
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 09 November 2023
Sec. Environmental Health and Exposome
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1186848
Background: The relationship between exposure to organophosphate esters (OPEs) and the risk of developing overactive bladder (OAB) is uncertain. The purpose of this study is to examine the potential link between urinary metabolites of organophosphate esters and OAB.
Method: Data from the National Health and Nutrition Examination Survey (NHANES) database of the 2011–2016 cycles were utilized. Four urinary metabolites of organophosphate esters: diphenyl phosphate (DPHP), bis (1,3-dichloro-2-propyl) phosphate (BDCPP), bis (2-chloroethyl) phosphate (BCEP), and dibutyl phosphate (DBUP) were included in the study. Multivariate logistic regression and restricted cubic spline (RCS) were used to evaluate the relationship between urinary OPEs metabolites and OAB. Interaction analysis was conducted on subgroups to confirm the findings.
Results: A total of 3,443 United States (US) adults aged 20 years or older were included in the study, of whom 597 participants were considered to have OAB. After adjusting for potential confounding factors, we found a positive association between DPHP and the risk of overactive bladder. The risk of overactive bladder increased with increasing DPHP concentrations compared with quartile 1 (quartile 2, OR = 1.19, 95% CI, 0.82–1.73, P = 0.34; quartile 3, OR = 1.67, 95% CI, 1.10–2.53, P = 0.02; Q4, OR = 1.75, 95% CI, 1.26–2.43, P = 0.002). However, after dividing the participants by gender, only the female group retained consistent results. Additionally, restricted cubic spline analysis revealed a nonlinear dose-response correlation between DPHP and OAB in female participants. In the subgroup analysis based on age, race, body mass index (BMI), recreational activity, smoking status, drinking status, hypertension, diabetes, and stroke, the interaction analysis revealed that the findings were uniform.
Conclusion: Our findings indicate that exposure to DPHP could elevate the risk of OAB in US adult females. Further experimental studies are needed to explore the underlying mechanism in the future.
As a substitute for customary brominated flame retardants (BFRs), organophosphate esters (OPEs) have been extensively utilized in an array of products, comprising building materials, electronics, and furniture (1, 2). Correspondingly, OPEs could also be implemented as plasticizers in different consumer products, like cosmetics and children's commodities (3, 4). With the gradual elimination of customary brominated flame retardants, the worldwide yield and utilization of organophosphates have grown enormously over the previous 15 years (5). The human body may be exposed to OPEs through indoor dust, drinking water, dietary intake, etc (6–8), which leads to constant human exposure to OPEs. And high concentration, long-term exposure to OPEs will lead to several adverse effects. Presently, animal experimental studies have shown that tris(1, 3dichloro-2-propyl) phosphate (TDCPP) can diminish serum thyroxine levels in chicken embryos (9). And exposure to higher concentrations of tris(1, 3-dichloro-2-isopropyl) phosphate (TDCIPP) is prone to hyperthyroidism in domestic cats (10). Tri-ortho-cresyl phosphate (TOCP) can destroy the spermatogenic epithelium of mouse testicles and reduce sperm density (11). It is noteworthy that some halogenated OPEs are carcinogenic to animals (12). Furthermore, studies indicate that OPEs increase the risk of cardiovascular disease, metabolic syndrome, urinary incontinence, and non-alcoholic fatty liver disease in adults (13–16). Meanwhile, in adolescents, it can also cause disorders of glucose metabolism, induce pre-diabetes, and lead to hypertension (17, 18).
Overactive bladder (OAB) was defined by the International Continence Society (ICS) in 2002 as a storage symptom syndrome characterized by “urgency, with or without urgency urinary incontinence (UUI), usually with increased daytime frequency and nocturia” (19). An epidemiological survey revealed that the overall prevalence of OAB in four European countries and Canada was 11.8%, with no significant difference between males and females (20). Surveys conducted in the United States indicate that the prevalence of OAB in the American population is 16.0% for males and 16.9% for females and that this increases with age (21). As a globally prevalent chronic disease, OAB significantly impacts patients' physical health and quality of life (22). Moreover, the healthcare costs of patients with OAB in the US population were >2.5 times higher than those of similar patients without OAB (23). However, the pathophysiology of OAB is currently unclear (24). Certain studies have indicated that OAB may be associated with risk factors such as obesity, smoking, alcohol consumption, reduced physical activity, diabetes, depression, and lower socioeconomic status (25–27). Previous research has demonstrated that exposure to environmental pollutants, including di-(2-ethylhexyl) phthalate, may elevate the probability of OAB (28). Nevertheless, no studies have concentrated on the correlation between exposure to OPEs and OAB or investigated the dose-response relationship between human metabolites of OPEs and OAB. Accordingly, we have utilized data from the National Health and Nutrition Examination Surveys (NHANES) from the 2011 to 2016 cycles to investigate the relationships.
The National Health and Nutrition Examination Survey (NHANES) is a continuous stratified multi-stage sampling program designed to evaluate the health and nutritional condition of adults and children in the United States, which involves a variety of health and nutrition measures. NHANES surveys a nationally representative sample of about 5,000 people each year, consisting mainly of interviews and physical examinations. The interview section includes demographic, socioeconomic, dietary, and health-related questions. The physical examination part includes physiological measurements, laboratory tests, etc. Written informed consent was obtained from each participant. The NHANES research project was reviewed and approved by the National Center for Health Statistics Research Board.
