- 1Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China
- 2Anhui Provincial Center for Disease Control and Prevention, Hefei, China
- 3Brown School, Washington University, St. Louis, MO, United States
- 4Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention, Nanning, China
- 5Hubei Provincial Center for Disease Control and Prevention, Wuhan, China
- 6Henan Provincial Center for Disease Control and Prevention, Zhengzhou, China
- 7Global and Tropical Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, NT, Australia
- 8College of Medicine and Public Health, Flinders University, Darwin, NT, Australia
- 9National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), NHC Key Laboratory of Parasite and Vector Biology, WHO Collaborating Center for Tropical Diseases, National Center for International Research on Tropical Diseases, Shanghai, China
Introduction: The provincial malaria diagnosis reference laboratories review and assess malaria cases diagnosed in health facilities for supporting the malaria elimination efforts and preventing re-transmission of imported malaria. The study aimed to evaluate the detection capability of malaria diagnosis in China from 2014 to 2021.
Methods: Data on malaria cases reported in the provincial-level administrative divisions (PLADs) of Anhui, Henan, Hubei, Guangxi, and Zhejiang from 2014 to 2021 were collected and analyzed.
Results: In total, 5,770 malaria cases were reported from 2014 to 2021, and 99.05% (5,715/5,770) were submitted to the provincial malaria diagnosis reference laboratories. The median time between malaria cases being reported and the samples being received by reference laboratories was 6 days (Interquartile range, IQR:3–12 days) from 2017 to 2021. Diagnosis of 5,680 samples in the laboratory were confirmed by provincial reference laboratories, including 3,970 cases of Plasmodium falciparum, 414 of P. vivax, 1,055 of P. ovale, 158 of P. malariae, 1 of P. knowlesi, and 82 of mixed infections. Plasmodium species of 5,141 confirmed cases were consistent with the initial diagnosis, with a species accuracy rate of 90.53% (5,141/5,679). The accuracy of P. falciparum diagnosis in health facilities was higher than that of non-falciparum species. The inconsistency between microscopy and nested polymerase chain reaction (nPCR) results of confirmatory diagnosis was mainly in malaria-positive versus malaria-negative cases, as well as in mixed versus single infection cases.
Conclusion: The provincial malaria diagnosis reference laboratories have played an important role in ensuring the accuracy and reliability of Plasmodium diagnosis in health facilities. However, the results of this study imply that capacity training for the identification of Plasmodium species in health facilities is warranted.
Introduction
Malaria is a vector-borne disease caused by parasites of Plasmodium species that are transmitted to people through the bites of infected female Anopheles mosquitoes and is one of the major public health problems worldwide. In 2021, there were 247 million estimated cases of malaria worldwide, and 619,000 estimated malaria-related deaths. The World Health Organization (WHO) African Region carries a disproportionately high share of the global malaria burden (1). After decades of efforts, China has not reported indigenous malaria cases since 2017 (2) and was certified malaria-free by the WHO on the 30th of June 2021. However, a large number of imported malaria cases have been reported annually in China (3).
Timely detection and identification of all malaria cases, the first line of defense against malaria in China, is essential to prevent transmission and deaths (4, 5). Methods used for malaria diagnosis in health facilities include microscopy and rapid diagnostic tests (RDTs), and RDTs were usually based on the detection of Plasmodium falciparum lactate dehydrogenase (pf-LDH) and Plasmodium lactate dehydrogenase (pan-pLDH) antigens. Since 2011, the China malaria diagnosis reference laboratory network based on provincial Centers for Disease Control and Prevention (CDCs) and the Institute of Parasitic Diseases (IPDs) has been set up in stages to review and assess malaria cases using microscopy and molecular diagnostic tools (6). The provincial malaria diagnosis reference laboratories are primarily responsible for case reviews, malaria diagnostic capacity training, and quality assurance of the performance of malaria case identification in health facilities (7). A number of measures to control the internal quality assessment of malaria diagnosis, such as routine reviews of blood samples, and provincial blind sample assessment, have been carried out continuously. Furthermore, technique competitions of parasitic diseases including malaria were held to promote diagnostic capacity in health facilities in the province (8). Likewise, the provincial reference laboratory diagnosis provides guidance for epidemiological investigation and standardized treatment of reported cases.
Anhui, Henan, and Hubei provinces are historically malaria-endemic provinces in central China (9–11). Guangxi Zhuang Autonomous Region and Zhejiang Province, located in the south and southeast of China, respectively, are the regions with large number of imported malaria cases (12, 13). In order to assess malaria diagnosis and explore strategy to improve diagnostic capacity, we analyzed in this study the accuracy of malaria diagnosis in the five provincial-level administrative divisions (PLADs) of Anhui, Henan, Hubei, Guangxi, and Zhejiang from 2014 to 2021.
Methods
Case diagnosis and report
According to WHO malaria terminology, an imported case corresponds to a patient in which the parasite has been detected in a diagnostic test and malaria infection was acquired outside the area where it was diagnosed (14). Clinicians should order microscopic examination of thick and thin blood smears, as well as a rapid diagnostic test (RDT) for malaria. Malaria is a notifiable infectious disease in China, and health facilities at each of the administrative level are required to report cases to the China Information System for Disease Control and Prevention (CISDCP) within 24 h of malaria diagnosis.
