
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 17 July 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1221788
Introduction: Pregnancy increases the risk of developing a severe illness due to COVID-19 infection. To the best of our knowledge, no previous study has been conducted on COVID-19 vaccine acceptance among pregnant women in Sudan. Hence, this study aimed to determine COVID-19 vaccination acceptance and its predictors among pregnant women.
Methods: A cross-sectional study was conducted among 623 pregnant women attending Gadarif maternity hospital in eastern Sudan through a structured questionnaire. Data were obtained on sociodemographic characteristics, obstetric and health-related characteristics, COVID-19 infection, and vaccination-related information, as well as beliefs about and acceptance of COVID-19 vaccination.
Results: COVID-19 vaccine acceptance among the pregnant women was 2.7%. The vaccine acceptance was higher if their husband’s education was secondary school or higher [adjusted odds ratio [AOR] 4.30, 95% confidence interval (CI) 1.11–16.65, p = 0.035] and discussion of COVID-19 vaccine with the pregnant women by a health care professional in the hospital (AOR 5.46, 95% CI 1.94–15.35, p < 0.001). The most common reasons for resistance to the vaccine were concerns about the side effects of the vaccine for the mother and her baby.
Conclusion: Acceptance of the COVID-19 vaccination among the pregnant women was very low. Discussions with pregnant women and their husbands by health care professionals regarding the safety of COVID-19 vaccine for the mother and her baby are highly recommended.
COVID-19 infections have been a major public health event since 2019 (1). The novel coronavirus has infected millions of people, disrupted health care systems, and caused global lockdowns (2). During the pandemic, the consistent use of face masks and maintenance of social distancing were identified as key preventive measures until mass vaccinations were initiated (3). Worldwide, once nations administered the vaccine, it substantially changed the course of the pandemic and saved millions of lives (4). Evidence has shown that pregnant women are likelier to become infected by SARS-CoV-2 (5). Pregnant women with COVID-19 infections are also at an elevated risk of severe illness and mortality (6, 7). Previous findings have shown that pregnant women hospitalized due to COVID-19 are likelier to be admitted to the intensive care unit (ICU), to require invasive ventilation, and to experience death compared with non-pregnant women with COVID-19 (8–11). Additionally, pregnant women with COVID-19 were found to have a higher risk of unfavorable birth outcomes, including stillbirth, preterm birth, cesarean delivery, and high rates of neonatal ICU admissions, compared with pregnant women without COVID-19 (12, 13). Limited evidence of vertical transmission of SARS-CoV-2 also exists (14). COVID-19 vaccination has been found to be the most efficient and effective way to prevent the transmission of SARS-CoV-2 to pregnant women and fetuses, as well as serious illness or other consequences, by producing immune responses during pregnancy (15, 16). The vaccine also protects the fetus or neonate against COVID-19 infection through the passive transplacental transfer of antibodies from the vaccinated mother during pregnancy (17). Thus, the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists have strongly recommended that pregnant women be vaccinated to prevent maternal and fetal morbidity and mortality (18–20).
Low vaccine uptake has become a growing challenge worldwide (21–24). According to the World Health Organization (WHO), vaccine hesitancy was one of the top 10 health threats worldwide before the outbreak of the COVID-19 pandemic in 2019 (25). A previous literature review showed varying results for global vaccine acceptance during pregnancy (26).
Sudan was the first country in the Middle East and North Africa to obtain the COVID-19 vaccine through the Vaccines Global Access (COVAX) facility through the GAVI vaccine alliance, the Coalition for Epidemic Preparedness Innovations, the WHO, and UNICEF, a key delivery partner, in March 2021 (4, 27). By the end of 2021, it was anticipated that Sudan would obtain a 17 million doses from the COVAX facility, which would cover 20% of the populace (27). Furthermore, it was projected that in excess of 38 million doses would be secured to encompass at the minimum 45% of the Sudanese demographic prior to the realization of herd immunity (27, 28). Sudan administered the COVID-19 vaccine in three phases. Frontline health care workers were vaccinated in the first phase (28). In the second phase, people over 45 years of age with chronic medical conditions, school staff and teachers, and high-risk people who could not avoid exposure to COVID-19 due to the nature of their jobs were vaccinated (28, 29). In the third phase, pregnant women, lactating mothers, children, adolescents up to 16 years, and 80 people aged 16–45 years were vaccinated (29). In phase 3 of the vaccination campaign, there was a moderate level of vaccine availability to cater to this targeted population subset, ranging from 21 to 50% (28, 29). The financing of the third phase of COVID-19 vaccination necessitated the Sudanese government’s willingness to allocate funds toward the initiative amid the ongoing economic crisis (27). Internal funding and support for the health care system in Sudan have been lacking, which has been compounded by the long-standing economic sanctions in effect for two decades until 2017 (27, 28). To achieve the objective of vaccinating 50% of the population, the Sudanese government had to collaborate with external organizations to obtain funding and assistance in enhancing access to vaccines among citizens. Failure to do so would exacerbate the COVID-19 situation in the country (28).
The current fertility rate in Sudan is 4.4 births per woman (30). Previous studies have revealed varying rates of COVID-19 vaccine acceptance among pregnant women (31, 32). The acceptance rate for the COVID-19 vaccine among the Sudanese population has been low (33). However, no previous study has been conducted on acceptance of the COVID-19 vaccine by pregnant women in Sudan. Hence, this study aimed to determine COVID-19 vaccination acceptance and its predictors among pregnant women.