Our analysis involved studying data from NHANES over 6 years (2011–2012, 2013–2014, 2015–2016) and included 29,902 participants. After excluding participants with missing data on OPE urine metabolites or urine creatinine (n = 22,761), incomplete data on OAB-related questionnaires (n = 2,447) and covariates (n = 654). Meanwhile, participants who were pregnant during the interview (n = 33), participants who reported a weak/failing kidney (n = 150), and participants with a history of cancer or malignancy (n = 414) were also excluded. Finally, a total of 3,443 participants were included in the study.
In NHANES, a random selection was made of one-third of participants older than 6 years for the measurement of organophosphate metabolites. Then 0.2 mL of the subject's urine was used for enzymatic hydrolysis of urinary conjugates of the target analytes, automated off-line solid phase extraction, reversed phase high-performance liquid chromatography separation, and isotope dilution-electrospray ionization tandem mass spectrometry detection (29). We included four major OPE urine metabolites in this study (15): Diphenyl phosphate(DPHP), Bis(1,3-dichloro-2-propyl)phosphate(BDCPP), Bis(2-chloroethyl) phosphate(BCEP), and Dibutyl phosphate(DBUP). According to the NHANES Analytic Guidelines, if the detection results of urine metabolites were below the limit of detection, the values were imputed by the square root of two. Meantime, to account for variation in urine sample volume, the OPEs were adjusted by urine creatinine using the values of OPEs/creatinine (μg/g) as the analytical variables.
According to the definition of OAB, the presence of OAB should be considered when the patient has urge urinary incontinence and nocturia. We used the following three questions from the Kidney Conditions-Urology questionnaire in NHANES: (1) During the past 12 months, have you leaked or lost control of even a small amount of urine with an urge or pressure to urinate and you couldn't get to the toilet fast enough? (2) How frequently does this occur? (3) During the past 30 days, how many times per night did you most typically get up to urinate, from the time you went to bed at night until the time you got up in the morning? We leveraged the table of the Criteria for Conversion of Symptom Frequencies Recorded in NHANES and Overactive Bladder Symptom Score (OABSS) Scores, as utilized by Shenhao Zhu et al. (30). To enhance the diagnosis of OAB, we further quantified overactive bladder symptoms by specific scoring refer to Table 1 for specific scoring criteria. Finally, each participant's overall OABSS score was obtained by adding the nocturia score and the urge urinary incontinence score. Individuals with a total score ≥3 were considered to have a diagnosis of overactive bladder disorder (30).
We used a Directed Acyclic Graph (DAG) (www.dagitty.net/dags.html) to show the hypothesized relations between OPEs, confounders, and overactive bladder outcomes (Supplementary Figure 1). According to the DAG, participants' sex, age, race, body mass index (BMI), education level, poverty income ratio (PIR), recreational activity, smoking, and drinking were included as confounding factors. We also carefully considered alternative DAG that included more variables, such as variables related to OAB or OPEs. Marital status, hypertension, and diabetes were also included as relevant variables.
To reduce the effects of the complex multistage sampling design of NHANES, we used appropriate sample weights to improve data precision, according to NHANES guidelines. Urinary creatinine-corrected metabolite concentrations of OPEs were natural-log transformed (ln-transformed) due to skewed distribution and categorized into four quartiles (Q1, Q2, Q3, and Q4). Demographic characteristics were expressed in weighted mean ± standard error (SE) for continuous variables and weighted percentage (%) for categorical variables by OAB status. Weighted t-tests for continuous variables and weighted chi-squared tests for categorical variables were used to assess the baseline characteristics of the participants by OAB status. Multivariable logistic regression analyses were utilized to explore the relationships between OPEs and OAB status. We constructed three logistic regression models: model 1 adjusted for no variables; model 2 adjusted for age, sex, race, education level, PIR, and BMI; model 3 further adjusted for recreational activity, smoking status, drinking status, hypertension, diabetes as well as stroke. In addition, in Model 3, we included OPEs urine concentration as a continuous variable and used a restricted cubic spline (RCS) to reveal the dose-response relationship between OPEs concentration and OAB risk. We then used subgroup analyses stratified by age, race, BMI, recreational activity, smoking status, drinking status, hypertension, diabetes, and stroke, and performed interaction analyses to examine whether there was a differential association between subgroups. All statistical analyses in this study were organized and analyzed using R software (R 4.2.3). Two-sided P < 0.05 was considered statistically significant.
Figure 1 illustrates the exposure levels of four distinct types of urinary, creatinine-adjusted organophosphate esters (OPEs) across 3,433 participants. The findings reveal that participants diagnosed with OAB present higher average exposure levels to DPHP, BDCPP, BCEP, and DBUP compared to those participants without the condition. Notably, among participants with overactive bladder, DPHP exhibited a markedly high exposure level of 1.71 ug/g, while DBUP demonstrated the lowest exposure level of 0.24 ug/g.
