
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 25 October 2023
Sec. Clinical Diabetes
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1181880
 Bing Sun1,2,3,4
Bing Sun1,2,3,4 Yimin Chen1,2,3,4
Yimin Chen1,2,3,4 Yulin Man1,2,3,4
Yulin Man1,2,3,4 Yu Fu1,2,3,4
Yu Fu1,2,3,4 Jianchang Lin1,2,3,4*
Jianchang Lin1,2,3,4* Zhaohong Chen1,2,3,4*
Zhaohong Chen1,2,3,4*Background: Diabetic foot-induced sepsis is a serious complication associated with increased disability and mortality in hospitalized patients. Early prediction of admission and detection effectively improve treatment options and prevent further deterioration. This study aims to evaluate the clinical value of the neutrophil-to-lymphocyte ratio (NLR) and prognostic nutritional index (PNI) to predict the risk of sepsis in patients with diabetic foot ulcers (DFU).
Methods: Retrospective analysis was performed on 216 patients who were admitted to the Fujian Medical University Union Hospital between January 2015 and December 2022. Patients with DFU were divided into the non-sepsis (n = 166) and the DFU-induced sepsis (n = 50) groups. The independent factors of DFU-induced sepsis were determined by univariate and multivariate logistic regression analyses. A receiver operating characteristic (ROC) curve was performed to compare the area under the curves (AUC) of PNI and NLR.
Results: Multivariate logistic regression analysis revealed that the PNI, NLR, international normalized ratio (INR), thrombin time (PT), and C-reactive protein (CRP) were independent prognostic factors for DFU-induced sepsis. After adjusting for potential confounders, the adjusted odds ratios of NLR for DFU-induced sepsis were 1.121 (1.072–1.172), 1.132 (1.077–1.189), and 1.080 (1.022–1.142), while those of PNI were 0.912 (0.873–0.953), 0.902 (0.856–0.950), and 1.004 (1.001–1.006). Moreover, the AUC of NLR was significantly greater than that of CRP (0.790, 95% CI: 0.689–0.891, p < 0.001 vs. 0.780, 95% CI: 0.686–0.873, p < 0.001).
Conclusion: NLR and PNI have been regarded as readily and independently predictive markers in patients with DFU-induced sepsis. NLR is critical for the early detection and effective treatment of DFU-induced sepsis and is superior to CRP.
Type 2 diabetes mellitus (T2DM) is characterized by metabolic disorders and a systemic chronic inflammatory response induced by prolonged hyperglycemia (1), which can cause a variety of consequences such as peripheral neuropathy, peripheral vascular disease, and foot ulcers (2). Patients with T2DM are more susceptible to the majority of infectious diseases (3). According to studies, infections such as lung infection, sepsis, urinary tract infection, and cellulitis are believed to be more common in patients with T2DM than non-diabetics (4). Diabetic foot ulcers (DFU) are major problems that affect both individuals and healthcare systems (4, 5). In addition to T2DM, other rare etiologies of foot ulcers include Hereditary Sensory and Autonomic Neuropathy (6) and infrapopliteal arterial disease (7). DFU is one of the most serious complications of diabetes, contributing significantly to disability and death (8). It is reported that the lifetime risk for a diabetic patient to develop a foot ulcer is 15–25% (9). Once developed, diabetic foot ulcers confer a high risk of below-knee amputation. Recent decades have witnessed an 85% increase in diabetes-related lower extremity amputations (10, 11). Parallel to the increasing prevalence of multidrug-resistant bacteria, patients with DFU encounter a markedly elevated risk of general infection, resulting in considerable morbidity and mortality (12). Beyond diabetes management, patients are additionally encumbered by foot-related complications of the disease (13).
Sepsis is a potentially fatal illness or complication induced by a defective host immune response to infection, with significant morbidity and death rates worldwide (14). Sepsis remains one of the most difficult and expensive diseases to treat (15), despite the continuous refinement of treatment strategies and advancements in medical equipment. Sepsis and sepsis shock are serious complications that occur in patients with DFU who are more susceptible to infection (16), increasing the risk of non-traumatic amputation, multiple organ failure, and even death.
The prognostic nutritional index (PNI) is a comprehensive and novel biomarker of inflammation based on albumin (ALB) levels and lymphocytes (17). The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are simple and composite markers of inflammation and nutritional status with high stability and usability. Several studies have suggested that PNI is a reliable biomarker for predicting early prognosis in patients with malignant tumors (18, 19). In addition, previous studies have demonstrated that PNI or NLR is a biomarker for predicting survival and mortality rates of patients with sepsis-induced acute kidney injury (AKI) (20, 21). In addition, procalcitonin (PCT) and C-reactive protein (CRP) are known to be used to diagnose sepsis and predict its severity and mortality in patients (22, 23). Nevertheless, these indicators were easily disturbed by myocardial damage, coronary artery disease, autoimmunity, and tumors (24). Since sepsis is an acute complication that affects survival rate, no research has been performed to assess the predictive usefulness of PNI and NLR in patients with DFU. Therefore, the purpose of this research is to understand the significance of the predictive values of PNI and NLR in DFU-induced sepsis.