This observational cross-sectional study was conducted among pregnant women aged 18–44 years who attended the Gadarif Maternity Hospital in eastern Sudan from July through September 2022. Gadarif, one of Sudan’s 18 states, has an area of 75,263 km2 and an estimated population of 1,827,181 (34, 35). This semi-desert tropical region is located in southeastern Sudan between latitudes 14 and 16 north and longitudes 35 and 36 east (34). Its main sources of income are farming, trading, and animal breeding (35, 36).
Face to face interviews was conducted using questionnaire which was framed according to previously published research and the objectives of this study (37–42). Three experts reviewed and approved the content validity of the questionnaire. Before the main study, the questionnaire was pilot tested on 20 respondents. The final version of the questionnaire was revised and approved by all the authors.
The dependent variable was assessed by the question “Do you intend to receive the COVID-19 vaccine?” (yes/no).
i. Sociodemographic information, including age, place of residence (i.e., urban or rural), level of education (lower than secondary school, secondary school, or higher), level of education of the pregnant woman’s husband (lower than secondary school, secondary school or higher), working status over the last 12 months (housewife or employed), residing with someone older than 60 years (yes/no).
ii. Obstetric and health-related characteristics, including parity, history of miscarriage (yes/no), gestational age in weeks, and history of comorbidity (i.e., diabetes mellitus, hypertension, thyroid disease, other, or none).
iii. COVID-19 infection and vaccination-related information, including history of COVID-19 infection before and during the current pregnancy; history of COVID-19 infection in the pregnant woman’s husband (yes/no); worry of COVID-19 infection during pregnancy (yes, no); and COVID-19 vaccine is safe and effective (yes, no); receipt of COVID-19 vaccine by the pregnant woman’s husband (yes or no); COVID-19 vaccine discussed with the pregnant woman by any hospital staff (i.e., midwife, doctor, unsure, or not yet discussed).
iv. Beliefs about and acceptance of COVID-19 vaccination, including the pregnant woman’s worry of side effects of the COVID-19 vaccination for herself (strongly agree, agree, disagree, or strongly disagree); the pregnant woman’s worry of side effects of COVID-19 vaccination on her baby (strongly agree, agree, disagree, or strongly disagree); inadequate information about the safety of the COVID-19 vaccine during pregnancy (strongly agree, agree, disagree, or strongly disagree); unsuitable times for scheduling an appointment to receive the COVID-19 vaccine during pregnancy (strongly agree, agree, disagree, or strongly disagree); the belief that COVID-19 in Gadarif is decreasing, and there is no need to be vaccinated against it (strongly agree, agree, disagree, or strongly disagree).
The sample size was calculated using OpenEpi software. The minimum sample size was 500 participants (95% confidence interval [CI], 80% statistical power, and 5% type I error). The rate of COVID-19 vaccination acceptance among pregnant women was assumed to be 50%, based on a lack of related studies in Sudan to attain the maximum sample size (30% expected incomplete and missing responses; Figure 1).
The ethics committee of Gadarif University approved the current study (reference number 15,06,2022). The purpose of this study was thoroughly explained to the participants, their informed consent was obtained, and their information was treated confidentially by the researchers. All study procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
The data were entered into an Excel sheet and checked to exclude duplicate answers, after which they were exported to STATA version 16 for statistical analysis. The categorized data are expressed as frequencies and percentage (%). The continuous data were not normally distributed and are expressed as medians and interquartile ranges (IQRs). Bivariate and multivariable analyses were conducted to determine associations between the independent variables (i.e., potential predictors) and the dependent variable (i.e., vaccine acceptance). Multiple logistic regressions included potential predictors with a value of p of <0.25 in the simple logistic regression. A value of p of <0.05 was considered robust evidence against the null hypothesis.
A total of 623 participants were included in this study. The median (IQR) age was 28 (24–32) years, the median (IQR) gestational age was 32 (27–38) weeks, and the median (IQR) parity was 3 (2–5). Two-thirds (66.4%) of the participants lived in rural areas. The numbers and proportions of the pregnant women and their husbands who had secondary school or higher-level education were 369 (59.2%) and 311 (49.9%), respectively. The majority (570, 91.5%) were housewife, participants with people over 60 years in their households numbered 434 (69.7%), and 27 (4.3%) of the pregnant women had comorbidities (Table 1). Only 17 (2.7%) of the pregnant women were willing to receive the COVID-19 vaccine, none of whom had a history of COVID-19 infection (Table 2). The results of the bivariate analysis showed that the pregnant women were likelier to receive the COVID-19 vaccine if their husbands had a secondary school education or higher (COR 4.86, 95% CI 1.38–17.07, p = 0.014), were employed (COR 3.49, 95% CI 1.09–11.14, p = 0.034), if their husbands had a history of COVID-19 infection (COR 9.97, 95% CI 1.94–50.97, p = 0.006), if they themselves believed that the COVID-19 vaccine was safe (COR 6.59, 95% CI 2.49–17.63, p < 0.001) and effective (COR 6.14, 95% CI 2.26–16.71, p < 0.001), and if hospital staff had discussed the COVID-19 vaccine with them (COR 5.46, 95% CI 1.94–15.35, p < 0.001; Table 3).