Reference laboratory confirmation
The CDC staff at the administrative county from where malaria cases were reported immediately conducted epidemiological investigation to identify the origins of malaria infection, as well as transported blood smears and samples to the provincial malaria diagnosis reference laboratories. Microscopic examination and nested polymerase chain reaction (nPCR) on each sample were performed by the provincial malaria diagnosis reference laboratories as described elsewhere (7, 15) to confirm the species, and the results of confirmatory diagnosis were subsequently fed back to county-level CDCs.
Criteria for confirmation of Plasmodium species in provincial reference laboratories: when Plasmodium was detected by microscopy only or nPCR only, the presence of Plasmodium was determined by using the results of the positive test method; when the results of microscopy and nPCR were inconsistent, the nPCR results overruled the results obtained by microscopy.
Data source and collection
Data on malaria cases reported in the five PLADs from 2014 to 2021 were collected through the China Information System for Disease Control and Prevention (CISDCP) and the Information System for Parasitic Disease Control and Prevention (ISPDCP), a subsystem of CISDCP. Moreover, the date of samples received by the provincial reference laboratory has been more fully recorded since 2017. Epidemiological history, countries visited, date of illness onset, diagnostic institutions, date of report, microscopy and RDTs results reported by health facilities, microscopy and nPCR results confirmed by the provincial reference laboratories were used for analysis.
Data analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS version 25; SPSS Inc., Chicago). Box plot was used to describe time interval between cases being reported and samples being received. The accuracy rates of Plasmodium species was expressed by the consistency between Plasmodium species diagnosed in health facilities and Plasmodium species confirmed by the provincial reference laboratory.
Results
General description
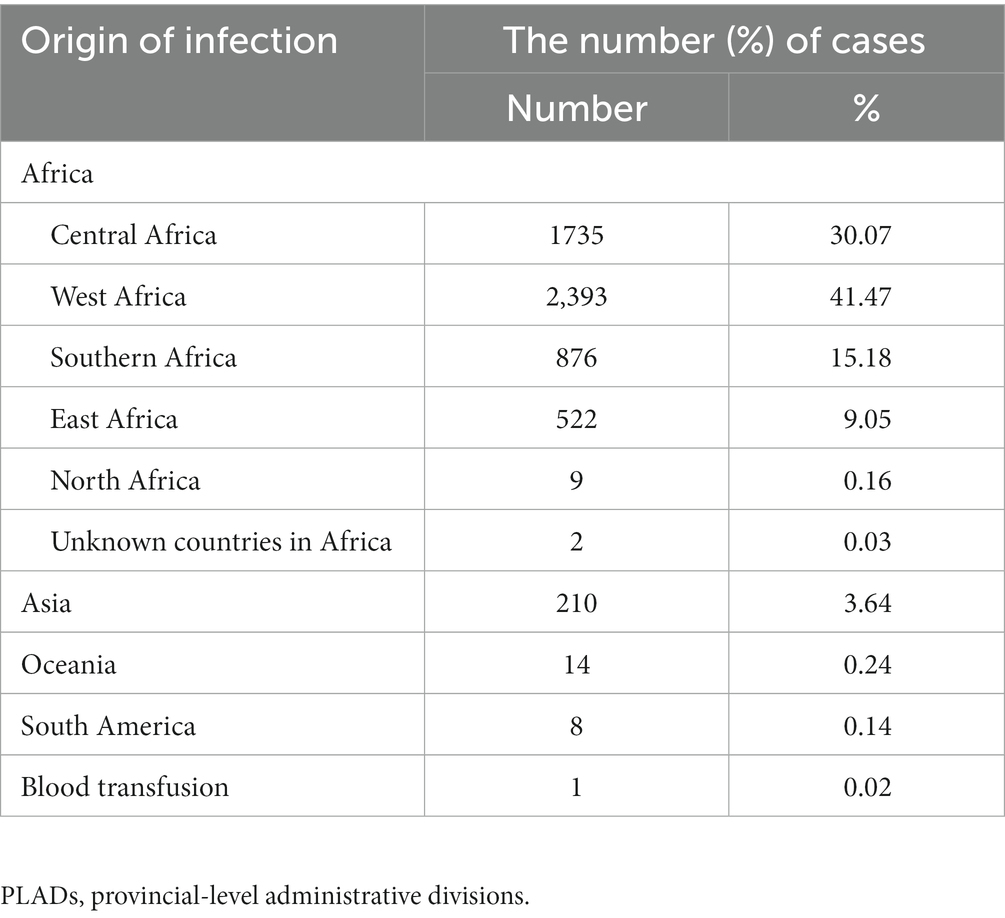
A total of 5,770 malaria cases were reported from 2014 to 2021 (743 in Anhui, 1,643 in Guangxi, 1,285 in Zhejiang, 832 in Hubei, and 1,267 in Henan), all of which were imported malaria cases, with the exception of one case of infection through blood transfusion in Guangxi. The number of malaria cases ranged from 139 to 1,035 in the years studied. Most infections (5,537, 95.96%) originated in Africa. Specifically, 41.47% (2,393/5,770) of the cases in this study were imported from the West African Region, followed by countries in Central Africa (1,735, 30.07%) and Southern Africa (876, 15.18%). Ghana (14.80%, 854/5770), Nigeria (12.13%, 700/5770), Cameroon (9.79%, 565/5770), and Angola (9.58%, 553/5770) were the main importing countries. Cases from other countries of Asia accounted for 3.64% (210/5,770) of the total cases (Table 1).
