- 1Department of Neuroscience and Rehabilitation, Institute of Psychiatry, University of Ferrara, Ferrara, Italy
- 2Programming and Management Control Service, Unit of Controls, St. Anna University-Hospital, Ferrara, Italy
- 3Programming and Management Control Service, Statistics Service, St. Anna University-Hospital, Ferrara, Italy
- 4Department of Neuroscience, University of Turin, Turin, Italy
- 5Division of Nephrology, Dialysis and Transplantation, Department of Internal Medicine, University of Genoa and IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 6Department of Medicine, University of Verona, Verona, Italy
- 7Nephrology and Dialysis Unit, St. Anna University-Hospital, Ferrara, Italy
- 8Nephrology and Dialysis Unit, Pederzoli Hospital, Verona, Italy
Introduction: Psychosocial factors frequently occur in kidney transplant recipients (KTRs), leading to behavioral alterations and reduced therapeutic adherence. However, the burden of psychosocial disorders on costs for KTRs is unknown. The aim of the study is to identify predictors of healthcare costs due to hospital admissions and emergency department access in KTRs.
Methods: This is a longitudinal observational study conducted on KTRs aged >18 years, excluding patients with an insufficient level of autonomy and cognitive disorder. KTRs underwent psychosocial assessment via two interviews, namely the Mini-International Neuropsychiatric Interview 6.0 (MINI 6.0) and the Diagnostic Criteria for Psychosomatic Research Interview (DCPR) and via the Edmonton Symptom Assessment System Revised (ESAS-R) scale, a self-administrated questionnaire. Sociodemographic data and healthcare costs for hospital admissions and emergency department access were collected in the 2016–2021 period. Psychosocial determinants were as follows: (1) ESAS-R psychological and physical score; (2) symptomatic clusters determined by DCPR (illness behavior cluster, somatization cluster, and personological cluster); and (3) ICD diagnosis of adjustment disorder, anxiety disorder, and mood disorder. A multivariate regression model was used to test the association between psychosocial determinants and total healthcare costs.
Results: A total of 134 KTRs were enrolled, of whom 90 (67%) were men with a mean age of 56 years. A preliminary analysis of healthcare costs highlighted that higher healthcare costs are correlated with worse outcomes and death (p<0.001). Somatization clusters (p = 0.020) and mood disorder (p < 0.001) were positively associated with costs due to total healthcare costs.
Conclusions: This study showed somatization and mood disorders could predict costs for hospital admissions and emergency department access and be possible risk factors for poor outcomes, including death, in KTRs.
1. Introduction
Kidney transplantation (KT) is the most desired therapy for stage 5 chronic kidney disease as for these patients it represents the most cost-effective treatment, improving quality of life and prolonging survival (1, 2). In spite of being less costly than dialysis (3, 4), KT is however related to substantial costs (5–8), which can also derive not only from health problems such as cardiovascular disease, infections, graft rejections, and neoplastic disease (9–11) but also from the indirect effects of psychological conditions, such as depression or anxiety (12).
KT is often accompanied by high patient expectations, but it is indeed a stressful condition both physically and mentally that requires special adaptations encompassing changes in a patient's personal and financial life, meeting possibly unrealistic expectations, the possibility of rehospitalizations, infections, graft rejections, and the necessity of long-term immunosuppression therapy (13, 14). Indeed, 25 to 40% of KT recipients (KTRs) have been found to develop mood and anxiety disorders in the post-transplant period (15–21) according to the traditional Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD). Furthermore, 60% of KTRs have shown some form of psychological distress when using the Diagnostic Criteria for Psychosomatic Research (DCPR) (22), a diagnostic and conceptual framework whose aim is to capture psychological dimensions and subthreshold syndromes (23, 24). These conditions are particularly relevant as they may generate dysfunctional illness behaviors (e.g., somatization, frequent attender behavior, and illness denial) that are associated with worse outcomes (12), medical non-adherence (25, 26), decreased quality of life, and increased costs (27, 28). More importantly, psychological conditions are both identifiable and treatable (22, 29–33), thus representing additional superfluous costs for the healthcare systems.