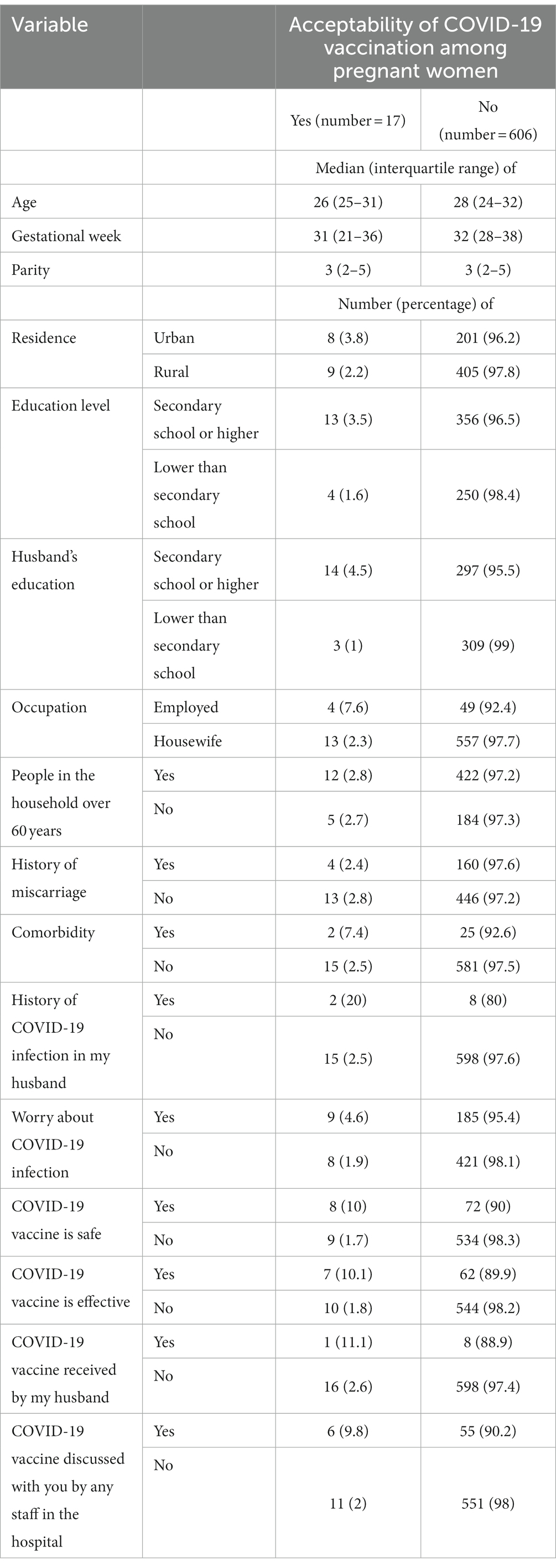
Table 2. Comparing different variables between women who accepted COVID-19 vaccination and women who did not.
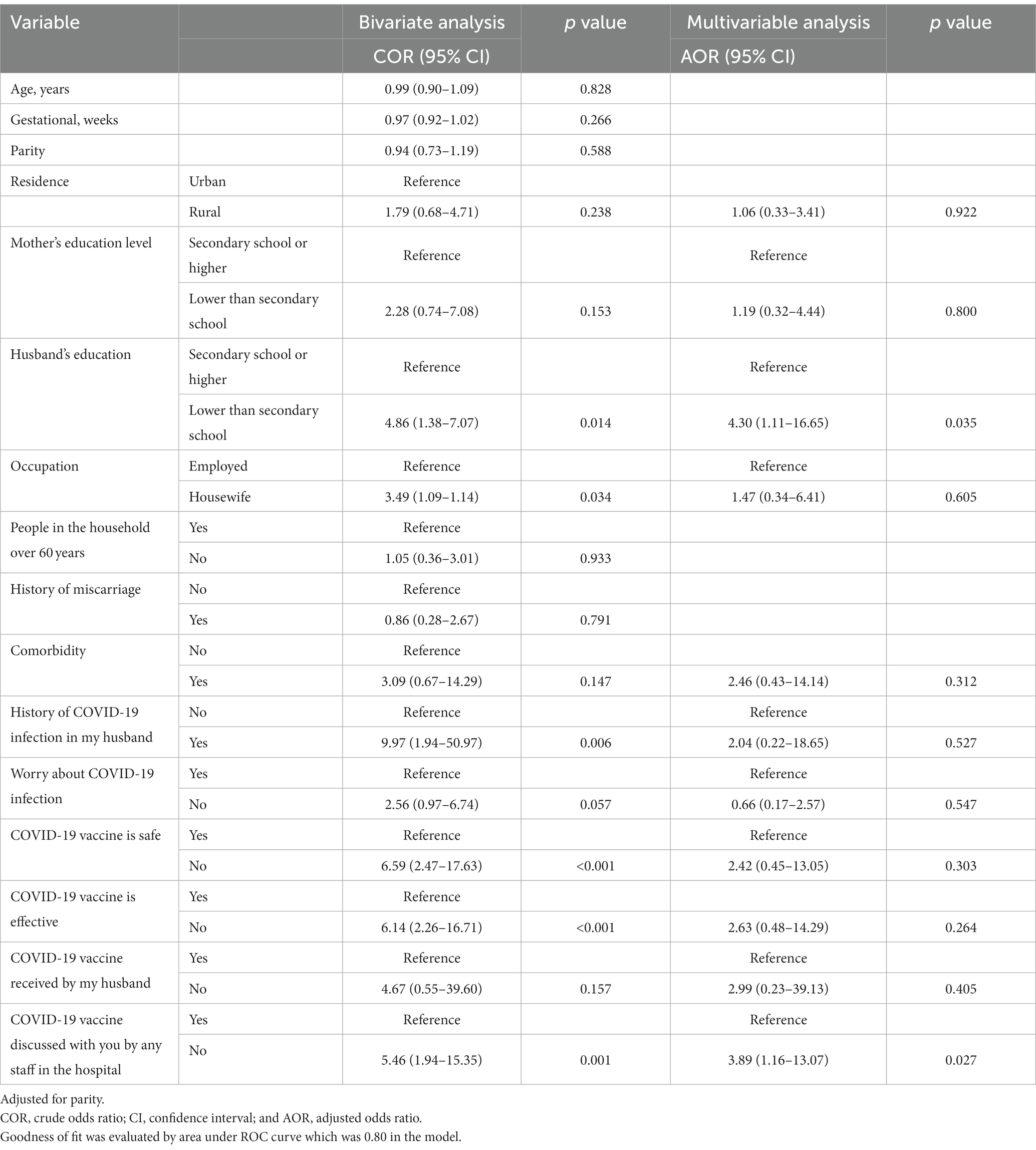
Table 3. Simple and multiple logistic regression analyses exploring the predictors of acceptability of COVID-19 vaccination among pregnant women.
