- 1Department of Biology, University of Waterloo, Waterloo, ON, Canada
- 2School of Public Health Sciences, University of Waterloo, Waterloo, ON, Canada
- 3Department of Kinesiology and Health Sciences, University of Waterloo, Waterloo, ON, Canada
- 4Department of Kinesiology, McMaster University, Hamilton, ON, Canada
- 5Department of Chemistry, University of Waterloo, Waterloo, ON, Canada
Objective: Visible minorities are disproportionately affected by musculoskeletal disorders (MSD) and other diseases; yet are largely underrepresented in health research. The purpose of this scoping review was to identify barriers and strategies associated with increasing recruitment of visible minorities in MSD research.
Methods: Electronic databases (MEDLINE, EMBASE, CINAHL, and PsycInfo) were searched. Search strategies used terms related to the concepts of ‘race/ethnicity’, ‘participation’, ‘research’ and ‘musculoskeletal’. All research designs were included. Two reviewers independently screened titles and abstracts, completed full-text reviews, and extracted data. Papers that did not focus on musculoskeletal research, include racial minorities, or focus on participation in research were excluded. Study characteristics (study location, design and methods; sample characteristics (size, age, sex and race); MSD of interest) as well as barriers and strategies to increasing participation of visible minorities in MSD research were extracted from each article and summarized in a table format.
Results: Of the 4,282 articles identified, 28 met inclusion criteria and were included. The majority were conducted in the United States (27 articles). Of the included studies, the groups of visible minorities represented were Black (25 articles), Hispanic (14 articles), Asian (6 articles), Indigenous (3 articles), Middle Eastern (1 article), and Multiracial (1 article). The most commonly cited barriers to research participation were mistrust, logistical barriers (e.g., transportation, inaccessible study location, financial constraints), and lack of awareness or understanding of research. Strategies for increasing diversity were ensuring benefit of participants, recruiting through sites serving the community of interest, and addressing logistical barriers.
Conclusion: Understanding the importance of diversity in MSD research, collaborating with communities of visible minorities, and addressing logistical barriers may be effective in reducing barriers to the participation of visible minorities in health research. This review presents strategies to aid researchers in increasing inclusion in MSD-related research.
Introduction
Musculoskeletal disorders (MSDs) are one of the leading causes of disability in Canada and affect 11 million (27.8%) Canadians; a prevalence projected to increase to 15 million by 2031 (1, 2). One of the most common MSDs is arthritis (3). Arthritis refers to health conditions that produce inflammation of joint tissues. There are over 100 forms of arthritis, with the most common including osteoarthritis (OA), rheumatoid arthritis, spondyloarthropathies and lupus (4). Arthritis creates more pain, depression, immobility, disability and unemployment than any other chronic condition (4, 5).
Race is a key risk factor for greater prevalence and severity of arthritis and other MSDs. Race is a social construct that reflects social, cultural and environmental experiences that can have biological implications on health (6–8). Visible minorities, defined in Canada as “persons, other than Aboriginal peoples, who are non-Caucasian in race or non-white in color” (9) are disproportionately impacted by MSDs including arthritis. In Canada, individuals identifying as Indigenous experience the highest prevalence of arthritis compared to any other racial group (10). In the United States, Black and Indigenous patients with arthritis experience worse pain intensity than White counterparts; yet are less likely to undergo joint replacement surgery and more likely to be recommended non-specific treatments such as opioids (8, 11, 12). This evidence emphasizes that health disparities among visible minorities are multifactorial and are not fully understood (13). In the context of systemic racism, interpersonal racism, colonialism, and implicit bias (14, 15), race likely influences health outcomes through multiple mechanisms: culturally appropriate and safe services, access to care, quality of care, and need of care due to stressors from psychosocial or environmental factors (8, 14, 15).
Underrepresentation of visible minorities in research contributes to these race-based health disparities by creating critical gaps in understanding the true underlying mechanisms and impacts of arthritis and other MSDs (13, 16–18). First, there is a paucity of research on the influence of race on health outcomes (8), which creates a gap in understanding how factors associated with race can be addressed to improve the identification and treatment of MSDs. Second, current health research includes samples of either unknown race, or predominantly White race, limiting our understanding of the generalizability of the findings. As of 2016, visible minorities make up 22.3% of Canadians (19); yet samples in health research rarely reflect this proportion of visible minorities. A scoping review by Khan and colleagues found that only 5 out of the 99 Canadian health studies examined used nationally representative data to study health (18). Of key concern, poor generalizability of research findings means that the true efficacy of diagnostic, prognostic and treatment approaches in visible minorities remains largely unknown (18). It is crucial to directly address these gaps to thoroughly understand health determinants where disparities exist (6). For example, the Kellgren-Lawrence grade (KLG) system used to indicate OA disease severity was developed based on predominantly White samples. The KLG systematically scores OA severity lower than self-reported OA pain intensity in Black compared to White patients (11). In the context of this finding, it is not surprising that Black patients are less likely than White patients to receive proper pain management for OA (20). A study utilizing machine learning showed that using a racially diverse dataset to assess OA severity was more accurate in predicting pain intensity in patients whose race is not White compared to KLG or other algorithms using non-diverse samples (11). This finding shows that diversifying datasets increases the generalizability of findings; and the underrepresentation of visible minorities in MSD research has produced a major gap. Thus, ensuring the participation of visible minorities in health research has potential to address race-based health disparities in MSDs.
By summarizing the existing research exploring racial representation in musculoskeletal health research, this paper will provide researchers and clinicians with information necessary to better engage visible minorities for their participation in research. The purpose of this scoping review is to highlight the unique barriers to research participation experienced by visible minorities; and identify strategies to increase recruitment and retention of visible minorities in MSD research. The findings aim to provide readers with tangible strategies to improve racial diversity in MSD research studies.
Materials and methods
A scoping review methodology was selected to examine the extent, range and nature of strategies used to engage visible minorities in MSD research from a heterogeneous set of studies. This report uses the methodological framework proposed by Arksey and O’Malley (21, 22) and modified by Levac (23). This scoping review did not critically appraise articles because articles included various research designs; e.g., narrative review to randomized clinical trial. This scoping review was not registered. A team of researchers were involved in identifying, extracting, and synthesizing data on barriers and strategies to engage visible minorities in musculoskeletal research, as recommended by the Arksey and O’Malley Framework (24).
Search strategy
The following electronic databases were searched from March 9 to March 12, 2021: MEDLINE (Ovid) 1946-2021, EMBASE (Ovid) 1974-2021, CINAHL (EBSCOhost) 1976-2021, and PsycInfo (ProQuest) 1800-2021. We also manually reviewed reference lists of eligible articles. The search strategies used keywords and MeSH terms related to ‘race/ethnicity’, ‘participation’, ‘research’ and ‘musculoskeletal’ and the search was limited to the English language. Search strategies were peer-reviewed by an information specialist (J. Stapleton) prior to the final implemented search (Supplementary material S1).