Data on 216 patients with DFU were collected retrospectively at the Fujian Medical University Union Hospital between January 2015 and December 2022. The inclusion criteria were as follows: (1) enrolled patients should be admitted without sepsis and (2) enrolled patients with DFU discharged from the hospital should have a DFU primary diagnosis code in the first or second diagnostic code. Furthermore, sepsis or sepsis shock should not be the first diagnosis code or be recorded before a DFU diagnosis code. The exclusion criteria were as follows: (1) patients younger than 18 years; (2) patients with missing clinical and laboratory data; (3) evidence of circulatory ulcers of the lower limbs caused by malnutrition, varicose veins, or tumors; and (4) evidence of foot ulcers caused by various diseases.
(1) Sequential organ failure assessment (SOFA) score ≥ 2 and probable infection are the criteria for sepsis according to the third international consensus (25).
(2) DFU (26): Diagnostic criteria for DFU are included in both the 2017 edition of the China Guide for Prevention and Control of Type 2 Diabetes and the China Guide for Prevention and Management of Diabetes (2019 edition).
The electronic medical record system of Fujian Medical University Union Hospital was used to collect all patient data. The following information was extracted: (1) demographic parameters such as age, sex, and body mass index (BMI); (2) vital signs such as systolic blood pressure (SBP) and diastolic blood pressure (DBP); (3) complications such as hypertension, cardiovascular disease, peripheral vascular disease, chronic liver disease (CLD), and chronic kidney disease; and (4) laboratory results obtained within 24 h of admission, such as procalcitonin (PCT), C-reactive protein (CRP), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), prognostic nutrition index (PNI), white blood cell (WBC) count, hemoglobin A1c (HbA1c), ALB, total bilirubin (TBIL), direct bilirubin (DBIL), activated partial thromboplastin time (APTT), prothrombin time (PT), international normalized ratio (INR), serum sodium, serum calcium, serum creatinine, aspartate aminotransferase (AST), ALT, blood urea nitrogen (BUN), PCT, and CRP. PNI was calculated as serum ALB (g/L) + 5 lymphocyte count (109/L) (27); and PLR and NLR were calculated by dividing absolute platelet count by absolute lymphocyte count and neutrophil count by absolute lymphocyte count, respectively (28).
Baseline characteristics and laboratory data of all patients were stratified based on whether they had sepsis or not. The research variables were divided into continuous and classified variables, and the Shapiro–Wilk test was used to determine whether the variables conformed to the normal distribution. Mean and standard deviation were used to express continuous variables with a normal distribution, while median and interquartile ranges were used to depict skewed distributions. The independent-sample test and the Wilcoxon Mann–Whitney test were used in univariate analysis to compare the continuous variables between the non-sepsis and the DFU-induced sepsis groups. The chi-square test or Fisher exact test was used to compare the frequency of classified variables as a percentage. The variables associated with DFU-induced sepsis were identified using multivariate logistic regression analysis, and the confounding factors were adjusted to determine the predictive value of the PNI and NLR on the occurrence of DFU-induced sepsis. The accuracy of various DFU-induced sepsis indicators was further investigated using receiver operating characteristic (ROC) curve analysis. To compare the area under the ROC curve (AUC), the z-test was used. SPSS 25.0 and R version 4.2.1 were used for all analyses, and the value of p of 0.05 was considered statistically significant.
Figure 1 illustrates the flow chart of patient registration. A total of 216 patients with DFU were categorized into two groups based on the research objective (whether sepsis eventually occurred). Among them, 50 patients were diagnosed with sepsis/septic shock. The patients’ ages ranged from 54 to 72 years, with an average age of 66 years. Women accounted for 14.8% of the total sample, while men comprised 85.2%. Table 1 presents the demographic and clinical characteristics of patients with DFU in both the non-sepsis and DFU-induced sepsis groups. There were no statistically significant differences in age, sex, BMI, SBP, DBP, qSOFA, or comorbidities such as cardiovascular disease, hypertension, diabetic peripheral neuropathy (DPN), diabetic retinopathy (DR), or chronic liver disease (CLD) (p > 0.05).
