- 1Department of Postpartum Rehabilitation, Maternal and Child Health Hospital of Hunan Province, Changsha, China
- 2Center for Reproductive Medicine, Maternal and Child Health Hospital of Hunan Province, Changsha, China
- 3Department of Maternal and Child Health, Xiangya School of Public Health, Central South University, Changsha, China
- 4Department of Public Health, Baoan Maternal and Child Health Hospital, Jinan University, Shenzhen, China
Background: Postpartum depression (PPD) is associated with several psychological and obstetric factors. Hepatitis B virus (HBV) infection has been linked with a high risk of depression, but little is known about the relationship between maternal HBV infection and PPD. We aimed to investigate the association between HBV infection and PPD.
Methods: This retrospective cohort study included 3,808 mothers who gave birth in a hospital in southern China. Self-reported Edinburgh Postnatal Depression Scale (EPDS) was used to assess PPD. Multivariate logistic regression was used to determine whether maternal HBV infection was associated with PPD risk.
Results: Of the 3,808 participants, 11.9% of mothers had PPD at 6 weeks postpartum. Two hundred and seventy-eight (7.3%) and 3,530 (92.7%) were in the HBV and control groups, respectively. Women with HBV infection were more likely to test positive for PPD (14.7 vs.11.7%). The multivariate logistic regression analysis showed that HBV-infected women did not have a significantly higher incidence of PPD (OR = 1.23; 95% CI, 0.82–1.84) than those without HBV infection in the study cohort. Parity and postpartum hemorrhage were found to be associated with PPD. In addition, our study showed that e antigen positivity was not associated with PPD risk (OR = 0.56, 95% CI 0.19–1.63).
Conclusions: To our knowledge, this is the first investigation of the relationship between maternal HBV infection and PPD. In a cohort of women without prior history or family history of mental illness, having HBV infection was not significantly associated with self-reporting of PPD compared to not having HBV infection.
1 Introduction
Hepatitis B virus (HBV) infection is a global public health issue (1–3). More than 240 million people have chronic HBV infection, which may develop into hepatitis, cirrhosis, and hepatocellular carcinoma without timely treatment (4). HBV prevalence was 7.18% in China, with approximately 93 million individuals infected with chronic HBV (5). A recent national study showed that the chronic HBV infection prevalence in Chinese pregnant women was 6.17% (6). Chronic hepatitis B is a contagious disease that transmits through blood, sexual activities, and mother-to-child transmission (MTCT). Therefore, patients with HBV infection often experience stigma and discrimination (7). People with chronic hepatitis B infection of any severity were associated with worse mental and physical health than healthy controls (8).
Postpartum depression (PPD), a common perinatal complication, affects 6.9–20% of puerperal women (9, 10), which significantly increases the morbidity and mortality of both mothers and children (11–15). Several studies have found that women who were diagnosed with PPD had an increased risk of future depression, suicide, or increased infant mortality (16–18). In addition, accumulated evidence showed that maternal PPD has deleterious effects on children's physical growth and mental development (19). Therefore, understanding the determinants of PPD and preventing its occurrence is vital.
Previous studies have demonstrated an association between other antenatal diagnoses and birth outcomes with PPD, including gestational diabetes (20), gestational hypertension (21), and preterm birth (22). The impact of maternal HBV infection on PPD is unknown. Previous study has examined the psychiatric effects of HBV infection outside of pregnancy and the puerperium (23). Researchers have suggested that comorbid depression is common in patients with HBV infection. For instance, a Vietnamese study showed that the prevalence of depressive symptoms in patients with chronic hepatitis B was 37.5% (24). Another study by Qureshi et al. reported that 58.6% patients with chronic hepatitis B had depression (25). In 2005, Atesci et al. assessed the incidence of mental disorders in 43 patients with HBV infection and 43 healthy controls, and reported that HBV-infected patients had significantly higher depression and anxiety scores than healthy controls (26). Similar result was found in the study conducted by Yilmaz et al. (27). The impact of maternal HBV infection on PPD, however, is still unknown. Hepatitis B e antigen (HBeAg) positivity has been frequently reported to be associated with MTCT of HBV (28). Therefore, we speculated that HBeAg positivity may increase the psychological burden on these mothers, resulting in a higher PPD risk.
Therefore, there is a need to evaluate the relationship between maternal HBV infection and PPD, and determine whether HBeAg positivity increases the risk of PPD among HBsAg-positive carriers. We conducted a large-scale retrospective cohort study to identify the potential association between HBV infection and PPD.
