
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 02 August 2023
Sec. Environmental Health and Exposome
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1173101
This article is part of the Research TopicDietary Exposures to Environmental Pollutants: Integrated Multimedia PerspectivesView all 16 articles
Background: Existing evidence indicates that exposure to per- and polyfluoroalkyl substances (PFASs) may increase the risk of hypertension, but the findings are inconsistent. Therefore, we aimed to explore the relationship between PFASs and hypertension through this systematic review and meta-analysis.
Methods: We searched PubMed, Embase, and the Web of Science databases for articles published in English that examined the relationship between PFASs and hypertension before 13 August 2022. The random effects model was used to aggregate the evaluation using Stata 15.0 for Windows. We also conducted subgroup analyses by region and hypertension definition. In addition, a sensitivity analysis was carried out to determine the robustness of the findings.
Results: The meta-analysis comprised 15 studies in total with 69,949 individuals. The risk of hypertension was substantially and positively correlated with exposure to perfluorooctane sulfonate (PFOS) (OR = 1.31, 95% CI: 1.14, 1.51), perfluorooctanoic acid (PFOA) (OR = 1.16, 95% CI: 1.07, 1.26), and perfluorohexane sulfonate (PFHxS) (OR = 1.04, 95% CI: 1.00, 1.09). However, perfluorononanoic acid (PFNA) exposure and hypertension were not significantly associated (OR = 1.08, 95% CI: 0.99, 1.17).
Conclusion: We evaluated the link between PFASs exposure and hypertension and discovered that higher levels of PFOS, PFOA, and PFHxS were correlated with an increased risk of hypertension. However, further high-quality population-based and pathophysiological investigations are required to shed light on the possible mechanism and demonstrate causation because of the considerable variability.
Systematic review registration: https://www.crd.york.ac.uk/prospero/ PROSPERO, registration number: CRD 42022358142.
Since the 1940s, per- and polyfluoroalkyl substances (PFASs) have been extensively used because of their surfactant and stability qualities in industrial processes and goods such as aerospace and military, automotive, aviation, textiles, leather, clothing, construction and household goods, electronics, fire protection, food processing, and medical supplies (1–3). Persistent organic pollutants (POPs), including PFASs are now widely found in the environment, animals, plants, and humans worldwide because of their extensive usage (4). Due to the production of fluorocarbon bonds, PFASs have a long half-life and biopersistence in humans. The typical serum half-life of PFASs ranges from 2.3 to 8.5 years (5). Due to their structural similarity to fatty acids, PFASs may interfere with the function of peroxisome proliferator-activated receptors (PPARs) and the signaling pathways that connect them to metabolic processes (6). Meanwhile, toxicological investigations have also indicated that PFASs exposure is connected with oxidative stress and endothelial dysfunction (7). Thus, the cardiovascular system is especially susceptible to the toxicity of PFASs. PFASs exposure is associated with an increased risk of cardiovascular disease (CVD) and peripheral artery disease (PAD) (8, 9), in addition to other CVD risk factors such as thyroid disease (10, 11), high total cholesterol and low-density lipoprotein (LDL) levels (12), a higher body mass index (13), and impaired glucose homeostasis.
According to the data from the World Burden of Disease (GBD), the increasing incidence of hypertension has emerged as a major contributor to global mortality (14–17). Hypertension is also a significant contributor to the development of cardiovascular disease and renal failure (18). Different environmental exposures, including nutrition, alcohol consumption, lifestyle, and environmental contaminants, have been found to have variable impacts on blood pressure (19–21) and have been implicated as essential and changeable risk factors for hypertension (22). Toxicological evidence shows that PFASs may contribute to hypertension by increasing oxidative stress and the generation of reactive oxygen species (23). Several cross-sectional studies have shown that there is a positive correlation between PFASs exposure (24–29) and hypertension, while some researchers have reported no correlation or even a negative correlation (30–38). Conclusions vary depending on the population studied and the specific PFASs, and substantial inconsistencies have been observed between multiple studies on the same type of PFASs. Furthermore, PFASs exposure has occurred worldwide but still varies among countries due to the diversity of potential sources and approaches (39). These results show that more research is needed to gather data and quantify the impact of lingering, alternative, and emergent fluorinated chemicals on the blood pressure health of the population.
A systematic review and meta-analysis were conducted to (1) review the evidence for the effect of PFASs exposure on hypertension in the population and (2) quantitatively assess the relationship between the concentration of specific PFASs in the blood and the risk of hypertension.
The review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD 42022358142) (https://www.crd.york.ac.uk/prospero/), which was conducted under the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (40). All the relevant studies in the database were searched from its establishment to 13 August 2022 to obtain all relevant articles from the PubMed, Embase, and Web of Science databases. Search keywords include exposure (per- and polyfluoroalkyl substances, PFOS, PFOA, PFNA, and PFHxS) and result (hypertension). A specific search strategy is added to the Supplementary material. Given the complexity and growing number of PFASs homologs, we manually scanned all the references in the collected research to obtain more relevant articles and ensure that all investigations were included.
We preliminarily screened the titles and abstracts, evaluated the full articles, and independently identified articles that met the criteria. The following epidemiological studies are included: (1) observational study design, such as case–control studies, cohort studies, and cross-sectional studies; (2) at least one type of PFASs exposure (such as PFOS, PFOA, PFNA, and PFHxS) is observed; (3) hypertension results; (4) a risk assessment is provided, including a 95% confidence interval (CI), ORs, RRs, or HRs. Exclusion criteria included studies that (1) are not full-text; (2) have pregnant women as participants; (3) have repetitive data; (4) take the form of laboratory research, non-human animal research, a letter, or a review; and (5) are of low quality.
Lv and An separately extracted data and evaluated the quality of each research project. Disagreements were discussed and resolved amicably. We retrieved the following data from every qualifying study: first author; publication year; research design; population characteristics (distribution of region, age, and gender); sample size; categories of PFASs; definition and diagnostic criteria of hypertension; maximum adjusted ORs, RRs, or HRs, 95% CI (41) corresponding adjustment covariates and so on.
