
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 11 May 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1170085
 Yang Liu1†
Yang Liu1† Yan-Hua Chai1†
Yan-Hua Chai1† Yi-Fan Wu1†
Yi-Fan Wu1† Yu-Wei Zhang1
Yu-Wei Zhang1 Ling Wang1
Ling Wang1 Ling Yang1
Ling Yang1 Yi-Han Shi1
Yi-Han Shi1 Le-Le Wang1
Le-Le Wang1 Li-Sha Zhang1
Li-Sha Zhang1 Yan Chen1
Yan Chen1 Rui Fan1
Rui Fan1 Yu-Hua Wen1
Yu-Hua Wen1 Heng Yang1
Heng Yang1 Li Li2
Li Li2 Yi-Han Liu1
Yi-Han Liu1 Hui-Zhen Zheng1
Hui-Zhen Zheng1 Ji-Jin Jiang1
Ji-Jin Jiang1 Hao Qian1
Hao Qian1 Ru-Jia Tao1
Ru-Jia Tao1 Ye-Chang Qian2
Ye-Chang Qian2 Ling-Wei Wang3,4*
Ling-Wei Wang3,4* Rong-Chang Chen3,4*
Rong-Chang Chen3,4* Jin-Fu Xu1*
Jin-Fu Xu1* Chen Wang5,6,7
Chen Wang5,6,7Purpose: The study aimed to identify potential risk factors for family transmission and to provide precautionary guidelines for the general public during novel Coronavirus disease 2019 (COVID-19) waves.
Methods: A retrospective cohort study with numerous COVID-19 patients recruited was conducted in Shanghai. Epidemiological data including transmission details, demographics, vaccination status, symptoms, comorbidities, antigen test, living environment, residential ventilation, disinfection and medical treatment of each participant were collected and risk factors for family transmission were determined.
Results: A total of 2,334 COVID-19 patients participated. Compared with non-cohabitation infected patients, cohabitated ones were younger (p = 0.019), more commonly unvaccinated (p = 0.048) or exposed to infections (p < 0.001), and had higher rates of symptoms (p = 0.003) or shared living room (p < 0.001). Risk factors analysis showed that the 2019-nCov antigen positive (OR = 1.86, 95%CI 1.40–2.48, p < 0.001), symptoms development (OR = 1.86, 95%CI 1.34–2.58, p < 0.001), direct contact exposure (OR = 1.47, 95%CI 1.09–1.96, p = 0.010) were independent risk factors for the cohabitant transmission of COVID-19, and a separate room with a separate toilet could reduce the risk of family transmission (OR = 0.62, 95%CI 0.41–0.92, p = 0.018).
Conclusion: Patients showing negative 2019-nCov antigen tests, being asymptomatic, living in a separate room with a separate toilet, or actively avoiding direct contact with cohabitants were at low risk of family transmission, and the study recommended that avoiding direct contact and residential disinfection could reduce the risk of all cohabitants within the same house being infected with COVID-19.
Coronavirus disease 2019 (COVID-19) is a highly contagious viral disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which had a catastrophic impact on global health, resulting in 656 million confirmed cases and a death toll up to 6.67 million worldwide till January 04, 2023 (Data from 2019ncov.chinacdc.cn) (1–5). The variant strain of Omicron is less virulent, but evolves toward highly contagious (6–9). Shanghai, the biggest city of China with a population of 25 million, has been suffering a wave of sub-lineage of the Omicron variant. Due to its high transmission rate, the pandemic has caused a dramatic increase in the number of confirmed cases. According to the Shanghai Municipal Health Commission, as of May 4, 2022, 593 336 cases have been identified, and 503 people have died with or from COVID-19 (10). The first co-infection case with BA.5.2.48 and BF.7.14 has recently been reported in China (11).
Many COVID-19 cases only showed mild symptoms or were asymptomatic, but a previous study showed us the transmission potential of asymptomatic patients (12). Thus, to limit the spread of the disease, there is an urgent need for an evolutionary strategy to manage COVID-19 patients. Studies had revealed that isolation of infected persons, mask use at home, disinfection and keeping social distancing were critical to reducing SARS-CoV-2 transmission in household settings (13–15). However, these had not been received much attention in the public health practice and we believed it deserved to be highlighted again.
Considering that the home may be the smallest unit of population aggregation, scientific home quarantine is important for epidemic prevention to reduce the consumption of medical resources during the pandemic (16–19). The WHO-China Joint Mission on COVID-19 urged prioritization of studies on risk factors for household transmission (20). Therefore, taking advantage of the event of Shanghai in 2022, this study aimed to analyze the exposure sources and indoor transmission of 2,334 infected patients during home quarantine prior to shelter admission, to identify potential factors affecting home quarantine efficiency, and ultimately to provide precautionary guidelines for the general public during COVID-19 waves.
A retrospective cohort study was conducted from 10 March 2022 to 30 April 2022. Patients who were confirmed with COVID-19 by positive nucleic acid test (a positive nucleic acid was defined as Ct value<35 (21)) and were transmitted to Fangcang shelter hospital were enrolled in this study. Relevant information including demographic data, exposure sources and transmission of each participant during home quarantine period before going to the shelter hospital were collected via telephone interviews and questionnaires. And potential factors affecting home quarantine efficiency were explored. The study was approved by the Research Ethics Committee of Shanghai Pulmonary Hospital (IRB number: L22–236). Written informed consents were waived by the Ethics Commission of the designated hospitals because of retrospectively study related to this emerging public health.
A standardized data collection spreadsheet was designed to obtain patients’ data from telephone interview and questionnaires (data in Supplementary File 2). All data collections were performed according to standardized protocol by researchers involved in this study. We defined cohabitation infection as the patient who became ill after direct contact with infected cohabitants, while non-cohabitation infection means that other cohabitants were not infected when the patient became infected, that is, she/he was the first person to be infected in the living environment. Two investigators independently reviewed the data collection forms to double check the validity of the data. Epidemiological data (upstream exposure source information, number of infected persons, downstream infected persons’ information, etc.), demographics (age, gender, BMI, occupation, educational level, vaccination information), symptoms onset (respiratory, gastrointestinal, neurological, etc.), comorbidities, 2019-nCov antigen test results, living environment, residential ventilation and disinfection, medical treatment were obtained for in-depth analysis.