2 Methods
2.1 Study design and population
This was a retrospective cohort study performed on mothers who gave birth at the Baoan Maternity and Child Health Hospital in Shenzhen, China, from October 2018 to June 2020. During the antenatal visit period, routine screening of hepatitis B surface antigen (HBsAg) and HBeAg status using enzyme-linked immunosorbent assay (ELISA) was performed on pregnant women. Women who returned to the hospital for a routine follow-up at 42-days postpartum were asked to undergo PPD screening. For the self-reporting of PPD, 96% pregnant women accepted in PPD screening, because it is a service paid by the government. Women aged 20 years or older who underwent HBV marker testing in this hospital were included in this study. Exclusion criteria are as follows: (1) multiple pregnancy; (2) stillbirth or birth defect; (3) presence of family history of mental illness; (4) having past psychiatric disorders; (5) refusing PPD test.
The present study was approved by the Institutional Review Board of the Baoan Maternal and Child Health Hospital, Jinan University. Informed consent was exempted due to the retrospective study design.
2.2 Exposure assessment
The exposure variable was the HBV infection. In the current study, HBV infection was defined as HBsAg positivity (6).
2.3 Outcomes
The primary outcome was PPD, measured using the Edinburgh Postnatal Depression Scale (EPDS). The EPDS consists of 10 items rated between 0 and 3. The total score varies from 0 to 30; the higher the score, the greater the PPDs risk. The reliability and validity of the EPDS for screening for PPD were assessed in Chinese population and the cut-off point of 10 was chose, with sensitivity of 0.82 and specificity of 0.86 (29). In our study, a score of 10 or more was considered an indicator of PPD, which is in line with previous studies (30).
2.4 Assessment of other variables
Sociodemographic data, including age, education, parity, pre-pregnancy weight, and height, were obtained from participants' electronic medical records. Information on gestational diabetes mellitus (GDM), placental abruption, postpartum blood loss, mode of delivery, gestational week, fetal sex, and postnatal conditions of the infants were extracted from the medical records. Postpartum hemorrhage is defined as a loss of blood equal to or greater than 500 mL within 24 h after delivery (31), and preterm birth as a gestational age less than 37 weeks (32).
2.5 Statistical analysis
Descriptive statistics were used to present the general characteristics of participants according to HBsAg status, such as means and standard deviations for continuous variables and percentages (%) for categorical variables. Student's t-tests or chi-square tests were used to test whether the distributions of continuous or categorical variables, respectively, differed by HBV infection status. The association between maternal HBV infection and PPD was assessed by using multiple logistic regression models. A two-sided 5% level was considered statistically significant. Statistical analyses were performed using SPSS (version 25.0, Chicago, IL, USA).
3 Results
3.1 Study patients
In total, 4,187 women with HBV infection conditions were identified. But 103 mothers who had stillbirth or malformation, and 67 participants with past psychiatric diseases or a family history of mental illness were excluded from this study. Subsequently, 92 subjects with multiple pregnancies and 117 participants with incomplete data on PPD were excluded. Finally, 3,808 puerperal women were included in the analysis, of which 453 (11.9%) were considered to have PPD. According to maternal HBV infection status, 278 (7.3%) HBV-infected women were assigned to the HBV group and 3,530 (92.8%) mothers without HBV infection were assigned to the control group.