The Newcastle–Ottawa Scale (NOS) was used in order to assess the level of methodological rigor present in case–control studies and cohort studies (42). The study's quality was evaluated based on its selection, comparability, exposure (in case–control studies), or result (in cohort studies). The highest score was 9, and research that scored ≥ 7 was considered high quality. The cross-sectional scale recommended by the Agency for Healthcare Research and Quality (AHRQ) was used to evaluate cross-sectional research (43). The scale consists of 11 items, with a maximum score of 11. The evaluation criteria are as follows: 0–3 = low quality, 4–7 = medium quality, and 8–11 = high quality.
The NTP/OHAT Risk of Bias Rating was also used to assess the quality of the included studies (44). Seven main domains were included: selection bias, confounding bias, attrition/exclusion bias, exposure characteristics, outcome representation, selective reporting bias, and conflict of interest. The criteria for risk of bias assessment are reported in Supplementary Table 3.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guideline was used to assess the confidence in the body of evidence (45), which evaluates eight criteria (risk of bias, indirectness, inconsistency, imprecision, publication bias, large magnitude of effect, dose–response, and confounding effect) to systematically assess the overall confidence in the evidence derived from the meta-analysis. Based on the overall assessment of reviewers, the method assigns the evidence a quality rating of “high,” “moderate,” “low,” or “very low.”
The ORs, RRs, and HRs, together with their respective 95% CIs, were derived using the maximum adjusted models in each research study. For these studies in which the categorical PFASs exposure dosage was variable and split into tertiles or quartiles, the fixed effect model combined the data, and the meta-analysis used the final pooled findings (46). The relevant effect values, such as ORs, RRs, or HRs, which may be integrated into the meta-analysis, were included. The effect size (ES) was calculated by ES = ln (OR), and the standard error (SE) of the effect size was calculated as SE = [ln (UC)–ln (LC)]/3.92 (UC and LC represent upper and lower confidence limits, respectively). The % weight represented the size of the information (i.e., sample size, number of events, and confidence interval) and was calculated as weight = 1/(SE2) (41). Heterogeneity in the research was tested using the I2 and P-values. A P-value of < 0.05 was regarded as heterogeneous. I2 statistics > 50% showed high heterogeneity, 25–50% moderate heterogeneity, and < 25% low heterogeneity. The fixed effect model was utilized for analysis when there was no significant heterogeneity (I2 < 50% or P > 0.05), otherwise, a random effect model was employed (46).
To determine the cause of the variation, a subgroup analysis was performed, stratified by geographic area and hypertension threshold. To evaluate the impact of missing studies and to identify the source and size of any heterogeneity in the results, sensitivity analyses were conducted by eliminating studies one by one from the analysis. Publication bias was evaluated using funnel plots, and the predicted findings were confirmed using Egger's test (47). The meta-analysis used Stata version 15.0 for Windows.
Following the search strategy, a total of 6,784 articles from these three online resources were reviewed. After removing the duplicates, we were left with 4,276 research articles. After evaluating the titles and abstracts, 34 articles were chosen for further consideration. Researchers discarded 19 articles because they did not meet the inclusion or exclusion criteria. These included one review, 10 studies without an applicable exposure or result, four studies conducted on pregnant women, and four studies that simply replicated previous findings. A total of 15 publications were included in the meta-analysis, as shown in Figure 1. There were a total of 71,059 participants in the studies that quantified PFASs levels in blood samples from 154 to 32,254 individuals. Table 1 shows the detailed article information.
The term “hypertension” alone has a wide range of interpretations. The majority of studies (10 of 15 included studies) use a blood pressure reading of 140/90 mm Hg as the diagnostic threshold for hypertension (24–27, 29, 30, 32, 35–37), which is in line with the guidelines of the American Heart Association and the Joint National Committee (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure). Four studies use a blood pressure reading of 130/85 mm Hg as the cutoff for hypertension (28, 33, 38), while another (31) uses a reading of 130/80 mm Hg since its subjects are adolescents. An individual study did not definitively establish the cutoff for hypertension, with the condition being defined as one that requires a medical professional's diagnosis (34).
The quality of the 15 studies that were suitable for inclusion was analyzed. The results showed that 11 of the cross-sectional studies were of high- or medium-quality (25–31, 33–36, 38), three of the cohort studies scored 7 (24, 29, 37), and one case–control study scored 8 (32), indicating that none of these studies was of poor quality. Supplementary Tables 1, 2 include further information.
The results of the risk of bias assessment are shown in Table 2. The risk of bias regarding attrition/exclusion, confounding, selection (exposure), and conflict was rated as “probably low” in all the included studies. Of the 15 studies, five were rated as “definitely low risk of bias”, seven were rated as “probably low”, and two were rated as “definitely high risk of bias” due to the use of self-reported cases. Selection bias was rated as “probably low” in all but one study. Overall, 13 studies and two studies were grouped as tier 1 and tier 2, respectively.