The results of the multivariable analysis showed that the variables that remained statistically significant predictors were as follows: husband’s education equal to secondary school or higher (adjusted odds ratio [AOR] 4.30, 95% CI 1.11–16.65 p = 0.035); and hospital staff discussed the COVID-19 vaccine with the pregnant women (COR 5.46, 95% CI 1.94–15.35, p < 0.001; Goodness of fit was evaluated by area under ROC curve which was 0.80 in the model; Table 3). Among the participants, the most frequent reason for not accepting the COVID-19 vaccination was concern about the side effects of the vaccine on the mother and her baby. The second most frequent reason was inadequate information about the safety of the vaccine, followed by the participants’ inability to find a suitable time to schedule an appointment for the vaccine, and the belief that the rate of COVID-19 infection was decreasing in the city of their residence (Table 4).
To the best of our knowledge, our study is the first to examine COVID-19 vaccine acceptance by pregnant women in Sudan. The findings showed a rate of COVID-19 vaccine acceptance in pregnant women of 2.7%, which is lower than the acceptance rate among African countries like Ethiopia (70.7%), South Africa (63.3%), and Cameroon (31%) (43–45). A systematic review and meta-analysis reported wide variations in countries around the globe for COVID-19 vaccine uptake among pregnant women, ranging from 7.0 to 68.7% (46). Contemporary research studies have reported an acceptance rate below 40% among pregnant women in India (18%), Singapore (30.3%), Ireland (38%), Turkey (37%), France (29.5%), and Japan (13.4%) (37, 47–51). On the other hand, China (77.4%), Thailand (60.8%), Saudi Arabia (68%), Qatar (75%), the United Kingdom (62.1%), Czechia (76.6%), Italy (84.5%), and the United States (76.2%) were reported to have more than a 60% acceptance rate among pregnant women (10, 32, 41, 42, 52–55).
The current study showed the lowest acceptance rate (2.7%) of COVID-19 vaccine uptake among pregnant women worldwide. The Africa CDC have reported a fully vaccinated rate for Sudan of 40.2% (56). However, our research indicates a notable lack of vaccination acceptance among pregnant women, which could result in a failure to reach the targeted phase 3 vaccination range of 21–50% in Sudan. This reluctance to receive a COVID-19 vaccination can be efficiently resolved by addressing the specific reasons that have been identified in our study. The primary concerns most commonly expressed by expectant mothers pertained to the safety of both their own health and that of their fetuses, thus corroborating the conclusions drawn from numerous antecedent studies (31, 37, 57–60). Additionally, a lack of sufficient information regarding the vaccine’s safety, as well as the inability to schedule a suitable time for the COVID-19 vaccination appointment during pregnancy, were cited as contributing factors in our research. The matter of vaccination trials or administration during pregnancy in Africa is shrouded in silence (61). The Pfizer-BioNTech vaccine, which has been approved, is not a live virus vaccination but rather an mRNA vaccine, and the Royal College of Obstetricians and Gynecologists has declared that there is insufficient evidence to support the routine use of COVID-19 vaccines for pregnant or breastfeeding women. Furthermore, women who plan to conceive within 3 months of receiving the first dose have been advised against getting vaccinated (62). The accelerated vaccine development process, which has employed modern technologies, has increasingly come under scrutiny (63). A survey conducted between August and December 2020, spanning 15 African countries, including Burkina Faso, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Gabon, Kenya, Malawi, Morocco, Niger, Nigeria, Senegal, South Africa, Sudan, Tunisia, and Uganda, polled over 15,000 adults aged 18 years and above. The Africa CDC collaborated with the London School of Hygiene & Tropical Medicine to conduct the survey, which revealed that a considerable majority (an average of 79%) of respondents in Africa would get vaccinated against COVID-19 if it was deemed safe and effective (64). Despite the absence of unequivocal proof regarding the timing and adverse effects of COVID-19 vaccination in pregnant women, no previous observational study or large case series has evinced any deleterious impact of the COVID-19 vaccine on the health of pregnant women or their neonates (65–71).
The findings of the present study showed an association between pregnant women whose husband’s education was equal to or higher than secondary level and a higher rate of COVID-19 vaccine acceptance. This can be rationalized by the indispensable role played by husbands in the process of making informed decisions pertaining to the administration of vaccines for women (72). This finding is similar to a study by Tefera et al. (73), who attributed the association to the ability of educated husbands to read and communicate to their wives news and social media messages related to the seriousness of COVID-19 infection and the positive effects of the vaccine. In Sudan, gender roles are characterized by a strong patriarchal influence, with rigid definitions in place. The male population is predominantly perceived as the primary breadwinners and decision-makers, while their female counterparts are predominantly viewed as homemakers. It is expected that husbands take on the economic responsibility of providing for their wives and children throughout their lifetimes (74).