Eligibility
We included studies that were published from 1964 to March 12, 2021, focused on MSD conditions and addressed barriers or strategies for recruitment of visible minorities. All research designs were included. The year 1964 was selected to reflect the implementation of the Helsinki declaration. We excluded studies that were not related to visible minorities, did not address recruitment in research, did not involve participants with musculoskeletal disorders, animal studies, gray literature (information produced outside traditional commercial or academic publishing and distribution), and studies where the full-text could not be accessed.
Selection process
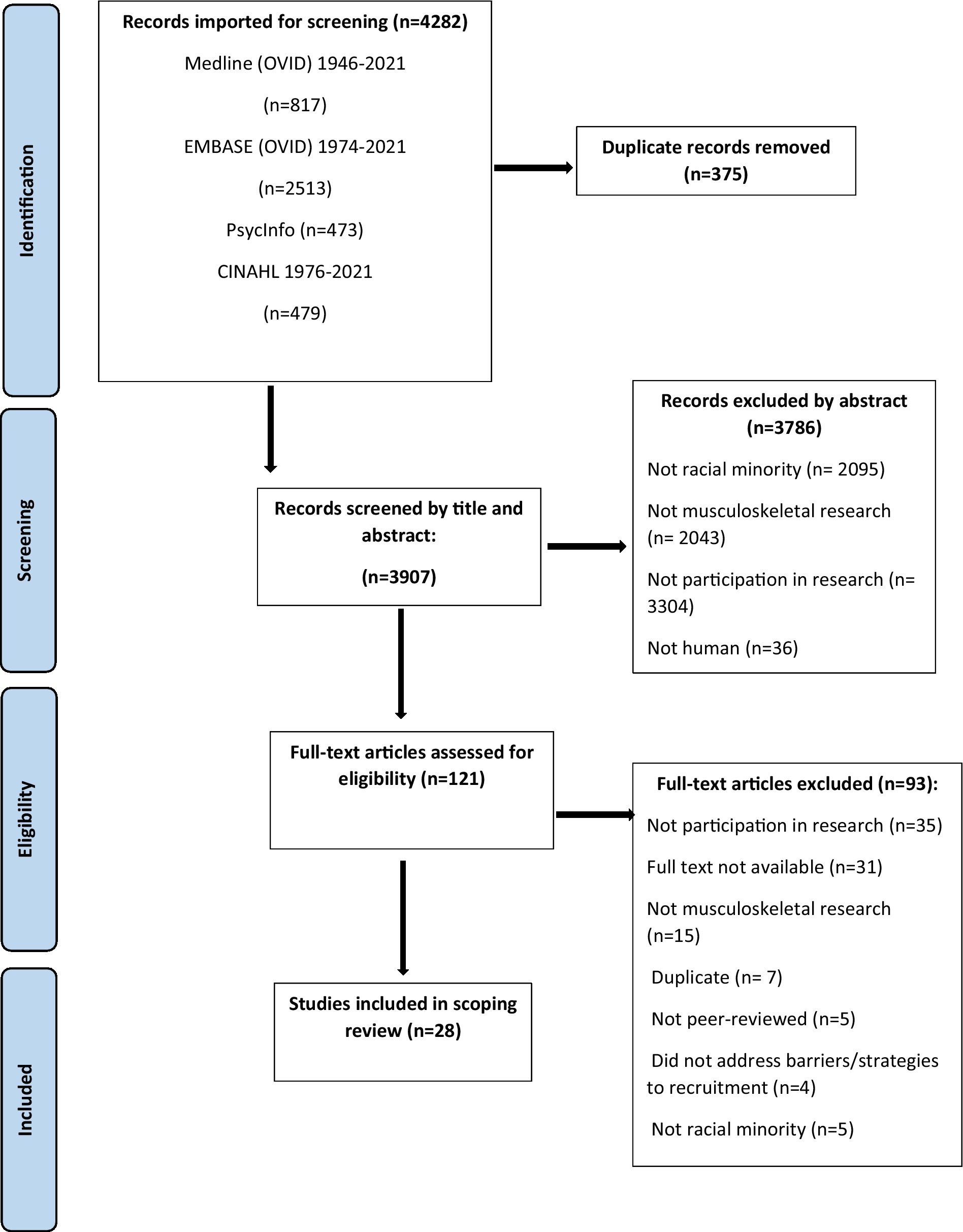
An overview of the selection process is represented in Figure 1. Title and abstracts were independently reviewed by two authors (DL and RA; DL and DR) for inclusion. Between-rater agreement was high for DL and RA [absolute agreement = 88.1% and relative agreement (Cohen’s Kappa value) = 0.41]; and DL and DR [absolute agreement = 94.3% and relative agreement (Cohen’s Kappa value) = 0.45]. Where there were disagreements, authors met with a third team member (MM) to reach consensus. Next, full-texts were imported into a data management software package (Covidence, Melbourne, Australia) and independently reviewed by DL and RA, then verified by DR. Duplicates were automatically removed by Covidence and manually identified by DL and RA.
Data extraction
The study team developed, pilot tested and revised a standardized form to chart data extracted from each study included in the scoping review. The following data were extracted: study location, design, and methods; sample characteristics (size, age, sex, and race); MSD of interest; and barriers and strategies to participation. These data were independently extracted and charted by two separate reviewers, where any conflicts were then resolved and verified by a third reviewer.
Synthesis of results
First, data regarding the characteristics of each study, sample characteristics, and disorder(s) of interest were collated and presented in a table to represent the scope and breadth of the evidence. Second, barriers to the participation of visible minorities in research identified in each study were compiled, then organized into major themes that emerged. Topics within these themes were presented in the order of frequency that was identified among the included studies. Strategies implemented in these studies to directly address these barriers were presented, again in the order of frequency. Led by DL and RA, all authors participated in establishing these major themes that emerged from the included studies. These themes on barriers and the corresponding strategies were summarized in a table.
