- Department of Immunization Program, Huangpu District Center for Disease Control and Prevention, Shanghai, China
Hand, foot and mouth disease (HFMD) is a kind of infectious disease caused by enterovirus infection. In this study we analysed the epidemiological characteristics and time trends of HFMD, vaccination status and vaccine protection effect assessment of EV71 vaccine from 2011 to 2021 in Huangpu District, Shanghai, China. HFMD cases showed a decreasing trend year by year from 2011 to 2021, from 122 cases reported in 2012 to 7 cases in 2020, and 12 cases in 2021. Etiological diagnosis was CV-A6 in 185 cases (29.8%), CV-A16 in 209 cases (33.7%), EV-A71 in 118 cases (19.0%) and other enteroviruses in 109 cases (17.6%). After the launch of EV71 vaccine, a total of 32,221 doses of EV71 vaccine were administered between 2016 and 2021. The case–control results showed that there was no evidence to support the effectiveness of EV71 vaccine, OR (95% CI) =0.52 (0.12 ~ 2.3), p = 0.37. The epidemic strains have changed. Surveillance and management of HFMD remain very important in the future and EV71 vaccine is considered to be included in National Immunization Program.
Introduction
Hand, foot and mouth disease (HFMD) is a kind of infectious disease caused by enterovirus infection, which mainly manifests as ulcerative herpes on the oral mucosa and blister-like rash on the extremities, which is common in children (1, 2). HFMD was listed as a notifiable infectious disease by the Ministry of Health of China in 2008 after an outbreak of HFMD which caused 23 deaths (3). The main pathogenic serotypes of HFMD include Coxsackievirus (CV) 4–7, 9, 10, 16 in group A, 1–3, 5 in group B, Enterovirus A71 (EV-A71) and partial serotypes of Echovirus. The common pathogens of HFMD in China are EV-A71, CV-A16 and some other enteroviruses (4, 5). However, the proportion of CV-A6 infections has gradually increased in recent years in China and around the world (6–8).The median annual incidence of HFMD was 153.78 per 100,000 (ranging from 120.79 to 205.06) in mainland China from 2011 to 2018 (9). China accounted for 87% (9.8 million/11.3 million) of all hand, foot, and mouth disease (HFMD) cases reported to WHO during 2010–2014 (10). EV-A71 is responsible for most of the severe HFMD cases and had the highest disease burden (11). HFMD not only costs, but also causes physical inconvenience and psychological pain to patients and their families. The invisible burden of these negative effects cannot be ignored. In 2016, Enterovirus Type 71 vaccine (EV71 vaccine) was approved for marketing in China (4). Currently, there are three manufacturers of EV71 vaccine on the market, including Wuhan Institute of Biological Products Co., Ltd. (Wuhan), Sinovac Biotech Co., Ltd. (Sinovac) and Institute of Medical Biology of Chinese Academy of Medical Sciences (CAMS) (12). In this study we analyzed the epidemiological characteristics and time trends of HFMD, vaccination status and vaccine protection effect assessment of EV71 vaccine in Huangpu District, Shanghai, China from 2011 to 2021. Our results can be used to adjust the prevention and control measures of HFMD and vaccination strategies.
Methods
Data collection
Exclusion and inclusion criteria
All HFMD cases were reported in China Disease Prevention and Control Information System due to the Law on the Prevention and Treatment of Infectious Diseases in China from 2008.
· Those who meet the diagnostic criteria of HFMD should be included, according to the National Health Commission’s Diagnosis and Treatment Guidelines for Hand, Foot and Mouth Disease.
· The time of onset was 2011–2021.
· Cases of HFMD have been laboratory diagnosed.
1. The specific PCR test of enterovirus (CV-A16, EV-A71, etc.) was positive.
2. Enterovirus was isolated and identified as CV-A16, EV-A71 or other enterovirus that can cause HFMD.
· Exclude clinically diagnosed cases without laboratory diagnosis.
Case information included population classification, age of onset, sex, onset time, diagnosis time, laboratory diagnosis, and pathogen classification. In the case reporting information system, the population was divided into: scattered children, preschool children, students and adults. In China, children who have not reached the age of kindergarten are usually referred to as scattered children.
Laboratory testing methods
The stool samples of HFMD cases were collected according to the “Technical Scheme for Collection and Testing of Hand, Foot and Mouth Disease Specimens,” and the etiological detection and typing were carried out by real-time PCR method. The etiological test results were divided into: EV-A71, CV-A16, CV-A6 and other enteroviruses.
Vaccination record
EV71 vaccination in Huangpu District of Shanghai from 2011 to 2021 was collected to analyze the relationship between EV71 vaccination and HFMD incidence trend. For HFMD laboratory-confirmed cases, the EV71 vaccination records were obtained by querying the Shanghai Immunization Information System. The full course of EV71 vaccination requires 2 doses, spaced 1 month apart. In this study, the completion of 2 doses of vaccination within 42 days before the onset of the disease was considered as a history of EV71 vaccination. Cases received one dose and vaccinated within 42 days before the onset of HFMD were considered to have no vaccination history. For some cases, if the vaccination is not in Shanghai, the vaccination record cannot be acquired in the Shanghai Immunization Information System. Therefore, the vaccination history of those cases was unknown and recorded as missing value.
