- 1Department of Family Health, Grawa Health Bureau, Grawa, Ethiopia
- 2School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 3School of Medicine, Obstetrics and Gynecology, Menelik Hospital, Addis Ababa, Ethiopia
- 4Resource Mobilization and Health Financing, East Hararghe Zonal Health Office, Harar, Ethiopia
- 5Revenue Authority, Dire Dawa City Administration, Dire Dawa, Ethiopia
- 6School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Background: Despite a dramatic decline in tuberculosis mortality over the past 10 years, tuberculosis is still the leading cause of death globally. In the last 2 years, tuberculosis has affected an estimated 10 million individuals, and 1.4 million people have died worldwide. In Ethiopia, the weight of the problem is less known in the study area. As a result, the purpose of this study was to assess food insecurity and associated factors among adult patients with tuberculosis attending public health facilities in Grawa district, Eastern Ethiopia.
Methods: A multicenter facility-based cross-sectional study was conducted from 01 March to 31 March 2022, among 488 randomly selected adult tuberculosis patients on treatment follow-up at public health facilities in Grawa district, Eastern Ethiopia. Data were collected using a pretested structured questionnaire through a face-to-face interview and document review, entered into EpiData version 3.1, and analyzed using SPSS version 25. The prevalence was reported using a 95% confidence interval (CI) and summary measures. Predictors were assessed using a multivariable logistic regression analysis model and reported using an adjusted odds ratio (AOR) with 95% CI. Statistical significance was declared at a p-value of <0.05.
Results: Overall, the prevalence of food insecurity among the study participants was 19.5%, with a 95% CI (15.8%, 23.2%). Factors such as being male [AOR = 0.58, 95% CI: (0.34, 0.97)], being married [AOR = 2.93, 95% CI: (1.33, 6.47)], being merchant [AOR = 0.22, 95% CI: (0.04, 0.67)], having low wealth quintiles [AOR = 2.10,95%CI:(1.04–4.23)], receiving anti-TB treatment for two or fewer months [AOR = 0.48, 95% CI: (0.26–0.91)], using khat [AOR = 2.18, 95% CI: (1.29, 3.70)], and owning livestock (AOR = 0.56, 95% CI: 0.29–0.94) were significantly associated with food insecurity.
Conclusions: According to this study, nearly one out of every five adults TB patients is food insecure. Factors such as being male, being married, being merchant, having low wealth quintiles, receiving anti-TB treatment for two or less months, those who chew mKhat and having a livestock were significantly associated with food insecurity. As a result, all stakeholders and concerned entities should prioritize improving the livelihood of TB patients through social security system programs, which are critical to the success of TB control and prevention efforts.
Introduction
Globally, 821 million people suffer from chronic food deprivation, and the situation has been worsening in most regions of the African continent (1). Insecurity is generally defined as a lack of regular access to enough safe and nutritious food for normal growth and development and an active and healthy life (1, 2). Food insecurity has a substantial impact on the health of populations. Meanwhile, TB continues to be a major cause of morbidity and mortality globally, and its association with access to food and nutrition has been acknowledged for a long time (3). Tuberculosis holds a prominent place in public health, in part because it is listed as among the top causes of premature death among the adult population (4). Tuberculosis is present in all countries and affects all age groups. However, it affects the world disproportionately, as the majority of TB cases and deaths occur in developing countries, where more than half of the cases are in economically productive age groups (5).
Despite a dramatic decline in tuberculosis mortality over the past 10 years, tuberculosis is still the leading cause of death globally. Malnutrition and tuberculosis are two examples of how food insecurity has an impact on mortality, treatment failure, and tuberculosis risk (6). In the last 2 years, tuberculosis has affected an estimated 10 million individuals who have contracted the disease, and 1.4 million people have died worldwide (7).
The majority of people in developing nations experience food insecurity and an unhealthy environment, which have an immediate impact on household food access, availability, and consumption (7). Inadequate food intake leads to poor nutritional status and impaired immune function. Food access, availability, and consumption in households are directly impacted by food insecurity and an unhealthy environment, which are prevalent among the majority of people in developing countries (8). Poor nutritional status and impaired immunity are affected by insufficient dietary consumption. It was detected that there is a vicious cycle between undernutrition and TB, according to which poor nutritional status increases the risk of tuberculosis (TB), which in turn can lead to undernutrition (9).
Almost half (1 million) of all TB patients worldwide are malnourished (10). The World Health Organization (WHO) recommends that all TB patients have a nutritional assessment and receive the proper nutritional therapy to reduce the incidence of undernutrition (11). A sufficient, wellbalanced diet is linked to better weight gain and quicker sputum conversion (12). Energy-dense supplements also increased lean body mass and athletic performance (13). Patients with co-infections displayed significant wasting due to these synergistic negative effects (14, 15). Moreover, behavioral factors are linked to undernutrition; for example, smoking increases the risk of undernutrition, which may be linked to decreased appetite and increased resting energy expenditure due to nicotine's effects on body metabolism (16).
On the other hand, undernutrition among TB patients was at risk for those with a low socioeconomic position, low educational status, being female, having a positive sputum smear, being unable to work functionally, and not receiving dietary counseling (17–19). Unexpectedly, TB is Ethiopia's third-leading cause of hospital fatalities and the eighth-leading cause of hospital admissions (20). Furthermore, a small number of studies revealed that a sizeable portion of TB patients are undernourished (21, 22). Poor anti-TB medication adherence has also been found to be a regular occurrence in patients who are malnourished, which may increase the chance of acquiring multi-drug resistance (MDR) type TB, one of the pressing public health issues currently facing Ethiopia and the world (11, 22).
A reduction in undernutrition in the general population could significantly lower the incidence of TB because it is a powerful predictor of active TB (23). Hence, enhancing TB patients' nutritional states is crucial to lowering the likelihood of comorbidities and accompanying mortality as well as adverse treatment outcomes. Moreover, studies demonstrating the scope and causes of undernutrition are crucial for improving early case identification and management, yet Ethiopian literature is scant. Even the already conducted studies have a more limited scope, which eventually reduces the generalizability of the conclusion (22, 24).
Ethiopia has a major share of the global incidence of TB and its related mortality and morbidity (25). With an estimated 219,186 new cases and 48,910 TB deaths, the country has been ranked among the top countries with a burden of tuberculosis (26). On the other hand, food insecurity emerges as a key problem and development challenge in Ethiopia, with the country being ranked among the top East African countries affected by hunger and undernourishment (21). Subsequently, the combined impacts of food insecurity and tuberculosis place further strain on already limited resources as affected individuals strive to meet the food demand.
Nowadays, any individual infected with TB in Ethiopia receives free treatment. As an integral part of TB care and control, the health sector should recognize and help address generalized malnutrition, food insecurity, and other socioeconomic determinants and consequences of TB (27). Understanding the level of food insecurity and its associated factors among adult TB patients is crucial in designing an appropriate intervention to address the bidirectional deteriorating effects. However, despite the high burden of TB and troubling food insecurity in Ethiopia, only a few studies have been conducted concerning food insecurity and associated risk factors among adult TB patients (24, 28). Moreover, these studies have been limited to other areas, with no consideration of eastern Ethiopia, which is overwhelmed by the recurrent impact of drought and pose the risk of food insecurity (11, 29, 30).
