
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 13 July 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1173482
This article is part of the Research Topic Knowledge and Behavioral Beliefs Related to Vaccination Hesitancy Among Healthcare Workers View all 11 articles
 Michelangelo Mercogliano1
Michelangelo Mercogliano1 Claudio Fiorilla1
Claudio Fiorilla1 Federica Esposito1
Federica Esposito1 Michele Sorrentino1
Michele Sorrentino1 Pasquale Domenico Mirizzi1
Pasquale Domenico Mirizzi1 Antonio Parisi1
Antonio Parisi1 Andrea Tajani1
Andrea Tajani1 Gaetano Buonocore2
Gaetano Buonocore2 Maria Triassi1,3
Maria Triassi1,3 Raffaele Palladino1,3,4*
Raffaele Palladino1,3,4*Introduction: In Europe, there is still suboptimal tetanus, diphtheria, and acellular pertussis (Tdap) booster coverage. This study aimed to assess coverage status, knowledge, and attitude on Tdap vaccination in healthcare workers (HcWs) of the University Hospital “Federico II” in Naples, Southern Italy, in 2022, to improve current vaccination strategies.
Methods: A cross-sectional study was conducted using a validated anonymous questionnaire. Knowledge and attitude were measured as scores. Multivariable logistic and linear regression models were employed to identify correlates of Tdap booster and knowledge and attitude toward the vaccination, as appropriate. Models were controlled for age, sex, profession, department, and job seniority.
Results: A total of 206 questionnaires were administered among HcWs, and 143 (69.4%) were medical doctors. In total, 71 (34.47%) HcWs received the Tdap booster. Those who have worked 5–9 years at the hospital had a 78% lower likelihood of being vaccinated with the Tdap booster (5–9 years—OR: 0.22, CI: 0.06 | 0.85) as compared with newly hired HcWs. No differences in the average knowledge score were found. Other healthcare workers had a lower attitude as compared to medical doctors (Other—Coef. −2.15; CI: −4.14 | −0.15) and, as compared with those who worked in a clinical department, those who worked in a diagnostic–therapeutic department or medical management had 3.1 and 2.0 lower attitude scores, on average, respectively (diagnostic–therapeutic—Coef. −3.12, CI: −5.13 | −1.12; public health—Coef. −1.98, CI: −3.41 | −0.56).
Discussion: The study findings support the necessity to implement public health strategies and improve knowledge and attitude toward vaccinations and specifically highlight the importance of Tdap booster every 10 years as a prevention tool to protect high-risk populations.
The burden of vaccine-preventable diseases is still a global concern. In the decade 2010–2019, epidemic outbreaks of pertussis have been reported in several countries worldwide (1), although this figure is in contrast with what has been observed in the past 3 years. For instance, in 2021, pertussis cases almost halved compared with previous years (2). In Europe, the cases reported in 2021 were 2,157 compared with more than 12,000 in 2020 (3). However, the main factor responsible for the observed reduced incidence in this period is likely to be the implementation of non-pharmaceutical interventions (NPI) to reduce the impact of the COVID-19 pandemic on Health Systems (e.g., the use of filtered masks, continuous hand hygiene, and contact ban) rather than specific preventive strategies for pertussis, such as tetanus, diphtheria, and acellular pertussis (Tdap) vaccination (4, 5).
Data provided by the World Health Organization show that globally the coverage of vaccination against pertussis among 1-year-old children has decreased from 2019 to 2021 by 5% (from 86 to 81%), with an estimated loss of approximately 25 million pediatric vaccinations (6). In Europe, although the reduction was more contained, a drop between 1 and 5% in the 0–24 months and 0–6 years of vaccination coverage between 2018 and 2021 has been documented (7). This reduction in vaccination coverage is worrisome and might considerably impact population health in the upcoming years of transition from pandemic to endemic. Despite a strong initial reduction in the incidence of respiratory infectious diseases, the implementation of NPIs has only a transient effect, with a backlash effect when lifted (8).
In Italy, according to the national vaccination plan, the primary cycle and the booster doses are provided free of charge (9), the official 2021 National Health System data reported that the average coverage for Tdap vaccinations in the 0–24 months population was 94 and 72–73% for the 0–18 years booster coverage, below the WHO threshold and with profound inter-regional differences (10).
The perdurance of vaccine protection is not established, hence, booster dose coverage is pivotal. Numerous studies evidence decreasing levels of anti-pertussis immunoglobulin G over time from vaccination, suggesting that immunity wanes in the years following the last dose of Tdap (11).