Laboratory markers were employed to evaluate the risk prediction for both patient groups. According to our findings, patients in the sepsis group had weaker coagulation and inflammatory responses than those in the non-sepsis group. Table 2 summarizes the univariate clinical and laboratory data within 24 h of admission. There were significant differences in WBC count, hemoglobin, INR, APTT, PT, DBIL, serum calcium, ALB, PCT, CRP, PLR, NLR, and PNI between the non-sepsis and sepsis groups. Multivariate binary logistic regression analysis was used to identify potential predictors of sepsis in patients with DFU. As presented in Table 3, CRP, INR, PT, NLR, and PNI were all independent predictors of sepsis in patients with DFU. After controlling for potential confounding factors, we developed several models to evaluate the independent effects of NLR and PNI in patients with DFU-induced sepsis. The adjusted odds ratios of NLR for DFU-induced sepsis were 1.121 (95% CI: 1.072–1.172), 1.132 (95% CI: 1.077–1.189), and 1.080 (95% CI: 1.022–1.142), respectively, while those of PNI were 0.912 (95% CI: 0.873–0.953), 0.902 (95% CI: 0.856–0.950), and 1.004 (95% CI: 1.001–1.006) (Table 4).
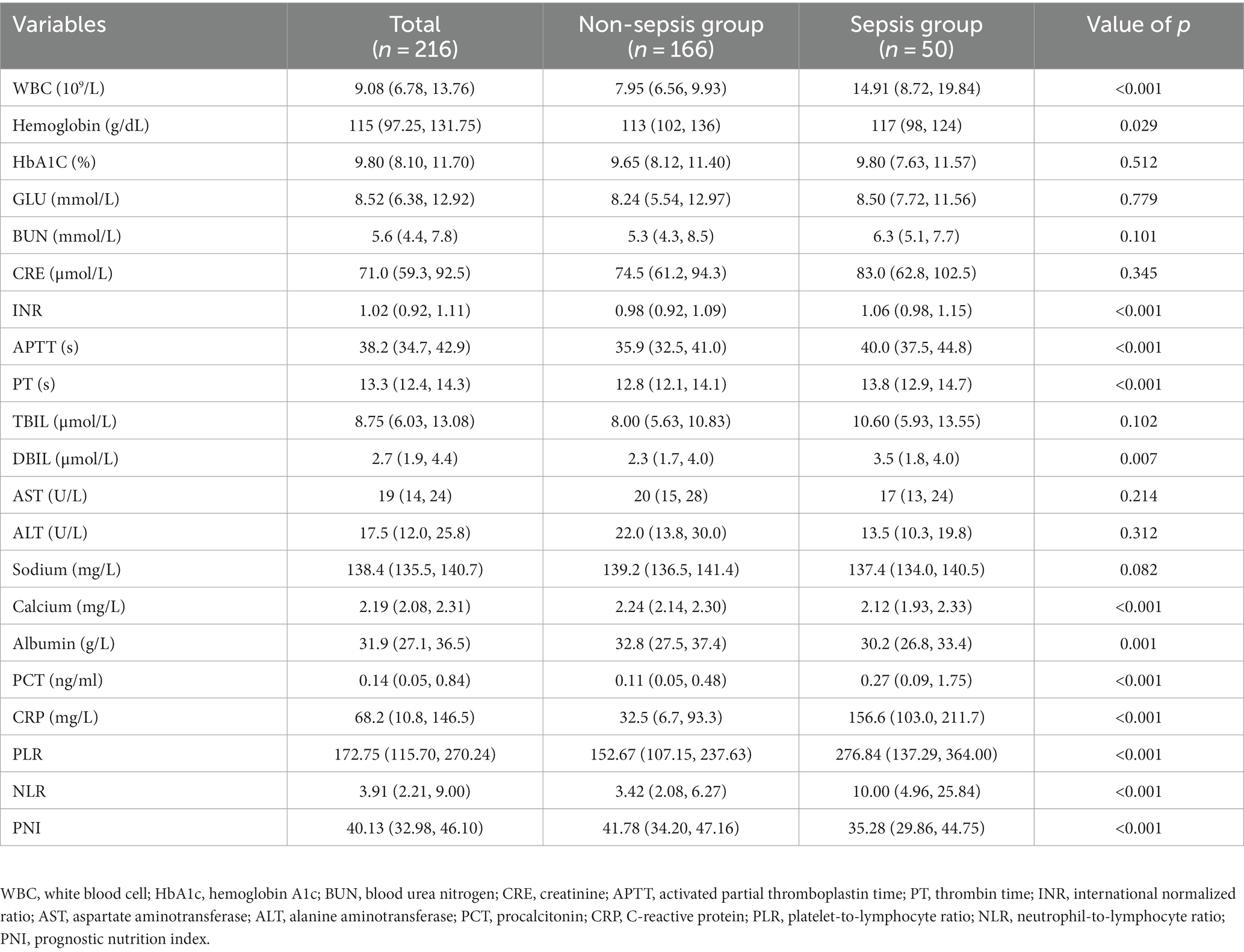
Table 2. Laboratory characteristics of patients with DFU between the non-sepsis group and sepsis group.
