
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 27 November 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1228048
This article is part of the Research TopicSexual Health Week 2022View all 7 articles
 Su Wang1,2,3†
Su Wang1,2,3† Li Ding1,2,3†
Li Ding1,2,3† Yixin Liu4†
Yixin Liu4† Zhaoyang Sun1,2,3†
Zhaoyang Sun1,2,3† Wenrong Jiang1,2,3
Wenrong Jiang1,2,3 Yingxin Miao1,2,3
Yingxin Miao1,2,3 Shiwen Wang1,2,3*
Shiwen Wang1,2,3* Jun Meng5*
Jun Meng5* Hu Zhao1,2,3*
Hu Zhao1,2,3*Background: Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae are the prevalent causes of several genital diseases worldwide; however, their characteristics in different genders have not been well documented in Shanghai. The aim of this study is to describe the prevalence of common pathogens among outpatients, considering variations by gender and age.
Methods: From January 1, 2016, to December 31, 2021, the urogenital swabs of 16216 outpatients aged 3–95 years from two general hospitals in Shanghai were collected. All participants' swabs were investigated for U. urealyticum, C. trachomatis, and N. gonorrhoeae by isothermal RNA-based simultaneous amplification and testing. The basic information of all participants was also recorded, including age and gender. The chi-square test was used to compare the prevalence between different genders, age groups, and infection patterns.
Results: There were 5,744 patients (35.42%) with positive samples whose ages ranged from 7 to 80 years (33.23 ± 8.63 years), and 62.14% of them were women. The most common pathogen detected was U. urealyticum (85.08%). The highest prevalence rate of all three pathogens was found in patients aged ≤ 20 years (40.53%, 95% confidence intervals [CI]: 33.80%-47.63%). The prevalent rate of U. urealyticum was higher in men (33.36%, 95% CI: 32.19%-34.55%). The overall prevalence rates of U. urealyticum, C. trachomatis, and N. gonorrhoeae were 30.14% (95% CI: 29.44%-30.85%), 6.00% (95% CI: 5.64%-6.38%), and 2.10% (95% CI: 1.89%-2.33%).
Conclusions: Ureaplasma urealyticum was the most prevalent pathogen in the population, and its prevalence decreased with age. Young men aged ≤ 20 years were more frequently infected. Regular screening for sexually transmitted pathogens in different genders and age groups are warranted, particularly in young men.
Sexually transmitted infection (STI) is one of the most common communicable conditions and has a profound impact on people's sexual and reproductive health (1, 2). More than 1 million new STI cases are reported each day worldwide, of which 50% occurred among young patients (3). Ureaplasma urealyticum (UU), Chlamydia trachomatis (CT), and Neisseria gonorrhoeae (NG) are the most common causative pathogens leading to bacterial STIs, and have gained increasing notoriety owing to their high prevalence (3, 4). About 70% of the sexually active individuals presented with UU in an ongoing carrier state (5). UU is a common commensal colonizing the genitourinary tract and often infects young people after unsafe sexual intercourse. UU infection can lead to infertility, abortion, and other fertility problems in women of childbearing age (6, 7). CT is an intracellular pathogen with an infection rate of 9.8% (8, 9) and of various serotypes (10). NG is the only one among the three pathogens that can be observed under the microscope by gram staining (11) and usually causes urethritis and cervicitis (9).
Timely and effective epidemiologic surveillance can help to make policies for the prevention, screening, and treatment of STIs (3, 12). In China, only a few studies have been conducted to monitor the prevalence of STI pathogens. For instance, a research conducted in Sichuan, which exclusively included female patients seeking care at the Department of Obstetrics and Gynecology, found that UU was identified as the leading cause of STIs (13). However, men are also important reservoirs of STI pathogens, and men who have sex with men (MSM) are seriously affected by STIs because of their particular sexual behavior (3). Therefore, a better understanding of the infection status and epidemiologic characteristics of these pathogens in both men and women is necessary.
This study included 16,216 outpatients attending healthcare facilities from January 1, 2016 to December 31, 2021 to update the prevalence and characteristics of UU, CT, and NG infections in Shanghai. We investigated the distribution of these species in different ages and genders. To our best knowledge, there is no study from Shanghai describing the epidemiologic characteristics of STI-related pathogens separately in men and women over such a long period.