3.2 Participant characteristics
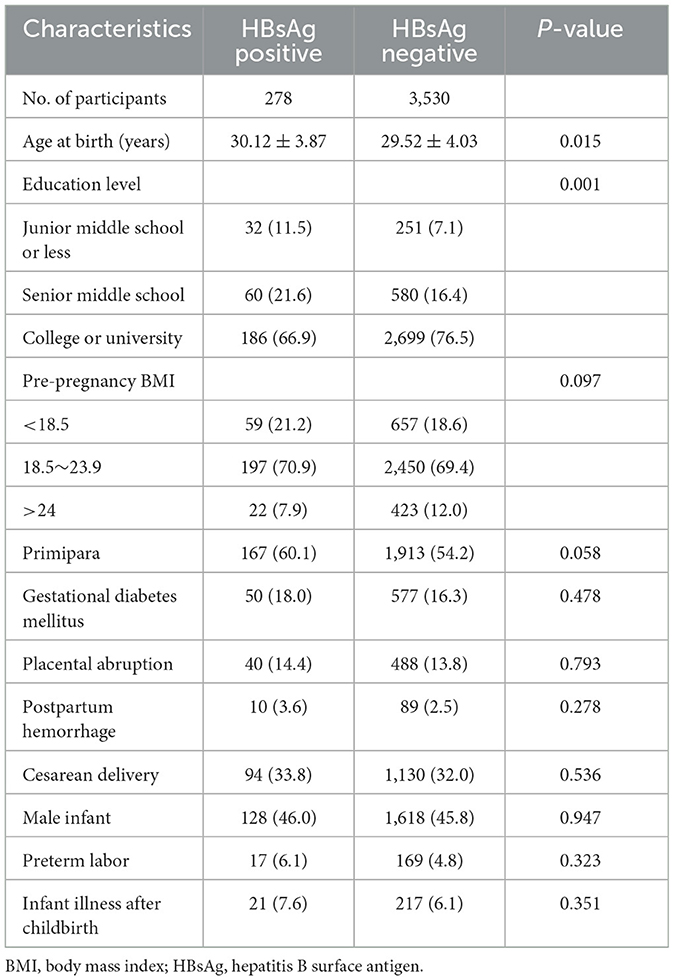
Table 1 presents the general characteristics, pregnancy complications, and delivery information of the two groups. Mothers with HBV infection were older and had a lower educational level. However, we did not observe significant differences in other pregnancy complications or delivery information between the two groups, such as pre-pregnancy BMI, parity, GDM, placental abruption, PHH, delivery mode, infant sex, preterm birth, or infant illness after childbirth.
3.3 Maternal hepatitis B virus infection and postpartum depression
The corresponding prevalence rates of PPD in the HBV and control groups were 14.7 and 11.7%, respectively. The prevalence of PPD was not significantly different between women with and without HBV infection (P > 0.05). Logistic regression analysis was used to evaluate the potential association between maternal HBV infection and PPD, the corresponding analysis results are presented in Table 2. The univariate analysis showed that primipara had a higher risk of PPD (OR = 1.34; 95% CI, 1.11–1.64; P = 0.003) compared with multipara. HBV infection (OR = 1.31; 95% CI, 0.93–1.85; P = 0.128) did not increase risk of developing PPD. The results of multivariate logistic regression analysis suggested that primipara (OR = 1.47; 95% CI, 1.15–1.89; P = 0.002) were more likely to develop PPD. In addition, mothers with postpartum hemorrhage were found to have a higher PPD risk (OR = 1.82; 95% CI, 1.02–3.24; P = 0.041). However, maternal HBV infection was not associated with the increased risk of PPD (OR = 1.23; 95% CI, 0.82–1.84; P = 0.320).
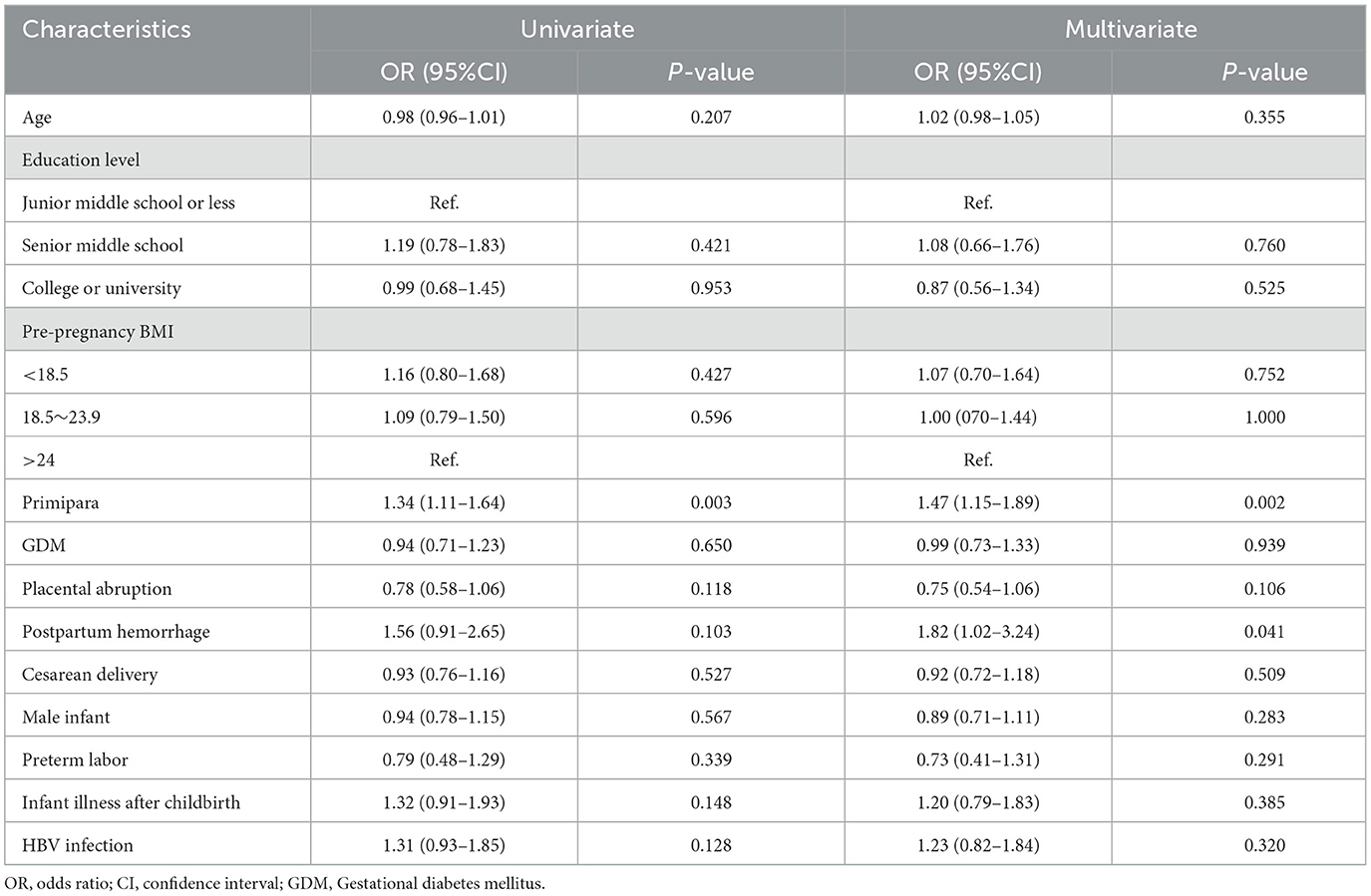
Table 2. Univariate and multivariate logistic regression analyses of factors related to postpartum depression.
3.4 HBeAg status and postpartum depression
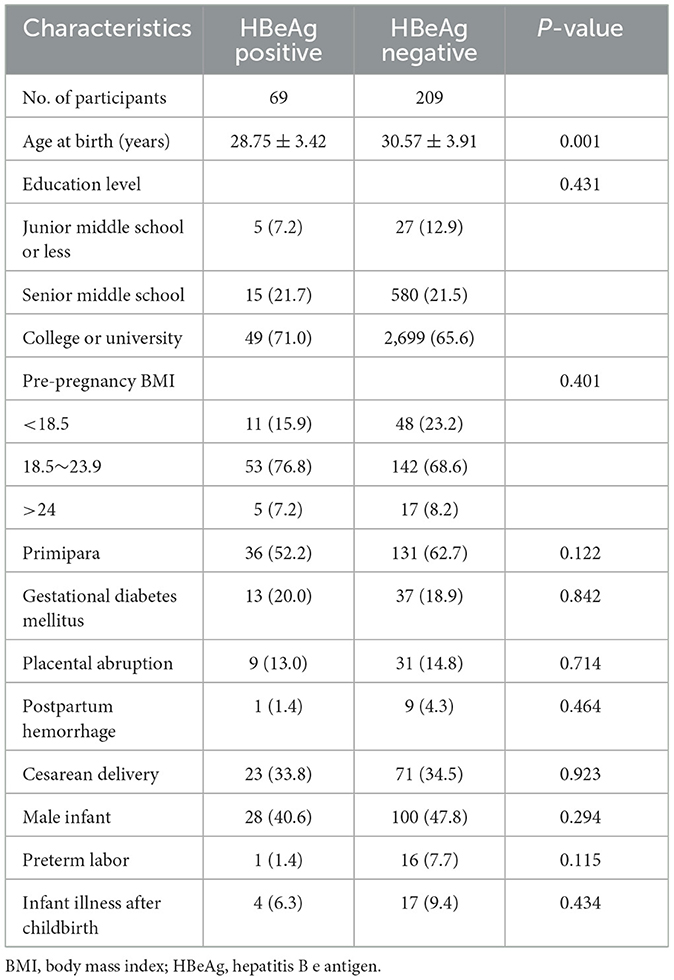
We further explored the impact of HBeAg status on maternal PPD in HBsAg-positive carriers. The results of the comparative analyses showed that HBeAg-positive mothers were younger than HBeAg-negative mothers (Table 3). Multivariable logistic regression analyses showed that HBeAg positivity (OR= 0.54, 95% CI 0.18–1.58, P = 0.261) was not significantly associated with an increased rate of PPD compared to HBeAg negativity.