Results
Study characteristics
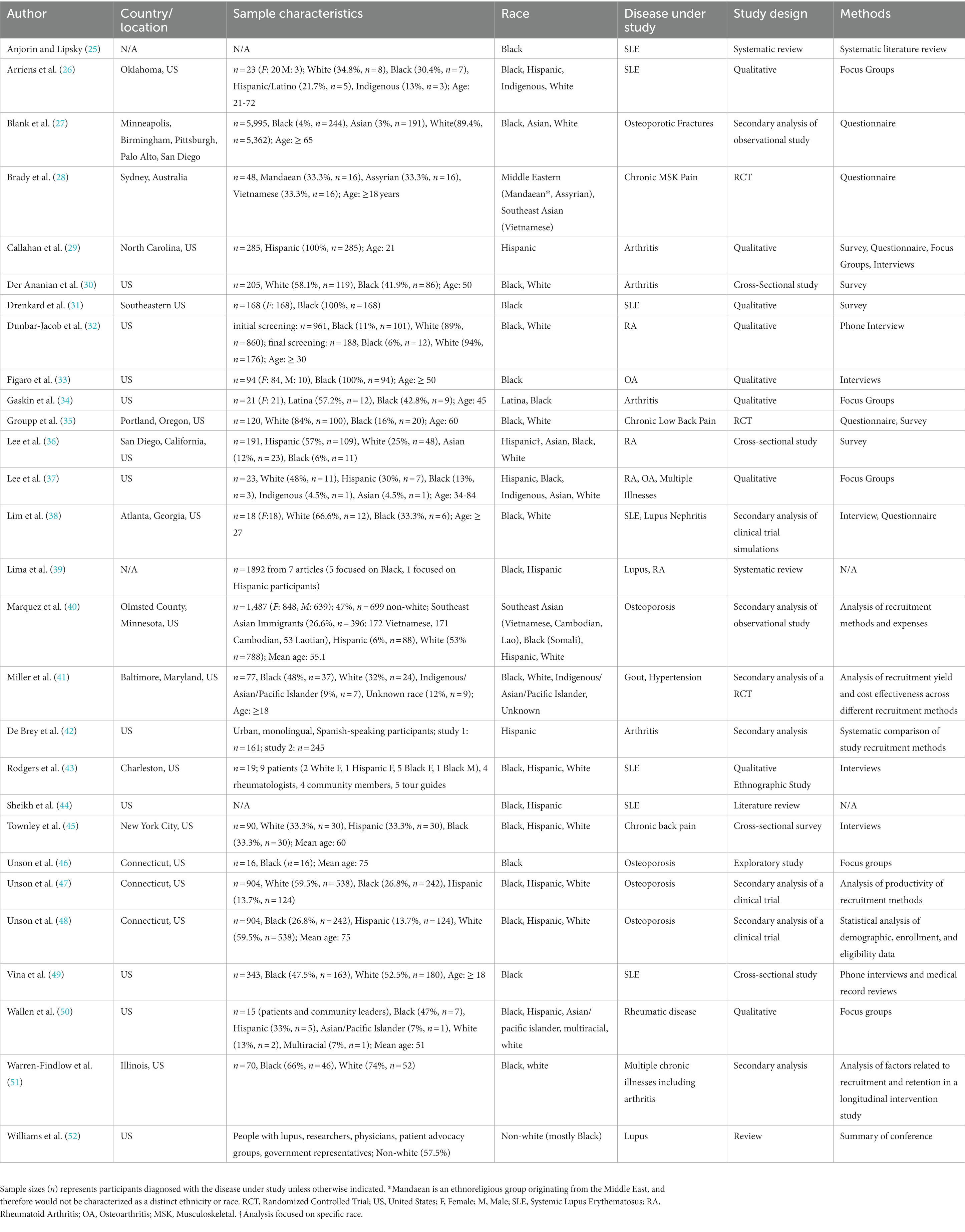
Of the 4,282 articles retrieved, 28 were included (Table 1). Almost all were located in the United States (US) (27 articles) with one in Australia. MSDs studied were systemic lupus erythematosus (SLE)1 (7 articles), osteoporosis (5 articles), arthritis (4 articles), rheumatoid arthritis (RA) or other rheumatic diseases (4 articles), lupus (3 articles), chronic back pain (2 articles), OA (2 articles), multiple or general MSD pain/illness (2 articles) and gout (1 article). Study designs included qualitative (9 articles), secondary analysis (8 articles), cross-sectional (4 articles), systematic or literature review (4 articles), randomized controlled trial (2 articles), and exploratory (1 article). A variety of methodologies were used, including analysis of recruitment methods/yield (6 articles), interviews (6 articles), questionnaires (5 articles), focus groups (5 articles), and surveys (5 articles). Races included Black (25 articles), Hispanic (14 articles), Asian (6 articles), Indigenous (3 articles), Middle Eastern (1 article), and Multiracial (1 article).
Barriers and strategies to participation in research
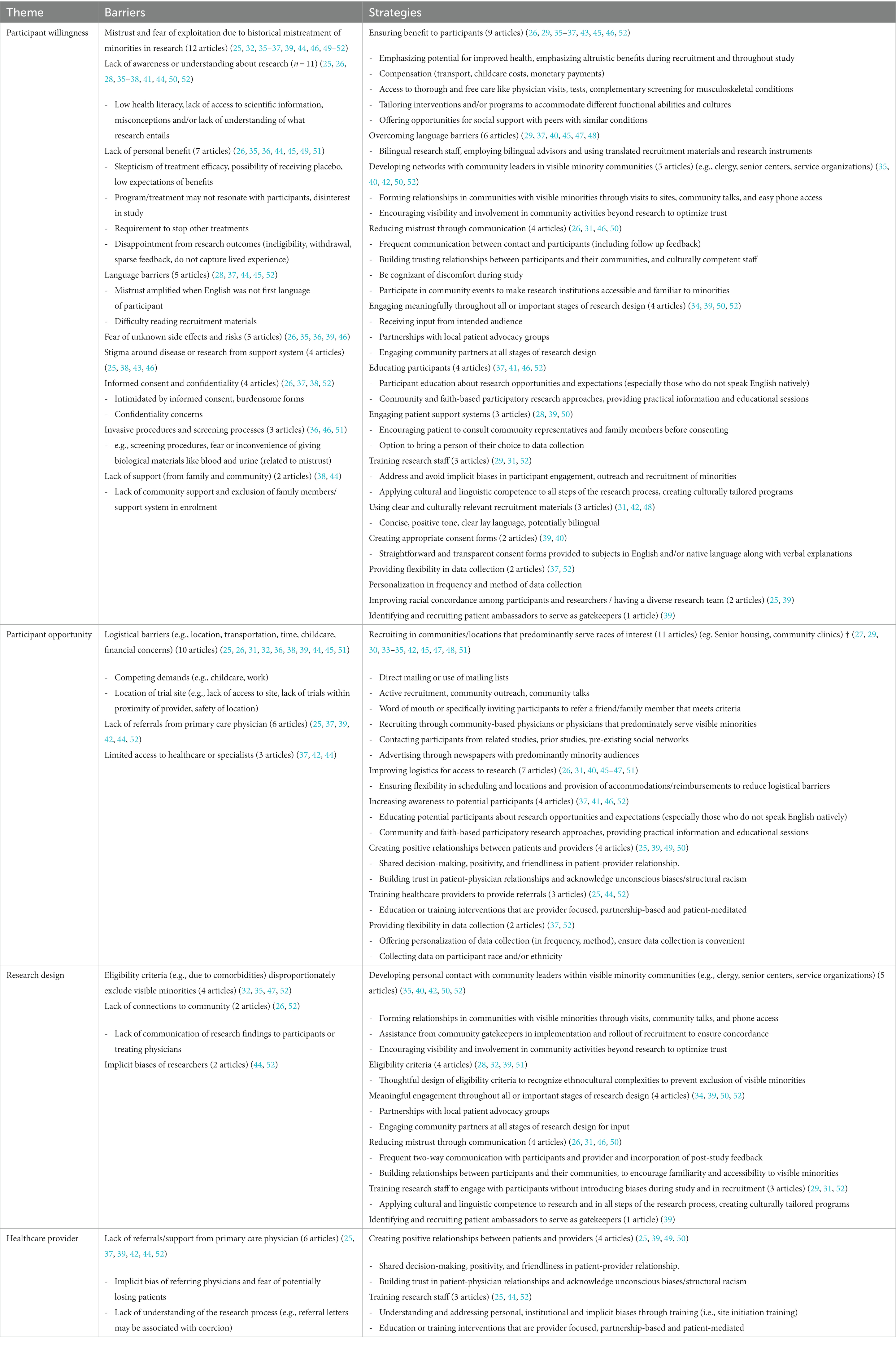
Barriers and strategies identified in the included articles were organized into the following four themes: Participant Willingness; Participant Opportunity; Research Design; and Healthcare Provider. These themes are defined and described below. Table 2 summarizes the barriers and strategies identified among the 28 included articles.
