
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Public Health , 20 October 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1181377
This article is part of the Research Topic Update on diagnostics, treatment, and prognosis on invasive fungal infections View all 7 articles
Lodderomyces elongisporus, a rare emerging pathogen, can cause fungemia often related to immunosuppression or intravenous devices. Herein, we report the case of a 58-year-old woman with subacute infective endocarditis due to Lodderomyces elongisporus identified by blood fungal culture and whole-genome sequencing, who was treated with antifungals, mitral replacement and endocardial vegetation removal surgery.
A 58-year-old woman was admitted to our hospital on September 8, 2022 (Day 1) with a 2-month history of repeated low fever, headache, dizziness, weakness in both lower limbs and excessive weight loss (10 kg). Before admission, she had been given a variety of anti-infective agents and antipyretic drugs (β-lactam/β-lactamase inhibitors, quinolones, and macrolides) at the local hospital to no avail. The patient gave no recent travel history. She had a history of rheumatoid arthritis without treatment, type 2 diabetes, and hypertension. This patient did not have intravenous devices or any complications of diabetes. Body temperature was 38.8°C, a systolic blowing noise was audible in the mitral valve auscultation area, and no superficial lymph nodes in the body were found. Laboratory tests showed a normal white blood cell count of 6.86 × 109/L (normal 3.5–9.5 × 109/L), with 67.2% neutrophils, rheumatoid factor (13.2 IU/mL, normal) and anti-streptolysin O (127.0 IU/mL, normal). Random blood glucose (19.45 mmol/L, normal <7.8), high-sensitivity C-reactive protein (33.96 mg/L; normal 0–5 mg/L), erythrocyte sedimentation rate (78 mm/h; normal 0–20), procalcitonin (0.30 ng/L; normal 0–0.1), and 1,3-β-D-glucan (245.33 pg/mL; normal 0–100) were elevated. Low levels of CD3+CD45+ T lymphocytes (919 cells/μL; normal 955-2860), CD3−CD19+ B lymphocytes (103 cells/μL; normal 95-560), CD3−CD16+CD56+ NK cells (35 cells/μL; normal 150–1,100), and CD3+CD16+CD56+ NKT cells (20 cells/μL; normal 40-300) were observed. The Aspergillus antigen test for serum galactomannan, CrAg lateral flow assay, T-SPOT TB, and parasite and tumor marker tests were negative. No other primary immunodeficiency diseases, human immunodeficiency virus infection, or antibody spectrum of autoimmune diseases was detected. Transthoracic echocardiography showed a vegetation lesion adherent to the patient's anterior mitral leaflet, which was concerning for infectious endocarditis (Day 3) (Figure 1A). Chest computed tomography (CT) revealed left atrial enlargement. Bacterial infective endocarditis was suspected. The patient was started on empirical antibacterial therapy with piperacillin-tazobactam, moxifloxacin and vancomycin. However, the patient's condition did not improve. Five days into the patient's hospital stay, her blood was positive for fungal culture (Day 5) (Figures 1B–E). Using whole-genome sequencing, the organism was identified as Lodderomyces elongisporus (L. elongisporus) (Supplemental Figure 1) from the blood. Antifungal susceptibility testing showed that L. elongisporus is sensitive to fluconazole, flucytosine, itraconazole, caspofungin, amphotericin B and voriconazole (Supplementary Table 1). Then, anti-infectious therapy was increased to caspofungin plus voriconazole for 2 weeks and sequential voriconazole treatment for 2 weeks. Subsequently, the patient's symptoms, including fever, headache, dizziness, and weakness, improved. However, transthoracic echocardiography showed that the vegetation lesion in the anterior mitral leaflet became larger. Thus, mitral replacement and endocardial vegetation removal surgery were performed (Day 30).