The proportion of symptomatic and asymptomatic COVID-19 patients and differential indicators between the two groups were evaluated. The sensitivity of the 2019-nCov antigen test and differential indicators between patients with positive antigen test and negative antigen test were determined. Comparisons between non-cohabitation infection patients and cohabitated infection patients were performed and analyzed, and a household transmission rate of the epidemic was reported. Risk factors for household transmission of SARS-CoV-2 Omicron variant during home quarantine were clarified.
The Kolmogorov–Smirnov test was applied for analyzing the distribution of quantitative variables. Quantitative data were presented as median (interquartile range) for non-normally distributed variables or mean (standard deviation) for normally distributed variables and qualitative data were presented as frequencies (percentages). Independent group t-test was applied to analyze quantitative variables that were normally distributed and homoscedasticity, and the Mann–Whitney U test was applied to analyze quantitative variables that were non-normally distributed or not homoscedasticity. Qualitative variables between the two groups were compared by using Chi-square test or Fisher’s exact test. In the multivariate analysis, the logistic regression was used to determine risk factors associated with household transmission of SARS-CoV-2 Omicron variant. Variables with significance level p < 0.1 in the univariate analysis were preliminary screened out and were entered into the multivariate regression model. The odds ratio (OR) and 95% confidence interval (CI) were calculated for the independent variables. For all analyses, p < 0.05 was considered statistically significant. All statistical analyses and diagramming were performed by SPSS (version 23.0, IBM), GraphPad Prism (version 8.0, GraphPad Software Inc), Origin Pro (version 2016, OriginLab) softwares.
A total of 2,334 confirmed COVID-19 patients were included. Patients were aged 1–91 years with a median age of 43 years (31–54), and 1,286 (55.1%) were male (Table 1). On the basis of their presentations prior to the examined day, 616 (26.4%) patients without any clinical signs or symptoms were classified as asymptomatic, while 1718 (73.6%) were classified as symptomatic. Compared with asymptomatic patients, symptomatic cases had higher rates of 2019-nCov antigen positive (283 [52.2%] vs. 1,249 [76.2%], p < 0.001) and household transmission (63 [9.9%] vs. 344 [20.0%], p < 0.001). A total of 2,181 (93.4%) patients completed the 2019-nCov antigen test at the same time as the nucleic acid test and the sensitivity of the 2019-nCov antigen test was 70.2% (1532) in our study. Compared with 2019-nCov antigen-negative patients, the proportion of 2019-nCov antigen-positive patients who had symptoms (390 [60.1%] vs. 1,249 [81.5%], p < 0.001), medical treatment (352 [54.2%] vs. 1,108 [72.3%], p < 0.001), and downstream cohabitants transmission (71 [10.9%] vs. 315 [20.6%], p < 0.001) were significantly higher (Supplementary Table 1).
