- 1Department of Public Health and Primary Care, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 2Department of Environment and Health Sciences, Technical University of Mombasa, Mombasa, Kenya
- 3International Centre for Reproductive Health, Mombasa, Kenya
- 4Aga Khan University Hospital, Nairobi, Kenya
Background: In the test and treat initiative, high-risk populations are screened for human immunodeficiency virus (HIV) infection and start early treatment if diagnosed positive. This study explores factors associated with willingness to initiate testing and immediate treatment among men who have sex with men (MSM) in Nairobi and its environs. The study was informed by a conceptual framework combining the AIDS Risk Reduction Model (ARRM) and the Modified Social Ecological Model.
Methods: This cross-sectional exploratory study targeted MSM (aged 18–60 years) reporting active engagement in anal or oral sex with men in Nairobi and its surrounding areas. Purposive sampling was used to identify data collection sites, and then snowballing was employed to reach the respondents. Data analysis was performed using SPSS version 23, and binary logistics regression was used for inferential analysis.
Results: Between July 2018 and June 2019, 391 MSM were recruited to fill out a self-administered questionnaire, out of which 345 complete questionnaires were analyzed. Never been tested for HIV, private/NGO as the facility of the last HIV test, and had unprotected anal sex were listed as the reasons for taking the most recent test, and the results of the most recent HIV test and seeking a post self-test confirmation were associated with a higher likelihood of accepting the immediate HIV test and treat initiative. Additionally, a preference for a health provider as the first source of support, belief in the efficacy of ARVs, and disclosure about being on ART were the other reasons. Additionally, being aged 25+ years, having more than 60 USD monthly income, and having inconsistent condom use during sex were associated with a higher likelihood of accepting the immediate HIV test and treat initiative. Barriers to the immediate test and treat strategy included stigma from healthcare providers and concerns about disruptions in lifestyle through antiretroviral therapy (ART) use.
Conclusion: Interventions aimed at increasing the HIV test and treat initiative in Kenya may need to take into account the demographic and social characteristics of MSM, including age, lack of habitual HIV testing, and lifestyle changes before and upon enrollment in ART. Projects should also consider working closely with healthcare facilities to strengthen treatment preparation, especially for asymptomatic MSM and those who may not be immediately willing to choose the test and treat strategy.
Background
Kenya had ~1.4 million adults living with the human immunodeficiency virus (HIV), and the HIV prevalence among men who have sex with men (MSM) was 18.2% in 2019 (1). One-third (33%) of all new HIV infections in Kenya occur among key populations, and HIV prevalence among MSM in Kenya is almost three times higher than the general population (2, 3). A steep reduction in incidences is required to halt the HIV epidemic in Kenya. UNAIDS aims to achieve 95–95–95 at a global level by 2030, and apart from Botswana (4), most of the other sub-Saharan African (SSA) nations are not on course to meet this UNAIDS target (5).
Early initiation of antiretroviral therapy (ART) significantly reduces HIV transmissions among couples (6, 7). Additionally, when HIV viral load is suppressed, HIV risk for transmission through anal sex is reduced significantly to zero (8–10). Test and treat is an intervention strategy aimed at eliminating HIV by reducing the spread of the virus to other people through screening at-risk populations for HIV and early treatment for the seropositive (11). The test and treat strategy offers two types of impact at the population level, namely, reduced opportunity for secondary transmission and (11) reduced infectiousness per contact. Mathematical modeling predicts that HIV incidences would significantly decline if the immediate test and treat option is provided throughout the population, even though HIV prevalence may not decrease and may increase (12).
HIV testing remains suboptimally implemented, especially within the MSM community. Many non-suppressed HIV-positive MSMs are asymptomatic with a high cluster of differentiation 4 (CD 4 cell) counts; such individuals may prove a challenge to test, engage in care, and virally suppress (13–16). However, the current challenge is finding HIV-infected individuals who are not receiving care and treatment and tailoring approaches that will ensure retention. A major challenge confronting HIV care and treatment programs in low- and middle-income countries is the late initiation of antiretroviral therapy (ART), challenges in retention, and high patient attrition sometimes due to stigma (17), and this situation may be even more dire among MSM. In addition, MSM in Africa has low engagement with the HIV treatment cascade despite improvements in HIV testing (18). The World Health Organization (WHO) released guidelines in 2015 for the immediate initiation of treatment for everyone diagnosed with HIV (19), which were adopted by Kenya in the same year (20). Additionally, the Ministry of Health (MOH) Kenya, in July 2016, released revised HIV prevention and treatment guidelines that called for immediate test and treat strategy. The new guidelines were received with mixed reactions, with many questions among the implementers about the feasibility of the strategy. Given these guidelines and the reactions to them, this study seeks to explore the HIV risk factors and other factors associated with willingness to immediately test and treat for HIV among the MSM in Nairobi, Kenya.
Methods
Study design and population
The conceptual framework combining the AIDS Risk Reduction Model (ARRM) and the Modified Social Ecological Model informed this study. A cross-sectional study design was used. All consenting adult men (18–60 years) who reported having engaged in consensual oral or anal sex with other men and living in Nairobi city and its neighboring Kiambu County were eligible to be part of the study population. The age limit of 18 years was chosen to reduce the ethical challenges of getting parental/guardian consent from minors. Identifying and recruiting the MSM is a doughty task due to the criminalization and stigmatization associated with homosexuality. Purposive sampling was used to identify 11 data collection points where the MSM community regularly meets because of the secretive nature of MSM activities as well as the criminalization of the same.