In Italy, tetanus, diphtheria, and acellular pertussis booster doses are recommended for adolescents and then every 10 years in adults to reduce the transmission and to protect the community, especially since Italy, in 2018, accounted for 39.1% of all notified cases of Tetanus in EU/EEA countries (11–14). Furthermore, in Italy, cases of pertussis have increased from 503 to 962 during 2015–18 (15), with a strong likelihood to be underreported (16). The implementation of the active offer to professional categories at risk is particularly important, given the high contagiousness of infectious diseases, such as pertussis to newborns, who have not yet been vaccinated (17).
Although healthcare workers (HcWs) are a target group to achieve high vaccination coverage (18), they usually show a low awareness of work-related risks (19) and can be a source of infection for susceptible patients and relatives, as well as other HcWs (20–23).
Despite the importance of reaching immunization targets for HcWs, there is a paucity of evidence related to the topic. A systematic review conducted in 2019 found only 28 studies that examined Tdap coverage on HcWs; in the included studies, the highest coverage rate observed was 63.9%, despite that, on average was just 40.0% (24).
This study aimed to estimate the Tdap coverage status in HcWs at the University Hospital “Federico II” in Naples, a large university hospital in Southern Italy, in 2022 and to assess knowledge and attitude on Tdap vaccination and their correlates to improve current vaccination strategies and implement prevention counseling in health surveillance.
This cross-sectional study has been conducted to estimate Tdap coverage, knowledge, and attitude toward vaccinations in HcWs. Data were collected through the administration of an anonymous questionnaire. All HcWs at the University Hospital “Federico II” of Naples, the largest university hospital in Southern Italy, were invited to participate in the study between October and December 2022. The study was approved by the University Hospital Ethical Committee (Prot. N. 00018993–11/08/2022) and conducted in accordance with good clinical practice and the Declaration of Helsinki.
Study variables were retrieved from a questionnaire that was adapted from a previously validated questionnaire (25). Before the questionnaire administration to our target population, it was discussed by a focus group composed of physicians and other healthcare workers to evaluate its comprehensibility and intelligibility. The questionnaire in its final form is available in the Supplementary Figure 1.
Study variables included the following sociodemographic characteristics: sex (male and female), age (up to 34 years old, 35 years, and older), and educational attainment (high school and below and degree and above). Additional variables related to the job status were as follows: profession (medical doctors, non-medical healthcare workers, such as nurses and healthcare assistants, and other healthcare workers including biologists and administrative staff), department (clinical, surgery, diagnostic–therapeutic, and medical management), job seniority (0–4 years, 5–9 years, and more than 10 years), and vaccination history (vaccinated against measles, mumps, rubella, hepatitis B, polio, chicken pox, Haemophilus influenzae, and tuberculosis; coded as yes/no/not sure). For vaccination history, a score of 3 was assigned to the answer “yes”, 2 to “not sure”, and 1 to “no” (26). Based on these answers, we constructed a score ranging from 8 to 24.
Outcome variables included the Tdap booster coverage in the past 10 years and the attitude and knowledge about vaccines. The knowledge section included 15 questions regarding recommended vaccinations. A score of 3 was assigned to the answer “yes”, 2 to “not sure”, and 1 to “no” (26). Based on these answers, we constructed a score ranging from 15 to 45. Attitude toward recommended vaccinations was measured as a score (ranging from 3 to 30) obtained through three questions regarding the perception of the risk of contracting an infection and the usefulness of vaccination for HcWs to protect themselves and patients. Each question comprised a scale from 1 to 10. The final score was obtained by summing up the three values.
Study population characteristics were summarized using descriptive statistics, as appropriate. Multivariable regression models controlled for gender, age, profession, education, department, and job seniority were employed to assess correlates of vaccination coverage, knowledge, and attitude. To better assess the contribution of each variable, we first controlled the regression model for gender and age (partially adjusted model), then we also included education, job, department, and job seniority (fully adjusted model). Only for boosters, we also considered a third model including knowledge, attitude, and vaccination history. Specifically, multivariable logistic regression models were employed for binary outcomes and linear regression models for continuous outcomes. The results are presented as odds ratios (ORs), statistical coefficients (Coef.), and 95% confidence intervals (95%CIs), as appropriate. The results were considered significant if the p-value was <0.05. Statistical analyses were performed using Stata MP 15.0 statistical software.
During the study period, 206 questionnaires were completed. The demographic characteristics of participants are presented in Table 1. In total, 50% of the sample participants were women: 26.8% were <35 years old and 43.2% were ≥35 years old. The majority of the sample participants, 93.7% (193), had a degree or higher education and 6.3% (13) did not. As per the job status, 69.4% of the subjects (143) were medical doctors, 18.9% (39) were non-medical HcWs, and the remaining workers were 11.6% (24). In total, 32.0% of the study population (66) was working in a clinical department, 15.0% (31) in a surgical department, 10.2% (21) in a diagnostic–therapeutic department, and the remaining 42.7% (88) in a medical management department. Most of the subjects, 74.8% (154), had worked for the university hospital for 0–4 years, 12.1% (25) for 5–9 years, and the remaining 13.1% (27) for 10 or more years.