The study was conducted in two hospitals, namely Ruijin Hospital and Huadong Hospital. Ruijin Hospital, Shanghai Jiao Tong University School of Medicine is a tertiary university-affiliated general hospital, located in the Huangpu district of Shanghai, which is a large metropolitan region with over 25 million inhabitants in China. It is a general 2200-bed hospital with emergency department, intensive care unit (ICU), surgery department and other departments, providing services to ~2.7 million patients annually. Huadong Hospital affiliated to Fudan University is also a tertiary university-affiliated general hospital in Jing'an district with 1,400 beds and different medical departments.
This descriptive and cross-sectional study was performed from January 2016 to December 2021 in these hospitals located in two different districts with the largest flow of people in the central city of Shanghai.
The participants were outpatients who came to Ruijin Hospital and Huadong Hospital from January 2016 to December 2021 to measure the presence of UU, CT, and NG infections. In total, 16,216 participants were enrolled according to the following inclusion criteria: (1) being outpatients during the study period; and (2) willing to provide urogenital tract samples. Pregnant women, patients treated for STIs within 2 weeks before testing, and those with a contraindication were excluded. All participants completed a data collection form to specify their basic information, including age, gender, clinical department, and diagnosis. The collected data were entered into a dataset in the laboratory information system (LIS) of the Department of Laboratory Medicine. Only the first positive test was reviewed and recorded for each outpatient per year.
The urogenital swabs of all participants were sampled by physicians. The samples were promptly put into the cell preservation medium to prevent the degradation of RNA samples. It was stored at 2–8°C and processed within 24 h using commercial kits (Shanghai Rendu Biotechnology Co., Ltd). The simultaneous amplification and testing (SAT) method was employed to detect UU, CT, and NG in each sample (14). The SAT method was divided into RNA extraction and amplification and was conducted commercial kits of Rendu Biotechnology Co., Ltd (Shanghai, China) (15). The RNA ion was extracted by the magnetic bead method. Then, a 30 μL RNA sample was mixed with 10 μL enzyme reagents as the 40 μL final system for amplification. All reactions were performed on the ABI 7500 detection system (Life Technologies, CA, USA) in the following conditions: 42°C for 60 s, 40 cycles. The target genes and internal controls were respectively detected by FAM and VIC fluorescent dyes. When a clear melting curve of the expected site (dt ≤ 35, Tm = 68 ± 5°C) was obtained, the results were considered positive. The lowest detection limit of the kit was 103 copies/test.
Two authors (SW and JM) independently extracted participants' data from LIS to ensure authenticity. We extracted data on gender, age, and detection results and recorded the number of participants with missing values. If the information was incomplete, we extracted the methods of imputation and any reported reasons for missing data. Disagreement was resolved by discussion with a third author. Descriptive statistics were used to describe the study population, frequency data, and the associations between variables (genders and age groups). The age groups were divided into ≤ 20, 21–30, 31–40, 41–50, 51–60, and >60 years. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was employed for statistical analysis. The percentages were calculated for categorical data. A 95% confidence interval (CI) was used to estimate prevalence rates, and the mean and standard deviation (mean ± SD) were used for continuous variables. Categorical variables were analyzed using Pearson's chi-square test with Fisher's exact test as appropriate. The prevalence trends in age groups were compared using Cochran-Armitage Test. A two-tailed p < 0.05 was considered statistically significant.
The ages of enrolled 16,216 participants ranged from 3 to 95 years (34.10 ± 9.29 years), and most participants were women (10,076, 62.14%). Most of the tested patients were 21–40 years old (13,288, 81.94%), and the highest number of tests was found in 2021 (5,447, 33.59%). Except for the Department of Gynecology and Andrology, the majority of patients were from the Department of Urology (3,985, 24.57%) and Reproductive Center (3,715, 22.91%) (Supplementary Table S1).
In total, 5,744 patients were infected (5,744, 35.42%), whose ages ranged from 7 to 80 years (33.23 ± 8.63 years). Patients were predominantly aged 21–30 (2,435, 42.39%) and 31–40 (2,290, 39.87%) years, and the highest positive detection rate (PDR) was in the ≤ 20 group (53.68%). Women constituted a greater proportion of patients (3,170, 55.19%), but the PDR among women was markedly lower than that in men (31.46 vs. 41.92%). Infertility was the most common complication in both men (25.33%) and women (17.57%) (Table 1).
