- 1Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, China
- 2Department of Epidemiology, School of Public Health, China Medical University, Shenyang, China
- 3Beijing Key Laboratory for HIV/AIDS Research, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 4Department of Infection, Zhengzhou Center for Disease Control and Prevention, Zhengzhou, China
- 5Department of AIDS/STD Control and Prevention, Tianjin Center for Disease Control and Prevention, Tianjin, China
- 6Department of Infection, The Second Hospital of Hohhot, Hohhot, China
- 7Department of Infection, Heilongjiang Provincial Hospital, Harbin, China
- 8Clinical Research Academy, Peking University Shenzhen Hospital, Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, China
Background: The coronavirus disease 2019 (COVID-19) pandemic has significantly affected the global population, with People Living with HIV (PLWH) being particularly vulnerable due to their compromised immune systems. Although vaccination is a crucial preventative measure against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, little is understood about the willingness of PLWH to receive a second COVID-19 booster dose and the factors that may influence this decision. This study investigates the willingness of PLWH in China to receive a second COVID-19 booster dose and its influencing factors, comparing these with a group of healthy individuals.
Methods: A multicenter cross-sectional study was conducted across five Chinese cities, namely, Beijing, Tianjin, Zhengzhou, Hohhot, and Harbin. Participants were recruited through five community-based organizations. Data were collected via participant self-administered questionnaires included demographic information, willingness to receive a second COVID-19 booster dose, and knowledge about HIV and COVID-19 vaccination. Factors influencing vaccination willingness were identified using multivariable logistic regression analyzes.
Results: A total of 156 PLWH and 151 healthy individuals were included in the study. After adjusting for potential confounders, it was found that PLWH demonstrated a lower willingness to receive a second COVID-19 booster dose compared to healthy individuals (77.6% vs. 88.7%, p = 0.009). Lower willingness was associated with HIV positive status (Adjusted Odds Ratio [AOR]: 0.39, 95%CI: 0.20, 0.75), perceived barriers (AOR: 0.05, 95%CI: 0.01, 0.26), and perceived severity (AOR: 0.32, 95%CI: 0.12, 0.90).
Conclusion: PLWH in China demonstrated a lower willingness to receive a second COVID-19 booster dose compared to healthy individuals. The findings suggest that perceptions and understanding of the COVID-19 vaccination and its necessity for protection against SARS-CoV-2 could influence this willingness. Efforts should be made to strengthen and disseminate knowledge about HIV and COVID-19 vaccinations among this population. In addition, developing interventions and policies that target specific subgroups and address misconceptions about vaccination could be instrumental in improving vaccination rates among PLWH.
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has escalated into a global pandemic, with over 750 million infections and 6.8 million deaths recorded as of February 1, 2023 (1). The impacts are particularly severe for specific populations, including people living with HIV (PLWH), who have been shown to have a higher risk of SARS-CoV-2 infection, serious illness, hospitalization, and death than the general population (2–6).
COVID-19 vaccines have been recognized as one of the most effective methods for preventing infection with SARS-CoV-2 and its variants (7, 8). Vaccination has significantly reduced the risk of SARS-CoV-2 infection and severe COVID-19 disease outcomes in PLWH (9, 10). However, the rise of new SARS-CoV-2 variants has necessitated booster doses of COVID-19 vaccines in some countries. Initial studies indicated the effectiveness of the first COVID-19 booster dose among PLWH against new variants such as Delta and Omicron (11–13). However, other research suggests that immunogenicity and the effectiveness of preventing severe outcomes with the first COVID-19 booster dose among PLWH may diminish over time, especially concerning the Omicron variant (14).
Given the potential decrease in immunogenicity and effectiveness over time, PLWH should receive a second COVID-19 booster dose at an appropriate time. Further research indicates enhanced immunogenicity and safety with the second COVID-19 booster dose in PLWH (15). In response to these findings, several countries, including China, now recommend a second booster dose for PLWH, along with other key populations such as individuals over the age of 60, high-risk groups, those with underlying health conditions, and particularly immunocompromised individuals (16–18).
In the past, significant hesitancy was observed among PLWH in China regarding full-dose COVID-19 vaccination. The vaccination coverage among this group was significantly lower than the international average for the PLWH population (6.2% vs. 63.5%) (19, 20). With the current promotion of the second COVID-19 booster dose both in China and globally, understanding the vaccination willingness of PLWH and exploring the relevant influencing factors have profound theoretical and practical implications for developing and promoting vaccination strategies.
According to the World Health Organization (WHO), it is still necessary for individuals who have previously been infected with SARS-CoV-2 to receive a second COVID-19 booster dose (21). However, only Uganda has reported vaccination rates and willingness to receive the first COVID-19 booster doses among PLWH (22). Given the significant differences in COVID-19 vaccination types and perceptions across countries, the results from other contexts cannot directly guide COVID-19 vaccination strategies for PLWH in China.
Previous studies have highlighted the concern about vaccine side effects as a significant factor influencing the hesitation of PLWH to receive the COVID-19 vaccine. Whether side effects after the first COVID-19 booster dose influence the willingness to receive the second booster dose remains unclear. Addressing these knowledge gaps would offer valuable insights to guide the administration of the second COVID-19 booster dose among PLWH.
The HBM is one of the most extensively utilized theories for understanding health and illness behaviors. The model is predicated on the understanding that a person’s belief in a personal threat of an illness or disease and belief in the effectiveness of the recommended health behavior or action will predict the likelihood that the person will adopt the behavior. The HBM has been previously employed to analyze COVID-19 complete vaccination willingness and behavior among cancer patients and PLWH, as well as in health education activities related to vaccine promotion (23, 24). Although applying HBM to COVID-19 vaccination could enhance our understanding of this health behavior, there is still a gap in the literature, particularly about COVID-19 booster vaccination among PLWH.
In this study, we developed a questionnaire based on the HBM to conduct an anonymous survey among the PLWH population in mainland China. This study aims to provide a theoretical basis for guiding the effective adjustment and implementation of vaccination strategies in our country and other nations in response to the continuously evolving disease situation.
2. Materials and methods
2.1. Study design and objective
This cross-sectional survey is derived from a registered prospective cohort study (the Chinese Clinical Trial Registry.ChiCTR2200058989). The prospective cohort study aimed to assess changes in immunogenicity and adverse reactions within 6 months following the first COVID-19 booster dose in China among PLWH. The prospective cohort study initially recruited both PLWH and healthy individuals in five Chinese cities (Beijing, Tianjin, Zhengzhou, Hohhot, and Harbin), with participant recruitment and selection criteria described in our previous work (25). Based on the cohort study, we further conducted a cross-sectional survey from December 2021 to March 2022. The present study has been approved by the Ethics Committee of Peking University Shenzhen Hospital (No. 2021-094).
2.2. Participants
In this study, the inclusion criteria for participants included: (1) aged between 18 and 65 years, (2) no history of SARS-CoV-2 infection, (3) having received full immunization (two doses of COVID-19 inactivated vaccine) and the first COVID-19 inactivated booster dose, (4) the second COVID-19 booster dose has not been vaccinated yet, and (5) willingness to participate in the study activities and having signed written informed consent. The HIV infection status was preliminarily self-reported by participants before attending this site study. We re-identified the HIV serostatus for PLWH using the Abbott ARCHITECT HIV Ag/Ab Combo assay, which has high sensitivity and specificity (S/CO ≥ 1.0, Reactive) at the study site. The exclusion criteria were: (1) interviewees with severe hearing loss, visual impairment, or intellectual disability and (2) major mental illness (schizophrenia or bipolar disorder) or neurocognitive impairment as assessed by the clinician.