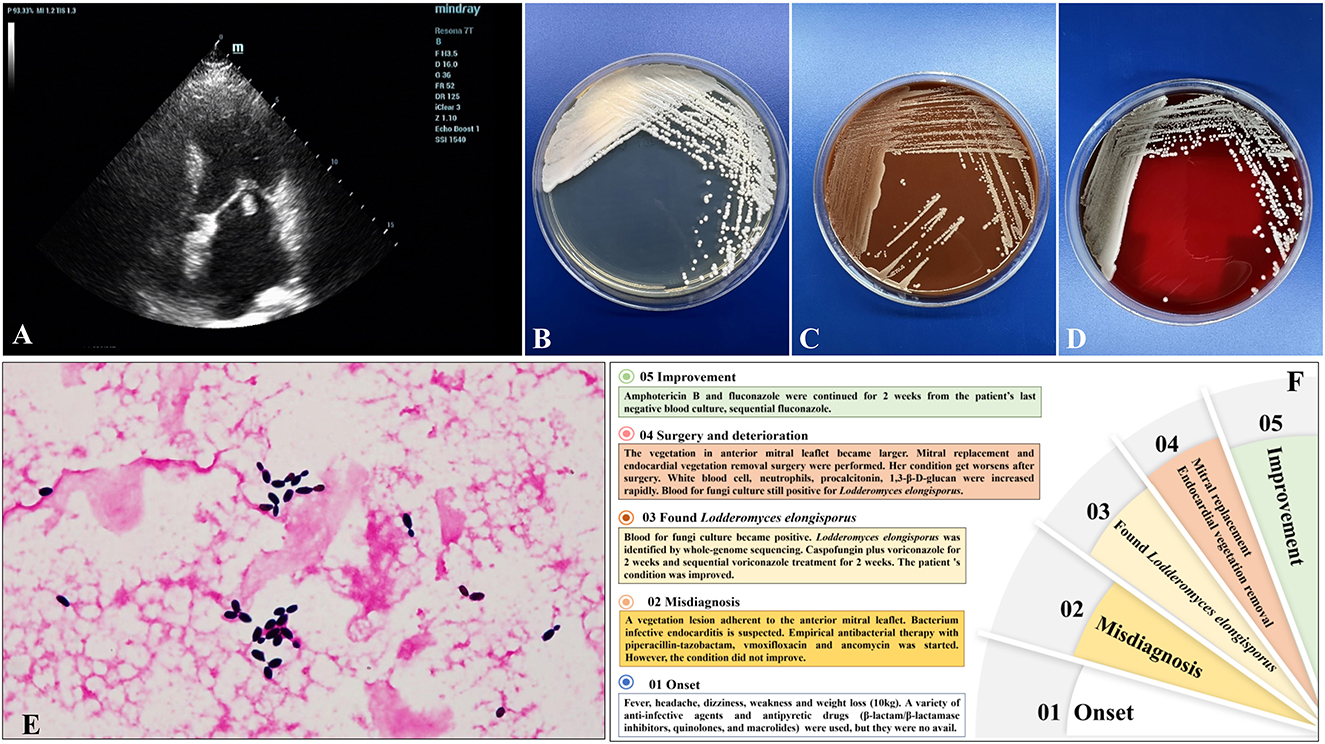
Figure 1. (A) Transthoracic echocardiography showed a vegetation lesion adherent to the patient's anterior mitral leaflet, which was concerning for infectiousendocarditis. (B–D) Culture and microscopic characteristics of L. elongisporusfrom the patient. Blood culture growth of cream-colored colonies on Sabouraud dextrose agar (B), chocolate agar (C), and Columbia CNA bloodagar plates (D) after 24 h of incubation at 35°C. (E) Microscopy of the blood smear revealed ellipsoidal to elongated blastoconidia of L. elongisporus (Gram stain, ×1,000). (F) The patient's disease development process.
The patient's condition worsened after surgery. The white blood cell count of 14.89 × 109/L (normal 3.5–9.5) with 78.4% neutrophils, procalcitonin (3.57 ng/L; normal 0-0.1), and 1,3-β-D-glucan (393.46 pg/mL; normal 0–100) increased rapidly. Blood for fungal culture was still positive for L. elongisporus (Day 39), and the result of antifungal susceptibility testing was the same as before. Then, liposomal amphotericin B (5 mg/kg.d, intravenous drip) was administered for 2 weeks, and fluconazole (400 mg/d, per os) continued for 4 weeks. The patient's blood culture turned negative, and the symptoms and signs improved observably (Day 57). After discharge from the hospital (Day 73), the patient received fluconazole for sequential antifungal therapy for the duration of the study (Figure 1F).