Sample collection
Among the 5,770 reported malaria cases, 5,715 samples (5,715/5,770, 99.05%) were submitted to the provincial malaria diagnosis reference laboratories. The remaining 55 cases that lacked blood samples retained the initial diagnosis results of health facilities, mainly occurring between 2014 and 2016. Among the 5,715 submitted samples, 5,645 consisted of both blood smears and blood samples, 30 consisted of blood smears only, and 40 consisted of blood samples only. A total of 5,680 cases were initially diagnosed in health facilities using microscopy and RDTs, while the remaining 35 were directly diagnosed by the provincial laboratories. Figure 1 depict the flow chart of samples submitted to the provincial reference laboratories.
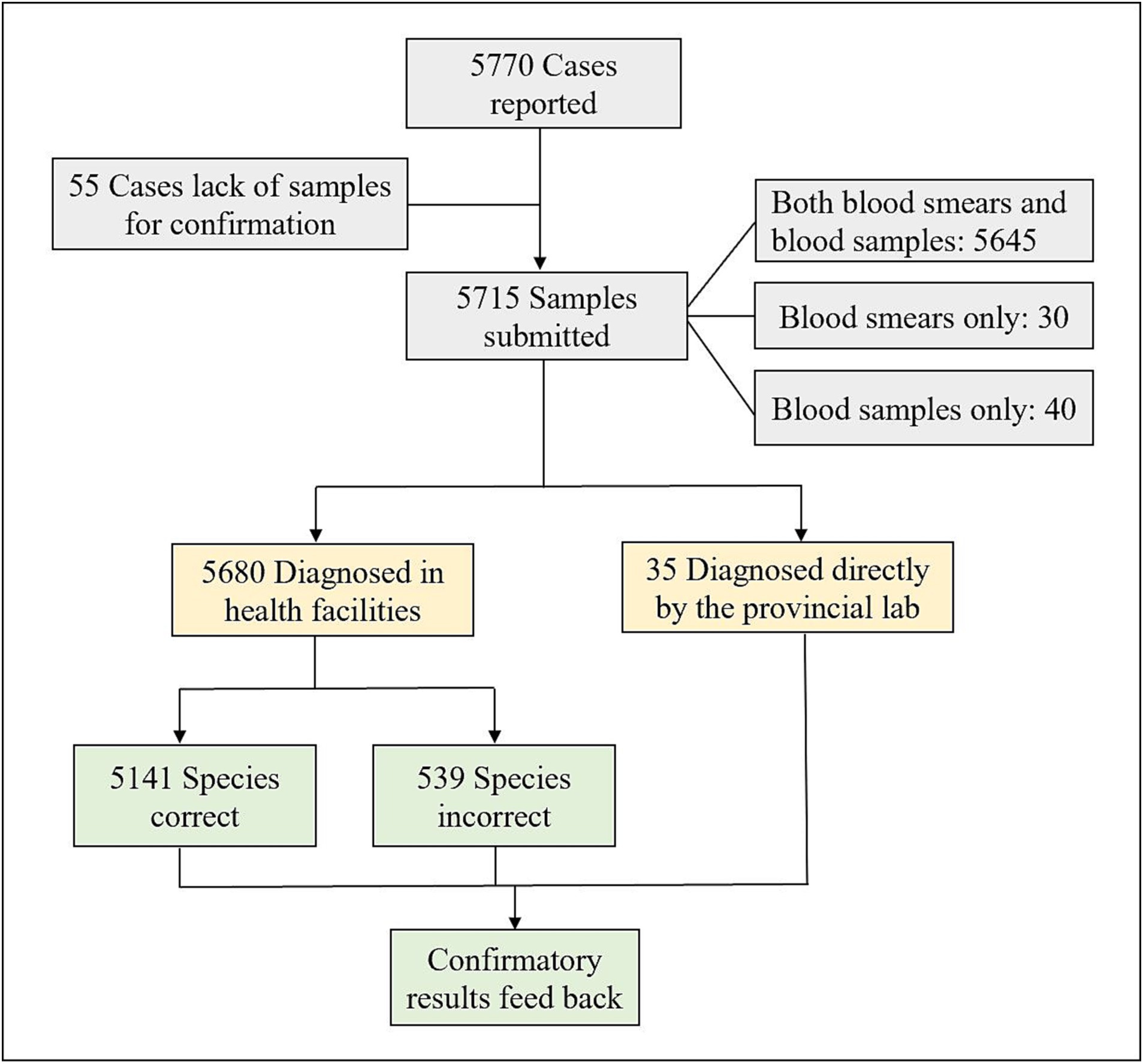
Figure 1. Flow chart of samples submitted to the reference laboratories for confirmation of malaria diagnosis in the five PLADs in China from 2014 to 2021.
Time interval between cases being reported and samples being received by the reference laboratories
From 2017 to 2021, the median interval between malaria cases being reported and samples being received by the provincial reference laboratories was 6 days (Interquartile range, IQR:3–12 days). Time interval in 2017 was longer than that in the 2018–2021 period (Figure 2). The interval for Plasmodium falciparum samples was longer than that for non-falciparum species samples, with the median interval being 7 (IQR:4–13 days) and 6 days (IQR:3–10 days), respectively. The interval for Plasmodium vivax samples was shorter than that for non-vivax species samples, with the median interval being 5 (IQR:3–9 days) and 7 days (IQR:4–13 days), respectively.