2.3. Study procedures
PLWH was recruited from five community-based organizations that collaborated with HIV clinical service providers and offered services to PLWH, one in each city. Recruitment advertisements were disseminated through WeChat public accounts, a widely used social media platform in China. Then, interested PLWH contacted project staff via social media and were briefly informed of the study’s purpose and procedure. Potential PLWH participants and the healthy control population received a detailed informed consent form. Upon signing, they were screened using inclusion criteria and a free HIV test through the HIV rapid test kit. Eligible HIV-negative individuals were also invited to participate in the study. Investigators issued an anonymous questionnaire through the online survey platform (Golden Data) at the prevaccination (before 2–4 weeks of receiving the first COVID-19 booster dose) and the fourth-week follow-up of the prospective cohort study to understand their feelings and willingness after the first COVID-19 booster dose. Questionnaires that did not meet the length (less than 100 s) to fill in the questionnaire and had logical errors (For instance: the time of COVID-19 vaccination was before the occurrence of COVID-19) were excluded.
2.4. Questionnaire
The questionnaire used in this survey consisted of five sections: Socio-demographic characteristics and health status; Adverse reactions after vaccination; Willingness to receive the second COVID-19 booster dose; HBM project; HIV-related information and immunization status.
To ensure effectiveness, all questions were constructed and evaluated by an expert team (including two public health experts and an epidemiologist specializing in infectious diseases).
In the HBM section, we set up 16 items across six dimensions, including perceived susceptibility (3 items), perceived severity (3 items), perceived harm (1 item), perceived benefits (2 items), behavioral cues (1 item), and self-efficacy (1 item). The score for each item ranged from 1 to 5, allocated to “strongly disagree, ““disagree, ““neutral, ““agree, “and “strongly agree.” The scale’s reliability was verified by Cronbach’s α coefficient (α = 0.835).
HIV-related information and immunization status included current HIV infection status, HIV infection time, ART conditions, the latest testing results of HIV viral load, and CD4 + T cell absolute count.
The questionnaire was anonymous, with a unique 6-digit number for each participant to protect privacy. A master list with identifiable information was saved on the principal investigator’s computer with password protection, accessible only to the principal investigator, and the data were encrypted and regularly backed up to prevent data loss or unauthorized access.
2.5. Sample size
This study aimed to evaluate Chinese PLWH’s willingness to vaccinate with the second COVID-19 booster dose relative to healthy individuals. Based on the results of published peer-reviewed studies in Greece, Italy, and China, it was estimated that the acceptance rate of the second COVID-19 booster dose among PLWH is 70%. The acceptance rate among the healthy control group is 85% (26–28). The confidence level of 1−α = 95% and the test efficacy 1−β = 0.8 were specified. After considering a 10% dropout rate, 270 participants were required, with the PLWH group and healthy individuals group allocated in a 1:1 ratio. The sample size was calculated using Power Analysis and Sample Size software (version 15.0.5).
2.6. Statistical analysis
For continuous variables, normality was assessed by the Shapiro–Wilk test. Variables conforming to normal distribution were analyzed using the t-test method, and for non-conforming variables, the Mann–Whitney test method was used. For categorical variables, the chi-square/Fisher method was used. A logistic regression analysis was performed to investigate the factors influencing vaccination willingness. First, a binary logistic regression analysis was performed for demographic characteristics information to obtain variables with p < 0.05. After that, the variables with p < 0.05 were added to the multivariable logistic regression analysis to correct for the bias introduced by background information. Associations between the independent variables of interest (i.e., variables at the individual, HBM project, and HIV-related information and immunization status) and the dependent variables were assessed by adjusted odds ratios (AORs) and 95% confidence intervals. Each AOR was obtained by fitting a logistic regression model involving an independent variable of interest and all significant background characteristics. All statistical analyzes were performed using SPSS software (version 25.0 IBM Corp., Armonk, NY). A two-tailed p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Background characteristics
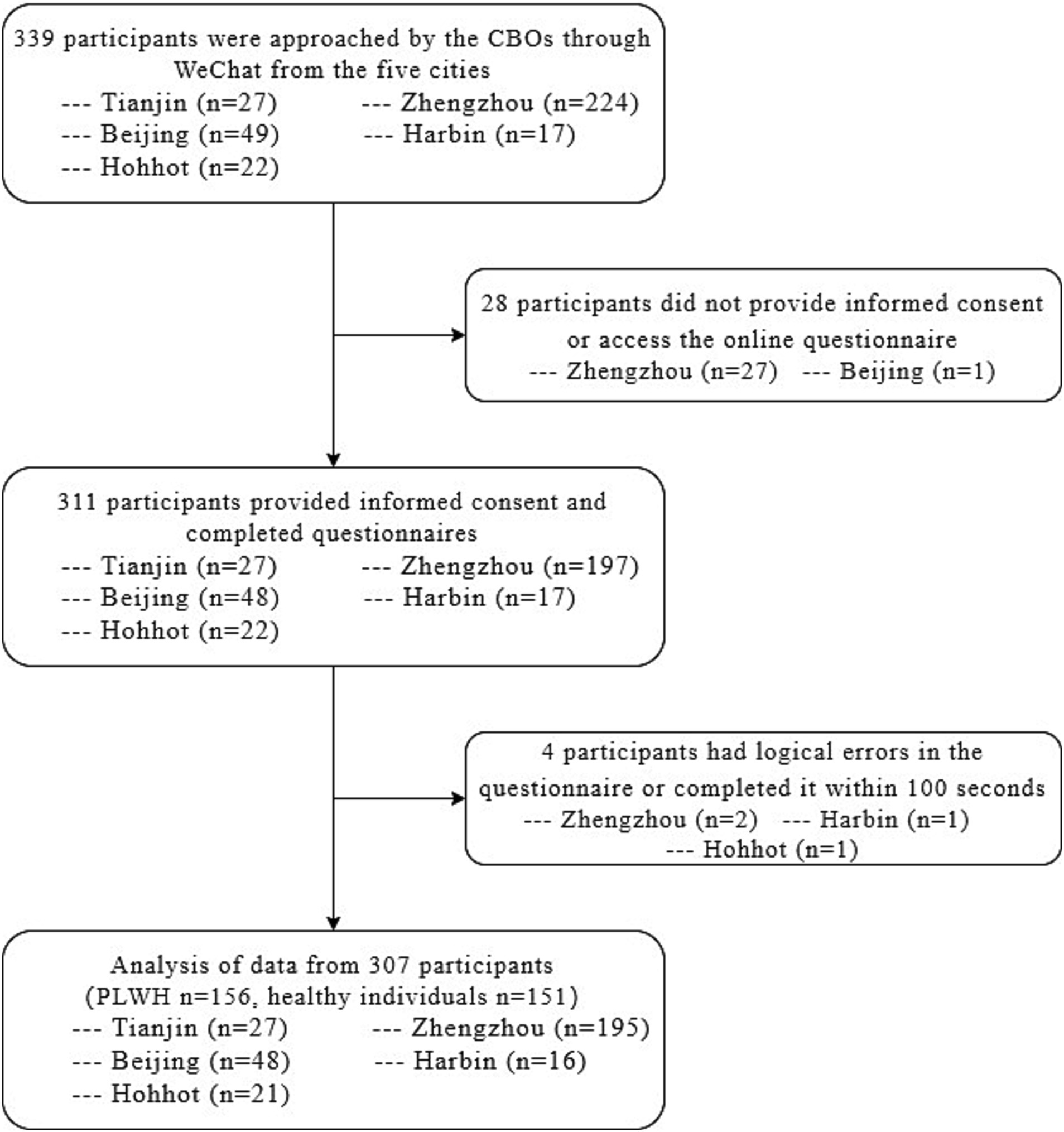
A total of 339 participants aged between 18 and 65 years old were approached for participation. Thirty-two participants were excluded from the study for four reasons: non-provision of informed consent, failure to complete the online questionnaire, presence of logical errors in the questionnaire responses, and inappropriate completion time. Of the remaining 307 participants, 50.81% were PLWH (156/307), and 49.19% were healthy individuals (151/307) (Figure 1).