While many studies have highlighted the detrimental effects of psychosocial conditions on KTRs, this is the first study with the intent to directly investigate the contribution of psychiatric and psychosocial diagnoses as identified by both ICD and DCPR systems on healthcare use costs in KTRs. Specifically, using linear regression models, we aimed to identify predictors of total healthcare costs due to hospital admissions and emergency department access.
2. Methods
A monocenter prospective observational longitudinal study was performed at the kidney transplant center of the Ferrara University Hospital from 2016 to 2021. The study was conducted according to the 1995 Declaration of Helsinki and its revisions (34). The Ethical Committee of the local academic hospital approved the protocol of the study (Protocol n: 151297, 2016). All participants signed written informed consent.
Inclusion criteria were age ≥ 18 years and being a recipient of a kidney from a cadaveric or living donor. Exclusion criteria were an insufficient level of autonomy (Karnofsky Performance Status Scale <50) and the presence of cognitive disorders (Mini-Mental State Examination <24). Two individual interviews, namely the Mini-International Neuropsychiatric Interview (MINI6.0) (35) and the Diagnostic Criteria for Psychosomatic Research Semi-Structured Interview (36) were administered by the same psychiatrist, an expert in psychosomatic research (L.Z.). A self-reporting instrument, the Edmonton Symptom Assessment System revised (ESAS-Revised) in the Italian language, was also filled in by patients. The characteristics of the above tools were extensively described elsewhere (37, 38). Briefly, the MINI6.0 is a structured diagnostic interview for assessing the major psychiatric disorders in ICD-10, which was used to make a psychiatric diagnosis. A DCPR semi-structured interview evaluates the presence of 12 syndromes divided into three different clusters: (1) abnormal illness behavior (AIB) (i.e., Disease Phobia, Health Anxiety, Illness Denial, and Thanatophobia); (2) somatization (i.e., Functional Somatic Symptoms Secondary to a Psychiatric Disorder, Persistent Somatization, Conversion Symptoms, and Anniversary Reaction); and (3) personological and psychological dimensions frequently diagnosable in KTRs (39) (i.e., Alexithymia, Type A Behavior, Irritable Mood, and Demoralization).
The ESAS-R is a pragmatic patient-centered symptom assessment tool with a visual analog scale, designed to assist in the assessment of six physical (i.e., pain, tiredness, nausea, drowsiness, lack of appetite, and shortness of breath) and four psychological (i.e., depression, anxiety, feeling of not being well, and emotional distress) symptoms. In particular, the physical symptoms are assessed objectively (i.e., pain is based on a knowledge of pain behaviors; shortness of breath as accelerated respirations causing patient distress; tiredness as lack of energy; lack of appetite, nausea, and drowsiness as the presence of eating, retching/vomiting, and sleep, respectively). The items can be summed in order to create subscales of psychological, physical, and total distress, which can be used to monitor symptoms and screen for mental and psychological disorders. It has been validated in dialysis patients (40) and kidney transplant cohorts (41). The Italian version shows an acceptable level of validity and good psychometric properties in KTRs (33, 42).
All data, including clinical characteristics and routine biochemistry, were collected from digital patients' archives.
The following variables were used as a measure of outcome: total healthcare costs due to hospital admissions in the 2016–2021 period; and total healthcare costs due to emergency department access in the 2016–2021 period. Costs, covered by Italy's National Health Service, were expressed in euros (€), the Italian currency, and were extracted from a hospital software database, searching for each patient record both the type of medical service delivered and the related amount charged across the period from 2016 to 2021.
As predictors of healthcare costs, we used the following psychosocial determinants, all measured before the outcome: ESAS, as a severity measure of physical and physiological symptoms; symptomatic clusters as measured by the DCPR; and clinical diagnosis according to MINI6.0 within the mood, anxiety, and adjustment disorder spectrum. Age (years), sex (men versus women), body mass index (BMI; kg/m2), time under dialysis before the transplant (months), transplant vintage (months), estimated glomerular filtration rate (eGFR) (ml/min), blood creatinine (mg/dl), blood albumin (g/dl), blood hemoglobin (g/dl), blood phosphate (mg/dl), blood calcium (mmol/l), blood inactive vitamin D (ng/ml), and past psychopathology (positive history versus no history) were entered as covariates in the analysis to control for variables that can affect healthcare use or somatic outcomes of the kidney transplant.
