- 1Pharmacy Service, Dr. Balmis General University Hospital, Alicante Institute for Health and Biomedical Research (ISABIAL), Alicante, Spain
- 2Pharmacy Service, Dr. Balmis General University Hospital, Alicante, Spain
- 3National School of Occupational Medicine, Carlos III Health Institute, Madrid, Spain
Background: This review wants to highlight the importance of computer programs used to control the steps in the management of dangerous drugs. It must be taken into account that there are phases in the process of handling dangerous medicines in pharmacy services that pose a risk to the healthcare personnel who handle them. Objective: To review the scientific literature to determine what computer programs have been used in the field of hospital pharmacy for the management of dangerous drugs (HDs).
Methods: The following electronic databases were searched from inception to July 30, 2021: MEDLINE (via PubMed), Embase, Cochrane Library, Scopus, Web of Science, Latin American and Caribbean Literature in Health Sciences (LILACS) and Medicine in Spanish (MEDES). The following terms were used in the search strategy: “Antineoplastic Agents,” “Cytostatic Agents,” “Hazardous Substances,” “Medical Informatics Applications,” “Mobile Applications,” “Software,” “Software Design,” and “Pharmacy Service, Hospital.”
Results: A total of 104 studies were retrieved form the databases, and 18 additional studies were obtained by manually searching the reference lists of the included studies and by consulting experts. Once the inclusion and exclusion criteria were applied, 26 studies were ultimately included in this review. Most of the applications described in the included studies were used for the management of antineoplastic drugs. The most commonly controlled stage was electronic prescription; 18 studies and 7 interventions carried out in the preparation stage focused on evaluating the accuracy of chemotherapy preparations.
Conclusion: Antineoplastic electronic prescription software was the most widely implemented software at the hospital level. No software was found to control the entire HD process. Only one of the selected studies measured safety events in workers who handle HDs. Moreover, health personnel were found to be satisfied with the implementation of this type of technology for daily work with these medications. All studies reviewed herein considered patient safety as their final objective. However, none of the studies evaluated the risk of HD exposure among workers.
1. Introduction
In 1979, Falck et al. (1) detected an elevated level of cellular mutagenicity in the urine of nurses who worked with cytostatic drugs; subsequently, there has been a growing concern regarding the safe handling of hazardous substances among health workers.
The concept of “hazardous drugs” (HDs), which was previously associated exclusively with cytostatic drugs, was introduced in 1990 by the American Society of Hospital Pharmacists (ASHP) and adopted in 2004 by the National Institute for Occupational Safety and Health (NIOSH), thus giving rise to the current internationally accepted definition: “any drug that presents in humans one or more of the following hazard criteria: carcinogenicity, teratogenicity or other developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity or those drugs with a structure or toxicity profile similar to other dangerous drugs” (2).
In 2014, the NIOSH classified HDs into three groups: antineoplastic drugs; nonantineoplastic drugs, with some criteria of danger; and drugs that present risk for the reproductive process, pregnant or lactating women but do not carry risk for the rest of the staff (3).
Since this classification, numerous studies have shown that HDs carry chemical risks for workers who handle them (4). However, recent efforts have focused on controlling the management of HDs to guarantee patient safety, i.e., the prescription, validation and administration of drugs, as well as avoiding potentially harmful medication-related errors. Technological tools have been very helpful for improving the safety of HDs and enhancing the efficiency of the system for managing these drugs, including the advent of electronic prescriptions, the identification of drugs with different types of codes and the use of intelligent infusion pumps (5).
The complexity and interdisciplinary nature of the HD manipulation process make it especially vulnerable to errors. Therefore, HDs are considered a high-risk therapy and can have serious sequelae for both the patient and the professional who handles them (6).
Efforts have been made to establish guidelines that guarantee the safe use of HD; however, there is no global consensus with respect to standards for preventing HD exposure (7).
Therefore, it is essential to standardize the processes, since when a protocol is based on clinical guidelines, variability decreases, thus improving quality and minimizing the risks associated with HD management (8).
Risk assessment plays a key role in the management and control of the PM process, since all measures that are adopted to guarantee the safety of the process are based on its results. A recent study on the perceived risk of exposure in the management of HDs in hospital units (9) recommended integrating HDs into a standardized management system to improve the safety of drugs; this type of risk management model is applicable to any health center.
Although such risk analyses are usually required (4) and there is a well-known need for them, few studies have described risk analyses for HDs; to date, the Hazardous Drug Consensus Group has established the only concrete methodological proposal to carry out a risk analysis with respect to HDs (10).
Therefore, it is essential to determine the risks associated with the HD management process, obtain the necessary information to adopt preventive measures, and minimize risks. It is essential to identify the main stages and operations of the HD management process as well as the preventive measures that can be applied to avoid occupational exposure to HDs (2). Previous studies have examined the hazards and risks associated with this process (4, 11) and the perception of the magnitude of the risk. The evaluation of the different parameters related to risk is especially important for the standardization of behaviors and practices in the area, with the aim of establishing a rational policy to protect the health of workers (12).
Due to the computerization of the processes, it is possible to obtain a considerable amount of information on the stages and operations of the HD manipulation process and obtain a (historical) report of current practices. Additionally, this type of analysis will enable us to identify risks and develop preventive measures that guarantee the safety of the whole process. Thus, the implementation and development of specific computer programs for the comprehensive management of HDs aims to reduce the risks associated with the manipulation of these substances.
Therefore, taking into account that technology has been shown to be very helpful in the management of HDs, the objective of this study was to review the scientific literature to determine which computer programs have been used in the field of hospital pharmacy for managing potential exposure to HDs. And in this way, obtain a point of reference on the current situation of workers who handle HD in hospital pharmacy services, as well as the preventive measures implemented through the computerization of processes and their evaluation. To achieve this objective, a systematic technique was used.
2. Materials and methods
2.1. Design
The current study used a cross-sectional descriptive design and a critical analysis of previous studies retrieved through systematic review. The structure of this review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the methodological framework proposed by Arksey and O’Malley (13) for scoping studies. Likewise, the methodological design of Arenas-Escaso et al. (14) was taken into consideration.
2.2. Source of data collection
The following electronic databases were searched: MEDLINE (via PubMed), Embase, Cochrane Library, Scopus, Web of Science, Latin American & Caribbean Health Sciences Literature (LILACS) and Medicine in Spanish (MEDES).
2.3. Unit of analysis
We examined the articles retrieved from the abovementioned databases.
2.4. Information processing
To select the search terms, we used the Thesaurus of Descriptors in Health Sciences (DeCS), which was developed by the Latin American and Caribbean Center for Medical Sciences Information (BIREME), and Medical Subject Headings (MeSH) terms, which were developed by the US National Library of Medicine.
Based on the hierarchy of both the thesaurus and the indexing files, the following search equations were considered adequate:
• Equation 1 - Dangerous drug.
“Antineoplastic Agents”(Mesh) OR “Antineoplastic Agent*”(Title/Abstract) OR “Antineoplastic Drug*”(Title/Abstract) OR “Antineoplastic*”(Title/Abstract) OR “Chemotherapeutic Anticancer Drug*”(Title/Abstract) OR “Antitumor Drug*”(Title/Abstract) OR “Cancer Chemotherapy Agent*”(Title/Abstract) OR “Cancer Chemotherapy Drug*”(Title/Abstract) OR “Chemotherapeutic Anticancer Agent*”(Title/Abstract) OR “Anticancer Agent*”(Title/Abstract) OR “Antitumor Agent*”(Title/Abstract) OR “Hazardous Substances”(Mesh) OR “Hazardous Material*”(Title/Abstract) OR “Hazardous Chemical*”(Title/Abstract) OR “Environmental Toxic Substance*”(Title/Abstract) OR “Toxic Environmental Substance*”(Title/Abstract) OR “Biohazard*”(Title/Abstract) OR “Cytostatic Agents”(MeSH Terms) OR “Cytostatic Agents*”(Title/Abstract) OR “Cytostatic*”(Title/Abstract) OR “Cytostatic Drug*”(Title/Abstract) OR “Hazardous Drug*”(Title/Abstract) OR “Chemotherapy”(Title/Abstract) OR “Chemotherapeutic Agent*”(Title/Abstract) OR “Chemotherapeutic Drug*”(Title/Abstract) OR “Antineoplastic Medication*”(Title/Abstract) OR “Anticancer Drug*”(Title/Abstract) OR “Highly Potent Drug*”(Title/Abstract).