Another important finding of our study was that the vaccination likelihood of pregnant women with whom any hospital staff discussed the potential benefits of the COVID-19 vaccine was higher compared with those who did not have that experience. This finding is consistent with the findings of recent studies by Riad et al. (10) in Czechia and Nganga et al. (75) in Kenya, which showed that the COVID-19 vaccine was likelier to be accepted by pregnant women if it was recommended by health care professionals (HCPs) and it was made accessible to them. The same findings were endorsed in a literature review by Wilson et al. (26). Therefore, it is highly recommended that HCPs be educated about evidence-based recommendations for maternal vaccinations during pregnancy to enable them to address vaccine-hesitant pregnant women (76, 77). The present study had the following limitations. Causal relationships could not be established because of the inherent limitations of the cross-sectional study design, the time factor in conducting the study during the COVID-19 pandemic produced limitations for properly validating the questionnaires, and the validation consisted only of expert judgment and a pilot study on 20 mothers, which is inadequate for proper psychometric validation of the tool.
Acceptance of the COVID-19 vaccination among the pregnant women in this study was extremely low. Discussions with pregnant women and their husbands by an HCP regarding the safety of the COVID-19 vaccine for both mother and baby are highly recommended. We also recommend that future vaccination campaigns focus on enhancing HCPs’ communication skills to ensure the engagement of pregnant women and promote vaccination uptake during pregnancy. The findings of our study could provide guidance for health care providers and policymakers in designing effective strategies to improve vaccine uptake during pregnancy in eastern Sudan.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The ethics committee of Gadarif University was approved the current study (reference number 15,06,2022). The patients/participants provided their written informed consent to participate in this study.
IA and OA-W conceived and designed the study. OO and SO prepared the manuscript. OO, IA, and SO supervised the study, and OA-W, RK, and IA contributed to the paper editing. All authors contributed to the article and approved the submitted version.
The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McCloskey, B, Zumla, A, Ippolito, G, Blumberg, L, Arbon, P, Cicero, A, et al. Mass gathering events and reducing further global spread of COVID-19: a political and public health dilemma. Lancet. (2020) 395:1096–9. doi: 10.1016/S0140-6736(20)30681-4
2. Chen, JM. Novel statistics predict the COVID-19 pandemic could terminate in 2022. J Med Virol. (2022) 94:2845–8. doi: 10.1002/jmv.27661
3. Corea, F, Folcarelli, L, Napoli, A, Del Giudice, GM, and Angelillo, IF. The impact of COVID-19 vaccination in changing the adherence to preventive measures: evidence from Italy. Vaccine. (2022) 10:777. doi: 10.3390/vaccines10050777
4. Watson, OJ, Barnsley, G, Toor, J, Hogan, AB, Winskill, P, and Ghani, AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. (2022) 22:1293–302. doi: 10.1016/S1473-3099(22)00320-6
5. Kotlar, B, Gerson, E, Petrillo, S, Langer, A, and Tiemeier, H. The impact of the COVID-19 pandemic on maternal and perinatal health: a scoping review. Reprod Health. (2021) 18:1–39. doi: 10.1186/s12978-021-01070-6
6. Riad, A, Jouzová, A, Üstün, B, Lagová, E, Hruban, L, Janku, P, et al. COVID-19 vaccine acceptance of pregnant and lactating women (PLW) in Czechia: an analytical cross-sectional study. Int J Environ Res. (2021) 18:13373. doi: 10.3390/ijerph182413373
7. Samannodi, M. COVID-19 vaccine acceptability among women who are pregnant or planning for pregnancy in Saudi Arabia: a cross-sectional study. Patient Prefer Adher. (2021) 15:2609–18. doi: 10.2147/PPA.S338932
8. Khalil, A, von Dadelszen, P, Draycott, T, Ugwumadu, A, O’Brien, P, and Magee, L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. (2020) 324:705–6. doi: 10.1001/jama.2020.12746
9. Lokken, EM, Huebner, EM, Taylor, GG, Hendrickson, S, Vanderhoeven, J, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington state. Am J Obstet Gynecol (2021) 225: e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221
10. Woodworth, KR, Olsen, EO, Neelam, V, Lewis, EL, Galang, RR, Oduyebo, T, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy-SET-net, 16 jurisdictions, march 29–October 14. MMWR Morb Mortal Wkly. (2020) 69:1635–40. doi: 10.15585/mmwr.mm6944e2
11. Zambrano, LD, Ellington, S, Strid, P, Galang, RR, Oduyebo, T, Tong, VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22–October 3. MMWR Morb Mortal Wkly Rep. (2020) 69:1641–7. doi: 10.15585/mmwr.mm6944e3
12. Ellington, S, Strid, P, Tong, VT, Woodworth, K, Galang, RR, Zambrano, LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22–June 7, 2020. Morb Mortal Wkly Rep. (2020) 69:769–75. doi: 10.15585/mmwr.mm6925a1
13. Villar, J, Ariff, S, Gunier, RB, Thiruvengadam, R, Rauch, S, Kholin, A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. (2021) 175:817–26. doi: 10.1001/jamapediatrics.2021.1050
14. Yang, R, Mei, H, Zheng, T, Fu, Q, Zhang, Y, Buka, S, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan. China BMC Med. (2020) 18:330. doi: 10.1186/s12916-020-01798-1
15. De Salazar, PM, Link, NB, Lamarca, K, and Santillana, M. High coverage COVID-19 mRNA vaccination rapidly controls SARS-CoV-2 transmission in long-term care facilities. Commun Med. (2021) 1:16. doi: 10.1038/s43856-021-00015-1
16. Garg, I, Shekhar, R, Sheikh, AB, and Pal, S. COVID-19 vaccine in pregnant and lactating women: a review of existing evidence and practice guidelines. Infect Dis Rep. (2021) 13:685–99. doi: 10.3390/idr13030064
17. Albrecht, M, and Arck, PC. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front Immunol. (2020) 11:555. doi: 10.3389/fimmu.2020.00555
18. Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding. Atlanta, GA, USA: Centers for Disease Control and Prevention (2021). 6p.