One-third of the sample (34.5%) had a Tdap vaccination booster over the past 10 years. The results from the multivariable logistic regression model showed that as compared with those with 0–4 years of employment at a university hospital, those with 5–9 years of job seniority had a 78% lower likelihood of being vaccinated with the booster dose (5–9 years—OR: 0.22, CI: 0.06 | 0.85) (Figure 1).
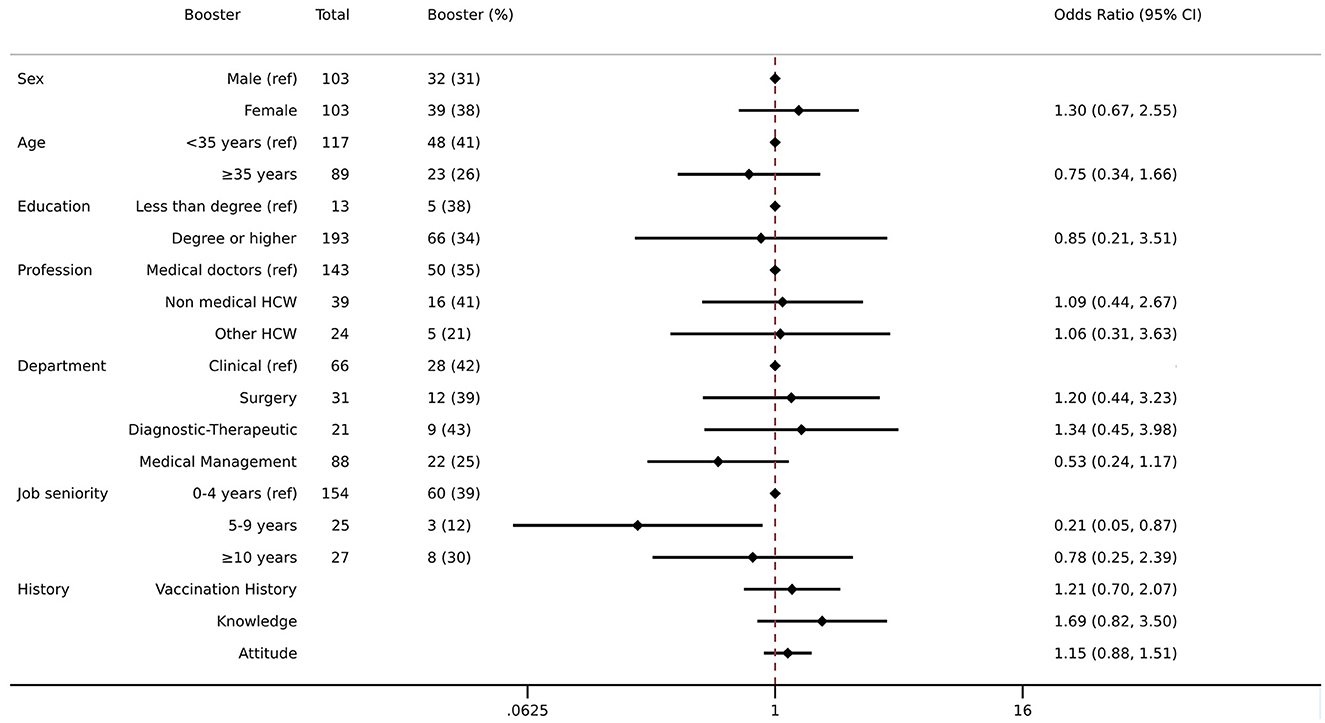
Figure 1. Association between demographic, job status, knowledge, attitude, vaccination history, and booster dose for Tdap. Multivariate logistic regression was employed including Tdap booster as an outcome variable and controlled for the following variables: sex, age, education, profession, department, job seniority, vaccination history, knowledge, and attitude. The results are presented as odds ratios (ORs) and 95% confidence intervals (95%CIs). The left column shows the crude number of those who received the booster Tdap and the proportion of them among the total.