Among the infected cases, 4,887 were positive for UU (85.08%), 973 for CT (16.94%), and 340 for NG (5.92%). Patients with UU infection were predominantly aged 21–30 years (41.99%) and were mostly women (58.09%). Among all participants, the overall prevalence of UU was 30.14% (95% CI: 29.44–30.85%), which was significantly higher than that of CT (6.00%, 95% CI: 5.64%-6.38%) or NG (2.10%, 95% CI: 1.89%-2.33%) (p < 0.0001). In men, the PDRs of UU, CT, and NG were 33.36% (95% CI: 32.19–34.55%), 8.36% (7.69–9.08%), and 4.51% (4.02–5.06%), respectively. Among women, the PDRs of UU, CT, and NG were 28.18% (27.31–29.07%), 4.57% (4.18–5.00%), and 0.63% (0.49–0.80%), respectively (Table 2).
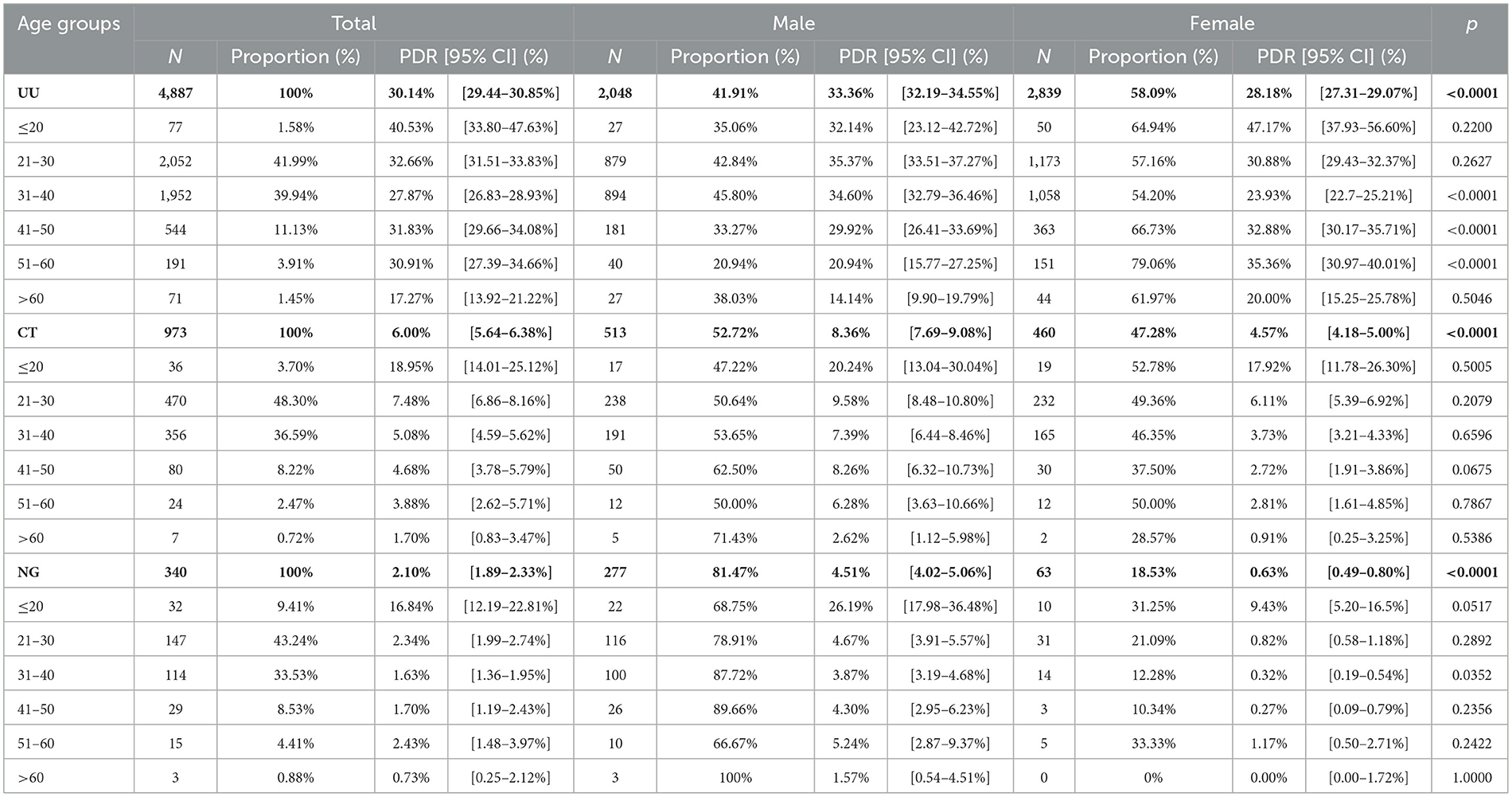
Table 2. The prevalence and distribution of genders in UU, CT, and NG positive patients in different genders and age groups.