Participant willingness
Participant willingness referred to a person’s inclination toward consenting to, or being ready, to engage as a participant in a research activity. Barriers included the following: mistrust, lack of awareness, lack of personal benefit, language, fear of unknown risks, stigma, confidentiality, invasive procedures, and lack of support. Mistrust was cited most frequently. In 2019, this mistrust was highlighted at a conference focused on recruiting underrepresented groups into clinical studies. The conference involved people with lupus, patient advocacy groups, physicians, trialists and social scientists. This conference identified a key issue contributing to lack of trust in research was historical abuses of non-white subjects by medical investigators (52). Knowledge and tangible benefits directly to participants supported willingness to participate in research among visible minorities. A qualitative study of serial focus groups with 16 Black women showed that those who articulated knowledge about their health condition and its treatment, and those who perceived personal benefit with fewer concerns of “being used,” were more likely to enroll in a clinical study (46). Across included studies, strategies identified to overcome these barriers included ensuring benefits to participants, addressing language barriers, building networks with community leaders, reducing mistrust, engaging visible minorities in research design, educating, implementing patient support systems, training staff on inclusion practices, tailoring recruitment materials and consent forms, providing flexible data collection, diversifying the research team, and engaging patient ambassadors. Providing participants with tangible benefits was the most common topic, with benefits to personal health, altruism, financial support, and social support as key examples. In a study conducted at the University of California, willingness to participate in a clinical trial among patients with rheumatoid arthritis (n = 144, 82 Hispanic and 36 Caucasian) were predicted by the following benefits: free blood test [odds ratio (OR) 3.66 (1.82-7.53)], more doctor visits [OR 3.39 (1.68-6.99)], earlier access to therapy [OR 2.95 (1.48-6.01)] and free treatment [OR 2.67 (1.33-5.44)] (36). Strategies to enhance understanding of the research process included providing free educational sessions with practical information and disease screening and community and/or faith-based participatory research.
Participant opportunity
Participant opportunity described the structure and circumstances of research that foster, or diminish, the ability of visible minorities to participate. Barriers included logistics, lack of referral, and limited access to healthcare or a specialist. Logistical concerns were the most frequently cited barrier. Challenges for potential participants included work and childcare demands, inaccessible study location and finances. For example, a simulated clinical trial (methods included informed consent; mock screening, drug dosing; debriefing interview) emphasized that transportation, financial issues, childcare and the need for strong community and online support were needed to facilitate participation of Black people in a clinical trial of drug therapy for lupus (38). Strategies to enhance participant opportunity included recruiting in communities of visible minorities, addressing logistical barriers, and improving awareness, positive healthcare provider-patient relationships, referrals, and flexibility. Callahan and colleagues integrated multiple of these strategies: 288 participants enrolled in a trial of a 6-week, evidence-based walking program for Hispanic adults with arthritis, where bilingual, trained research staff conducted site-specific recruitment practices within Hispanic spaces (e.g., churches, consulate) and aided in reading and completing study materials. At 6 weeks, 233 (82%) participants completed the follow-up measures (29).
Research design
Research design refers to decisions made in the focus, methodology and interpretation of research activities, spanning from defining study objectives to disseminating findings. Barriers identified in the included articles were eligibility criteria, lack of community connections and implicit biases. The most frequently cited barrier incorporated in research design was eligibility criteria that disproportionately excludes visible minorities from becoming a research participant. In a study of people with rheumatoid arthritis, Dunbar-Jacob and colleagues reported that Black potential participants were more likely to be excluded due to comorbid conditions compared White potential participants (22.4% versus 13.1%) (32). Strategies identified included connection with community leaders, thoughtful eligibility criteria, engagement of visible minorities throughout research design, communication, training researchers and patient ambassadors. Multiple approaches to foster connection with community leaders were identified, ranging from educational presentations, community activities outside of research, and formal partnership. For example, a community-based participatory research approach was used to explore how to conduct health behavior research for people with arthritis in an urban setting (50). Informal and formal discussions with researchers, community leaders and patients enabled sharing in expertise, decision-making and ownership of the research process. This approach emphasized that researchers must be open to change based on the input of community leaders and patients (50).
Healthcare provider
This theme referred to the knowledge, experience, biases, and actions of healthcare providers that can foster, or discourage, the participation of visible minorities in clinical research. Topics in this healthcare provider theme influence participant willingness and participant opportunity. The key barrier identified in this review was that visible minorities were less likely to receive referrals or support from their primary physician to engage in research. In a narrative review focused on lupus, Sheikh and colleagues highlighted literature citing that healthcare providers lack familiarity and access to clinical trials (e.g., study information, eligibility criteria, and principal investigators), may have implicit biases (e.g., beliefs about adherence to trial protocols, negative impact on relationships with patients), and lack the resources to counsel patients about clinical trials (e.g., time, connection with principal investigator, proximity to trial site) (44). Training healthcare providers was identified as a strategy. Not only can training overcome the barrier of familiarity and access to research opportunities, training may also create an inclusive environment and promote positive patient-provider relationships; for example, through shared decision-making and acknowledging then addressing biases.