Statistic methods
The cases were summarized and analyzed according to the time of onset and type of diagnosis, and the characteristics and trends of HFMD during 2011–2021 were described. Chi-square test was used to compare the differences of HFMD cases among different factors. According to the laboratory diagnosis results, the case group was diagnosed with EV-A71, and the control group was diagnosed with other enteric pathogens. Whether there was a history of EV71 vaccination as a risk factor, a case–control study was conducted to analyze the protective effect of EV71 vaccine. All statistical analyses were performed using SPSS version 18.0, p < 0.05 or 95% confidence interval (95% CI) excluding 0 was considered statistically significant.
Results
Epidemiological characteristics
From 2011 to 2021, a total of 621 laboratory-confirmed cases of HFMD were reported in Huangpu District, Shanghai, and no severe and fatal cases were reported. The mean age at onset was 4.1 years, the median age at onset was 3.7 years, the youngest was 2 months old, and the oldest was 26 years old. There were 368 males (59.3%) and 253 females (40.7%), with a sex ratio of 1.45:1. 290 (46.7%) were preschool children, 257 (41.4%) were scattered children, 73 (11.8%) were students, and 1 (0.1%) was an adult. 415 people (66.8%) had not received EV71 vaccine, 72 people (11.6%) had received EV71 vaccine, and 134 people (21.6%) could not find the vaccination records.
Seasonal trend
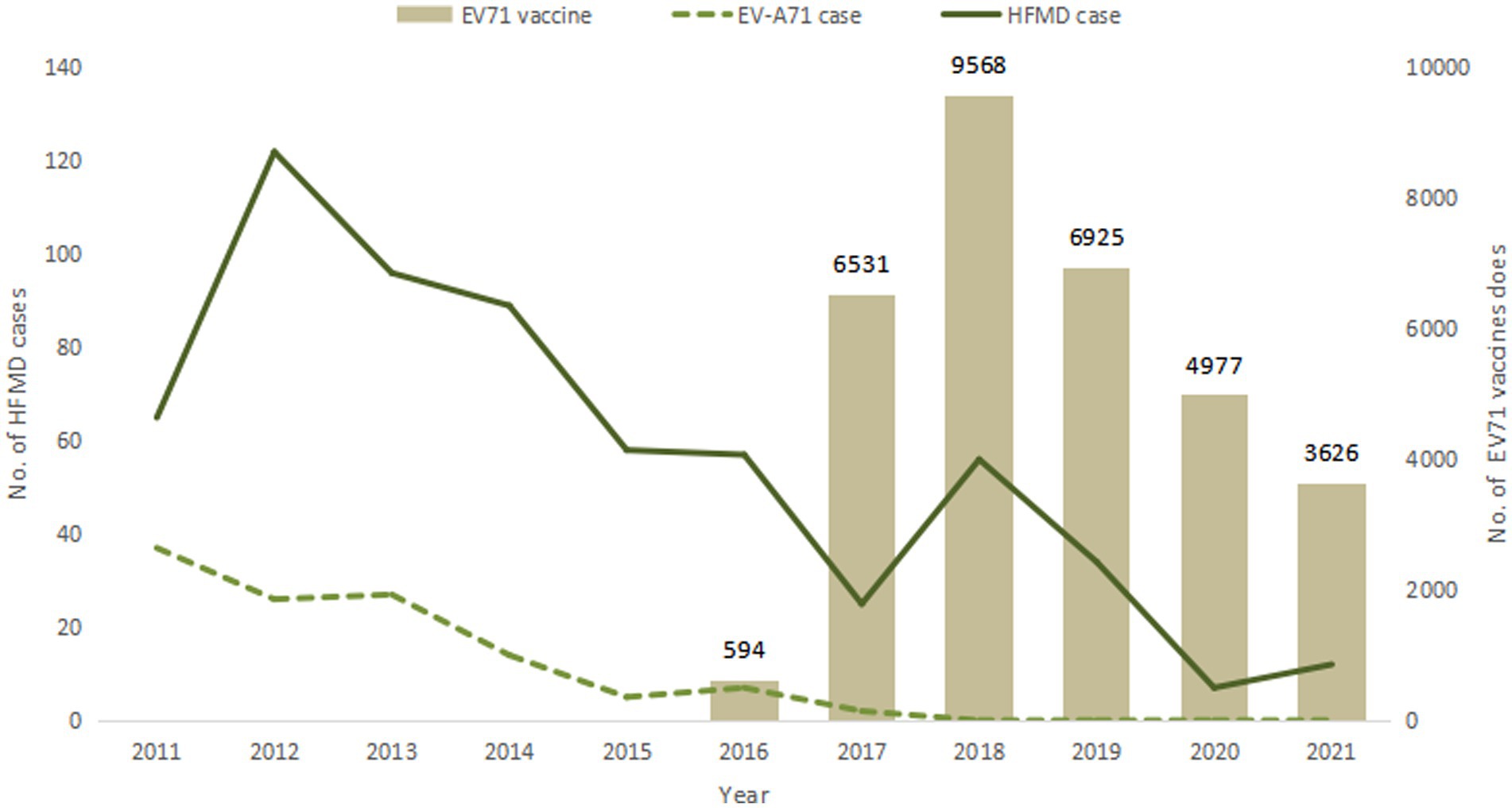
According to the reported onset year, HFMD cases showed a decreasing trend year by year from 2011 to 2021, from 122 cases reported in 2012 to 7 cases in 2020, and 12 cases in 2021. Among them, EV-A71 HFMD cases were reported most in 2011, with 37 cases, and then showed a downward trend. There was no EV-A71 HFMD case from 2018 to 2021. The month with the least reported cases was February (8 cases, accounting for 1.3%), the main peak of number of reported cases was from May to July, the second peak was from September to November. Most cases were reported in June (128 cases, 20.6%), May (88 cases, 14.2%) and July (75 cases, 12.1%) as shown in Figure 1.