Patients were mostly 21–30 years old for all infections (UU: 41.99%; CT: 48.30%; NG: 43.24%). However, the highest PDRs of UU, CT, and NG infections were found in patients aged ≤ 20 years (40.53% [95% CI: 33.80–47.63%], 18.95% [14.01–25.12%], and 16.84% [12.19–22.81%]). The prevalence of UU infection significantly decreased with aging (p < 0.0001) (Figure 1).
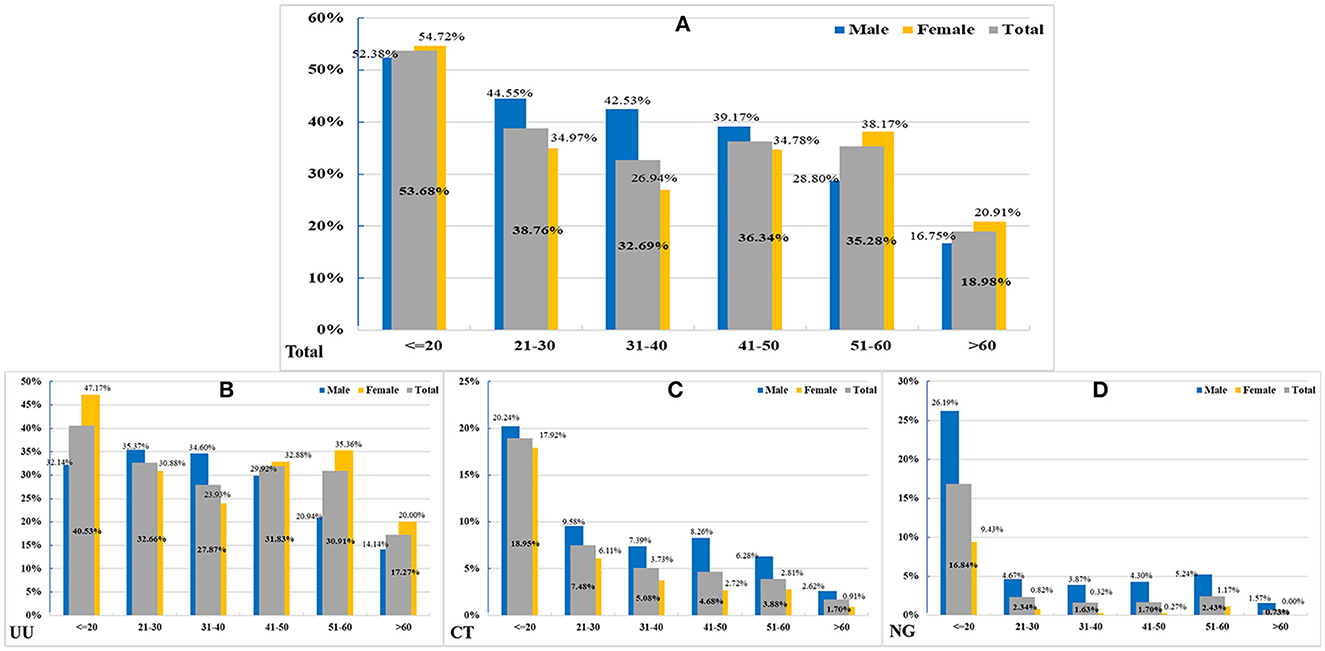
Figure 1. The positive detection rates (PDRs) in different age groups. (A) In 5,744 total positive patients in different genders (Z = 4.3292, P < 0.0001). (B) In 4,887 patients with UU infection (Z = 4.5677, P < 0.0001). (C) In 973 patients with CT infection (Z = 1.0633, P = 0.2877). (D) in 340 patients with NG infection (Z = 1.6276, P = 0.1036).