• Equation 2 - Medical Informatics Applications.
“Medical Informatics Applications”(Mesh) OR “Medical Informatics Application*”(Title/Abstract) OR “Online System*”(Title/Abstract) OR “Clinical Informatic*”(Title/Abstract) OR “Health Informatic*”(Title/Abstract) OR “Medical Data Processing”(Title/Abstract) OR “Medical Informatic*”(Title/Abstract) OR “Medical Informatics Computing”(Title/Abstract) OR “Public Health Informatic*”(Title/Abstract) OR “Mobile Applications”(Mesh) OR “Mobile Application*”(Title/Abstract) OR “Mobile App*”(Title/Abstract) OR “Portable Electronic App*”(Title/Abstract) OR “Portable Electronic Application*”(Title/Abstract) OR “Portable Software App*”(Title/Abstract) OR “Portable Software Application*”(Title/Abstract) OR “Tablet Application*”(Title/Abstract) OR “Software”(Mesh) OR “Computer Software”(Title/Abstract) OR “Computer Program*”(Title/Abstract) OR “Software Tool*”(Title/Abstract) OR “Software Engineering*”(Title/Abstract) OR “Computer Applications Software*”(Title/Abstract) OR “Computer Software Application*”(Title/Abstract) OR “Software Design”(Mesh) OR “Software Design”(Title/Abstract).
• Equation 3 - Hospital Pharmacy.
“Pharmacy Service, Hospital”(Mesh) OR “Hospital Pharmacy Service*”(Title/Abstract) OR “Hospital Pharmacy Service*”(Title/Abstract) OR “Hospital Pharmaceutical Service*”(Title/Abstract) OR “Clinical Pharmacy Service*”(Title/Abstract) OR “Hospital Pharmacies”(Title/Abstract) OR “Hospital Pharmacy”(Title/Abstract).
The final search equation was developed for use in the MEDLINE database via PubMed by using Boolean operators to combine the 3 proposed equations (Equation 1 AND Equation 2 AND Equation 3).
This strategy was subsequently adapted to the characteristics of each databases, and the databases were searched from inception to July 30, 2021 (the final equations, in each bibliographic database, can be consulted in Supplementary Annex 1). Additionally, the references lists of the included studies were manually searched to identify additional eligible articles. Furthermore, experts on the current subject were contacted in an attempt to identify gray literature (materials and research produced by organizations outside the traditional commercial or academic publications that are disseminated through other distribution channels).
2.5. Final selection processing
For the review and critical analysis, the articles that met the following criteria were chosen:
• Inclusion: relevant to the objectives of the current research; an original article, published in peer-reviewed journals and written in English, Spanish or Portuguese.
• Exclusion: articles for which the full text could not be found, and articles that did not specify a relationship between the intervention and the outcome under study (causality criterion).
Relevant articles were selected by two authors of the present review (SC-B. and JS-V.). To validate the inclusion of the articles, it was established that the interrater agreement (kappa index = IK) should be greater than 0.60 (15). Provided that this condition was met, disagreements would be resolved by consensus among all the authors of the review.
2.6. Stages of the HD integral management process
The comprehensive management stages that were taken into account for the comprehensive management of the entire HD process were those included in the work by Bernabeu-Martínez et al. (4). Likewise, from this work the flowcharts of said management were obtained.
2.7. Document correction, level of evidence and grade of recommendation
The structural validity of the articles was assessed using the Strengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (16), which contains a list of 22 essential aspects that should be described in each article. For each selected article, one point was assigned for each item present. When an item was composed of several sections, these were evaluated independently, with the same value assigned to each item, and then, an average was calculated (this being the final result of that item) in such a way that in no case exceeded the total score of one point per item.
To determine the level of evidence and its degree of recommendation, the Scottish Intercollegiate Guidelines Network Grading Review Group (SIGN) recommendations (17) were used.
2.8. Data extraction
The control of the correction of the data was performed by double tables that allowed the detection of the deviations and their correction through newly consulting the original data.
Duplicate records were excluded using the multiplatform program ZOTERO, a bibliographic reference manager developed by the Center for History and New Media of George Mason University.
To determine the timeliness of the studies, the Burton-Kebler half-period (BK) and the price index (IP) were calculated.
The articles were grouped according to the variables under study to systematize and facilitate the understanding of the results. The following data were extracted: first author, year of publication, country where the work was developed, substance studied, software application used, stages, subject to control, type of intervention performed and main results motivated by the effect of the intervention.
2.9. Data analysis
The data obtained from the reviewed studies are presented as frequencies and percentages.
To determine the BK, the median age was calculated according to the time range analyzed, and the PI was calculated by determining the percentage of articles with an age of less than 5 years.
The IK value was used to determine the interrater agreement with respect to eh inclusion of each article. Interrater agreement is considered good when IK > 60% (good or very good agreement).
The scores of the STROBE questionnaire were analyzed using the median, maximum and minimum values. The evolution of this score, in relation to the years of publication, was obtained by Pearson’s correlation analysis.
2.10. Ethical aspects
All data were obtained from articles accepted for review. Therefore, and in accordance with Law 14/2007 on biomedical research, the approval of the ethics committee was not necessary due to the use of secondary data.
3. Results
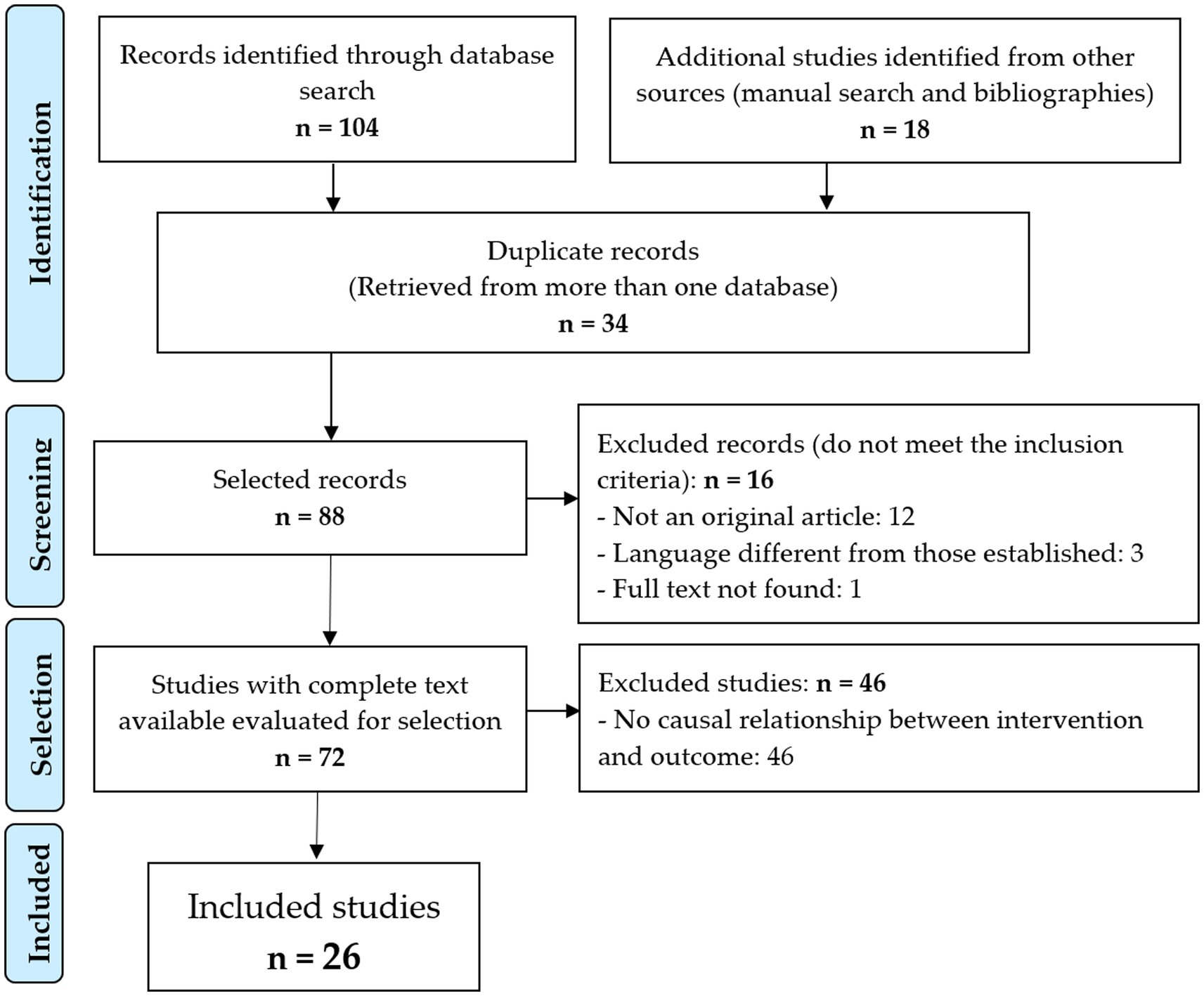
When applying the search criteria, a total of 104 references were retrieved, including 41 (38.32%) from MEDLINE (via PubMed), 20 (19.23%) from Embase, SCOPUS and Web of Science, and 1 (0.96%). from the Cochrane Library, LILACS and MEDES. An additional 18 studies were identified by manually searching the references lists of the included articles and by consulting experts.