The average knowledge score was 36.94 (CI: 35.93|37.95) out of 45. No differences in the average knowledge score were found between sub-groups (Figure 2). The average attitude score toward vaccination was 23.16 (CI: 22.59| 23.73) out of 30. When compared with medical doctors, other HcWs had a lower attitude score of 2.2, on average, (other—Coef. −2.15 on 30; CI: −4.14 | −0.15) and when compared with those who worked in a clinical department, on average, those who worked in a diagnostic–therapeutic department or medical management had lower attitude scores of 3.1 and 2.0, respectively (diagnostic–therapeutic—Coef. −3.12 on 30, CI: −5.13 | −1.12; medical management—Coef. −1.98 on 30, CI: −3.41 | −0.56) (Figure 2).
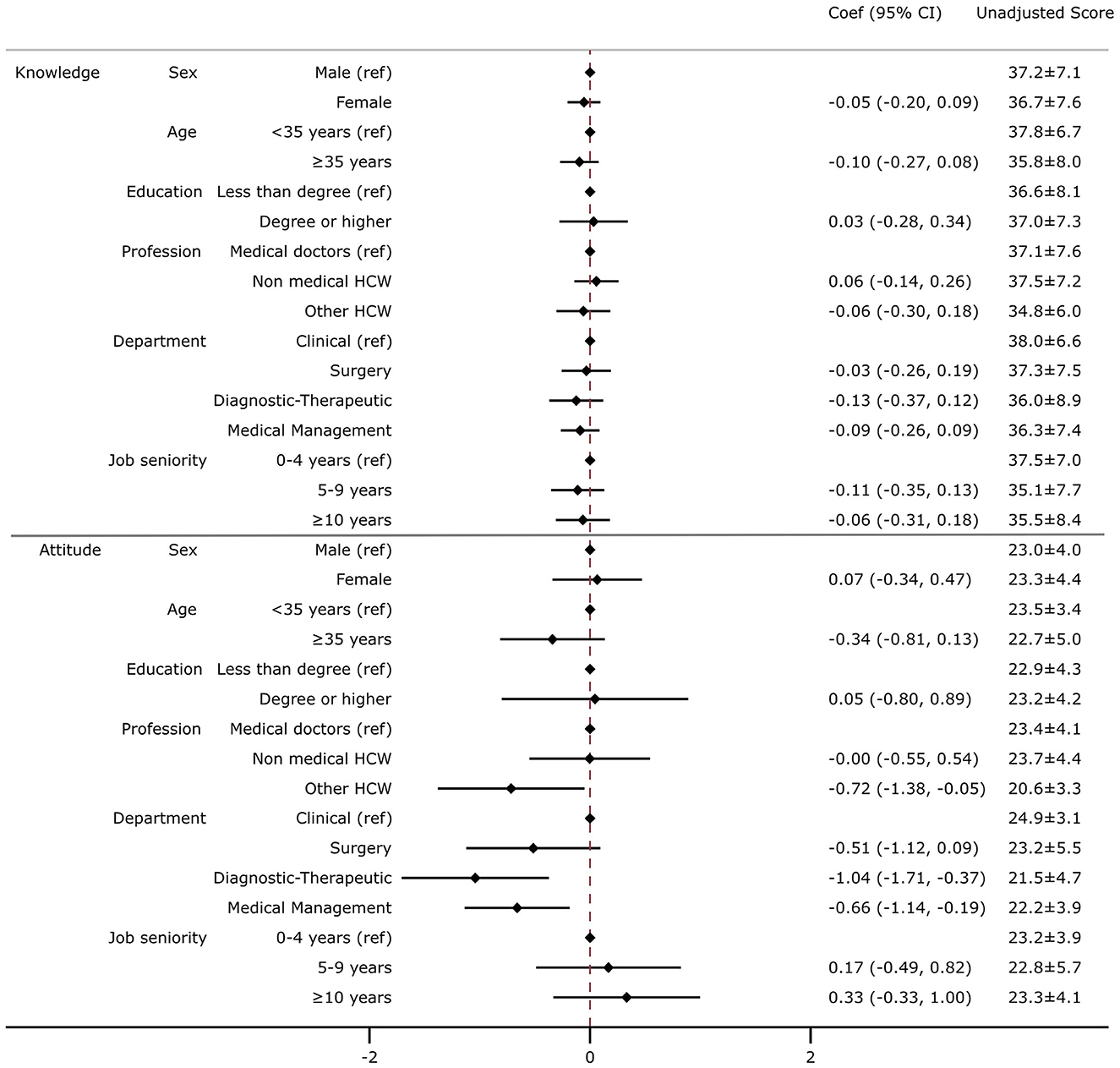
Figure 2. Association between demographic, job status, and knowledge or attitude toward vaccination. Multivariate linear regression was employed including knowledge (top) or attitude (bottom) as an outcome variable and controlled for the following variables: sex, age, education, profession, department, and job seniority. The results are presented as a coefficient (Coef) and 95% confidence intervals (95% CIs). In the right column, the unadjusted average score and standard deviation for attitude and knowledge are displayed. The knowledge score was calculated considering the average score of 15 questions regarding recommended vaccination (with a score ranging from 1 to 3 for each question, with a final score ranging from 15 to 45). The attitude score was calculated considering the average score of three questions regarding the perception of the risk of contracting an infection and the usefulness of vaccination for HcWs to protect themselves and patients (with a score ranging from 1 to 10 for each question, with a final score ranging from 3 to 30).