In this study, we investigated the upstream potential exposure factors of patients and found that of the 2,334 patients enrolled, 698 (29.9%) patients were infected from their cohabitants, and 1,636 (70.1%) patients were infected from non-cohabitants. The diagnostic time line for the two groups was shown in Figure 1A. Compared with patients with non-cohabitation infection, patients with cohabitated infection were younger (43 [32–54] vs. 41 [29–54], p = 0.019), more common being unvaccinated (176 [10.9%] vs. 100 [14.3%], p = 0.048) and having direct contact exposure (1,054 [68.9%] vs. 552 [82.4%], p < 0.001), had a higher rate of symptoms (1,175 [71.8%] vs. 543 [77.8%], p = 0.003) and needed more medical treatment (1,050 [64.2%] vs. 486 [69.6%], p = 0.011), as well as having a higher proportion of sharing living room (989 [65.1%] vs. 511 [76.5%], p < 0.001) (Table 2, Figures 1B–E). We further analyzed the age of the upstream transmission source population, the enrolled patient population, and the downstream infected population, and found that downstream infected patients were significantly younger than upstream population and all enrolled patients (38 [25–54] vs. 46 [33–55], p < 0.001; 38 [25–54] vs. 43 [31–54], p < 0.001) (Supplementary Figure S1). In addition, the household transmission rate of patients with cohabitated infection was lower than that of patients with non-cohabitation infection (84 [12.0%] vs. 321 [19.6%], p < 0.001) (Table 2).
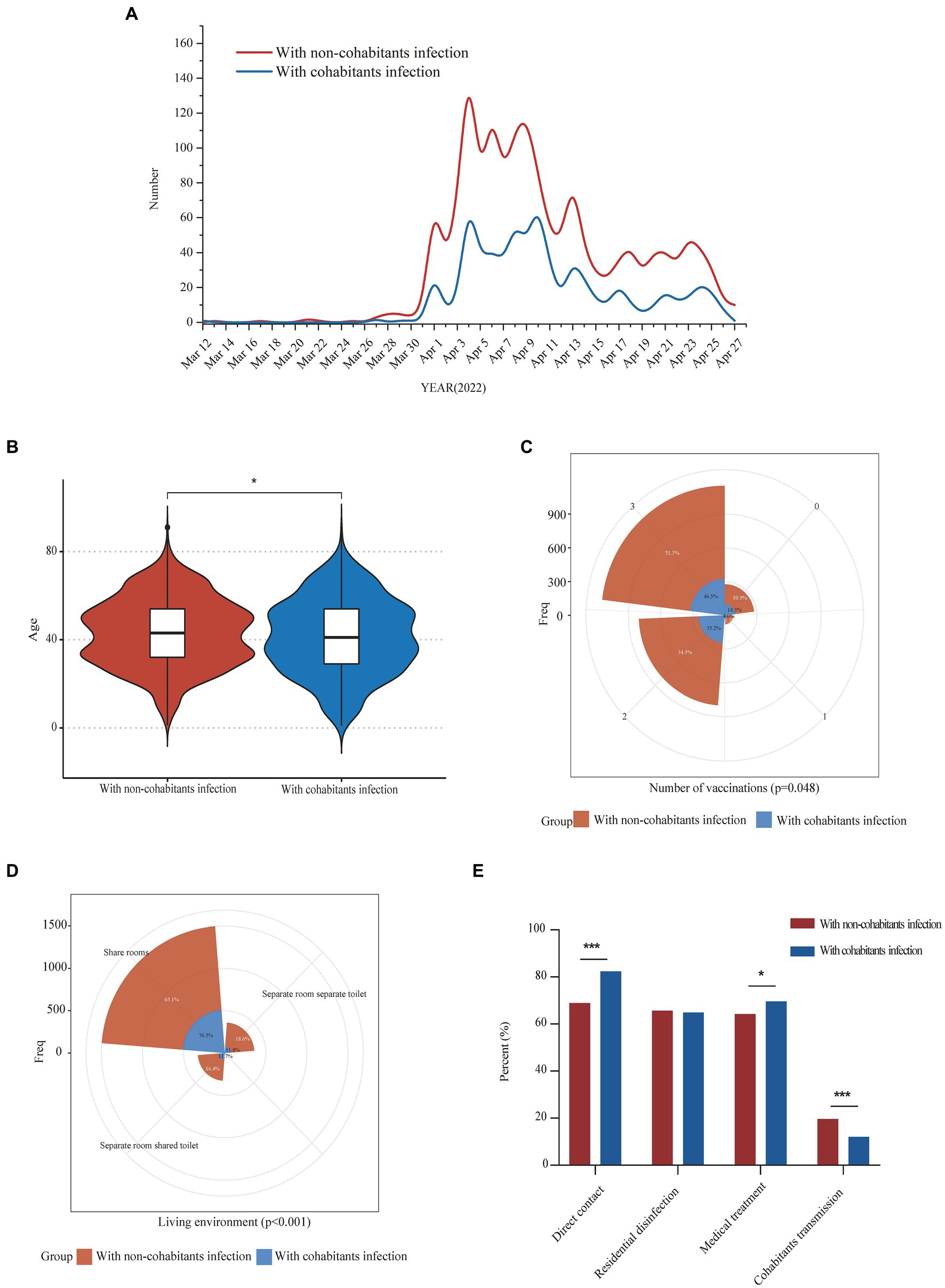
Figure 1. Epidemiological characteristics comparison of COVID-19 patients with infectious exposure from cohabitants and non-cohabitants. Panel (A) showed the daily numbers of COVID-19 patients with infectious exposure from cohabitants and non-cohabitants from 12 March 2022 to 27 April 2022; Comparisons of differences in age, vaccination frequency and living environment between the two groups were shown in panel (B–D) respectively; Differences in the proportion of direct contact exposure, residential disinfection, medical treatment and downstream cohabitants transmission between the two groups were shown in panel (E). *p < 0.05; **p < 0.01; ***p < 0.001.
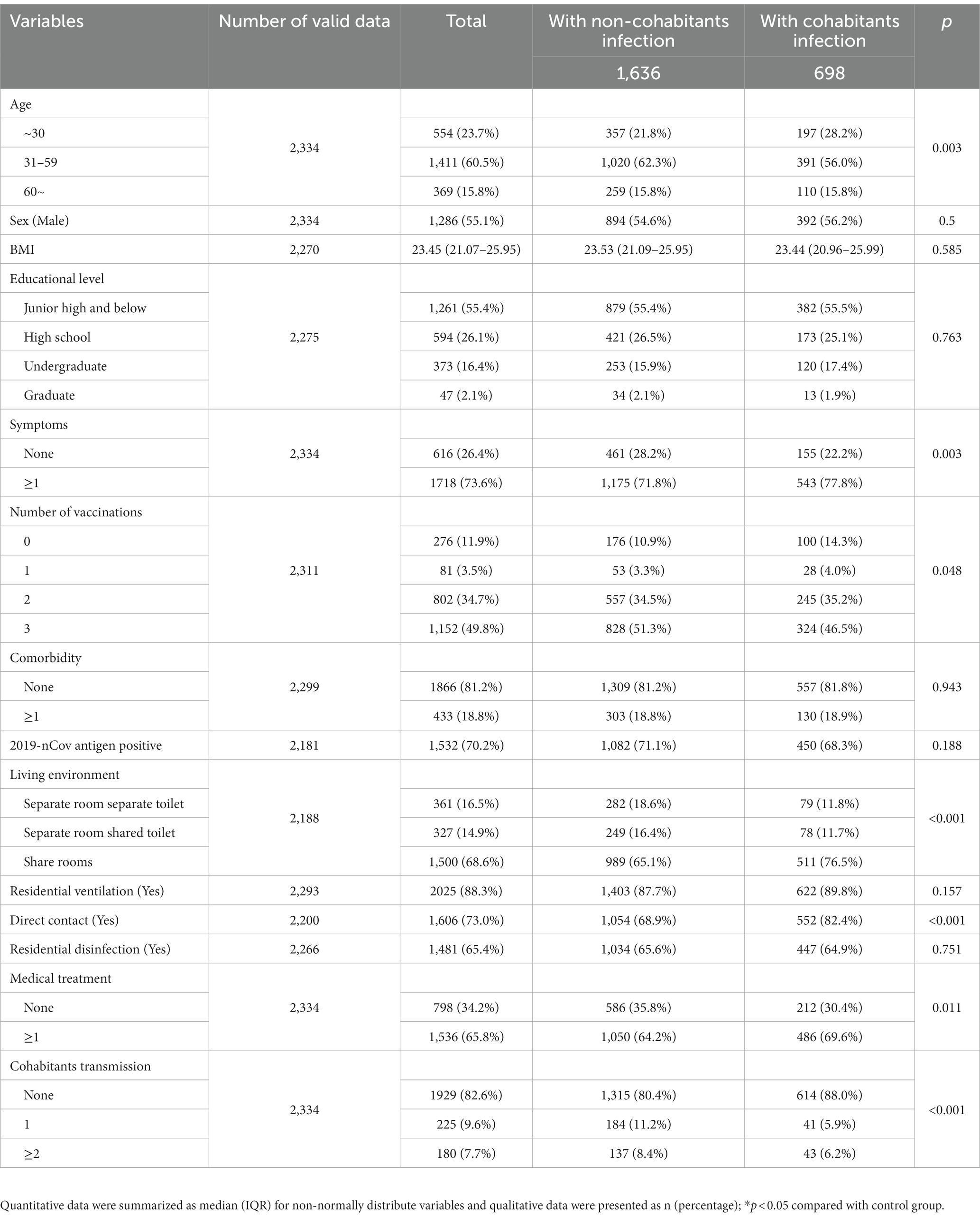
Table 2. Comparative analysis of baseline characteristics of COVID-19 patients with infectious exposure from cohabitants and non-cohabitants.