After excluding the 34 duplicate records and applying the inclusion and exclusion criteria (Figure 1), 26 original articles (18–43) were included for review and critical analysis (Table 1). The interrater agreement for the selection of the studies among the evaluators was 80.48% (p < 0.001).
The selected articles presented an obsolescence, according to the BK semiperiod (8 years), with a PI of 15.4%.
When evaluating the document correction of the included articles through the STROBE questionnaire, the scores ranged from 6.5 (32.5% compliance) to 19.1 (95.5% compliance), with a median of 14.3 (see Table 2). There was a weak, nonsignificant linear trend over time (R2 = 7.1; p = 0.187).
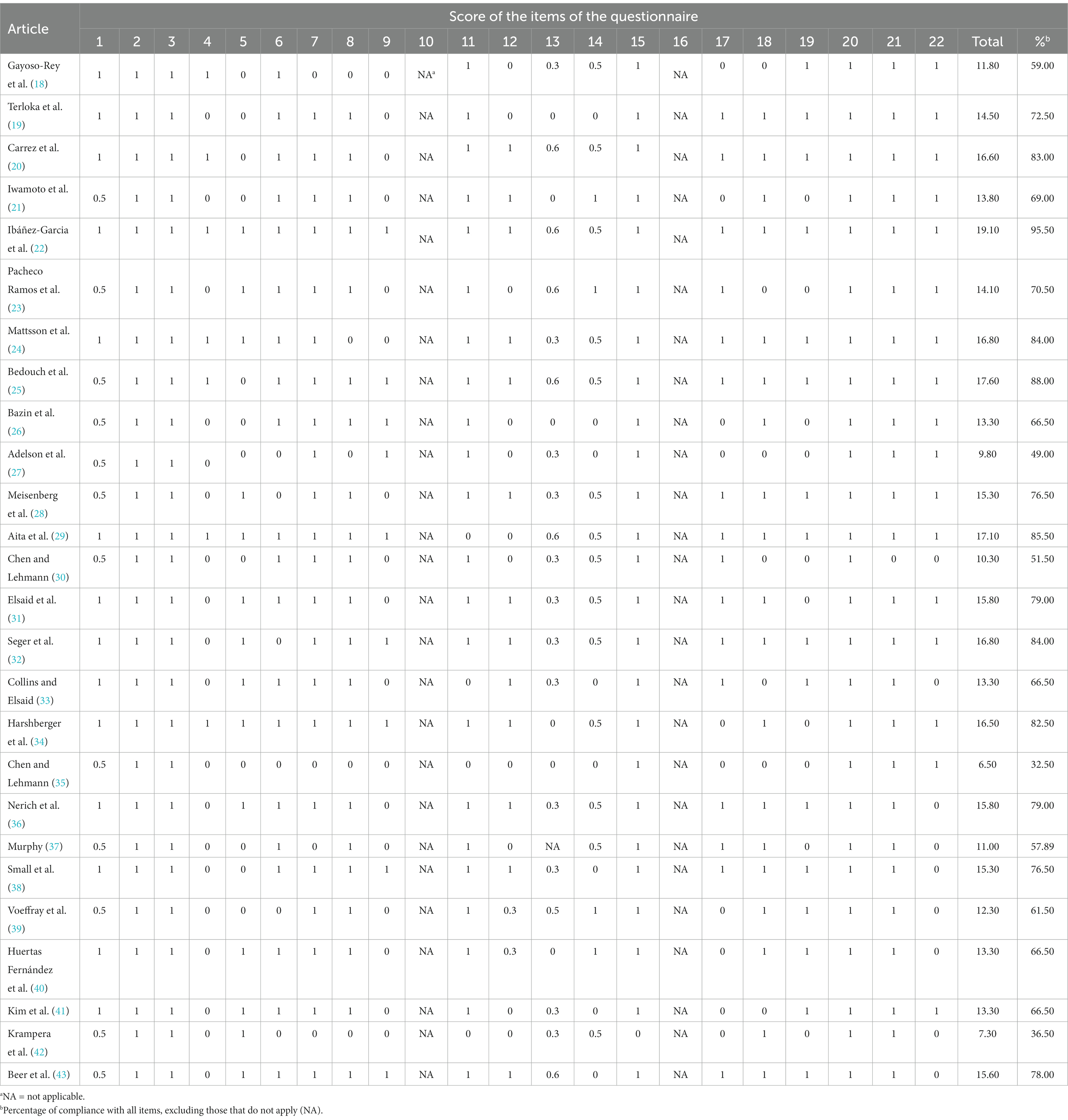
Table 2. Analysis of the documentary quality of the studies through the 22 assessment items of the STROBE guide.
According to the SIGN criteria, this level of evidence among the included studies was 2 ++ (systematic reviews with a high probability that the relationship is causal) with recommendation grade of B (a body of evidence that includes studies directly applicable to the target population and that demonstrate overall consistency of the results).
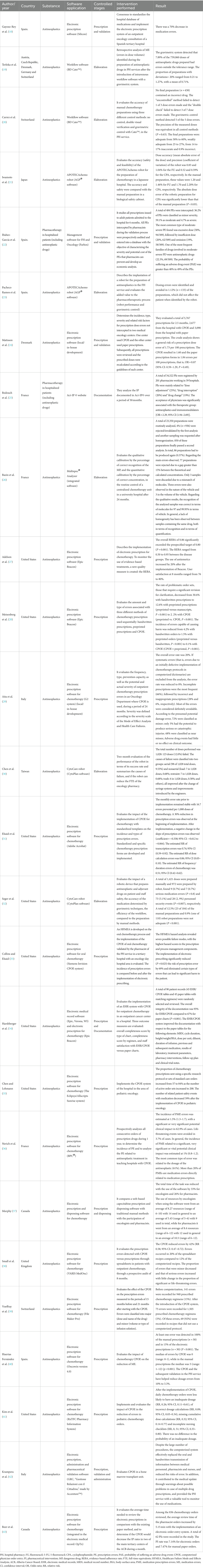
Eight studies were published in English (27, 28, 31–35, 41), and 4 studies were published in Spanish (18, 22, 23, 40).
3.1. Controlled hazardous substance
All studies examined the management of antineoplastic drugs. Two studies (22, 25) were focused on the management of pharmacotherapy among hospitalized patients, although they also included antineoplastic drugs.