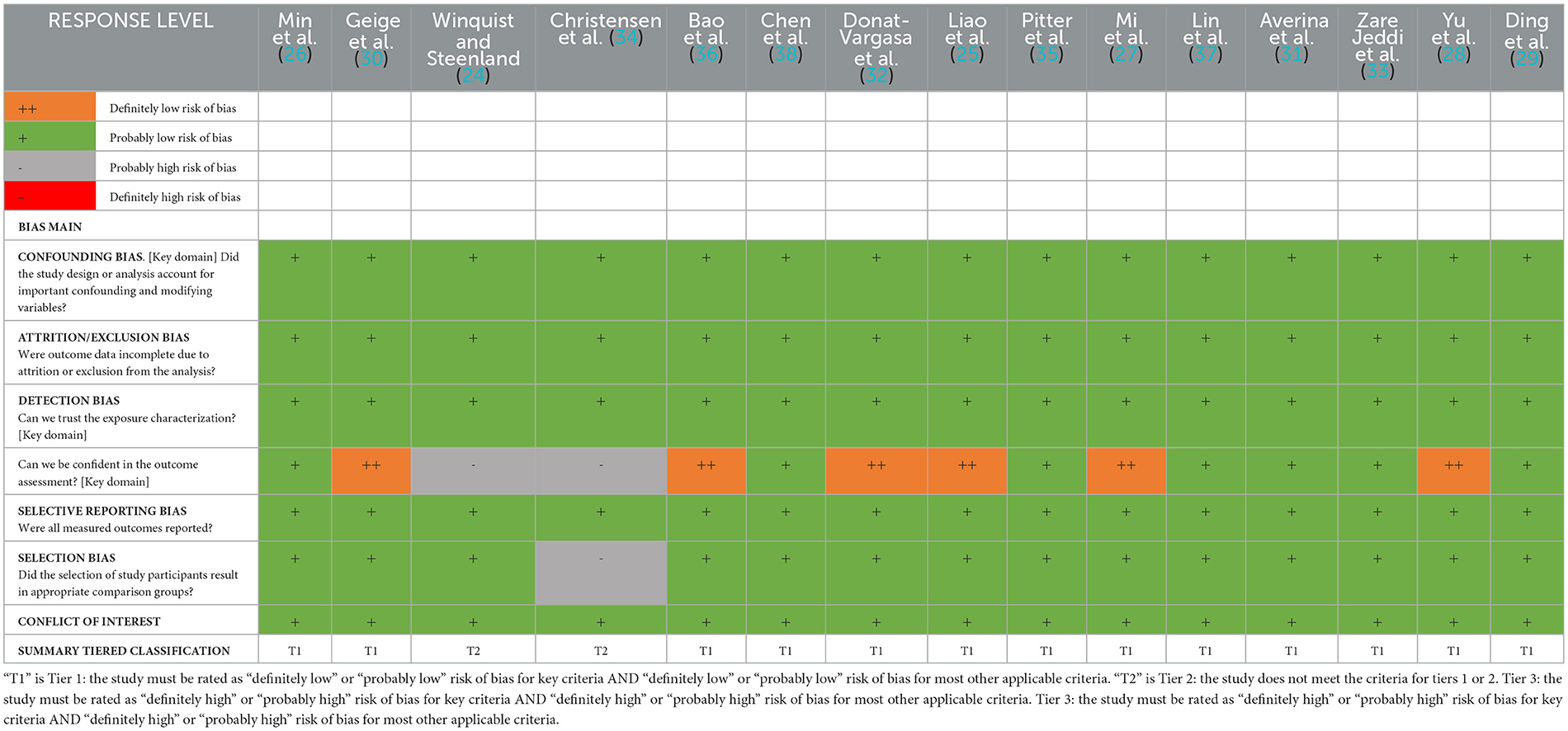
Table 2. Summary of risk of bias domains for individual studies examining associations between PFAS and hypertension.
Based on cross-sectional and case–control studies, the overall strength of evidence for the association between PFASs and hypertension was “limited”, and the direction of effect was inconsistent across most studies. However, for PFASs combined with hypertension events, we rated the overall strength of evidence as “moderate”. The majority of PFASs–hypertension combinations assessed exhibited consistent statistically significant positive evidence of an association, and all studies included in the meta-analysis were “moderate.” These results provide some epidemiologic proof that PFASs may increase the risk of hypertension.
To determine whether PFASs exposure was associated with hypertension, 15 studies were analyzed, as shown in Figure 2. This included 15 outcomes for PFOA exposure, 14 outcomes for PFOS exposure, 11 outcomes for PFHxS exposure, and 10 outcomes for PFNA exposure.
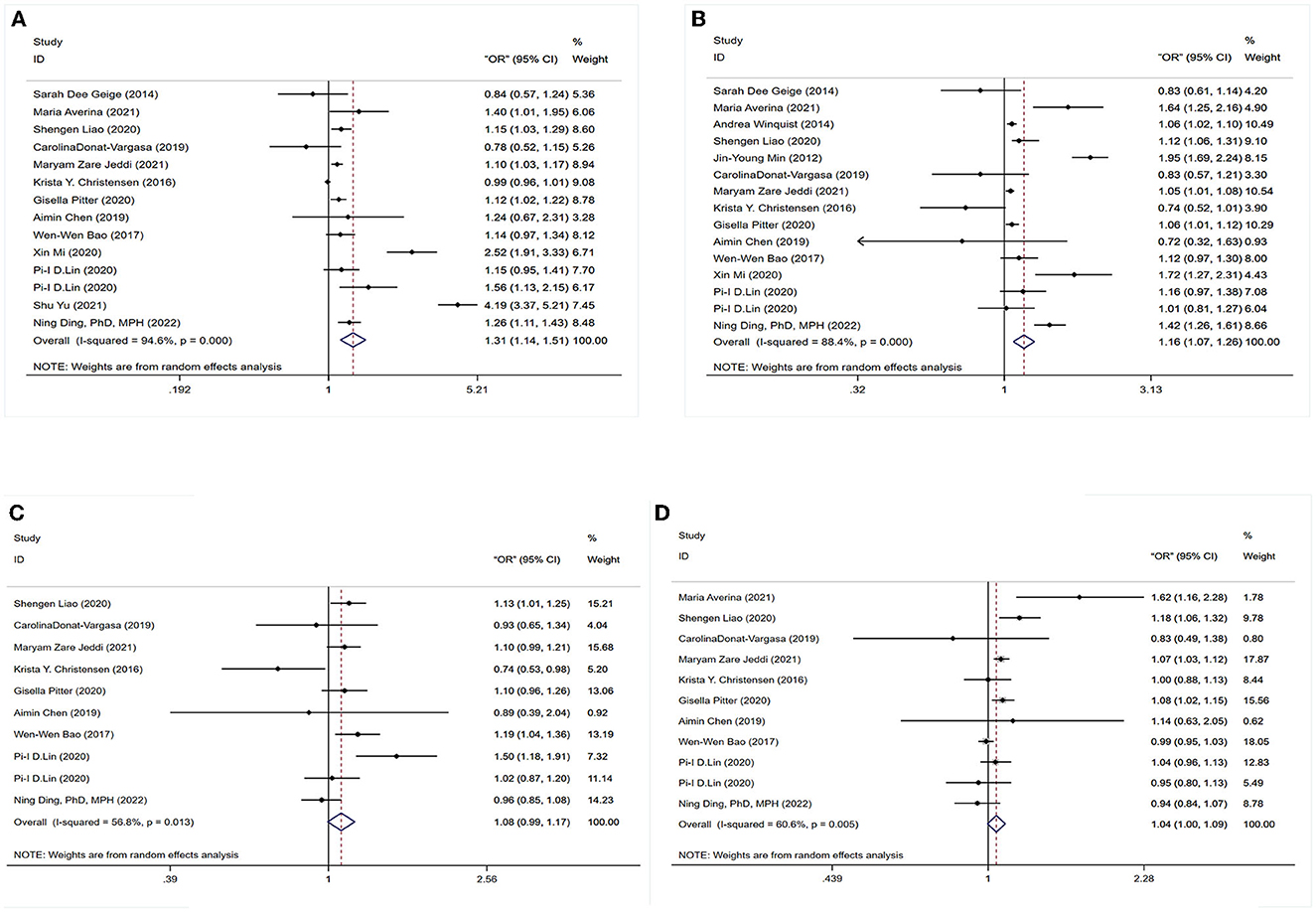
Figure 2. Forest plot of the association between exposure to PFAS with hypertension. (A) PFOS; (B) PFOA; (C) PFNA; (D) PFHxS.