4 Discussion
To the best of our knowledge, this is the first study to assess the relationship between maternal HBV infection and PPD in a large Chinese population. Our study showed that 11.9% of the mothers were considered as PPD at 6 weeks postpartum. We did not observe a greater risk of PPD in mothers with HBV infection. In addition, HBeAg positivity did not increase PPD risk among mothers living with HBV. Remarkably, our study suggested that parity and postpartum hemorrhage were associated with the risk of PPD.
This study found that 11.9% of Chinese postpartum women had PPD, slightly lower than the results of a meta-analysis conducted by Nisar et al., which reported a PPD prevalence of 14.8% in women from mainland China (33). The possible reason is that the latter analysis included studies where participants had a past history of mental illness. In addition, Liu et al. conducted a cross-sectional study in Shanghai, China, and found that 23.2% of women had depressive symptoms at 6 weeks postpartum (34), which is approximately twice the proportion in our study. Possible explanations for the difference in the results includes the study design and sampling method. Their study was a cross-sectional study based on convenience sampling, higher participation rate of women with high-risk pregnancies or complications may contribute to a higher prevalence. Our study was a hospital-based cohort study, and excluded those mothers with stillbirth, birth defect, past psychiatric diseases, or family history of mental illness that may increase the risk of PPD, leading to a lower prevalence of PPD.
A cross-sectional study conducted by Liu et al. suggested that hepatitis B self-awareness was significantly associated with depression in a Chinese study of 500,000 adults (35). Additionally, several studies found that people with chronic hepatitis B had higher depressive scores (26, 27). However, a case-control study conducted by Arslan et al. showed no difference in depression and anxiety between HBV-infected children and healthy control children (36). Similarly, Gale's study did not find a significant association of hepatitis B and depression in adult population (37). Our study showed that maternal HBV infection did not increase the risk of PPD. Therefore, the association between HBV infection and depression differs greatly across studies. It is known that specific populations have higher rates of mental health disorders with internalized stigma contributing to this, such as those with HIV/AIDS (38). It is possible that internalized stigma, common in patients with HBV may also contribute to increased risk of depression (39). Several reasons may contribute to explain the result difference between our study and Liu' study. First, participants with higher education in our study accounted for 66.9%, which is much higher than the 5.9% reported by Liu et al. (35). The HBV infection rate of 7.3% in our study is also 2.3 times that of their study (3.2%). A recent review reported that a higher education level, having a family member with HBV infection, and knowing someone with HBV infection are associated with the reduced stigma (40). Second, Interferon, the common drug for the treatment of hepatitis B, has a side effect of depression (41). But it is a contraindication for pregnancy women to receive Interferon therapy. Third, free antenatal education was offered to expectant mothers in China, which protects the attenders from adverse mental health outcomes (42).
The positive status of HBeAg in the serum is considered a credible marker of viral replication, which is associated with a higher risk of HBV MTCT. Our study indicated that HBeAg positivity was not associated with PPD risk. Viral load and antiviral use during pregnancy were identified as strong predictors of HBV MTCT (43). However, our study lacks data on viral load and antiviral use, which prevents us assessing the association between HBV MTCT and PPD directly. Therefore, this may be an important area for future studies to evaluate.
Consistent with our study, previous study indicated that primiparity was a risk factor for PPD during the first month postpartum (44). The main reason is that primipara may have many concerns, such as baby care, crying, and feeding, which are strongly associated with depressive symptoms in the early postpartum period (45, 46). In addition, our study suggested that women with postpartum hemorrhage still had higher risk of PPD. Similar results could be found in the study by Parry-Smith et al. (47) and our previous research (48). Postpartum hemorrhage usually leads to an increase in hospital stay and comorbidities, which may exacerbate the psychological burden of women who have suffered from postpartum hemorrhage.
Our study has several strengths. First, the cohort study design that enabled us to assess HBV infection status before PPD screening, which contributed to understanding the association between HBV infection and PPD. Second, data from a large sample cohort were used, which yielded more convincing results. However, the following limitations should be considered. First, the assessment of PPD was based on a self-reported scale instead of a psychiatric examination, although the EPDS is a validated tool for screening PPD with high sensitivity and specificity. Second, some confounding variables that may contribute to the risk of PPD were not considered, such as social support (from a husband, parents, or friends). Third, our study excluded patients with past history of mental illness, family history, or infants with birth defects, which may underestimate the impacts of HBV infection on PPD. Fourth, one-fifth participants was excluded because some women receiving PPD screening did not have a prenatal examination in this hospital during pregnancy, resulting in missing data on HBsAg and HBeAg.