3.2. Computer application used
Seventeen studies described computer software related to electronic prescription (18, 24, 27–29, 31, 33–43). The article by Murphy (37) also described software for managing and dispersing drugs, and the article by Krampera et al. (42) described the administration and validation of drugs. In addition, Harshberger et al. (34) described the use of electronic medical records software.
Terloka et al. (19) and Carrez et al. (20) described workflow software, and Ibáñez-Garcia et al. (22) described a program for the managing antineoplastic drugs in hospital pharmacies.
Bedouch et al. (25) described a website, and 4 other studies described the use of robots for the development of HDs: 2 APOTECAchemo robots (AGP® software) (21, 23) and 2 CytoCare robots (CytoPlan software) (30, 32). Finally, 1 study described a Multispec® analyzer (integrated software) (26).
Stages of the controlled hazardous substance management process.
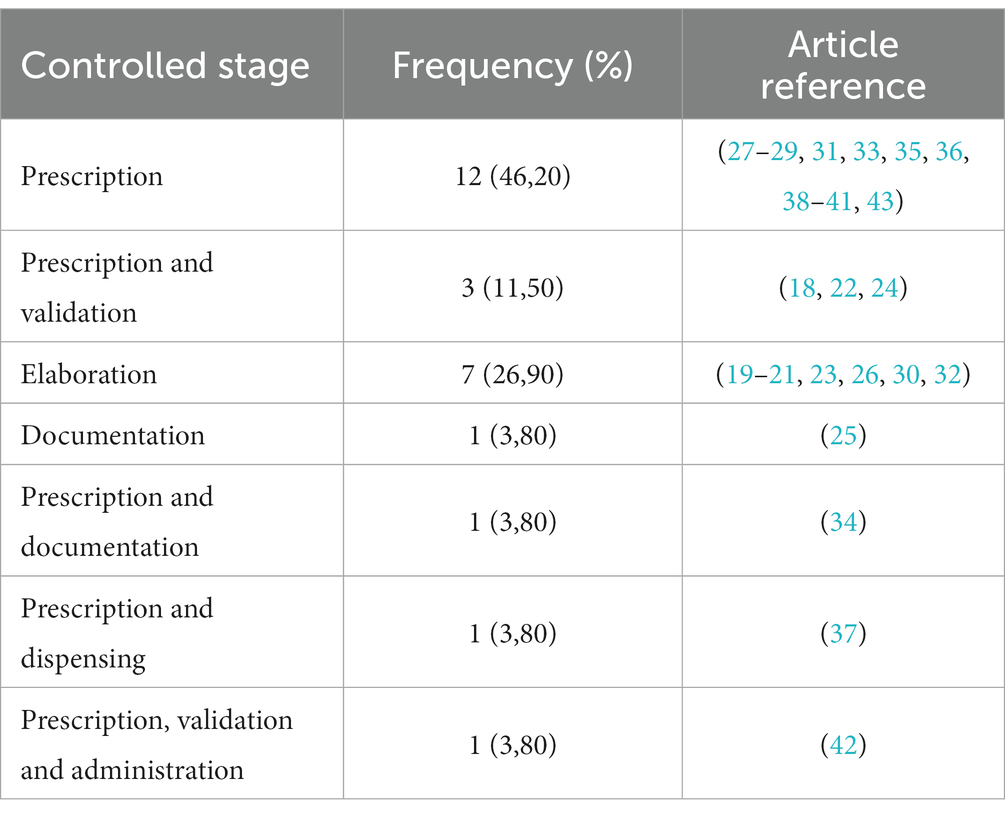
Among the reviewed studies, none reported using a standardized system that ensured the integral management of HD (see Table 3).
3.3. Main interventions carried out
Of the 19 interventions related to electronic prescriptions (18, 22, 24, 25, 27–29, 31, 33–43), six studies described the implementation of an electronic prescription system (18, 27, 31, 34, 35, 41).
The most commonly reported results were the incidence and/or type of electronic prescription error (22, 24, 25, 28, 29, 31, 33, 35, 36, 38–42). Six articles compared these data with previous methods, i.e., paper prescription (24, 28, 39–42), preprinted prescriptions (28) and spreadsheets (38).
Other outcomes included the severity (22, 24, 29), the associated risk factors (24, 26), the cost (22) and the ability to prevent prescription errors (29). Pharmaceutical interventions were also analyzed with respect to the process of validating prescribed treatments (22, 25); the evidence-based adherence rate (REBA) (compliance with clinical guidelines) (26); the correct completion of medical history, clinical and electronic prescription (34); and the time and resources used in the process of prescribing and validating oncological treatments (37, 43).
In two studies, an analysis of “mode of effect and failures” was conducted to define the severity of prescription errors (29) and examine the process of oral chemotherapy (33). In two others, a survey of staff satisfaction with electronic prescription versus paper prescription was conducted (27, 34).
All investigations were carried out in oncological inpatients and/or outpatients, except for 2 studies that also included nononcological patients (22, 25), 1 study that included pediatric oncological patients (40) and 1 study that focused on bone marrow transplantation patients (42).
The 7 interventions performed in the development stage focused on quantitatively evaluating the accuracy of the chemotherapy preparations (19–21, 23, 26, 30, 32) when incorporating the software. Among these interventions, 3 compared the software with the previous methods, i.e., manual preparations without control or double visual verification versus gravimetric control (20) and manual preparations versus preparation robot (21, 32). Only one study qualitatively evaluated chemotherapy preparations (26).
Finally, 3 interventions evaluated the performance of a robot (23, 30, 32); one in one of these studies, the robots also prepared the adjuvant medication (32).
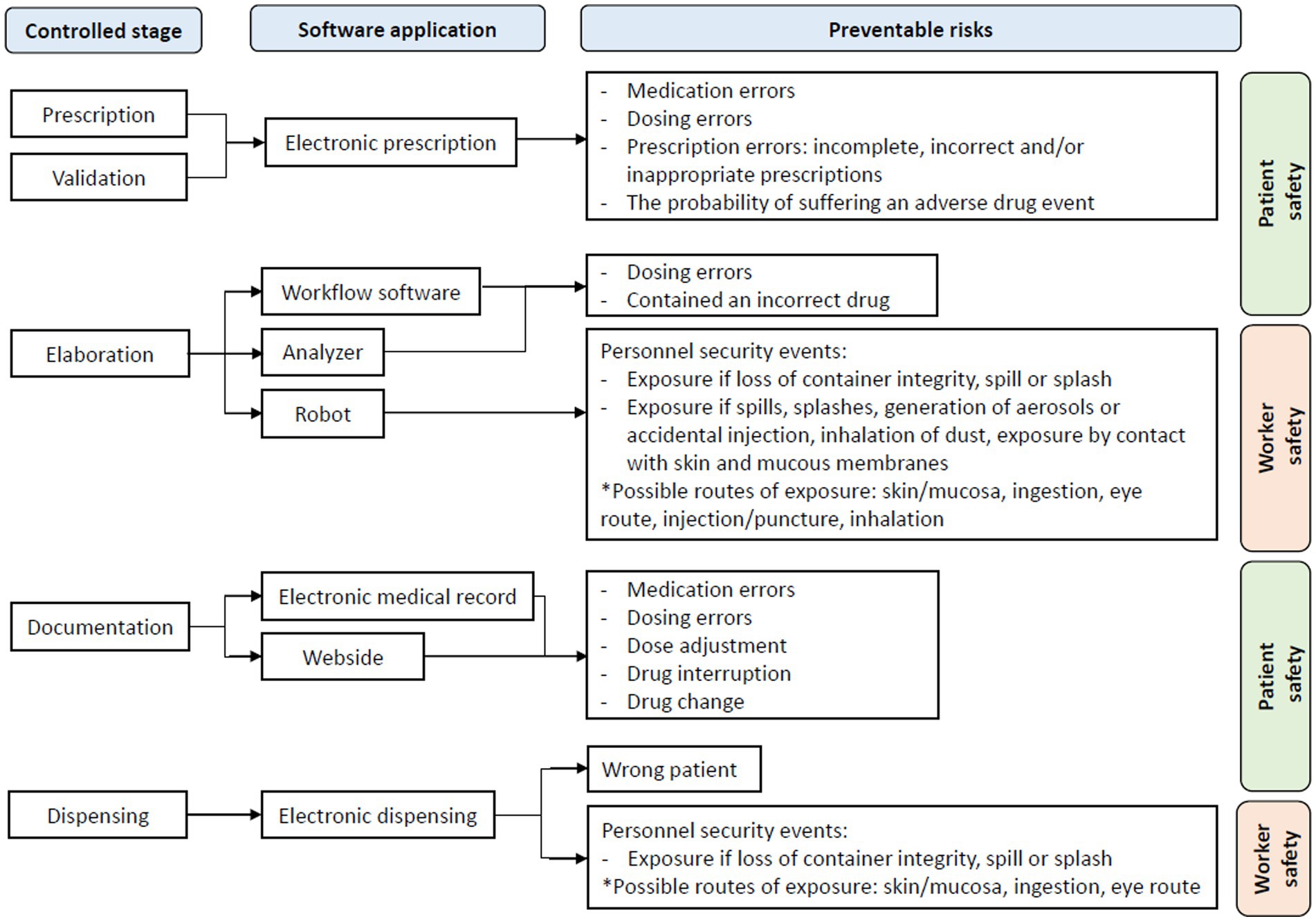
Figure 2 visually proposes the risks that should be prevented in each of the stages that have been studied in Table 3.