Sampling techniques
The study reached 391 MSM who responded to the self-administered questionnaires, of which 345 had complete information. Places and venues frequented by the MSM community, such as safe spaces/drop-in centers, hotels, and massage parlors, were identified through purposive sampling. Other studies have suggested that in a given geographical environment, the same population is likely to be captured in different spaces within that environment. The snowball/chain referral method was used to reach respondents, as key/hidden populations typically have the same/similar set of networks and physical spaces they feel safe in and frequent. This method, despite its inherent flaws, was the most optimal to use to identify and reach this hidden population. Missing data were handled through a listwise approach that omitted those cases with the missing data and analyzed the remaining data. Listwise is a default option for analysis in most statistical software packages, including the Statistical Package for the Social Sciences (SPSS), which we used for the analysis (21).
Data collection
Data collection tools were developed by consulting experts and the MSM and pretested with 45 respondents for comprehension, relevance, and completeness. Data collection for the study took place in the months of July 2018 to June 2019 after obtaining informed consent from the interviewees. English paper-based self-administered structured questionnaires were distributed. The questionnaires were structured in three sections: demographics and socio-economic status of the interviewees formed the first section; followed by questions on HIV risk behaviors in the second section; and the final section had questions on willingness, uptake of immediate HIV test and treat option, and its associated factors.
Measures
The dependent variable for the study was a willingness to start ART immediately. The following socio-demographics were collected: age, place of birth, religion, education level, marital status, employment status, and income. Beyond that, the following risk factors were investigated: identifying as MSM/MSW, number of sexual partners, preference for top or bottom sexual positions, condom use, condom use after alcohol/hard drugs, and type of lubricant. The following variables were of interest in the willingness and uptake of the immediate HIV test and treat initiative: never tested for HIV; type of facility for the last HIV test; reason for the most recent test; never heard of the window period; if HIV positive through HIV self-test, would you go for a confirmatory test; would you go for counseling after positive result; if positive test result, who would you want to approach first to seek support; have you ever experienced stigma from healthcare providers; do you think ARV would affect or disrupt your lifestyle; and if you were on ART, would you prefer to have a treatment “buddy.” All of the above were binary variables.
Data analysis and management
The data analysis process began with numbering the questionnaires. The data cleaning and management was conducted on a Microsoft Excel 2016 spreadsheet, which was then exported to IBM SPSS 23. In the SPSS Version 23.0 software database, variables were labeled and analyses were conducted. Proportions and their 95% confidence interval were calculated. Data that had inconsistencies between different sections were excluded from the study. Cross-tabulation was performed. Bivariate analysis was performed. The prevalence odds ratio (POR) and adjusted POR were calculated. Multivariable analysis was performed, including variables statistically significant on bivariate analysis or those known to have an impact on the variable of interest. A p-value of < 0.05 was considered statistically significant.
Ethical considerations
The study obtained ethical clearance from the Ghent University approval number (PA 2016/009) and the Ethics Review Committee for Mount Kenya University (approval number: MKU/ERC/0463). The researchers also tried to ensure full compliance with the Declaration of Helsinki as the study involved human respondents. The study obtained informed consent from the interviewees. All identifiers that would potentially help to identify the interviewee in the data were omitted, and unique identifiers were used during data processing. Data were only accessible to researchers doing data analysis in secure databases and computers. The research team underwent comprehensive training on research ethics before the commencement of the study.
Results
Respondent demographic and socio-economic information
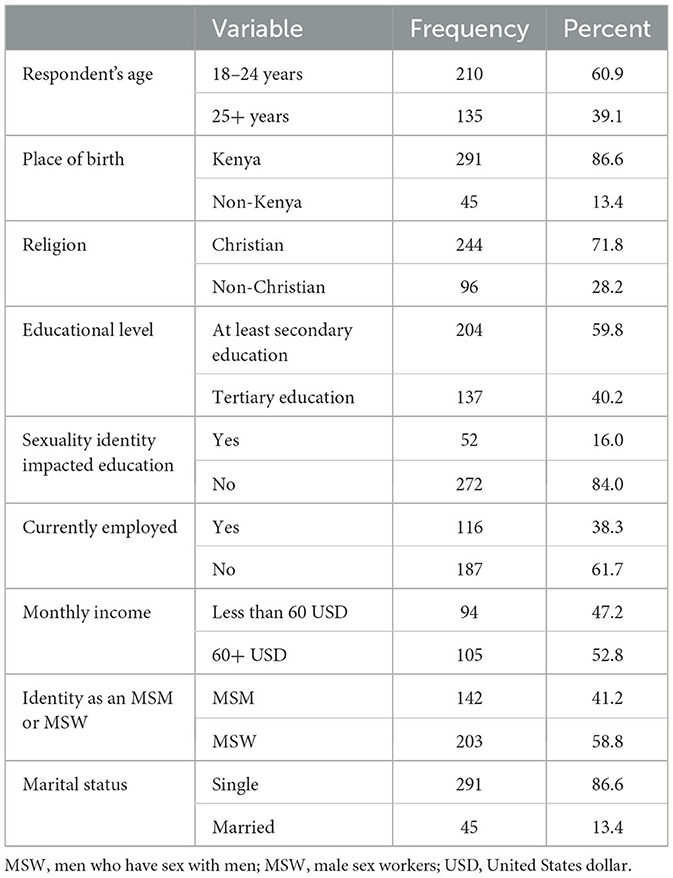
A majority of the respondents (64%) were under 25 years old, single (86.6%), and unemployed (61.7%). While all respondents had attained at least secondary education, their education levels were generally high (at least secondary and above). In total, 16% of the participants indicated that their education had been impacted by their sexual identity. More than half of respondents (58.8%) identified themselves as MSMs who engage in sex work (MSW; Table 1).