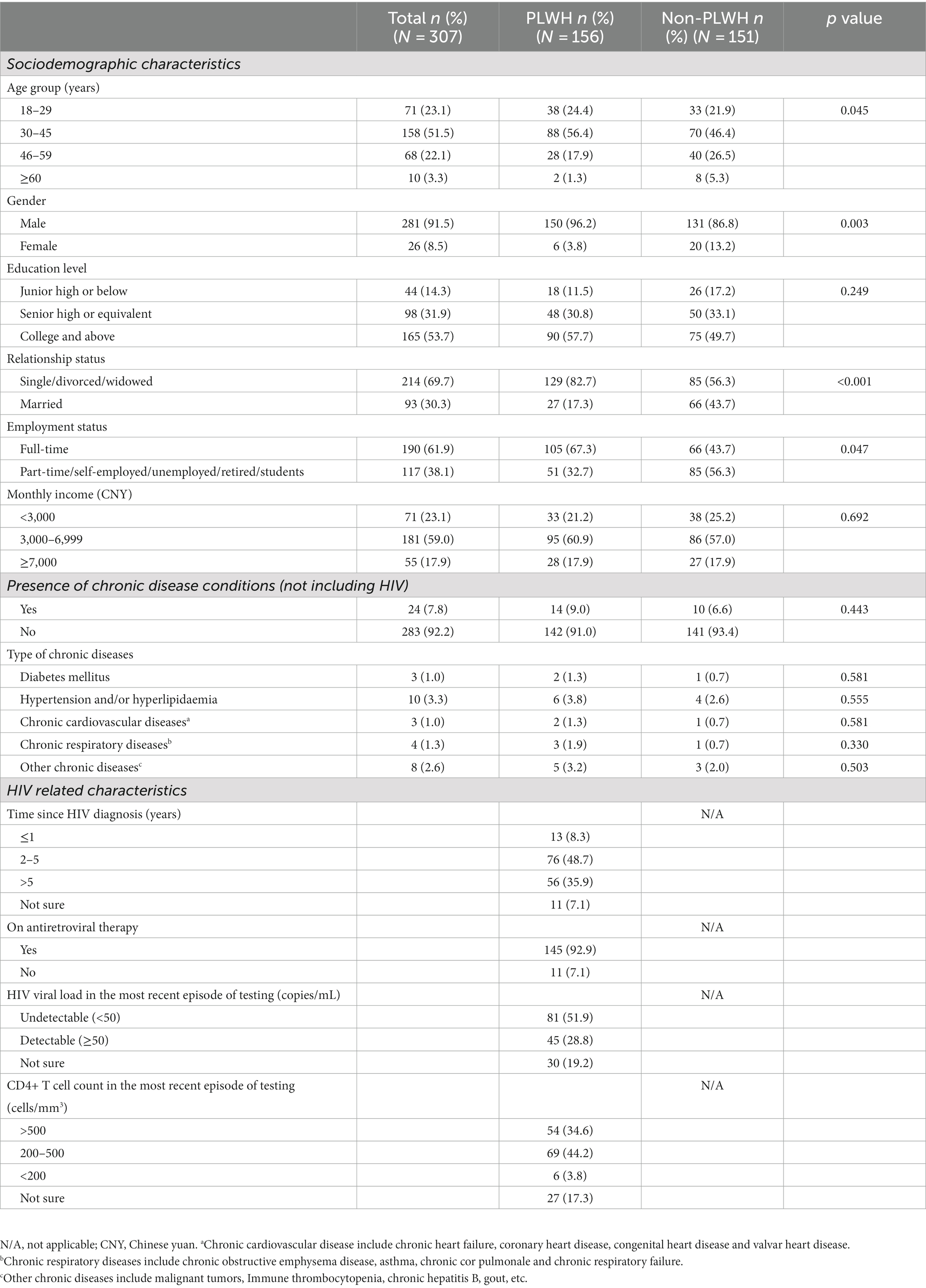
All participants had been vaccinated with the preliminary schedule of two doses of inactivated COVID-19 before recruitment for this study. On average, they received the first COVID-19 booster dose 203 days after receiving the initial two doses. The PLWH group had a significantly higher proportion of males (96.2% vs. 86.8%, p = 0.003) and individuals aged 30–45 (56.4% vs. 46.4%, p = 0.045) than the healthy individual group. Conversely, the healthy individual group had a significantly lower proportion of single/divorced/widowed (56.3% vs. 82.7%, p < 0.001) and engaged in full-time work (43.7% vs. 67.3%, p = 0.047) than the PLWH group. Regarding HIV-related information and immunization status, 84.6% of PLWH had been infected with HIV for over 2 years, with the majority (92.9%) receiving ART. 51.9% of PLWHs reported that their last HIV viral load test was undetectable, and 78.8% of PLWHs reported that their last CD4+ T cell count was over 200 cells/mm3. There were no significant differences (p > 0.05) between the PLWH and healthy individual group regarding education level, monthly income, or prevalence of chronic underlying diseases. More details of the background characteristics can be found in Table 1.
3.2. Vaccination intention and adverse reactions
A significant difference was observed in the willingness to receive the second COVID-19 booster dose between the PLWH group and the healthy individual group (77.6% vs. 88.7%, p = 0.009).
Regarding adverse reactions within 1 month of receiving the first COVID-19 booster dose, 5.1% of the PLWH group and 2.0% of the healthy individual group reported adverse reactions. The primary adverse reactions were local, with 4.5% of the PLWH group and 2.0% of the healthy individual group experiencing them. In the PLWH group, the main complaint was pain at the inoculation site (4.6%), while in the healthy individual group, the main complaint was redness at the inoculation site (2.0%). No significant difference was found between the two groups in the incidence of adverse reactions, local adverse reactions, and systemic adverse reactions (p > 0.05). Table 2 presents the specific details of the adverse reactions.