The association between PFOS exposure and hypertension was investigated in 13 studies (10 cross-sectional, one case–control, and two cohort studies). A combined OR estimate of 1.31 (95% CI: 1.14, 1.51) was calculated. We employed a random effects model to examine the connection between PFOS exposure and hypertension due to the significant heterogeneity of the studies (I2 = 94.6%, P < 0.05), as shown in Figure 2A.
In total, 11 cross-sectional studies, one case–control study, and three cohort studies were retrieved to examine the correlation between PFOA exposure and hypertension. The overall findings revealed that being exposed to PFOA increased the risk of hypertension (OR = 1.16, 95% CI: 1.07, 1.26). A random effects model was used due to the significant heterogeneity of the included studies (I2 = 88.4%, P < 0.05), as shown in Figure 2B.
No statistically significant association between PFNA exposure and hypertension was found in six cross-sectional studies, one case–control study, and two cohort studies. As a whole, we found a merged evaluation of 1.08 (95% CI: 0.99, 1.17). In addition, a random effects model was adopted since the studies had high heterogeneity (I2 = 6.8%, P < 0.05), as shown in Figure 2C.
The association between PFHxS exposure and hypertension risk was examined in seven cross-sectional investigations, one case–control study, and two cohort studies. A positive relationship (OR = 1.04, 95% CI: 1.00, 1.09) was found between PFHxS exposure and the risk of hypertension with a random effect model because of high heterogeneity (I2 = 60.6%, P < 0.05), as shown in Figure 2D.
Exposure to PFOS, PFOA, and PFHxS was observed to have a positive and statistically significant connection with hypertension, but exposure to PFNA did not. We conducted a further subgroup analysis based on geography and hypertension thresholds to delve deeper into the correlation studies. Stratified by region, the pooled evaluated OR of PFOA and hypertension was 1.13 (95% CI: 1.03, 1.24) for Non-American region and 1.15 (95% CI: 0.97, 1.35) for American region. Then, the pooled estimate OR of PFNA and hypertension was 1.11 (95% CI: 1.04, 1.19) for non-America and 1.05 (95% CI: 0.90,1.23) for America. In addition, a subgroup analysis by hypertension threshold revealed a positive association between PFOS and PFOA exposure and the development of hypertension (OR = 1.19, 95% CI: 1.05, 1.28; OR = 1.15, 95% CI: 1.03, 1.28) for 140/90 mmHg, but no statistically significant association (OR = 1.58, 95% CI: 0.90, 2.78; OR = 1.28, 95% CI: 0.83, 1.98) for non-140/90 mmHg. All results are shown in Table 3.