Despite the commitment of various stakeholders and the government, the prevalence and determinants of food security, as well as tuberculosis, which are thought to be the direct and underlying causes of undernutrition, continue to be a problem. Moreover, there are a few studies conducted in Ethiopia that pointed out that food insecurity among Tb patients is a major widespread problem (11, 21, 31). Furthermore, there is a paucity of documented evidence regarding the problems under study generally at the county level and particularly at the study area level, so the purpose of this study was to assess factors associated with food insecurity among adult tuberculosis patients attending public health facilities in Grawa district, Eastern Ethiopia.
Methods and materials
Study design, setting, and period
A multicenter facility-based cross-sectional study was conducted from 01 March to 31 March 2022, among 488 randomly selected adult tuberculosis patients on treatment follow-up at public health facilities in Grawa district, Eastern Ethiopia. Regarding the location, Grawa is located in Eastern Ethiopia, 580 km from Ethiopia's capital, Addis Ababa. According to projections from the 2007 national census, the district will have a total population of 349,543 (179,248 men and 170,295 women) by 2020. The district has 45 health posts, 9 health centers, and 1 general hospital. From those district health facilities, six health centers and one hospital provided tuberculosis laboratory services. We used the STROBE cross-sectional checklist when writing our report (32).
Eligibility criteria
All adult tuberculosis patients who had follow-up treatment in Grawa district public health facilities during the study period were considered the source population, whereas all adult TB patients who had follow-up treatment in randomly selected public health facilities and were available during the data collection period were regarded as the study population. The study excluded adult tuberculosis patients who were critically ill and unable to provide the necessary information during data collection.
Data collection methods
An interviewer-administered, pretested, structured questionnaire adapted from the Household Food Insecurity Access Scale (HFIAS) for the measurement of food access was developed by USAID (33) and related published literature that contextualized the study objectives (34). It contains socio-demographic and economic characteristics, household-related conditions, substance use-related behavior, healthcare services, and comorbidity-related characteristics. The data were collected by six BSc Nurse Health professionals who fluently speak Afaan Oromo (the local language). Supervision was conducted by the principal investigator and two public health experts familiar with the study setting. Data collectors and supervisors were trained for 2 days on ethics, tool sampling, and data collection procedures. The data collection process was supervised on a daily basis, and timely feedback was communicated to the data collectors. First, we translated it while keeping the purpose of the questionnaire and the intent of the questions in mind. It was conducted by group members who speak both languages fluently translate it. To ensure the translation's accuracy, the questionnaire was translated back into English by someone who had not seen the original version and was unfamiliar with the questionnaire's context. The back-translated version is then compared to the original, and any meaning differences are corrected. After that, to ensure a cross-validity, we tried to interview a set of respondents in English and another set in the local language such as Afaan Oromo and Amharic, and their answers were then compared to detect differences in understanding. Finally, pretesting was conducted to identify questions that are poorly understood, ambiguous, or elicit hostile or other undesirable responses. We attempted to conduct a pretest using the already-translated questionnaire. We tried to implement all the steps in pretesting such as obtaining an evaluation of a questionnaire and testing the revised questionnaire through its paces on friends, colleagues, and so on. Moreover, when choosing a tool, reliability and validity must be taken into account. The consistency with which an instrument produces the same results across multiple trials is referred to as its reliability. The degree to which an instrument measures what it was designed to measure is known as its validity. Statistically, we performed Cronbach's alpha, which is a measure used to assess the quality of our employed instruments. The result was 0.87, which was within acceptable ranges.
Variables and their measurement
Food security
A situation in which all people at all times have physical, social, and economic access to sufficient, safe, and nutritious foods that meet their dietary needs and food preferences for an active and healthy life (35).
Food insecurity
The lack of regular access to enough safe and nutritious food for normal growth and development and active and healthy life. This may be due to the unavailability of food and/or lack of resources to obtain food (36).
Food secure
May report worrying or being anxious about household food supply, but only rarely. Otherwise, the household does not experience any other conditions of inadequate food access.
Current substance use
Taking any substance such as alcohol, hashish, shisha, tobacco, and khat in the last 1 month prior to the study.
Ever substance use
Taking any substance such as alcohol, hashish, shisha, smoking cigarettes, and khat at least once in lifetime.
Bias
There were a number of biases involved while conducting this research, and the researchers took explicit measures to avoid them. One of the biases was social desirability, and to avoid it, the researchers paraphrase the questions in a way that was not socially desirable.
Sample size determination and sampling procedures
The sampling size was computed using the single population proportion formula, considering the following assumptions: 28.0% as the prevalence of undernutrition among adults with TB in southwest Ethiopia (28), a 95% level of confidence, and a 5% margin of error. A 10% non-response rate was also added to get a minimum sample size of 488.
To gather the study subjects, a multi-stage sampling technique was used. First, seven health centers from nine health centers in the Grawa district were selected by simple random sampling. Next, the total number of TB patients on follow-up treatment in each health institution was taken from the registration book of the patient to obtain a sampling frame (a list of eligible participants) before the actual data collection period. The probability-based proportional sampling technique was used to allocate the total sample size proportionally to each respective health institution. Then, each patient was given a unique code. Finally, study participants were selected using a simple random sampling technique (Figure 1).