5 Conclusions
In summary, this study is the first to explore the relationship between maternal HBV infection and PPD, although HBV infection did not increase the risk of PPD in puerperium women with no psychiatric or family history. The absence of an association may be explained by the diversity of sociological, behavioral, and psychological factors that affect the mental health of puerperal women. However, there were a number of issues that would be worth addressing in future research, e.g., HBeAg positivity, prior history of mental health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Baoan Maternal and Child Health Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WH and XW did the statistical analysis and drafted the initial manuscript. ZY, YG, and XL contributed to assist with data collection and revised the manuscript. LM took part in the sample collection. SP contributed to the critical revision of the article. All authors contributed significantly to this work. All authors have contributed to and have approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China, Grant Number (82103861), the Natural Science Foundation of Hunan Province, China (2021JJ40809), Hunan placental Medicine Clinical Medical Research Center (20220511-1003), Open Project of Key Laboratory of Environment and Health, Ministry of Education (2021GWKFJJ01), Hubei Key Laboratory of Food Nutrition and Safety (FNS-HBKL2020A01), NHC Key Laboratory of Birth Defect for Research and Prevention (Hunan Provincial Maternal and Child Health Care Hospital), No. KF2021013, and Central South University Graduate Student Independent Exploration and Innovation Project (2023ZZTS0601).
Acknowledgments
We thank our colleagues in collaborated hospitals who contributed to data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis b virus infection: a systematic review of data published between 1965 and 2013. Lancet. (2015) 386:1546–55. doi: 10.1016/s0140-6736(15)61412-x
2. Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet. (2014) 384:2053–63. doi: 10.1016/s0140-6736(14)60220-8
3. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen D-S, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. (2018) 3:383–403. doi: 10.1016/s2468-1253(18)30056-6
4. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. (2018) 319:1802–13. doi: 10.1001/jama.2018.3795
5. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China–declining Hbv prevalence due to hepatitis B vaccination. Vaccine. (2009) 27:6550–7. doi: 10.1016/j.vaccine.2009.08.048
6. Liu J, Wang X, Wang Q, Qiao Y, Jin X, Li Z, et al. Hepatitis B virus infection among 90 million pregnant women in 2853 Chinese counties, 2015-2020: a national observational study. Lancet Reg Health West Pac. (2021) 16:100267. doi: 10.1016/j.lanwpc.2021.100267
7. Huang J, Guan ML, Balch J, Wu E, Rao H, Lin A, et al. Survey of hepatitis B knowledge and stigma among chronically infected patients and uninfected persons in Beijing, China. Liver Int. (2016) 36:1595–603. doi: 10.1111/liv.13168
8. Altindag A, Cadirci D, Sirmatel F. Depression and health related quality of life in non-cirrhotic chronic hepatitis B patients and hepatitis B carriers. Neurosciences. (2009) 14:56–9.
9. Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. (2014) 384:1775–88. doi: 10.1016/S0140-6736(14)61276-9
10. Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu Rev Med. (2019) 70:183–96. doi: 10.1146/annurev-med-041217-011106
11. Shi P, Ren H, Li H, Dai Q. Maternal depression and suicide at immediate prenatal and early postpartum periods and psychosocial risk factors. Psychiatry Res. (2018) 261:298–306. doi: 10.1016/j.psychres.2017.12.085
12. Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. (2016) 3:973–82. doi: 10.1016/S2215-0366(16)30284-X
13. Farias-Antunez S, Xavier MO, Santos IS. Effect of maternal postpartum depression on offspring's growth. J Affect Disord. (2018) 228:143–52. doi: 10.1016/j.jad.2017.12.013
14. Closa-Monasterolo R, Gispert-Llaurado M, Canals J, Luque V, Zaragoza-Jordana M, Koletzko B, et al. The effect of postpartum depression and current mental health problems of the mother on child behaviour at eight years. Matern Child Health J. (2017) 21:1563–72. doi: 10.1007/s10995-017-2288-x
15. Jacques N, de Mola CL, Joseph G, Mesenburg MA, da Silveira MF. Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: a systematic review and meta-analysis. J Affect Disord. (2019) 243:201–8. doi: 10.1016/j.jad.2018.09.055
16. Briggs J, Scott R. Murder (infanticide) in post-partum depression: the case of Akon Guode. J Law Med. (2021) 28:855–82.