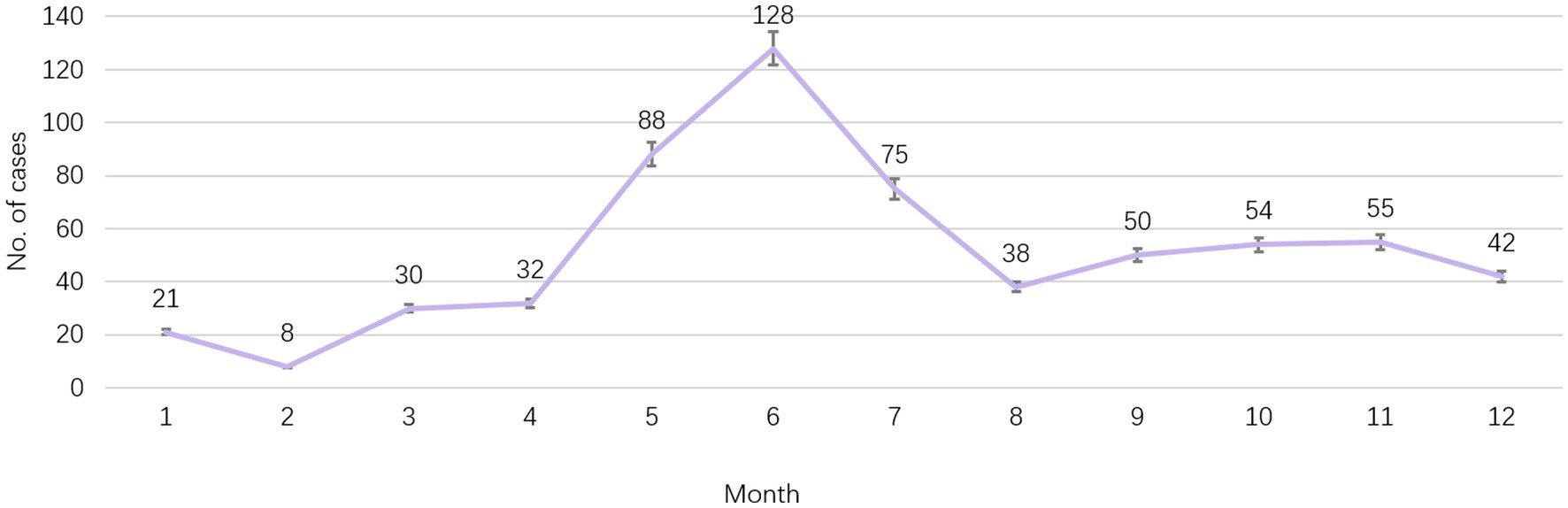
Figure 1. Seasonal trend of report cases of HFMD in Huangpu District, Shanghai, China from 2011 to 2021.
Etiological diagnosis
Etiological diagnosis was CV-A6 in 185 cases (29.8%), CV-A16 in 209 cases (33.7%), EV-A71 in 118 cases (19.0%) and other enteroviruses in 109 cases (17.6%). The proportion of EV-A71 decreased year by year, while that of CV-A6 increased year by year. All cases reported in 2020 (7 cases) were diagnosed as CV-A6, and the specific composition ratio was shown in Figure 2.