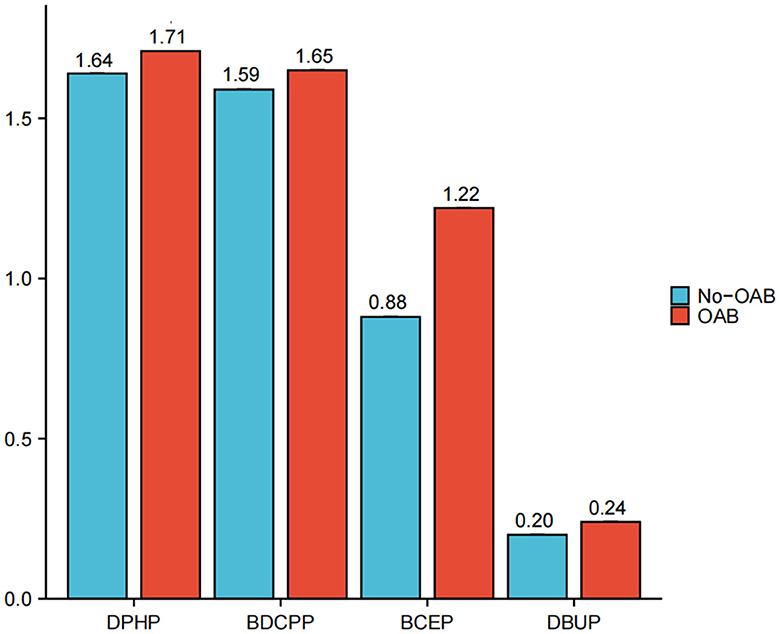
Figure 1. Urinary creatinine-corrected OPE metabolites exposure levels among 3,433 participants. Diphenyl phosphate (DPHP), Bis(1,3-dichloro-2-propyl) phosphate (BDCPP), Bis(2-chloroethyl) phosphate (BCEP), Dibutyl phosphate (DBUP).
As shown in Table 2, in this study, a total of 3,433 people, 1,715 males and 1,718 females, were included. The prevalence of OAB was 17.39%, and statistically significant differences were found between the two groups in the distributions of age, race, marital status, educational levels, BMI, PIR, smoking status, drinking status, recreational activity, stroke, hypertension, and diabetes. Participants with OAB status were older than those without OAB. In addition, women, white, people with a college degree or above, obesity, drinking, physical inactivity, and high blood pressure were more likely to have overactive bladder.
Table 3 displays the results of multivariate logistic regression analyses. The results showed a positive relationship between the exposure level of DPHP and the risk of OAB. This association is significant in our model 1 (OR = 1.11; 95% CI = 1.02–1.22, P = 0.02) and model 2 (OR = 1.18; 95% CI = 1.06–1.32, P = 0.004). Furthermore, in Model 3, after adjusting for all covariates, these increases remained statistically significant (OR = 1.19; 95% CI = 1.06–1.32, P = 0.004). At the same time, we convert four types of OPEs from a continuous variable to a categorical variable (quartiles) for further analysis. After adjusting for all confounding factors, we observed a 1.67-fold and 1.75-fold increase in the risk of OAB in the DPHP Q3 and Q4 groups compared with the DPHP Q1 group (Q3 vs. Q1, OR = 1.67, 95%CI: 1.10–2.53, P = 0.02; Q4 vs. Q1, OR = 1.75,95%CI: 1.26–2.43). However, no statistically significant associations were observed between BDCPP, BCEP, and DBUP and OAB. Meanwhile, after dividing the participants by gender, only the female group retained the consistent results (OR = 1.05; 95% CI, 0.90–1.23; P = 0.04 in model 1, OR = 1.16; 95% CI: 0.98–1.36; P = 0.03 in model 2 and OR = 1.16; 95% CI, 0.98–1.36; P = 0.01 in model 3) (Supplementary Table 1). In contrast, no statistically significant association was observed between DPHP and OAB in the individual male group (Supplementary Table 2).
The restricted cubic spline depicted the dose-response relationship analysis between the concentrations of DPHP and OAB in Figure 2. We detected that the risk of OAB gradually increased as DPHP increased (P for overall = 0.002; P for nonlinearity = 0.053). Meanwhile, a linear negative correlation between DBUP and OAB was observed (P for overall = 0.006; P for nonlinearity = 0.833). However, no linear or nonlinear associations were observed between BDCPP and BCEP and OAB. (Both p for overall > 0.05, both p for nonlinearity > 0.05). In addition, as shown in Supplementary Figure 2, a nonlinear correlation between DPHP and OAB was observed in females (P for overall = 0.002; P for nonlinearity = 0.002). Piecewise regression analysis demonstrated that the inflection point of In(DPHP) was 0.458 (DPHP = 1.581 ug/g) in female participants. Specifically, when the urinary creatinine adjusted DPHP was <1.581 ug/g, the risk of OAB increased with the level of exposure to DPHP. However, when urine creatinine adjusted DPHP is >1.581 ug/g, the risk of OAB is a downward trend. However, in male participants, no linear or non-linear association between DPHP and OAB was observed (P for overall > 0.05, P for nonlinearity > 0.05).