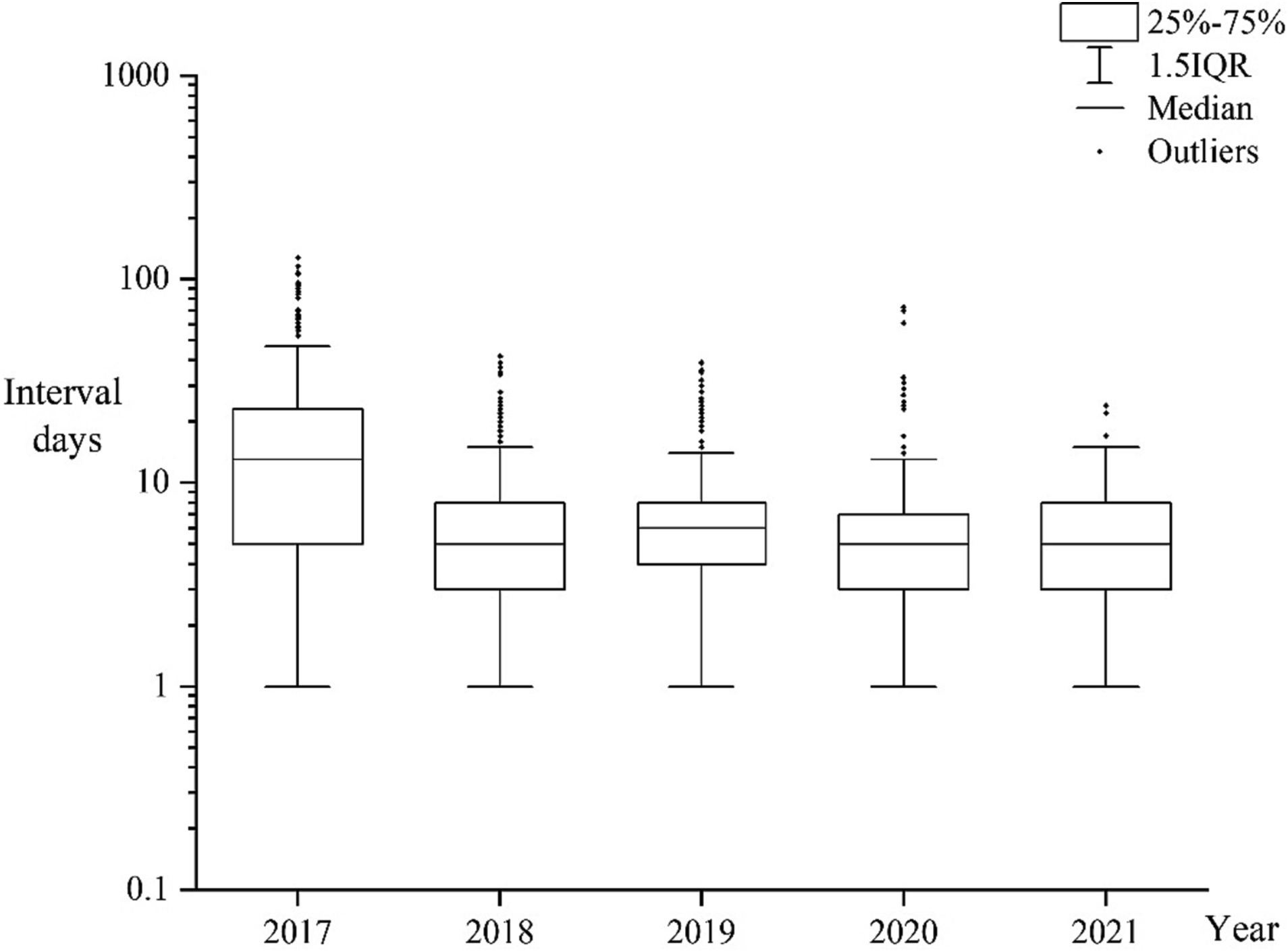
Figure 2. Box plot representing time interval between cases being reported and samples being received by the reference laboratories from 2017 to 2021.
Malaria cases initially diagnosed in health facilities
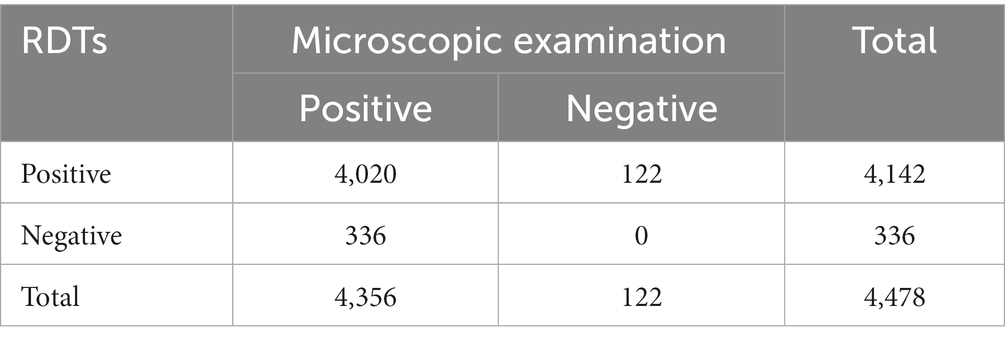
For the 5,680 malaria cases initially diagnosed in health facilities, results of 4,478 cases were obtained using both microscopy and RDTs. The malaria-positivity rates obtained by microscopy and RDTs at health facilities were 97.28% (4,356/4,478) and 92.50% (4,142/4,478), respectively. The consistency between the two methods was 89.77% (4,020/4,478) (Table 2).