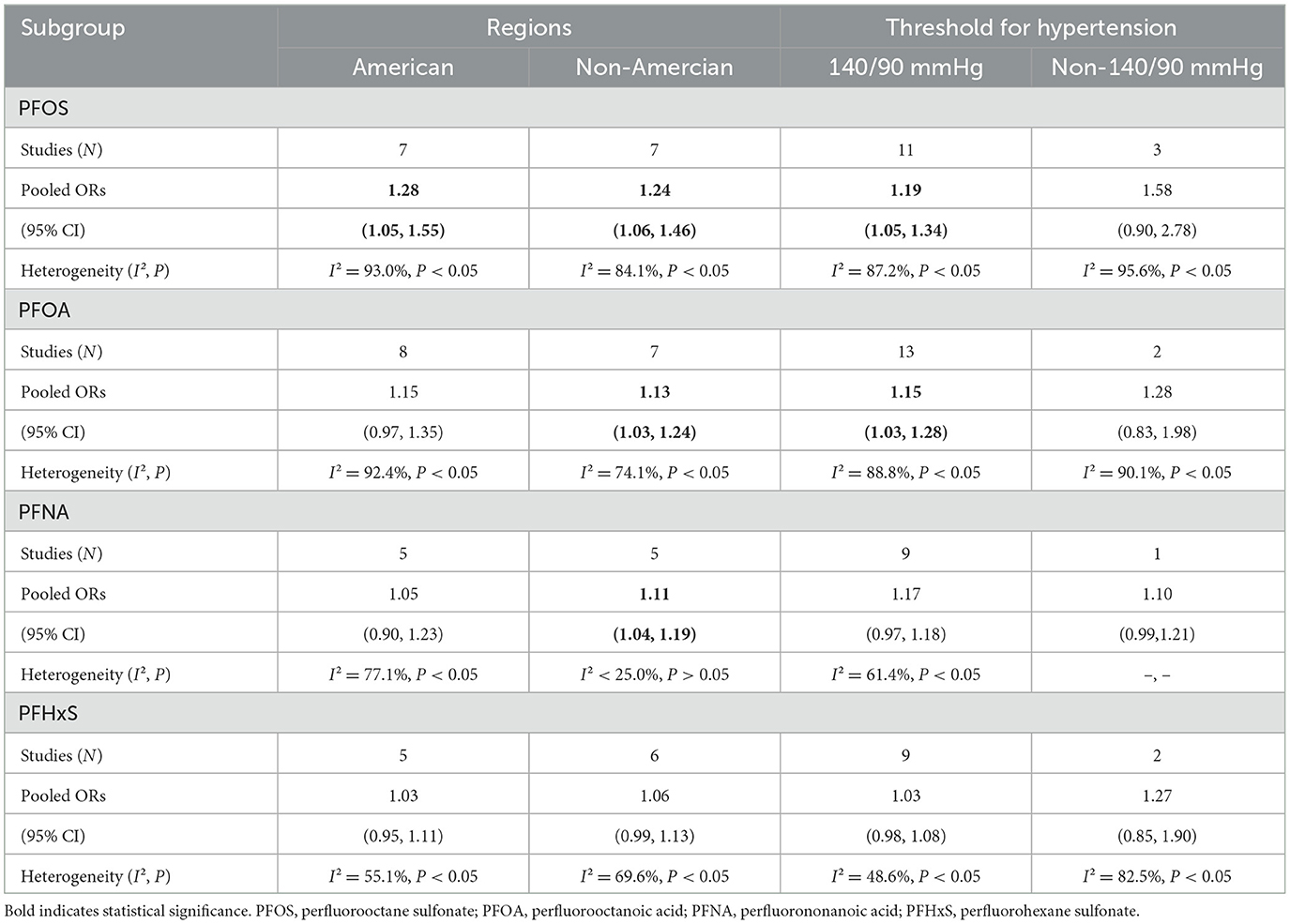
Table 3. Subgroup analysis of PFASs (PFOA, PFOS, PFNA, and PFHxS) exposure and risk of hypertension.
The correlation between PFOA, PFNA, and hypertension was investigated, and the results showed no substantial publication bias (P = 0.26 for PFOA, P = 0.56 for PFNA, P = 0.67 for PFHxS). However, publication bias was present in the meta-analysis evaluating the association between PFOS and hypertension (P = 0.028). A sensitivity analysis was conducted by eliminating specific articles one by one to assess the stability of the findings and establish that any research did not influence them. The results for PFOA, PFNA, and PFHxS are consistent to some degree. The odds ratio (OR) between PFOS exposure and hypertension increased but remained positively associated (OR = 1.20, 95% CI: 1.16, 1.25), except for the research by Christensen et al. (34). As shown in Figure 3, however, when the trimming and filling approach was used, the result was reversed (OR = 1.04, 95% CI: 0.89, 1.20), indicating that the robustness of the meta-analysis between PFOS and hypertension is poor, and the source of the disagreement must be explained.
In this systematic review and meta-analysis, we first summarize all the current evidence on the risk of PFASs exposure for hypertension. The same type of research on PFASs was collected for analysis in order to summarize their relevance in this study. According to our results, there was a significant positive association between exposure to PFOS, PFOA, and PFHxS and an increased risk of hypertension in the population, but no association between PFNA and hypertension. Our study is the first meta-analysis to investigate the association between PFASs exposure and the risk of hypertension in a population. It has implications for reducing the risk of hypertension in populations living in areas contaminated with PFASs.
The results revealed a substantial positive relationship between PFOA (OR = 1.31, 95% CI: 1.14, 1.51) and PFOS (OR = 1.16, 95% CI: 1.07, 1.26) exposure and the risk of hypertension. Our findings are consistent with the nine previously published studies (25, 27–29, 31, 33, 35–37) that found a positive connection between PFOS or PFOA exposure and hypertension. A negative link between PFOS and hypertension was found in three studies (30, 32, 34), although the results were not statistically significant. Uncontrolled variables, such as diet, comorbidities, physiological features of distinct subpopulations, and family history of hypertension, may be to blame for this discrepancy. For PFHxS, we found a relationship between exposure and hypertension (OR = 1.04, 95% CI: 1.00, 1.09). Our findings are consistent with those of previous research showing that prolonged exposure to high levels of PFHxS increases blood pressure in the general population (25, 27, 31, 35). Although not statistically significant, some studies have shown an inverse correlation between PFHxS and hypertension (29, 33, 34, 36, 37). It is essential to consider the possibility that this discrepancy is due to random chance or confounding factors in the study design or treatment pharmacokinetics. In light of this discrepancy, more studies are needed to establish a causal relationship between PFHxS and hypertension. Contrary to expectations, we found no evidence that PFNA exposure increased the risk of developing hypertension (OR = 1.08, 95% CI: 0.99, 1.17). Studies by Pitter et al. (35) (OR = 1.1, 95% CI: 0.96, 1.26), Lin et al. (37) (OR = 1.00, 95% CI: 0.76, 1.32), Donat-Vargas et al. (32) (OR = 0.9, 95% CI: 0.52, 1.57), and Ding et al. (29) (OR = 1.00, 95% CI: 0.83, 1.19) were consistent with our findings. Three other studies reported positive associations between hypertension and PFNA: one by Liao et al. (25) (OR = 1.18, 95% CI: 1.01, 1.38), one by Zare Jeddi et al. (33) (OR = 1.07, 95% CI: 1.03, 1.12), and one by Bao et al. (36) (OR = 1.19, 95% CI: 1.04, 1.36). However, one study using the NHANCE database found that PFNA exposure was linked to a reduced incidence of hypertension (34) (OR = 0.74, 95% CI: 0.53, 0.98). This finding was not consistent with ours. These discrepancies may be related to the failure to consider other confounding factors that may be strongly associated with hypertension, such as ethnicity, diet, family history, exercise habits, and the local prevalence of hypertension. In addition, this discrepancy highlights the need for further investigation of the effects of PFNA exposure on hypertension.