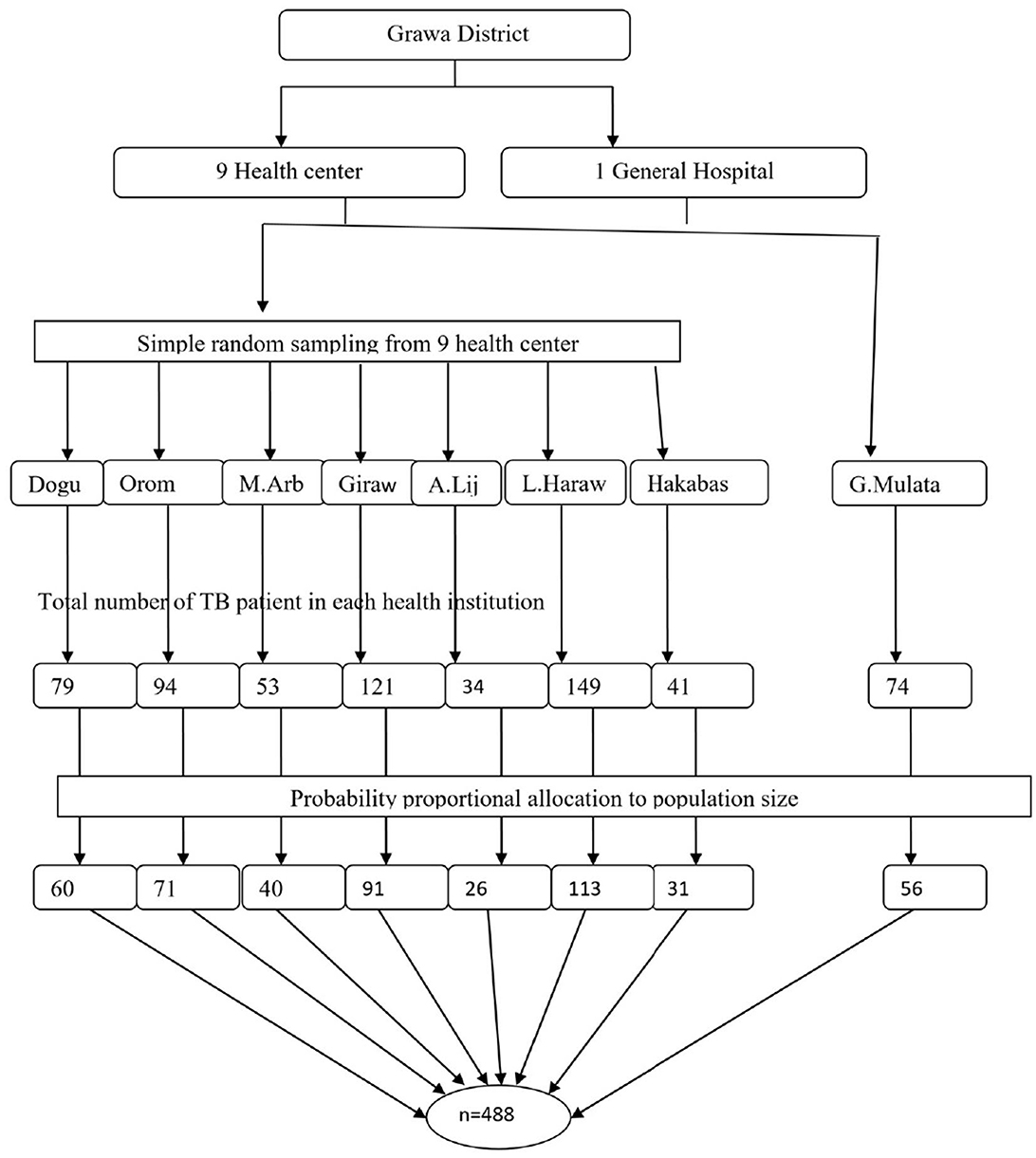
Figure 1. Schematic presentation of sample selection procedures of food insecurity among adult TB patients in Grawa District, Eastern Ethiopia.
Data quality control
The questionnaire was initially prepared in English and then translated into the local languages by a bilingual expert (Afaan Oromo). Then, it was translated back into an English version to ensure its consistency. The data collectors and supervisor received training on the data collection tool and procedures. Before the actual study data collection, a pretest was conducted among 5% of the study participants in similar settings. The investigators and experienced research supervisors provided regular supervision.
Data processing and analysis
First, the collected data were checked for completeness and consistency. Then, they were cleaned, coded, and entered into EpiData version 3.1 for further analysis. The entered data were exported to SPSS version 25 for analysis. Descriptive and summary statistics were conducted and reported using frequency tables and figures. A binary logistic regression model was fitted to check for an association between independent variables and the outcome variable. The model's fitness was checked by Hosmer–Lemeshow statistics and Omnibus tests. A multivariable analysis was performed to identify the true predictors of the outcome variables. A multi-collinearity test was carried out to check the presence of correlation between independent variables by using the standard error and co-linearity statistics, and no collinearity effects were detected. Thus, the value of the variance inflation factor (VIF) was 0.951. The direction and strength of the statistical association were measured by the odds ratio (OR) along with the 95% confidence interval (CI). A p-value of 0.05 was considered to be statistically significant in both bivariable and multivariable analyses.
Results
Socioeconomic and demographic characteristics
A total of 482 adult TB patients who were on treatment follow-up participated in the study, resulting in a response rate of 98.8%. The mean age of the study participants was 34.34 (SD ± 11.9) years, with 18 and 65 years being the minimum and maximum ages, respectively. Above half (53.5%) of the study participants were men. Most of the study participants 448 (92.9%) and 461 (95.6%) belonged to the Muslim religion and Oromo ethnicity, respectively. The majority, 411 (85.3%) of the participants, were rural dwellers. A total of 322 (62.2%) study participants had no formal education, and 311 (64.5%) of them were living with their spouses. Nearly a quarter, or 113 (23.4%), of the study participants were categorized as being in the high-wealth quintile (Table 1).
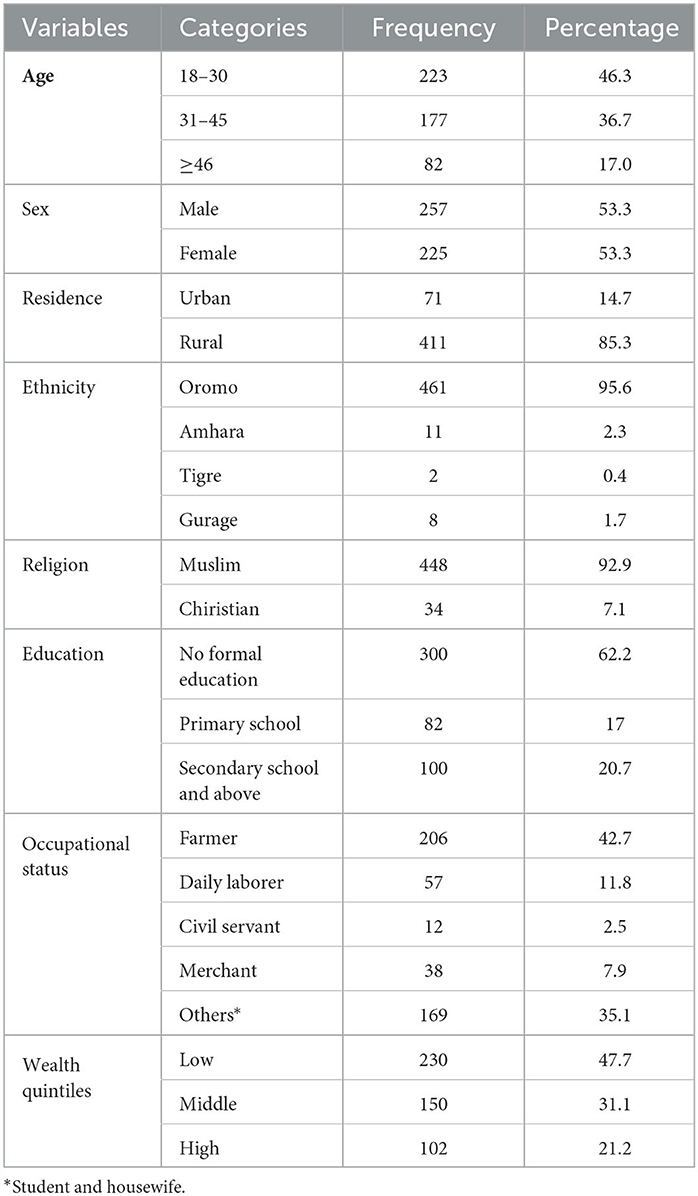
Table 1. Sociodemographic characteristics of adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022 (n = 482).
As shown in Figure 2, among 482 study participants, 311 (64.5%) were married, and 9 (1.9%) were widowed.