19. Rasmussen, SA, and Jamieson, DJ. Pregnancy, postpartum care, and COVID-19 vaccination in 2021. JAMA. (2021) 325:1099–100. doi: 10.1001/jama.2021.1683
20. American College of Obstetricians and Gynecologists ACOG and SMFM recommended COVID-19 vaccination for pregnant individuals. Washington, DC, USA: American College of Obstetricians and Gynecologists. (2021).
21. Stojanovic, J, Boucher, VG, Gagne, M, Gupta, S, Joyal-Desmarais, K, Paduano, S, et al. Global trends and correlates of COVID-19 vaccination hesitancy: findings from the iCARE study. Vaccine. (2021) 9:661. doi: 10.3390/vaccines9060661
22. Boyon, N. (2023). COVID-19 vaccination intent is decreasing globally. Ipsos and World Economic Forum. Available at: https://www.ipsos.com/en/global-attitudes-covid-19-vaccine-october-2020 (Accessed April 8, 2023).
23. Troiano, G, and Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health. (2021) 194:245–51. doi: 10.1016/j.puhe.2021.02.025
24. Sallam, M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccine. (2021) 9:160. doi: 10.3390/vaccines9020160
25. World Health Organization (2023). Ten threats to global health in 2019. Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (Accessed April 5, 2023).
26. Wilson, RJ, Paterson, P, Jarrett, C, and Larson, HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. (2015) 33:6420–9. doi: 10.1016/j.vaccine.2015.08.046
27. UNICEF (2021). Sudan receives the first delivery of COVID-19 vaccines with over 800,000 doses (2021 March). Available at: https://www.unicef.org/press-releases/sudan-receives-first-delivery-covid-19-vaccines-over-800000-doses
28. Musa, MB, Elmahi, OK, Modber, MA, Yagoub, FE, Dafallah, AA, Musa, SS, et al. The fight against the COVID-19 pandemic: vaccination challenges in Sudan. Public Health Pract. (2021) 2:100205. doi: 10.1016/j.puhip.2021.100205
29. Republic of Sudan (2023). Federal ministry, national deployment and vaccination plan for COVID-19 vaccines. Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/sudan_covid_nvdp_final.pdf (Accessed March 1, 2023).
30. World Data Atlas (2023). Sudan demographics: Total fertility rate. Available at: https://knoema.com/atlas/Sudan/Fertility-rate#:~:text=In%202022%2C%20fertility%20rate%20for,births%20per%20woman%20in%202022 (Accessed March 1, 2023).
31. Skjefte, M, Ngirbabul, M, Akeju, O, Escudero, D, Hernandez-Diaz, S, Wyszynski, DF, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. (2021) 36:197–211. doi: 10.1007/s10654-021-00728-6
32. Sutton, D, D’Alton, M, Zhang, Y, Kahe, K, Cepin, A, Goffman, D, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. (2021) 3:100403. doi: 10.1016/j.ajogmf.2021.100403
33. Mohmmed, HA, Alawad, RA, Awad, AK, and Alobied, AA. Knowledge, attitude, and acceptance regarding COVID-19 vaccines in Sudan. Front Public Health. (2022) 10:954810. doi: 10.3389/fpubh.2022.954810
34. Eldigail, MH, Adam, GK, Babiker, RA, Khalid, F, Adam, IA, Omer, OH, et al. Prevalence of dengue fever virus antibodies and associated risk factors among residents of El-Gadarif state. Sudan BMC Public Health. (2018) 18:921. doi: 10.1186/s12889-018-5853-3