Results of confirmatory diagnosis obtained by reference laboratory
A total of 5,680 samples submitted to the provincial reference laboratories were initially diagnosed in health facilities, including 3,970 cases of P. falciparum, 414 cases of P. vivax, 1,055 cases of P. ovale, 158 cases of P. malariae, 82 cases with mixed Plasmodium species infections, and 1 case of P. knowlesi. The confirmation of Plasmodium species in 5,141 cases was consistent with the initial diagnosis (Figure 1), including 3,867 cases of P. falciparum, 370 cases of P. vivax, 769 cases of P. ovale, 102 cases of P. malariae, and 33 cases with mixed species infections, with an accuracy rate of 90.53% (5,141/5,679) (one case of P. knowlesi was not included; Table 3). The accuracy rates for species identification of P. falciparum, P. vivax, P. ovale, P. malariae and mixed infections were 97.41% (3,867/3,970), 89.37% (370/414), 72.89% (769/1,055), 64.56% (102/158), and 40.24% (33/82), respectively, and the accuracy of P. falciparum diagnosis was higher than that of non-falciparum cases. Comparison of species identification between the five PLADs showed that the accuracy of Plasmodium species identified in health facilities in Guangxi Zhuang Autonomous Region was higher than that in the other four provinces.
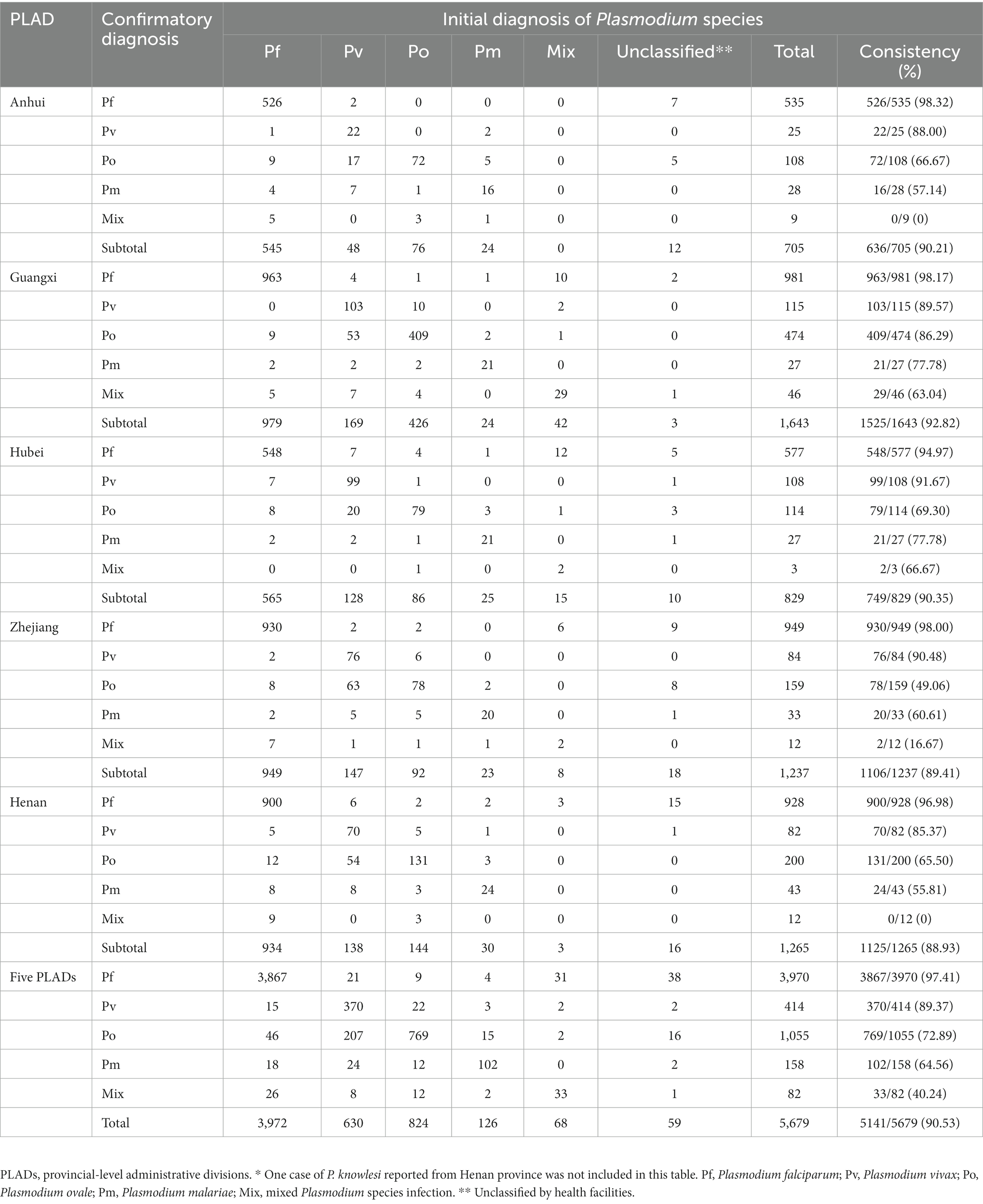
Table 3. Comparison of Plasmodium species initially diagnosed in the health facilities with those confirmed by the reference laboratories*.