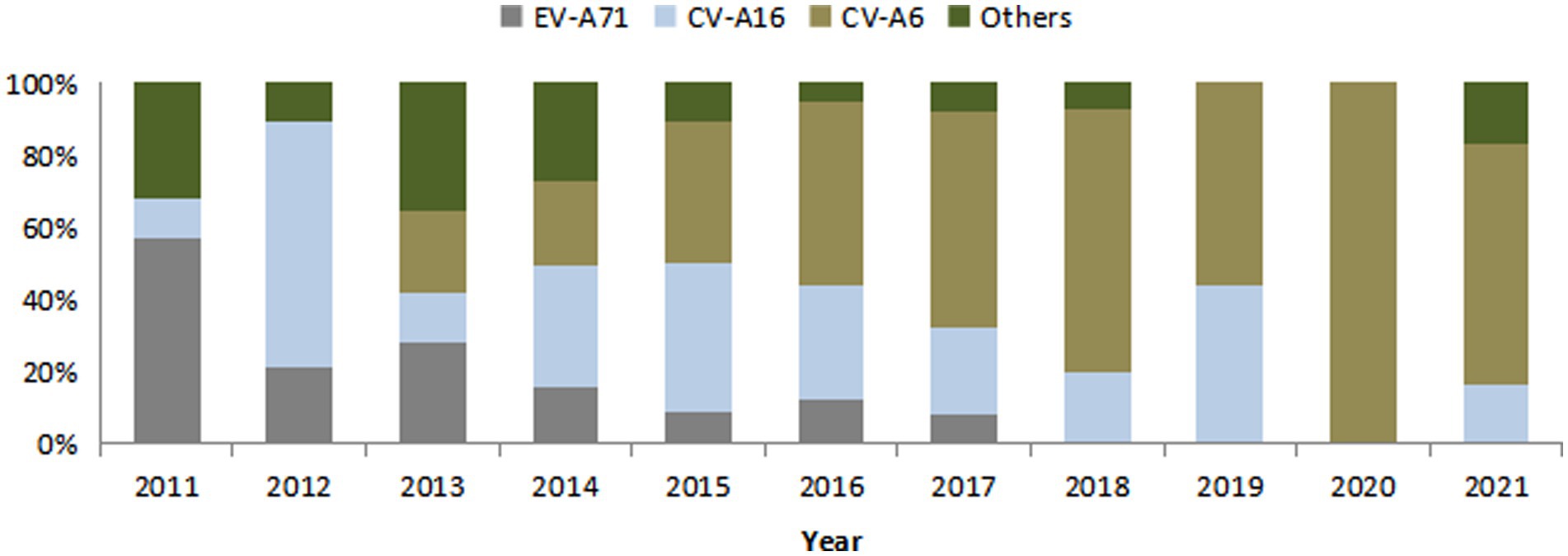
Figure 2. Composition of pathogenic diagnostic classification of HFMD cases in Huangpu District, Shanghai, China from 2011 to 2021.
The mean age of onset for each etiological diagnosis type was as follows: 4.4 years for CV-A6, 4.1 years for CV-A16, and 3.7 years for EV-A71 and other enteroviruses. The mean age of onset was statistically significant in the classification of etiological diagnosis, F = 3.02, p = 0.03. The influencing factors of gender, EV71 vaccination history and different population classification on the composition of etiological diagnosis types were analyzed, and the results were shown in Table 1. The influencing factor of EV71 vaccination history on the composition of etiological diagnosis types was statistically significant (p < 0.05).
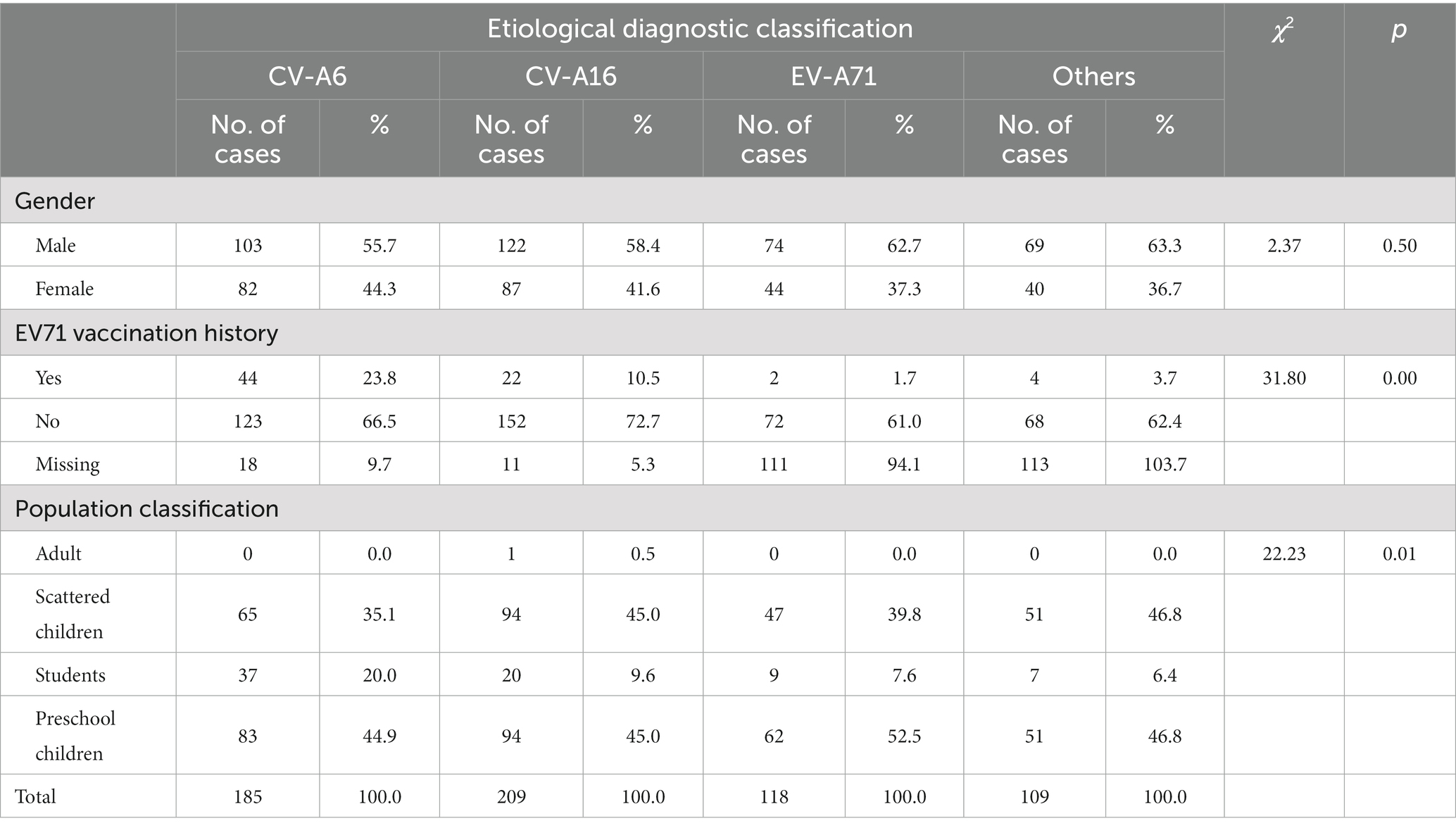
Table 1. Composition and influencing factors of diagnostic classification of HFMD cases from 2011 to 2021.
Vaccination status
After the launch of EV71 vaccine, a total of 32,221 doses of EV71 vaccine were administered between 2016 and 2021, with the largest amount of 9,568 doses in 2018. The vaccines were produced by three manufacturers, including 23,007 doses (71.4%) of CAMS vaccine, 6,120 doses (19.0%) of Wuhan vaccine, and 3,094 doses (9.6%) of Sinovac vaccine. The number of HFMD cases was compared with the amount of vaccination as shown in Figure 3.
Effectiveness of EV71 vaccine
According to the etiological diagnosis classification, the confirmed EV-A71 cases were used as the case group, other cases were used as the control group, and the risk factor was effective EV71 vaccination history for case–control analysis. The proportion of vaccination history in the case group was 2.7%, and the proportion of vaccination history in the control group was 5.1%. The case–control results showed that there was no evidence to support the effectiveness of EV71 vaccine, OR (95% CI) was 0.52 (0.12 ~ 2.3), p = 0.37.
Discussion
HFMD cases showed a decreasing trend year by year from 2011 to 2021.After the launch of EV71 vaccine. The epidemic strains have changed. The proportion of CV-A6, CV-A16 and other enterovirus types increased significantly. The case–control results showed that EV71 vaccination had no effect on the incidence. Surveillance and management of HFMD remain very important in the future and EV71 vaccine is considered to be included in National Immunization Program.