2.1. Statistical analysis
Data were entered in Excel, then coded and analyzed using the Statistical Package for Social Sciences (SPSS) version 28. All tests were two-tailed, with alpha set at p < 0.05.
Descriptive statistics were reported as means with standard deviation and range, or as counts and percentages. A regression model to test the association of our predictors to the outcomes was used. A preliminary exploration of the association of each variable with the outcomes, using univariate linear regression models, was performed. Afterward, a stepwise multivariable regression model to evaluate the association of our predictors with the outcomes were tested, by taking into account the considered covariates with the significance level for removal fixed at p < 0.10. In the model, discrete variables were entered as continuous values while nominal variables were entered as dichotomous [absent (0) vs. present (1)] values.
The minimum required sample size for multiple regression, given a desired power of 80% at alpha = 0.05 with 21 predictors and aiming at detecting an effect size of f2 = 0.20, was 124 participants. The calculation was carried out according to Soper (43). Multicollinearity was measured with the variance inflation factor (VIF), using a cut-off of 2.5 as a threshold to consider the presence of multicollinearity that could affect the regression model (44). As an effect size of the linear regression model, we used Cohen's f2, according to the formula: f2 = R2/(1–R2). By convention (45), f2 effect sizes of 0.02, 0.15, and 0.35 are considered small, medium, and large, respectively.
3. Results
Overall, 134 kidney transplant recipients, of whom only 10 were from living donors, were included in the longitudinal study, of which 90 (67%) were men and 44 (33%) were women. Nine patients declined to participate (six for work or family reasons and three because of health reasons). Men and women did not differ in demographic characteristics, biochemical values, and healthcare costs except for eGFR, blood creatinine, and personological cluster (Table 1). In fact, eGFR was on average higher in women (76.8 ± 27.2) than in men (58.6 ± 21.02): t = 3.90; p < 0.001. Conversely, blood creatinine was on average higher in men (1.5 ± 0.5) than in women (1.2 ± 0.4): t = −3.49; p < 0.001. Men [n = 51 (57%)] were more likely than women (n = 15 (34%)] to have a personological cluster (χ2 = 5.15; df = 1; p = 0.023). Above all, kidney transplant patients were Caucasians, coming from local districts.
