
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 02 November 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1187948
This article is part of the Research Topic SARS-CoV-2: Virology, Epidemiology, Diagnosis, Pathogenesis, and Control View all 24 articles
 Adisu Asefa1*
Adisu Asefa1* Nitsuh Derjachew2
Nitsuh Derjachew2 Abebe Muche Belete1
Abebe Muche Belete1 Feredegn Talargia1
Feredegn Talargia1 Daniel Molla Melese1
Daniel Molla Melese1 Bekalu Getachew3
Bekalu Getachew3Background of the study: One of the best medical approaches for halting the spread of infectious diseases is vaccination. During the COVID-19 pandemic, healthcare workers (HCWs) were a high-risk population. Due to their susceptibility in terms of their working environment, front-line healthcare personnel should receive vaccinations before others.
Objective: The purpose of this study was to assess the adverse reactions to COVID-19 vaccines among Ethiopian healthcare professionals in 2022.
Methods: A facility-based cross-sectional study design was conducted in Addis Ababa Health Facilities, Ethiopia. A total of 290 health professionals who were vaccinated during the study period were involved. Data entry was done by Epidata (version 3.1) and analyzed using SPSS software version 26. Bivariable analysis was conducted and a p value of less than 0.25 was selected for further multivariable analysis. A p value of 0.05 was considered statistically significant at a 95% confidence level.
Results: A total of 277 study participants were successfully involved in the study, yielding a response rate of 95.5%. The study participants comprised 123 (44.4%) women and 154 (55.6%) men. The majority of them (202, 72.9%) had received the Oxford AstraZeneca vaccine. Among the 277 study participants, 142 (51.3%) had developed adverse reactions associated with vaccination. Of these, 81 (29.2%) had moderate adverse reactions. Only 2 (0.7%) had developed adverse reactions that led to hospitalization. The most reported short-term adverse reactions were injection site pain (151, 54.5%), headache (114, 41.2%), fever (104, 37.5%), fatigability and tiredness (94, 33.9%), chills (92, 33.2%), muscle pain (79, 28.5%), and decreased sleep quality (34, 12.3%). The multivariable logistic regression showed that the odds of having an adverse reaction were 1.501 times higher among women than men (AOR = 1.501, 95% CI [1.08, 2.754]).
Conclusion and recommendations: This study revealed that adverse effects following the COVID-19 vaccine were moderate in magnitude and minimal in severity. This study showed that adverse reactions that led to hospitalization were rare. Based on the findings of this study, it is recommended that national, multicenter, prospective, and randomized studies be conducted to assess the independent association of each vaccine.
One of the best medical approaches for halting the spread of infectious diseases is vaccination. To protect communities from COVID-19 and prevent further economic hardship, safe and effective SARS-CoV-2 vaccinations are required (1).
A 94.1% efficacy of the SARS-CoV-2 vaccine (mRNA-1273) has been confirmed, and the first human clinical study of the vaccine began in March 2020 in the United States. However, the SARS-CoV-2 vaccine’s global uptake is still insufficient for herd immunity (2).
Healthcare workers (HCWs) are a high-risk population during the COVID-19 pandemic. This subpopulation has a 9–11 times higher infection risk than the general population (3). In China, a total of 1,433 healthcare workers (HCWs) received vaccinations, and 135 of them reported adverse reactions (9.4%) (4).
According to a study done in India, 98.2% of people experienced adverse effects following immunization. In this study, generalized weakness, local pain, or swelling at the injection site were some of the side effects that were frequently experienced after vaccination. In this study, women (67.7%) were more likely than men (32.3%) to experience detrimental impacts when working as healthcare professionals (5).
The Centers for Disease Control and Prevention (CDC) and other studies have shown that symptoms at the injection site (swelling, pain, and redness) and systemic effects (back pain, fatigue, headache, muscle pain, joint pain, chills, fever, and nausea) were connected to post-COVID-19 vaccination (6).
According to a Chinese study, the two most common complaints were weakness (74, 5.2%) and headache/dizziness (58, 4.0%). The most often reported side effects associated with the COVID-19 vaccination include headache, weariness, muscle and joint pain, fever and chills, and soreness at the injection site (7).
According to a study from Nigeria, participants who had previously experienced an adverse reaction to a medication or vaccination were younger (40 years old), had received two doses, and reported experiencing symptoms more frequently. Approximately 71.1% of the 295 vaccine recipients in Nigeria who participated in the trial experienced at least one side effect (8).