We searched PubMed, Web of Science, Embase, and BIOSIS Library for original case reports and cohort studies published in English on Lodderomyces elongisporus (L. elongisporus) between January 1, 1985, and December 31, 2022. We used the keywords “Lodderomyces elongisporus” and “L. elongisporus”. The references of the retrieved articles were reviewed for additional relevant citations. The abstracts of all identified articles were viewed, and the full-text versions of relevant articles were retrieved for data extraction and analysis.
The inclusion criteria for articles and cases in the systematic literature review were as follows: (1) original case reports or cohort studies on L. elongisporus published in English between January 1, 1985, and December 31, 2022; and (2) articles describing definitive etiological evidence for L. elongisporus based on pathological and culture proof.
The clinical course of infection was divided into the following two categories: (1) survival (complete or partial improvement of clinical symptoms after antifungal treatment); and (2) death.
The following data were extracted: geographical distribution, demographics, pathogen proof, clinical signs and symptoms, involvement sites, diagnosis, treatment, outcomes and minimum inhibitory concentration (MIC) values for antifungal drugs for L. elongisporus. If a case was reported in more than one publication, the most recent article was used for data extraction.
The high-quality reads were assembled using the SPAdes software, version 3.15.2 (1), default pipeline. The genome assembly was evaluated by QUAST software, version 5.0.2. The completeness of genome assemblies was evaluated in BUSCO software, version 5.4.3 (2), against the database of fungi_odb10 (or saccharomycetes_odb10) (OrthoDB; https://www.orthodb.org). Genome annotations were performed using Funannotate software, version 1.8.13 (3). The sequencing data have been uploaded to NCBI (PRJNA954074).
L. elongisporus, a rare emerging pathogen that is an ascomycetous yeast, can cause fungemia often related to immunosuppression or intravenous devices (4). To date, 23 invasive L. elongisporus infection patients have been reported, including 22 with fungemia and 1 with meningitis (Table 1) (4–16). Notably, only one patient was a neonate, and the other 22 cases occurred in adult patients. In addition to bloodstream infections, L. elongisporus was also present in the central nervous system and intracranial vascular system in 7 patients. Only 3 patients (patients 1 and 11 in the systematic literature review and the present patient) had infective endocarditis (4, 14). All 3 patients presented with cardiac valve vegetations adherent to the prosthetic aortic valve, anterior aorta and mitral valve. Two patients were diagnosed with blood-positive fungal cultures and identified as having L. elongisporus by PCR amplification or PCR amplification, followed by DNA sequencing (PCR sequencing) of the internal transcribed spacer (ITS) region and/or the D1/D2 domains of ribosomal ®DNA or by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF). In our present patient, diagnosis with blood-positive fungal culture and identification of L. elongisporus was achieved through mNGS.