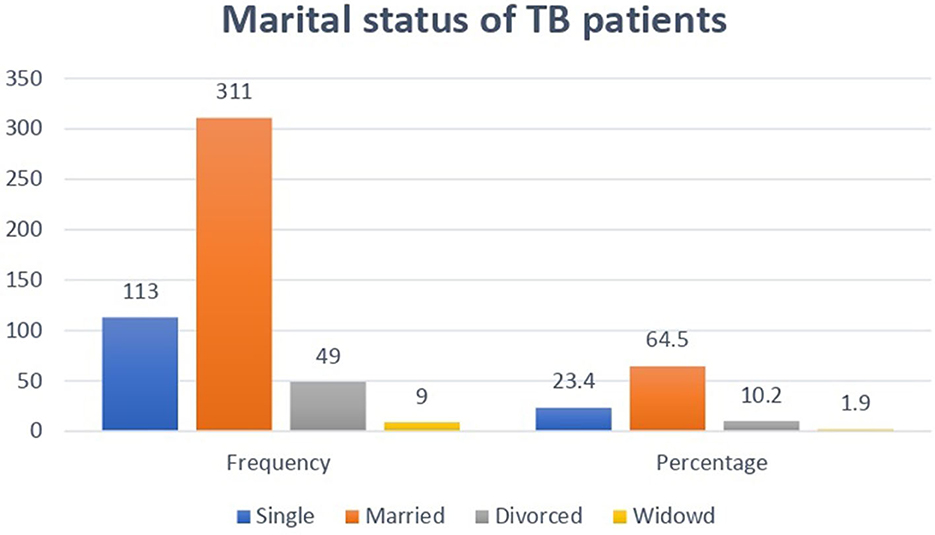
Figure 2. Distribution of marital status among adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022.
Household characteristics of the study participants
A total of 98 (20.3%) study subjects live alone in the household, whereas 308 (63.9%) study subjects live with four or more family members. The majority 322 (83.9%) of the households were monogamous. Nearly three-quarters 359 (74.5%) of the households had livestock, and 357 had farmland. Concerning food aid received from different organization, 22 (4.6%) households were beneficiaries of food support programs. Most of the 434 households (90%) have a latrine, and more than a quarter 134 (27.8%) of households have no window (Table 2).
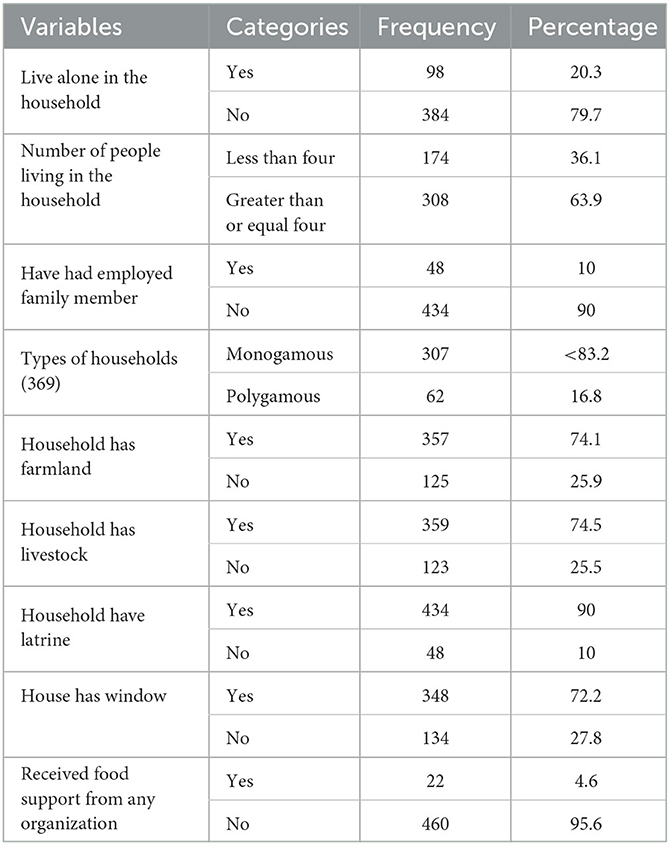
Table 2. Household characteristics of adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022 (n = 482).
Health history of adult TB patients
More than three-quarters of the study subjects 371 (76.9%) were diagnosed with and treated for pulmonary tuberculosis, while the remaining 111 (23.1%) were diagnosed and treated for extrapulmonary tuberculosis. At the time of diagnosis, more than half of the cases 256 (53.2%) had a +1-sputum smear grade. In terms of treatment duration, 358 (74.3%) of patients received anti-TB treatment for more than 2 months, and 35 (7.3%) reported skipping anti-TB medication since starting treatment. The vast majority of respondents, 405 (84.1%), had no diagnosed comorbidity. Concerning the impact of health education on treatment adherence, the majority 401 (83.2%) of study subjects reported receiving health education from health professionals since enrolling in the treatment (Table 3).
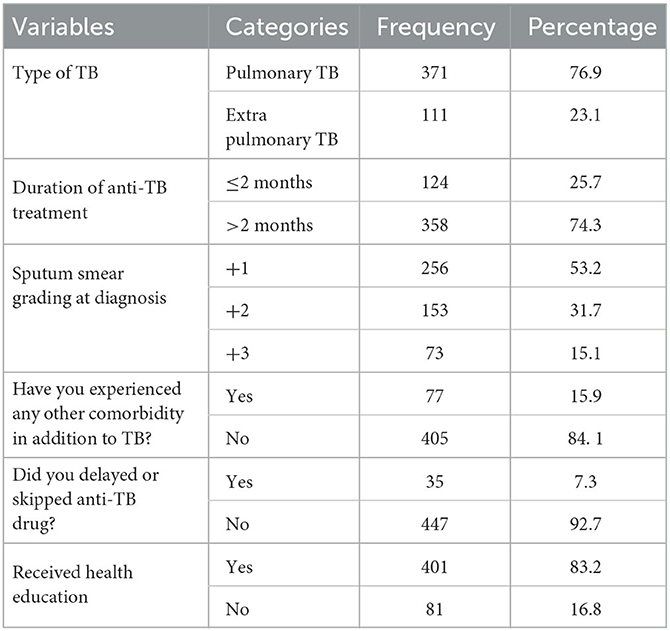
Table 3. Health history of adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022 (n = 482).
Diet and lifestyle conditions of the study participants
Almost a quarter 118 (24.5%) of study participants reported eating fruit the week before the interview, while 152 (31.5%) reported eating vegetables. More than half of the respondents, 262 (54.4%), reported using khat, and 152 (31.3%) were smoking at the time of the treatment follow-up (Table 4).