In our cross-sectional study, conducted in the University Hospital “Federico II” of Naples, the largest university hospital in Southern Italy, we found that only one-third (34.5%) of the study population had a booster vaccination for Tdap, with a lower likelihood of receiving a booster dose for those with a 5–9 year employment history when compared with those employed for <5 years. No differences were found regarding the vaccination knowledge between sub-groups, while attitude toward vaccination was lower in the other HcWs (administrative employees, biologists) when compared with medical doctors and in HcWs employed in diagnostic–therapeutic and medical management departments when compared with clinical departments.
Overall, the prevalence rate of Tdap booster vaccination in the sample was as low as 34.47%. This evidence, although in the lower range, has been reported in similar studies conducted in the USA with values ranging from 34.7 to 47.2% (27–29) and in Turkey (36% of HcWs with at least one booster dose in the past) (30). Interestingly, we found no sex differences in the proportion of Tdap boosters received, although the previous literature suggested that HcWs of the female sex were more likely to receive the Tdap (31–33). We also found a weak positive association between younger age and the likelihood of Tdap booster vaccination (Supplementary Table 1), which, however, was not confirmed in the fully adjusted model. However, this evidence has been confirmed by previous studies conducted in similar settings (24, 29, 34, 35).
In the partially adjusted model, younger participants, as compared with those participants of 35 years and older, had a higher knowledge regarding recommended vaccines for the HcWs (Supplementary Table 2), although this was a weak association not confirmed in the fully adjusted model. This finding might be explained by the shorter time period since obtaining their degree. Furthermore, this evidence is consistent with a study conducted in similar settings (36).
Attitude toward vaccination varied according to occupation. In line with previous evidence (29), we found that medical doctors had significantly higher attitude than other HcWs, which might also be explained by their perception of being at high risk and the frequency of contacts with other high-risk groups, i.e., patients (37). We also found that attitude toward vaccination was higher for HcWs working in clinical departments, where the intensity of contact with high-risk patients is higher when compared with those working in diagnostic and medical management departments, which is in line with recent evidence conducted in similar settings (30, 38–41).
We conducted our research on HcWs working in the largest university hospital in Southern Italy. Hence, the results might be generalized to similar healthcare settings in the country. However, several considerations merit discussion. First, responses may be influenced by difficulty in recalling their vaccination status, particularly for pediatric vaccinations. However, when recall bias is equally distributed in every study participant, the overall effect of the bias on study findings is reduced (42). Second, although the questionnaire was designed to be anonymous, responses or the lack of participation may have been influenced by the fear of the vaccinations or being targeted for vaccination campaigns, especially after the COVID-19 pandemic and the decision by the Italian NHS to enforce the COVID-19 vaccination for HcWs. Third, this specific analysis was based on a relatively small sample, and the results might be influenced by possible selection bias, as only personnel more willing to share their experiences might have decided to participate. Finally, another limitation of the study was to assess knowledge in a yes-no-don't know system. Although this approach might limit the precision of the outcome derivation, this choice was made to avoid altering the original questionnaire.
Healthcare workers are a high-risk population for infectious disease exposure and transmission. Low vaccine coverage for HcWs can lead to severe disease outbreaks, decreasing productivity, increasing absenteeism, and is also costly to the health system (43–46). Improving attitude and belief regarding vaccination among HcWs is important to avoid drops in the vaccination coverage rates and may also influence patients' responses to immunization campaigns (47). Our findings highlighted the importance to implement effective information and communication strategies, mostly among more experienced staff, to refresh and update information regarding vaccination in HcWs. Specifically, tailored strategies should be undertaken to improve Tdap booster coverage because, although the booster is offered free of charge in line with the national vaccination plan, there is no monitoring strategy in place as the quantitative serum immunoglobulin test is not included as a minimum requirement in the protocol of health surveillance for HcWs.