Demographic and HIV risk factors associated with willingness to start ART immediately
Study respondents aged 25+ years (75.6%) were more likely to consider immediate HIV testing and treatment at POR 1.06 (1.03, 1.42). Monthly income had statistical significance with a POR of 0.93 (1.09, 1.79). There were no differences in the proportion of Kenyans (77.3%) and non-Kenyans (73.33%) in regard to immediate HIV testing and treatment. There was no observed statistical difference between Christians (79.5%) and non-Christians (70.8%) in accepting treatment immediately. Respondents earning above 60 USD (79.0%) reported willingness to test and treat with a POR of 0.93 (1.09, 1.79); this indicates that >60 USD income negatively influences willingness to test and treat, implying reduced chances of respondents with monthly income above 60 USD willingness to test and treat by 0.07 times. While there were no statistical differences observed between self-identified MSM (76.06%) and MSW, a higher proportion of MSW who were using condoms inconsistently [95 (72.5%)] reported that they would be willing to immediately enter into ART, with a POR of 1.1 (1.25, 1.97), implying that they were 0.1 times more likely to willingly be initiated into ART (Table 2).
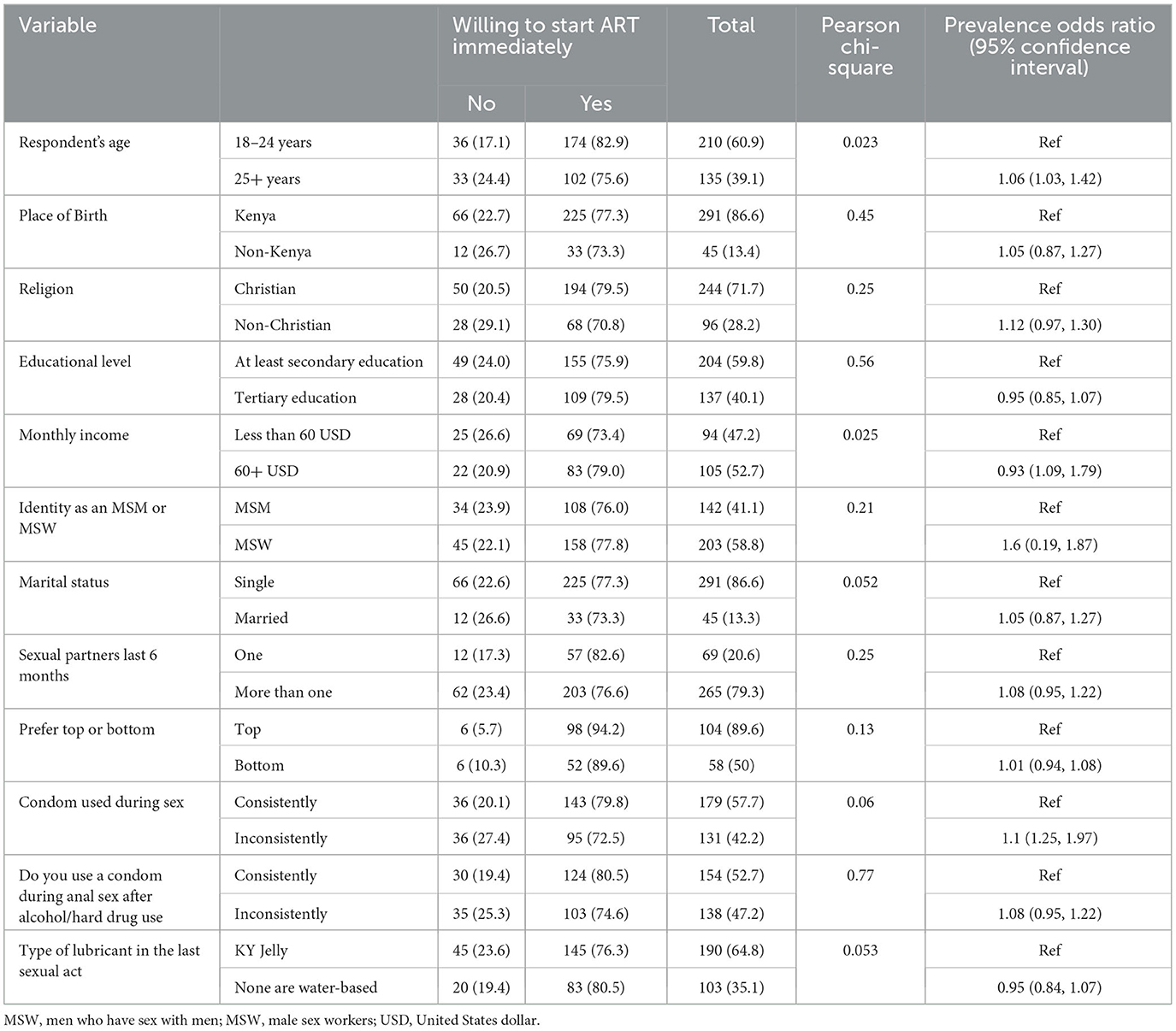
Table 2. Bivariate analysis of demographic and HIV risk factors associated with willingness to start ART immediately.