Discussion
This scoping review identified barriers that contribute to underrepresentation of visible minorities in MSD research, including mistrust, a lack of awareness of research activities, as well as inaccessible research practices. Strategies to overcome these barriers emphasized directly addressing participant willingness and opportunity; meaningful community engagement; research activities that bolster recruitment and address logistical barriers; and positive relationships of healthcare providers with visible minorities. This scoping review also highlighted that the vast majority of research regarding underrepresentation took place in the United States. While invaluable, the heterogeneity within and between minority groups and the uniqueness of healthcare and research environments within and between countries suggests the experiences summarized in this review may not be widely generalizable.
The most frequently cited barriers were mistrust, logistics, and lack of awareness or understanding of research. Mistrust among visible minorities from various racial backgrounds reflected multiple contributors, including a history of exploitation in clinical or research environments based on race, as well as negative personal experiences and opinions from trusted community members such as family. Mistrust was emphasized in Black participants, stemming from a history of exploitation in research such as the Tuskegee study (which involved purposefully and deceptively withholding antibiotics for syphilis among Black participants to document the natural history of this disease) (53). Mistrust was further reinforced by negative experiences in health care and prevailing discrimination and exploitation (53). Black participants were more likely to believe that research findings will reinforce negative stereotypes about their race and expose them to unnecessary risks (53). We anticipate that mistrust would also be a significant barrier for other groups that have experienced a history of exploitation and/or colonization, such as Indigenous Peoples in Canada. Goodman (54) highlighted that Indigenous Peoples in Canada who participated in research experienced mistrust of researchers, lack of transparency, lack of benefit to the community, and were subjected to questionable research practices (54). Several barriers such as stigma, fear of unknown risks, invasive procedures, and confidentiality concerns may also be associated with mistrust. Additionally, language barriers, lack of understanding, and barriers related to opportunity were commonly identified by Hispanic participants. Studies that aimed to recruit Hispanic participants prioritized language accessibility and employing bilingual research staff (29, 42).
Existing theories that underscore the barriers confronted by visible minorities in societal systems, including healthcare and academic institutions, can help researchers understand the need to implement strategies for improving diversity in MSD research. Critical Race Theory (CRT) and the Public Health Critical Race praxis (PHCRP) introduced by Ford (55) are useful to consider because these theories provide a set of principles to understand inequities and identify strategies to address or eliminate factors contributing to inequities in health outcomes (55). To truly address underrepresentation, researchers must make advances toward breaking down structural racism and racial inequities. To make these advances, researchers can consider (i) gaining understanding of the impact of implicit biases and the importance of diversity in clinical research; (ii) acting through adequate training of research staff and thoughtful research design (e.g., ensuring benefit to participants, recruiting through sites serving the community of interest, addressing logistical barriers through flexibility in scheduling and locations, and reimbursements); (iii) collaborating with communities to meet needs and translate findings and; (iv) developing meaningful community partnerships to reduce health disparities. For example, working with communities and involvement of community leaders in the research process was emphasized as an important strategy (35, 40, 42, 50, 52). These goals of understanding, acting, collaborating and meaningful partnership can be conceived as existing on a continuum, with engagement that progressively requires more involvement from underrepresented communities. Designing research specifically to reduce inequity requires meaningful partnerships with visible minorities, such as that achieved through participatory action research. It is not feasible to implement all of these strategies at once. Researchers can begin by collecting data on race within existing research and begin understanding implicit racial biases and the role of these biases on limiting research impact.
As researchers progress on the continuum of improving diversity in MSD research, partnering with visible minority communities may have the largest impact on recruitment and meaningful engagement of participants. Community-engaged research approaches include “a process of working collaboratively with groups of people who are affiliated by geographic proximity, special interests, or similar situations with respect to issues affecting their well-being” (56). This involves engagement through partnerships with community members throughout the research process (57), such as recruiting through community sites. Engagement can also reduce mistrust, ensure benefit to participants and their communities, improve awareness, and overcome logistical barriers. In a study observing over-researched Indigenous Peoples in Canada, participants reported feeling left uninformed of research outcomes and objectives after information was taken, and that benefits were preserved for audiences outside of the community being researched (54). Lack of personal or tangible benefits reflects inadequate communication between researchers and communities of visible minorities. Further, disinterest of potential participants in a study may reflect a lack of culturally-important input that would be valued by the community.
A key resource to operationalize strategies to improve representation of visible minorities in MSD research is the PROGRESS-Plus framework. PROGRESS-Plus (Place of residence, Race/ ethnicity/ culture /language, Occupation, Gender, Religion, Education, Socio-economic status, Social capital and “Plus” that includes other context-specific factors) identifies social determinants and how they may influence recruitment, experiences, and unique needs of visible minorities in research participation (58). Nonetheless, because little data were found, it is unclear whether these strategies could be effective among other visible minority groups or in other contexts (To further support researchers interested in improving representation, we list resources in Supplementary material S2). Future work to aid researchers and clinicians could include exploring the effectiveness of implementing strategies presented in this review, as well as in frameworks such as PROGRESS-Plus in directly addressing underrepresentation in MSD research.
Limitations and future work
The included studies did not include a broad range of visible minority groups. Visible minorities represented were mainly Black and Hispanic. Due to underrepresentation of visible minorities in Canadian studies, we do not have sufficient data to fully reflect the experiences of visible minorities that live in Canada, including Indigenous, South Asian, and East Asian, among others. Although 3 studies included Indigenous participants, data derived from these participants were not presented separately from the remaining sample and therefore we were unable to identify perspectives specific to this group. Therefore, the results may not recognize barriers experienced by Indigenous Peoples that reflect interactions with healthcare. The systems of care for Indigenous Peoples of Canada, which historically involved segregated healthcare facilities, were developed on a foundation of colonialism and racism constituted by the Indian Act (59). Furthermore, diversity exists within and between different Indigenous communities, suggesting barriers are likely unique to regional communities and experiences. A community-specific approach is likely required to enhance representation of visible minorities. Variations exist in beliefs and experiences among individual participants and between different communities or races; there is evidence that different barriers and strategies may be emphasized in one racial group over another (60). Knowing that there is no one-size-fits-all approach for all communities, it is critical to tailor strategies to the community of interest (40).
Future work should focus on identifying barriers and strategies in MSD research that reflects a broader range of visible minorities, for example Indigenous, South Asian, and East Asian groups living in Westernized countries. The intersectional experiences (i.e., the interconnectedness of social categorizations, such as race, gender, socioeconomic status, and other factors that are overlapping and interdependent) of minorities may not be reflected since only race was assessed. Future research should provide actionable steps for improving diversity in health research, with consideration of how intersections of race and other social determinants may influence participation.