To evaluate the predictive value of NLR and PNI for the incidence of DFU-induced sepsis, ROC curve analysis was performed, as shown in Figures 2, 3. Table 5 displays the results of the AUC with a 95% confidence interval in ROC analysis. The AUC of the NLR was 0.790 (95% CI: 0.689–0.891, p < 0.001), which was significantly greater than that of the CRP (0.780, 95% CI: 0.686–0.873, p < 0.001). Additionally, the AUC of PNI was a substantial predictor for DFU-induced sepsis (0.702, 95%CI: 0.619–0.785, p < 0.001).
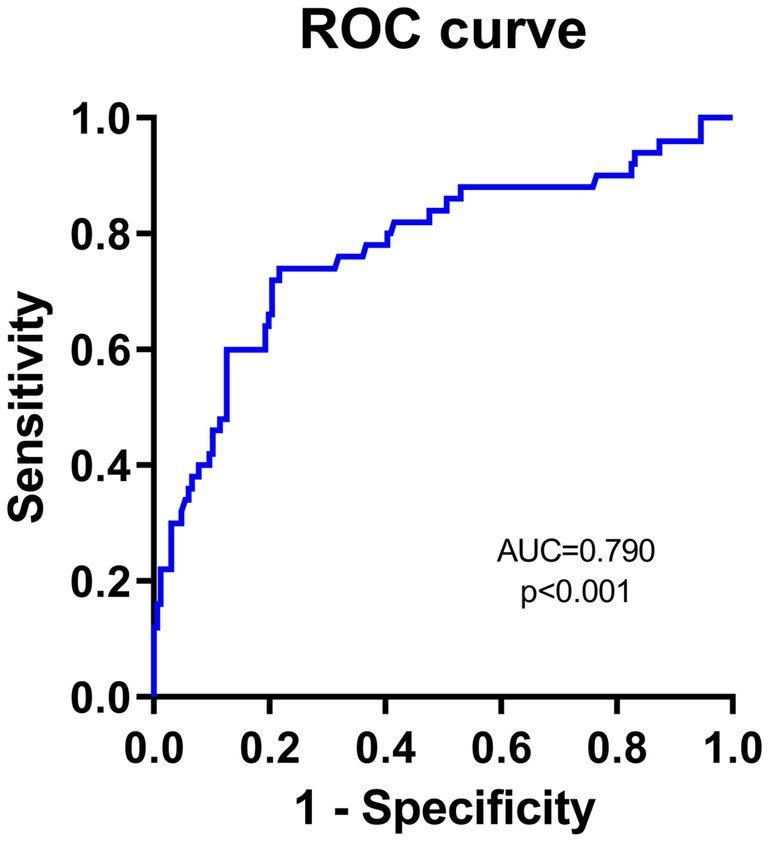
Figure 2. Receiver operating characteristic curve analyses of independent predictors for DFU-induced sepsis. The ROC curve of NLR in predicting DFU patients with sepsis.
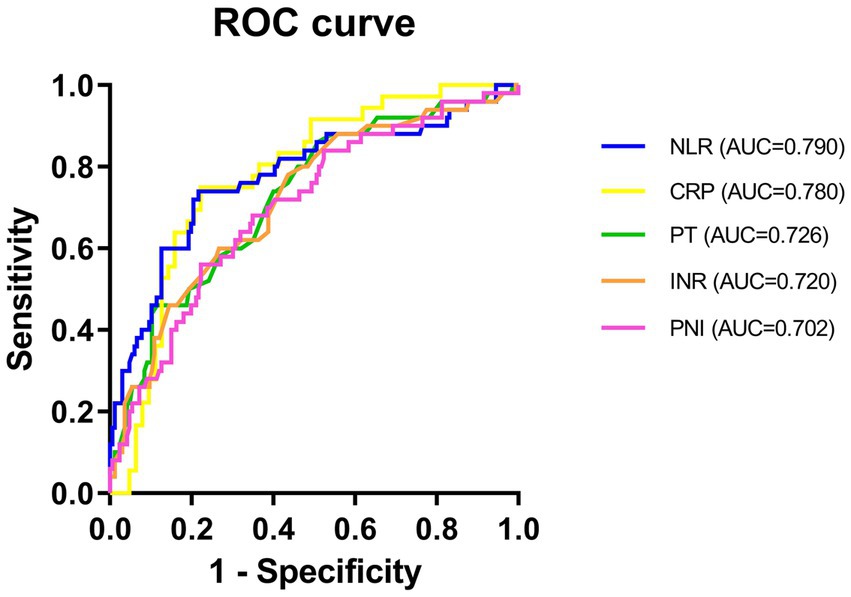
Figure 3. ROC curve of NLR, CRP, INR, PT, and PNI in predicting patients with DFU-induced sepsis. PT, thrombin time; INR, international normalized ratio; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutrition index.