Double confirmation of plasmodium species
Of the malaria samples submitted to reference laboratories, 5,645 cases were double confirmed using both microscopy and nPCR, 30 cases were confirmed by microscopy with blood smears only, and 40 cases were confirmed by nPCR with blood samples only. The consistency between microscopy and nPCR confirmation was 95.09% (5,368/5,645). Among the 277 inconsistent cases, 0.55% (31/5,645) were confirmed as malaria-positive using microscopy, but malaria-negative using nPCR, and 3.60% (203/5,645) were confirmed as malaria-negative using microscopy, but malaria-positive using nPCR. The remaining 43 cases (0.76%, 43/5645) were inconsistent in Plasmodium species identification, including 15 identified as P. vivax using microscopy versus P. ovale using nPCR, and 28 as single infections with P. falciparum, P. vivax, P. ovale or P. malariae using microscopy versus mixed infections using nPCR.
Discussion
The malaria diagnosis reference laboratory network has provided technical support for identifying malaria cases during its elimination and prevention of re-transmission of imported malaria in China. Malaria diagnosis in China still faces many challenges, including improvement of microscopy competency at lower levels (7) and promotion awareness of malaria diagnosis in an elimination setting (16).
Microscopy is still the gold standard method for malaria diagnosis (17), although it is time-consuming and requires extensive technical experience. Malaria RDTs have been widely used in primary health facilities due to their simplicity and convenience (18). In fact, more than 78% of the total diagnoses were made using both microscopy and RDTs in the five PLADs, and microscopy showed better sensitivity than RDTs. Malaria RDTs are usually most effective in cases of P. falciparum (19). Jean et al. revealed that the sensitivity of RDTs was 93% (90.6–94.2%) for P. vivax in French Amazonia (20); whereas RDTs still showed low sensitivity and specificity for P. ovale and P. malariae in various other diagnostic contexts (21). In addition, malaria RDTs based on P. falciparum histidine-rich protein 2 (HRP2) raised malaria public health concerns because HRP2 deletions have been reported in different parts of the world (22). In view of their limitations, malaria RDTs are suggested to be complemented by microscopy to detect malaria, rather than being used as an alternative to microscopy in non-endemic areas (23, 24).
The accuracy for species identification initially diagnosed in the five PLADs was 90.51% from 2014 to 2021, which was higher than the 62–66% accuracy reported in China in 2015 (7), but similar to the 88.8% reported in Jiangsu Province in 2017 (25) and the 92.6% reported in Yunnan Province from 2013 to 2018 (26). Moreover, the accuracy of P. falciparum diagnosis remained above 94% in all five PLADs, but showed poorer accuracy for species identification of P. ovale and P. malariae. This may be due to the distribution of imported malaria species reported in China over recent years (27, 28), with P. falciparum as the predominant species, increased cases of P. ovale, and decreased cases of P. vivax. Consequently, the diagnostic capacity of health facilities for P. falciparum was maintained at a high level, whereas the capacity for non-falciparum species declined. Further comparison of species identification between the five PLADs showed that the accuracy of malaria diagnosis in Guangxi Zhuang Autonomous Region was higher than that in the other four provinces. This may be related to migrant workers returning from Africa (i.e., the Republic of Ghana) to Shanglin County of Guangxi in clusters which resulted in a significant increase in the number of malaria cases in 2013 (12). Therefore, the high prevalence of malaria cases at these might be advantageous in maintaining the microscopic ability to identify malaria in a stable state.
Case detection and accurate identification of Plasmodium species directly affect the implementation of malaria prevention and control measures (29). According to the national technical program for malaria elimination, the provincial reference laboratories for malaria diagnosis are required to provide timely feedback on results of confirmatory diagnosis to the administrative county from where malaria cases were reported. The results showed that the time interval from cases being reported to samples being received by the reference laboratories was shorter in 2018 to 2021 than that in 2017. Given the risk of local transmission caused by P. vivax (30) due to suitable vectors and conditions in historically malaria-endemic areas in China, timely detection of P. vivax for foci investigation and disposal to prevent re-transmission is of crucial importance. As shown in this study, the time interval was shorter for P. vivax than that for non-vivax species. Another reason to pay attention to P. vivax is that P. vivax forms dormant in the liver (hypnozoites) that can cause relapses and requires primaquine for radical cure (31), which remains a challenge to diagnosis and treatment.
The inconsistency between microscopy and nPCR confirmation at provincial reference laboratories was mainly in determining malaria positivity or negativity. Explanations for this included low parasite density affecting the ability to detect malaria by microscopy (32), some patients with illness history self-medicated after their return from overseas resulting in low detectability of viable parasites in the bloodstream by microscopy, or non-optimal sampling time of blood smears and samples for diagnosis (33). In addition, some differences in reference laboratory confirmatory diagnosis showed inconsistencies between mixed and single infections, as well as between P. ovale and P. vivax. Therefore, it is necessary to strengthen training to distinguish the morphology of Plasmodium species mentioned above and improve the detection ability of microscopists in samples with low parasitemia.
Our study has two main limitations. First, the study was retrospective; hence, some information may have gone missing during the study period, such as the time interval of samples received by the reference laboratories from 2014 to 2016. Secondly, the database only included reported malaria-positive cases; those initially diagnosed as malaria with confirmed malaria-negative results were not included in the analysis.