There were 405 (17.4%) cases in which patients transmitted the disease to their cohabitants (Figure 2A). The multivariate analysis of independent factors associated with household transmission of SARS-CoV-2 Omicron variant during home quarantine was showed in Table 3. Independent risk factors including 2019-nCov antigen positive (OR = 1.86, 95%CI 1.40–2.48, p < 0.001), symptoms development (OR = 1.86, 95%CI 1.34–2.58, p < 0.001), direct contact exposure (OR = 1.47, 95%CI 1.09–1.96, p = 0.010) were associated with increased household transmission of the disease, while having a separate room with a separate toilet could reduce the risk of household transmission during home quarantine (OR = 0.62, 95%CI 0.41–0.92, p = 0.018). Compared with patients who had no infection to cohabitants, patients that transmitted the disease to their cohabitants had higher rates of symptoms (1,374 [71.2%] vs. 344 [84.9%], p < 0.001) (Figure 2B), 2019-nCov antigen positive (1,217 [67.8%] vs. 315 [81.6%], p < 0.001), direct contact exposure (1,285 [68.3%] vs. 321 [79.5%], p < 0.001) (Figure 2C), as well as a higher proportion of patients with living environment of a sharing room (1,193 [66.3%] vs. 307 [78.9%], p < 0.001) (Figure 2D). Considering that living environment significantly affected the spread of the disease among the cohabitants during home quarantine, we divided all patients into two groups with or without a separate room and a separate toilet, and analyzed the difference in the household transmission rates between the two groups. Results showed that the downstream household transmission rate of patients without a separate room and a separate toilet was significantly increased compared with that of patients with a separate room and a separate toilet (371 [18.8%] vs. 34 [9.4%], p < 0.001) (Figure 2E).
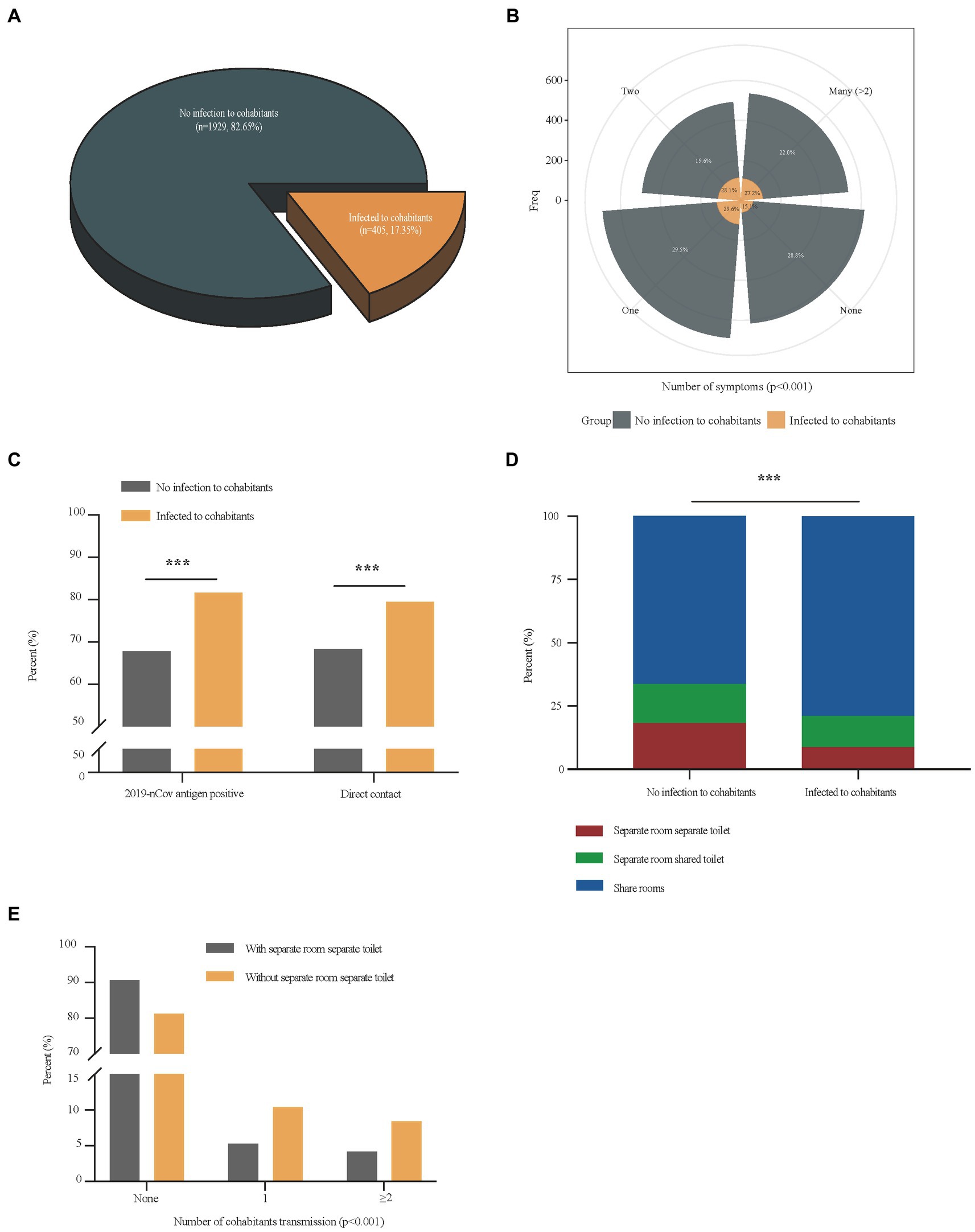
Figure 2. Comparisons of differential factors of COVID-19 patients with and without cohabitation transmission. Panel (A) showed the proportion of patients who transmitted the disease to their cohabitants in total patients; Differences in the number of symptoms, and the proportion of 2019-nCov antigen positive, direct contact exposure and living environments between COVID-19 patients with cohabitants transmission and those without cohabitants transmission were shown in panel (B–D); Differences in the number of cohabitants transmission between COVID-19 patients with separate room separate toilet and those without separate room separate toilet was shown in panel (E). ***p < 0.001.