This study is the first study to demonstrate that sepsis in patients with DFU can be predicted by specific inflammatory markers. Our research yielded several important findings, such as NLR, PLR, and PNI were risk factors for sepsis in both patients without sepsis and those with DFU-induced sepsis; however, only NLR and PNI were independent risk factors for higher morbidity in DFU-induced sepsis. In addition, it was observed that higher NLR and lower PNI at admission are associated with DFU-sepsis morbidity.
Sepsis is the leading cause of death worldwide, with a mortality rate of more than 10%. It is caused by the host’s maladaptive response to infection (29). The diabetes-related complication is a major cause of hospitalization, disability, and death. Several studies have reported that patients with T2DM have a 2–6 times higher risk of sepsis (30) and a higher risk of sepsis-related morbidity and mortality (31) when compared with age-matched patients with non-T2DM. Furthermore, drug-resistant pathogen colonization may occur more frequently in patients with T2DM (32). The pathophysiological mechanism of DFU is still debated. Previous studies have identified diabetic foot as one of the serious complications caused by pathophysiological changes such as inflammation, endothelial dysfunction, and blood coagulation imbalance (33). The presence of immune cells in an environment of chronic hyperglycemia and hypertriglyceridemia caused chronic immune system disorders and impaired responses to acute infections and sepsis in patients with DFU. In addition, the coexistence of DFU and severe sepsis compromised RBC deformability, worsened microcirculation, accelerated the progression of organ dysfunction, and even worsened the long-term prognosis of patients with DFU with general infection and sepsis (34, 35). Sepsis has been reported to be an independent risk factor for lower limb amputations in patients with DFU (36), resulting in increased hospital readmissions, amputation rates, morbidity, and mortality (37). Therefore, early detection of high-risk patients with DFU-induced sepsis is critical for prediction and prevention.
PNI, which has a significant impact on the patient’s nutritional and immune status, was estimated using ALB levels and the total number of peripheral blood lymphocytes (18). Nutritional status is commonly used to indicate health status and predict infection. In patients with cancer, PNI is a reliable prognostic factor. Furthermore, PNI has emerged as a prognostic biomarker for patients with acute ischemic stroke (38), sepsis-induced AKI (20), and coronary heart disease (39). Li et al. reported that the presence and severity of neonatal septicemia were negatively and independently correlated with PNI (40). Patients with DFU-induced sepsis had significantly lower PNI than those without sepsis (41.78, 34.20–47.16 vs. 35.28, 29.86–44.75, respectively, p < 0.001).
Immunologic function, inflammation, and nutritional status play crucial roles in the pathogenesis of sepsis. NLR is a well-established biomarker of systemic inflammation that is essential for sepsis diagnosis and prognosis. Due to the following primary reasons, an aberrant inflammatory response resulted in severe infections and sepsis: 1. Neutrophils secrete pro-inflammatory mediators, which induce endothelium and organ damage, as well as an increase in the risk of severe consequences and limb amputation (41); 2. Increased platelet levels, one of the inflammatory mediators, may indicate prothrombotic activity and chronic inflammatory conditions by stimulating megakaryocytes (42, 43); and 3. Lymphocytes can exert anti-inflammatory effects by secreting anti-inflammatory substances such as interleukin-10. As such, when sustaining oxidative DNA damage in hyperglycemic conditions, lymphocytes can predispose the body toward an immunosuppressive state (44). The systemic inflammatory response causes changes in neutrophil, monocyte, and platelet counts. Numerous studies have suggested that NLR and PLR may predict systemic inflammation (45) and that these markers are highly sensitive to numerous diseases (46) and readily accessible in clinical practice (47, 48). Furthermore, NLR has a significant role in predicting COVID-19 pneumonia in patients with T2DM (49). Despite the close association between inflammation and DFU, no previous studies have focused on the role of NLR and PLR in assessing and predicting the presence of DFU-induced sepsis.
Numerous studies have shown that malnutrition, immunology, and inflammation all play important roles in the initiation and progression of diabetes (8), especially with regard to its long-term consequences. Higher levels of inflammatory mediators and catabolic hormones stimulate catabolism while weakening anabolism in DFU patients, resulting in severe malnutrition, immunosuppression, and worsened inflammatory responses. In this study, the immune-nutritional marker PNI (AUC = 0.702) and the inflammatory marker NLR (AUC = 0.790) have been shown to increase the accuracy of predicting the development of sepsis. It should be noted that in our study, PCT could not be utilized as an independent predictor of sepsis in DFU patients, and there was no significant difference in multivariate logistic regression analysis. This phenomenon could be explained by PCT being less sensitive to early local infection, mild infection, and chronic inflammatory responses but more sensitive to acute systemic inflammatory response syndrome. According to studies, PCT is an independent prognostic factor for all-cause mortality in patients with sepsis (50). Furthermore, PCT can be used to diagnose bacterial sepsis or septic shock (51) and is a valuable marker for the diagnosis of patients with T2DM who have DFU infection (52). Therefore, more samples need to be collected to confirm the early predictive effect of PCT.