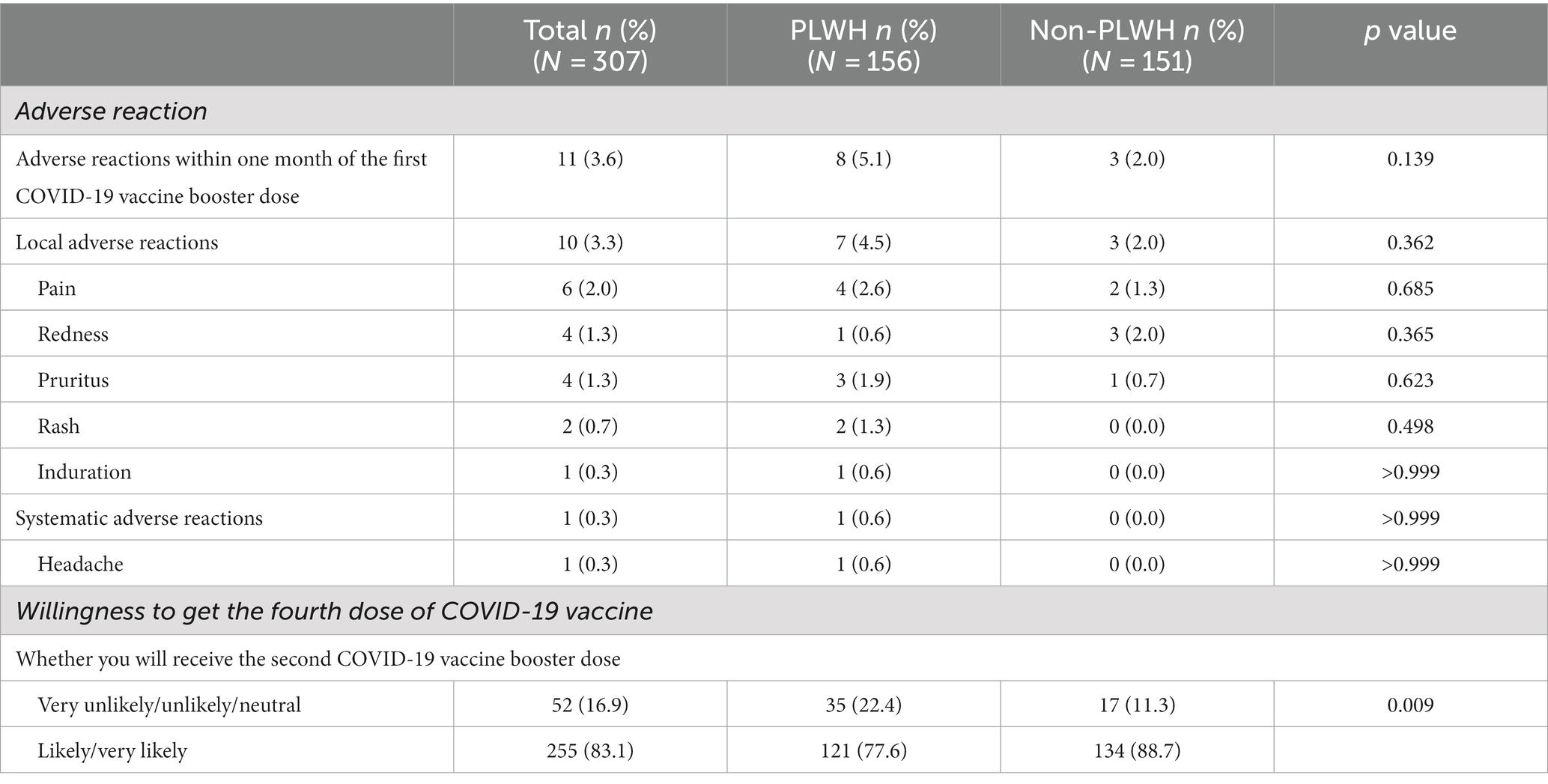
Table 2. Adverse reaction after the first COVID-19 booster dose and the willingness regarding the second COVID-19 booster dose (N = 307).
3.3. Health belief model measures
Table 3 presents the attitudes of all participants regarding the six primary dimensions of the HBM and the specific items in each dimension. In five dimensions—perceived benefit, perceived susceptibility, perceived severity, action clues, and self-efficacy—the PLWH group scored significantly lower than the healthy individual group (p < 0.001). Conversely, in the dimension of perceived barriers, the PLWH group scored significantly higher than the healthy individual group (7.1% vs. 1.3%, p = 0.004). These results suggest that the PLWH group may face more obstacles and be less motivated to receive the COVID-19 booster than the healthy individuals group.
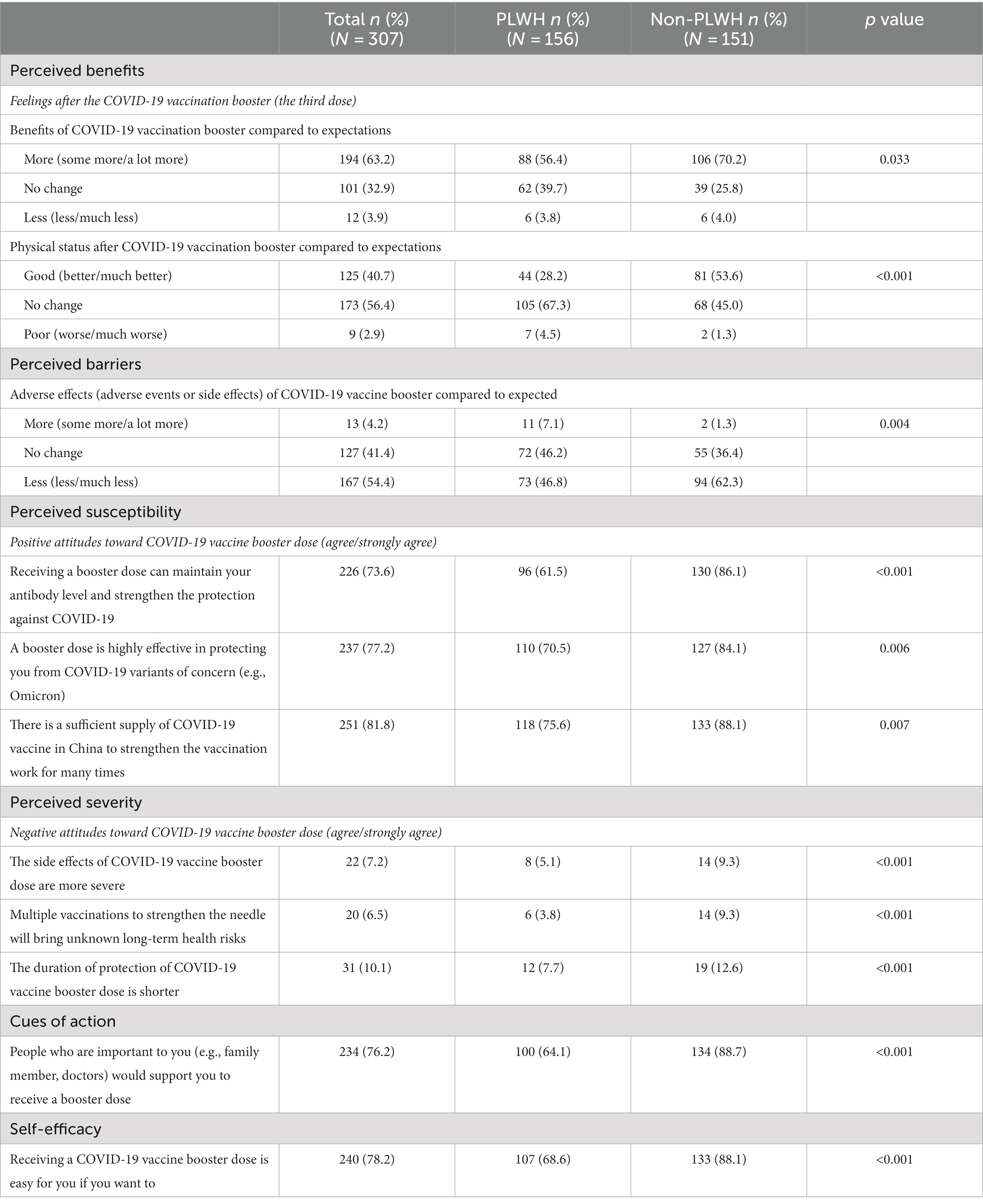
Table 3. HBM items: perceived susceptibility, perceived severity, perceived benefits, perceived barriers, cues of action, and self-efficacy (N = 307).