Uptake of immediate test and treat and other associated factors
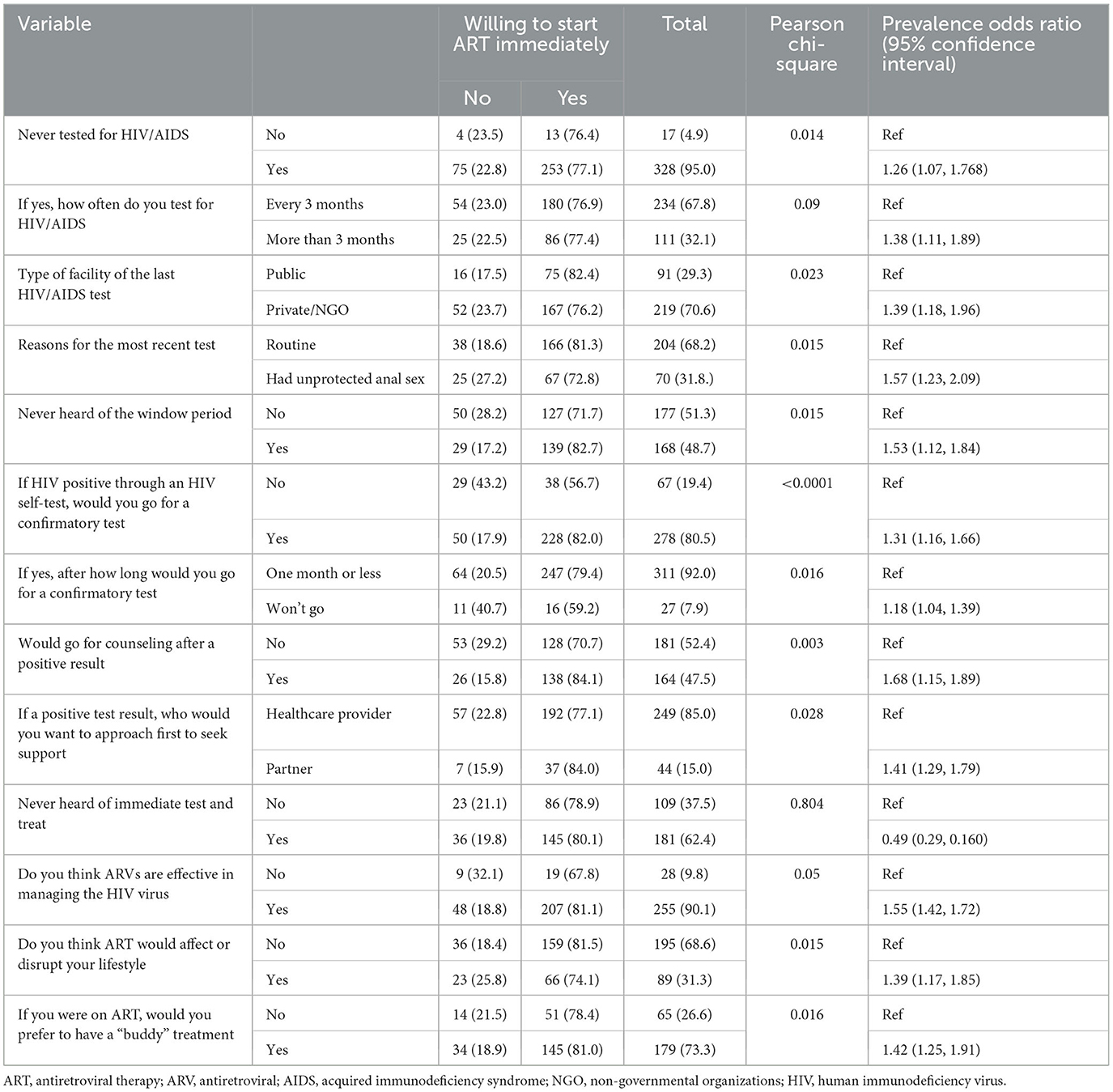
Previous experience with HIV testing had a statistically significant POR of 1.26 (1.07, 1.77), indicating that the respondents who had never tested were 1.26 times more likely to start ART immediately; frequency of HIV testing had a statistically significant POR of 1.38 (1.11, 1.89), indicating that the respondents who routinely tested were 1.38 times more likely to start ART immediately; and the respondents type of facility visited in the last HIV/AIDS test was significant at POR 1.39 (1.18, 1.96), implying that the type of facility in the last visit was 1.39 times more likely to influence immediate ART treatment. The study respondents' reason for the most recent test, had a significant POR of 1.57 (1.23, 2.09), implying that the participant's reason for the most recent test was 1.57 times more likely to influence immediate ART start. The research participants who had never heard of the window period had a significant POR of 1.53 (1.12, 1.84), demonstrating that awareness of the window period was 1.53 times more likely to influence immediate ART start. The willingness to confirm HIV positive self-test had a statistically significant POR of 1.31 (1.16, 1.66), suggesting that the willingness to confirm HIV positive self-test was 0.31 times more likely to influence the immediate test of ART. The respondents who went for a confirmatory test after undertaking an HIV self-test within a month had a significant POR of 1.18 (1.04, 1.39), indicating that their willingness to undertake confirmatory testing was 0.18 times more likely to influence an immediate ART start. Respondents' willingness to go for counseling after a positive result had a statistically significant POR of 1.68 (1.15, 1.89), implying that the participants' willingness to go for counseling after a positive result was 0.68 times more likely to influence immediate ART start. Participants' opinion, in case of a positive test result, as to whom they would prefer to approach to seek support, was a significant POR of 1.41 (1.29, 1.79), meaning that the person approached first by the participants in case of a positive test result would influence immediate ART start 1.79 times. The respondents' opinion on the effectiveness of ART in managing HIV/AIDS was a significant POR of 1.55 (1.42, 1.72), implying that the respondents' opinion on the effectiveness of ART in managing HIV/AIDS was 0.55 times more likely to influence immediate ART start. Participants' opinion on the effect of ART on their lifestyle was statistically significant at 1.39 (1.17, 1.85), indicating that the participants' opinion on the effect of ART on their lifestyle was 1.39 times more likely to influence their immediate ART start. Participants' preference to have treatment buddies had a statistically significant POR of 1.42 (1.25, 1.91), meaning that treatment buddy preference by the study participants would influence the immediate start of ART 1.42 times. The above binary regression test was conducted at a 95% confidence interval (Table 3).