The association between hypertension and any PFASs (PFOS, PFOA, PFNA, and PFHxS) (OR = 1.26, 95% CI: 1.12, 1.41) was reported in a cohort-based study based on electronic health records (48). Higher blood PFASs concentrations were also related to an increased risk of hypertension (HR = 1.71, 95% CI: 1.15, 2.54) (29), according to a recently published cohort study with a mean follow-up of 12.4 years. At the same time, a cross-sectional study of adolescents in northern Norway showed that total PFASs were positively associated with hypertension, with OR=2.24 95% CI: 1.10, 4.54 (31). Moreover, a study based on the Study of Women's Health Across the Nation reported that in a mixed model, there were positive associations between n-PFOS (β = 0.051), Sm-PFOS (β = 0.115), n-PFOA (β = 0.032), PFNA (β = 0.086), and PFHxS (β = −0.074) (29). This is not entirely consistent with the results of our study. The choice of the statistical model, the definition of hypertension, the degree of exposure, and the susceptibility of different populations may have contributed to this difference (49, 50).
It was shown that in the further stratified analysis, grouping according to the hypertension threshold, PFASs exposure, and hypertension were not significantly correlated with the non-140/90 group, which may be due to the lack of studies in the non-140/90 group (PFOS, N = 3; PFOA, N = 2; PFNA, N = 1; PFHxS, N = 2). Studies were categorized by country in order to identify regional variations in the association between PFASs exposure and hypertension. Notably, PFOA, PFNA, and PFHxS were not associated with hypertension in the United States. Regional differences in lifestyle, socioeconomic level, local diets, the local incidence of hypertension, average blood pressure, and ethnic adaptability to PFASs exposure may obscure the association between PFASs exposure and hypertension.
While there are various potential processes linking community exposure to PFASs to an increased risk of hypertension, the mechanism of the relationship between PFASs and blood pressure is still unclear. PFASs have been linked to increased oxidative stress in the liver and endothelial cells (23, 51, 52). Inadequate production of nitric oxide and increased production of superoxide, both byproducts of oxidative stress, may contribute to an increase in blood pressure in the process of attenuating vasodilation. Consequently, PFAS-induced oxidative stress may increase the need for homocysteine methyl donors, which in turn may reduce the effectiveness of the body's natural ability to dilate blood vessels (51, 52). The presence of PFASs may also have a secondary effect on blood pressure, according to another theory. Numerous human (53, 54) and animal studies have revealed that reduced nephron endowment and glucocorticoid excess contribute to hypertension. In a recent animal model of hypertension, prenatal exposure to PFASs was shown to reduce nephron endowment and increase renal glucocorticoid receptor (GR) gene expression in the offspring of mothers who were exposed to the investigated chemicals during pregnancy (55). Upregulation of GRs augments the action of glucocorticoids, and the sclerotic and stress-induced natriuretic cycle initiated by a reduction in nephrons may contribute to hypertension. These mechanisms may cooperate with angiotensin II to boost proximal tubular sodium reabsorption (56). As a result, elevated serum PFASs levels may contribute to an indirect increase in blood pressure, especially in the presence of elevated glucocorticoids and diminished nephrons. In conclusion, PFASs exposure has been associated with a possible increase in the incidence of hypertension. Nevertheless, its mechanism in the human body remains unclear and needs further research.
The research has several strengths. First, the number of hypertension patients in our study is greater than that in smaller studies. With such large samples, we could thoroughly explore the link between PFASs and hypertension risk and conduct a nuanced subgroup analysis. Second, to more thoroughly evaluate the association between exposure to hypertension and various PFASs, we used a fixed effects model to pool data from studies in which the PFASs exposure dosage was categorically variable, such as split into tertiles or quartiles. The findings of this meta-analysis can be trusted since none of the 15 studies used to compile it were of poor quality.
Despite these benefits, there are several caveats to our research. First, many studies looked at “residual PFASs” (PFOS and PFOA) and found that the possible relationship could be completely recognized. However, there is a lack of research on “surrogate PFASs”, or polyfluoroalkyl compounds such as F-53B and OBS, which results in a high degree of variability or a hampered capacity to discover possible correlations. Therefore, the lack of association we observed calls for a thorough explanation and further research. At the same time, PFASs exposure occurs worldwide but differs from country to country due to the wide variety of potential sources and routes of exposure. In addition, because each study's population, models, statistical techniques, and adjustments for numerous confounding variables are unique, each study's conclusions may differ. Finally, a recent study highlighted the importance of PFASs isomers and enantiomers (57). Due to their structural differences, different PFASs isomers and enantiomers may have different harmful health consequences. However, due to the need for data, we instead focused on the general direction of previous studies in this area. As the volume of research continues to grow, we will be able to better categorize our results using more precise diagnostic methods. Despite these obstacles, a meta-analysis can answer numerous questions and shed light on the causes of variability in study findings, pointing the way to new avenues of inquiry.
Our understanding of the relationship between PFASs exposure and hypertension has been strengthened by this meta-analysis, which demonstrates a positive association between PFOS, PFOA, and PFHxS exposure and hypertension but no relationship between PFNA and hypertension. In order to manage hypertension and further lower the prevalence of cardiovascular disease and stroke, people should seriously consider reducing environmental PFASs pollution and PFASs exposure. To further understand these mechanisms, further research should be encouraged.
Publicly available datasets were analyzed in this study. This data can be found here: Pubmed, Embase, and Web Of Science.
FX and XS: integrity of the data, the accuracy of the data analysis, and study concept and design. FX, ZA, and JL: data extraction and analysis. FX: drafting of the manuscript. ZA and JL: study supervision. HG, XL, ZA, JL, XS, HS, and YL: critical revision. All authors reviewed and revised the manuscript, approved the final version for publication, and accepted responsibility for all aspects of the manuscript.