Another study conducted on Ghanaian healthcare workers showed that 528 (80.7%) of the participants reported having adverse reactions. The most common adverse effects among Ghanaian healthcare workers were generalized weakness (32.0%), headache (27.3%), and fever (19.1%) (9). According to a study, healthcare workers aged 35–39 and 40–44 had reduced probabilities of adverse reactions compared to those aged 25–29. Analgesics used by medical personnel before immunization reduced the risk of negative reactions (9).
A study done in Togo revealed that out of 1,639 medical professionals, 71.6% of participants reported at least one adverse effect (10). According to a study done in Ethiopia, 510 (75.7%) medical professionals who received the vaccination reported injection site symptoms of pain (65.48%) and discomfort (57.9%) (11).
Since evidence of the adverse effects of all vaccines given in Ethiopia is scarce, this study was conducted to quickly document adverse events to reassure the population. This study was intended to assess adverse reactions following COVID-19 vaccination and their associated factors among healthcare professionals working in Ethiopia.
A facility-based cross-sectional study was carried out among healthcare professionals working in Addis Ababa Public Health Facilities, Ethiopia from February 10, 2022 to June 10, 2022.
According to a previous study conducted in Ethiopia, 75.8% of healthcare professionals who received the Oxford AstraZeneca vaccine reported injection site symptoms of pain and tenderness (11). Based on this assumption, the minimum sample size required for this study was determined using the single population proportion formula.
Taking p = 75.8%, 5% level of precision (d) with a 95% confidence interval, and a 10% non-response rate was added. Since the source population was 4,471, the population correction formula was utilized. The final sample size was = 290.
From a total of 11 Governmental Hospitals in Addis Ababa, three were selected by simple random sampling technique (lottery method). The selected Governmental Hospitals included St. Paul’s Millennium Medical College, Yekatit 12 Hospital Medical College, and Eka Kotebe General Hospital. Based on the data from the Addis Ababa Health Bureau and the Federal Ministry of Health, the total number of healthcare professionals working in Addis Ababa governmental hospitals was 4,471.
A systematic random sampling technique was employed after using proportional allocation. The sampling fraction was: 4,471/290 = 15. The first sample was selected using a simple random sampling technique. Then, every 15 healthcare professionals were included in the study from each of the governmental hospitals until the calculated sample size was achieved.
Adverse reactions following COVID-19 vaccine.
Socio-demographic factors (age, sex, and educational status), behavioral factors (alcohol drinking, cigarette smoking, khat chewing, and drugs used for any other chronic illnesses), and other factors (type and dose of vaccine, presence of chronic illnesses, and COVID-19 result before or after vaccination).
Data were collected using a structured questionnaire adapted from different literature. The questionnaire includes four parts; socio-demographic characteristics, medical history, behavioral factors, and vaccination status. The questionnaire was prepared in the English language and translated into Amharic and then back into English. Five BSc health professionals were recruited to collect the data and two BSc/MSc health professionals supervised the data collection process. Timely supervision was undertaken by the principal investigator during the data collection period.
Adverse reactions: unintended pharmacologic reactions that occur when medication or vaccine is administered correctly.
Mild adverse reaction: HCPs who stayed at home to rest and who also took painkillers.
Moderate adverse reaction: HCPs who attended health institutions but did not require hospitalization.
Severe adverse reaction: HCPs who were admitted to hospital and received the required health care services.
The data were entered into EPI data manager version 3.3 and analyzed using IBM SPSS Statistics version 22. Model fitness was also checked using the Hosmer and Lemeshow test. A summary of descriptive statistics was computed for most variables. A binary logistic regression analysis model was utilized. A point estimate of Odds ratio (OR) with a 95% confidence interval (CI) was determined to assess the strength of association between independent and dependent variables. For all statistically significant tests, value of p < 0.05 was used as a cut-off point.
Out of 290 study participants, 277 were successfully involved in the study, yielding a response rate of 95.5%. The study participants comprised 123 (44.4%) women and 154 (55.6%) men. The study participants’ ages ranged from 22 to 54 years, with mean and standard deviations of 31 and ± 6.46 years, respectively. Most of the participants (127, 45.8%) were nurses in the profession. The majority (266, 96%) had no chronic diseases (Table 1).
The majority (159, 57.4%) of study participants’ previous COVID-19 results were negative. All of the study participants were vaccinated. Among the vaccinated, 39 (14.1%) were infected by the virus (Figure 1).
The majority of them (202, 72.9%) had received the Oxford AstraZeneca vaccine. Only 11 (4%) took Sinopharm. Most of the participants (208, 75.1%) received two doses of the COVID-19 vaccine. A total of 249 had no allergies to any types of food or medicines. Only 5 (1.8%) had used substances (Figure 2).