Multivariate analysis on uptake of immediate test and treat and associated factors
The covariates that had a statistically significant influence on willingness to immediately test and treat at the 5% level of significance were: respondent's age; never tested for HIV/AIDS; if never tested for HIV/AIDS, how often do you test for HIV/AIDS; type of facility last tested; reason for most recent test; never heard of window period; willingness to undertake a confirmatory test within a month; if yes, after how long would you go for a confirmatory test; and if tested HIV positive through HIV self-test, willingness to undergo counseling after positive HIV result. If a positive test result, who would you want to approach first to seek support if diagnosed with HIV; ARVs are effective in managing HIV; ARV would affect or disrupt lifestyle; experience stigma from healthcare providers; and preference for having treatment “buddy” if on ART were significantly associated with willingness to immediately test and treat with p-values of < 0.05. The above data can be found on Table 4.
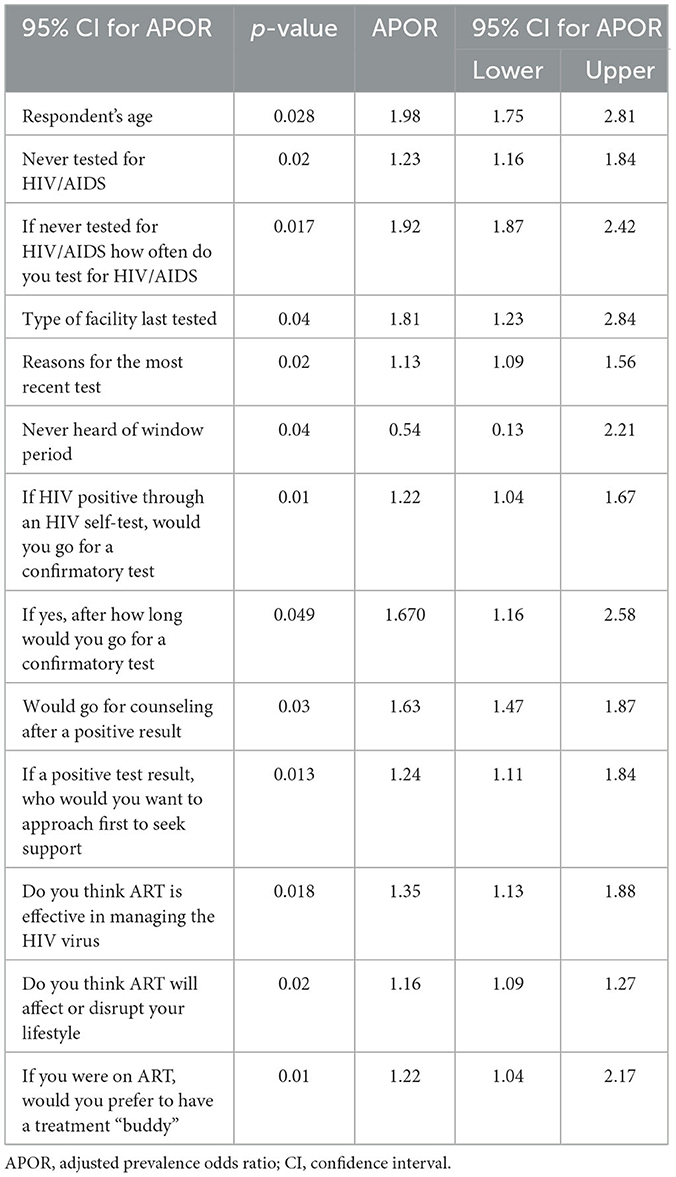
Table 4. Multivariate binary logistics regression analysis of covariates on attitudes toward uptake of immediate test and treat and predictors.
Discussion
The findings suggest that age is associated with willingness to test and treat; a higher proportion of the respondents who were willing to enter into ART immediately after a positive HIV test result were young and possibly single MSM aged 18–24 years. There were statistically significant associations between immediate test and treat and having never tested for HIV. Respondents who tested in private or NGO facilities appeared more willing to test and treat, suggesting that the type of facility for the last HIV test has an influence on willingness to test and treat. Additionally, other factors that may influence the immediate uptake of ART include the reason for the most recent test, the results of the most recent HIV test, and seeking a confirmatory test upon a positive HIV result through a self-test. Barriers to immediately taking up test and treat included concerns about disruptions in lifestyle through ART use and stigma from healthcare providers.