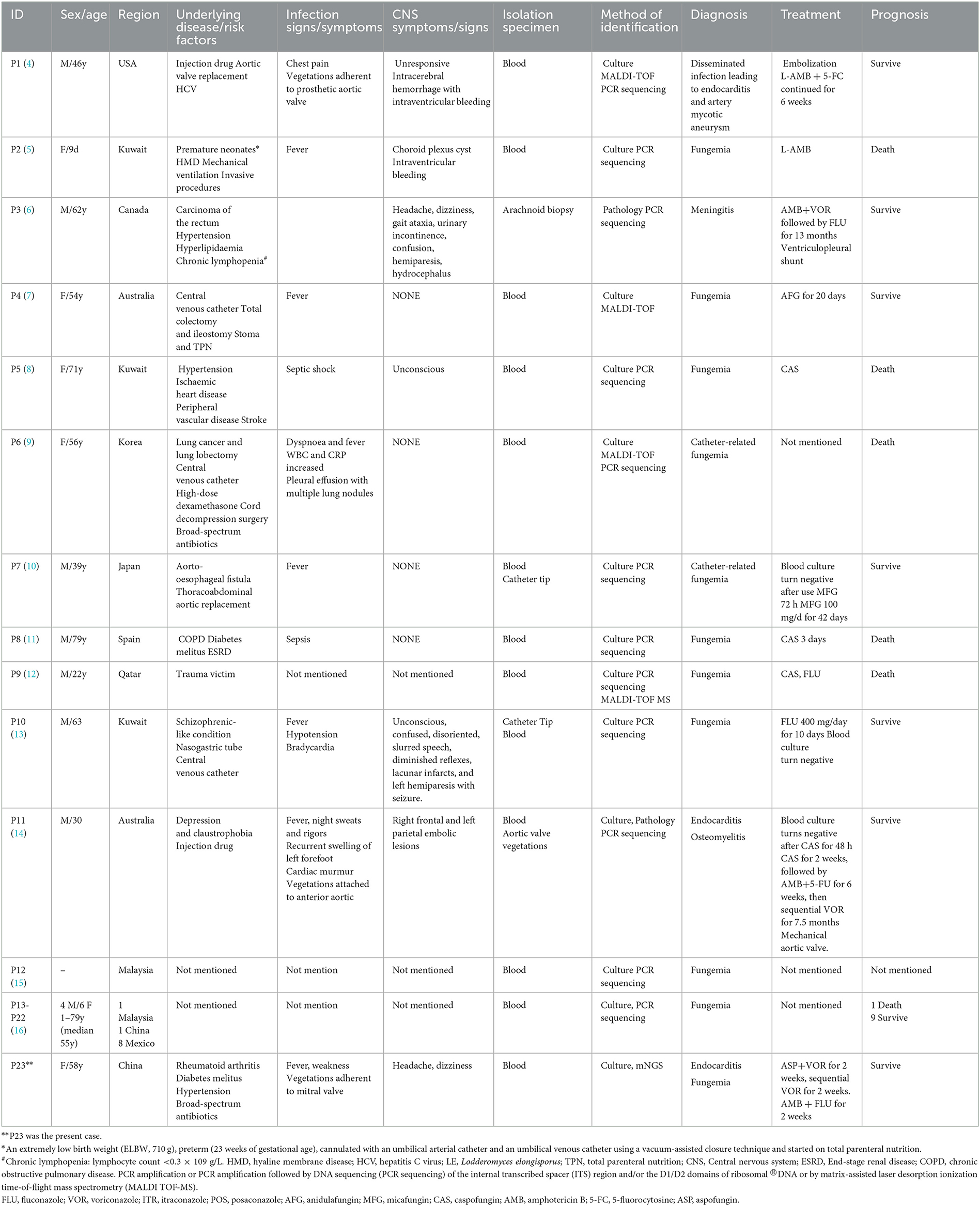
Table 1. Demographic and clinical characteristics of 23 cases of Lodderomyces elongisporus fungemia.
In this case report and review of the literature, only 2 patients were not clearly identified for the existence of underlying disease/risk factors (15, 16). The underlying disease/risk factors were as follows: surgery and trauma (6 patients), cardiovascular and cerebrovascular diseases (6 patients), central venous catheterization (3 patients), injection drugs (2 patients), cancer (2 patients), diabetes mellitus (2 patients), broad-spectrum antibiotics (2 patients), and mental illness (2 patients). Each case of these diseases included high-dose dexamethasone, nasogastric tube, stoma and total parenteral nutrition, mechanical ventilation, aorto-esophageal fistula, end-stage renal disease, rheumatoid arthritis, premature neonates, hyaline membrane disease, chronic lymphopenia, hepatitis C virus and chronic obstructive pulmonary disease. These results suggested that, in addition to the previously discussed immunosuppression and intravenous devices underlying diseases, surgery and trauma, cardiovascular and cerebrovascular diseases, cancer, diabetes mellitus and broad-spectrum antibiotics, vigilance against L. elongisporus infection is also needed.
There is currently a lack of consensus and guidelines for recognized treatment of L. elongisporus infection. The crude mortality rate in this study for invasive L. elongisporus infection was 27.27% (6/22). The minimum inhibitory concentration values for 23 strains of L. elongisporus indicated that they were sensitive to common antifungal drugs. It is worth noting that, when regular antifungal therapy for L. elongisporus endocarditis is not effective, surgical intervention should be considered. In addition, it is essential to incorporate prospective future projects or study subjects pertaining to the proper identification of L. elongisporus, as well as risk factors, surveillance, epidemiological investigations and standardized diagnosis and treatment.
In this study, we utilized the mNGS method for pathogen detection in a particular sample. The high-quality reads obtained were assembled using the default pipeline of SPAdes software, version 3.15.2 (1). We evaluated the genome assembly using QUAST software, version 5.0.2, and assessed the completeness of the genome assembly using BUSCO software, version 5.4.3 (2), against the fungi_odb10 (or saccharomycetes_odb10) database from OrthoDB (OrthoDB | E Zdobnov lab). We performed genome annotations using Funannotate software, version 1.8.13 (3).