3.4. Results obtained as a cause of the intervention
The decrease in medication errors due to electronic prescribing (the most common intervention) was highlighted in most studies (18, 24, 28, 29, 31, 33, 35, 38, 40, 42), association in 5 (28, 31, 33, 38, 41).
The most common type of prescription error was dosage error (22, 24, 25, 29, 31, 36, 40, 41), followed by a lack of integrity in the prescriptions (29, 34). The majority of prescription errors were classified as minor or mild (22, 29); most moderate-to-severe errors were dosage errors (22).
The studies that evaluated the time it takes for the prescription and its validation (37, 43) showed that the use of the software reduced the task time by 33% for oncologists and 26% for pharmacists (37). On the other hand, one study observed that task time increased by 5.15 min (43).
A greater number of dosing errors were detected due to the implementation of gravimetric systems (19, 20), a processing robot (23) and a sample analyzer (26). Dosing errors were reduced with the use of processing robots compared to manual preparations, with this statistically significant difference (21, 32).
The only study that measured personnel safety events observed a reduction in such events (32); two studies assessed staff satisfaction with electronic prescribing (27, 34).
4. Discussion
According to the recommendations on the objectives of a systematic review (44), the current review synthesized information related to interventions for managing HDs in hospital pharmacy services and the software used in the management process. This review aimed to provide relevant information to the scientific community to promote new interventions for worker protection. The results indicate that although software is used for the prescription and preparation of HDs – mainly antineoplastic drugs –no study described a software that controlled the integral management of HDs within the hospital pharmacy services.
The development of new technologies, especially derived from Web 2.0, has led the information and communication industries to play an increasingly important role in a global economy. For this reason, there are studies that conclude that the future development of this industry must take into account the privacy and control of the personal data of its patients and users (45).
Thus, other studies have concluded that mHealth apps could be used to predict the behavior of patients in the face of preventive recommendations and to monitor the symptoms of users in home care (46).
The obsolescence of the articles reviewed was similar to that found in a previous systematic review related to occupational health and exposure to HDs (2, 47). This is confirmed by the low percentage of articles included herein that were published within the past 5 years, thus indicating the need for updated findings.
The evaluation of the document correction of the studies included in this review was performed using STROBE and did not reveal any temporal evolution. Typically, the most recent articles present better results and are linked to the progressive implementation of quality questionnaires. In fact, the oldest studies did not usually follow these quality guidelines; for example, the first documents on STROBE date from 2004, and their use was gradual (48). It should be noted that in the vast majority of the included studies, all the measures adopted to address potential sources of bias were not specified, nor were additional interaction of sensitivity analyses performed. All this is the consequence of not having obtained higher scores.
The level of evidence and grade of recommendation that this study would obtain according to the SIGN criteria are consistent with those observed in previous studies (49). Despite seeking a consistent cause-effect relationship, since intervention studies were examined, this circumstance was not always fulfilled. Some types of studies are more prone to bias than others (49). It is known that many studies of occupational health and safety are still not based on the highest-quality evidence (50). This may be due to the limitation of certain designs of primary studies, such as clinical trials, which are considered robust but may not be adequate to evaluate interventions in occupational health because they generally present very long-term effects. In this review, the management and control of HDs are not the most studied causes.
The predominance of American studies in reviews is widely observed in the scientific literature. The power of the country’s universities and the significant public and private funding of its institutions and research centers contribute to this. Furthermore, most of the included studies were published in English because this language is predominant in health science publications (51). Moreover, the number of English-language journals contained in the main bibliographic databases is very high, and publishing in these journals increases an article’s chances of citation (52).
Almost all computer applications described in the reviewed studies were dedicated to controlling antineoplastic drugs, a logical situation since they are drugs classified as high risk, and their incorrect use presents a greater probability of causing serious or even fatal damage to patients (53). Antineoplastic drugs are the second most common cause of death due to medication errors (54), so they are a priority in all clinical safety programs that are established in hospitals to improve the safety of drug use. The U.S. National Quality Forum included the “improvement of the safety of high-risk drugs” among the 30 fundamental safety practices for widespread implementation in all hospitals (55).
Similarly, it is important to consider the high complexity of high-risk drugs when they are used in hospitals, so it is necessary to intervene in each and every one of their stages, highlighting packaging, storage, prescription, validation, preparation, and dispensing. Administration and disposal, with many of these stages being the responsibility of the hospital pharmacy service (56).
Therefore, it is essential to carry out specific practices that avoid errors and do not cause adverse effects in patients. For this, and thanks to the development of new technologies, programs and tools have been designed and implemented to improve drug safety and reduce possible errors caused by the human factor (57). It should be noted that two meta-analyses found that electronic prescribing reduced medication-related errors by half (58, 59).
In the present review, the most commonly used computer application was the one for electronic prescription and, second, those for preparation. This result was predictable since it has long been known that the complexity of antineoplastic treatments means that most medication errors are found in the stages of prescription (60), administration and preparation (61, 62). These errors (together with their toxicity associated with treatment) may have a severe impact on patients. If, in addition, it is taken into account that medication errors are preventable in most cases (63, 64), it is normal that technological solutions have focused on these stages, reducing their complexity, such as standardization and simplification of protocols and procedures, automation of calculations (drug dose according to weight, body surface, renal function, among others), incorporation of restrictions that prevent unauthorized processes (implementing multiple alarms in the prescription: variation of dose between two cycles, excess of maximum and accumulated dose, improbable interval between two prescriptions, etc.) and decreasing the possibility of human error (gravimetric quality control, processing and dispensing robots, etc.). These solutions have led to a significant reduction in medication-related errors.
This predominance of publications focused on prescription software is consistent with the general trend of efforts and resources focused on patient safety with mandatory quality standards and rather than worker safety, possibly due to a lack of worker safety regulations (2).
Other computer applications examined in this review were those involved in the development, specifically in the quantitative and qualitative quality control of antineoplastic preparations intended for administration; these applications also aimed to increase patient safety. Here, we highlight the difficult challenge of designing an accurate and efficient preparation method that also boasts a high level of safety with the aim of preventing HD exposure among health workers. The use of robots in drug preparation is a partial solution to the manual preparation, as it helps to avoid or reduce HD exposure (in the form of spills, exposure to aerosols and needle sticks) as well as reduce the stress of preparation, human error and lack of traceability of the preparation.
Health workers are exposed to the toxic effects of these HDs while preparing or administering chemotherapy (9). There are no equal measures for their protection or for the protection of patients, since the latter assume their risk of exposure to HDs to cure their disease, while it is not necessary for workers to assume that risk.