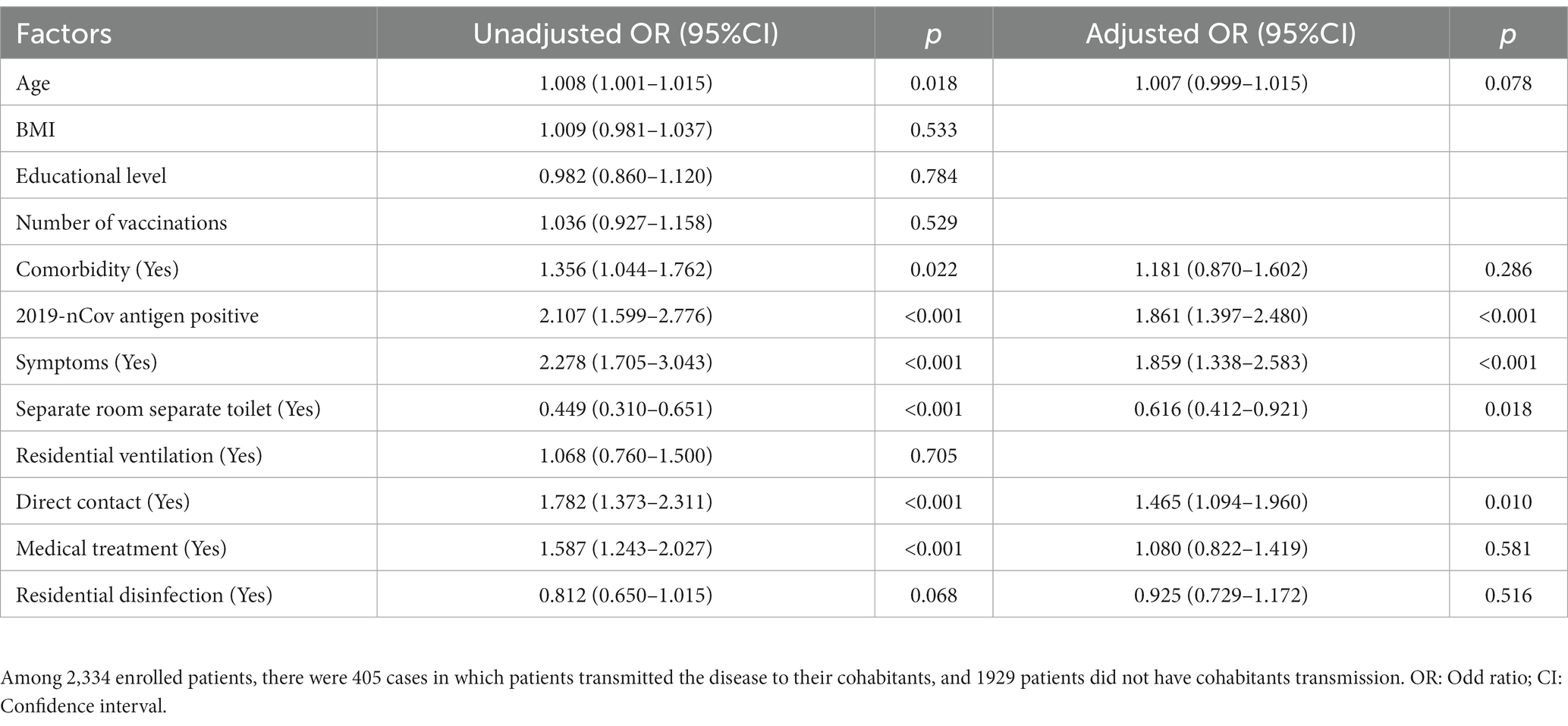
Table 3. Multivariate analysis of independent factors associated with cohabitants transmission of COVID-19.
Of the 2,334 patients enrolled in the study, all cohabitants of 811 (34.7%) patients were confirmed with COVID-19 during home quarantine. Important characteristics of these 811 patients were summarized in Supplementary Table S2. Patients were aged 1–89 years, with a median age of 41 years (30–54), and 479 (59.1%) were male. Among them, 386 (47.6%) patients had completed three doses of vaccination, 605 (74.6%) patients had symptoms, 138 (17.2%) patients had comorbidity, 532 (65.9%) patients had taken medical treatment, only 94 (12.5%) patients had a living environment of a separate room and a separate toilet, and up to 631 (78.3%) patients had direct contact exposure with their cohabitants. In this population, the positive rate of 2019-nCov antigen test of patients was 71.6% (544), and the cohabitants transmission rate of these patients was 21.6% (175). The multivariate results of independent factors associated with COVID-19 infection in all cohabitants within the same house during home quarantine were shown in Table 4. Direct contact exposure with cohabitants (OR = 1.36, 95%CI 1.09–1.71, p = 0.008) was an independent risk factor for the virus infection in all cohabitants within the same house during home quarantine, while residential disinfection could reduce this risk (OR = 0.78, 95%CI 0.63–0.95, p = 0.016).