To determine the closest match to the sample, we aligned the assembled scaffolds to the abvf (archaea, bacteria, viruses, fungi) database from the NT and ITS databases. In both cases, the results showed that the sample was closest to Lodderomyces elongisporus. To confirm our conclusion, we calculated the values of ANI (average nucleotide identity) and dDDH (digital DNA–DNA hybridization) among the species, which also indicated a close match to Lodderomyces elongisporus.
Furthermore, we used reads to map the abvf database directly with blastn (17) and Kraken (18)-Bracken (19). The classification results revealed that most of the reads were labeled Lodderomyces elongisporus, providing additional support for our conclusion.
We conducted a phylogenetic analysis to determine the relationships of the sample L. elongisporus in the Saccharomycetales order. The results provided important insights into the evolutionary relationships of L. elongisporus within the Saccharomycetales order and its position among other fungi. This information could be useful for understanding the genetic diversity and evolutionary history of fungal species, as well as for developing effective classification schemes and identifying potential targets for drug development.
The use of mNGS technology in clinical pathogen detection demonstrated high accuracy in detecting fungi. This technology allowed for a comprehensive and precise analysis of the sample's genome assembly, as well as identification of the closest match to Lodderomyces elongisporus. The findings suggested that mNGS has great potential as a valuable tool for clinical pathogen detection and research.
The results of our study suggested that mNGS could be a valuable tool for clinical pathogen detection and open new avenues for research in the field. Moreover, mNGS could provide important data on pathogen epidemiology and evolution for infectious disease surveillance. By analyzing large amounts of pathogen genomic data, a deeper understanding of the pathogen's evolutionary history, spread pattern, and interaction with the host can be achieved. This information is significant for developing effective prevention and control strategies and researching new vaccines.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The Ethics Review Board of the First Affiliated Hospital of Guangzhou Medical University (reference number 2022051) approved the study, which was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from the individual(s) and/or minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
YQ, ZL, and FY were in charge of writing and editing this manuscript. YM, JG, and DS were responsible for the etiological examination and antimicrobial susceptibility test. YS, ZW, and JH were responsible for the collection and follow-up of the case data. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China [grant numbers NSFC 82202544 and Science and Technology Program of Guangzhou (202201020537)].
First, we would like to thank the patients whose data were collected and the nurses and clinical staff who took care of these patients. In addition, we would like to thank the staff of the Respiratory Medicine Department of the Hospital, the clinical laboratory staff, and the Department of State Key Laboratory of Respiratory Disease staff for its valuable assistance. Furthermore, we would also like to thank the AJE team for English language editing. We would also like to thank the HUGO biotech team for next-generation sequencing data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1181377/full#supplementary-material
Supplementary Figure 1. Phylogenetic tree of Lodderomyces elongisporus (sample) in the Saccharomycetales order, using representative genome strains from the RefSeq database (ftp://ftp.ncbi.nlm.nih.gov/refseq/) and an outgroup species Trichoderma of ascomycetes. The tree was constructed based on single-copy genes, and the series of values over the branches corresponds to ML bootstrap values.
Supplementary Figure 2. Antimicrobial susceptibility testing of minimum inhibitory concentration values (μg/ml) for the L. elongisporus strain by Etest in a fungal susceptibility plate (Jiangmen Kailin Trading Co., Ltd.) in 35 °C for 48 hours.
1. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. (2012) 19:455–77. doi: 10.1089/cmb.2012.0021
2. Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. (2015) 31:3210–2.
3. Li WC, Wang TF. PacBio long-read sequencing, assembly, and funannotate reannotation of the complete genome of trichoderma reesei QM6a. Methods Mol Biol. (2021) 2234:311–329.