Our study has a few limitations, which are as follows: (1) This is a single-center, retrospective study with a small sample size. Therefore, more prospective studies are required to validate our findings and confirm the predictive effectiveness of NLR and PNI in DFU-induced sepsis. (2) While investigating the risk factors for sepsis in patients with DFU, not all of them were considered. (3) Only the initial serological indexes within 24 h of admission were included; however, changes in the index of patients with DFU during hospitalization could not be dynamically analyzed. (4) Owing to the lack of corresponding follow-up data, we were unable to analyze the contribution of risk factors to the survival status on follow-up of patients.
In clinics, assessing the risk of developing sepsis in DFU and conducting a hospital-based study have remained an ongoing challenge. In our study, CRP, INR, PT, NLR, and PNI were observed to be independent predictors of sepsis in patients with DFU. Furthermore, the inflammatory marker NLR has a higher diagnostic value than the conventional marker CRP, indicating that they may have complementary benefits and improve the accuracy of early DFU-induced sepsis prediction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Fujian Medical University Union Hospital (No. 2022KY222). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants in accordance with the national legislation and the institutional requirements. No potentially identifiable human images or data are presented in the manuscript.
BS conceived of the study and drafted the manuscript. YC, YM, and YF gathered and processed the data. JL, ZC conception, supervision, and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Clinical Specialty Discipline Construction Programme of the 14th Five-Year Plan for Fujian Medical Development, China [(2021)76], and Fujian Provincial Key Laboratory of Burn and Trauma, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tai, N, Wong, FS, and Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. (2015) 16:55–65. doi: 10.1007/s11154-015-9309-0
2. Vincent, JL, Jones, G, David, S, Olariu, E, and Cadwell, KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. (2019) 23:196. doi: 10.1186/s13054-019-2478-6
3. Monge, L, Gnavi, R, Carna, P, Broglio, F, Boffano, GM, and Giorda, CB. Incidence of hospitalization and mortality in patients with diabetic foot regardless of amputation: a population study. Acta Diabetol. (2020) 57:221–8. doi: 10.1007/s00592-019-01412-8
4. Schuetz, P, Castro, P, and Shapiro, NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care. (2011) 34:771–8. doi: 10.2337/dc10-1185
5. Gherman, D, Dumitrescu, CI, Ciocan, A, and Melincovici, CS. Histopathological changes in major amputations due to diabetic foot - a review. Romanian J Morphol Embryol. (2018) 59:699–702.
6. Mathur, DK, Prakash, C, Bhargava, P, Paliwal, V, and Mathur, K. Hereditary sensory and autonomic neuropathy presenting with mutilating trophic ulcers. Wounds. (2018) 30:E25–8.
7. Mohapatra, A, Henry, JC, Avgerinos, ED, Chaer, RA, Leers, SA, Boitet, A, et al. Heel wounds predict mortality but not amputation after Infrapopliteal revascularization. Ann Vasc Surg. (2018) 51:78–85. doi: 10.1016/j.avsg.2017.11.072
8. Hoffstad, O, Mitra, N, Walsh, J, and Margolis, DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care. (2015) 38:1852–7. doi: 10.2337/dc15-0536
9. Bondor, CI, Veresiu, IA, Florea, B, Vinik, EJ, Vinik, AI, and Gavan, NA. Epidemiology of diabetic foot ulcers and amputations in Romania: results of a cross-sectional quality of life questionnaire based survey. J Diabetes Res. (2016) 2016:5439521–7. doi: 10.1155/2016/5439521
10. Kim, TG, Moon, SY, Park, MS, Kwon, SS, Jung, KJ, Lee, T, et al. Factors affecting length of hospital stay and mortality in infected diabetic foot ulcers undergoing surgical drainage without major amputation. J Korean Med Sci. (2016) 31:120–4. doi: 10.3346/jkms.2016.31.1.120
11. Nather, A, Cao, S, Chen, JLW, and Low, AY. Prevention of diabetic foot complications. Singap Med J. (2018) 59:291–4. doi: 10.11622/smedj.2018069
12. Wukich, DK, Raspovic, KM, Jupiter, DC, Heineman, N, Ahn, J, Johnson, MJ, et al. Amputation and infection are the greatest fears in patients with diabetes foot complications. J Diabetes Complicat. (2022) 36:108222. doi: 10.1016/j.jdiacomp.2022.108222
13. Jansen, RB, Jorgensen, B, Holstein, PE, Moller, KK, and Svendsen, OL. Mortality and complications after treatment of acute diabetic Charcot foot. J Diabetes Complicat. (2018) 32:1141–7. doi: 10.1016/j.jdiacomp.2018.09.013
14. Fortini, A, Faraone, A, Meini, S, Bettucchi, M, Longo, B, Valoriani, B, et al. Italian Federation of Hospital Internists - Sepsis collaboration Group of the Tuscany R. Validity of "Sepsis-3" criteria in identifying patients with community-onset sepsis in internal medicine wards; a prospective, multicenter study. Eur J Intern Med. (2021) 85:92–7. doi: 10.1016/j.ejim.2020.12.025
15. Shorr, AF, and Zilberberg, MD. Sepsis and septic shock: evolving evidence, evolving paradigms. Semin Respir Crit Care Med. (2022) 43:039–45. doi: 10.1055/s-0041-1740975
16. Berg, D, and Gerlach, H. Recent advances in understanding and managing sepsis. F1000Res. (2018) 7:1570. doi: 10.12688/f1000research.15758.1.