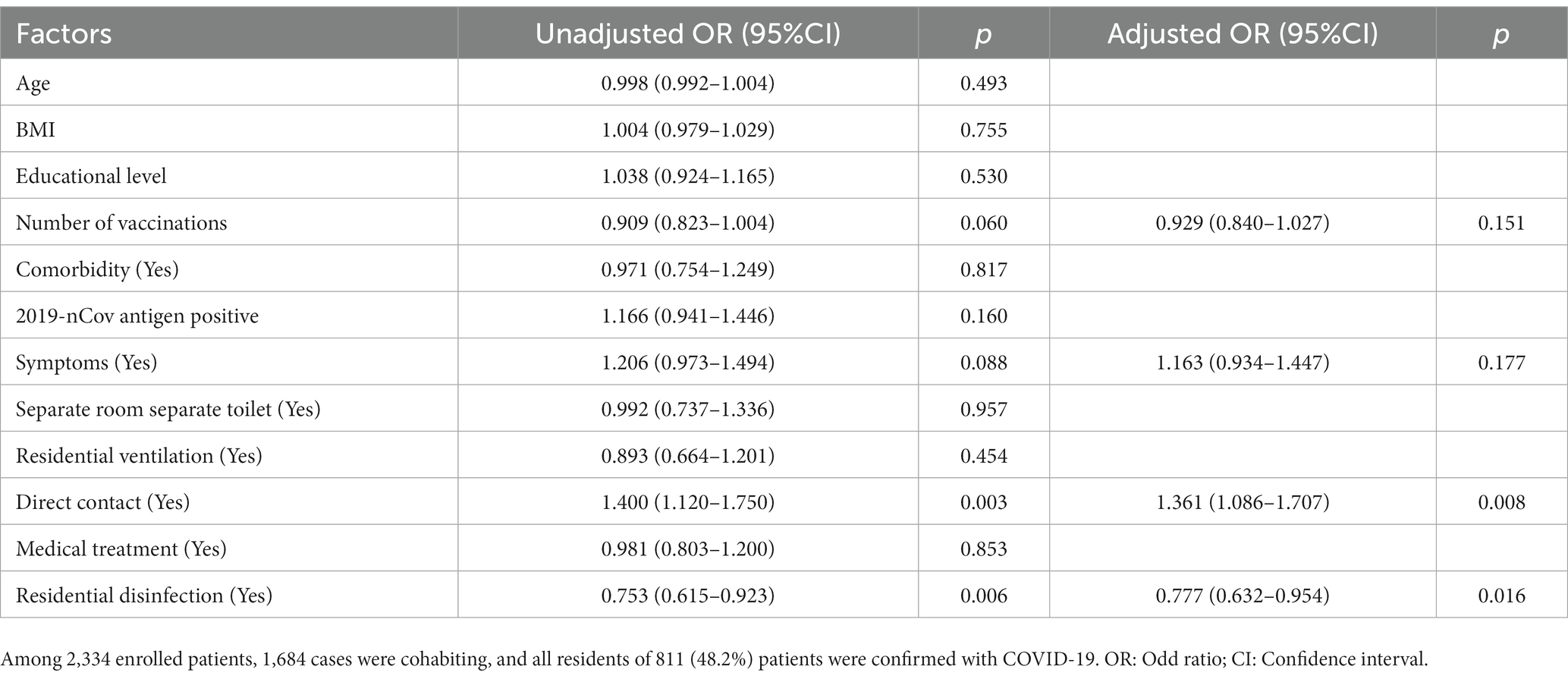
Table 4. Multivariate analysis of independent factors associated with COVID-19 infection in all cohabitants within the same house during home quarantine.
It may lead to secondary or multiple transmission of the COVID-19 if effective measures were not taken when families were infected. Studies showed that the outbreak of infection in a large population over a short time could easily lead to the emergence of new mutant strains. Minimizing the prevalence of SARS-CoV-2 will reduce chances of forming recombinant lineages with genetic combinations that may increase the adaptability of the virus potentially (6, 22). It is particularly important to protect vulnerable groups such as the older adults, children and pregnant women by reducing household transmission of the disease effectively. And the Lancet was calling for the creation of a strong resilient health system and international preparedness strategies to control the pandemic (23). Thus, our study analyzed and determined risk factors associated with household transmission of SARS-CoV-2 Omicron variant through a large-scale epidemiological survey of 2,234 patients. Based on the results, we found a group of patients who were at low risk of transmission. They were patients with 2019-nCov antigen negative, no relevant clinical symptoms, living in a separate room with a separate toilet, and actively avoiding direct contact with cohabitants.
SARS-CoV-2 is more transmissible in households than SARS-CoV and Middle East respiratory syndrome coronavirus (24). The long incubation period and high presymptomatic contagiousness of COVID-19 make transmission among family members a special risk (25, 26). Evidence on the spread of SARS-CoV-2 Omicron variant during home quarantine has been reported in different countries, while characteristics of family transmission of the COVID-19 pandemic and important factors associated with it were still poorly understood (5, 13, 27, 28). In this study, we observed that the household transmission rate of the Omicron variant was 17.4%, which was much lower than that reported in the United States (52.7%) and South Korea (50.0%) (13, 29). In addition, Madewell et al. systematically analyzed the household secondary attack rates of Omicron variant from seven foreign studies and found that the overall household secondary attack rate was 42.7% (35.4–50.4%) (30), which was also higher than that in Shanghai in 2022. The heterogeneity in household transmission rates in different regions may be due to differences in control measures, surveillance practices, and household crowding.
Studies had revealed that many factors could influence the secondary transmission of SARS-CoV-2 in households, including isolation of infected persons, mask use at home, disinfection and social distancing (13, 14). In addition to these factors, our study showed that 2019-nCov antigen positive and symptoms development were independent risk factors for family transmission of the disease. Moreover, the study recommended that avoiding direct contact and residential disinfection could reduce the risk of all cohabitants within the same house being infected with SARS-CoV-2 Omicron variant. These will inform precautionary guidelines for families to reduce indoor transmission in areas where there is high community transmission for COVID-19.
It had been reported that viral load in asymptomatic patients was comparable to that in symptomatic patients, suggesting the transmission potential of asymptomatic patients (12, 31). However, Li et al. found that asymptomatic patients were less likely to infect others than symptomatic cases, and symptomatic cases were more infectious during the incubation period than those during the symptomatic period (20). These were consistent with our finding that symptomatic cases had higher rates of household transmission than that of asymptomatic patients. Antigen-based rapid tests have important diagnostic value early in the course of disease, which showed relatively high sensitivity in SARS-CoV-2 diagnosis in the early phase of infection (32). It had been reported that the average sensitivity of commercial antigen assays was higher in symptomatic compared to asymptomatic participants (33), and antigen-based rapid diagnostic test sensitivity was higher in the first 7 days after symptom onset than that in asymptomatic patients (34). Similarly, symptomatic patients had a higher proportion of positive 2019-nCov antigen testing than asymptomatic patients in this study. These may be related to the different courses of the disease in the two groups of patients when the antigen testing was performed. The above provided possible explanations why 2019-nCov antigen positive, symptomatic patients were at a higher risk of household transmission during home quarantine.
The spread of the novel coronavirus was associated with exposure to fomites, aerosols, and droplets, especially in the crowded or confined spaces (8, 35). It had been reported that the risk and probability of being caught by the indoor COVID-19 disease increased in time, particularly in the downstream of a localized infectious person (36). A previous study provided the evidence of the effectiveness of social distancing in preventing COVID-19 (14), which was consistent with our findings that direct contact exposure could increase the risk of home transmission, and the living conditions of a separate room with a separate toilet could protect the cohabitants from infection. Randomized clinical trials had shown that hand hygiene alone did not prevent respiratory transmissible viruses, but the combination of masks did work (37). Importantly, our study showed that, except for the uncontrollable conditions of the patient, it could greatly reduce the spread of the disease during home quarantine by improving hygiene measures such as avoiding direct contact with cohabitants and actively disinfecting the house, and providing separate living space for infected cohabitants, thereby greatly reducing the risk that all of the family members becoming infected.