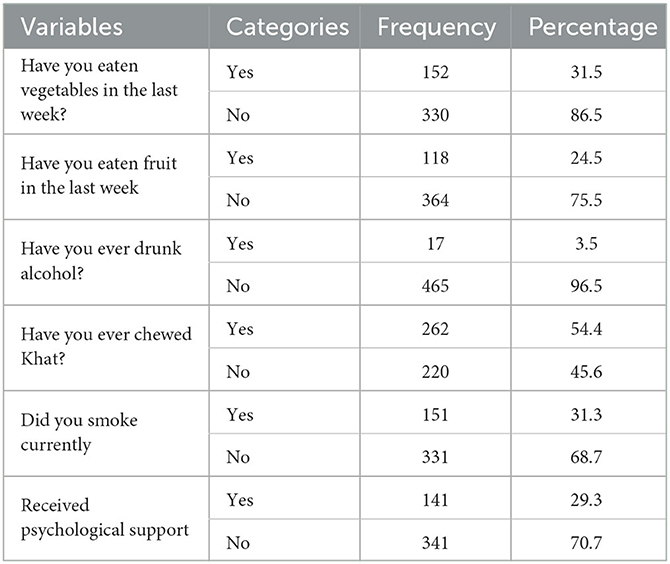
Table 4. Diet and lifestyle conditions of adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022 (n = 482).
Level of food insecurity
According to the household food insecurity access scale, 94 patients were food insecure, with an overall proportion of 19.5% (95% CI: 15.8 to 23.2%). A total of 21 (22.3%), 44 (46.8%), and 29 (30.9%) TB patients with food insecurity had mild, moderate, and severe food insecurity, respectively. Nearly 72 (41.9%) patients reported being concerned about not having enough food to eat in the household, and 38 (7.9%) patients reported that household members went to bed hungry (Figure 3).
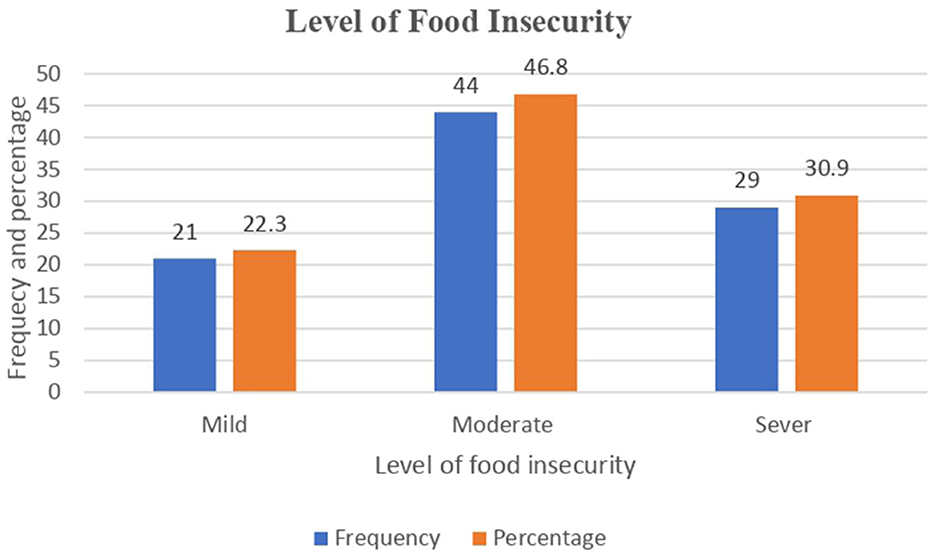
Figure 3. Level of food insecurity among adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022.
Factors associated with food insecurity among the study participants
In the bivariable analysis, predictor variables such as the sex of the patient, marital status, wealth quintiles, being an urban resident, the khat chewing condition, duration of anti-TB treatment, having farmland, and the presence of livestock in the households were significantly associated with food insecurity among TB patients. However, in the final model of multivariable logistic regression analysis, predictor variables such as being male, being married, being a merchant, having low wealth quintiles, the duration of anti-TB treatment, chewing khat, and having livestock were factors that remained significantly associated with food insecurity.
Accordingly, male counterparts were 42% less likely to experience food insecurity as compared to their female counterparts [AOR = 0.58, 95% CI: 0.34–0.97]. Similarly, the likelihood of food insecurity was nearly three times higher among married TB patients (AOR = 2.93, 95% CI: 1.33–6.47). Furthermore, merchants were 78% less likely to experience food insecurity than their counterparts [AOR = 0.22, 95% CI: 0.04–0.67]. Likewise, the odds of having food insecurity were 2.10 times higher among TB patients who were in the lowest wealth quintile [AOR = 2.10, 95% CI: 1.04–4.23]. Furthermore, when compared to those who received anti-TB treatment for more than 2 months, those who received treatment for 2 months or less were 52% less likely to be food insecure [AOR = 0.48, 95% CI: 0.26, 0.91]. TB patients who chewed khat, on the other hand, were 2.18 times more likely to be food insecure than those who did not [AOR = 2.18, 95% CI: 1.29, 3.70]. Furthermore, TB patients with livestock in their households were 44% less likely to be food insecure than those without livestock [AOR = 0.56, 95% CI: 0.29, 0.94] (Table 5).
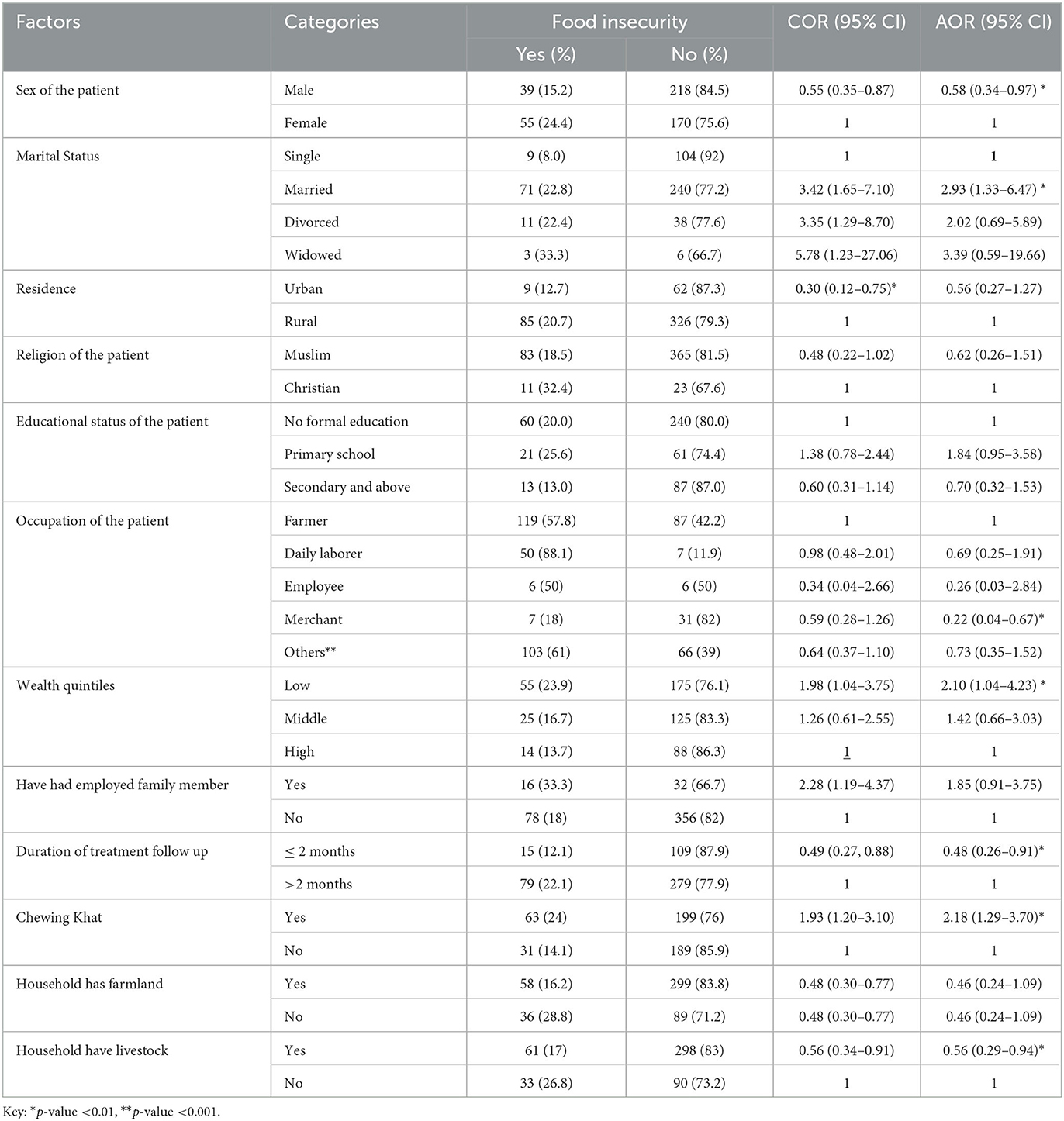
Table 5. Bi-variable and multivariable logistic regression analysis of factors associated with adult TB patients undergoing treatment in public health facilities of Grawa District, Eastern Ethiopia, 2022 (n = 482).