While the use of automation technology is increasing, it is not being implemented at the rate that would be needed since most hospitals continue to work with manual preparation and dispensing methods. There are several reasons for this delay, including the high price of this technology and the lack of strict standards for employee safety and environmental control. However, there is legislation that supports the safety of the environment (65).
It would not be appropriate to end this section without recognizing that, currently, there are other management applications of some parts of the HD process that have not appeared in the review or that have not been evaluated in the scientific literature.
Regarding the stages of the HD management process, as already mentioned, in the reviewed studies, no software was found that would perform the integral management of the entire HD process controlling all its stages, and in addition, there is no traceability. of the processes or minimization of the risks associated with these drugs.
At this point, attention was drawn to the lack of publications related to the rest of the stages of HDs other than prescription, which are also very important in the process of managing these drugs, both due to their complexity and risk of exposure as well as their costs (including materials, equipment and labor), such as the preparation, dispensing and disposal of HDs waste, and that their responsibility falls mainly on hospital pharmacy services and their workers. In addition, these steps carry risks both for patients (errors in preparation, dispensing, etc.) and for workers (mainly exposure to HD).
Most of the interventions related to electronic prescribing published results of incidence of prescription errors (dosing error predominant), showing, in all cases, a decrease after the implementation of electronic prescribing, giving strength to this safety measure.
Regarding the preparation stage, articles that used a processing robot for the preparation of HDs were predominant, followed by gravimetric control software. All their observed results showed that the technology improved the accuracy of the preparations, so the widespread implementation of these software is essential to continue improving the quality of the preparations.
4.1. Critical analysis of the authors
It was noteworthy that in the studies on the development of HDs wherein a robot was used, the risk of exposure among workers was not evaluated. This was especially true when this circumstance was one of the arguments for the implementation of robots. Compulsory quality standards are available for the preparation of certain drugs, but there are no specific standards for the prevention of HD exposure among health workers who preparing or administering these drugs.
Consistent with the findings of Bernabeu-Martínez et al. (4), dangerous drugs should be integrated into a standardized management system to improve the safety of the patients and health professionals while enhancing the efficiency of resources and reducing the risk of the processes, thereby guaranteeing the quality and safety of the HD management process. Carrying out a risk assessment in accordance with a systematic methodology and using a preventive approach would allow us to calibrate the probability of occurrence and the severity of any adverse event.
Only 2 studies examined the level of satisfaction with the implementation of the computer application among workers; specifically, these studies examined satisfaction with the use of electronic prescription. The current authors consider it important and necessary to measure worker satisfaction, despite that fact that most of the selected studies neglected to do so.
4.2. Limitations
Although systematic reviews should be based on studies with follow-ups and studies with a high level of scientific rigor, all relevant studies were included in this analysis. The results of the present review are limited by the shortcomings of each included study. Furthermore, as in many other studies related to occupational health, it is nearly impossible to included only studies with high-level designs and high levels of evidence (51, 66).
The high rate of nonrelevant articles retrieved compared to the number of articles that were ultimately included (28) can be considered a possible limitation of this review. It is important to consider that the Scopus and Web of Science databases yielded documents that were ultimately irrelevant; this phenomenon could be due to the lack of indexing (the search was performed using text to search the title, abstract and keywords). This high level of “documentary noise” has been observed in previous systematic reviews (2, 67). In addition, although an exhaustive search was conducted, it is possible that some relevant studies were not identified.
5. Conclusion
Among all the software developed for the management of HDs, the electronic prescription software for antineoplastic drugs was the most widely implemented software at the hospital level.
No software was found to control the entire HD process.
Only one of the selected studies measured safety events in workers who handle HDs. Health professionals reported being satisfied with the implementation of this type of technology for their daily work with HDs.
All studies reviewed herein considered patient safety as their final objective. However, none of the studies evaluated the risk of exposure to HDs among workers.
For all these reasons, in line with the work of Bernabeu-Martínez et al. (2), it would be convenient to apply information and communication technologies to manage the processes that involve HD in a more complete and simple way and avoid the associated risks.
Author contributions
JS-V and PG-S supervised and carried out the conception of the work. SC-B and JS-V designed the study, collected the data, and prepared the database. SC-B, PG-S, and JS-V analyzed, interpreted the data, and edited the first draft. All authors participated equally in the critical review and editing of the article, read and agreed to the published version of the manuscript, and contributed substantially to the present study.
Funding
Partial financial support for translation and publication was received from the Alicante Biomedical and Health Research Institute (ISABIAL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1233264/full#supplementary-material
Abbreviations
HDs, dangerous drugs; LILACS, Latin American and Caribbean Literature in Health Sciences; MEDES, Medicine in Spanish; ASHP, American Society of Hospital Pharmacists; NIOSH, National Institute for Occupational Safety and Health; DeCS, Descriptors in Health Sciences; BIREME, Latin American and Caribbean Center for Medical Sciences Information; MeSH, Medical Subject Headings; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; STROBE, Strengthening the Reporting of OBservational studies in Epidemiology; SIGN, Scottish Intercollegiate Guidelines Network Grading Review Group; BK, Burton-Kebler; IP, price index; REBA, evidence-based adherence rate.
References
1. Falck, K , Gröhn, P , Sorsa, M , Vainio, H , Heinonen, E , and Holsti, LR . Mutagenicity in urine of nurses handling cytostatic drugs. Lancet. (1979) 1:1250–1. doi: 10.1016/s0140-6736(79)91939-1
2. Bernabeu-Martínez, MA , Ramos Merino, M , Santos Gago, JM , Álvarez Sabucedo, LM , Wanden-Berghe, C , and Sanz-Valero, J . Guidelines for safe handling of hazardous drugs: a systematic review. PLoS One. (2018) 13:e0197172. doi: 10.1371/journal.pone.0197172
3. National Institute of occupational safety and health (NIOSH) . NIOSH alert: preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings. Atlanta: NIOSH (2004). Available at: https://bit.ly/3pg2YDx (Accessed September 16, 2021).
4. Bernabeu-Martínez, MÁ , García-Salom, P , Burgos-San José, A , Navarro-Ruiz, A , Sanz-Valero, J , and Wanden-Berghe, C . Consensus to identify the dangerous drugs risks in hospital pharmacy services. Farm Hosp. (2020) 44:51–61. doi: 10.7399/fh.11290
5. Shahmoradi, L , Safdari, R , Ahmadi, H , and Zahmatkeshan, M . Clinical decision support systems-based interventions to improve medication outcomes: a systematic literature review on features and effects. Med J Islam Repub Iran. (2021) 35:27. doi: 10.47176/mjiri.35.27
6. Johnson, PE , Dahlman, G , Eng, K , Garg, R , Gottlieb, S , Hoffman, JM, et al. NCCN oncology risk evaluation and mitigation strategies white paper: recommendations for stakeholders. J Natl Compr Cancer Netw. (2010) 8:S-7–S-27. doi: 10.6004/jnccn.2010.0135
7. Erce, A ed. Preventing occupational exposure to cytotoxic and other hazardous drugs: European policy recommendations. Brussels: Rodhe Public Policy (2016).
8. Martínez Gabarrón, J , Sanz-Valero, J , and Wanden-Berghe, C . Information systems in clinical pharmacy applied to parenteral nutrition management and traceability: a systematic review. Farm Hosp. (2017) 41:89–104. doi: 10.7399/fh.2017.41.1.10610
9. Bernabeu-Martínez, MÁ , Sánchez-Tormo, J , García-Salom, P , Sanz-Valero, J , and Wanden-Berghe, C . Perception of risk of exposure in the management of hazardous drugs in home hospitalization and hospital units. PLoS One. (2021) 16:e0253909. doi: 10.1371/journal.pone.0253909
10. Hazardous Drug Consensus Group (HDGC) . Consensus statement on the handling of hazardous drugs per USP chapter <800>. Cary, NC: Accreditation Commission for Health Care (2017). Available at: https://bit.ly/37pzVTO (Accessed September 16, 2021).