Conclusion
Compared to White counterparts, visible minorities experience a greater prevalence and disease severity of MSDs, including various forms of arthritis. Yet, visible minorities are largely underrepresented in clinical research. Underrepresentation in clinical research creates critical gaps in understanding underlying illness mechanisms, disease impacts, and treatment efficacy, which all may vary due to race-related factors. This scoping review sought to identify barriers to research participation by visible minorities; and highlight strategies implemented in MSD research to overcome these barriers. Among 28 studies included in the review, the most commonly cited barriers to research participation were mistrust, logistical barriers, and lack of awareness or understanding of research. Strategies for increasing diversity included ensuring benefit to participants, recruiting through sites serving the community of interest, and addressing logistical barriers. This scoping review provides health researchers with strategies for improving representation among participants and making MSD health research more equitable to better address the health of a diverse population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DL, RA, DR, NI, SH, AB, and MM conducted the conception, design, analysis, and interpretation. DL, RA, DR, and MM conducted the article selection and data extraction. DL, RA, DR, NI, AB, and MM conducted the drafting and critical review for intellectual content. DL, RA, DR, NI, SH, AB, and MM conducted the approval of final manuscript. All authors contributed to the article and approved the submitted version.
Funding
MM is supported by the Arthritis Society Stars Mid-Career Development Award funded by the Canadian Institute of Health Research-Institute of Musculoskeletal Health and Arthritis. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (353715).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1211520/full#supplementary-material
Footnotes
1. ^Systemic Lupus Erythematosus is the most common form of lupus. Lupus is a chronic auto-immune disease.
References
1. Orthopaedic Care Strategy Group. Backgrounder report: Building a collective policy agenda for musculoskeletal health and mobility. Orthopaedic Care Strategy Group (2010).
2. Kopec, JA, Cibere, J, Sayre, EC, Li, LC, Lacaille, D, and Esdaile, JM. Descriptive epidemiology of musculoskeletal disorders in Canada: data from the global burden of disease study. Osteoarthr Cartil. (2019) 27:S259. doi: 10.1016/j.joca.2019.02.629
3. Mackay, C, Canizares, M, Davis, AM, and Badley, EM. Health care utilization for musculoskeletal disorders. (2010) Available at: https://onlinelibrary.wiley.com/doi/10.1002/acr.20064.
4. Life with arthritis in Canada. A personal and public health challenge. Available at: https://www.canada.ca/en/public-health/services/chronic-diseases/arthritis/life-arthritis-canada-a-personal-public-health-challenge.html. (Accessed Apr 10, 2023).
5. Health Canada. Arthritis in Canada: An ongoing challenge. (2003). Available at: https://publications.gc.ca/Collection/H39-4-14-2003E.pdf. (Accessed Apr 10, 2023).
6. Shanawani, H, Dame, L, Schwartz, DA, and Cook-Deegan, R. Non-reporting and inconsistent reporting of race and ethnicity in articles that claim associations among genotype, outcome, and race or ethnicity. J Med Ethics. (2006) 32:724–8. doi: 10.1136/jme.2005.014456
7. Perry, M, Baumbauer, K, Young, EE, Dorsey, SG, Taylor, JY, and Starkweather, AR. The influence of race, ethnicity and genetic variants on postoperative pain intensity: an integrative literature review. Pain Manag Nurs. (2019) 20:198–206. doi: 10.1016/j.pmn.2018.11.002
8. Thompson, KA, Terry, EL, Sibille, KT, Gossett, EW, Ross, EN, Bartley, EJ, et al. At the intersection of ethnicity/race and poverty: knee pain and physical function. J Racial Ethn Health Disparities. (2019) 6:1131–43. doi: 10.1007/s40615-019-00615-7
9. Minister of Justice. Employment equity act. Ottawa; (1995). Available at: https://laws-lois.justice.gc.ca/eng/acts/E-5.401/section-3.html. (Accessed Mar 10, 2022).
10. Ramraj, C, Shahidi, FV, Darity, W, Kawachi, I, Zuberi, D, and Siddiqi, A. Equally inequitable? A cross-national comparative study of racial health inequalities in the United States and Canada. Soc Sci Med. (2016) 161:19–26. doi: 10.1016/j.socscimed.2016.05.028
11. Pierson, E, Cutler, DM, Leskovec, J, Mullainathan, S, and Obermeyer, Z. An algorithmic approach to reducing unexplained pain disparities in underserved populations. Nat Med. (2021) 27:136–40. doi: 10.1038/s41591-020-01192-7
12. Skinner, J, Weinstein, JN, Sporer, SM, and Wennberg, JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. (2003) 349:1350–9. doi: 10.1056/NEJMsa021569
13. Nnorom, O, Findlay, N, Lee-Foon, NK, Jain, AA, Ziegler, CP, Scott, FE, et al. Dying to learn: a scoping review of breast and cervical cancer studies focusing on black Canadian women. J Health Care Poor Underserved. (2019) 30:1331–59. doi: 10.1353/hpu.2019.0100
14. Canadian Institute for Health Information. Proposed standards for race-based and indigenous identity data collection and health reporting in Canada. Toronto, Canada: Canadian Institute for Health Information (2020).
15. Weinstein, JN, Geller, A, Negussie, Y, and Baciu, A. Communities in action: pathways to health equity. Washington, DC: National Academies Press, 104–111. (2017).
16. Dickmann, LJ, and Schutzman, JL. Racial and ethnic composition of Cancer clinical drug trials: How diverse are we? Oncologist. (2018) 23:243–6. doi: 10.1634/theoncologist.2017-0237
17. Flores, LE, Frontera, WR, Andrasik, MP, del Rio, C, Mondríguez-González, A, Price, SA, et al. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw Open. (2021) 4:e2037640. doi: 10.1001/jamanetworkopen.2020.37640
18. Khan, M, Kobayashi, K, Lee, SM, Vang, Z, Khan, M, Kobayashi, K, et al. (In)visible minorities in Canadian health data and Research. (2015) Available at: https://ir.lib.uwo.ca/pclchttps://ir.lib.uwo.ca/pclc/vol3/iss1/5. (Accessed Mar 10, 2022).
19. Statistics Canada. Aboriginal peoples in Canada: Key results from the 2016 census. (2017) Available at: https://www150.statcan.gc.ca/n1/daily-quotidien/171025/dq171025a-eng.htm. (Accessed Mar 10, 2022).
