in Nigeria and Lusaka, Zambia (2 years vs. 1 year). Retention was generally higher in the ATT cohort than in the BTT cohort, with an average retention rate of over 90%. However, the overall retention rate in the province was 83%. This is higher than reported in some African studies (41, 42) and recently in Lusaka, Zambia (20).
The enrollment age was younger for the ATT cohort than the BTT cohort, which is expected to indicate an effective and well-implemented policy. This was even evident with an average ART initiation turn-around time of 0 days compared to the BTT cohort, where the average ART initiation turn-around time was 14 days. This translated into good treatment outcomes, including clinical and immunological outcomes. For example, compared to the BTT cohort, a higher proportion of clients in the ATT cohort were in stage 1 of the WHO stage, had higher baseline CD4 counts, better attrition factors, and their BMI improved remarkably. However, the viral suppression and CD4 count seemed to be better before the test-and-treat policy. This could be because individuals under the ATT policy were relatively younger than those in BTT, and it has been well-studied that young people struggle to achieve viral suppression (43–45).
The majority of clients in the ATT cohort were retained on ART and still alive, and very few had stopped treatment, transferred out, lost to follow-up, or died. Our findings align with several clinical trials that assessed rapid universal treatment (46–49) and demonstrated that initiating individuals on ART immediately after an HIV diagnosis not only effectively retains PWH in care in real world settings but also enhances clinical outcomes, such as reducing mortality. On the contrary, US researchers observed similar rates of loss to follow-up between those who began ART on the same day and those who started based on their CD4 counts (13). These differences might be attributed to the varying sample sizes, with our study having greater statistical power. Additionally, recent interventions targeting a reduction in HIV loss to follow-up could also account for this discrepancy.
Mortality was significantly reduced during the ATT phase compared to the time before the universal test-and-treat policy, which is in line with what was observed in Ethiopia (50). One possible reason for this may be that, under BTT policies, most individuals had to reach an advanced WHO clinical stage before initiating ART (40, 51). Similarly, we observed that a greater number of participants were in the more severe WHO clinical stages before the implementation of the test-and-treat policy. The recipients of care ATT were younger than those BTT, which could partly explain the differences in death rates.
Our findings are not just the mere effect of implementing the test-and-treat policy among PWH; this program required implementing several programmatic adjustments, financial support, and increasing human resources to enhance care and support all structures involved in the treatment cascade of PWH, including counseling and testing services. In the real world, testing and treating all PWH to achieve viral suppression is possible when the whole ART cascade of care is enhanced.
4.1. Limitations of the study
Missing data led to delays in data abstraction. Some characteristics, such as viral load and CD4 count, were missing. We did not collect data on alcohol consumption, HIV disclosure status, year of enrollment for each participant, distance to the health facility, or adherence counseling, which could have helped explain retention rates among our study participants. Additionally, we could not get the patients’ perspectives regarding UTT policy. However, the study was able to quantify retention in HIV care and clinical outcomes before and after the universal test-and-treat policy.
4.2. Strengths of the study
We used a large sample size in our study compared to similar studies. Hence, this study was highly powered, and the results may be applicable to PWH living in Zambia. The methodology and statistical analysis were rigorous. The multicenter model and sampling methods used strengthened the study and minimized bias, providing a comprehensive overview of the whole program in the province.
5. Conclusion
Our data strongly indicate that the test-and-treat policy implemented in 2016 was significantly more effective in improving retention in HIV care and treatment compared to the BTT period. Additionally, clinical outcomes (alive and on treatment, transferred out of the facility, lost to follow-up, stopped treatment, and death) improved remarkably to acceptable levels in the ATT cohort. Factors that significantly influenced retention included the treatment policy, older age, location of the facility, not being married, high baseline BMI, and worse WHO stages. Hence, interventions targeting young individuals, urban care recipients, non-married people, and those with symptomatic WHO stages and a low BMI are encouraged.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was obtained from the Macha Research Trust Institution Review Board (IRB), and the Zambia National Health Research Authority (ZNHRA). This project was also reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures. In this study, we analyzed de-identified data from healthcare facilities. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was a retrospective study.
Author contributions
BH and SMa drafted the manuscript. BH and IF conducted all statistical analyses. BH, SMu, LS, MS, JM, SKu, SKa, CC, IF, CB, and SMa participated in data collection. BM, KM, and NK guided study design and draft review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of “Southern Province Health Office grant number NU2GGH002045.