Discussion
This study pointed out that 19.5% of the 95% CI (15.8%, 23.2%) study participants were found to be food insecure, of whom 22.3%, 46.8%, and 30.9% were mildly, moderately, and severely food insecure, respectively. Factors such as being male, being married, being a merchant, low wealth quintile, those who chew khat, duration of the anti-TB treatment, and presence of livestock in the households were identified as predictors of food insecurity.
In this study, nearly 19.5% of tuberculosis patients were food insecure. The findings from this study are in harmony with those of studies conducted in Vietnam (22%) (37) and South Africa (21%) (38). The similarities could be due to the fact that the two studies use a similar strategy for laboratory techniques and have a similar social structure. However, the current level of food insecurity is much higher than in previous studies conducted in different settings, like Sri Lanka (6%) (34). The discrepancy might be due to the variation in socioeconomic status and extended social security programs such as food safety nets among the study areas. In addition, seasonal variability when the studies were conducted could explain the observed difference among the findings. On the contrary, this finding was relatively lower than studies conducted elsewhere, such as in Indonesia (64%) (39), southwest Ethiopia (949.3%) (28), and south India (34.1%) (39). A possible justification could be the difference in the socioeconomic status among the study participants.
This study pointed out that being male was the strongest predictor of food insecurity among TB patients. Thus, male counterparts were 42% less likely to experience food security. This finding is supported by studies conducted in different settings, such as South Africa (38), the Somali Region (31), Adama (40), and low-income countries (41). The possible justification could be attributed to the fact that men have greater access to social capital and pathways out of crisis; for instance, their income pays off previous debts and secures new farm loans, whereas women frequently face severe time constraints due to their household food-security roles. Furthermore, gender roles may be a plausible explanation for this association. The majority of rural women in developing countries face financial and land-control constraints (41, 42). This may limit their ability to purchase high-demand food and reduce production, worsening food insecurity among them.
In the final model of multivariable analysis, marital status was found to be associated with food insecurity. Thus, those study participants who married were 2.93 times more likely to experience food insecurity as compared to their counterparts. Similar findings were reported from the studies conducted in Sri Lanka (34). The possible justification could be that a married couple has children who share a few resources, which absolutely plays a pivotal role in establishing and creating food insecurity in that specific household.
The current study found that low wealth quintile were another factor that was independently associated with food insecurity. Patients with tuberculosis in the lowest wealth quintile were more likely to be food insecure than those in the highest wealth quintile. This finding is in harmony with the study done in the Kembata Tembaro zone, southern Ethiopia (43), and south India (39), which found that TB patients with a low monthly income had twice the odds of experiencing household food insecurity as their high-income counterparts. This could be due to the fact that a low income limits an individual's purchasing power for food, potentially exacerbating food insecurity. Furthermore, catastrophic health expenditure as a result of tuberculosis diagnosis and treatment can exacerbate food insecurity in low-income groups throughout the disease's course (44). Similarly, a significant association was found between food insecurity and occupation in the current study, with merchants having lower odds of experiencing food insecurity than farmers. Thus, merchants were 78% less likely to be food insecure than their counterparts. This is in line with the findings of previous studies conducted in Nigeria and Ethiopia (45, 46). This could be due to merchants' purchase and access to power. Furthermore, they have a higher income than the others, allowing them to live a normal life free of food insecurity.
Furthermore, khat consumption was found to be significantly associated with food insecurity in this study. Patients who reported chewing khat had a higher risk of food insecurity than non-chewers. Several previous studies have found that food insecurity is significantly associated with substance use habits, which is consistent with the current findings (47–50). The current study's findings of increased food insecurity among khat chewers may be due to the negative effects of khat consumption on the household economy. Khat is a major cash crop and source of income for millions of households in eastern Ethiopia (51). It is frequently blamed for worsening food insecurity by diverting money to buy khat, complicating the chewer work culture (52), and displacing food crops (53). The current study's finding of an increased risk of food insecurity among khat chewers among TB patients may help researchers better understand how khat chewing affects the healthcare continuum in the study settings.
The current study also revealed that the duration of anti-TB treatment was significantly associated with food insecurity. Patients with TB who received anti-TB treatment for 2 months or less had lower odds of experiencing food insecurity compared with those who received anti-TB treatment for more than months. In contrast to the current finding, the study conducted in Burkina Faso reported no significant association between the duration of anti-TB treatment and undernutrition (54). A possible explanation for the association in the present findings could be from the perspective of the “health shocks notion,” where being on treatment for a longer duration resulted in extra expenditure incurred to receive healthcare and a loss in working days, which in turn adversely affected household earnings and food security (55, 56).
Finally, this study showed that study participants who had livestock were 44% less likely to experience food insecurity as compared to their counterparts. This is supported by the studies conducted in Ghana (57). This is because livestock is critical to food systems facing these emerging global challenges. Smallholders rely on livestock for income and labor-saving, productive assets. Livestock also helps with nutrition because animal-based foods are essential, especially in reducing child stunting in developing countries (58).
Implications and limitations of study
This study plays a pivotal role in clearly showing the prevalence of food security among immune-compromised individuals such as TB patients. It also points out the factors that influence the problems under study. In this study, due to the nature of the study design, it would be impossible to determine the causal relationship between the variable and the outcome in the analysis. Moreover, the study failed to consider seasonal variability in effect which could bias the level of food insecurity.