17. Bullock, AF, Greenley, SL, McKenzie, GAG, Paton, LW, and Johnson, MJ. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur J Clin Nutr. (2020) 74:1519–35. doi: 10.1038/s41430-020-0629-0
18. Mirili, C, Yilmaz, A, Demirkan, S, Bilici, M, and Basol, TS. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. (2019) 24:1301–10. doi: 10.1007/s10147-019-01461-7
19. Yamamoto, T, Kawada, K, and Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal Cancer patients. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22158002
20. Xie, T, Xin, Q, Chen, R, Zhang, X, Zhang, F, Ren, H, et al. Clinical value of prognostic nutritional index and neutrophil-to-lymphocyte ratio in prediction of the development of Sepsis-induced kidney injury. Dis Markers. (2022) 2022:1449758–9. doi: 10.1155/2022/1449758
21. Hu, Y, Cao, Q, Wang, H, Yang, Y, Xiong, Y, Li, X, et al. Prognostic nutritional index predicts acute kidney injury and mortality of patients in the coronary care unit. Exp Ther Med. (2021) 21:123. doi: 10.3892/etm.2020.9555
22. Jekarl, DW, Lee, S, Kim, M, Kim, Y, Woo, SH, and Lee, WJ. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. (2019) 33:e22996. doi: 10.1002/jcla.22996
23. Liang, P, and Yu, F. Value of CRP, PCT, and NLR in prediction of severity and prognosis of patients with bloodstream infections and Sepsis. Front Surg. (2022) 9:857218. doi: 10.3389/fsurg.2022.857218
24. Paudel, R, Dogra, P, Montgomery-Yates, AA, and Coz, YA. Procalcitonin: a promising tool or just another overhyped test? Int J Med Sci. (2020) 17:332–7. doi: 10.7150/ijms.39367
25. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
26. Ma, YN, Zhang, LX, Hu, YY, and Shi, TL. Nomogram model for predicting the risk of multidrug-resistant Bacteria infection in diabetic foot patients. Infect Drug Resist. (2021) 14:627–37. doi: 10.2147/IDR.S287852
27. Gao, X, Pan, Y, Zhou, L, Li, Y, Lin, B, and Zheng, Y. The fib-PNI-MLR score, an integrative model of coagulation cascades, nutrition status, and systemic inflammatory response, predicts urological outcomes after surgery in patients with non-metastatic renal cell carcinoma. Front Oncol. (2020) 10:555152. doi: 10.3389/fonc.2020.555152
28. Mandaliya, H, Jones, M, Oldmeadow, C, and Nordman, II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.16
29. Costantini, E, Carlin, M, Porta, M, and Brizzi, MF. Type 2 diabetes mellitus and sepsis: state of the art, certainties and missing evidence. Acta Diabetol. (2021) 58:1139–51. doi: 10.1007/s00592-021-01728-4
30. Tiwari, S, Pratyush, DD, Gahlot, A, and Singh, SK. Sepsis in diabetes: a bad duo. Diabetes Metab Syndr. (2011) 5:222–7. doi: 10.1016/j.dsx.2012.02.026
31. Muller, LM, Gorter, KJ, Hak, E, Goudzwaard, WL, Schellevis, FG, Hoepelman, AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. (2005) 41:281–8. doi: 10.1086/431587
32. Frydrych, LM, Fattahi, F, He, K, Ward, PA, and Delano, MJ. Diabetes and Sepsis: risk, recurrence, and ruination. Front Endocrinol. (2017) 8:271. doi: 10.3389/fendo.2017.00271
33. Huang, Y, Zhong, Z, and Liu, F. The Association of Coagulation Indicators and Coagulant Agents with 30-day mortality of critical diabetics. Clin Appl Thromb Hemost. (2021) 27:10760296211026385. doi: 10.1177/10760296211026385
34. Morbach, S, Furchert, H, Groblinghoff, U, Hoffmeier, H, Kersten, K, Klauke, GT, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. (2012) 35:2021–7. doi: 10.2337/dc12-0200
35. Brownrigg, JR, Griffin, M, Hughes, CO, Jones, KG, Patel, N, Thompson, MM, et al. Influence of foot ulceration on cause-specific mortality in patients with diabetes mellitus. J Vasc Surg. (2014) 60:982–986.e3. doi: 10.1016/j.jvs.2014.04.052
36. Gurney, JK, Stanley, J, York, S, Rosenbaum, D, and Sarfati, D. Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia. (2018) 61:626–35. doi: 10.1007/s00125-017-4488-8