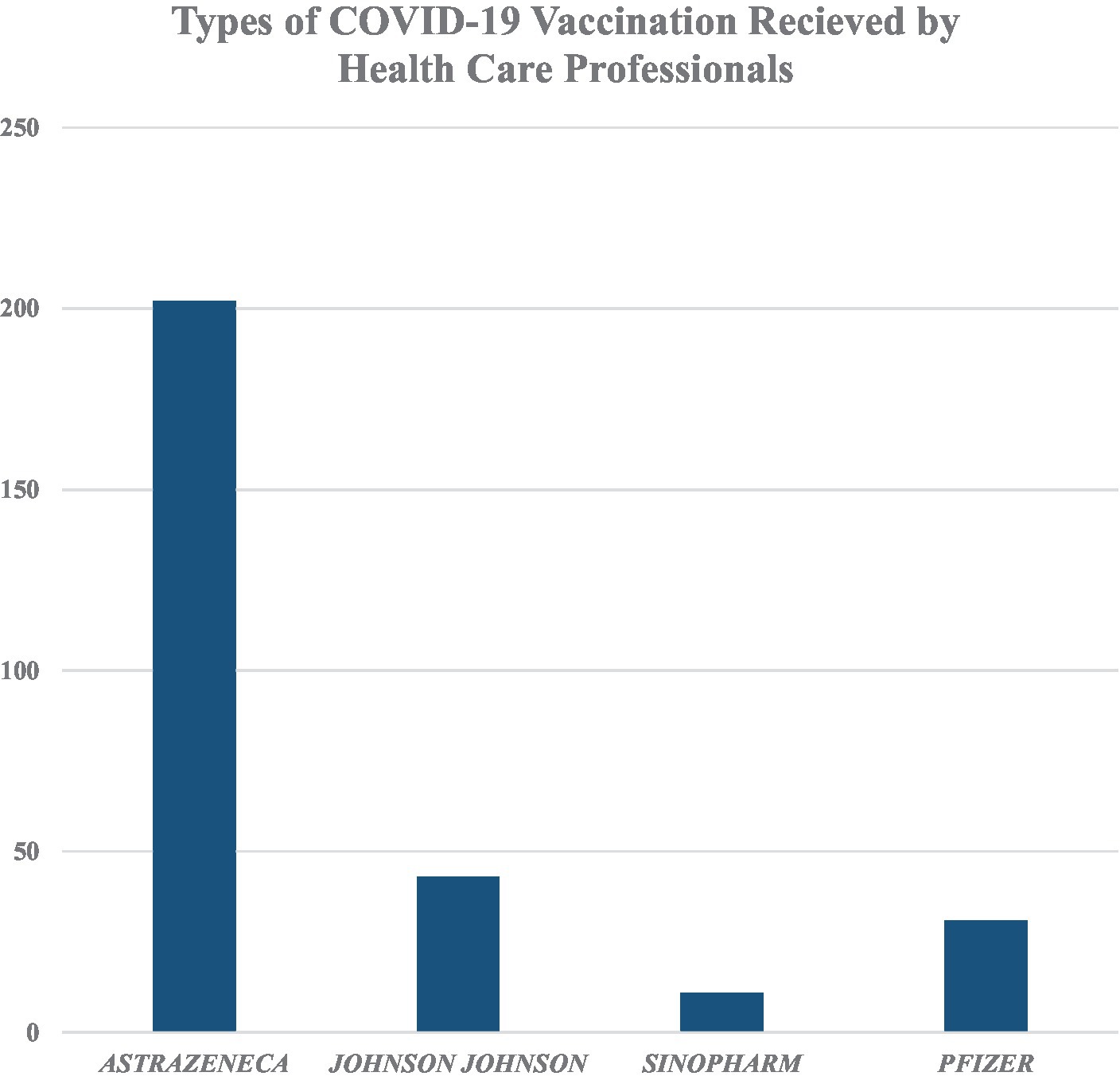
Figure 2. Types of COVID-19 vaccination received by healthcare professionals working in Addis Ababa Public Health Facilities, Ethiopia.
Among the 277 study participants, 142 (51.3%) had developed adverse reactions associated with vaccination. Of these, 81 (29.2%) had moderate adverse reactions. Only 2 (0.7%) had developed adverse reactions that led to hospitalization (Figure 3). Among those who had adverse reactions, 70 (25.3%) developed adverse reaction symptoms within 5–8 h of vaccine administration. In the majority (79, 28.5%), the symptoms lasted for 1–3 days. Of the study participants who had received the COVID-19 vaccine, only 2 (0.7%) were diagnosed with thrombosis. Most of the study participants (245, 88.4%) recommended having the COVID-19 vaccine to others.