Women constituted most (80.71%) of the 757 infected individuals who had no symptoms or complications (ages range: 7–78 years; 33.34 ± 9.47 years). A strong gender disparity was found in 41–50, 51–60, and >60 groups (p < 0.05) (Table 3). There were 428 (7.45%) cases of co-infection (ages range: 14–66 years, 29.98 ± 8.27 years), of which 57.01% were men. The most frequent co-infection pattern was UU+CT (68.46%), with patients aged 21–30 years having the highest incidence (56.31%) (Table 4). Among cases with CT or NG infection, the detection rates of UU were higher (32.99%, 26.18%) (Figure 2).
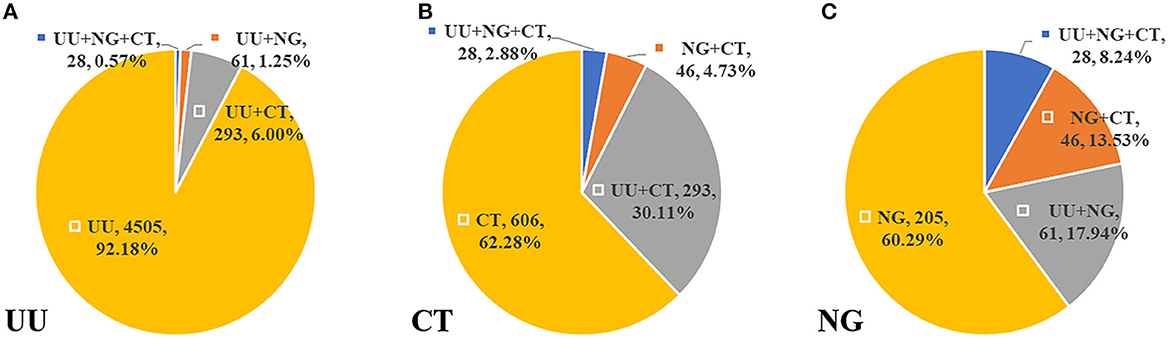
Figure 2. The distribution of co-infection patterns (A) in 4,887 UU infected cases. (B) In 973 CT infected cases. (C) In 340 NG infected cases.
The burden of STIs in China has increased in the past decades (1, 3, 16). STIs are transmitted predominantly via unprotected sexual contact and are closely associated with several urogenital diseases (12), but all STIs are preventable, and most can be effectively cured or treated in case of timely diagnosis (3). Therefore, early detection and surveillance are key cornerstones for controlling STIs. However, most studies only focused on the distribution of pathogens in women, especially in women from the Department of Obstetrics and Gynecology (17), and the long-term epidemiologic features of STI-related pathogens in Shanghai have been rarely reported (18, 19). Enrolling 16,216 outpatients from Shanghai, we described the prevalence of UU, CT, and NG infections from 2016 to 2021 and analyzed the infection types between different genders and age groups to facilitate the development of effective surveillance and prevention strategies for these infections.
Our results revealed the substantial increase in the number of tested women, which may also contribute to the difference in prevalences of STIs among men and women. Accordingly, our study suggested that the monitoring should also be strengthened in men. The prevalence of STIs in our study (35.42%) was higher than that among US adults (about 20% in 2018) (2), indicating that periodic STI surveillance is needed in Shanghai. Researchers have clarified that UU infection can cause inflammation of the female reproductive system (13). Perinatal UU and CT infections can cause abortion, premature delivery, and low birth weight (8, 13). UU and CT are frequent among infertile men and decrease the quality of semen (7, 20, 21). In this study, up to 25% of men with STIs exhibited infertility and adverse pregnancy outcomes, which were the most common complications. However, the latest Chinese guidelines did not recommend screening for STI-related pathogens among infertile couples (22). Hence, we suggest a screening program for infertile couples, especially for men.
Additionally, the overall prevalence rates of UU, CT, and NG in Shanghai were all higher than those from developed countries (2, 23–25), which may be attributed to effective control measures in these countries, such as popularization of sexual knowledge, attention to sexual hygiene, and regular screening of high-risk people. The proportion and prevalence of UU infection were both significantly higher than those of CT and NG infections, which was consistent with previous studies (13, 26, 27). In a study from Beijing, which included patients from 2013 to 2016, the prevalence of the three pathogens was similar to our findings, but in our study, UU and NG infections were more prevalent in men (28). These results indicate that the prevalence may vary in different regions, and the distribution of pathogens can be different in different populations.