4. Thompson CM, Warner N, Hurt CB, Alby K, Miller MB. Closing the brief case: a case of prosthetic valve endocarditis due to Lodderomyces elongisporus. J Clin Microbiol. (2021) 59:e01227–20. doi: 10.1128/JCM.01227-20
5. Asadzadeh M, Al-Sweih N, Ahmad S, Khan S, Alfouzan W, Joseph L. Fatal Lodderomyces elongisporus fungemia in a premature, extremely low-birth-weight neonate. J Fungi. (2022) 8:906. doi: 10.3390/jof8090906
6. Dear T, Joe Yu Y, Pandey S, Fuller J, Devlin MK. The first described case of Lodderomyces elongisporus meningitis. J Assoc Med Microbiol Infect Dis Can. (2021) 6:221–8. doi: 10.3138/jammi-2021-0006
7. Koh B, Halliday C, Chan R. Concurrent bloodstream infection with Lodderomyces elongisporus and Candida parapsilosis. Med Mycol Case Rep. (2020) 28:23–5. doi: 10.1016/j.mmcr.2020.03.007
8. Al-Obaid K, Ahmad S, Joseph L, Khan Z. Lodderomyces elongisporus: a bloodstream pathogen of greater clinical significance. New Microbes New Infect. (2018) 26:20–4. doi: 10.1016/j.nmni.2018.07.004
9. Hatanaka S, Nakamura I, Fukushima S, Ohkusu K, Matsumoto T. Catheter-related bloodstream infection due to Lodderomyces elongisporus in a patient with lung cancer. Ann Lab Med. (2018) 38:182–4. doi: 10.3343/alm.2018.38.2.182
10. Hatanaka S, Nakamura I, Fukushima S, Ohkusu K, Matsumoto T. Catheter-related bloodstream infection due to Lodderomyces elongisporus. Jpn J Infect Dis. (2016) 69:520–2. doi: 10.7883/yoken.JJID.2015.307
11. Fernández-Ruiz M, Guinea J, Puig-Asensio M, Zaragoza Ó, Almirante B, Cuenca-Estrella M, et al. CANDIPOP Project; GEIH-GEMICOMED (SEIMC) and REIPI. Fungemia due to rare opportunistic yeasts: data from a population-based surveillance in Spain. Med Mycol. (2017) 55:125–36. doi: 10.1093/mmy/myw055
12. Taj-Aldeen SJ, AbdulWahab A, Kolecka A, Deshmukh A, Meis JF, Boekhout T. Uncommon opportunistic yeast bloodstream infections from Qatar. Med Mycol. (2014) 52:552–6. doi: 10.1093/mmycol/myu016
13. Ahmad S, Khan ZU, Johny M, Ashour NM, Al-Tourah WH, Joseph L, et al. Isolation of Lodderomyces elongisporus from the catheter tip of a fungemia patient in the middle east. Case Rep Med. (2013) 2013:560406. doi: 10.1155/2013/560406
14. Daveson KL, Woods ML. Lodderomyces elongisporus endocarditis in an intravenous drug user: a new entity in fungal endocarditis. J Med Microbiol. (2012) 61:1338–40. doi: 10.1099/jmm.0.047548-0
15. Minea B, Nastasa V, Moraru RF, Kolecka A, Flonta MM, Marincu I, et al. Species distribution and susceptibility profile to fluconazole, voriconazole and MXP-4509 of 551 clinical yeast isolates from a Romanian multi-centre study. Eur J Clin Microbiol Infect Dis. (2015) 34:367–83. doi: 10.1007/s10096-014-2240-6
16. Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J Clin Microbiol. (2008) 46:374–6. doi: 10.1128/JCM.01790-07
17. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. (1990) 215:403–10. doi: 10.1016/S0022-2836(05)80360-2
18. Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken. Genome Biol. (2019) 20:257. doi: 10.1186/s13059-019-1891-0
Keywords: Lodderomyces elongisporus, infective endocarditis, central nervous system infection, invasive fungal disease, fungemia
Citation: Qiu Y, Shi Y, Mai Y, Wu Z, Guan J, Huang J, Su D, Ye F and Li Z (2023) Subacute infective endocarditis due to Lodderomyces elongisporus: a case report and review of the literature. Front. Public Health 11:1181377. doi: 10.3389/fpubh.2023.1181377
Received: 07 March 2023; Accepted: 17 April 2023;
Published: 20 October 2023.
Edited by:
Zhimin Tao, Jiangsu University, ChinaReviewed by:
Mohammad Asadullah Asadzadeh, Kuwait University, KuwaitCopyright © 2023 Qiu, Shi, Mai, Wu, Guan, Huang, Su, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ye, dHUyNzYwMjVAZ2lyZC5jbg==; eWVmZW5nQGdpcmQuY24=; Zhengtu Li, dHUyNzYwMjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.