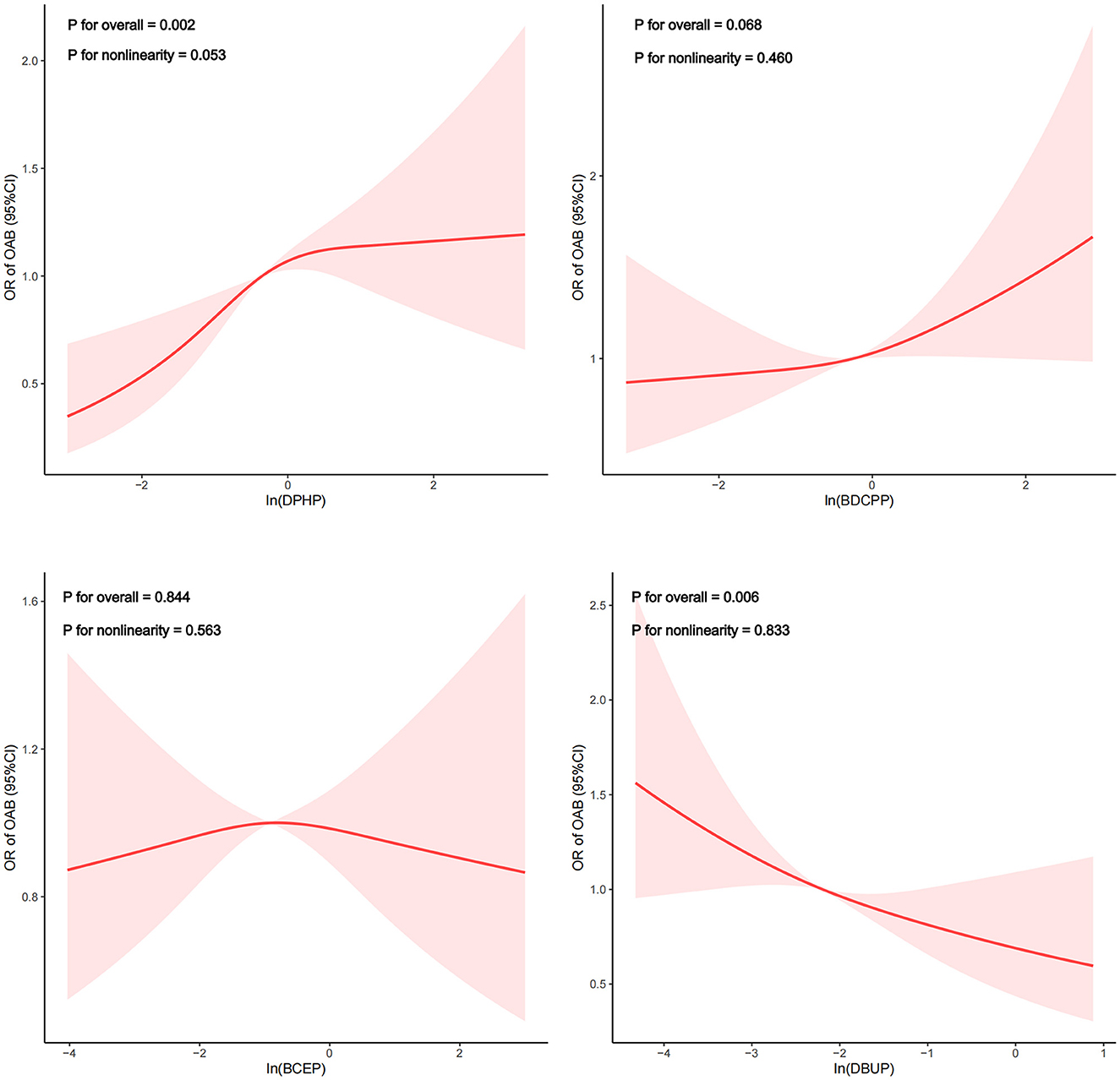
Figure 2. Dose-response relationship analysis between OPE metabolites and overactive bladder. Restricted cubic spline plots of the association between ln-transformed concentration of OPEs and OAB. RCS regression was adjusted for age, sex, race, marital status, educational levels, BMI, PIR, smoking status, drinking status, recreational activity, stroke, hypertension and diabetes (Model 3). The red or blue solid line represents ORs, red or blue shaded region represents 95 % CI.
We further adopted stratified analysis to assess whether the correlation of DPHP with OAB was stable in different subgroups (Figure 3). After adjusting for the covariates, we found that there was no significant difference between OAB and DPHP within the subgroup. In detail, the associations between DPHP and the risk of OAB remained consistent in different subgroups by age, race, BMI, recreational activity, smoking status, drinking status, hypertension, diabetes, and stroke.