Acknowledgments
The authors thank the data abstractors, Monde Chiyabe and Aviness Lishimpi, for their administrative work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not represent the official views of the Centers for Disease Control and Prevention.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1244125/full#supplementary-material
References
1. Harries, AD, Zachariah, R, Lawn, SD, and Rosen, S. Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Tropical Med Int Health. (2010) 15:70–5. doi: 10.1111/j.1365-3156.2010.02506.x
2. Lifson, AR, Grund, B, Gardner, EM, Kaplan, R, Denning, E, Engen, N, et al. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. (2017) 31:953–63. doi: 10.1097/QAD.0000000000001417
3. WHO Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. (2015) Available at: https://www.who.int/publications-detail-redirect/9789241509565 (Accessed July 19, 2023)
4. World Health Organization Progress report 2016: prevent HIV, test and treat all: WHO support for country impact. (2016). Available at: https://apps.who.int/iris/handle/10665/251713 (Accessed July 19, 2023).
5. Geng, EH, and Havlir, DV. The science of rapid start—From the when to the how of antiretroviral initiation. PLoS Med. (2017) 14:e1002358. doi: 10.1371/journal.pmed.1002358
6. Iwuji, CC, Orne-Gliemann, J, Larmarange, J, Balestre, E, Thiebaut, R, Tanser, F, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV. (2018) 5:e116–25. doi: 10.1016/S2352-3018(17)30205-9
7. Lofgren, SM, Tsui, S, Atuyambe, L, Ankunda, L, Komuhendo, R, Wamala, N, et al. Barriers to HIV care in Uganda and implications for universal test-and-treat: a qualitative study. AIDS Care. (2022) 34:597–605. doi: 10.1080/09540121.2021.1946000
8. Labhardt, ND, Ringera, I, Lejone, TI, Klimkait, T, Muhairwe, J, Amstutz, A, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA. (2018) 319:1103–12. doi: 10.1001/jama.2018.1818
9. TEMPRANO ANRS 12136 Study GroupDanel, C, Moh, R, Gabillard, D, Badje, A, Le Carrou, J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. (2015) 373:808–22. doi: 10.1056/NEJMoa1507198
10. INSIGHT START Study GroupLundgren, JD, Babiker, AG, Gordin, F, Emery, S, Grund, B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. (2015) 373:795–807. doi: 10.1056/NEJMoa1506816
11. Grinsztejn, B, Hosseinipour, MC, Ribaudo, HJ, Swindells, S, Eron, J, Chen, YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. (2014) 14:281–90. doi: 10.1016/S1473-3099(13)70692-3
12. Tanser, F, Bärnighausen, T, Grapsa, E, Zaidi, J, and Newell, M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. (2013) 339:966–71. doi: 10.1126/science.1228160
13. Pilcher, CD, Ospina-Norvell, C, Dasgupta, A, Jones, D, Hartogensis, W, Torres, S, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr. (2017) 74:44–51. doi: 10.1097/QAI.0000000000001134
14. Rosen, S, Maskew, M, Fox, MP, Nyoni, C, Mongwenyana, C, Malete, G, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: The RapIT randomized controlled trial. PLoS Med. (2016) 13:e1002015. doi: 10.1371/journal.pmed.1002015
15. Cohen, MS, Chen, YQ, McCauley, M, Gamble, T, Hosseinipour, MC, Kumarasamy, N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. (2016) 375:830–9. doi: 10.1056/NEJMoa1600693
16. Oldenburg, CE, Bor, J, Harling, G, Tanser, F, Mutevedzi, T, Shahmanesh, M, et al. Impact of early antiretroviral therapy eligibility on HIV acquisition: household-level evidence from rural South Africa. AIDS. (2018) 32:635–43. doi: 10.1097/QAD.0000000000001737
17. Hayes, RJ, Donnell, D, Floyd, S, Mandla, N, Bwalya, J, Sabapathy, K, et al. Effect of universal testing and treatment on HIV incidence—HPTN 071 (PopART). N Engl J Med. (2019) 381:207–18. doi: 10.1056/NEJMoa1814556
18. UNAIDS Fast-Track strategy to end the AIDS epidemic by 2023. (2023). Available at: https://www.unaids.org/en/resources/campaigns/World-AIDS-Day-Report-2014 (Accessed July 7, 2023).