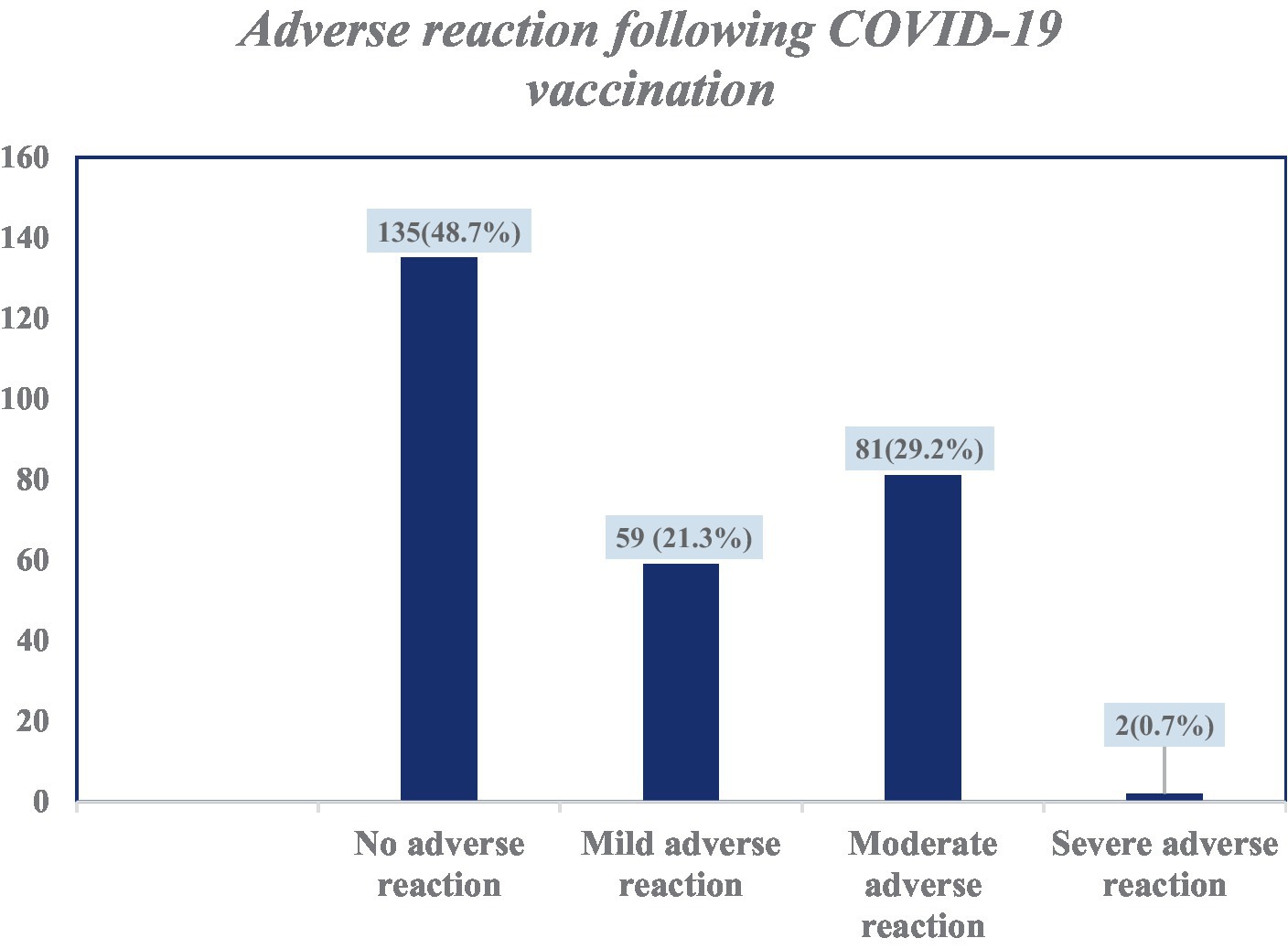
Figure 3. Categories of adverse reactions following COVID-19 vaccination among health care professionals in Addis Ababa, Ethiopia.
The most reported short-term adverse reactions were injection site pain 151 (54.5%), headache 114 (41.2%), fever 104 (37.5%), fatigability and tiredness 94 (33.9%), chills 92 (33.2%), muscle pain 79 (28.5%), and decreased sleep quality 34 (12.3%; Table 2).