3.4. Factors associated with willingness to receive the second COVID-19 booster dose
Table 4 shows the results from the univariate analysis. Notably, willingness to receive a second COVID-19 booster dose was higher among those aged 18 to 29 years (90.1%) compared to those aged 30 years and older (79.1, 88.2, 60.0%). Similarly, those with a monthly income of 3,000 to 6,999 Yuan were more willing to receive the booster dose (87.8%) compared to those earning less than 3,000 Yuan (77.5%) and more than 7,000 Yuan (74.5%).
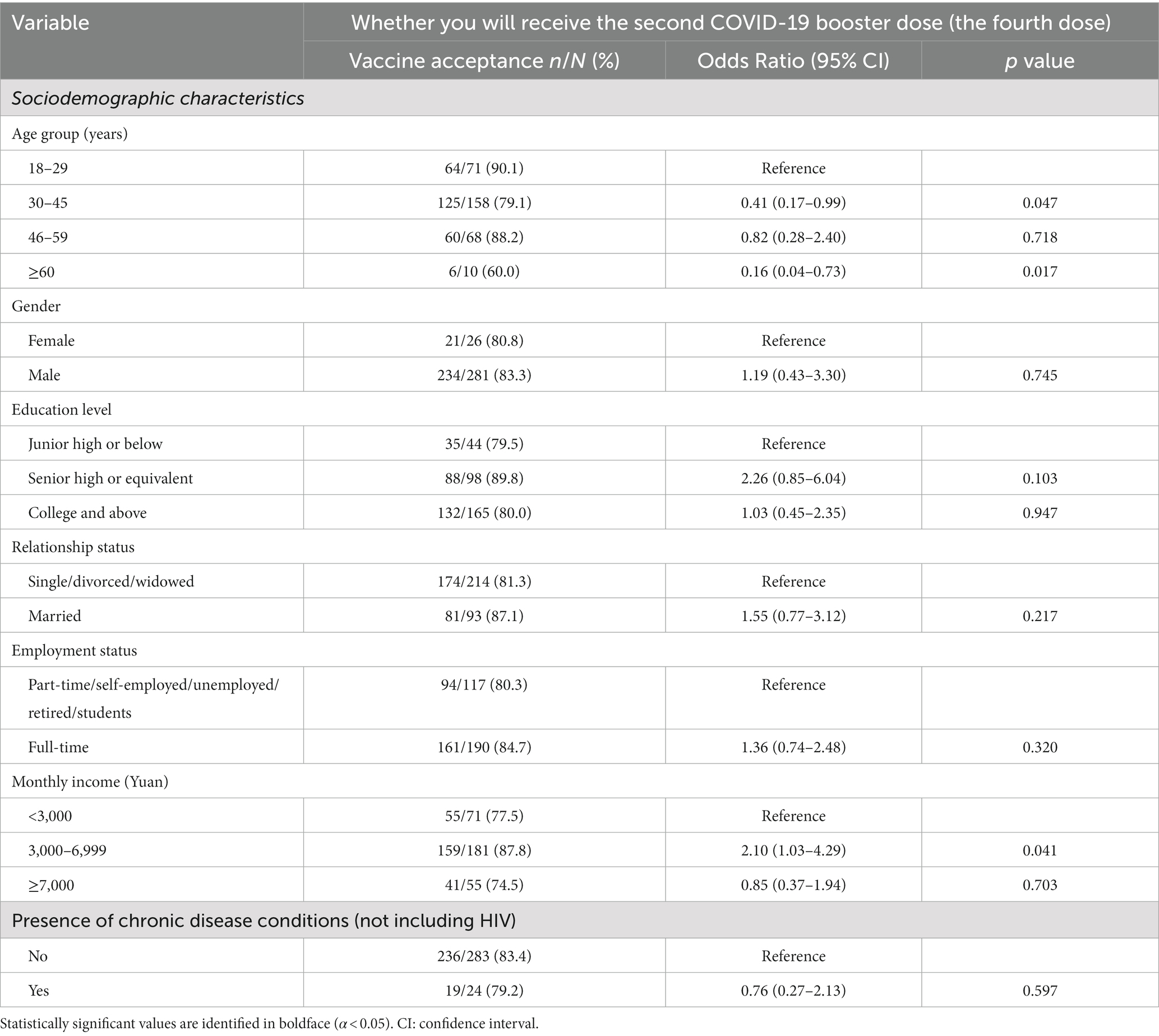
Table 4. Univariate logistic regression of participants’ sociodemographic characteristics (N = 307).
After adjusting for statistically significant sociodemographic characteristics, the outcome of lower willingness to receive the second booster dose was independently associated with HIV positivity (AOR: 0.39, 95%CI: 0.20, 0.75), perceived barriers (indicating the expectation of more adverse effects from the COVID-19 vaccine booster) (AOR: 0.05, 95%CI: 0.01, 0.26), and perceived severity (referring to negative attitudes toward the COVID-19 vaccine booster dose) (AOR: 0.32, 95%CI: 0.12, 0.89).
Conversely, a higher inclination towards receiving the second booster dose was associated with perceived benefits (indicating the expectation of more benefits from the COVID-19 vaccine booster) (AOR: 18.57, 95%CI: 4.02, 85.83) and (referring to better physical status after the vaccination) (AOR: 33.37, 95%CI: 4.22, 263.91). Furthermore, consistent with perceived benefits, a stronger inclination towards receiving the second booster dose showed positive correlations (AOR > 1 for all aforementioned variables) with perceived susceptibility, cues to action, self-efficacy, and detectable HIV viral load. Detailed information (e.g., AOR and 95% CI) can be referenced in Table 5.