20. Reyes, AM, and Katz, JN. Racial/ethnic and socioeconomic disparities in osteoarthritis management. Rheum Dis Clin N Am. (2021) 47:21–40. doi: 10.1016/j.rdc.2020.09.006
21. Arksey, H, and O’malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
22. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
23. Levac, D, Colquhoun, H, and O’Brien, KK. Scoping studies: advancing the methodology. Implement Sci. (2010) 5:69. doi: 10.1186/1748-5908-5-69
24. Westphaln, KK, Regoeczi, W, Masotya, M, Vazquez-Westphaln, B, Lounsbury, K, McDavid, L, et al. From Arksey and O’Malley and beyond: customizations to enhance a team-based, mixed approach to scoping review methodology. MethodsX. (2021) 8:101375. doi: 10.1016/j.mex.2021.101375
25. Anjorin, A, and Lipsky, P. Engaging African ancestry participants in SLE clinical trials. Lupus Sci Med. (2018) 5:297. doi: 10.1136/lupus-2018-000297
26. Arriens, C, Aberle, T, Carthen, F, Kamp, S, Thanou, A, Chakravarty, E, et al. Lupus patient decisions about clinical trial participation: a qualitative evaluation of perceptions, facilitators and barriers. Lupus Sci Med. (2020) 7:360. doi: 10.1136/lupus-2019-000360
27. Blank, JB, Cawthon, PM, Carrion-Petersen, ML, Harper, L, Johnson, JP, Mitson, E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Control Clin Trials. (2005) 26:557–68. doi: 10.1016/j.cct.2005.05.005
28. Brady, B, Veljanova, I, Schabrun, S, and Chipchase, L. Integrating culturally informed approaches into physiotherapy assessment and treatment of chronic pain: a pilot randomised controlled trial. BMJ Open. (2018) 8:21999. doi: 10.1136/bmjopen-2018-021999
29. Callahan, LF, Rivadeneira, A, Altpeter, M, Vilen, L, Cleveland, RJ, Sepulveda, VE, et al. Evaluation of the Arthritis Foundation’s Camine con gusto program for Hispanic adults with arthritis. Hispanic Health Care Int. (2016) 14:132–40. doi: 10.1177/1540415316665202
30. der Ananian, CA, Churan, C, and Adams, MA. Correlates of physical activity among blacks and whites with arthritis. Health Values. (2015) 39:562–72. doi: 10.5993/AJHB.39.4.13
31. Drenkard, C, Easley, K, Bao, G, Dunlop-Thomas, C, Lim, SS, and Brady, T. Overcoming barriers to recruitment and retention of African–American women with SLE in behavioural interventions: lessons learnt from the WELL study. Lupus Sci Med. (2020) 7:e000391. doi: 10.1136/lupus-2020-000391
32. Dunbar-Jacob, J, Holmes, JL, Sereika, S, Kent Kwoh, C, Burke, LE, Starz, TW, et al. Factors associated with attrition of African Americans during the recruitment phase of a clinical trial examining adherence among individuals with rheumatoid arthritis. Arthritis Care Res (Hoboken). (2004) 51:422–8. doi: 10.1002/art.20411
33. Figaro, MK, Williams-Russo, P, and Allegrante, JP. Expectation and outlook: the impact of patient preference on arthritis care among African Americans. J Ambul Care Manage. (2005) 28:41–8. doi: 10.1097/00004479-200501000-00006
34. Gaskin, DJ, Karmarkar, TD, Maurer, A, Bucay-Harari, L, Casillas, G, Gittens, A, et al. Potential role of cost and quality of life in treatment decisions for arthritis-related knee pain in African American and Latina women. Arthritis Care Res (Hoboken). (2020) 72:692–8. doi: 10.1002/acr.23903
35. Groupp, E, Haas, M, Fairweather, A, Ganger, B, and Attwood, M. Recruiting seniors with chronic low Back pain for a randomized controlled trial of a self-management program. J Manip Physiol Ther. (2005) 28:97–102. doi: 10.1016/j.jmpt.2005.01.004
36. Lee, SJ, Lenert, L, Weisman, S, and Kavanaugh, A. Factors affecting rheumatoid arthritis patients’ decisions to participate in clinical trials. J Rheumatol. (2005) 32:2317–25.
37. Lee, SB, Zak, A, Iversen, MD, Polletta, VL, Shadick, NA, and Solomon, DH. Participation in clinical research registries: a focus group study examining views from patients with arthritis and other chronic illnesses. Arthritis Care Res (Hoboken). (2016) 68:974–80. doi: 10.1002/acr.22767
38. Lim, SS, Kivitz, AJ, McKinnell, D, Pierson, ME, and O’Brien, FS. Simulating clinical trial visits yields patient insights into study design and recruitment. Patient Prefer Adherence. (2017) 11:1295–307. doi: 10.2147/PPA.S137416
39. Lima, K, Phillip, CR, Williams, J, Peterson, J, Feldman, CH, and Ramsey-Goldman, R. Factors associated with participation in rheumatic disease–related research among underrepresented populations: a qualitative systematic review. Arthritis Care Res (Hoboken). (2020) 72:1481–9. doi: 10.1002/acr.24036
40. Marquez, MA, Muhs, JM, Tosomeen, A, Riggs, BL, and Melton, LJ. Costs and strategies in minority recruitment for osteoporosis research. J Bone Miner Res. (2003) 18:3–8. doi: 10.1359/jbmr.2003.18.1.3
41. Miller, HN, Charleston, J, Wu, B, Gleason, K, White, K, Dennison Himmelfarb, CR, et al. Use of electronic recruitment methods in a clinical trial of adults with gout. Clin Trials. (2021) 18:92–103. doi: 10.1177/1740774520956969
42. De Brey, V, and Gonzalez, VM. Recruiting for arthritis studies in hard-to-reach populations: a comparison of methods used in an urban Spanish-speaking community. Arthritis Rheum. (1997) 10:64–71. doi: 10.1002/art.1790100110
43. Rodgers, W, Williams, EM, Smalls, BL, Singleton, T, Tennessee, A, Kamen, D, et al. Treating systemic lupus erythematosus (Sle): the impact of historical environmental context on healthcare perceptions and decision-making in Charleston, South Carolina. Int J Environ Res Public Health. (2020) 17:2285. doi: 10.3390/ijerph17072285
44. Sheikh, SZ, McCalla, S, Wanty, NI, Stephens, J, and Holtz, KD. The state of lupus clinical trials: minority participation needed. J Clin Med. (2019) 8:1245. doi: 10.3390/jcm8081245
45. Townley, S, Papaleontiou, M, Amanfo, L, Henderson, CR, Pillemer, K, Beissner, K, et al. Preparing to implement a self-management program for Back pain in new York City senior centers: what do prospective consumers think? Pain Med. (2010) 11:405–15. doi: 10.1111/j.1526-4637.2009.00783.x
46. Unson, CG, Dunbar, N, Curry, L, Kenyon, L, and Prestwood, K. The effects of knowledge, attitudes, and significant others on decisions to enroll in a clinical trial on osteoporosis: implications for recruitment of older African-American women. J Natl Med Assoc. (2001) 93:392–401.