Young age has been verified as a strong risk factor for STIs (29). Our results also showed that almost 80% of UU, CT, and NG infections occurred in young individuals aged 21–40 years, similar to those reported from Beijing and Zhejiang, China (28, 30). Nevertheless, we also found a higher prevalence in men than in women in the 21–40-year-old group, suggesting that men aged 21–40 years should undergo UU, CT, and NG screening. Notably, the UU infection was more prevalent among women aged 41–60 years, possibly due to the unique structure of genital tracts and endocrine factors among these women (28). In women aged more than 40 years, STI detection can be considered a supplement to routine physical examination.
In our study, a considerable proportion of infected people, especially women (nearly 20%), had no clinical symptoms, which is consistent with previous findings claiming that female STI is more likely to be asymptomatic (3, 31). Notably, the mean age of co-infected patients (29.98 years) was lower than that of all infected patients (33.23 years), and men were significantly more susceptible to mixed infection, which may be related to the openness to sexuality and the increasing number of young MSM (YMSM) (32, 33). These might reflect a lack of sexual protection and the necessity of sex education, especially among YMSM. Some studies have reported the relationships between STI pathogens. For instance, STI pathogens can increase the risk of HIV acquisition and transmission (28); UU is associated with the persistence of HPV infection and early cytological changes of the cervix (34); and CT colonization contributes to UU or NG infection (30). In our study population, the UU+CT co-infection pattern was dominant, especially among women, where UU played a major role in co-infections. In our study, the distribution of co-infection patterns in women was similar to that in Zhejiang (30). The high prevalence in patients aged 21–30 years suggests that surveillance should be strengthened in this population, and people in their 40s should be cautious of multiple STIs.
In general, UU accounted for the highest proportion of STIs, but its prevalence decreased with aging. The prevalence and infection types varied in age and gender groups. People in their 20s and 30s were most infected with UU, CT, and NG, and the three pathogens were all more prevalent in men than in women. Our findings suggest the screening of UU, CT, and NG for high-risk populations, such as young men, asymptomatic women over 40 years old, and infertile couples. In addition, we illuminated the relationship between gender and STIs to promote the early diagnosis and treatment of STIs and avoid adverse outcomes.
To the best of our knowledge, this was the first study investigating the prevalence of UU, CT, and NG infections among outpatients in Shanghai and analyzing their distribution in different gender and age groups. Information on education, STI history, sexual orientation, condom usage, and sexual partners was not collected, which is the main limitation of this study. The prevalence reported by this study might not be comprehensive enough; thus, further studies are needed to assess the risk factors for these STI-related pathogens in Shanghai and other regions in China.
We demonstrated the high prevalence of STIs in Shanghai and the predominant role of UU. Young men aged ≤ 20 years showed the highest STI prevalence. Different screening, prevention, and treatment strategies are needed for different gender and age groups.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
This study was approved by the Ethics Committee of Huadong Hospital Affiliated to Fudan University (Ethics Approval Number: 20190112). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SuW, HZ, and ShW contributed to conception and design of the study. SuW and JM organized the database. SuW, LD, YL, ZS, WJ, and YM performed the statistical analysis. SuW wrote the first draft of the manuscript. HZ, JM, and ShW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82272987, 82172933, and 81902380), Shanghai Rising-Star Program (Grant No. 23QA1403000), Shanghai Science and Technology Commission (Grant Nos. 21ZR1421700 and 21Y11900900), Shanghai Municipal Health Commission (Grant Nos. 20184Y0032 and 20204Y0296), and Huadong Hospital Affiliated to Fudan University (Grant Nos. ZDXK2212, GZRPY010Y, QMRC2208, and JYRC2207).
We would like to thank the Department of Laboratory Medicine at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine of for excellent laboratory provision and technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1228048/full#supplementary-material
1. Ma W, Chen Z, Niu S. Advances and challenges in sexually transmitted infections prevention among men who have sex with men in Asia. Curr Opin Infect Dis. (2022) 36:26–34. doi: 10.1097/QCO.0000000000000892
2. Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA. (2022) 327:161–72. doi: 10.1001/jama.2021.23487