In the present study, we found that only one-third of the HcWs employed at the University Hospital “Federico II” of Naples, the largest academic hospital in Southern Italy, had a Tdap vaccination booster in the past 10 years. Longer employment history was associated with a lower likelihood of receiving the Tdap booster. Medical doctors had a higher attitude toward vaccination than other HcWs. Our findings support the need to implement public health strategies to improve information and awareness toward vaccinations and specifically highlight the importance of actively including the Tdap booster every 10 years as a prevention tool to protect high-risk populations.
The raw data supporting the conclusions of this article will be made available by the authors upon request and approbation from the ethical committee.
The studies involving human participants were reviewed and approved by University Hospital Ethical Committee of “Federico II”. The patients/participants provided their written informed consent to participate in this study.
RP and MT conceived the study and devised the study methodology and supervised the study. MM, CF, FE, MS, PM, and AP contributed to the acquisition of data for the study. RP and MM performed the formal data analysis. RP, MM, CF, FE, and MS wrote the first draft of the manuscript. RP had final responsibility for the decision to submit for publication. All authors reviewed and edited the manuscript, contributed to the article, and approved the submitted version.
This study was partly funded by SANOFI ITALIA. The founder played no role in the acquisition of data, statistical analysis, and preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1173482/full#supplementary-material
1. WHO. Pertussis vaccines: WHO position paper, August 2015—recommendations. Vaccine. (2016) 34:1423–25. doi: 10.1016/j.vaccine.2015.10.136
2. Pertussis Reported Cases and Incidence. Available online at: https://immunizationdata.who.int/pages/incidence/PERTUSSIS.html?CODE=Global&YEAR= (accessed 10, January 2023).
3. ECDC. ECDC Surveillance Atlas of Infectious Diseases. Available online at: https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=38 (accessed June 8, 2023).
4. Zhang Q. Benefits of COVID-19 non-pharmaceutical interventions on the prevention of other notifiable infectious diseases. Lancet Reg Health West Pac. (2021) 17:100303. doi: 10.1016/j.lanwpc.2021.100303
5. Oh DY, Buda S, Biere B, Reiche J, Schlosser F, Duwe S, et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January - September 2020: Analysis of national surveillance data. Lancet Reg Health Eur. (2021) 6:100112. doi: 10.1016/j.lanepe.2021.100112
6. Immunization Vaccines and Biologicals. Available online at: https://www.who.int/data/gho/data/themes/immunization (accessed 10, January 2023).
7. WHO. WHO Immunization Data. https://immunizationdata.who.int/compare.html?COMPARISON=type1__WIISE/MT_AD_COV_LONG+type2__WIISE/MT_AD_COV_LONG+option1__DTP_coverage+option2__DTP_PLUS_coverage&CODE=EUR&YEAR= (accessed June 8, 2023).
8. Baker RE, Saad-Roy CM, Park SW, Farrar J, Metcalf CJE, Grenfell BT. Long-term benefits of nonpharmaceutical interventions for endemic infections are shaped by respiratory pathogen dynamics. Proc Nat Acad Sci. (2022). 119:e2208895119. doi: 10.1073/pnas.2208895119
9. Donzelli A, Bellavite P, Demicheli V. [Epidemiology of pertussis and prevention strategies: problems and perspectives]. Epidemiol Prev. (2019) 43:83–91.
10. Md S,. Dati Coperture Vaccinali 2021. (2021). Available online at: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 (accessed June 8, 2023).
11. Esposito S, Principi N. Immunization against pertussis in adolescents and adults. Clin Microbiol Infect. (2016) 22:S89–95. doi: 10.1016/j.cmi.2016.01.003
12. Centre for Disease Prevention E. Annual Epidemiological Report for 2018 – Tetanus. Solna: Centre for Disease Prevention E.
13. America Academy of Pediatrics Commitee on Infectious Diseases. Prevention of Pertussis Among Adolescents: Recommendations For Use Of Tetanus Toxoid, Reduced Diphtheria Toxoid, And Acellular Pertussis (Tdap) vaccine. Pediatrics. (2006). 117:965–78. doi: 10.1542/peds.2005-3038
14. Zepp F, Heininger U, Mertsola J, Bernatowska E, Guiso N, Roord J, et al. Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect Dis. (2011) 11:557–70. doi: 10.1016/S1473-3099(11)70007-X
15. EpiCentro, - Istituto Superiore di Sanità,. Pertosse - Aspetti epidemiologici. Available online at: https://www.epicentro.iss.it/pertosse/epidemiologia (accessed June 8, 2023).