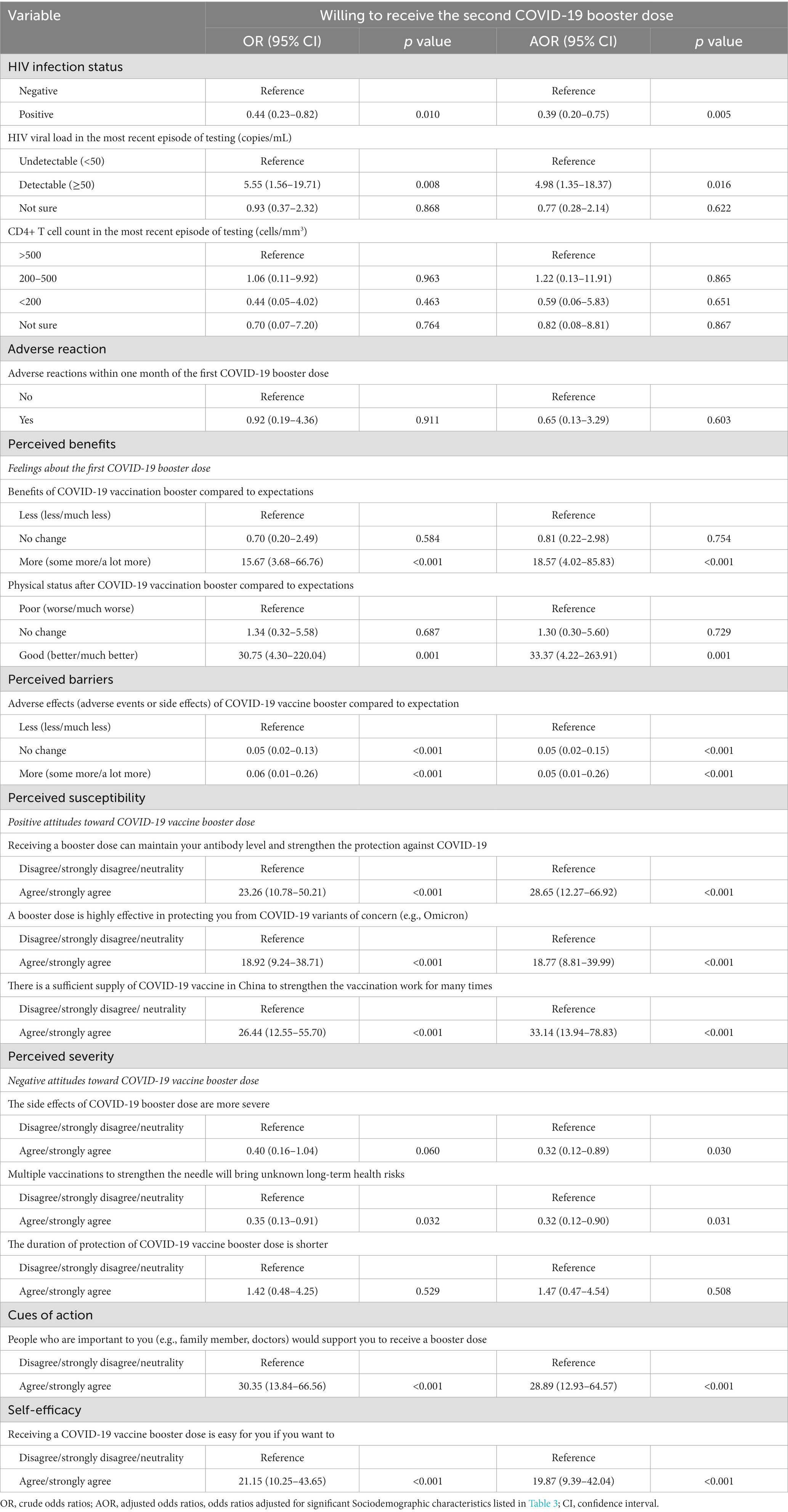
Table 5. Univariate and multivariable analysis of factors associated with willing to receive the second COVID-19 booster dose (N = 307).
4. Discussion
To our knowledge, this is the first multicenter cross-sectional study to explore the willingness of PLWH to receive a second COVID-19 booster dose and its influencing factors in China. Our findings suggest that PLWH were more hesitant to receive a second COVID-19 booster dose than the healthy population. The reasons for this hesitation appear to be multifactorial, with HIV infection status, more significant than expected adverse effects after the first COVID-19 booster dose, and negative attitudes toward the COVID-19 vaccine booster dose being the main factors contributing to vaccine hesitancy. Our findings provide important insights into the willingness of PLWH in China to receive a second COVID-19 booster dose and the associated factors influencing this decision. Moreover, our results could inform both the theoretical framework and practical measures for institutions aiming to understand and address the vaccination intentions of PLWH and the factors influencing them. This, in turn, may assist in designing more effective public health interventions and educational campaigns for PLWH, aiming to boost vaccination coverage and minimize the risk of co-infection and severe clinical outcomes.
Our study observed a lower willingness among PLWH to receive a second COVID-19 booster dose compared to full immunization and the first COVID-19 booster dose reported in the United States, Italy, and Latin America and the Caribbean (78–86.2%) (29–31). One possible explanation for this disparity might be that over time, China’s measures to control COVID-19 have not diminished, yet the prolonged duration of such controls has engendered a sense of fatigue among the population. Consequently, this has led to PLWH beginning to underestimate the pathogenic potential of SARS-CoV-2 and its variants. At the same time, our study corroborates previous findings in healthy individuals in China indicating a high willingness to receive a second COVID-19 booster dose (81.1% vs. 88.7%) (32). However, our study, after adjusting for potential confounders, revealed a significant association between HIV status and vaccine hesitancy for the second COVID-19 booster dose. This persistent vaccine hesitancy among PLWH in China warrants further investigation, despite demonstrated safety and preventative efficacy of the fourth COVID-19 dose and ongoing promotion by relevant health departments (15). It suggests the need for targeted interventions and education to address the factors contributing to this hesitancy.
To date, abundant studies investigating COVID-19 vaccine acceptance among PLWH has generated valuable insights into the underlying influencing factors. For instance, a cross-sectional study demonstrated that negative attitudes towards prime vaccines was associated with the diminished likelihood of vaccine acceptance in China (33). Conversely, individuals with positive perceptions of the prime COVID-19 vaccine exhibited higher rates of acceptance. Furthermore, a positive association between the booster vaccine acceptance and beliefs in the safety, benefits, and accessibility of the booster vaccine was also proved in Uganda (22). Our study further demonstrated that negative attitudes towards vaccines and perceived barriers were both associated with reduced acceptance of the second COVID-19 booster Dose. Similar results were found in immunocompromised cancer patients, which strengthened the necessity of vaccination among specific populations (34, 35).
Our study is the first to investigate the relationship between the willingness of PLWH in China to receive the second COVID-19 booster dose and the six main dimensions of the HBM. These dimensions include perceived susceptibility, perceived severity, perceived benefits, perceived barriers, cues to action, and self-efficacy. Our findings indicate that perceived barriers negatively correlate with vaccine willingness, suggesting fears and misconceptions may dissuade PLWH from receiving the booster dose (36). On the contrary, perceived benefits were positively associated with vaccine willingness, highlighting the potential impact of understanding the benefits of vaccination in promoting vaccine acceptance. Interestingly, we found a positive correlation between perceived susceptibility and vaccine willingness, suggesting that individuals at risk of contracting COVID-19 may be more willing to get vaccinated. However, perceived severity was negatively associated with vaccine willingness, which could indicate that those who perceive COVID-19 as a severe disease may have heightened fears about the safety of vaccines (37). We also noted a positive correlation between self-efficacy and preventive behavior, reinforcing that individual belief in their ability to take preventive measures successfully can influence their willingness to vaccinate (38, 39). Finally, our findings showed a positive correlation between cues to action and vaccine willingness. This implies that support and encouragement from family, friends, and doctors could be critical in promoting vaccination among PLWH (33, 40).
In light of these findings, health departments in China should amplify their efforts to communicate the benefits of the second COVID-19 booster dose. This includes providing clear and reassuring information about the vaccine’s safety, encouraging social support networks to promote vaccination, and fostering a sense of self-efficacy among PLWH. Addressing these factors can reduce vaccine hesitancy and increase the second COVID-19 booster dose uptake among PLWH.
Our multivariable logistic regression analysis revealed that PLWH with a detectable HIV viral load (≥50 copies/mL) demonstrated a higher willingness to receive the second COVID-19 booster dose than those with an undetectable viral load (<50 copies/mL). This result diverges from a US study, which reported a higher willingness to vaccinate among PLWH with an undetectable HIV viral load (29). The discrepancy could be attributed to differences in study design, participant demographics, cultural attitudes towards vaccination, or the methodology of obtaining HIV viral load data. However, the impact of these factors should be further investigated in future studies.