It is important to note that ~51% of all new HIV cases in Kenya occur among adolescents and young people (15–24 years) (2, 3). Given the population demographics in Kenya (22), the willingness of young people (and specifically MSM) to enter into immediate ART is important in reducing the incidence of HIV in the coming generations. While there were no significant differences between MSM and MSW with respect to willingness to immediately enter into treatment, higher proportions of MSW than MSM were willing to enter into treatment, and this is critical as there is evidence that MSW is at a higher risk of infection than MSM (23–26).
The importance of immediate initiation into ART in reducing HIV incidence has been demonstrated by previous studies (27–32). Habitual HIV testing, awareness of test and treat, and willingness to protect self and others upon a positive test result, were also associated with the willingness to immediately enter into treatment, suggesting that knowledge of available options as well as personal attributes are potentially critical factors in the willingness to immediately test and treat.
Study findings also suggest that support systems are important in the quest for immediate treatment upon a positive HIV result, and this finding confirms those from other studies (33–38). Evidence also suggests that quality of care was associated with willingness to enter treatment upon a positive HIV result. There is evidence that positive provider attitudes are contributing to the willingness to immediately test and treat (39–44). Facilities that possibly had fewer judgmental providers (such as private and NGO facilities) were associated with more willingness to enter into ART than government/public facilities. The possibility of seeking support from providers, the availability of posttest counseling services, having a treatment buddy, and the ability to disclose to a partner that one was on ART were all associated with willingness to immediately enter into ART upon testing positive for HIV.
A number of factors would prevent the MSM from enrolling in care and treatment immediately after testing HIV positive, which include fear of ART affecting or disrupting lifestyle, side effects, and a poor service provider attitude toward stigma. Being tested, knowing their status, and starting ART treatment immediately posed a challenge to many participants, who felt that more time would be needed to prepare and be able to integrate the treatment regimen into their daily life schedules (32).
Our study presents crucial findings that would help better refine and implement the immediate test and treat initiative in Kenya, especially within the MSM community. This study had the following limitations. First, the respondents for this study were recruited from MSM-friendly projects. The projects are likely to provide sensitizations and training related to immediate testing and treating. Such selection bias can limit our generalizability. Second, due to the non-probability sampling techniques used during recruitment, MSM who participated in the study may not be representative of MSM in Nairobi and its surrounding areas.
Conclusion
This study explored the facilitating and inhibiting factors concerning the immediate test and treat strategy among the MSM community in Nairobi, Kenya. Interventions aimed at increasing the acceptance of the immediate test and treat initiative in Kenya may need to take into account the demographic and social characteristics of MSM, which include age, lack of habitual HIV testing, addressing lifestyle changes before and upon enrollment in ART, and safe environments for MSM. Projects should also consider working closely with healthcare facilities to strengthen treatment preparation, especially for asymptomatic MSM, and ensure close follow-ups for MSM under immediate test and treat programs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the study obtained ethical clearance from the Ghent University Approval number (PA 2016/009) and the Ethics Review Committee for Mount Kenya University (Approval number: MKU/ERC/0463). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KN was responsible for the formulation of the study, conducting data collection, analyzing, and developing the article. PG and MT reviewed the manuscript and provided technical advice. All authors read, approved the final manuscript, and made significant contributions to the conceptualization and design of this study.
Acknowledgments
We thank all the participants for supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed herein belong to the authors alone.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1228709/full#supplementary-material
Abbreviations
ART, antiretroviral therapy; AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; HIVST, human immunodeficiency virus self-test; MSM, men who have sex with men; MSW, men who are sex workers.
References
1. Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS data 2019. Science. (2019) 268:350. doi: 10.1126/science.7716530
2. The National Syndemic Diseases Control Council (NSDCC). HIV Situation in Kenya. (2021). Available online at: https://nsdcc.go.ke/download/hiv-situation-in-kenya/ (accessed August 04, 2023).