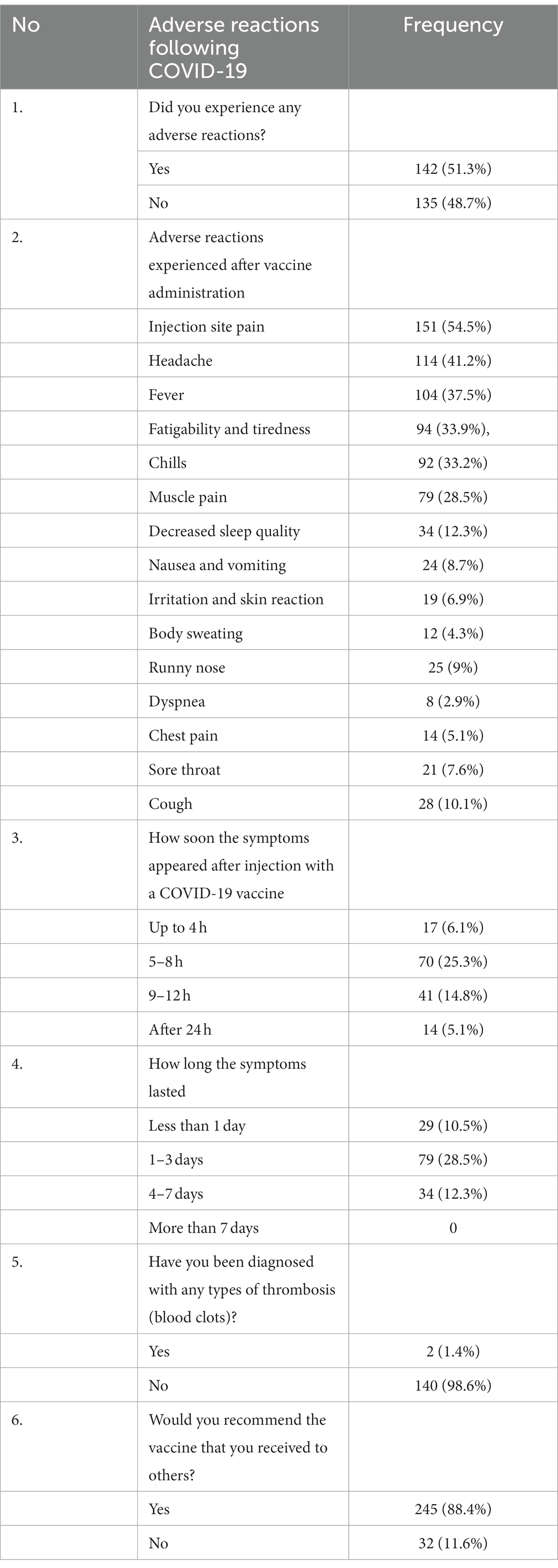
Table 2. Adverse reaction after COVID-19 vaccine administration among health care professionals in Addis Ababa, Ethiopia.
All sociodemographic characteristics were entered into bivariate logistic regression. Age group (p value = 0.23), gender (p value = 0.017), job category (p value = 0.031), and underlying chronic diseases (p value = 0.320). Based on a binary regression result, the odds of having adverse reactions were 1.799 times higher among women than men (COR = 1.799, 95% CI [1.13, 2.906]). The odds of adverse reactions were 66% less likely among study participants who had received two doses of the COVID-19 vaccine than among those who had received it once (COR = 0.337, 95% CI [0.117, 0.975]).
There was no statistically significant association with adverse reactions related to the specific types of COVID-19 vaccine [Oxford AstraZeneca (COR = 1.385, 95% CI [0.814, 2.359]), Johnson & Johnson (COR = 0.90, 95% CI [0.469, 1.727]), Sinopharm (COR = 0.00), and Pfizer (COR = 2.169, 95% CI [0.981, 4.797])].
Multivariable logistic regression analysis has shown that the odds of having adverse reactions were 1.501 times higher among women than men (AOR = 1.501, 95% CI [1.08, 2.754]; Table 3).