19. Alhaj, M, Amberbir, A, Singogo, E, Banda, V, van Lettow, M, Matengeni, A, et al. Retention on antiretroviral therapy during Universal Test and Treat implementation in Zomba district, Malawi: a retrospective cohort study. J Int AIDS Soc. (2019) 22:e25239. doi: 10.1002/jia2.25239
20. Mody, A, Sikazwe, I, Namwase, AS, Wa Mwanza, M, Savory, T, Mwila, A, et al. Effects of implementing universal and rapid HIV treatment on initiation of antiretroviral therapy and retention in care in Zambia: a natural experiment using regression discontinuity. Lancet HIV. (2021) 8:e755–65. doi: 10.1016/S2352-3018(21)00186-7
21. Mugenyi, L, Nanfuka, M, Byawaka, J, Agaba, C, Mijumbi, A, Kagimu, D, et al. Effect of universal test and treat on retention and mortality among people living with HIV-infection in Uganda: An interrupted time series analysis. PLoS One. (2022) 17:e0268226. doi: 10.1371/journal.pone.0268226
22. Baisley, K, Orne-Gliemann, J, Larmarange, J, Plazy, M, Collier, D, Dreyer, J, et al. Early HIV treatment and survival over six years of observation in the ANRS 12249 treatment as prevention trial. HIV Med. (2022) 23:922–8. doi: 10.1111/hiv.13263
23. Hirasen, K, Fox, MP, Hendrickson, CJ, Sineke, T, and Onoya, D. HIV treatment outcomes among patients initiated on antiretroviral therapy pre and post-universal test and treat guidelines in South Africa. Ther Clin Risk Manag. (2020) 16:169–80. doi: 10.2147/TCRM.S227290
24. Kimanga, DO, Oramisi, VA, Hassan, AS, Mugambi, MK, Miruka, FO, Muthoka, KJ, et al. Uptake and effect of universal test-and-treat on twelve months retention and initial virologic suppression in routine HIV program in Kenya. PLoS One. (2022) 17:e0277675. doi: 10.1371/journal.pone.0277675
25. Chauke, P, Huma, M, and Madiba, S. Lost to follow up rate in the first year of ART in adults initiated in a universal test and treat programme: a retrospective cohort study in Ekurhuleni District. Pan Afr Med J. (2020) 37:198. doi: 10.11604/pamj.2020.37.198.25294
26. Nicol, E, Basera, W, Mukumbang, FC, Cheyip, M, Mthethwa, S, Lombard, C, et al. Linkage to HIV care and early retention in care rates in the universal test-and-treat era: a population-based prospective study in KwaZulu-Natal. South Africa AIDS Behav. (2023) 27:1068–81. doi: 10.1007/s10461-022-03844-w
27. Rosen, S, Maskew, M, Larson, BA, Brennan, AT, Tsikhutsu, I, Fox, MP, et al. Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya. PLoS Med. (2019) 16:e1002912. doi: 10.1371/journal.pmed.1002912
28. Gosset, A, Protopopescu, C, Larmarange, J, Orne-Gliemann, J, McGrath, N, Pillay, D, et al. Retention in care trajectories of HIV-positive individuals participating in a universal test-and-treat program in Rural South Africa (ANRS 12249 TasP Trial). J Acquir Immune Defic Syndr. (2019) 80:375–85. doi: 10.1097/QAI.0000000000001938
29. UN (2023). Goal 3: Ensure healthy lives and promote well-being for all at all ages. United Nations Sustainable Development https://www.un.org/sustainabledevelopment/health/ (Accessed July 11, 2023)
30. WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. (2021). Available at: https://www.who.int/publications-detail-redirect/9789240031593 (Accessed July 11, 2023).