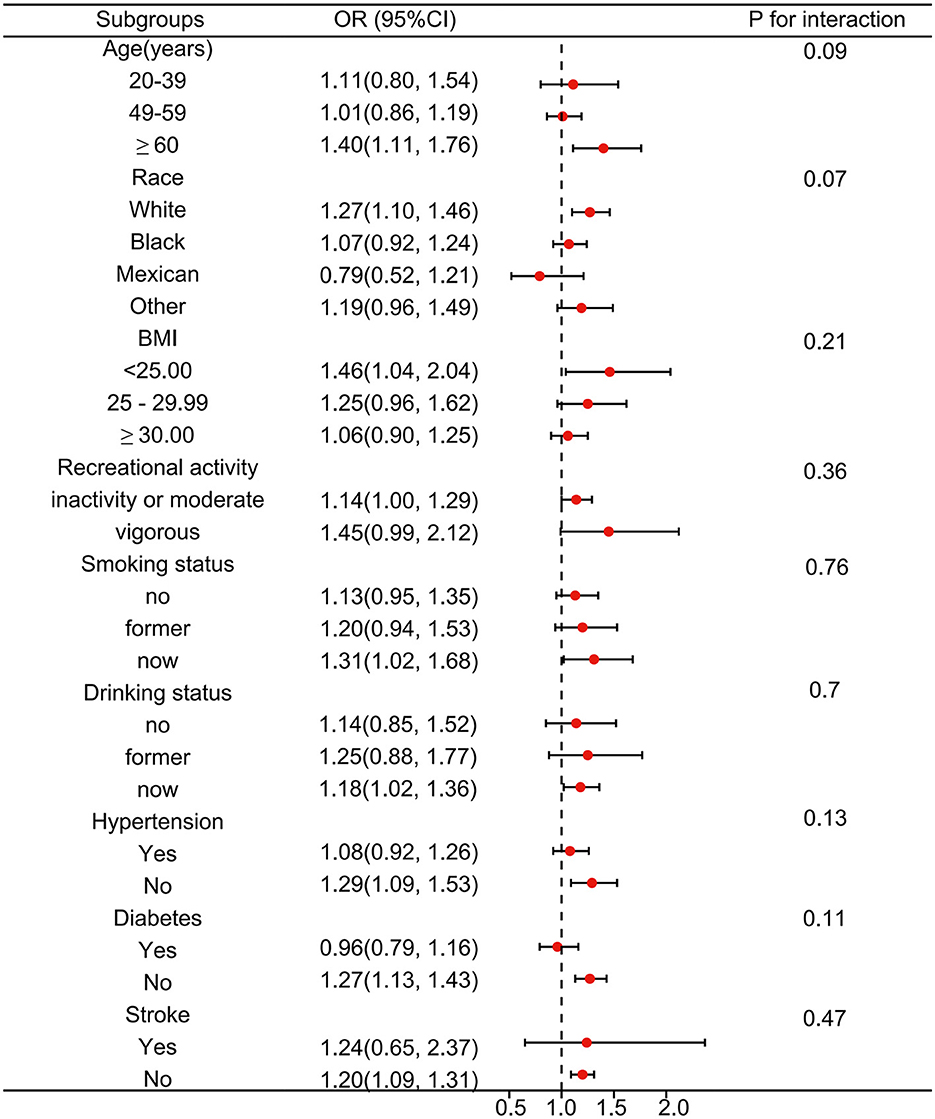
Figure 3. Subgroup analysis between Diphenyl phosphate (DPHP) and overactive bladder. Analyses were adjusted for age, sex, race, marital status, educational levels, BMI, PIR, smoking status, drinking status, recreational activity, stroke, hypertension and diabetes.
Overactive bladder is a common urological disease, which not only brings a significant negative impact on a patient's quality of life but also leads to an economic loss of productivity and higher health care costs (31). Therefore, identifying the risk factors of OAB is of great significance for its prevention of it. To the best of our knowledge, our study is the first to examine the relationship between metabolites of OPEs and OAB. In this nationally representative study, we found a strong association between DPHP and OAB. Univariate and multivariate logistic regression results showed that exposure to high levels of DPHP was associated with a higher risk of OAB. When we converted DPHP from a continuous variable to a categorical variable, we found that higher DPHP exposure levels were significantly associated with higher OAB risk compared to lower DPHP exposure levels. However, when stratified by sex, this association remained consistent only among female participants. Dose-response analyses of DPHP and OAB indicated a nonlinear association between DPHP and OAB in female participants. Subgroup analysis by age, race, BMI, recreational activity, smoking status, drinking status, hypertension, diabetes, and stroke also showed no effect on the stability of the relationship between DPHP and OAB.
Research conducted over the past decade has demonstrated that OPEs have become almost ubiquitous in a range of microenvironments and populations worldwide. Exposure can arise from many common sources, including consumer products, construction materials, and food packaging (32). It is persistent in the environment and toxic to humans and animals. Numerous studies have demonstrated that OPEs may cause various side effects, including neurotoxicity, carcinogenesis, endocrine disrupting activity, reproductive, and developmental toxicity, among others (33–35). However, research examining the relationship between OPE exposure and bladder dysfunction in humans is limited. A recent analysis of the NHANES database by He et al. (15) found that urine metabolites of OPEs were linked to a higher odds ratio for mixed incontinence in females. Nonetheless, the precise mechanism by which OPEs impact bladder function remains unclear.
At present, it is increasingly evident that OPEs are a type of endocrine disruptor (36). It is hypothesized that OPEs may impact bladder function by interfering with endocrine pathways. Currently, there is scientific consensus that metabolic syndrome (MetS) and OAB may have shared pathophysiologies (37). A study conducted by Kai Luo et al. showed that exposure to OPEs elevated the risk of MetS in adults (14). At the same time, Yacong Bo and Kai Luo et al. discovered a positive correlation between organophosphate esters (OPEs) and insulin resistance in adults and adolescents (17, 38). Animal studies have also shown that OPE exposure can lead to insulin resistance (39). Uzun et al. (40) observed a link between insulin resistance and overactive bladder (OAB) in the population. Additionally, in an obese mice model, insulin resistance in the bladder mucosa impeded detrusor relaxation and contributed to bladder overactivity (41, 42). Therefore, OPEs might lead to overactive bladder by causing insulin resistance of the bladder mucosa.