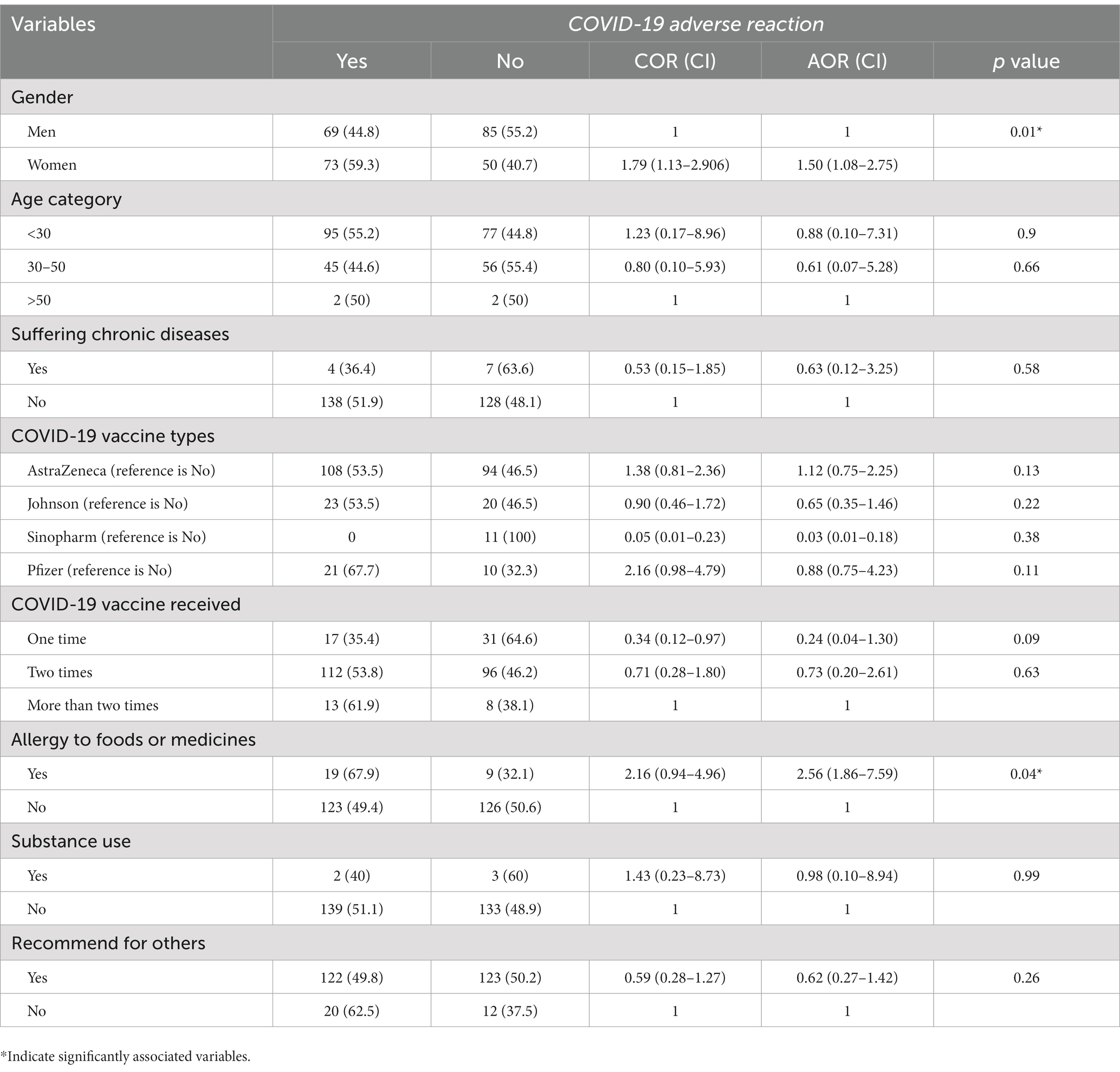
Table 3. Factors associated with the occurrence of adverse reactions among healthcare professionals in Addis Ababa, Ethiopia.
During the COVID-19 pandemic, healthcare professionals were among the high-risk populations. Due to their susceptibility in terms of their working conditions, front-line healthcare personnel were given priority when it came to vaccination (3). Evidence of the adverse effects of the COVID-19 vaccines administered in Ethiopia is scarce.
Among the study participants, 51.3% had developed adverse reactions associated with vaccination. The current study’s findings are lower than those of other studies conducted in Ghana, which showed that the prevalence of adverse reactions among study participants was 80.7% (9); in Togo, it was 71.6% (10); and in UAE, it was 64.8% (12). These differences in the prevalence of adverse reactions could be due to variation in sample size and socioeconomic status.
The major adverse effects reported by the COVID-19 vaccine recipients were pain at the site of injection (47%), fatigue and drowsiness (28.2%), and joint/muscle pain (23.1%), followed by headache (17.7%) and fever (14.4%). A survey based on a mobile self-report questionnaire to assess the prevalence and characteristics of adverse reactions following the first dose of the ChAdOx1 nCoV-19 Vaccine and the BNT162b2 vaccine was conducted among healthcare workers in South Korea. Of the 5,589 healthcare workers in the ChAdOx1 nCoV-19 group, the overall adverse reaction rate was 93%. Approximately, half of the ChAdOx1 nCoV-19 group reported moderate or severe grade events (13).