16. Bagordo F, Grassi T, Savio M, Rota MC, Baldovin T, Vicentini C, et al. Assessment of pertussis underreporting in Italy. J Clin Med. (2023) 12:1732. doi: 10.3390/jcm12051732
17. Gabutti G, Cetin I, Conversano M, Costantino C, Durando P, Giuffrida S, et al. Experts' opinion for improving pertussis vaccination rates in adolescents and adults: a call to action. Int J Environ Res Public Health. (2022) 19:4412. doi: 10.3390/ijerph19074412
18. Ozisik L, Tanriover MD. Vaccinating healthcare workers: level of implementation, barriers and proposal for evidence-based policies in Turkey. Hum Vaccin Immunother. (2017) 13:1198–206. doi: 10.1080/21645515.2016.1269992
19. Loulergue P, Fonteneau L, Armengaud JB, Momcilovic S, Levy-Brühl D, Launay O, et al. Vaccine coverage of healthcare students in hospitals of the Paris region in 2009: the Studyvax survey. Vaccine. (2013) 31:2835–8. doi: 10.1016/j.vaccine.2013.04.004
20. Wright SW, Decker MD, Edwards KM. Incidence of pertussis infection in healthcare workers. Infect. Cont. Hosp. Epidemiol. (1999) 20:120–3. doi: 10.1086/501593
21. Deville JG, Cherry JD, Christenson PD, Pineda E, Leach CT, Kuhls TL, et al. Frequency of unrecognized Bordetella pertussis infections in adults. Clin Infect Dis. (1995) 21:639–42. doi: 10.1093/clinids/21.3.639
22. Sandora TJ, Gidengil CA, Lee GM. Pertussis vaccination for health care workers. Clin Microbiol Rev. (2008) 21:426–34. doi: 10.1128/CMR.00003-08
23. Boulay BR, Murray CJ, Ptak J, Kirkland KB, Montero J, Talbot EA, et al. An outbreak of pertussis in a hematology-oncology care unit: implications for adult vaccination policy. Infect Cont Hosp Epidemiol. (2006) 27:92–5. doi: 10.1086/500420
24. Randi BA, Miyaji KT, Lara AN, Ibrahim KY, Infante V, Rodrigues CCM, et al. Low tetanus-diphtheria-acellular pertussis (Tdap) vaccine coverage among healthcare workers in a quaternary university hospital in São Paulo, Brazil: need for continuous surveillance and implementation of active strategies. Braz J Infect Dis. (2019) 23:231–6. doi: 10.1016/j.bjid.2019.06.007
25. D'Alessandro A, Napolitano F, D'Ambrosio A, Angelillo IF. Vaccination knowledge and acceptability among pregnant women in Italy. Hum Vaccin Immunother. (2018) 14:1573–9. doi: 10.1080/21645515.2018.1483809
26. Médicins, du Monde,. The KAP Survey Model (Knowledge, Attitudes, and Practices). Available online at:https://www.spring-nutrition.org/publications/tool-summaries/kap-survey-model-knowledge-attitudes-and-practices (accessed June 8, 2023).
27. O'Halloran AC, Lu P jun, Meyer SA, Williams WW, Schumacher PK, Sussell AL, et al. Tdap vaccination among healthcare personnel-−21 states, 2013. Am J Prevent Med. (2018) 54:119–123. doi: 10.1016/j.amepre.2017.09.017
28. Advisory Advisory Committee on Immunization Practices AC, for Disease Control C, (CDC) P. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. (2011) 60:1–45.
29. Srivastav A, Black CL, Lu PJ, Zhang J, Liang JL, Greby SM, et al. Tdap vaccination among healthcare personnel, internet panel survey, 2012–2014. Am J Prev Med. (2017) 53:537–46. doi: 10.1016/j.amepre.2017.04.002
30. Seyman D, Keskin AS, Küçükateş E, Ceylan MR, Kul G, Tosun S, et al. Healthcare personnel's attitude and coverage about tetanus vaccination in Turkey: a multicenter study. Hum Vacc Immunother. (2022). 18. doi: 10.1080/21645515.2021.2014732
31. Karadag FY, Saglam ZA. Assessment of the factors influencing primary care physicians' approach to vaccination of adult risk groups in Istanbul, Turkey. PeerJ. (2019) 7:e7516. doi: 10.7717/peerj.7516
32. Napolitano F, Bianco A, D'Alessandro A, Papadopoli R, Angelillo IF. Healthcare workers' knowledge, beliefs, and coverage regarding vaccinations in critical care units in Italy. Vaccine. (2019) 37:6900–6. doi: 10.1016/j.vaccine.2019.09.053
33. Çatakli T, Duyan-Çamurdan A, Aksakal-Baran FN, Güven AE, Beyazova U. Attitudes of physicians concerning vaccines not included in the national immunization schedule. Turk J Pediatr. (2018) 60:290. doi: 10.24953/turkjped.2018.03.009