This study has important practical implications, as it found that the willingness of PLWH to receive the second COVID-19 booster dose in China is notably lower than that of the general adult population. It identifies inhibiting factors such as perception barriers and negative attitudes, suggesting a need for targeted educational campaigns to enhance booster vaccine coverage among PLWH.
However, several limitations in our study should be acknowledged: First, as with all cross-sectional studies, establishing causal relationships between independent variables and different outcomes of interest is impossible. Longitudinal studies or randomized controlled trials would be needed to examine causal relationships. Second, subjectively self-administered questionnaires may introduce recall bias, which is difficult to avoid considering the need for anonymity in our study. Future research could consider using alternative methods, such as structured interviews or electronic data collection, to minimize this bias. Third, while most of the items and scales used in this study were self-constructed based on those used in the general population, the external validation of these measures was limited. Further research should seek to validate these measures against established scales or through other external validation methods. Finally, there were variations in the distribution of sociodemographic characteristics between the two groups. Although we adjusted for these characteristics in the multivariable logistic regression model, their potential impact on the study results should be considered. Future studies could explore the potential influence of these characteristics on vaccine willingness more comprehensively and consider other statistical techniques, such as propensity score matching, to address these imbalances.
5. Conclusion
In conclusion, our study highlights the lower willingness of Chinese PLWH to receive a second COVID-19 booster dose compared to healthy individuals. Concerns about adverse effects and negative attitudes toward the booster dose primarily drive this reluctance. Strengthening and promoting knowledge about HIV and COVID-19 vaccination, including the importance of vaccine protection against SARS-CoV-2, is crucial. Based on the findings of this study, targeted interventions should be implemented to increase the willingness of PLWH to receive the second COVID-19 booster dose. This may include tailored education and communication strategies, providing comprehensive information and support, and engaging community resources to address the specific concerns and needs of PLWH.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JX was responsible for the conceptualization of the study and funding acquisition. XL was responsible for data curation and analysis. JX, MS, SL, MY, BS, YQ, and LW were responsible for project administration and securing resources. JX and YW were responsible for supervision and reviewing and editing the article. XL and YW were responsible for writing the original draft of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Shenzhen Science and Technology Innovation Committee Projects (No. JCYJ20220818102817038) and the Scientific Research Foundation of Peking University Shenzhen Hospital (No. KYQD2022216).
Acknowledgments
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XZ declared a past co-authorship with the authors YQ, MY, and JX to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PLWH, people living with HIV; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; AOR, adjusted odds ratio; WHO, world health organization; HBM, health belief model.
References
1. World Health Organization. Coronavirus disease (2019) (COVID-19). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
2. Ssentongo, P, Heilbrunn, ES, Ssentongo, AE, Advani, S, Chinchilli, VM, Nunez, JJ, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. (2021) 11:6283. doi: 10.1038/s41598-021-85359-3
3. Venturas, J, Zamparini, J, Shaddock, E, Stacey, S, Murray, L, Richards, GA, et al. Comparison of outcomes in HIV-positive and HIV-negative patients with COVID-19. J Infect. (2021) 83:217–27. doi: 10.1016/j.jinf.2021.05.020
4. Bertagnolio, S, Thwin, SS, Silva, R, Nagarajan, S, Jassat, W, Fowler, R, et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO global clinical platform of COVID-19. Lancet HIV. (2022) 9:e486–95. doi: 10.1016/S2352-3018(22)00097-2
5. Lee, KW, Yap, SF, Ngeow, YF, and Lye, MS. COVID-19 in people living with HIV: a systematic review and Meta-analysis. Int J Environ Res Public Health. (2021) 18:3554. doi: 10.3390/ijerph18073554
6. Bhaskaran, K, Rentsch, CT, MacKenna, B, Schultze, A, Mehrkar, A, Bates, CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. (2021) 8:e24–32. doi: 10.1016/S2352-3018(20)30305-2
7. Delany, I, Rappuoli, R, and De Gregorio, E. Vaccines for the 21st century. EMBO Mol Med. (2014) 6:708–20. doi: 10.1002/emmm.201403876
8. Cochrane Emergency and Critical Care GroupGraña, C, Ghosn, L, Evrenoglou, T, Jarde, A, Minozzi, S, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. (2023) 12:CD015477. doi: 10.1002/14651858.CD015477
9. Yin, J, Chen, Y, Li, Y, Wang, C, and Zhang, X. Immunogenicity and efficacy of COVID-19 vaccines in people living with HIV: a systematic review and meta-analysis. Int J Infect Dis. (2022) 124:212–23. doi: 10.1016/j.ijid.2022.10.005
10. Fowokan, A, Samji, H, Puyat, JH, Janjua, NZ, Wilton, J, Wong, J, et al. Effectiveness of COVID-19 vaccines in people living with HIV in British Columbia and comparisons with a matched HIV-negative cohort: a test-negative design. Int J Infect Dis. (2023) 127:162–70. doi: 10.1016/j.ijid.2022.11.035
11. Zhan, H, Gao, H, Liu, Y, Zhang, X, Li, H, Li, X, et al. Booster shot of inactivated SARS-CoV-2 vaccine induces potent immune responses in people living with HIV. J Med Virol. (2023) 95:e28428. doi: 10.1002/jmv.28428
12. Yan, Y, Davgadorj, C, Lyu, C, Zhang, S, and Qiu, Y. Immunogenicity of a third dose of inactivated COVID-19 vaccine in people living with HIV-1, HBV, and tuberculosis during the omicron variant epidemic: a cross-sectional study. J Infect. (2022) 85:e109–11. doi: 10.1016/j.jinf.2022.06.032
13. Vergori, A, Cozzi Lepri, A, Cicalini, S, Matusali, G, Bordoni, V, Lanini, S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun. (2022) 13:4922. doi: 10.1038/s41467-022-32263-7
14. Ao, L, Lu, T, Cao, Y, Chen, Z, Wang, Y, Li, Z, et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect. (2022) 11:1126–34. doi: 10.1080/22221751.2022.2059401
15. Cheung, PK, Lapointe, HR, Sang, Y, Ennis, S, Mwimanzi, F, Speckmaier, S, et al. SARS-CoV-2 live virus neutralization after four COVID-19 vaccine doses in people with HIV receiving suppressive antiretroviral therapy. AIDS. (2023) 37:F11–8. doi: 10.1097/QAD.0000000000003519
16. Bar-On, YM, Goldberg, Y, Mandel, M, Bodenheimer, O, Amir, O, Freedman, L, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. (2022) 386:1712–20. doi: 10.1056/NEJMoa2201570
17. U.S. Food and Drug Administration. U.S. Food and Drug Administration coronavirus (COVID-19) update: FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and
18. The Comprehensive Group of the Joint Prevention and Control Mechanism. The Comprehensive Group of the Joint Prevention and Control Mechanism of the state council in response to the epidemic situation of COVID-19: novel coronavirus vaccine second dose enhanced immunization implementation plan Available at: www.gov.cn/xinwen/2022-12/14/content_5731899.htm
19. Yang, J, Yu, M, Fu, G, Lan, G, Li, L, Qiao, Y, et al. COVID-19 vaccination uptake among a Nationwide sample of people living with HIV during the early phase of vaccine rollout in China. Front Med (Lausanne). (2022) 9:822680. doi: 10.3389/fmed.2022.822680
20. Tesoriero, JM, Patterson, W, Daskalakis, D, Chicoine, J, Morne, J, Braunstein, S, et al. Notes from the field: COVID-19 vaccination among persons living with diagnosed HIV infection - New York, October 2021. MMWR Morb Mortal Wkly Rep. (2022) 71:182–4. doi: 10.15585/mmwr.mm7105a4
21. World Health O. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing: Interim guidance, first issued 8 January 2021, updated 15 June 2021, updated 19 November 2021, updated 21 January 2022, updated 18 august 2022. Geneva: World Health Organization; 2022 2022. Contract no.: WHO/2019-nCoV/vaccines/SAGE_recommendation/BNT162b2/2022.2.