11. Bernabeu Martinez, MÁ , García Salom, P , Burgos San José, A , Navarro Ruiz, A , Sanz Valero, J , and Wanden-Berghe, C . Development of the management of the general process of the handling of hazardous drugs in the home hospitalization units. Hosp Domic. (2019) 3:9–23. doi: 10.22585/hospdomic.v3i1.62
12. Ness, SLR , Mascarenhas, MÁ , Arbo, MD , Tonietto, BD , Cestonaro, LV , Dos Santos, NG, et al. Occupational exposure assessment in professionals who manipulate and administer antineoplastic drugs in a university hospital in southern Brazil. J Oncol Pharm Pract. (2021) 27:1205–13. doi: 10.1177/10781552211003638
13. Arksey, H , and O’Malley, L . Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
14. Arenas-Escaso, JF , Folgado-Fernández, JÁ , and Palos-Sanchez, PR . Digital disconnection as an opportunity for the tourism business: a bibliometric analysis. Emerg Sci J. (2022) 6:1100–13. doi: 10.28991/ESJ-2022-06-05-013
15. Wanden-Berghe, C , and Sanz-Valero, J . Systematic reviews in nutrition: standardized methodology. Br J Nutr. (2012) 107:S3–7. doi: 10.1017/S0007114512001432
16. von Elm, E , Altman, DG , Egger, M , Pocock, SJ , Gøtzsche, PC , Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Gac Sanit. (2008) 22:144–50. doi: 10.1157/13119325
17. Harbour, R , and Miller, J . A new system for grading recommendations in evidence based guidelines. BMJ. (2001) 323:334–6. doi: 10.1136/bmj.323.7308.334
18. Gayoso-Rey, M , Romero-Ventosa, EY , Leboreiro-Enríquez, B , Álvarez-Sánchez, MJ , Gonzalo, LB , García-Comesaña, J, et al. Standardization consensus of a hospital drug database: an efficient tool. Ther Innov Regul Sci. (2020) 54:85–92. doi: 10.1007/s43441-019-00032-2
19. Terkola, R , Czejka, M , and Bérubé, J . Evaluation of real-time data obtained from gravimetric preparation of antineoplastic agents shows medication errors with possible critical therapeutic impact: results of a large-scale, multicentre, multinational, retrospective study. J Clin Pharm Ther. (2017) 42:446–53. doi: 10.1111/jcpt.12529
20. Carrez, L , Bouchoud, L , Fleury-Souverain, S , Combescure, C , Falaschi, L , Sadeghipour, F, et al. Reliability of chemotherapy preparation processes: evaluating independent double-checking and computer-assisted gravimetric control. J Oncol Pharm Pract. (2017) 23:83–92. doi: 10.1177/1078155215620001
21. Iwamoto, T , Morikawa, T , Hioki, M , Sudo, H , Paolucci, D , and Okuda, M . Performance evaluation of the compounding robot, APOTECAchemo, for injectable anticancer drugs in a Japanese hospital. J Pharm Health Care Sci. (2017) 3:12. doi: 10.1186/s40780-017-0081-z
22. Ibáñez-Garcia, S , Rodriguez-Gonzalez, CG , Martin-Barbero, ML , Sanjurjo-Saez, M , and Herranz-Alonso, A, iPharma . Adding value through pharmacy validation: a safety and cost perspective. J Eval Clin Pract. (2016) 22:253–60. doi: 10.1111/jep.12466
23. Pacheco Ramos, M , De La, P , Arenaza Peña, AE , Santiago Pérez, A , Bilbao Gómez-Martino, C , Zamora Barrios, MD, et al. Implementation of a robot for the preparation of antineoplastic drugs in the pharmacy service. Farm Hosp. (2015) 39:137–46. doi: 10.7399/fh.2015.39.3.7497
24. Mattsson, TO , Holm, B , Michelsen, H , Knudsen, JL , Brixen, K , and Herrstedt, J . Non-intercepted dose errors in prescribing anti-neoplastic treatment: a prospective, comparative cohort study. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:981–6. doi: 10.1093/annonc/mdv032
25. Bedouch, P , Sylvoz, N , Charpiat, B , Juste, M , Roubille, R , Rose, F-X, et al. Trends in pharmacists’ medication order review in French hospitals from 2006 to 2009: analysis of pharmacists’ interventions from the act-IP© website observatory. J Clin Pharm Ther. (2015) 40:32–40. doi: 10.1111/jcpt.12214
26. Bazin, C , Vieillard, V , Astier, A , and Paul, M . Implementation of real-time identification analysis and quantification of chemotherapies preparations with a multispec(®) analyser. Ann Pharm Fr. (2014) 72:33–40. doi: 10.1016/j.pharma.2013.09.006
27. Adelson, KB , Qiu, YC , Evangelista, M , Spencer-Cisek, P , Whipple, C , and Holcombe, RF . Implementation of electronic chemotherapy ordering: an opportunity to improve evidence-based oncology care. J Oncol Pract. (2014) 10:e113–9. doi: 10.1200/JOP.2013.001184
28. Meisenberg, BR , Wright, RR , and Brady-Copertino, CJ . Reduction in chemotherapy order errors with computerized physician order entry. J Oncol Pract. (2014) 10:e5–9. doi: 10.1200/JOP.2013.000903
29. Aita, M , Belvedere, O , De Carlo, E , Deroma, L , De Pauli, F , Gurrieri, L, et al. Chemotherapy prescribing errors: an observational study on the role of information technology and computerized physician order entry systems. BMC Health Serv Res. (2013) 13:522. doi: 10.1186/1472-6963-13-522
30. Chen, W-H , Shen, L-J , Guan, R-J , and Wu, F-LL . Assessment of an automatic robotic arm for dispensing of chemotherapy in a 2500-bed medical center. J Formos Med Assoc. (2013) 112:193–200. doi: 10.1016/j.jfma.2011.11.026
31. Elsaid, K , Truong, T , Monckeberg, M , McCarthy, H , Butera, J , and Collins, C . Impact of electronic chemotherapy order forms on prescribing errors at an urban medical center: results from an interrupted time-series analysis. Int J Qual Health Care J Int Soc Qual Health Care. (2013) 25:656–63. doi: 10.1093/intqhc/mzt067
32. Seger, AC , Churchill, WW , Keohane, CA , Belisle, CD , Wong, ST , Sylvester, KW, et al. Impact of robotic antineoplastic preparation on safety, workflow, and costs. J Oncol Pract. (2012) 8:344–9. doi: 10.1200/JOP.2012.000600
33. Collins, CM , and Elsaid, KA . Using an enhanced oral chemotherapy computerized provider order entry system to reduce prescribing errors and improve safety. Int J Qual Health Care. (2011) 23:36–43. doi: 10.1093/intqhc/mzq066
34. Harshberger, CA , Harper, AJ , Carro, GW , Spath, WE , Hui, WC , Lawton, JM, et al. Outcomes of computerized physician order entry in an electronic health record after implementation in an outpatient oncology setting. J Oncol Pract. (2011) 7:233–7. doi: 10.1200/JOP.2011.000261
35. Chen, AR , and Lehmann, CU . Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. J Oncol Pract. (2011) 7:218–22. doi: 10.1200/JOP.2011.000344
36. Nerich, V , Limat, S , Demarchi, M , Borg, C , Rohrlich, PS , Deconinck, E, et al. Computerized physician order entry of injectable antineoplastic drugs: an epidemiologic study of prescribing medication errors. Int J Med Inf. (2010) 79:699–706. doi: 10.1016/j.ijmedinf.2010.07.003
37. Murphy, KC . Assessing software impact on clinical workflow and resource utilization. Stud Health Technol Inform. (2009) 143:309–14. doi: 10.3233/978-1-58603-979-0-309
38. Small, MDC , Barrett, A , and Price, GM . The impact of computerized prescribing on error rate in a department of oncology/hematology. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract. (2008) 14:181–7. doi: 10.1177/1078155208094453
39. Voeffray, M , Pannatier, A , Stupp, R , Fucina, N , Leyvraz, S , and Wasserfallen, J-B . Effect of computerisation on the quality and safety of chemotherapy prescription. Qual Saf Health Care. (2006) 15:418–21. doi: 10.1136/qshc.2005.016808