In addition, we found that patients with cohabitation infection were younger than those with non-cohabitation infection. The phenomenon may be partially due to a lack of self-protect awareness, but the main reason should be that most older patients were responsible for the normal life of their families and were more likely to go out to the gathering places such as supermarkets or farmers’ markets to buy daily necessities, which increases the risk of infection. While most young patients were less active, and their infection mainly came from contacting with their infected cohabitants. Moreover, the living environment of these people was mainly of sharing room, and the proportion of isolated conditions with a separate room and a separate toilet was very small, making it difficult to avoid contact with infected cohabitants. These indicated that home quarantine conditions played a very important role in the spread of the epidemic within the household. It is noteworthy that the number of vaccinations was not an independent risk factor of home transmission in our study. Similarly, previous studies observed in some countries that, the vaccination offered less protect against the spread of disease, since some portions of vaccinated people were not totally immunized (38, 39).
Our study has several limitations. Telephone interviews have inherent limitations, including recall bias. In addition, we could not collect baseline information on uninfected cohabitants of enrolled patients, and therefore could not do a comparative analysis. Besides, the study could only define that the non-cohabitation infection originated from social contact, and could not exclude other infection risks.
In conclusion, important factors revealed in this study, including negative 2019-nCov antigen tests, absence of associated clinical symptoms, living in a separate room with a separate toilet, and active avoidance of direct contact with cohabitants, will inform precautionary guidelines for all families to reduce household transmission during the waves of COVID-19 pandemic, and the study recommended that avoiding direct contact and residential disinfection could reduce the risk of all cohabitants within the same house being infected with COVID-19.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: written informed consents were waived by the Ethics Commission of the designated hospitals because of retrospectively study related to this emerging public health.
YL, Y-HC, and Y-FW: data analysis and these authors have contributed equally to this work. CW and J-FX: supervision, concept, and design. YL, Y-HC, Y-FW, Y-WZ, LW, LY, Y-HS, L-LW, L-SZ, YC, RF, Y-HW, HY, LL, Y-HL, H-ZZ, J-JJ, HQ, R-JT, and Y-CQ: acquisition and interpretation of data. YL, J-FX, and CW: drafting of the manuscript. L-WW, R-CC, J-FX, and CW: critical revision of the manuscript for important intellectual content and administrative, technical, or material support. All authors contributed to the article and approved the submitted version.
The authors would like to express our sincere thanks to all the hospital staffs and all the patients for their contributions to the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1170085/full#supplementary-material
1. Cascella, M, Rajnik, M, Aleem, A, Dulebohn, SC, and Di Napoli, R. Features, evaluation, and treatment of coronavirus (COVID-19) In: StatPearls Publishing Copyright © 2022, editor. StatPearls. Treasure Island, FL: StatPearls Publishing (2022)
2. To, KK, Sridhar, S, Chiu, KH, Hung, DL, Li, X, Hung, IF, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. (2021) 10:507–35. doi: 10.1080/22221751.2021.1898291
3. Guan, WJ, Ni, ZY, Hu, Y, Liang, WH, Ou, CQ, He, JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
4. Aouissi, HA, Hamimes, A, Ababsa, M, Bianco, L, Napoli, C, Kebaili, FK, et al. Bayesian modeling of COVID-19 to classify the infection and death rates in a specific duration: the case of Algerian provinces. Int J Environ Res Public Health. (2022) 19:9586. doi: 10.3390/ijerph19159586
5. Leveau, CM, Aouissi, HA, and Kebaili, FK. Spatial diffusion of COVID-19 in Algeria during the third wave. GeoJournal. (2022) 88:1175–80. doi: 10.1007/s10708-022-10608-5
6. Ren, SY, Wang, WB, Gao, RD, and Zhou, AM. Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. (2022) 10:1–11. doi: 10.12998/wjcc.v10.i1.1
7. Liu, Y, Ning, Z, Chen, Y, Guo, M, Liu, Y, Gali, NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. (2020) 582:557–60. doi: 10.1038/s41586-020-2271-3
8. Greenhalgh, T, Jimenez, JL, Prather, KA, Tufekci, Z, Fisman, D, and Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. (2021) 397:1603–5. doi: 10.1016/s0140-6736(21)00869-2
9. Wiersinga, WJ, Rhodes, A, Cheng, AC, Peacock, SJ, and Prescott, HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 324:782–93. doi: 10.1001/jama.2020.12839
10. Zhang, X, Zhang, W, and Chen, S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. (2022) 399:2011–2. doi: 10.1016/S0140-6736(22)00838-8
11. Su, K, Huang, Y, Chen, X, Liu, F, Yan, Q, Jiang, X, et al. The first case of co-infection with omicron subvariants BA.5.2.48 and BF.7.14 – Chongqing municipality, China, February 2023. China CDC Wkly. (2023) 5:255–7. doi: 10.46234/ccdcw2023.046
12. Han, MS, Seong, MW, Kim, N, Shin, S, Cho, SI, Park, H, et al. Viral RNA load in mildly symptomatic and asymptomatic children with COVID-19, Seoul, South Korea. Emerg Infect Dis. (2020) 26:2497–9. doi: 10.3201/eid2610.202449
13. Baker, JM, Nakayama, JY, O'Hegarty, M, McGowan, A, Teran, RA, Bart, SM, et al. SARS-CoV-2 B.1.1.529 (omicron) variant transmission within households – four U.S. jurisdictions, November 2021–February 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:341–6. doi: 10.15585/mmwr.mm7109e1
14. Wang, Y, Tian, H, Zhang, L, Zhang, M, Guo, D, Wu, W, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. (2020) 5:e002794. doi: 10.1136/bmjgh-2020-002794
15. Goodwin, L, Hayward, T, Krishan, P, Nolan, G, Nundy, M, Ostrishko, K, et al. Which factors influence the extent of indoor transmission of SARS-CoV-2? a rapid evidence review. J Glob Health. (2021) 11:10002. doi: 10.7189/jogh.11.10002
16. Denford, S, Morton, K, Horwood, J, de Garang, R, and Yardley, L. Preventing within household transmission of Covid-19: is the provision of accommodation to support self-isolation feasible and acceptable? BMC Public Health. (2021) 21:1641. doi: 10.1186/s12889-021-11666-z
17. Al-Tawfiq, JA, Kheir, H, Al-Dakheel, T, Al-Qahtani, S, AlKhadra, H, Sarhan, A, et al. COVID-19 home monitoring program: healthcare innovation in developing, maintaining, and impacting the outcome of SARS-CoV-2 infected patients. Travel Med Infect Dis. (2021) 43:102089. doi: 10.1016/j.tmaid.2021.102089
18. Li, H, Peng, YY, and Lu, JP. Investigation and analysis of 108 cases of home isolated patients with mild COVID-19. Disaster Med Public Health Prep. (2021) 15:e8–e11. doi: 10.1017/dmp.2020.296
19. Bulfone, TC, Malekinejad, M, Rutherford, GW, and Razani, N. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis. (2021) 223:550–61. doi: 10.1093/infdis/jiaa742
20. Li, F, Li, YY, Liu, MJ, Fang, LQ, Dean, NE, Wong, GWK, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. (2021) 21:617–28. doi: 10.1016/s1473-3099(20)30981-6
21. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 9). (2022). Available at: http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm
22. Jackson, B, Boni, MF, Bull, MJ, Colleran, A, Colquhoun, RM, Darby, AC, et al. Generation and transmission of interlineage recombinants in the SARS-CoV-2 pandemic. Cell. (2021) 184:5179–5188.e8. doi: 10.1016/j.cell.2021.08.014
23. The, L. COVID-19: the next phase and beyond. Lancet. (2022) 399:1753. doi: 10.1016/s0140-6736(22)00817-0
24. Jing, QL, Liu, MJ, Zhang, ZB, Fang, LQ, Yuan, J, Zhang, AR, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. (2020) 20:1141–50. doi: 10.1016/s1473-3099(20)30471-0
25. Ayaz, CM, Dizman, GT, Metan, G, Alp, A, and Unal, S. Out-patient management of patients with COVID-19 on home isolation. Infez Med. (2020) 28:351–6.