Another possible mechanism is that OPEs may increase the risk of overactive bladder by affecting human sex hormone levels. Previous studies have demonstrated that male participants with higher DPHP urine levels had lower total testosterone and estradiol levels (43). Gao et al. (44) also found that some organophosphate esters such as TPhP and EHDPP may reduce estradiol levels in women of childbearing age. However, testosterone and estrogen can bind to corresponding receptors in the bladder and pelvic floor muscles to further affect bladder function (45, 46). The mechanism by which testosterone and estrogen affect bladder function was further elucidated in the mouse model. Kiril L and Georgi V Petkov discovered that testosterone and estradiol can lower the excitability of detrusor smooth muscle cells by directly activating BK channels through a nongenomic mechanism (47, 48). Other studies have demonstrated that reduced testosterone can cause fibrosis of the bladder wall in rats and impact the release of urinary mediators in the bladder, resulting in bladder dysfunction (49, 50). Additionally, animal studies have illustrated that estradiol may reverse urothelial damage, inflammatory cell infiltration, and muscular atrophy (51). Therefore, prolonged exposure to OPEs could result in reduced levels of estrogen and testosterone in the body, ultimately causing bladder dysfunction.
This study has several strengths and limitations. The main advantage is that the study includes a large population sample, which can be representative of the characteristics of the national population. In addition, we considered appropriate sampling weights in the analysis to reduce the bias of oversampling, which made our conclusions more reliable. This study also has some limitations. First, This is a cross-sectional survey, and the causal relationship between OPEs and the occurrence of OAB cannot be determined. Second, The information provided by NHANES on overactive bladder is incomplete and the diagnosis of OAB is mainly based on questionnaire form, which reduces accuracy. Finally, we cannot determine whether there are potential confounding factors that were not involved in the study.
Our results suggest that exposure to high levels of DPHP may increase the risk of OAB in US adult females. Further experimental studies are needed to explore its underlying mechanism in the future.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
WL and M-EL wrote, revised, and reviewed the manuscript, drafted the study design, and supervised all processes. HW and ZW performed the statistical analysis and interpreted the analysis. WZ wrote, revised, and reviewed the manuscript. All authors have reviewed the results and approved the submission of the manuscript.
The authors thank Jing Zhang (Shanghai Fifth People's Hospital) for providing statistical methodology consultation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1186848/full#supplementary-material
1. Blum A, Behl M, Birnbaum L, Diamond ML, Phillips A, Singla V, et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett. (2019) 6:638–49. doi: 10.1021/acs.estlett.9b00582
2. Kemmlein S, Herzke D, Law RJ. BFR—governmental testing programme. Environ Int. (2003) 29:781–92. doi: 10.1016/S0160-4120(03)00112-0
3. Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. (2016) 86:45–51. doi: 10.1016/j.envint.2015.10.005
4. Hammel SC, Zhang S, Lorenzo AM, Eichner B, Stapleton HM, Hoffman K. Young infants' exposure to organophosphate esters: breast milk as a potential source of exposure. Environ Int. (2020) 143:106009. doi: 10.1016/j.envint.2020.106009
5. Zhang Q, Wang Y, Zhang C, Yao Y, Wang L, Sun H, et al. Review of organophosphate esters in soil: implications for the potential source, transfer, and transformation mechanism. Environ Res. (2022) 204:112122. doi: 10.1016/j.envres.2021.112122
6. Zhao L, Zhang Y, Deng Y, Jian K, Li J, Ya M, et al. Traditional and emerging organophosphate esters (OPEs) in indoor dust of Nanjing, eastern China: Occurrence, human exposure, and risk assessment. Sci Total Environ. (2020) 712:136494. doi: 10.1016/j.scitotenv.2020.136494
7. Bi R, Su G. Dietary intake assessment of known and unknown organophosphate esters (OPEs) in foodstuffs via high-resolution mass spectrometry. Sci Total Environ. (2023) 854:158452. doi: 10.1016/j.scitotenv.2022.158452
8. Huang J, Gao Z, Hu G, Su G. Non-target screening and risk assessment of organophosphate esters (OPEs) in drinking water resource water, surface water, groundwater, and seawater. Environ Int. (2022) 168:107443. doi: 10.1016/j.envint.2022.107443
9. Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, et al. In Ovo effects of two organophosphate flame retardants–TCPP and TDCPP–on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. (2013) 134:92–102. doi: 10.1093/toxsci/kft100