22. Muhindo, R, Okoboi, S, Kiragga, A, King, R, Arinaitwe, WJ, and Castelnuovo, B. COVID-19 vaccine acceptability, and uptake among people living with HIV in Uganda. PLoS One. (2022) 17:e0278692. doi: 10.1371/journal.pone.0278692
23. Lyons, N, Bhagwandeen, B, and Edwards, J. Factors affecting COVID-19 vaccination intentions among patients attending a large HIV treatment Clinic in Trinidad Using Constructs of the health belief model. Vaccines (Basel). (2022) 11:4. doi: 10.3390/vaccines11010004
24. Servidio, R, Malvaso, A, Vizza, D, Valente, M, Campagna, MR, Iacono, ML, et al. The intention to get COVID-19 vaccine and vaccine uptake among cancer patients: an extension of the theory of planned behaviour (TPB). Support Care Cancer. (2022) 30:7973–82. doi: 10.1007/s00520-022-07238-5
25. Wang, Y, Qiao, Y, Huo, Y, Wang, L, Liang, S, Yu, M, et al. The safety and immunogenicity of a two-dose schedule of CoronaVac, and the immune persistence of vaccination for six months, in people living with HIV: a multicenter prospective cohort study. Front Immunol. (2023) 14:1129651. doi: 10.3389/fimmu.2023.1129651
26. Galanis, P, Vraka, I, Katsiroumpa, A, Siskou, O, Konstantakopoulou, O, Katsoulas, T, et al. Predictors of willingness of the general public to receive a second COVID-19 booster dose or a new COVID-19 vaccine: a cross-sectional study in Greece. Vaccines (Basel). (2022) 10:1061. doi: 10.3390/vaccines10071061
27. Della Polla, G, Miraglia Del Giudice, G, Folcarelli, L, Napoli, A, and Angelillo, IF. Willingness to accept a second COVID-19 vaccination booster dose among healthcare workers in Italy. Front Public Health. (2022) 10:1051035. doi: 10.3389/fpubh.2022.1051035
28. Wu, S, Ming, F, Xing, Z, Zhang, Z, Zhu, S, Guo, W, et al. COVID-19 vaccination willingness among people living with HIV in Wuhan, China. Front Public Health. (2022) 10:883453. doi: 10.3389/fpubh.2022.883453
29. Wickersham, JA, Meyer, JP, Shenoi, S, Altice, FL, Barakat, LA, Virata, M, et al. Willingness to be vaccinated against COVID-19 among people with HIV in the United States: results from a National Survey. Front Med (Lausanne). (2022) 9:886936. doi: 10.3389/fmed.2022.886936
30. Bert, F, Pivi, A, Russotto, A, Mollero, B, Voglino, G, Orofino, G, et al. COVID-19 vaccination among HIV+ patients: an Italian cross-sectional survey. Vaccines (Basel). (2022) 10:1438. doi: 10.3390/vaccines10091438
31. Alarcón-Braga, EA, Hernandez-Bustamante, EA, Salazar-Valdivia, FE, Valdez-Cornejo, VA, Mosquera-Rojas, MD, Ulloque-Badaracco, JR, et al. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: a systematic review and meta-analysis. Travel Med Infect Dis. (2022) 49:102369. doi: 10.1016/j.tmaid.2022.102369
32. Qin, C, du, M, Wang, Y, Liu, Q, Yan, W, Tao, L, et al. Assessing acceptability of the fourth dose against COVID-19 among Chinese adults: a population-based survey. Hum Vaccin Immunother. (2023) 19:2186108. doi: 10.1080/21645515.2023.2186108
33. Huang, X, Yu, M, Fu, G, Lan, G, Li, L, Yang, J, et al. Willingness to receive COVID-19 vaccination among people living with HIV and AIDS in China: Nationwide cross-sectional online survey. JMIR Public Health Surveill. (2021) 7:e31125. doi: 10.2196/31125
34. Zhang, L, Yang, J, Su, R, du, X, Wang, Y, Chen, S, et al. Concerns related to the interactions between COVID-19 vaccination and cancer/cancer treatment were barriers to complete primary vaccination series among Chinese cancer patients: a multicentre cross-sectional survey. Hum Vaccin Immunother. (2023) 19:2222648. doi: 10.1080/21645515.2023.2222648
35. Wang, Y, Zhang, L, Chen, S, Lan, X, Song, M, Su, R, et al. Hesitancy to receive the booster doses of COVID-19 vaccine among Cancer patients in China: a multicenter cross-sectional survey - four PLADs, China, 2022. China CDC Weekly. (2023) 5:223–8. doi: 10.46234/ccdcw2023.041
36. Qin, C, Yan, W, du, M, Liu, Q, Tao, L, Liu, M, et al. Acceptance of the COVID-19 vaccine booster dose and associated factors among the elderly in China based on the health belief model (HBM): a national cross-sectional study. Front Public Health. (2022) 10:986916. doi: 10.3389/fpubh.2022.986916
37. Lin, Y, Hu, Z, Zhao, Q, Alias, H, Danaee, M, and Wong, LP. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. (2020) 14:e0008961. doi: 10.1371/journal.pntd.0008961
38. Orji, R, Vassileva, J, and Mandryk, R. Towards an effective health interventions design: an extension of the health belief model. Online J Public Health Inform. (2012) 4:ojphi.v4i3.4321. doi: 10.5210/ojphi.v4i3.4321
39. Chen, H, Li, X, Gao, J, Liu, X, Mao, Y, Wang, R, et al. Health belief model perspective on the control of COVID-19 vaccine hesitancy and the promotion of vaccination in China: web-based cross-sectional study. J Med Internet Res. (2021) 23:e29329. doi: 10.2196/29329
Keywords: COVID-19 vaccines, PLWH, health belief model, willingness, booster dose
Citation: Lan X, Su B, Liang S, Yu M, Qiao Y, Wang L, Song M, Wang Y and Xu J (2023) Willingness of people living with HIV to receive a second COVID-19 booster dose: a multicenter cross-sectional study in China. Front. Public Health. 11:1227277. doi: 10.3389/fpubh.2023.1227277
Edited by:
Severino Jefferson Ribeiro da Silva, University of Toronto, CanadaReviewed by:
Zixin Wang, The Chinese University of Hong Kong, ChinaXiangjun Zhang, University of Tennessee Health Science Center (UTHSC), United States
Copyright © 2023 Lan, Su, Liang, Yu, Qiao, Wang, Song, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Xu, eGpqY211QDE2My5jb20=; Yuxiao Wang, d2FuZ3l1eGlhbzEyMTNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xinquan Lan
Xinquan Lan Bin Su
Bin Su Shijie Liang4
Shijie Liang4 Yuxiao Wang
Yuxiao Wang Junjie Xu
Junjie Xu