4. Gaolathe T, Wirth KE, Holme MP, Makhema J, Moyo S, Chakalisa U, et al. Botswana's progress toward achieving the 2020 UNAIDS 90–90–90 antiretroviral treatment and virologic suppression goals: results of a population-based survey. Lancet HIV. (2017) 3:221–30. doi: 10.1016/S2352-3018(16)00037-0
5. Sabapathy K, Balzer L, Larmarange J, Block L, Floyd S, Iwuji C, et al. Achieving the UNAIDS 90–90-90 targets: a comparative analysis of four large community randomised trials delivering universal testing and treatment to reduce HIV transmission in sub-Saharan Africa. BMC Public Health. (2022) 22:2333. doi: 10.1186/s12889-022-14713-5
6. Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA. (2023) 329:63–84. doi: 10.1001/jama.2022.22246
7. Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. Jo of Antimicrob Chemother. (2022) 77:290–302. doi: 10.1093/jac/dkab324
8. Mugenyi L, Hansen CH, Mayaud P, Seeley J, Newton R, Nanfuka M, et al. Effect of the ‘universal test and treat' policy on the characteristics of persons registering for HIV care and initiating antiretroviral therapy in Uganda. Front Public Health. (2023) 11:1187274. doi: 10.3389/fpubh.2023.1187274
9. Mugenyi L, Nanfuka M, Byawaka J, Agaba C, Mijumbi A, Kagimu D, et al. Effect of universal test and treat on retention and mortality among people living with HIV-infection in Uganda: an interrupted time series analysis. PLoS ONE. (2022) 17:e0268226. doi: 10.1371/journal.pone.0268226
10. Kimanga DO, Oramisi VA, Hassan AS, Mugambi MK, Miruka FO, Muthoka KJ, et al. Uptake and effect of universal test-and-treat on twelve months retention and initial virologic suppression in routine HIV program in Kenya. PLoS ONE. (2022) 17:e0277675. doi: 10.1371/journal.pone.0277675
11. Nah K, Nishiura H, Tsuchiya N, Sun X, Asai Y, Imamura A, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. (2017) 14:1–11. doi: 10.1186/s12976-017-0062-9
12. Shafer LA, Nsubuga RN, Chapman R, O'Brien K, Mayanja BN, White RG, et al. The dual impact of antiretroviral therapy and sexual behaviour changes on HIV epidemiologic trends in Uganda: a modelling study. Sex Transm Infect. (2014) 90:423–9. doi: 10.1136/sextrans-2013-051219
13. Farquhar C, Masyuko S, Mugo P. Social network–based strategies to improve uptake of HIV testing and linkage to care among men who have sex with men in Sub-Saharan Africa. JAMA Netw Open. (2022) 5:e220155. doi: 10.1001/jamanetworkopen.2022.0155
14. McKetchnie SM, White B, Fontenot H, Dormitzer J, Psaros C, Fitch C, et al. Perspectives of young men who have sex with men on PrEP adherence and peer navigation: a qualitative study. Arch Sex Behav. (2023) 1–13. doi: 10.1007/s10508-023-02579-6
15. Sterrett-Hong EM, Crosby R, Johnson M, Mayo-Wilson LJ, Arroyo C, Machinga R, et al. Socio-ecological influences on HIV care engagement: perspectives of young black men who have sex with men living with HIV in the southern US. J Racial Ethn Health Disparities. (2023) 10:1798–808. doi: 10.1007/s40615-022-01364-w
16. Biello KB, Chan PA, Holcomb R, Ndoye CD, Valente PK, Maynard M, et al. PrEPare for work: a pilot randomized controlled trial of an intervention to optimize HIV PrEP outcomes among male sex workers. AIDS Behav. (2023) 27:3294–305. doi: 10.1007/s10461-023-04050-y
17. Matovu JKB, Choko AT, Korte JE, Conserve DF. Assessing the power of HIV self-testing in unreachable populations in sub-Saharan Africa. Front Public Health. (2022) 10:1078729. doi: 10.3389/fpubh.2022.1078729
18. Stannah J, Dale E, Elmes J, Staunton R, Beyrer C, Mitchell KM, et al. HIV testing and engagement with the HIV treatment cascade among men who have sex with men in Africa: a systematic review and meta-analysis. Lancet HIV. (2019) 6:e769–87. doi: 10.1016/S2352-3018(19)30239-5
19. WHO. Guideline on When to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV Guidelines. Geneva: WHO (2015).
22. NCPD UNFPA. The State of Kenya Population 2020: Zero Harmful Practices-Accelerating the Promise of ICPD25, 1–52. Available online at: https://docplayer.net/189943933-The-state-of-kenya-population-2020.html
23. Okal J, Luchters S, Geibel S, Chersich MF, Lango D, Temmerman M, et al. Social context, sexual risk perceptions and stigma: HIV vulnerability among male sex workers in Mombasa, Kenya. Cult Health Sex. (2009) 11:811–26. doi: 10.1080/13691050902906488
24. Morse EV, Simon PM, Osofsky HJ, Balson PM, Gaumer HR. The male street prostitute: a vector for transmission of HIV infection into the heterosexual world. Soc Sci Med. (1991) 32:535–9. doi: 10.1016/0277-9536(91)90287-M
25. Belza MJ. Risk of HIV infection among male sex workers in Spain. Sex Transm Infect. (2005) 81:85–8. doi: 10.1136/sti.2003.008649
26. Vuylsteke B, Semde G, Sika L, Crucitti T, Traore VE, Buve A, et al. High prevalence of HIV and sexually transmitted infections among male sex workers in Abidjan, Côte d'Ivoire: need for services tailored to their needs. Sex Transm Infect. (2012) 88:288–93. doi: 10.1136/sextrans-2011-050276
27. Koenig SP, Dorvil N, Dévieux JG, Hedt-Gauthier BL, Riviere C, Faustin M, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. (2017) 14:1–15. doi: 10.1371/journal.pmed.1002357