40. Huertas Fernández, MJ , Baena-Cañada, JM , Martínez Bautista, MJ , Arriola Arellano, E , and García Palacios, MV . Impact of computerised chemotherapy prescriptions on the prevention of medication errors. Clin Transl Oncol. (2006) 8:821–5. doi: 10.1007/s12094-006-0138-1
41. Kim, GR , Chen, AR , Arceci, RJ , Mitchell, SH , Kokoszka, KM , Daniel, D, et al. Error reduction in pediatric chemotherapy: computerized order entry and failure modes and effects analysis. Arch Pediatr Adolesc Med. (2006) 160:495–8. doi: 10.1001/archpedi.160.5.495
42. Krampera, M , Venturini, F , Benedetti, F , Oliani, A , Carolei, S , Visco, C, et al. Computer-based drug management in a bone marrow transplant unit: a suitable tool for multiple prescriptions even in critical conditions. Br J Haematol. (2004) 125:50–7. doi: 10.1111/j.1365-2141.2004.04855.x
43. Beer, J , Dobish, R , and Chambers, C . Physician order entry: a mixed blessing to pharmacy? J Oncol Pharm Pract. (2002) 8:119–26. doi: 10.1191/1078155202jp099oa
44. Hagger, MS . What makes a ‘good’ review article? Some reflections and recommendations. Health Psychol Rev. (2012) 6:141–6. doi: 10.1080/17437199.2012.705556
45. Palos-Sanchez, PR , Saura, JR , Rios Martin, MÁ , and Aguayo-Camacho, M . Toward a better understanding of the intention to use mHealth apps: exploratory study. JMIR Mhealth Uhealth. (2021) 9:e27021. doi: 10.2196/27021
46. Palos-Sanchez, PR , Saura, JR , and Ayestaran, R . An exploratory approach to the adoption process of bitcoin by business executives. Mathematics. (2021) 9:1–23. doi: 10.1007/978-3-319-99590-8_7
47. Domingo-Pueyo, A , Sanz-Valero, J , and Wanden-Berghe, C . Disorders induced by direct occupational exposure to noise: systematic review. Noise Health. (2016) 18:229–39. doi: 10.4103/1463-1741.192479
48. Glasziou, P , Vandenbroucke, JP , and Chalmers, I . Assessing the quality of research. BMJ. (2004) 328:39–41. doi: 10.1136/bmj.328.7430.39
49. Manterola, C , Asenjo-Lobos, C , and Otzen, T . Hierarchy of evidence: levels of evidence and grades of recommendation from current use. Rev Chil Infectologia Organo Soc Chil Infectologia. (2014) 31:705–18. doi: 10.4067/S0716-10182014000600011
50. Teufer, B , Ebenberger, A , Affengruber, L , Kien, C , Klerings, I , Szelag, M, et al. Evidence-based occupational health and safety interventions: a comprehensive overview of reviews. BMJ Open. (2019) 9:e032528. doi: 10.1136/bmjopen-2019-032528
51. Barriocanal-Gómez, P , Del Pozo-Díez, CM , Kudryavtseva, O , Portillo Chicano, I , and Sanz-Valero, J . Effects derived from occupational exposure to hazardous substances in pregnant working women: systematic review. Arch Prev Riesgos Laborales. (2021) 24:263–96. doi: 10.12961/aprl.2021.24.03.04
52. Sánchez-Moya, J , Sanz-Valero, J , and Lopez-Pintor, E . Community pharmacy interventions in adult patients receiving home health care: an exploratory review. Hosp Domic. (2020) 4:209. doi: 10.22585/hospdomic.v4i4.113
53. Cohen, MR , Smetzer, JL , Tuohy, NR , and Kilo, CM . High-alert medications: safeguarding against errors In: MR Cohen , editor. Medication errors. Washington, DC: American Pharmaceutical Association (2007). 317–411.
54. Phillips, J , Beam, S , Brinker, A , Holquist, C , Honig, P , Lee, LY, et al. Retrospective analysis of mortalities associated with medication errors. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. (2001) 58:1835–41. doi: 10.1093/ajhp/58.19.1835
55. The National Quality Forum . Safe practices for better healthcare: a copsensus report (document NQFCR-05-03). Washington, DC: National Quality Forum (2003).
56. European Medicines Agency . Good practice guide on recording, coding, reporting and assessment of medication errors. London: European Medicines Agency (2015). Available at: https://bit.ly/3ee9Nz6 (Accessed December 12, 2021).
57. European Society of Oncology Pharmacy . Quality standard for the oncology pharmacy service - QUAPOS 6. Hamburg: European Society of Oncology Pharmacy (2018). Available at: https://bit.ly/32gfQRh (Accessed December 12, 2021).
58. Shamliyan, TA , Duval, S , Du, J , and Kane, RL . Just what the doctor ordered: review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res. (2008) 43:32–53. doi: 10.1111/j.1475-6773.2007.00751.x
59. Radley, DC , Wasserman, MR , Olsho, LE , Shoemaker, SJ , Spranca, MD , and Bradshaw, B . Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. (2013) 20:470–6. doi: 10.1136/amiajnl-2012-001241
60. Leape, LL , Bates, DW , Cullen, DJ , Cooper, J , Demonaco, HJ , Gallivan, T, et al. Systems analysis of adverse drug events: ADE prevention study group. JAMA. (1995) 274:35–43. doi: 10.1001/jama.1995.03530010049034
61. Escoms, MC , Cabañas, MJ , Oliveras, M , Hidalgo, E , and Barroso, C . Errors evolution and analysis in antineoplastic drug preparation during one year. Pharm World Sci. (1996) 18:178–81. doi: 10.1007/BF00820729
62. Limat, S , Drouhin, JP , Demesmay, K , Tissot, E , Jacquet, M , and Woronoff-Lemsi, MC . Incidence and risk factors of preparation errors in a centralized cytotoxic preparation unit. Pharm World Sci. (2001) 23:102–6. doi: 10.1023/a:1011252132478
63. Aranaz, JM , Aibar, C , Gea, MT , and León, MT . Adverse effects in hospital healthcare: a critical review. Med Clin. (2004) 123:21–5. doi: 10.1016/s0025-7753(04)74399-7
64. Michel, P , Quenon, JL , Djihoud, A , Tricaud-Vialle, S , and de Sarasqueta, AM . French national survey of inpatient adverse events prospectively assessed with ward staff. Qual Saf Health Care. (2007) 16:369–77. doi: 10.1136/qshc.2005.016964
65. García, SP . Hazardous drugs. Med Segur Trab. (2021) 67:6–10. doi: 10.4321/S0465-546X2021000100001
66. Muñoz-Cobo-Orosa, B , Varela-Serrano, C , Rodriguez-Ledott, M , and Sanz-Valero, J . Malignant skin neoplasms in workers in the fishing industry: systematic review. Arch Prev Riesgos Laborales. (2021) 24:47–61. doi: 10.12961/aprl.2021.24.01.05
Keywords: antineoplastic agents, cytostatic agents, hazardous substances, medical informatics applications, software, Hospital Pharmacy, patient safety, occupational health
Citation: Climent-Ballester S, García-Salom P and Sanz-Valero J (2023) Computer programs used in the field of hospital pharmacy for the management of dangerous drugs: systematic review of literature. Front. Public Health. 11:1233264. doi: 10.3389/fpubh.2023.1233264
Edited by:
Pedro R. Palos Sanchez, Sevilla University, SpainReviewed by:
Victor Garro Abarca, Instituto Tecnológico de Costa Rica (ITCR), Costa RicaJosé A. Folgado-Fernández, University of Extremadura, Spain
Mario Alberto Rojas Sánchez, Instituto Tecnológico de Costa Rica (ITCR), Costa Rica
Copyright © 2023 Climent-Ballester, García-Salom and Sanz-Valero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seira Climent-Ballester, c2VpcmEuY2xpbWVudGJhbGxlc3RlckBnbWFpbC5jb20=
 Seira Climent-Ballester
Seira Climent-Ballester Pedro García-Salom
Pedro García-Salom Javier Sanz-Valero3
Javier Sanz-Valero3