Conclusion
The findings of this study showed that the accuracy of species identification by health facilities was higher than 90% in the five PLADs from 2014 to 2021, but fell short in the detection of P. ovale and P. malariae. Capacity training for the identification of Plasmodium species in health facilities needs to be strengthened. Multiple test approaches including microscopy and nPCR have enabled correct diagnosis of malaria, thus contributing to malaria elimination in China. The provincial malaria diagnosis reference laboratories have played an important role in ensuring the accuracy and reliability of malaria diagnosis in the elimination phase, as well as in the consolidation phase of malaria elimination.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
SZL, WR, and DQW conceived and designed the study. JJJ, YS, HY, JX, YL, LCS, XXW, and SNL contributed to data collection, finding interpretation, and the writing. XZ and JM performed the analyses. XZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by China–Africa cooperation project on malaria control under the Project No. 2020-C4-0002-3 and the Program of the Chinese Center for Tropical Diseases Research (No. 131031104000160004); UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) Small Grant (WHO Reference 2021/1104003-0); and the Medical Research Program of Zhejiang Province (No. 2022KY723).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
WHO, World Health Organization; CDCs, Centers for Disease Control and Prevention; IPDs, Institute of Parasitic Diseases; PLADs, Provincial-level administrative divisions; RDTs, Rapid Diagnostic Tests; nPCR, Nested polymerase chain reaction; CISDCP, China Information System for Disease Control and Prevention; ISPDCP, Information System for Parasitic Disease Control and Prevention; IQR, Interquartile range; HRP2, Histidine-rich protein 2.
References
2. Feng, J, Zhang, L, Huang, F, Yin, JH, Tu, H, Xia, ZG, et al. Ready for malaria elimination: zero indigenous case reported in the People's Republic of China. Malar J. (2018) 17:315. doi: 10.1186/s12936-018-2444-9
3. Zhang, SS, Feng, J, Zhang, L, Ren, X, Geoffroy, E, Manguin, S, et al. Imported malaria cases in former endemic and non-malaria endemic areas in China: are there differences in case profile and time to response? Infect Dis Poverty. (2019) 8:61. doi: 10.1186/s40249-019-0571-3
4. Ding, GS, Zhu, GD, Cao, CQ, Miao, P, Cao, YY, Wang, WM, et al. The challenge of maintaining microscopist capacity at basic levels for malaria elimination in Jiangsu Province, China. BMC Public Health. (2018) 18:489. doi: 10.1186/s12889-018-5307-y
5. Zhang, T, Xu, X, Jiang, JJ, Yu, C, Tian, CC, Xie, QX, et al. Risk factors of severe imported malaria in Anhui province, China. Acta Trop. (2019) 197:104934. doi: 10.1016/j.actatropica.2019.02.020
6. Yin, JH, Li, M, Yan, H, Zhou, SS, and Xia, ZG. Laboratory diagnosis for malaria in the elimination phase in China: efforts and challenges. Front Med. (2022) 16:10–6. doi: 10.1007/s11684-021-0889-7
7. Yin, JH, Yan, H, Huang, F, Li, M, Xiao, HH, Zhou, SS, et al. Establishing a China malaria diagnosis reference laboratory network for malaria elimination. Malar J. (2015) 14:40. doi: 10.1186/s12936-015-0556-z
8. Wu, JL, Tu, ZW, Pei, JS, Zhang, HX, Zhu, H, Cai, SX, et al. Competition results of parasitic disease control techniques among technician in Hubei Province in 2015. Chin J Parasitol Parasit Dis. (2016) 34:154–156, 160.
9. Li, WD, Zhang, T, Xu, X, Jiang, JJ, Yu, C, Tian, CC, et al. Problems associated with the diagnosis of imported malaria in Anhui Province, China. Am J Trop Med Hyg. (2020) 102:142–6. doi: 10.4269/ajtmh.19-0471
10. Liu, Y, He, ZQ, Wang, D, Hu, YB, Qian, D, Yang, CY, et al. One health approach to improve the malaria elimination programme in Henan Province. Adv Parasitol. (2022) 116:153–86. doi: 10.1016/bs.apar.2022.02.001
11. Xia, J, Wu, DN, Zhu, H, Wan, L, Zhang, J, Lin, W, et al. Course of malaria control and elimination in Hubei Province. Chin J Parasitol Parasit Dis. (2021) 39:565–71. doi: 10.12140/j.issn.1000-7423.2021.05.001
12. Feng, J, Xiao, HH, Xia, ZG, Zhang, L, and Xiao, N. Analysis of malaria epidemiological characteristics in the People's Republic of China, 2004–2013. Am J Trop Med Hyg. (2015) 93:293–9. doi: 10.4269/ajtmh.14-0733
13. Zhang, X, Yao, LN, Sun, JM, Pan, JR, Chen, HL, and Ruan, W. Malaria in southeastern China from 2012 to 2016: analysis of imported cases. Am J Trop Med Hyg. (2018) 98:1107–12. doi: 10.4269/ajtmh.17-0476
15. Snounou, G, Viriyakosol, S, Zhu, XP, Jarra, W, Pinheiro, L, Rosario, V, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. (1993) 61:315–20. doi: 10.1016/0166-6851(93)90077-B