In the current study, only 0.7% had developed adverse reactions that led to hospitalization. Comparable findings were noted in a study conducted in Southern Ethiopia, which showed that 1.1% had severe adverse reactions (14), and in Togo, where 1% were found to have been hospitalized (10). These comparable findings from different studies might implicate the rare occurrence of severe adverse reactions associated with the COVID-19 vaccine.
The most reported short-term adverse reactions were headache (41.2%), fever (37.5%), fatigability and tiredness (33.9%), chills (33.2%), muscle pain (28.5%), and decreased sleep quality (12.3%). These findings are comparable with other studies conducted in Ghana (9), Togo (10), and Southern Ethiopia (14). The similarities could be due to the wide scale use of the AstraZeneca vaccine in this population.
The present study revealed that only 1.4% had been diagnosed with thrombosis (blood clots). This finding contradicts a study conducted in Ethiopia, which showed none of the study participants reported laboratory-confirmed blood clotting problems (11). However, a systematic review and meta-analysis study indicated that venous thrombosis due to the COVID-19 vaccine was 28 per 100,000 doses (15). Similarly, other systematic reviews and exploratory analysis studies indicated the presence of venous thrombosis due to the COVID-19 vaccine (16). According to this systematic review and exploratory analysis study, the pathophysiology behind venous thrombosis is explained as follows: “New experimental studies have assumed that thrombosis is related to a soluble adenoviral protein spike variant, originating from splicing events, which cause important endothelial inflammatory events, and binding to endothelial cells expressing ACE2” (16) (p.2).
Multivariable logistic regression analysis showed that the odds of experiencing adverse reactions were 1.501 times higher among women than men. Similar findings were noted in a study conducted in Togo (10). This result may be explained by a greater immunological response brought on by estrogen (6) or other unidentified immunologic differences between men and women (10).
This study revealed no statistically significant correlation between the different COVID-19 vaccination types and associated adverse reactions. These results suggest that unfavorable reactions to a vaccine are not influenced by the type of vaccine.
In this study, adverse reactions following the COVID-19 vaccine were moderate in magnitude and minimal in severity. This study revealed that 51.3% of participants had developed adverse reactions associated with vaccination. The majority of the study participants (72.9%) had received the AstraZeneca vaccine. The most reported short-term adverse reactions following vaccination were headache, fever, fatigability and tiredness, chills, and muscle pain. Less than 1% (0.7%) had developed adverse reactions that led to hospitalization. The present study revealed that the occurrence of thrombosis (blood clots) was rare. In the current study, the odds of having adverse reactions were higher among women than men. The type of COVID-19 vaccine had no significant association with adverse reactions.
Based on our findings, we recommend health professionals receive any of the COVID-19 vaccines without fear or hesitancy since severe adverse reactions were found to be rare. Future national, multicenter, prospective, and randomized study should be conducted to assess the independent association of each vaccine with adverse reactions. Our results show that women were more likely to develop adverse reactions than men. Therefore, future randomized control studies should investigate this association clearly.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted after obtaining ethical approval letters from the (IRB) of the College of Medicine, Asrat Woldeyes Health Science Campus, Debre Berhan University, and Addis Ababa Health Bureau complied with the Declaration of Helsinki. A permission letter was obtained from the study hospitals. The data were collected after obtaining written informed consent from the study participants. To keep confidentiality, codes were used and unauthorized persons did not have access to the data.
AA contributed to conception or design, data collection, acquisition, analysis, or interpretation, drafted the manuscript, and critically revised the final manuscript. ND contributed to conception or design and data collection, and drafted the manuscript. AB, FT, and DM contributed to acquisition, analysis, or interpretation, drafted the manuscript, and critically revised the final manuscript. BG drafted the manuscript and critically revised the final manuscript. All authors contributed to the article and approved the submitted version.
We would like to express our great appreciation and thank St. Paul’s Millennium Medical College, Yekatit 12 Hospital Medical College, Eka Kotebe General Hospital, and Addis Ababa Health Bureau for their kind cooperation and support. We also acknowledge Debre Berhan University and Gamby Medical and Business College for their kind cooperation in conducting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ADRs, Adverse drug reactions; AEFI, Adverse events following immunization; AEs, Adverse effects; CDC, Center for Disease Control and Prevention; CVST, Cerebral venous sinus thrombosis; EMA, European medicines agency; HCPs, Health care professional; NDVP, National deployment and vaccination plan; WHO, World Health Organization.