34. Lu P jun, Graitcer SB, O'Halloran A, Liang JL. Tetanus, diphtheria and acellular pertussis (Tdap) vaccination among healthcare personnel-United States, 2011. Vaccine. (2014) 32:572–8. doi: 10.1016/j.vaccine.2013.11.077
35. Lu PJ, Euler GL. Influenza, hepatitis B, and tetanus vaccination coverage among health care personnel in the United States. Am J Infect Cont. (2011) 39:488–94. doi: 10.1016/j.ajic.2010.10.009
36. Montagna MT, Giglio O de, Napoli C, Fasano F, Diella G, Donnoli R, et al. Adherence to vaccination policy among public health professionals: results of a national survey in Italy. Vaccines. (2020) 8:379. doi: 10.3390/vaccines8030379
37. Jiang L, Ng HL, Ho HJ, Leo YS, Prem K, Cook AR, et al. Contacts of healthcare workers, patients and visitors in general wards in Singapore. Epidemiol Infect. (2017) 145:3085–95. doi: 10.1017/S0950268817002035
38. Alicino C, Iudici R, Barberis I, Paganino C, Cacciani R, Zacconi M, et al. Influenza vaccination among healthcare workers in Italy. Hum Vaccin Immunother. (2015) 11:95–100. doi: 10.4161/hv.34362
39. Maggiore ULR, Scala C, Toletone A, Debarbieri N, Perria M, D'Amico B, et al. Susceptibility to vaccine-preventable diseases and vaccination adherence among healthcare workers in Italy: a cross-sectional survey at a regional acute-care university hospital and a systematic review. Hum Vaccin Immunother. (2017) 13:470–6. doi: 10.1080/21645515.2017.1264746
40. Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, et al. Healthcare workers' (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. (2023) 99:520–8. doi: 10.1136/postgradmedj-2021-140195
41. RICCò M, Vezzosi L, Gualerzi G, Bragazzi NL, Balzarini F. Pertussis immunization in healthcare workers working in pediatric settings: knowledge, Attitudes and Practices (KAP) of Occupational Physicians. Preliminary results from a web-based survey (2017). J Prev Med Hyg. (2020) 61: E66–75. doi: 10.15167/2421-4248/jpmh2020.61.1.1155
42. Khare SR, Vedel I. Recall bias and reduction measures: an example in primary health care service utilization. Fam Pract. (2019) 36:672–6. doi: 10.1093/fampra/cmz042
43. Genovese C, Picerno IAM, Trimarchi G, Cannavò G, Egitto G, Cosenza B, et al. Vaccination coverage in healthcare workers: a multicenter cross-sectional study in Italy. J Prev Med Hyg. (2019) 60:E12–7. doi: 10.15167/2421-4248/jpmh2019.60.1.1097
44. Calugar A, Ortega-Sanchez IR, Tiwari T, Oakes L, Jahre JA, Murphy TV. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin Infect Dis. (2006) 42:981–8. doi: 10.1086/500321
45. Baggett HC, Duchin JS, Shelton W, Zerr DM, Heath J, Ortega-Sanchez IR, et al. Two nosocomial pertussis outbreaks and their associated costs—King County, Washington, 2004. Infect Cont Hosp Epidemiol. (2007) 28:537–43. doi: 10.1086/513497
46. Gianino MM, Politano G, Scarmozzino A, Charrier L, Testa M, Giacomelli S, et al. Estimation of sickness absenteeism among Italian healthcare workers during seasonal influenza epidemics. PLoS ONE. (2017) 12:e0182510. doi: 10.1371/journal.pone.0182510
Keywords: Tdap, vaccine, pertussis, knowledge, questionnaire, attitude, booster, healthcare
Citation: Mercogliano M, Fiorilla C, Esposito F, Sorrentino M, Mirizzi PD, Parisi A, Tajani A, Buonocore G, Triassi M and Palladino R (2023) Knowledge and attitude factors associated with the prevalence of Tdap (tetanus, diphtheria, and acellular pertussis) booster vaccination in healthcare workers in a large academic hospital in Southern Italy in 2022: a cross-sectional study. Front. Public Health 11:1173482. doi: 10.3389/fpubh.2023.1173482
Received: 24 February 2023; Accepted: 13 June 2023;
Published: 13 July 2023.
Edited by:
Alessandro Muzzi, GlaxoSmithKline, ItalyReviewed by:
Zhen Sun, University of South China, ChinaCopyright © 2023 Mercogliano, Fiorilla, Esposito, Sorrentino, Mirizzi, Parisi, Tajani, Buonocore, Triassi and Palladino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Palladino, cmFmZmFlbGUucGFsbGFkaW5vQHVuaW5hLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.