26. Little, P, Read, RC, Amlôt, R, Chadborn, T, Rice, C, Bostock, J, et al. Reducing risks from coronavirus transmission in the home-the role of viral load. BMJ. (2020) 369:m1728. doi: 10.1136/bmj.m1728
27. Abad-Corpa, E, Sánchez-López, D, and Moreno-Casbas, MT. Scoping review about the recommendations for home isolation in the COVID-19 pandemic. Enferm Clin. (2021) 31:S94–9. doi: 10.1016/j.enfcli.2020.05.007
28. Lopez Bernal, J, Panagiotopoulos, N, Byers, C, Garcia Vilaplana, T, Boddington, N, Zhang, XS, et al. Transmission dynamics of COVID-19 in household and community settings in the United Kingdom, January to march 2020. Euro Surveill. (2022) 27:20015. doi: 10.2807/1560-7917.Es.2022.27.15.2001551
29. Song, JS, Lee, J, Kim, M, Jeong, HS, Kim, MS, Kim, SG, et al. Serial intervals and household transmission of SARS-CoV-2 omicron variant, South Korea, 2021. Emerg Infect Dis. (2022) 28:756–9. doi: 10.3201/eid2803.212607
30. Madewell, ZJ, Yang, Y, Longini, IM Jr, Halloran, ME, and Dean, NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open. (2022) 5:e229317. doi: 10.1001/jamanetworkopen.2022.9317
31. Zou, L, Ruan, F, Huang, M, Liang, L, Huang, H, Hong, Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. (2020) 382:1177–9. doi: 10.1056/NEJMc2001737
32. Diao, B, Wen, K, Zhang, J, Chen, J, Han, C, Chen, Y, et al. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect. (2021) 27:289.e1–4. doi: 10.1016/j.cmi.2020.09.057
33. Dinnes, J, Sharma, P, Berhane, S, van Wyk, SS, Nyaaba, N, Domen, J, et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. (2022) 7:CD013705. doi: 10.1002/14651858.CD013705.pub3
34. Boum, Y, Fai, KN, Nikolay, B, Mboringong, AB, Bebell, LM, Ndifon, M, et al. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis. (2021) 21:1089–96. doi: 10.1016/s1473-3099(21)00132-8
35. Zhang, X, Wu, J, Smith, LM, Li, X, Yancey, O, Franzblau, A, et al. Monitoring SARS-CoV-2 in air and on surfaces and estimating infection risk in buildings and buses on a university campus. J Expo Sci Environ Epidemiol. (2022) 32:751–8. doi: 10.1038/s41370-022-00442-9
36. Turkyilmazoglu, M. Indoor transmission of airborne viral aerosol with a simplistic reaction-diffusion model. Eur Phys J Spec Top. (2022) 231:3591–601. doi: 10.1140/epjs/s11734-022-00614-6
37. Wong, VW, Cowling, BJ, and Aiello, AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol Infect. (2014) 142:922–32. doi: 10.1017/s095026881400003x
38. Turkyilmazoglu, M. An extended epidemic model with vaccination: weak-immune SIRVI. Physica A. (2022) 598:127429. doi: 10.1016/j.physa.2022.127429
Keywords: COVID-19, omicron, indoor transmission, risk factor, home quarantine
Citation: Liu Y, Chai Y-H, Wu Y-F, Zhang Y-W, Wang L, Yang L, Shi Y-H, Wang L-L, Zhang L-S, Chen Y, Fan R, Wen Y-H, Yang H, Li L, Liu Y-H, Zheng H-Z, Jiang J-J, Qian H, Tao R-J, Qian Y-C, Wang L-W, Chen R-C, Xu J-F and Wang C (2023) Risk factors associated with indoor transmission during home quarantine of COVID-19 patients. Front. Public Health. 11:1170085. doi: 10.3389/fpubh.2023.1170085
Received: 20 February 2023; Accepted: 13 April 2023;
Published: 11 May 2023.
Edited by:
Hasim Altan, Prince Mohammad bin Fahd University, Saudi ArabiaReviewed by:
Mustafa Turkyilmazoglu, Hacettepe University, TürkiyeCopyright © 2023 Liu, Chai, Wu, Zhang, Wang, Yang, Shi, Wang, Zhang, Chen, Fan, Wen, Yang, Li, Liu, Zheng, Jiang, Qian, Tao, Qian, Wang, Chen, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Wei Wang, bGltZXlAc2luYS5jb20=; Rong-Chang Chen, Y2hlbnJjQHZpcC4xNjMuY29t; Jin-Fu Xu, amZ4dUB0b25namkuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.