35. Gedaref Sudan (2023). Available at: https://www.britannica.com/place/Al-Qadarif (Accessed March 4, 2023).
36. Ahmed, A, Mahmoud, I, Eldigail, M, Elhassan, R, and Weaver, S. The emergence of Rift Valley fever in Gedaref state urges the need for a cross-border one health strategy and enforcement of the international health regulations. Pathogens. (2021) 10:885. doi: 10.3390/pathogens10070885
37. Goncu Ayhan, S, Oluklu, D, Atalay, A, Menekse Beser, D, Tanacan, A, Moraloglu Tekin, O, et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. (2021) 154:291–6. doi: 10.1002/ijgo.13713
38. Bagalb, AS, Almazrou, D, Albraiki, AA, Alflaih, LI, and Bamunif, LO. COVID-19 vaccine acceptance among pregnant and lactating women in Saudi Arabia. Cureus. (2022) 14:e32133. doi: 10.7759/cureus.32133
39. Taye, EB, Taye, ZW, Muche, HA, Tsega, NT, Haile, TT, and Tiguh, AE. COVID-19 vaccine acceptance and associated factors among women attending antenatal and postnatal care in Central Gondar zone public hospitals. Clin Epidemiol Glob Health. (2022) 14:100993. doi: 10.1016/j.cegh.2022.100993
40. Chekol Abebe, E, Ayalew Tiruneh, G, Asmare Adela, G, Mengie Ayele, T, Tilahun Muche, Z, Behaile, T, et al. COVID-19 vaccine uptake and associated factors among pregnant women attending antenatal care in Debre Tabor public health institutions: a cross-sectional study. Front Public Health. (2022) 10:919494. doi: 10.3389/fpubh.2022.919494
41. Pairat, K, and Phaloprakarn, C. Acceptance of COVID-19 vaccination during pregnancy among Thai pregnant women and their spouses: a prospective survey. Reprod Health. (2022) 19:74. doi: 10.1186/s12978-022-01383-0
42. Ghamri, RA, Othman, SS, Alhiniah, MH, Alelyani, RH, Badawi, AM, and Alshahrani, AA. Acceptance of COVID-19 vaccine and associated factors among pregnant women in Saudi Arabia. Patient Prefer Adher. (2022) 16:861–73. doi: 10.2147/PPA.S357653
43. Mose, A, and Yeshaneh, A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: institutional-based cross-sectional study. Int J Gen Med. (2021) 14:2385–95. doi: 10.2147/IJGM.S314346
44. Hoque, AM, Buckus, S, Hoque, M, Hoque, ME, and Van Hal, G. COVID-19 vaccine acceptability among pregnant women at a primary health care facility in Durban, South Africa. Eur J Med Health Sci. (2020) 2:493. doi: 10.24018/ejmed.2020.2.5.493
45. Gunawardhana, N, Baecher, K, Boutwell, A, Pekwarake, S, Kifem, M, Ngong, MG, et al. COVID-19 vaccine acceptance and perceived risk among pregnant and non-pregnant adults in Cameroon. PLOS One. (2022) 17:e0274541. doi: 10.1371/journal.pone.0274541
46. Galanis, P, Vraka, I, Siskou, O, Konstantakopoulou, O, Katsiroumpa, A, and Kaitelidou, D. Uptake of COVID-19 vaccines among pregnant women: a systematic review and meta-analysis. Vaccine. (2022) 10:766. doi: 10.3390/vaccines10050766
47. Singh, P, Chauhan, M, Verma, M, Malhotra, V, Chaudhary, S, and Yadav, R. Understanding of COVID-19 vaccination in pregnant female–hesitancy and acceptance: a prospective observational study. Asian J Med Sci. (2022) 13:11–6. doi: 10.3126/ajms.v13i11.46597
48. Jayagobi, PA, Ong, C, Thai, YK, Lim, CC, Jiun, SM, Koon, KL, et al. Perceptions and acceptance of COVID-19 vaccine among pregnant and lactating women in Singapore: a cross-sectional study. MedRxiv [Preprint]. (2021) doi: 10.1101/2021.06.29.21259741v1
49. Geoghegan, S, Stephens, LC, Feemster, KA, Drew, RJ, Eogan, M, and Butler, KM. “This choice does not just affect me.” attitudes of pregnant women toward COVID-19 vaccines: a mixed-methods study. Hum Vaccin Immunother. (2021) 17:3371–6. doi: 10.1080/21645515.2021.1924018
50. Egloff, C, Couffignal, C, Cordier, AG, Deruelle, P, Sibiude, J, Anselem, O, et al. Pregnant women’s perceptions of the COVID-19 vaccine: a French survey. PLoS One. (2022) 17:e0263512. doi: 10.1371/journal.pone.0263512
51. Hosokawa, Y, Okawa, S, Hori, A, Morisaki, N, Takahashi, Y, Fujiwara, T, et al. The prevalence of COVID-19 vaccination and vaccine hesitancy in pregnant women: an internet-based cross-sectional study in Japan. J Epidemiol. (2022) 32:188–94. doi: 10.2188/jea.JE20210458
52. Tao, L, Wang, R, Han, N, Liu, J, Yuan, C, Deng, L, et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother. (2021) 17:2378–88. doi: 10.1080/21645515.2021.1892432
53. Mohan, S, Reagu, S, Lindow, S, and Alabdulla, M. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J Perinat Med. (2021) 49:678–85. doi: 10.1515/jpm-2021-0069
54. Skirrow, H, Barnett, S, Bell, S, Riaposova, L, Mounier-Jack, S, Kampmann, B, et al. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. (2022) 22:33. doi: 10.1186/s12884-021-04321-3
55. Mappa, I, Luviso, M, Distefano, FA, Carbone, L, Maruotti, GM, and Rizzo, G. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonat Med. (2022) 35:6302–5. doi: 10.1080/14767058.2021.1910672
56. COVID-19 Vaccination (2023). Africa CDC. Available at: https://africacdc.org/covid-19-vaccination/ (Accessed June 14, 2023).
57. Khubchandani, J, Sharma, S, Price, JH, Wiblishauser, MJ, Sharma, M, and Webb, FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health. (2021) 46:270–7. doi: 10.1007/s10900-020-00958-x
58. Ceulemans, M, Foulon, V, Panchaud, A, Winterfeld, U, Pomar, L, Lambelet, V, et al. Vaccine willingness and impact of the COVID-19 pandemic on women’s perinatal experiences and practices: a multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res. (2021) 18:3367. doi: 10.3390/ijerph18073367
59. Ahlers-Schmidt, CR, Hervey, AM, Neil, T, Kuhlmann, S, and Kuhlmann, Z. Concerns of women regarding pregnancy and childbirth during the COVID19 pandemic. Patient Educ Couns. (2020) 103:2578–82. doi: 10.1016/j.pec.2020.09.031
60. Battarbee, AN, Stockwell, MS, Varner, M, Newes-Adeyi, G, Daugherty, M, Gyamfi-Bannerman, C, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during august–December 2020. Am J Perinatol. (2022) 39:75–83. doi: 10.1055/s-0041-1735878
61. abc News (2020). Adjoa Smalls-Mantey Pregnant? Allergies? Immunocompromised? You are still eligible for the COVID 19 vaccine. Available at: https://preprod.abcnews.go.com/Health/pregnant-allergies-immunocompromised-eligible-covid-19-vaccine/story?id=74722712 (Accessed December 19, 2020).