16. Perera, R, Wickremasinghe, R, Newby, G, Caldera, A, Fernando, D, and Mendis, K. Malaria control, elimination, and prevention as components of health security: a review. Am J Trop Med Hyg. (2022) 107:747–53. doi: 10.4269/ajtmh.22-0038
17. Feleke, DG, Alemu, Y, and Yemanebirhane, N. Performance of rapid diagnostic tests, microscopy, loop-mediated isothermal amplification (LAMP) and PCR for malaria diagnosis in Ethiopia: a systematic review and meta-analysis. Malar J. (2021) 20:384. doi: 10.1186/s12936-021-03923-8
18. Liang, D, Jin, JJ, Wang, WM, Cao, YY, Zhu, GD, Zhou, HY, et al. Evaluating the implementation of rapid diagnostic tests in a malaria elimination setting. Infect Dis Poverty. (2020) 9:84. doi: 10.1186/s40249-020-00702-6
19. Loomans, L, Conesa Botella, A, D'Hondt, A, Verschueren, J, Bossche, D, Van Esbroeck, M, et al. Accuracy of malaria diagnosis by clinical laboratories in Belgium. Malar J. (2019) 18:104. doi: 10.1186/s12936-019-2731-0
20. Pujo, JM, Houcke, S, Lemmonier, S, Portecop, P, Fremery, A, Blanchet, D, et al. Accuracy of SD malaria Ag P.f/Pan(R) as a rapid diagnostic test in French Amazonia. Malar J. (2021) 20:369. doi: 10.1186/s12936-021-03902-z
21. Maltha, J, Gillet, P, and Jacobs, J. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect. (2013) 19:408–15. doi: 10.1111/1469-0691.12152
22. Nolder, D, Stewart, L, Tucker, J, Ibrahim, A, Gray, A, Corrah, T, et al. Failure of rapid diagnostic tests in plasmodium falciparum malaria cases among travelers to the UK and Ireland: identification and characterisation of the parasites. Int J Infect Dis. (2021) 108:137–44. doi: 10.1016/j.ijid.2021.05.008
23. Askling, HH, Bruneel, F, Burchard, G, Castelli, F, Chiodini, PL, Grobusch, MP, et al. Management of imported malaria in Europe. Malar J. (2012) 11:328. doi: 10.1186/1475-2875-11-328
24. Bailey, JW, Williams, J, Bain, BJ, Parker-Williams, J, and Chiodini, PL. Guideline: the laboratory diagnosis of malaria. General Haematology task force of the British Committee for Standards in Haematology. Br J Haematol. (2013) 163:573–80. doi: 10.1111/bjh.12572
25. Xu, S, Gu, YP, Cao, YY, Wang, WM, Tang, JX, Li, JL, et al. Results analysis of Jiangsu provincial malaria diagnostic reference laboratory in 2017. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. (2018) 30:630–4. doi: 10.16250/j.32.1374.2018267
26. Dong, Y, Deng, Y, Xu, YC, Chen, MN, Wei, C, Zhang, CL, et al. Analysis of initial laboratory diagnosis of malaria and its accuracy compared with re-testing from 2013 to 2018 in Yunnan Province, China. Malar J. (2020) 19:409. doi: 10.1186/s12936-020-03477-1
27. Zhou, S, Li, ZJ, Cotter, C, Zheng, CJ, Zhang, Q, Li, HZ, et al. Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar J. (2016) 15:39. doi: 10.1186/s12936-016-1093-0
28. Zhang, L, Feng, J, Zhang, SS, Xia, ZG, and Zhou, SS. The progress of national malaria elimination and epidemiological characteristics of malaria in China in 2017. Chin J Parasitol Parasit Dis. (2018) 36:201–9.
29. malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. (2011) 8:e1000396. doi: 10.1371/journal.pmed.1000396
30. Feng, J, Xiao, HH, Zhang, L, Yan, H, Feng, XY, Fang, W, et al. The plasmodium vivax in China: decreased in local cases but increased imported cases from Southeast Asia and Africa. Sci Rep. (2015) 5:8847. doi: 10.1038/srep08847
32. Dahal, P, Khanal, B, Rai, K, Kattel, V, Yadav, S, and Bhattarai, NR. Challenges in laboratory diagnosis of malaria in a low-resource country at tertiary care in Eastern Nepal: a comparative study of conventional vs. molecular methodologies. J Trop Med. (2021) 2021:1–9. doi: 10.1155/2021/3811318
Keywords: malaria, laboratory diagnosis, accuracy, reference laboratory, China
Citation: Zhang X, Jiang J, Sui Y, Yan H, Xia J, Liu Y, Sun L, Wang X, Marfurt J, Lu S, Li S, Ruan W and Wang D (2023) Evaluation of performance for malaria diagnosis in health facilities by five provincial reference laboratories of China. Front. Public Health. 11:1243642. doi: 10.3389/fpubh.2023.1243642
Edited by:
Nuno Sepulveda, Warsaw University of Technology, PolandReviewed by:
Mbong Eta Ngole, University of Yaounde I, CameroonSusanta Kumar Ghosh, National Institute of Malaria Research (ICMR), India
Copyright © 2023 Zhang, Jiang, Sui, Yan, Xia, Liu, Sun, Wang, Marfurt, Lu, Li, Ruan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ruan, d3J1YW5AY2RjLnpqLmNu; Duoquan Wang, d2FuZ2RxQG5pcGQuY2hpbmFjZGMuY24=
 Xuan Zhang
Xuan Zhang Jingjing Jiang2
Jingjing Jiang2 Xiaoxiao Wang
Xiaoxiao Wang Shenning Lu
Shenning Lu Shizhu Li
Shizhu Li Wei Ruan
Wei Ruan Duoquan Wang
Duoquan Wang