31. Bantie, B, Seid, A, Kerebeh, G, Alebel, A, and Dessie, G. Loss to follow-up in “test and treat era” and its predictors among HIV-positive adults receiving ART in Northwest Ethiopia: Institution-based cohort study. Front Public Health. (2022) 10:876430. doi: 10.3389/fpubh.2022.876430
32. Lb, B, Dv, H, Ayieko, J, Mwangwa, F, Owaraganise, A, Kwarisiima, D, et al. High levels of retention in care with streamlined care and universal test-and-treat in East Africa. AIDS. (2016) 30:2855–64. doi: 10.1097/QAD.0000000000001250
33. Elgalib, A, Shah, S, Al-Wahaibi, A, Al-Habsi, Z, Al-Fouri, M, Lau, R, et al. Retention in HIV care and factors associated with loss to follow-up in Oman: a countrywide study from the Middle East. AIDS Care. (2022) 34:568–74. doi: 10.1080/09540121.2021.1916871
34. Genberg, BL, Lee, H, Hogan, JW, Some, F, Wachira, J, Wu, XK, et al. Point of diagnosis and patient retention in HIV care in Western Kenya. J Acquir Immune Defic Syndr. (2018) 78:383–9. doi: 10.1097/QAI.0000000000001703
35. Muwanguzi, M, Lugobe, HM, Ssemwanga, E, Lule, AP, Atwiine, E, Kirabira, V, et al. Retention in HIV care and associated factors among youths aged 15-24 years in rural southwestern Uganda. BMC Public Health. (2021) 21:1489. doi: 10.1186/s12889-021-11547-5
36. Koenig, SP, Dorvil, N, Dévieux, JG, Hedt-Gauthier, BL, Riviere, C, Faustin, M, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. (2017) 14:e1002357. doi: 10.1371/journal.pmed.1002357
37. MOH Zambia HIV Consolidated Guidelines 2022—Google Search. (2022). Available at: https://www.google.com/search?q=zambia+hiv+consolidated+guidelines+2022&sxsrf=AB5stBj3aym3sJLiXO8wa1IYMczwEkWTLA%3A1689099566629&source=hp&ei=Lp2tZM6rJP2ChbIPjtaTsAI&iflsig=AD69kcEAAAAAZK2rPgeOaPnMOy2_jxqD7Avh-JpER5z4&oq=Zambia+HIV+consolidated+&gs_lcp=Cgdnd3Mtd2l6EAEYADIFCAAQgAQyBQgAEIAEMggIABCKBRCGAzIICAAQigUQhgMyCAgAEIoFEIYDOgcIIxCKBRAnOggIABCABBCxAzoECAAQAzoRCC4QgwEQxwEQsQMQ0QMQgAQ6CwguEIAEELEDEIMBOgsIABCABBCxAxCDAToLCAAQigUQsQMQgwE6EQguEIAEELEDEIMBEMcBENEDOggILhCABBCxAzoFCC4QgAQ6BwgAEIAEEAo6DgguEBYQHhDHARDRAxAKOgYIABAWEB46BwgAEA0QgAQ6DQguEA0QgAQQxwEQ0QM6CAgAEAgQHhANOgYIABAIEB46BQgAEKIEOgUIIRCgAVAAWMu8AWDF3QFoAHAAeACAAcsGiAGtcJIBCzMtMjMuMTAuMi4xmAEAoAEB&sclient=gws-wiz (Accessed July 11, 2023).
38. Dupont, WD, and Plummer, WD. Power and sample size calculations. A review and computer program. Control Clin Trials. (1990) 11:116–28. doi: 10.1016/0197-2456(90)90005-m
39. Raffi, F, Esser, S, Nunnari, G, Pérez-Valero, I, and Waters, L. Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens. HIV Med. (2016) 17:3–16. doi: 10.1111/hiv.12440
40. Stafford, KA, Odafe, SF, Lo, J, Ibrahim, R, Ehoche, A, Niyang, M, et al. Evaluation of the clinical outcomes of the test and treat strategy to implement treat all in nigeria: results from the nigeria multicenter ART study. PLoS One. (2019) 14:e0218555. doi: 10.1371/journal.pone.0218555
41. Abebe Moges, N, Olubukola, A, Micheal, O, and Berhane, Y. HIV patients retention and attrition in care and their determinants in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. (2020) 20:439. doi: 10.1186/s12879-020-05168-3
42. Prinapori, R, Giannini, B, Riccardi, N, Bovis, F, Giacomini, M, Setti, M, et al. Predictors of retention in care in HIV-infected patients in a large hospital cohort in Italy. Epidemiol Infect. (2018) 146:606–11. doi: 10.1017/S0950268817003107