10. Poutasse CM, Herbstman JB, Peterson ME, Gordon J, Soboroff PH, Holmes D, et al. Silicone Pet Tags Associate Tris(1,3-dichloro-2-isopropyl) Phosphate Exposures with Feline Hyperthyroidism. Environ Sci Technol. (2019) 53:9203–13. doi: 10.1021/acs.est.9b02226
11. Chen J-X, Xu L-L, Mei J-H, Yu X-B, Kuang H-B, Liu H-Y, et al. Involvement of neuropathy target esterase in tri-ortho-cresyl phosphate-induced testicular spermatogenesis failure and growth inhibition of spermatogonial stem cells in mice. Toxicol Lett. (2012) 211:54–61. doi: 10.1016/j.toxlet.2012.03.004
12. van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. (2012) 88:1119–53. doi: 10.1016/j.chemosphere.2012.03.067
13. Guo X, Wu B, Xia W, Gao J, Xie P, Feng L, et al. Association of organophosphate ester exposure with cardiovascular disease among US adults: cross-sectional findings from the 2011–2018 National Health and Nutrition Examination Survey. Chemosphere. (2022) 308:136428. doi: 10.1016/j.chemosphere.2022.136428
14. Luo K, Zhang R, Aimuzi R, Wang Y, Nian M, Zhang J. Exposure to Organophosphate esters and metabolic syndrome in adults. Environ Int. (2020) 143:105941. doi: 10.1016/j.envint.2020.105941
15. He M, Jin K, Qiu S, Liao X, Zheng X, Chen Z, et al. The associations between organophosphate esters and urinary incontinence in the general US population. Environ Sci Pollut Res. (2022) 29:10400–7. doi: 10.1007/s11356-021-14153-5
16. Chai H, Hu W, Dai Y, Zhu X, Qian P, Zhu J. Environmental exposure to organophosphate esters and suspected non-alcoholic fatty liver disease among US adults: a mixture analysis. Front Public Health. (2022) 10:995649. doi: 10.3389/fpubh.2022.995649
17. Luo K, Aimuzi R, Wang Y, Nian M, Zhang J. Urinary organophosphate esters metabolites, glucose homeostasis and prediabetes in adolescents. Environ Pollut. (2020) 267:115607. doi: 10.1016/j.envpol.2020.115607
18. Guo X, Ke Y, Wu B, Song Q, Sun C, Li Y, et al. Exploratory analysis of the association between organophosphate ester mixtures with high blood pressure of children and adolescents aged 8–17 years: cross-sectional findings from the National Health and Nutrition Examination Survey. Environ Sci Pollut Res Published online October 29. (2022). doi: 10.1007/s11356-022-23740-z
19. Abrams P, Cardozo L, Fall M, Griffits D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. (2002) 187:116–26. doi: 10.1067/mob.2002.125704
20. Irwin DE, Milsom I, Hunskaar S, Reiley K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. (2006) 50:1306–15. doi: 10.1016/j.eururo.2006.09.019
21. Stewart W, Van Rooyen J, Cundiff G, Abrams P, Herzong AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. (2003) 20:327–36. doi: 10.1007/s00345-002-0301-4
22. Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. (2003) 92:948–54. doi: 10.1111/j.1464-410X.2003.04527.x
23. Durden E, Walker D, Gray S, Fowler R, Juneau P, Gooch K. The economic burden of overactive bladder (OAB) and its effects on the costs associated with other chronic, age-related comorbidities in the United States. Neurourol Urodyn. (2018) 37:1641–9. doi: 10.1002/nau.23513
24. Peyronnet B, Mironska E, Chapple C, Cardozo L, Oelke M, Dmochowski R, et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. (2019) 75:988–1000. doi: 10.1016/j.eururo.2019.02.038
25. Wang Y, Xu K, Hu H, Wang X, Na Y, Kang X. Prevalence, risk factors, and impact on health related quality of life of overactive bladder in China. Neurourol Urodyn. (2011) 30:1448–55. doi: 10.1002/nau.21072
26. Mckellar K, Bellin E, Schoenbaum E, Abraham N. Prevalence, risk factors, and treatment for overactive bladder in a racially diverse population. Urology. (2019) 126:70–5. doi: 10.1016/j.urology.2018.12.021
27. Hirayama A, Torimoto K, Mastusita C, Okomato N, Morikawa M, Tanaka N, et al. Risk factors for new-onset overactive bladder in older subjects: results of the Fujiwara-kyo study. Urology. (2012) 80:71–6. doi: 10.1016/j.urology.2012.04.019
28. Yang L, Liu Z, Peng Z, Song P, Zhou J, Wang L, et al. Exposure to DEHP is potential to increase the risk of overactive bladder, evidence from NHANES 2003–2008. Environ Sci Pollut Res. (2022) 29:89643–51. doi: 10.1007/s11356-022-22092-y
29. Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, Ospina M. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere. (2019) 235:481–91. doi: 10.1016/j.chemosphere.2019.06.181
30. Zhu S, Wang Z, Tao Z, Wang S, Wang Z. Relationship between marijuana use and overactive bladder (OAB): a cross-sectional research of NHANES 2005 to 2018. Am J Med. (2023) 136:72–8. doi: 10.1016/j.amjmed.2022.08.031
31. Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract. (2013) 67:1015–33. doi: 10.1111/ijcp.12164
32. Doherty BT, Hammel SC, Daniels JL, Stapleton HM, Hoffman K. Organophosphate esters: are these flame retardants and plasticizers affecting children's health? Curr Envir Health Rpt. (2019) 6:201–13. doi: 10.1007/s40572-019-00258-0