37. Peng, B, Min, R, Liao, Y, and Yu, A. Development of predictive nomograms for clinical use to quantify the risk of amputation in patients with diabetic foot ulcer. J Diabetes Res. (2021) 2021:6621035–9. doi: 10.1155/2021/6621035
38. Yuan, K, Zhu, S, Wang, H, Chen, J, Zhang, X, Xu, P, et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin Nutr. (2021) 40:2535–42. doi: 10.1016/j.clnu.2021.04.018
39. Raposeiras Roubin, S, Abu Assi, E, Cespon Fernandez, M, Barreiro Pardal, C, Lizancos Castro, A, Parada, JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 76:828–40. doi: 10.1016/j.jacc.2020.06.058
40. Li, T, Qi, M, Dong, G, Li, X, Xu, Z, Wei, Y, et al. Clinical value of prognostic nutritional index in prediction of the presence and severity of neonatal Sepsis. J Inflamm Res. (2021) 14:7181–90. doi: 10.2147/JIR.S343992
41. Roy, R, Zayas, J, Singh, SK, Delgado, K, Wood, SJ, Mohamed, MF, et al. Overriding impaired FPR chemotaxis signaling in diabetic neutrophil stimulates infection control in murine diabetic wound. elife. (2022) 11:11. doi: 10.7554/eLife.72071
42. Demirdal, T, and Sen, P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res Clin Pract. (2018) 144:118–25. doi: 10.1016/j.diabres.2018.08.009
43. Bakogiannis, C, Sachse, M, Stamatelopoulos, K, and Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. (2019) 122:154157. doi: 10.1016/j.cyto.2017.09.013
44. Walzik, D, Joisten, N, Zacher, J, and Zimmer, P. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. (2021) 121:1803–14. doi: 10.1007/s00421-021-04668-7
45. Serban, D, Papanas, N, Dascalu, AM, Kempler, P, Raz, I, Rizvi, AA, et al. Significance of neutrophil to lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR) in diabetic foot ulcer and potential new therapeutic targets. Int J Low Extrem Wounds. (2021):15347346211057742. doi: 10.1177/15347346211057742
46. Medzhitov, R. Origin and physiological roles of inflammation. Nature. (2008) 454:428–35. doi: 10.1038/nature07201
47. Verdoia, M, Schaffer, A, Barbieri, L, Aimaretti, G, Marino, P, Sinigaglia, F, et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. (2015) 41:304–11. doi: 10.1016/j.diabet.2015.01.001
48. Sen, V, Bozkurt, IH, Aydogdu, O, Yonguc, T, Yarimoglu, S, Sen, P, et al. Significance of preoperative neutrophil-lymphocyte count ratio on predicting postoperative sepsis after percutaneous nephrolithotomy. Kaohsiung J Med Sci. (2016) 32:507–13. doi: 10.1016/j.kjms.2016.08.008
49. Petrakis, V, Panagopoulos, P, Trypsianis, G, Papazoglou, D, and Papanas, N. Fasting plasma glucose increase and neutrophil-to-lymphocyte ratio as risk predictors of clinical outcome of COVID-19 pneumonia in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. (2023) 131:194–7. doi: 10.1055/a-2009-6937
50. Kyriazopoulou, E, Liaskou-Antoniou, L, Adamis, G, Panagaki, A, Melachroinopoulos, N, Drakou, E, et al. Procalcitonin to reduce long-term infection-associated adverse events in Sepsis. a randomized trial. Am J Respir Crit Care Med. (2021) 203:202–10. doi: 10.1164/rccm.202004-1201OC
51. Song, J, Park, DW, Moon, S, Cho, HJ, Park, JH, Seok, H, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. (2019) 19:968. doi: 10.1186/s12879-019-4618-7
Keywords: diabetic foot ulcers, NLR, PNI, sepsis, T2DM
Citation: Sun B, Chen Y, Man Y, Fu Y, Lin J and Chen Z (2023) Clinical value of neutrophil-to-lymphocyte ratio and prognostic nutritional index on prediction of occurrence and development of diabetic foot-induced sepsis. Front. Public Health. 11:1181880. doi: 10.3389/fpubh.2023.1181880
Received: 08 March 2023; Accepted: 05 October 2023;
Published: 25 October 2023.
Edited by:
Mehmet Ali Eren, Harran University, TürkiyeReviewed by:
Guanghua Zhai, Nanjing Medical University, ChinaCopyright © 2023 Sun, Chen, Man, Fu, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianchang Lin, Mzk4NTc0OTc1QHFxLmNvbQ==; Zhaohong Chen, ZG9jdG9yY3poQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.