This study was supported by grants from the National Natural Science Fund of China (21976050) and the Science and Technology Program of Hebei Province (21377779D).
We thank the participants for joining our study and the reviewers for their valuable suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The findings, interpretations, and conclusions expressed in this study do not necessarily reflect the views of the agencies mentioned.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1173101/full#supplementary-material
1. Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect. (2007) 115:1596–602. doi: 10.1289/ehp.10598
2. Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ Health. (2017) 16:115. doi: 10.1186/s12940-017-0332-3
3. Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, et al. Fluorinated compounds in US fast food packaging. Environ Sci Technol Lett. (2017) 4:105–11. doi: 10.1021/acs.estlett.6b00435
4. Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. (2006) 40:3463–73. doi: 10.1021/es052580b
5. Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. (2007) 115:1298–305. doi: 10.1289/ehp.10009
6. Bijland S, Rensen PC, Pieterman EJ, Maas ACE, van der Hoorn JW, van Erk MJ, et al. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-leiden CETP mice. Toxicol Sci. (2011) 123:290–303. doi: 10.1093/toxsci/kfr142
7. DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol. (2009) 39:76–94. doi: 10.1080/10408440802209804
8. Shankar A, Xiao J, Ducatman A. Perfluorooctanoic acid and cardiovascular disease in US adults. Arch Intern Med. (2012) 172:1397–403. doi: 10.1001/archinternmed.2012.3393
9. Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ Int. (2018) 119:37–46. doi: 10.1016/j.envint.2018.05.051
10. Melzer D, Rice N, Depledge MH, Henley WE. Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the US National Health and Nutrition Examination Survey. Environ Health Perspect. (2010) 118:686–92. doi: 10.1289/ehp.0901584
11. Shrestha S, Bloom MS, Yucel R, Seegal RF, Wu Q, Kannan K, et al. Perfluoroalkyl substances and thyroid function in older adults. Environ Int. (2015) 75:206–14. doi: 10.1016/j.envint.2014.11.018
12. Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environ Int. (2019) 129:343–53. doi: 10.1016/j.envint.2019.05.027
13. Nelson JW, Hatch EE. Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general US population. Environ Health Perspect. (2010) 118:197–202. doi: 10.1289/ehp.0901165
14. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
15. Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
16. Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. (2017) 70:736–42. doi: 10.1161/HYPERTENSIONAHA.117.09801
17. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2224–60. doi: 10.1016/S0140-6736(12)61766-8
18. Yang BY, Guo Y, Bloom MS, Xiao X, Qian ZM, Liu E et al. Ambient PM1 air pollution, blood pressure, and hypertension: Insights from the 33 Communities Chinese Health Study. Environ Res. (2019) 170:252–9. doi: 10.1016/j.envres.2018.12.047
19. Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J Urol. (2017) 198:858–63. doi: 10.1016/j.juro.2017.03.124
20. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. (1983) 67:968–77. doi: 10.1161/01.cir.67.5.968
21. Liu QS, Hao F, Sun Z, Long Y, Zhou Q, Jiang G. Perfluorohexadecanoic acid increases paracellular permeability in endothelial cells through the activation of plasma kallikrein-kinin system. Chemosphere. (2018) 190:191–200. doi: 10.1016/j.chemosphere.2017.10.002
22. Wang YY Li Q, Guo Y, Zhou H, Wang QM, Shen HP. et al. Long-term exposure to airborne particulate matter of 1 μm or less and blood pressure in healthy young adults: A national study with 12 million pregnancy planners. Environ Res. (2020) 184:109113. doi: 10.1016/j.envres.2020.109113.
23. Yao X, Zhong L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat Res. (2005) 587:38–44. doi: 10.1016/j.mrgentox.2005.07.010
24. Winquist A, Steenland K. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect. (2014) 122:1299–305. doi: 10.1289/ehp.1307943
25. Liao S, Yao W, Cheang I, Tang X, Yin T, Lu X, et al. Association between perfluoroalkyl acids and the prevalence of hypertension among US adults. Ecotoxicol Environ Saf. (2020) 196:110589. doi: 10.1016/j.ecoenv.2020.110589
26. Min JY, Lee KJ, Park JB, Min KB. Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in US adults. Occup Environ Med. (2012) 69:658–62. doi: 10.1136/oemed-2011-100288
27. Mi X, Yang YQ, Zeeshan M, Wang ZB, Zeng XY, Zhou Y, et al. Serum levels of per- and polyfluoroalkyl substances alternatives and blood pressure by sex status: isomers of C8 health project in China. Chemosphere. (2020) 261:127691. doi: 10.1016/j.chemosphere.2020.127691
28. Yu S, Feng WR, Liang ZM, Zeng XY, Bloom MS, Hu GC, et al. Perfluorooctane sulfonate alternatives and metabolic syndrome in adults: new evidence from the isomers of C8 health project in China. Environ Pollut. (2021) 283:117078. doi: 10.1016/j.envpol.2021.117078
29. Ding N, Karvonen-Gutierrez CA, Mukherjee B, Calafat AM, Harlow SD, Park SK. Per- and polyfluoroalkyl substances and incident hypertension in multi-racial/ethnic women: the study of women's health across the nation. Hypertension. (2022) 79:1876–86. doi: 10.1161/HYPERTENSIONAHA.121.18809
30. Geiger SD, Xiao J, Shankar A. No association between perfluoroalkyl chemicals and hypertension in children. Integr Blood Press Control. (2014) 7:1–7. doi: 10.2147/IBPC.S47660
31. Averina M, Brox J, Huber S, Furberg AS. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. Fit Futures study Environ Res. (2021) 195:110740. doi: 10.1016/j.envres.2021.110740
32. Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Koponen J, et al. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environ Int. (2019) 124:58–65. doi: 10.1016/j.envint.2019.01.007
33. Zare Jeddi M, Dalla Zuanna T, Barbieri G, Fabricio ASC, Daprà F, Fletcher T, et al. Associations of perfluoroalkyl substances with prevalence of metabolic syndrome in highly exposed young adult community residents-a cross-sectional study in Veneto Region, Italy. Int J Environ Res Public Health. (2021) 18:1194. doi: 10.3390/ijerph18031194
34. Christensen KY, Raymond M, Thompson BA, Anderson HA. Perfluoroalkyl substances in older male anglers in Wisconsin. Environ Int. (2016) 91:312–8. doi: 10.1016/j.envint.2016.03.012
35. Pitter G, Zare Jeddi M, Barbieri G, Gion M, Fabricio ASC, Daprà F, et al. Perfluoroalkyl substances are associated with elevated blood pressure and hypertension in highly exposed young adults. Environ Health. (2020) 19:102. doi: 10.1186/s12940-020-00656-0
36. Bao WW, Qian ZM, Geiger SD, Liu E, Liu Y, Wang SQ, et al. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: Isomers of C8 Health Project in China. Sci Tot Environ. (2017) 607–8:1304–12. doi: 10.1016/j.scitotenv.2017.07.124
37. Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. Per- and polyfluoroalkyl substances and blood pressure in pre-diabetic adults-cross-sectional and longitudinal analyses of the diabetes prevention program outcomes study. Environ Int. (2020) 137:105573. doi: 10.1016/j.envint.2020.105573
38. Chen A, Jandarov R, Zhou L, Calafat AM, Zhang G, Urbina EM, et al. Association of perfluoroalkyl substances exposure with cardiometabolic traits in an island population of the eastern Adriatic coast of Croatia. Sci Tot Environ. (2019) 683:29–36. doi: 10.1016/j.scitotenv.2019.05.250
39. Blake BE, Fenton SE. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology. (2020) 443:152565. doi: 10.1016/j.tox.2020.152565
40. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
41. Wang J, Zhang J, Fan Y, Li Z, Tao C, Yan W, et al. Association between per- and polyfluoroalkyl substances and risk of gestational diabetes mellitus. Int J Hyg Environ Health. (2022) 240:113904. doi: 10.1016/j.ijheh.2021.113904
42. Zhang YZ, Wang B, Wang W, Li WC, Huang J, Deng SB, et al. Occurrence and source apportionment of per- and poly-fluorinated compounds (PFCs) in North Canal Basin, Beijing. Sci Rep. (2016) 6:36683. doi: 10.1038/srep36683
43. Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. (2007) 36:666–76. doi: 10.1093/ije/dym018
44. Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. (2014) 122:711–8. doi: 10.1289/ehp.1307972
45. Morgan RL, Beverly B, Ghersi D. GRADE guidelines for environmental and occupational health: a new series of articles in environment international. Environ Int. (2019) 128:11–2. doi: 10.1016/j.envint.2019.04.016
46. Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
47. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
48. Ward-Caviness CK, Moyer J, Weaver A, Devlin R, Diaz-Sanchez D. Associations between PFAS occurrence and multimorbidity as observed in an electronic health record cohort. Environ Epidemiol. (2022) 6:e217. doi: 10.1097/EE9.0000000000000217
49. Zhao J, Li A, Mei Y, Zhou Q, Li Y, Li K, et al. The association of arsenic exposure with hypertension and blood pressure: a systematic review and dose-response meta-analysis. Environ Pollut. (2021) 289:117914. doi: 10.1016/j.envpol.2021.117914
50. Li A, Zhou Q, Mei Y, Zhao J, Zhao M. Xu, et al. Novel strategies for assessing associations between selenium biomarkers and cardiometabolic risk factors: concentration, visit-to-visit variability, or individual mean? Evidence from a repeated-measures study of older adults with high selenium. Front Nutr. (2022) 9:838613. doi: 10.3389/fnut.2022.838613
51. Huang Q, Zhang J, Martin FL, Peng S, Tian M, Mu X, et al. Perfluorooctanoic acid induces apoptosis through the p53-dependent mitochondrial pathway in human hepatic cells: a proteomic study. Toxicol Lett. (2013) 223:211–20. doi: 10.1016/j.toxlet.2013.09.002
52. Panaretakis T, Shabalina IG, Grandér D, Shoshan MC, DePierre JW. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol. (2001) 173:56–64. doi: 10.1006/taap.2001.9159
53. Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, et al. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. (2017) 28:313–20. doi: 10.1681/ASN.2016020154
54. Gurusinghe S, Brown RD, Cai X, Samuel CS, Ricardo SD, Thomas MC, et al. Does a nephron deficit exacerbate the renal and cardiovascular effects of obesity? PLoS ONE. (2013) 8:e73095. doi: 10.1371/journal.pone.0073095
55. Rogers JM, Ellis-Hutchings RG, Grey BE, Zucker RM, Norwood J Jr., Grace CE, et al. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol Sci. (2014) 137:436–46. doi: 10.1093/toxsci/kft248
56. Brem AS. Insights into glucocorticoid-associated hypertension. Am J Kidney Dis. (2001) 37:1–10. doi: 10.1053/ajkd.2001.20637
Keywords: per-and polyfluoroalkyl substances, hypertension, high blood pressure, meta-analysis, environmental pollutants
Citation: Xiao F, An Z, Lv J, Sun X, Sun H, Liu Y, Liu X and Guo H (2023) Association between per- and polyfluoroalkyl substances and risk of hypertension: a systematic review and meta-analysis. Front. Public Health 11:1173101. doi: 10.3389/fpubh.2023.1173101
Received: 24 February 2023; Accepted: 27 June 2023;
Published: 02 August 2023.
Edited by:
Qun Xu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Cheng-Yang Hu, Anhui Medical University, ChinaCopyright © 2023 Xiao, An, Lv, Sun, Sun, Liu, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehui Liu, bHhoMTk3NjIwMDZAMTYzLmNvbQ==; Huicai Guo, aHVpY2FpZ3VvQGhlYm11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.