Conclusion
According to this study, nearly one out of every five adults TB patients is food insecure. Factors such as being male, being married, being merchant, having low wealth quintiles, receiving anti-TB treatment for two or less months, those who chew mKhat and having a livestock were significantly associated with food insecurity. As a result, all stakeholders and concerned entities should prioritize improving the livelihood of TB patients through social security system programs, which are critical to the success of TB control and prevention efforts.
Data availability statement
The datasets used for this study are available from the corresponding authors upon reasonable request.
Ethics statement
Ethical approval was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of Haramaya University, College of Health and Medical Sciences. Support letters from the College of Health and Medical Sciences were submitted to the selected health facilities where the study was conducted. After getting all permission letters from responsible bodies, informed, voluntary, written and signed consent was obtained from the study participants.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
Haramaya University provided financial support for this study. However, the funding agency had no role in the collection, analysis, and interpretation of the data, as well as the writing up of the manuscript.
Acknowledgments
The authors are very thankful to Haramaya University, the heads and staff of the College of Health and Medical Sciences, the study participants, data collectors, and supervisors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. WHO, WHO policy on collaborative. TB/HIV activities: guidelines for national programmes and other stakeholders. (2012).
3. Podewils LJ, Holtz T, Riekstina V, Skripconoka V, Zarovska E, Kirvelaite G, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. (2011) 139:113–20. doi: 10.1017/S0950268810000907
4. Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. (2008) 8:233–43. doi: 10.1016/S1473-3099(07)70291-8
5. Okhovat-Isfahani B, Bitaraf S, Mansournia MA, Doosti-Irani A. Inequality in the global incidence and prevalence of tuberculosis (TB) and TB/HIV according to the human development index. Med J Islam Repub Iran. (2019) 33:45. doi: 10.47176/mjiri.33.45
6. Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. (2019) 19:182. doi: 10.1186/s12890-019-0953-0
7. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global tuberculosis report—reflections on the global tb burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113 Suppl 1:S7–S12. doi: 10.1016/j.ijid.2021.02.107
8. FAO I, UNICEF, WFP, WHO. The State of Food Security and Nutrition in the World (SOFI). Building climate resilience for food security and nutrition (2018).
9. Zachariah R, Spielmann MP, Harries AD, Salaniponi FML. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. (2002) 96:291–4. doi: 10.1016/S0035-9203(02)90103-3
10. WHO. Guideline : Nutritional care and support for patients with tuberculosis. World Heal Organ [Internet]. (2013). Available online at: http://apps.who.int/iris/bitstream/10665/94836/1/9789241506410_eng.pdf (accessed April 23, 2023).
11. Gemechu F, Zemedu L, Yousuf J. Determinants of farm household food security in Hawi Gudina district, West Hararghe zone, Oromia Regional National State, Ethiopia. J Agricult Exten Rural Develop Bull World Health Organ. (2016) 8:12–8. doi: 10.5897/JAERD2014.0660
12. Paton NI, Chua Y-K, Earnest A, Chee CBE. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. Am J Clin Nutr. (2004) 80:460–5. doi: 10.1093/ajcn/80.2.460
13. Gebremichael E, Seyoum WM, Ishimwe B, Sataer G. Lake surface area expansion: insights into the role of volcano-tectonic processes, Lake Beseka, East Africa. J Hydrol Reg Stud. (2022) 41:101093. doi: 10.1016/j.ejrh.2022.101093
14. Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010-50: cure, care, social development. Lancet. (2010) 375:1814–29. doi: 10.1016/S0140-6736(10)60483-7
15. Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol. (2012) 176:409–22. doi: 10.1093/aje/kws007
16. Collins LC, Walker J, Stamford BA. Smoking multiple high- versus low-nicotine cigarettes: impact on resting energy expenditure. Metabolism. (1996) 45:923–6. doi: 10.1016/S0026-0495(96)90256-5
17. Indupalli AS, Sirwar SB, Shaikh B. Nutritional status of tuberculosis cases registered under tuberculosis unit of Gulbarga city north Karnataka, India. Jama. (2013) 2:616–9. doi: 10.21746/IJBIO.2013.03.0027
18. Guadie FF. AB assessment of nutritional status and associated factors among adult tb patients on directly observed treatment of short course in health facilities at Adama Town, East Shewa Zone, Ethiopia. Sch Pr Pr J. (2016) 1:23. Available online at: http://www.scholarpractitionerjournals.com/article/assessment-nutritional-status-and-associated-factors-among-adult-tb-patients-directly
19. Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. (2009) 2:122–6.
20. Seyoum B, Demissie M, Worku A, Bekele S, Aseffa A. Prevalence and drug resistance patterns of mycobacterium tuberculosis among new smear positive pulmonary tuberculosis patients in Eastern Ethiopia. Tuberc Res Treat. (2014) 2014:753492. doi: 10.1155/2014/753492
21. Endalkachew K, Ferede YM, Derso T, Kebede A. Prevalence and associated factors of undernutrition among adult TB patients attending Amhara National Regional State hospitals, Northwest Ethiopia. J Clin Tuberc Other Mycobact Dis. (2022) 26:100291. doi: 10.1016/j.jctube.2021.100291
22. World Health O. The World health report: 2000: health systems: improving performance. Geneva: World Health Organization (2000).
24. Dargie B, Tesfaye G, Worku A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: a cross-sectional study. BMC Nutrition. (2016) 2:7. doi: 10.1186/s40795-016-0046-x
25. WHO. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. (2012).
26. Deribew A, Deribe K, Dejene T, Tessema GA, Melaku YA, Lakew Y, et al. Tuberculosis Burden in Ethiopia from 1990 to 2016: evidence from the Global Burden of Diseases. Study Ethiop J Health Sci. (2018) 28:519–28. doi: 10.4314/ejhs.v28i5.2
27. Trivedi N, Abhijit G, Singh B, Anuj A, Sachin S, Anuj M, et al. Nutritional care and support for patients with tuberculosis: A review on guidelines issued by WHO. Pharma Res. (2013) 10:54–74. Available online at: https://apps.who.int/iris/handle/10665/94836
28. Soboka M, Tesfaye M, Adorjan K, Krahl W, Tesfaye E, Yitayih Y, et al. Effect of food insecurity on mental health of patients with tuberculosis in Southwest Ethiopia: a prospective cohort study. BMJ Open. (2021) 11:e045434. doi: 10.1136/bmjopen-2020-045434
29. Mulugeta M, Tiruneh G, Alemu ZA. Magnitude and associated factors of household food insecurity in Fedis Woreda East Hararghe zone, Oromia region, Ethiopia. Agricult Food Secur J Agricult Exten Rur Develop. (2018) 7:1–8. doi: 10.1186/s40066-017-0140-6
30. Tesfaye W, Seifu L. Climate change perception and choice of adaptation strategies. Int J Clim Change Strateg Manage. (2016) 8:253–70. doi: 10.1108/IJCCSM-01-2014-0017
31. Muse AI, Osman MO, Ibrahim AM, Wedajo GT, Daud FI, Abate KH. Undernutrition and associated factors among adult tuberculosis patients in Jigjiga Public Health Facilities, Somali Region, East, Ethiopia. Res Rep Trop Med. (2021) 12:123–33. doi: 10.2147/RRTM.S311476
32. von Elm E AD, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies (2019).
33. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide: version 3. (2007).
34. Jayasuriya NANL, Derore INK. Food Security and Nutrition Among the Tuberculosis Infected Patients. A case study among patients at the chest clinic in Sri Lanka. (2015).
35. CLAY. Food security:concept and measurement. Paper for FAO Expert Consultation on Trade and Food security. Conceptualizing the Linkage, Rome July, 2002. (2002).
37. Nhung NT, Van NTB, Van Cuong N, Duong TTQ, Nhat TT, Hang TTT, et al. Antimicrobial residues and resistance against critically important antimicrobials in non-typhoidal Salmonella from meat sold at wet markets and supermarkets in Vietnam. Int J Food Microbiol. (2018) 266:301–9. doi: 10.1016/j.ijfoodmicro.2017.12.015
38. Tomita A, Ramlall S, Naidu T, Mthembu SS, Padayatchi N, Burns JK. Major depression and household food insecurity among individuals with multidrug-resistant tuberculosis (MDR-TB) in South Africa. Soc Psychiatry Psychiatr Epidemiol. (2019) 54:387–93. doi: 10.1007/s00127-019-01669-y
39. Ayiraveetil R, Sarkar S, Chinnakali P, Jeyashree K, Vijayageetha M, Thekkur P, et al. Household food insecurity among patients with pulmonary tuberculosis and its associated factors in South India: a cross-sectional analysis. BMJ Open. (2020) 10:e033798. doi: 10.1136/bmjopen-2019-033798
40. Guadie FF, Assaminew PPJ. Assessment of nutritional status and associated factors among adult TB patients on directly observed treatment of short course in health facilities at Adama Town, East Shewa Zone, Ethiopia. (2016).
41. Botreau HC. Gender Inequalities and Food Insecurity: Ten years after the food price crisis, why are women farmers still food-insecure?. RelReliefWeb. (2019) 9:1. doi: 10.1016/bs.af2s.2020.09.001
42. Negesse A, Jara D, Temesgen H, Dessie G, Getaneh T, Mulugeta H, et al. The impact of being of the female gender for household head on the prevalence of food insecurity in Ethiopia: a systematic-review and meta-analysis. Public Health Rev. (2020) 41:15. doi: 10.1186/s40985-020-00131-8
43. Yohannes G, Wolka E, Bati T, Yohannes T. Household food insecurity and coping strategies among rural households in Kedida Gamela District, Kembata-Tembaro zone, Southern Ethiopia: mixed-methods concurrent triangulation design. BMC Nutrition. (2023) 9:4. doi: 10.1186/s40795-022-00663-z
44. Division CT. Guidance document: nutritional care and support for patients with tuberculosis in India. Ministry of Health and Family Welfare New Delhi, India (2017).
45. Sholeye OO, Animasahun VJ, Salako AA, Oyewole BK. Household food insecurity among people living with HIV in Sagamu, Nigeria: a preliminary study. Nutr Health. (2017) 23:95–102. doi: 10.1177/0260106017704798
46. Tadesse Tantu A, Demissie Gamebo T, Kuma Sheno B, Yohannis Kabalo M. Household food insecurity and associated factors among households in Wolaita Sodo town 2015. Agriculture & Food Security. (2017) 6:19. doi: 10.1186/s40066-017-0098-4
47. Sreeramareddy CT, Ramakrishnareddy N, Rahman M, Mir IA. Prevalence of tobacco use and perceptions of student health professionals about cessation training: results from Global Health Professions Students Survey. BMJ Open. (2018) 8:e017477. doi: 10.1136/bmjopen-2017-017477
48. Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lönnroth K, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). a systematic review. BMC Public Health. (2009) 9:450. doi: 10.1186/1471-2458-9-450
49. Strike C, Rudzinski K, Patterson J, Millson M. Frequent food insecurity among injection drug users: correlates and concerns. BMC Public Health. (2012) 12:1058. doi: 10.1186/1471-2458-12-1058
50. Sreeramareddy CT, Ramakrishnareddy N. Association of adult tobacco use with household food access insecurity: results from Nepal demographic and health survey 2011. BMC Public Health. (2017) 18:48. doi: 10.1186/s12889-017-4579-y
51. Hailu T. The conceptualization of khat, its production and its socio-economic implications in Eastern Ethiopia. Int J Educ Cult Soc. (2019) 4:54–9. doi: 10.11648/j.ijecs.20190403.12
52. Gudata ZG, Cochrane L, Imana G. An assessment of khat consumption habit and its linkage to household economies and work culture: the case of Harar city. PLoS One. (2019) 14:e0224606. doi: 10.1371/journal.pone.0224606
53. Ademe BW, Coates J, Dalsgaard A, Brimer L, Lema TB. Khat production and consumption; its implication on land area used for crop production and crop variety production among rural. Household of Ethiopia. (2017) 5:148–54. doi: 10.12691/jfs-5-4-5
54. Musuenge BB, Poda GG, Chen PC. Nutritional status of patients with tuberculosis and associated factors in the health centre region of burkina Faso. Nutrients. (2020) 12:9. doi: 10.3390/nu12092540
55. Alam K, Mahal A. Economic impacts of health shocks on households in low and middle income countries: a review of the literature. Global Health. (2014) 10:21. doi: 10.1186/1744-8603-10-21
56. Nhung NV, Hoa NB, Anh NT, Anh LTN, Siroka A, Lönnroth K, et al. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int J Tuberc Lung Dis. (2018) 22:983–90. doi: 10.5588/ijtld.17.0859
57. Nuvey FS, Nortey PA, Addo KK, Addo-Lartey A, Kreppel K, Houngbedji CA, et al. Farm-related determinants of food insecurity among livestock dependent households in two agrarian districts with varying rainfall patterns in Ghana. Jama. (2022) 6:23. doi: 10.3389/fsufs.2022.743600
Keywords: prevalence, food insecurity, tuberculosis, associated factors, Ethiopia
Citation: Abdulla JH, Gebremichael B, Maruta MB, Yuye I, Mohammed A, Debella A and Mussa I (2023) Nearly one out of every five adult TB patients suffered from food insecurity in Grawa District, Eastern Ethiopia: a multicenter facility-based cross-sectional study. Front. Public Health 11:1177618. doi: 10.3389/fpubh.2023.1177618
Received: 01 March 2023; Accepted: 11 May 2023;
Published: 09 June 2023.
Edited by:
Carla Sofia e Sá Farinha, New University of Lisbon, PortugalReviewed by:
Mojtaba Vaismoradi, Nord University, NorwaySeok Shin Tan, Monash University Malaysia, Malaysia
Shailaja S. Patil, BLDE University, India
Copyright © 2023 Abdulla, Gebremichael, Maruta, Yuye, Mohammed, Debella and Mussa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibsa Mussa, aWJzYW11c3NhOTNAZ21haWwuY29t
†ORCID: Ibsa Mussa orcid.org/0000-0002-6318-4092
 Jabir H. Abdulla1
Jabir H. Abdulla1 Berhe Gebremichael
Berhe Gebremichael Adera Debella
Adera Debella Ibsa Mussa
Ibsa Mussa