17. Stewart DE, Vigod S. Postpartum depression. N Engl J Med. (2016) 375:2177–86. doi: 10.1056/NEJMcp1607649
18. Lee YL, Tien Y, Bai YS, Lin CK, Yin CS, Chung CH, et al. Association of postpartum depression with maternal suicide: a nationwide population-based study. Int J Environ Res Public Health. (2022) 19:5118. doi: 10.3390/ijerph19095118
19. Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. (2014) 384:1800–19. doi: 10.1016/S0140-6736(14)61277-0
20. Shuffrey LC, Lucchini M, Morales S, Sania A, Hockett C, Barrett E, et al. Gestational diabetes mellitus, prenatal maternal depression, and risk for postpartum depression: an environmental influences on child health outcomes (echo) study. BMC Pregnancy Childbirth. (2022) 22:758. doi: 10.1186/s12884-022-05049-4
21. Koutra K, Vassilaki M, Georgiou V, Koutis A, Bitsios P, Kogevinas M, et al. Pregnancy, perinatal and postpartum complications as determinants of postpartum depression: the rhea mother-child cohort in Crete, Greece. Epidemiol Psychiatr Sci. (2018) 27:244–55. doi: 10.1017/S2045796016001062
22. Genova F, Neri E, Trombini E, Stella M, Agostini F. Severity of preterm birth and perinatal depressive symptoms in mothers and fathers: trajectories over the first postpartum year. J Affect Disord. (2022) 298(Pt A):182–9. doi: 10.1016/j.jad.2021.10.080
23. Gupta R, Avasthi A, Chawla YK, Grover S. Psychiatric morbidity, fatigue, stigma and quality of life of patients with hepatitis B infection. J Clin Exp Hepatol. (2020) 10:429–41. doi: 10.1016/j.jceh.2020.04.003
24. Vu TTM, Le TV, Dang AK, Nguyen LH, Nguyen BC, Tran BX, et al. Socioeconomic vulnerability to depressive symptoms in patients with chronic hepatitis B. Int J Environ Res Public Health. (2019) 16:255. doi: 10.3390/ijerph16020255
25. Qureshi MO, Khokhar N, Shafqat F. Severity of depression in hepatitis B and hepatitis C patients. J Coll Physicians Surg Pak. (2012) 22:632–4. doi: 10.2012/JCPSP.632634
26. Atesci FC, Cetin BC, Oguzhanoglu NK, Karadag F, Turgut H. Psychiatric disorders and functioning in hepatitis B virus carriers. Psychosomatics. (2005) 46:142–7. doi: 10.1176/appi.psy.46.2.142
27. Yilmaz A, Ucmak F, Dönmezdil S, Kaya MC, Tekin R, Günes M, et al. Somatosensory amplification, anxiety, and depression in patients with hepatitis B: impact on functionality. Medicine. (2016) 95:e3779. doi: 10.1097/MD.0000000000003779
28. Lu Y, Song Y, Zhai X, Zhu F, Liu J, Chang Z, et al. Maternal hepatitis B E antigen can be an indicator for antiviral prophylaxis of perinatal transmission of hepatitis B virus. Emerg Microbes Infect. (2021) 10:555–64. doi: 10.1080/22221751.2021.1899055
29. Lee DT, Yip SK, Chiu HF, Leung TY, Chan KP, Chau IO, et al. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the edinburgh postnatal depression scale. Br J Psychiatry. (1998) 172:433–7. doi: 10.1192/bjp.172.5.433
30. Peng S, Lai X, Du Y, Meng L, Gan Y, Zhang X. Prevalence and risk factors of postpartum depression in China: a hospital-based cross-sectional study. J Affect Disord. (2021) 282:1096–100. doi: 10.1016/j.jad.2021.01.012
31. Abdul-Kadir R, McLintock C, Ducloy AS, El-Refaey H, England A, Federici AB, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion. (2014) 54:1756–68. doi: 10.1111/trf.12550
32. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
33. Nisar A, Yin J, Waqas A, Bai X, Wang D, Rahman A, et al. Prevalence of perinatal depression and its determinants in mainland China: a systematic review and meta-analysis. J Affect Disord. (2020) 277:1022–37. doi: 10.1016/j.jad.2020.07.046
34. Liu Y, Guo N, Li T, Zhuang W, Jiang H. Prevalence and associated factors of postpartum anxiety and depression symptoms among women in Shanghai, China. J Affect Disord. (2020) 274:848–56. doi: 10.1016/j.jad.2020.05.028
35. Liu Y, Tang K, Long J, Zhao C. The association between hepatitis B self-awareness and depression: exploring the modifying effects of socio-economic factors. J Viral Hepat. (2017) 24:330–6. doi: 10.1111/jvh.12647
36. Arslan N, Büyükgebiz B, Oztürk Y, Akay AP. Depression and anxiety in chronic hepatitis B: effect of hepatitis B virus infection on psychological state in childhood. Turk J Pediatr. (2003) 45:26–8.