HFMD can occur throughout the year, with obvious seasonal characteristics, and the overall bimodal distribution. The results of this study are consistent with southern China, Hong Kong, and Taiwan (13–15). May to July is the main peak of HFMD incidence, and September–November is the secondary peak, probably because the distribution of enteroviruses is related to changes in natural factors such as seasons, climate, and humidity (13, 16). The incidence level decreased significantly in August, which may be related to the summer vacation and the reduction of children’s gathering in schools. In this study we found that the bimodal pattern of HFMD prevalence did not change after vaccination, suggesting that enterovirus infection is affected by climate, and vaccination of EV71 vaccine did not affect this pattern. From 2020 to 2021, there were only 7 and 12 HFMD cases, and no peak was observed, which was related to the implementation of COVID-19 prevention and control measures. Beginning in 2020, with the pandemic of COVID-19, gathering and movement have greatly reduced, and a series of personal protective measures have been implemented, such as wearing masks, strengthening hand hygiene, and maintaining social distance, classroom ventilation and environmental disinfection measures. These series of measures have raised public health awareness and improved healthy behaviors, reducing the occurrence of HFMD to a certain extent.
The male–female sex ratio of HFMD cases was 1.45:1, which may be because boys are active and more willing to participate in outdoor activities, which greatly increases the probability of exposure and infection, but there is no statistically significant difference in the pathogenic test results between males and females, indicating that the population is generally susceptible to HFMD. HFMD cases were mainly children in kindergartens and scattered children, accounting for 88.08% which was consistent with the results of other studies taken in China (27 months of age, 2008–2012) (13), Taiwan (under 4 years old, 1998–2005) (14), Hangzhou, China (preschool children, 2016–2018) (17) and Singapore (under 4 years old, 2001–2007) (18). This is due to the fact that young children have low immunity level, poor hygiene awareness and habits, and are more susceptible to enteroviruses (13, 19), resulting in the spread of HFMD. Infants under 6 months of age have fewer infections, most likely because of the protection of maternally transmitted antibodies (20, 21). And adults can acquire durable immunity through latent infection (13).