47. Unson, CG, Ohannessian, C, Kenyon, L, Case, A, Reisine, S, and Prestwood, K. Barriers to eligibility and enrolment among older women in a clinical trial on osteoporosis: effects of ethnicity and SES. J Aging Health. (2004) 16:426–43. doi: 10.1177/0898264304264211
48. Unson, CG, Ohannessian, C, Kenyon, L, Fenster, J, Reisine, S, and Prestwood, K. Strategies for enrolling diverse older women in an osteoporosis trial. J Aging Health. (2004) 16:669–87. doi: 10.1177/0898264304268588
49. Vina, ER, Utset, TO, Hannon, MJ, Masi, CM, Roberts, NJ, and Kwoh, CK. Racial differences in treatment preferences among lupus patients: a two-site study. Clin Exp Rheumatol. (2014) 32:680–8.
50. Wallen, GR, Middleton, KR, Miller-Davis, C, Tataw-Ayuketah, G, Todaro, A, Rivera-Goba, M, et al. Patients’ and community leaders’ perceptions regarding conducting health behavior research in a diverse, urban clinic specializing in rheumatic diseases. Prog Community Health Partnersh. (2012) 6:405–15. doi: 10.1353/cpr.2012.0052
51. Warren-Findlow, J, Prohaska, TR, and Freedman, D. Challenges and opportunities in recruiting and retaining underrepresented populations into health promotion research. Gerontologist. (2003) 43:37–46. doi: 10.1093/geront/43.suppl_1.37
52. Williams, JN, Dall'Era, M, Lim, SS, Feldman, CH, Arntsen, KA, Blazer, AD, et al. Increasing ancestral diversity in systemic lupus erythematosus clinical studies. Arthritis Care Res (Hoboken). (2022) 74:420–6. doi: 10.1002/acr.24474
53. Scharff, DP, Mathews, KJ, Jackson, P, Hoffsuemmer, J, Martin, E, and Edwards, D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. (2010) 21:879–97. doi: 10.1353/hpu.0.0323
54. Goodman, A, Morgan, R, Kuehlke, R, Kastor, S, Fleming, K, and Boyd, J. “We’ve been researched to death”: exploring the research experiences of urban indigenous peoples in Vancouver, Canada. Int Indig Policy J. (2018) 9:3. doi: 10.18584/iipj.2018.9.2.3
55. Ford, CL, and Airhihenbuwa, CO. Critical race theory, race equity, and public health: toward antiracism praxis. Am J Public Health. (2010) 100:S30–5. doi: 10.2105/AJPH.2009.171058
56. Community Engagement Key Function Committee Task Force. Principles of community engagement clinical and translational science awards consortium community engagement key function committee task force on the principles of community engagement. Community Engagement Key Function Committee Task Force (2011).
57. Wieland, ML, Njeru, JW, Alahdab, F, Doubeni, CA, and Sia, IG. Community-engaged approaches for minority recruitment into clinical research: a scoping review of the literature. Mayo Clin Proc. (2021) 96:733–43. doi: 10.1016/j.mayocp.2020.03.028
58. O’Neill, J, Tabish, H, Welch, V, Petticrew, M, Pottie, K, Clarke, M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. (2014) 67:56–64. doi: 10.1016/j.jclinepi.2013.08.005
59. Richmond, CAM, and Cook, C. Creating conditions for Canadian aboriginal health equity: the promise of healthy public policy. Public Health Rev. (2016) 37:2. doi: 10.1186/s40985-016-0016-5
60. George, S, Duran, N, and Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific islanders. Am J Public Health. (2014) 104:e16–31. doi: 10.2105/AJPH.2013.301706
61. Ontario Human Rights Commission. Collecting human rights-based data. (2009) Available at: https://www.ohrc.on.ca/en/count-me-collecting-human-rights-based-data. (Accessed Mar 10, 2022).
62. Project Implicit. The implicit association test. (2011). Available at: https://implicit.harvard.edu/implicit/takeatest.html. (Accessed Mar 10, 2022).
63. Farooqi, A, Raghavan, R, Jutlla, K, Patel, N, Akroyd, C, Desai, B, et al. Increasing participation of black, Asian and minority ethnic (BAME) groups in health and social care research. BMC Med Res Methodol. (2018) 22:17.
64. University of Waterloo. Resources and guides for indigenous research research University of Waterloo. Available at: https://uwaterloo.ca/research/research-equity-and-inclusion/research-equity-diversity-and-inclusion-redi-council/resources-and-guides-indigenous-research. (Accessed Mar 10, 2022).
65. Treweek, S, Banister, K, Bower, P, Cotton, S, Devane, D, Gardner, HR, et al. Developing the INCLUDE ethnicity framework–a tool to help trialists design trials that better reflect the communities they serve. Trials. (2021) 22:337. doi: 10.1186/s13063-021-05276-8
66. Swann, SA, Campbell, AR, Nicholson, VJ, and MCM, M. Meaningful community collaboration in research practicable methods to overcome barriers to effective collaboration during a pandemic. 340 BC MediCal J. (2020) 62:340.
67. Matsui, EC, Perry, TT, and Adamson, AS. An antiracist framework for racial and ethnic health disparities research. Longit Stud Child Health Dev. (2020) 146:2020018572. doi: 10.1542/peds.2020-018572
68. National Collaborating Centre for Determinants of Health. Key public health resources for anti-racism action: a curated list. Antigonish, NS: National Collaborating Centre for Determinants of Health (2018).
69. Government of Canada. Best practices in equity, diversity and inclusion in research. (2021) Available at: https://www.sshrc-crsh.gc.ca/funding-financement/nfrf-fnfr/edi-eng.aspx?wbdisable=true. (Accessed Mar 10, 2022).
Keywords: race, ethnicity, diversity, minority, research strategies, recruitment, patient participation, clinical research
Citation: Le D, Almaw RD, Rinaldi D, Ivanochko NK, Harris S, Benjamin A and Maly MR (2023) Barriers and strategies for recruiting participants who identify as racial minorities in musculoskeletal health research: a scoping review. Front. Public Health. 11:1211520. doi: 10.3389/fpubh.2023.1211520
Edited by:
Chakema Carmack, University of Houston, United StatesReviewed by:
Simon Robert Stones, Envision Pharma Group, United KingdomCandace S. Brown, University of North Carolina at Charlotte, United States
Copyright © 2023 Le, Almaw, Rinaldi, Ivanochko, Harris, Benjamin and Maly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica R. Maly, bXJtYWx5QHV3YXRlcmxvby5jYQ==
 Denise Le1
Denise Le1 Rachel D. Almaw
Rachel D. Almaw Monica R. Maly
Monica R. Maly