43. Cherutich, P, Kim, AA, Kellogg, TA, Sherr, K, Waruru, A, De Cock, KM, et al. Detectable HIV viral load in Kenya: data from a population-based survey. PLoS One. (2016) 11:e0154318. doi: 10.1371/journal.pone.0154318
44. Jiamsakul, A, Kariminia, A, Althoff, KN, Cesar, C, Cortes, CP, Davies, M-A, et al. HIV viral load suppression in adults and children receiving antiretroviral therapy-results from the IeDEA collaboration. J Acquir Immune Defic Syndr. (2017) 76:319–29. doi: 10.1097/QAI.0000000000001499
45. Abimiku, A, Ramadhani, HO, Moloney, M, Stafford, KA, Chang, JC-W, Patel, HK, et al. Factors associated with viral suppression among adults living with HIV on antiretroviral therapy in Nigeria: Analysis of a population-based survey, 2018. HIV Med. (2023) 24:827–37. doi: 10.1111/hiv.13485
46. Perriat, D, Balzer, L, Hayes, R, Lockman, S, Walsh, F, Ayles, H, et al. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc. (2018) 21:e25048. doi: 10.1002/jia2.25048
47. Havlir, D, Lockman, S, Ayles, H, Larmarange, J, Chamie, G, Gaolathe, T, et al. What do the Universal Test and Treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc. (2020) 23:e25455. doi: 10.1002/jia2.25455
48. Khan, S, Spiegelman, D, Walsh, F, Mazibuko, S, Pasipamire, M, Chai, B, et al. Early access to antiretroviral therapy versus standard of care among HIV-positive participants in Eswatini in the public health sector: the MaxART stepped-wedge randomized controlled trial. J Int AIDS Soc. (2020) 23:e25610. doi: 10.1002/jia2.25610
49. Lebelonyane, R, Bachanas, P, Block, L, Ussery, F, Abrams, W, Roland, M, et al. Rapid antiretroviral therapy initiation in the Botswana Combination Prevention Project: a quasi-experimental before and after study. Lancet HIV. (2020) 7:e545–53. doi: 10.1016/S2352-3018(20)30187-9
50. Girum, T, Yasin, F, Wasie, A, Shumbej, T, Bekele, F, and Zeleke, B. The effect of “universal test and treat” program on HIV treatment outcomes and patient survival among a cohort of adults taking antiretroviral treatment (ART) in low income settings of Gurage zone, South Ethiopia. AIDS Res Ther. (2020) 17:19. doi: 10.1186/s12981-020-00274-3
51. Ingabire, PM, Semitala, F, Kamya, MR, and Nakanjako, D. Delayed antiretroviral therapy (ART) initiation among hospitalized adults in a resource-limited settings: a challenge to the global target of ART for 90% of HIV-infected individuals. AIDS Res Treat. (2019) 2019:1832152. doi: 10.1155/2019/1832152
Keywords: retention, HIV, testing, antiretroviral therapy, Zambia, clinical outcomes, test-and-treat, Southern Province
Citation: Hamooya BM, Mutembo S, Muyunda B, Mweebo K, Kancheya N, Sikazwe L, Sakala M, Mvula J, Kunda S, Kabesha S, Cheelo C, Fwemba I, Banda C and Masenga SK (2023) HIV test-and-treat policy improves clinical outcomes in Zambian adults from Southern Province: a multicenter retrospective cohort study. Front. Public Health. 11:1244125. doi: 10.3389/fpubh.2023.1244125
Edited by:
Katri Jalava, Royal Veterinary College (RVC), United KingdomReviewed by:
Wei Li Adeline Koay, Children’s National Hospital, United StatesKalina Andreevska, Sofia University, Bulgaria
Joshua Murphy, Independent researcher, Randburg, South Africa
Copyright © 2023 Hamooya, Mutembo, Muyunda, Mweebo, Kancheya, Sikazwe, Sakala, Mvula, Kunda, Kabesha, Cheelo, Fwemba, Banda and Masenga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sepiso K. Masenga, c2VwaXNvbWFzZW5nYUBnbWFpbC5jb20=
 Benson M. Hamooya
Benson M. Hamooya Simon Mutembo2
Simon Mutembo2 Shem Kabesha
Shem Kabesha Isaac Fwemba
Isaac Fwemba Sepiso K. Masenga
Sepiso K. Masenga