EV71 vaccine, as the first HFMD vaccine independently developed by China and launched in the world. It is currently the only vaccine that can effectively prevent HFMD, which made an important step in the prevention and treatment of HFMD. The EV71 vaccine is mainly suitable for children aged 6 months to 5 years old. The basic immunization program is two doses with a one-month interval in between. At present, EV17 vaccine is not included in the National Immunization Program (NIP), and the amount of vaccination doses are relatively small compared to other NIP vaccines. Many children even receive only one dose instead of the recommended two doses (19). The vaccination coverage of EV71 vaccine may increase if the vaccine was included in NIP and the vaccine costs covered by medical insurance in the future. This study showed that in 2011, EV-A71 virus was the main causative pathogen of HFMD. After the launch of EV71 vaccine in 2016, the HFMD etiological monitoring results showed that the epidemic strains have changed, and the proportion of EV-A71 type HFMD decreased significantly and no EV-A71 cases after 2018. Meanwhile, the proportion of CV-A6, CV-A16 and other enterovirus types increased significantly. Currently, the proportion of other enteroviruses such as CV-A6 in HFMD cases is increasing in China and worldwide (7, 22, 23). Thus, continuous monitoring of HFMD pathogen spectrum should be carried out, monitoring of CV-A6, CV-A16 and other enteroviruses should be strengthened and emphasized.
After school-age children are vaccinated against EV-A71, they can obtain personal immunity, thereby forming an immune barrier in the population and preventing the spread of EV-A71 virus in the population. Pre-market clinical trials of the vaccine have shown that vaccination of EV71 vaccine can prevent more than 90% of HFMD caused by EV-A71 virus, and the protection rate against severe HFMD can reach 100% (24–26). Although the results of case–control analysis showed no statistically significant difference, EV71 vaccine had no obvious protective effect on HFMD, but no EV-A71 type HFMD occurred since 2018, suggesting that there may be population protective effect of EV71 vaccine (6, 27). An immunogenicity analysis of EV71 vaccine in healthy children showed that the seropositive rate of was still 94.34% after 5 years of vaccination, indicating good persistence of the immunity generated by vaccination (28). Since EV71 vaccine does not have cross-protection effect against other viruses (24, 27, 29), children are still at risk of infected by other serotypes of HFMD after vaccination of EV71 vaccine, and this risk should not be ignored. The results of epidemiological studies on HFMD showed that CV-A6 and CV-A16 were two major non-EV-A71 enteroviruses causing HFMD, Xiao Dan Meng’s study in Hubei, China in 2016–2017 showed that CV-A6 accounted for 59.54% (30), Jiratchaya Puenpa’s study in Thailand in 2012 also showed that CV-A6 was the dominant pathogen accounted for 33.5% (31), and Thi NguyenHoa-Tran’s study showed that CV-A6 and CV-A16 were two major non-EV-A71 enteroviruses causing HFMD in Vietnam, 2008–2017 (32). At present, the pathogenic spectrum of HFMD-associated enteroviruses has changed, CV-A6, CV-A10 and other serotypes have gradually replaced EV-A71 and CV-A16 as the main pathogens. The EV71 vaccine cannot prevent HFMD caused by other serotypes. Faced with the changes of HFMD epidemic strains, the development of multivalent vaccines containing EV-A71 and other major serotypes has become a recognized means of preventing HFMD, controlling the spread of enteroviruses, and delaying the emergence of mutations and recombinant strains (33). Bivalent (CV-A6 + A10, EV-A71 + CV-A16) and trivalent (EV-A71 + CV-A16 + A6 and CV-A16 + A6 + A10) vaccine candidates are currently in clinical trials (34–36). In addition to traditional inactivated vaccines and live attenuated vaccines, there are also technical routes such as VLP vaccines, recombinant VP1 protein vaccines, and synthetic peptide vaccines (12).
Research on multivalent vaccines for HFMD will continue to rely on ongoing monitoring of enterovirus epidemiology and changes in pathogenic spectrum. Therefore, the surveillance and management of HFMD remain very important in the future. Currently, HFMD management in Shanghai mainly relies on pediatric outpatient clinics in large general hospitals for case reporting and sampling. Starting from 2022, pediatric outpatient clinics in community health service centers will open, which will improve the timeliness and scope of case detection and surveillance to a certain extent. At the same time, it is more important to evaluate the quality of surveillance work and training of staff in pediatric outpatient clinic.
Limitations
The reported number of HFMD cases is only a part of the actual number of infections and cases in the population. It may be because the symptoms after infection are mild and they do not go to the hospital, and the EV-A71 type HFMD in the population has decreased significantly. As HFMD cases primarily occurred in children, the decreasing time trend reported by case counts might be associated with the decreasing trend of the total number of children in Shanghai, which might not reflect the actual temporal trend. Therefore, the evaluation of the protective effect of the vaccine requires further research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Review Board of Huangpu District Center for Disease Control and Prevention (No. 2022HPLL01). Anonymity was guaranteed. All methods were performed in accordance with the relevant guidelines and regulations. Consent to participate is not applicable.