1. Sadoff, J, Le Gars, M, Shukarev, G, Heerwegh, D, Truyers, C, de Groot, AM, et al. Interim results of a phase 1–2a trial of Ad26. COV2. S Covid-19 vaccine. N Engl J Med. (2021) 384:1824–35. doi: 10.1056/NEJMoa2034201
2. Baden, LR, El Sahly, HM, Essink, B, Kotloff, K, Frey, S, Novak, R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
3. He, Z, Ren, L, Yang, J, Guo, L, Feng, L, Ma, C, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. (2021) 397:1075–84. doi: 10.1016/S0140-6736(21)00238-5
4. Ye, X, Ye, W, Yu, J, Gao, Y, Ren, Z, Chen, L, et al. (2021). The landscape of COVID-19 vaccination among healthcare workers at the first round of COVID-19 vaccination in China: willingness, acceptance and self-reported adverse effects. medRxiv [Preprint]. doi: 10.1101/2021.05.15.21257094
5. Sharma, A, Jain, M, and Vigarniya, M. Acceptance and adverse effects following COVID-19 vaccination among the health care workers at a health care Centre in the most backward district of India. J Fam Med Prim Care. (2022) 11:3224–9. doi: 10.4103/jfmpc.jfmpc_2370_21
6. Gee, J, Marquez, P, Su, J, Calvert, GM, Liu, R, Myers, T, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morb Mortal Wkly Rep. (2021) 70:283–8. doi: 10.15585/mmwr.mm7008e3
7. World Health Organization (WHO) (2021). Coronavirus disease (COVID-19): vaccines safety. Available at: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety (Accessed March 26, 2021).
8. Okezie, KC. Knowledge, Awareness and incidence of adverse events following immunization with astrazeneca covid-19 vaccine among healthcare professionals in north central zone of Nigeria. Int J Collab Res Intern Med Public Health. (2022) 14:001–5.
9. Serwaa, D, Osei-Boakye, F, Nkansah, C, Ahiatrogah, S, Lamptey, E, Abdulai, R, et al. Non-life-threatening adverse reactions from COVID-19 vaccine; a cross-sectional study with self-reported symptoms among Ghanaian healthcare workers. Hum Vaccin Immunother. (2021) 17:3881–6. doi: 10.1080/21645515.2021.1963600
10. Konu, YR, Gbeasor-Komlanvi, FA, Yerima, M, Sadio, AJ, Tchankoni, MK, Zida-Compaore, WI, et al. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Archiv Public Health. (2021) 79:1–9. doi: 10.1186/s13690-021-00741-x
11. Solomon, Y, Eshete, T, Mekasha, B, and Assefa, W. COVID-19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in low-income country: Ethiopia. J Multidiscip Healthc. (2021) 14:2577–85. doi: 10.2147/JMDH.S331140
12. Ganesan, S, Al Ketbi, LM, Al Kaabi, N, Al Mansoori, M, Al Maskari, NN, Al Shamsi, MS, et al. Vaccine side effects following COVID-19 vaccination among the residents of the UAE—an observational study. Front Public Health. (2022) 10:876336. doi: 10.3389/fpubh.2022.876336
13. Bae, S, Lee, YW, Lim, SY, Lee, JH, Lim, JS, Lee, S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. (2021) 36:1–4. doi: 10.3346/jkms.2021.36.e115
14. Zewude, B, Habtegiorgis, T, Hizkeal, A, Dela, T, and Siraw, G. Perceptions and experiences of COVID-19 vaccine side-effects among healthcare workers in southern Ethiopia: a cross-sectional study. Pragmat Observ Res. (2021) 12:131–45. doi: 10.2147/POR.S344848
15. Kim, AY, Woo, W, Yon, DK, Lee, SW, Yang, JW, Kim, JH, et al. Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a systematic review and Meta-analysis. Int J Infect Dis. (2022) 119:130–9. doi: 10.1016/j.ijid.2022.03.034
Keywords: COVID-19 vaccine, healthcare professionals, vaccine, Oxford AstraZeneca, corona virus
Citation: Asefa A, Derjachew N, Belete AM, Talargia F, Melese DM and Getachew B (2023) Adverse reactions following COVID-19 vaccine among healthcare professionals working in Ethiopia: a facility-based cross-sectional study. Front. Public Health. 11:1187948. doi: 10.3389/fpubh.2023.1187948
Received: 16 March 2023; Accepted: 13 October 2023;
Published: 02 November 2023.
Edited by:
Ana Afonso, NOVA University of Lisbon, PortugalCopyright © 2023 Asefa, Derjachew, Belete, Talargia, Melese and Getachew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adisu Asefa, c2FkYW1hc2VmYWRiQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.