3. WHO. Sexually Transmitted Infections (Stis) (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed July 20, 2022).
4. Taku O, Brink A, Meiring TL, Phohlo K, Businge CB, Mbulawa ZZA, et al. Detection of sexually transmitted pathogens and co-infection with human papillomavirus in women residing in rural Eastern Cape, South Africa. PeerJ. (2021) 9:e10793. doi: 10.7717/peerj.10793
5. Abad S, Neira E, Vinansaca L, Escandon S, Neira VA. Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in asymptomatic women from urban-peripheral and rural populations of Cuenca, Ecuador. Infect Dis Rep. (2022) 14:646–54. doi: 10.3390/idr14050070
6. Beeton ML, Payne MS, Jones L. The Role of Ureaplasma spp. in the development of nongonococcal urethritis and infertility among men. Clin Microbiol Rev. (2019) 18:32. doi: 10.1128/CMR.00137-18
7. Paira DA, Olivera C, Tissera AD, Molina RI, Olmedo JJ, Rivero VE, et al. Ureaplasma urealyticum and Mycoplasma hominis urogenital infections associate with semen inflammation and decreased sperm quality. J Leukoc Biol. (2023) 113:18–26. doi: 10.1093/jleuko/qiac006
8. Ahmadi A, Ramazanzadeh R, Sayehmiri K, Sayehmiri F, Amirmozafari N. Association of Chlamydia trachomatis infections with preterm delivery; a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2018) 18:240. doi: 10.1186/s12884-018-1868-0
9. Heijne JCM, van den Broek IVF, Bruisten SM, van Bergen JEA, de Graaf H, van Benthem BHB, et al. National prevalence estimates of chlamydia and gonorrhoea in the Netherlands. Sex Transm Infect. (2019) 95:53–9. doi: 10.1136/sextrans-2017-053478
10. Murray SM, McKay PF. Chlamydia trachomatis: cell biology, immunology and vaccination. Vaccine. (2021) 39:2965–75. doi: 10.1016/j.vaccine.2021.03.043
11. Savitskaya VY, Monakhova MV, Iakushkina IV, Borovikova II, Kubareva EA. Neisseria gonorrhoeae: DNA repair systems and their role in pathogenesis. Biochem Biokhimiia. (2022) 87:965–82. doi: 10.1134/S0006297922090097
12. Jenkins WD, Williams LD, Pearson WS. Sexually transmitted infection epidemiology and care in rural areas: a narrative review. Sex Transm Dis. (2021) 48:e236–40. doi: 10.1097/OLQ.0000000000001512
13. Liu T, Lai SY, Zhou W, Liu YL, Chen SS, Jiang YM, et al. Analysis of Ureaplasma urealyticum, Chlamydia trachomatis, Mycoplasma genitalium and Neisseria gonorrhoeae infections among obstetrics and gynecological outpatients in southwest China: a retrospective study. BMC Infect Dis. (2022) 22:283. doi: 10.1186/s12879-021-06966-z
14. Li T, Shi T, Sun Y, Zhou K, Huang Z, Wang P, et al. Application of real-time simultaneous amplification and testing method to accurately and rapidly detect extra-pulmonary tuberculosis. BMC Infect Dis. (2020) 20:1–6. doi: 10.1186/s12879-020-05036-0
15. Qing L, Song QX, Feng JL, Li HY, Liu G, Jiang HH, et al. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Ureaplasma urealyticum infections using a novel isothermal simultaneous RNA amplification testing method in infertile males. Ann Clin Microbiol Antimicrob. (2017) 16:45. doi: 10.1186/s12941-017-0220-2
16. Yang S, Wu J, Ding C, Cui Y, Zhou Y, Li Y, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. (2017) 17:716–25. doi: 10.1016/S1473-3099(17)30227-X
17. Sameni F, Zadehmodarres S, Dabiri H, Khaledi M, Nezamzadeh F. Evaluation of Ureaplasma urealyticum, Chlamydia trachomatis, Mycoplasma genitalium and Neisseria gonorrhoeae in infertile women compared to pregnant women. J Obstet Gynaecol. (2022) 42:2151–5. doi: 10.1080/01443615.2022.2035328
18. Chen XS, Gong XD, Liang GJ, Zhang GC. Epidemiologic trends of sexually transmitted diseases in China. Sex Transm Dis. (2000) 27:138–42. doi: 10.1097/00007435-200003000-00003
19. Han Y, Chen S, Xu W, Shi M, Chen K, Liu J, et al. A Nationwide survey on detection of chlamydia trachomatis in health facilities in China. Sex Transm Dis. (2023) 50:420–4. doi: 10.1097/OLQ.0000000000001799
20. Ahmadi K, Moosavian M, Mardaneh J, Pouresmaeil O, Afzali M. Prevalence of Chlamydia trachomatis, Ureaplasma parvum and Mycoplasma genitalium in infertile couples and the effect on semen parameters. Ethiop J Health Sci. (2023) 33:133–42. doi: 10.4314/ejhs.v33i1.17
21. Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. (2013) 26:231–40. doi: 10.1097/QCO.0b013e328360db58
22. Chen ZJ, Liu JY, Huang HF, Qiao J, Zhou CQ, Huang GN, et al. Guideline on diagnosis of infertility. Chin J Obstet Gynecol. (2019) 54:505–11.
23. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. (2015) 10:e0143304. doi: 10.1371/journal.pone.0143304
24. Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the national surveys of sexual attitudes and lifestyles (Natsal). Lancet. (2013) 382:1795–806. doi: 10.1016/S0140-6736(13)61947-9
25. Zeidan AR, Strey K, Vargas MN, Reveles KR. Sexually transmitted infection laboratory testing and education trends in US outpatient physician offices, 2009-2016. Family Med Commun Health. (2021) 9:3. doi: 10.1136/fmch-2021-000914
26. Dehghan A, Pourmand MR, Salimi V, Asbagh FA, Foroushani AR, Sadeghi K, et al. The effects of Chlamydia trachomatis, Mycoplasma hominis, and Ureaplasma urealyticum loads on semen quality: detection and quantitative analysis. Microb Pathog. (2022) 169:105676. doi: 10.1016/j.micpath.2022.105676
27. Paira DA, Molina G, Tissera AD, Olivera C, Molina RI, Motrich RD, et al. Results from a large cross-sectional study assessing Chlamydia trachomatis, Ureaplasma spp. and Mycoplasma hominis urogenital infections in patients with primary infertility. Sci Rep. (2021) 11:13655. doi: 10.1038/s41598-021-93318-1
28. Liang YY, Zhai HY, Li ZJ, Jin X, Chen Y. Prevalence of Ureaplasma urealyticum, Chlamydia trachomatis, Neisseria gonorrhoeae and herpes simplex virus in Beijing, China. Epidemiol Infect. (2019) 147:e59. doi: 10.1017/S0950268818003163
29. Vasilevsky S, Greub G, Nardelli-Haefliger D, Baud D. Genital Chlamydia trachomatis: understanding the roles of innate and adaptive immunity in vaccine research. Clin Microbiol Rev. (2014) 27:346–70. doi: 10.1128/CMR.00105-13
30. Cai S, Pan J, Duan D, Yu C, Yang Z, Zou J, et al. Prevalence of Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae in gynecological outpatients, Taizhou, China. J Clin Lab Anal. (2020) 34:e23072. doi: 10.1002/jcla.23072
31. Marovt M, Keše D, Kotar T, Kmet N, Miljković J, Šoba B, et al. Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis. (2015) 34:1237–45. doi: 10.1007/s10096-015-2351-8
32. Association CFP Network CY Health TURCfP. Report on the Sexual and Reproductive Health of College Students in China, 2019–2020 (2020). Available online at: https://www.douban.com/note/761288103/?type=rec&start=120&_i=8963177fGMUTaH
33. Jordan SJ, Toh E, Williams JA, Fortenberry L, LaPradd ML, Katz BP. Aetiology and prevalence of mixed-infections and mono-infections in non-gonococcal urethritis in men: a case-control study. Sex Transm Infect. (2020) 96:306–11. doi: 10.1136/sextrans-2019-054121
Keywords: Ureaplasma urealyticum, Chlamydia trachomatis, Neisseria gonorrhoeae, urogenital tract, outpatients
Citation: Wang S, Ding L, Liu Y, Sun Z, Jiang W, Miao Y, Wang S, Meng J and Zhao H (2023) Characteristics of common pathogens of urogenital tract among outpatients in Shanghai, China from 2016 to 2021. Front. Public Health 11:1228048. doi: 10.3389/fpubh.2023.1228048
Received: 24 May 2023; Accepted: 07 November 2023;
Published: 27 November 2023.
Edited by:
Abdulbasit Musa Seid, Royal Children's Hospital, AustraliaCopyright © 2023 Wang, Ding, Liu, Sun, Jiang, Miao, Wang, Meng and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Zhao, aHViZXJ0emhhb0AxNjMuY29t; Jun Meng, bWo0MDU2M0ByamguY29tLmNu; Shiwen Wang, d2FuZ3NoaXdlbjE5ODZAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.