Author contributions
JW and SZ wrote the main manuscript text and JW prepared all figures and tables. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Yangtze River Delta Regional Leading Talents Research Project on Immunization (CSJP032).
Acknowledgments
The authors would like to thank staff of Huangpu CDC, who facilitated data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu, GQ, Tan, H, Li, CH, and Bai, LQ. Incidence of hand, foot and mouth disease and effect of EV71 vaccine vaccination in Chenzhou city of Hunan province, 2013-2018. Chin J Public Health. (2020) 36:385–8. doi: 10.11847/zgggws1127276
2. Xu, ZY, Wang, X, Zhang, LP, Lyu, J, Li, XC, Liu, N, et al. Health and economic evaluation of entervirus 71 vaccine immunization on HFMD in Minhang District of Shanghai from 2016 to 2018. Shanghai J Prev Med. (2021) 33:404–9. doi: 10.19428/j.cnki.sjpm.2021.19835
3. Zhang, Y, Zhu, Z, Yang, W, Ren, J, Tan, X, Wang, Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. (2010) 7:94. doi: 10.1186/1743-422X-7-94
4. Li, XL, Lu, WW, Zhang, ZY, Hao, CS, Guo, HJ, Zhang, YT, et al. Development of inactivated enterovirus 71 vaccine (vero cell). Chin J Viral Dis. (2018) 8:445–50.
5. Dong, XQ, He, M, Qiao, MK, ShI, LM, Zhang, HY, and Du, XF. Epidemiological and pathogenic characteristics of HFMD in Nanjing, 2017-2019. J Trop Med. (2021) 21:1483–6.
6. Li, JS, Dong, XG, Qin, M, Feng, HR, Yang, JY, Li, RX, et al. Outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 in a Juku in Fengtai District, Beijing, China, 2015. Springerplus. (2016) 5:1650. doi: 10.1186/s40064-016-3307-x
7. Kimmis, BD, Downing, C, and Tyring, S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis. (2018) 102:353–6.
8. Xing, Y, and Cheng, ZY. Epidemiological and etiological characteristics of hand-foot-mouth disease in children aged 0-5 years in Jilin from 2011 to 2020. Matern Child Health Care China. (2021) 36:5526–9. doi: 10.19829/j.zgfybj
9. Wu, Y, Wang, T, Zhao, M, Dong, S, Wang, S, and Shi, J. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the province level in mainland China, 2011-2018. PLoS One (2022) 17:e0270061. doi: 10.1371/journal.pone.0270061
10. Wu, JT, Jit, M, Zheng, Y, Leung, K, Xing, W, Yang, J, et al. Routine pediatric enterovirus 71 vaccination in China: a cost-effectiveness analysis. PLoS Med. (2016) 13:e1001975. doi: 10.1371/journal.pmed.1001975
11. Wang, H, Jiang, SQ, Chen, C, Lu, Y, Wang, DH, Li, MX, et al. Analysis of disease economic burden of outpatients with different types of hand, foot and mouth disease in Guangzhou. Mod Prev Med. (2022) 49:152–7.
12. Jin, WP, Qian, SS, and Shen, S. Progress in research on hand, foot and mouth disease-related enteroviruses and vaccines. Chin J Biol. (2021) 34:1273–83.
13. Xing, WJ, Liao, QH, Cécile, V, Zhang, J, Sun, JL, Wu, JT, et al. Epidemiological characteristics of hand-foot-and-mouth disease in China, 2008-2012. Lancet Infect Dis. (2014) 14:308–18. doi: 10.1016/S1473-3099(13)70342-6
14. Chen, SC, Chang, HL, Yan, TR, Cheng, YT, and Chen, KT. An eight-year study of epidemiologic features of enterovirus 71 infection in Taiwan. Am J Trop Med Hyg. (2007) 77:188–91. doi: 10.4269/ajtmh.2007.77.188
15. Ma, E, Chan, KC, Cheng, P, Wong, C, and Chuang, SK. The enterovirus 71 epidemic in 2008--public health implications for Hong Kong. Int J Infect Dis. (2010) 14:e775–80. doi: 10.1016/j.ijid.2010.02.2265
16. Wang, P, Goggins, WB, and Chan, EY. Hand, foot and mouth disease in Hong Kong: a time-series analysis on its relationship with weather. PLoS One. (2016) 11:e0161006. doi: 10.1371/journal.pone.0161006
17. Wang, J, Zhou, J, Xie, G, Zheng, S, Lou, B, Chen, Y, et al. The epidemiological and clinical characteristics of hand, foot, and mouth disease in Hangzhou, China, 2016 to 2018. Clin Pediatr (Phila). (2020) 59:656–62. doi: 10.1177/0009922820910822
18. Ang, LW, Koh, BK, Chan, KP, Chua, LT, James, L, and Goh, KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann Acad Med Singap. (2009) 38:106–12. doi: 10.47102/annals-acadmedsg.V38N2p106
19. Liu, J, Yan, XL, Xie, ZX, Huang, SH, and Xu, CY. Etiological characteristics influencing factors for hand, foot and mouth disease in children from 2012 to 2017. Chin J Nosocomiol. (2019) 29:2037–40.