37. Gale SD, Berrett AN, Erickson LD, Brown BL, Hedges DW. Association between virus exposure and depression in US adults. Psychiatry Res. (2018) 261:73–9. doi: 10.1016/j.psychres.2017.12.037
38. Zeng C, Li L, Hong YA, Zhang H, Babbitt AW, Liu C, et al. A structural equation model of perceived and internalized stigma, depression, and suicidal status among people living with HIV/AIDS. BMC Public Health. (2018) 18:138. doi: 10.1186/s12889-018-5053-1
39. Smith-Palmer J, Cerri K, Sbarigia U, Chan EKH, Pollock RF, Valentine WJ, et al. Impact of stigma on people living with chronic hepatitis B. Patient Relat Outcome Meas. (2020) 11:95–107. doi: 10.2147/PROM.S226936
40. Jin D, Treloar C, Brener L. Hepatitis B virus related stigma among chinese living in mainland china: a scoping review. Psychol Health Med. (2021) 27:1760–73. doi: 10.1080/13548506.2021.1944651
41. Pinto EF, Andrade C. Interferon-related depression: a primer on mechanisms, treatment, and prevention of a common clinical problem. Curr Neuropharmacol. (2016) 14:743–8. doi: 10.2174/1570159X14666160106155129
42. Chen YL, Tseng CH, Cheong ML, Lien YJ, Wang SH, Chang CM, et al. Associations between antenatal education program and mental health outcomes in Taiwan: a population-based cohort study. Psychiatry Res. (2023) 322:115128. doi: 10.1016/j.psychres.2023.115128
43. Peng S, Wan Z, Liu T, Zhu H, Du Y. Incidence and risk factors of intrauterine transmission among pregnant women with chronic hepatitis B virus infection. J Clin Gastroenterol. (2019) 53:51–7. doi: 10.1097/MCG.0000000000001001
44. Iwata H, Mori E, Sakajo A, Aoki K, Maehara K, Tamakoshi K. Prevalence of postpartum depressive symptoms during the first 6 months postpartum: association with maternal age and parity. J Affect Disord. (2016) 203:227–32. doi: 10.1016/j.jad.2016.06.002
45. Chen TL, Chien LY. Feeding self-efficacy and feeding outcome expectancy mediate the association between maternal depressive symptoms and responsive feeding. Acta Psychol. (2022) 230:103755. doi: 10.1016/j.actpsy.2022.103755
46. Ölmestig TK, Siersma V, Birkmose AR, Kragstrup J, Ertmann RK. Infant crying problems related to maternal depressive and anxiety symptoms during pregnancy: a prospective cohort study. BMC Pregnancy Childbirth. (2021) 21:777. doi: 10.1186/s12884-021-04252-z
47. Parry-Smith W, Okoth K, Subramanian A, Gokhale KM, Chandan JS, Humpston C, et al. Postpartum haemorrhage and risk of mental ill health: a population-based longitudinal study using linked primary and secondary care databases. J Psychiatr Res. (2021) 137:419–25. doi: 10.1016/j.jpsychires.2021.03.022
Keywords: postpartum depression, HBV, obstetric factors, EPDS, PPD
Citation: Huang W, Wu X, Yao Z, Gu Y, Lai X, Meng L and Peng S (2023) Investigating the relationship between hepatitis B virus infection and postpartum depression in Chinese women: a retrospective cohort study. Front. Public Health 11:1214151. doi: 10.3389/fpubh.2023.1214151
Received: 29 April 2023; Accepted: 07 November 2023;
Published: 29 November 2023.
Edited by:
Yang Zhang, Capital Medical University, ChinaReviewed by:
Loredana Sabina Cornelia Manolescu, Carol Davila University of Medicine and Pharmacy, RomaniaMichelle Giles, Monash University, Australia
Naomi Whyler, Monash University, Australia, in collaboration with reviewer MG
Copyright © 2023 Huang, Wu, Yao, Gu, Lai, Meng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songxu Peng, Z3d4eXBzeEAxNjMuY29t
 Wei Huang1
Wei Huang1 Songxu Peng
Songxu Peng