28. De Cock KM, Barker JL, Baggaley R, El Sadr WM. Where are the positives? HIV testing in sub-Saharan Africa in the era of test and treat. Aids. (2019) 33:349–52. doi: 10.1097/QAD.0000000000002096
29. De Anda S, Njoroge A, Njuguna I, Dunbar MD, Abuna F, Macharia P, et al. Predictors of first-time and repeat HIV testing among HIV-positive individuals in Kenya. J Acquir Immune Defic Syndr. (2020) 85:399–407. doi: 10.1097/QAI.0000000000002469
30. Colasanti J, Sumitani J, Mehta CC, Zhang Y, Nguyen ML, Rio CD, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the southern United States. Open Forum Infect Dis. (2018) 5:1–8. doi: 10.1093/ofid/ofy104
31. Labhardt ND, Ringera I, Lejone TI, Klimkait T, Muhairwe J, Amstutz A, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA. (2018) 319:1103–12. doi: 10.1001/jama.2018.1818
32. Mbonye M, Seeley J, Nalugya R, Kiwanuka T, Bagiire D, Mugyenyi M, et al. Test and treat: the early experiences in a clinic serving women at high risk of HIV infection in Kampala. AIDS Care. (2016) 28:33–8. doi: 10.1080/09540121.2016.1164804
33. Nyoni T, Sallah YH, Okumu M, Byansi W, Lipsey K, Small E, et al. The effectiveness of treatment supporter interventions in antiretroviral treatment adherence in sub-Saharan Africa: a systematic review and meta-analysis. AIDS Care. (2020) 32:214–27. doi: 10.1080/09540121.2020.1742870
34. Bogart LM, Phaladze N, Green HD, Klein DJ, Kgotlaetsile K, Lekoko B, et al. Role of support reciprocity in HIV viral suppression among people living with HIV and their treatment partners in botswana. Int J Behav Med. (2021) 2021:1–10. doi: 10.1007/s12529-021-10021-1
35. Penukonda V, Utz T, Perry NS, Ware D, Brennan-Ing M, Meanley S, et al. Viral suppression among middle-aged and aging MSM living with HIV: partnership type and quality. PLoS ONE. (2021) 16:e0258032. doi: 10.1371/journal.pone.0258032
36. Bogart LM, Mosepele M, Phaladze N, Lekoko B, Klein DJ, MacCarthy S, et al. A Social network analysis of HIV treatment partners and patient viral suppression in Botswana. J Acquir Immune Defic Syndr. (2018) 78:183. doi: 10.1097/QAI.0000000000001661
37. Nakigozi G, Makumbi FE, Bwanika JB, Atuyambe L, Reynolds SJ, Kigozi G, et al. Impact of patient-selected care buddies on adherence to HIV care, disease progression and conduct of daily life among pre-antiretroviral HIV-infected patients in Rakai, Uganda: a randomized controlled trial. J Acquir Immune Defic Syndr. (2015) 70:75. doi: 10.1097/QAI.0000000000000710
38. Masquillier C, Wouters E, Mortelmans D, le Roux Booysen F. The impact of community support initiatives on the stigma experienced by people living with HIV/AIDS in South Africa. AIDS Behav. (2014) 19:214–26. doi: 10.1007/s10461-014-0865-1
39. Brown LB, Getahun M, Ayieko J, Kwarisiima D, Owaraganise A, Atukunda M, et al. Factors predictive of successful retention in care among HIV-infected men in a universal test-and-treat setting in Uganda and Kenya: a mixed methods analysis. PLoS ONE. (2019) 14:e0210126. doi: 10.1371/journal.pone.0210126
40. Magaço A, Dovel K, Cataldo F, Nhassengo P, Hoffman R, Nerua L, et al. ‘Good health' as a barrier and facilitator to ART initiation: a qualitative study in the era of test-and-treat in Mozambique. Cult Health Sex. (2019) 21:1059–73. doi: 10.1080/13691058.2018.1535091
41. Pell C, Reis R, Dlamini N, Moyer E, Vernooij E. ‘Then her neighbour will not know her status': how health providers advocate antiretroviral therapy under universal test and treat. Int Health. (2019) 11:36–41. doi: 10.1093/inthealth/ihy058
42. Magnus M, Herwehe J, Murtaza-Rossini M, Reine P, Cuffie D, Gruber D, et al. Linking and retaining HIV patients in care: the importance of provider attitudes and behaviors. AIDS Patient Care STDS. (2013) 27:297–303. doi: 10.1089/apc.2012.0423
43. Nhassengo P, Cataldo F, Magaço A, Hoffman RM, Nerua L, Saide M, et al. Barriers and facilitators to the uptake of test and treat in mozambique: a qualitative study on patient and provider perceptions. PLoS ONE. (2018) 13:e0205919. doi: 10.1371/journal.pone.0205919
44. Boyer S, Iwuji C, Gosset A, Protopopescu C, Okesola N, Plazy M, et al. Factors associated with antiretroviral treatment initiation amongst HIV-positive individuals linked to care within a universal test and treat programme: early findings of the ANRS 12249 TasP trial in rural South Africa. ADS Care. (2016) 28:39–51. doi: 10.1080/09540121.2016.1164808
Keywords: ART, HIV, HIVST, immedate test and treat, MSM, MSW
Citation: Ndungu K, Gichangi P and Temmerman M (2024) Exploring the willingness toward HIV immediate test and treat among MSM in Nairobi and its environs: a cross-sectional study. Front. Public Health 11:1228709. doi: 10.3389/fpubh.2023.1228709
Received: 25 May 2023; Accepted: 23 October 2023;
Published: 03 January 2024.
Edited by:
Mathieu Nacher, INSERM CIC1424 Centre d'Investigation Clinique Antilles Guyane, FranceReviewed by:
Erma Sulistyaningsih, University of Jember, IndonesiaCyrus Mugo Wachira, Kenyatta National Hospital, Kenya
Copyright © 2024 Ndungu, Gichangi and Temmerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kingori Ndungu, a2luZ29yaWluZHVuZ3VAZ21haWwuY29t
 Kingori Ndungu
Kingori Ndungu Peter Gichangi
Peter Gichangi Marleen Temmerman
Marleen Temmerman