20. Wang, SM, Ho, TS, Lin, HC, Lei, HY, Wang, JR, and Liu, CC. Reemerging of enterovirus 71 in Taiwan: the age impact on disease severity. Eur J Clin Microbiol Infect Dis. (2012) 31:1219–24. doi: 10.1007/s10096-011-1432-6
21. Zhu, FC, Liang, ZL, Meng, FY, Zeng, Y, Mao, QY, Chu, K, et al. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS One. (2012) 7:e37206. doi: 10.1371/journal.pone.0037206
22. Li, Y, Chang, Z, Wu, P, Liao, Q, Liu, F, Zheng, Y, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. (2018) 24:1902–6. doi: 10.3201/eid2410.171953
23. Bian, L, Wang, Y, Yao, X, Mao, Q, Xu, M, and Liang, Z. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti-Infect Ther. (2015) 13:1061–71. doi: 10.1586/14787210.2015.1058156
24. Zhu, FC, Meng, FY, Li, JX, Li, XL, Mao, QY, Tao, H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2013) 381:2024–32. doi: 10.1016/S0140-6736(13)61049-1
25. Zhu, F, Xu, W, Xia, J, Liang, Z, Liu, Y, Zhang, X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. (2014) 370:818–28. doi: 10.1056/NEJMoa1304923
26. Li, R, Liu, L, Mo, Z, Wang, X, Xia, J, Liang, Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. (2014) 370:829–37. doi: 10.1056/NEJMoa1303224
27. Li, Y, Zhou, Y, Cheng, Y, Wu, P, Zhou, C, Cui, P, et al. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017–18: a test-negative case-control study. Lancet Child Adolesc Health. (2019) 3:697–704. doi: 10.1016/S2352-4642(19)30185-3
28. Hu, Y, Zeng, G, Chu, K, Zhang, J, Han, W, Zhang, Y, et al. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: a further observation. Hum Vaccin Immunother. (2018) 14:1517–23. doi: 10.1080/21645515.2018.1442997
29. Wang, X, An, Z, Huo, D, Jia, L, Li, J, Yang, Y, et al. Enterovirus A71 vaccine effectiveness in preventing enterovirus A71 infection among medically-attended hand, foot, and mouth disease cases, Beijing, China. Hum Vaccin Immunother. (2019) 15:1183–90. doi: 10.1080/21645515.2019.1581539
30. Meng, XD, Tong, Y, Wei, ZN, Wang, L, Mai, JY, Wu, Y, et al. Epidemical and etiological study on hand, foot and mouth disease following EV-A71 vaccination in Xiangyang, China. Sci Rep. (2020) 10:20909. doi: 10.1038/s41598-020-77768-7
31. Puenpa, J, Mauleekoonphairoj, J, Linsuwanon, P, Suwannakarn, K, Chieochansin, T, Korkong, S, et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. (2014) 9:e98888. doi: 10.1371/journal.pone.0098888
32. Hoa-Tran, TN, Dao, ATH, Nguyen, AT, Kataoka, C, Takemura, T, Pham, CH, et al. Coxsackieviruses A6 and A16 associated with hand, foot, and mouth disease in Vietnam, 2008-2017: essential information for rational vaccine design. Vaccine. (2020) 38:8273–85. doi: 10.1016/j.vaccine.2020.11.031
33. Aswathyraj, S, Arunkumar, G, Alidjinou, EK, and Hober, D. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol. (2016) 205:397–407. doi: 10.1007/s00430-016-0465-y
34. Fang, CY, and Liu, CC. Recent development of enterovirus a vaccine candidates for the prevention of hand, foot, and mouth disease. Expert Rev Vaccines. (2018) 17:819–31. doi: 10.1080/14760584.2018.1510326
35. Fan, S, Liao, Y, Jiang, G, Wang, L, Zhao, H, Yu, L, et al. Efficacy of an inactivated bivalent vaccine for enterovirus 71 and coxsackievirus A16 in mice immunized intradermally. Vaccine. (2021) 39:596–604. doi: 10.1016/j.vaccine.2020.11.070
Keywords: HFMD, epidemiology, EV71 vaccine, vaccination status, seasonal trend
Citation: Wang J and Zhang S (2023) Epidemiological characteristics and trends of hand-foot-mouth disease in Shanghai, China from 2011 to 2021. Front. Public Health. 11:1162209. doi: 10.3389/fpubh.2023.1162209
Edited by:
Tianfeng He, Ningbo Municipal Center for Disease Control and Prevention, ChinaReviewed by:
Muruganandam Nagarajan, Regional Medical Research Centre (ICMR), IndiaLefei Han, Shanghai Jiao Tong University, China
Copyright © 2023 Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Zhang, emhhbmdzaGFuQGhwY2RjLnNoLmNu
 Jing Wang
Jing Wang Shan Zhang
Shan Zhang