33. Sutha J, Anila PA, Umamaheswari S, Ramesh M, Narayanasamy A, Poopal R-K, et al. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP). Environ Sci Pollut Res. (2020) 27:34369–87. doi: 10.1007/s11356-020-09527-0
34. Yao C, Yang H, Li Y. A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity. Sci Total Environ. (2021) 795:148837. doi: 10.1016/j.scitotenv.2021.148837
35. Hales BF, Robaire B. Effects of brominated and organophosphate ester flame retardants on male reproduction. Andrology. (2020) 8:915–23. doi: 10.1111/andr.12789
36. Zhang Q, Ji C, Yin X, Yan L, Lu M, Zhao M. Thyroid hormone-disrupting activity and ecological risk assessment of phosphorus-containing flame retardants by in vitro, in vivo and in silico approaches. Environ Pollut. (2016) 210:27–33. doi: 10.1016/j.envpol.2015.11.051
37. Hsu LN, Hu JC, Chen PY, Lee WC, Chuang YC. Metabolic syndrome and overactive bladder syndrome may share common pathophysiologies. Biomedicines. (2022) 10:1957. doi: 10.3390/biomedicines10081957
38. Bo Y, Zhu Y. Organophosphate esters exposure in relation to glucose homeostasis and type 2 diabetes in adults: a national cross-sectional study from the national health and nutrition survey. Chemosphere. (2022) 301:134669. doi: 10.1016/j.chemosphere.2022.134669
39. Krumm EA, Patel VJ, Tillery TS, Yasrebi A, Shen J, Guo GL, et al. Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicol Sci. (2018) 162:212–24. doi: 10.1093/toxsci/kfx238
40. Uzun H, Yilmaz A, Kemik A, Zorba OU, Kalkan M. Association of insulin resistance with overactive bladder in female patients. Int Neurourol J. (2012) 16:181–6. doi: 10.5213/inj.2012.16.4.181
41. Leiria LO, Sollon C, Báu FR, Mónica FZ, D'Ancona CL, De Nucci G, et al. Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J Physiol. (2013) 591:2259–73. doi: 10.1113/jphysiol.2013.251843
42. Lee WC, Wu KLH, Tain YL, Leu S, Cheng YT, Chan JYH. Impaired insulin signaling at the bladder mucosa facilitates metabolic syndrome-associated bladder overactivity in rats with maternal and post-weaning fructose exposure. J Formos Med Assoc. (2023) 122:258–66. doi: 10.1016/j.jfma.2022.09.013
43. Chen Z, Qiu S, Zhang C, Zhan Y, Liu L, Bao Y, et al. Association of urinary organophosphate esters level with sex steroid hormones levels in adult males: a nationwide study, NHANES 2013–2014. Andrology. (2022) 10:567–75. doi: 10.1111/andr.13149
44. Gao F, Zhang X, Shen X, Zhao F, Shen H, Hu J. Exposure assessment of aryl-organophosphate esters based on specific urinary biomarkers and their associations with reproductive hormone homeostasis disruption in women of childbearing age. Environ Int. (2022) 169:107503. doi: 10.1016/j.envint.2022.107503
45. Do we need to know more about the effects of hormones on lower urinary tract dysfunction? In: ICI-RS 2014 - Hanna-Mitchell - Neurourology and Urodynamics - Wiley Online Library. (2016). Available online at: https://onlinelibrary.wiley.com/doi/10.1002/nau.22809 (accessed March 9, 2023).
46. Prevalence and Risk Factors for Urinary Incontinence in Women With Type 2 Diabetes and Impaired Fasting Glucose. In: Diabetes Care. Arlington County: American Diabetes Association. Available online at: https://diabetesjournals.org/care/article/29/6/1307/24959/Prevalence-and-Risk-Factors-for-Urinary (accessed March 9, 2023).
47. Gv P. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regulat integrat Comparat Physiol. (2014) 307:6. doi: 10.1152/ajpregu.00142.2014
48. Hristov K, Parajuli S, Provence A, Petkov GV. Testosterone decreases urinary bladder smooth muscle excitability via novel signaling mechanism involving direct activation of the BK channels. Am J Physiol Renal Physiol. (2016) 311:F1253–F1259. doi: 10.1152/ajprenal.00238.2016
49. de Barros CAV, Lorenzetti F, Ortiz V, Dambros M. Testosterone supplementation's effects on age-related bladder remodeling – experimental study in rats. Aging Male. (2013) 16:102–7. doi: 10.3109/13685538.2013.807426
50. Bravo G, Massa H, Rose'Meyer R, Chess-Williams R, McDermott C, Sellers DJ. Effect of short-term androgen deficiency on bladder contractility and urothelial mediator release Naunyn-Schmiedeberg's. Arch Pharmacol. (2017) 390:547–56. doi: 10.1007/s00210-017-1355-6
Keywords: organophosphate esters (OPEs), overactive bladder (OAB), NHANES, nocturia, urge urinary incontinence
Citation: Lin W, Wang H, Wu Z, Zhang W and Lin M-E (2023) Associations between exposure to organophosphate esters and overactive bladder in U.S. adults: a cross-sectional study. Front. Public Health 11:1186848. doi: 10.3389/fpubh.2023.1186848
Received: 15 March 2023; Accepted: 20 October 2023;
Published: 09 November 2023.
Edited by:
Aimin Yang, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Mojtaba Keikha, Kerman University of Medical Sciences, IranCopyright © 2023 Lin, Wang, Wu, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-En Lin, bWVfbGluMjBAMTYzLmNvbQ==; Wei Zhang, dmlhZ3JhbWFuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.