62. RCOG (2021). Updated advice on COVID-19 vaccination in pregnancy and women who are breastfeeding. Available at: https://www.rcog.org.uk/en/news/updated-advice-on-covid-19-vaccination-in-pregnancyand-women-who-are-breastfeeding (Accessed February 9, 2021).
63. Kihara, AB. Vaccination against COVID-19 in Africa. Int J Gynaecol Obstet. (2021) 153:186–7. doi: 10.1002/ijgo.13646
64. Africa Centres for Disease Control and Prevention (2020). Majority of Africans would take a safe and effective COVID 19 vaccine. Available at: https://africacdc.org/newsitem/majority-of-africans-would-take-a-safe-and-effective-covid-19-vaccine/ (Accessed December 19, 2020).
65. Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html#:~:text=CDC%20recommends%20COVID%2D19%20vaccines,is%20time%20to%20get%20one (Accessed April 4, 2023).
66. Kharbanda, EO, and Vazquez-Benitez, G. COVID-19 mRNA vaccines during pregnancy: new evidence to help address vaccine hesitancy. JAMA. (2022) 327:1451–3. doi: 10.1001/jama.2022.2459
67. Bookstein Peretz, S, Regev, N, Novick, L, Nachshol, M, Goffer, E, Ben-David, A, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. (2021) 58:450–6. doi: 10.1002/uog.23729
68. Bowman, CJ, Bouressam, M, Campion, SN, Cappon, GD, Catlin, NR, Cutler, MW, et al. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, an mRNA-based COVID-19 vaccine. Reprod Toxicol. (2021) 103:28–35. doi: 10.1016/j.reprotox.2021.05.007
69. Shimabukuro, TT, Kim, SY, Myers, TR, Moro, PL, Oduyebo, T, Panagiotakopoulos, L, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. (2021) 385:1535–1536. doi: 10.1056/NEJMc2113516
70. Trostle, ME, Limaye, MA, Avtushka, V, Lighter, JL, Penfield, CA, and Roman, AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. (2021) 3:100464. doi: 10.1016/j.ajogmf.2021.100464
71. Zauche, LH, Wallace, B, Smoots, AN, Olson, CK, Oduyebo, T, Kim, SY, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. (2021) 385:1533–5. doi: 10.1056/NEJMc2113891
72. Butler, N, Roldan de Jong, T, Muzzulini, B, and Tulloch, O. Key considerations: improving uptake of the COVID-19 vaccine amongst women in South Sudan. Soc Sci Hum Act. (2022) 1–15. doi: 10.19088/SSHAP.2022.006
73. Tefera, Z, and Assefaw, M. A mixed-methods study of COVID-19 vaccine acceptance and its determinants among pregnant women in Northeast Ethiopia. Patient Prefer Adher. (2022) 16:2287–99. doi: 10.2147/PPA.S374217
74. North Sudanese Culture-Family–Cultural Atlas (2023). Available at: https://culturalatlas.sbs.com.au/north-sudanese-culture/north-sudanese-culture-family (Accessed June 14, 2023).
75. Nganga, SW, Otieno, NA, Adero, M, Ouma, D, Chaves, SS, Verani, JR, et al. Patient and provider perspectives on how trust influences maternal vaccine acceptance among pregnant women in Kenya. BMC Health Serv Res. (2019) 19:1–3. doi: 10.1186/s12913-019-4537-8
76. Wilcox, CR, Bottrell, K, Paterson, P, Schulz, WS, Vandrevala, T, Larson, HJ, et al. Influenza and pertussis vaccination in pregnancy: portrayal in online media articles and perceptions of pregnant women and healthcare professionals. Vaccine. (2018) 36:7625–31. doi: 10.1016/j.vaccine.2018.10.092
77. Wilcox, CR, Calvert, A, Metz, J, Kilich, E, MacLeod, R, Beadon, K, et al. Determinants of influenza and pertussis vaccination uptake in pregnancy: a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr Infect Dis J. (2019) 38:625–30. doi: 10.1097/INF.0000000000002242
Keywords: COVID-19 vaccine, pregnancy, COVID-19, acceptance, Sudan
Citation: Omar SM, Osman OS, Khalil R, Al-Wutayd O and Adam I (2023) COVID-19 vaccine acceptance among pregnant women: a hospital-based cross-sectional study in Sudan. Front. Public Health. 11:1221788. doi: 10.3389/fpubh.2023.1221788
Received: 12 May 2023; Accepted: 03 July 2023;
Published: 17 July 2023.
Edited by:
Zhiliang Hu, Nanjing Second Hospital, ChinaReviewed by:
Anita Walker, Nanjing Medical University, ChinaCopyright © 2023 Omar, Osman, Khalil, Al-Wutayd and Adam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osama Al-Wutayd, by5hbHd1dGF5ZEBxdS5lZHUuc2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.