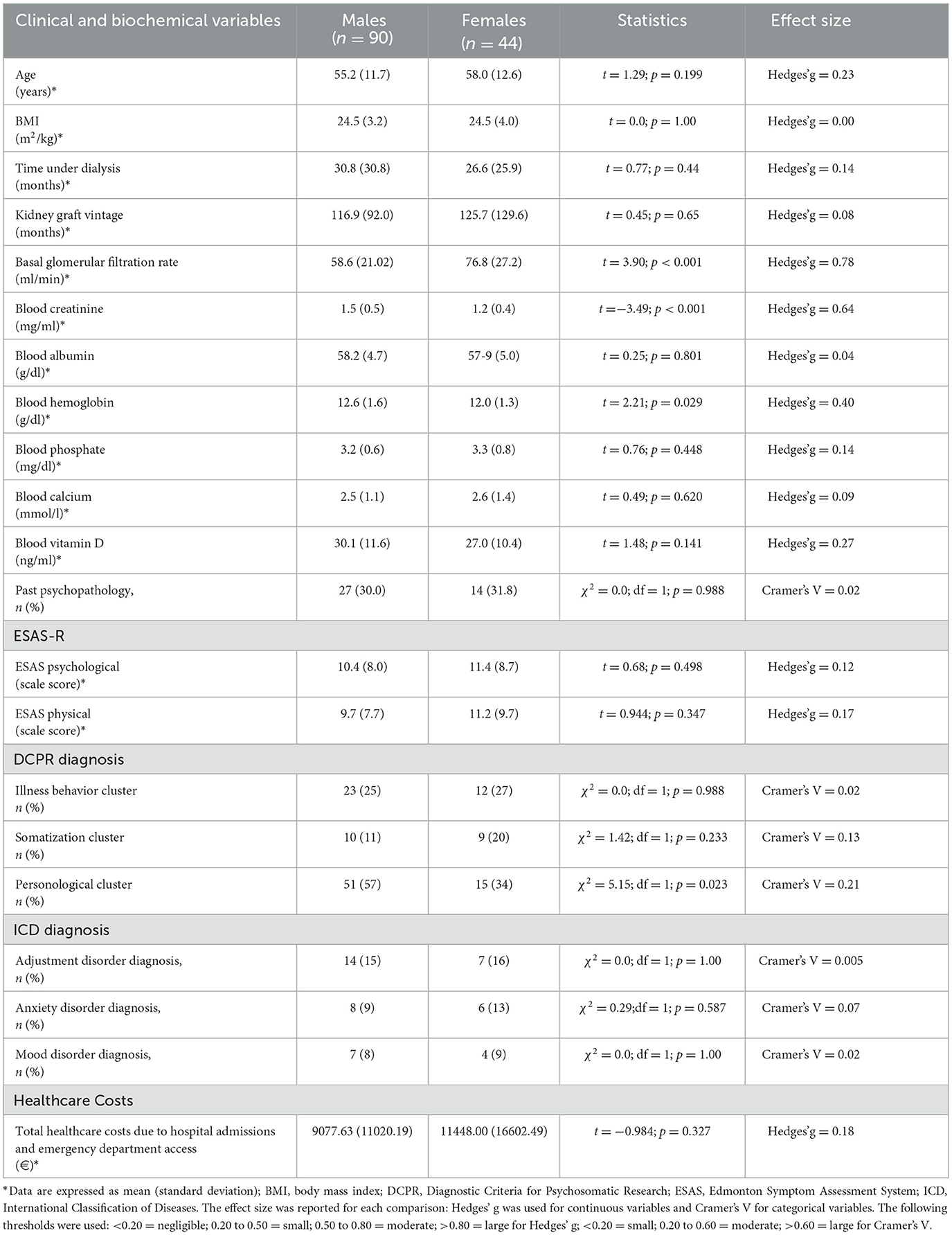
Table 1. Distribution in the sample (n = 134) of the variables included in the study according to sex.
At the end of the 2016–2021 observational period, the sample included 28 participants (21%) who had died. In preliminary investigations, increasing costs were related to an increased chance of a worse outcome, such as death (Table 2). Hence, healthcare use costs, either for hospital admission or emergency department access, represented an indicator of pejorative trajectories after a kidney transplant; in other words, higher costs were associated with poorer health.
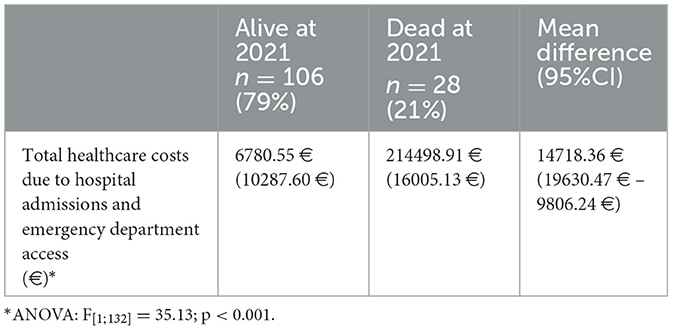
Table 2. Outcome of kidney transplant according to health care use costs in the 2015–2021 observational period.
The results of the univariate linear regression model are reported in Table 3. The beta can be interpreted as the increase (or decrease) in the outcome for each score point of a discrete variable or the presence of a nominal variable. For example, each year of age imports an increase of €216.61 in the total healthcare cost for hospital admissions. Hence, older people had higher healthcare use for hospital admissions, and as higher total healthcare costs for hospital admissions were related to a higher risk of death, they were also exposed to a greater risk of death. The standardized beta describes the strength of the association between the predictor and the outcome, and it is measured in units of standard deviation. The role of age was non-negligible as the change of 1 standard deviation in its value corresponded to a 19.9% of standard deviation in the dependent variable. Overall, only a minority of the predictors were related to the outcomes in a statistically significant manner. The presence of a mood disorder had the greatest impact on the outcomes and was associated with the largest increase in healthcare costs for both hospital admissions and emergency department access. It also had the largest association with the outcomes.
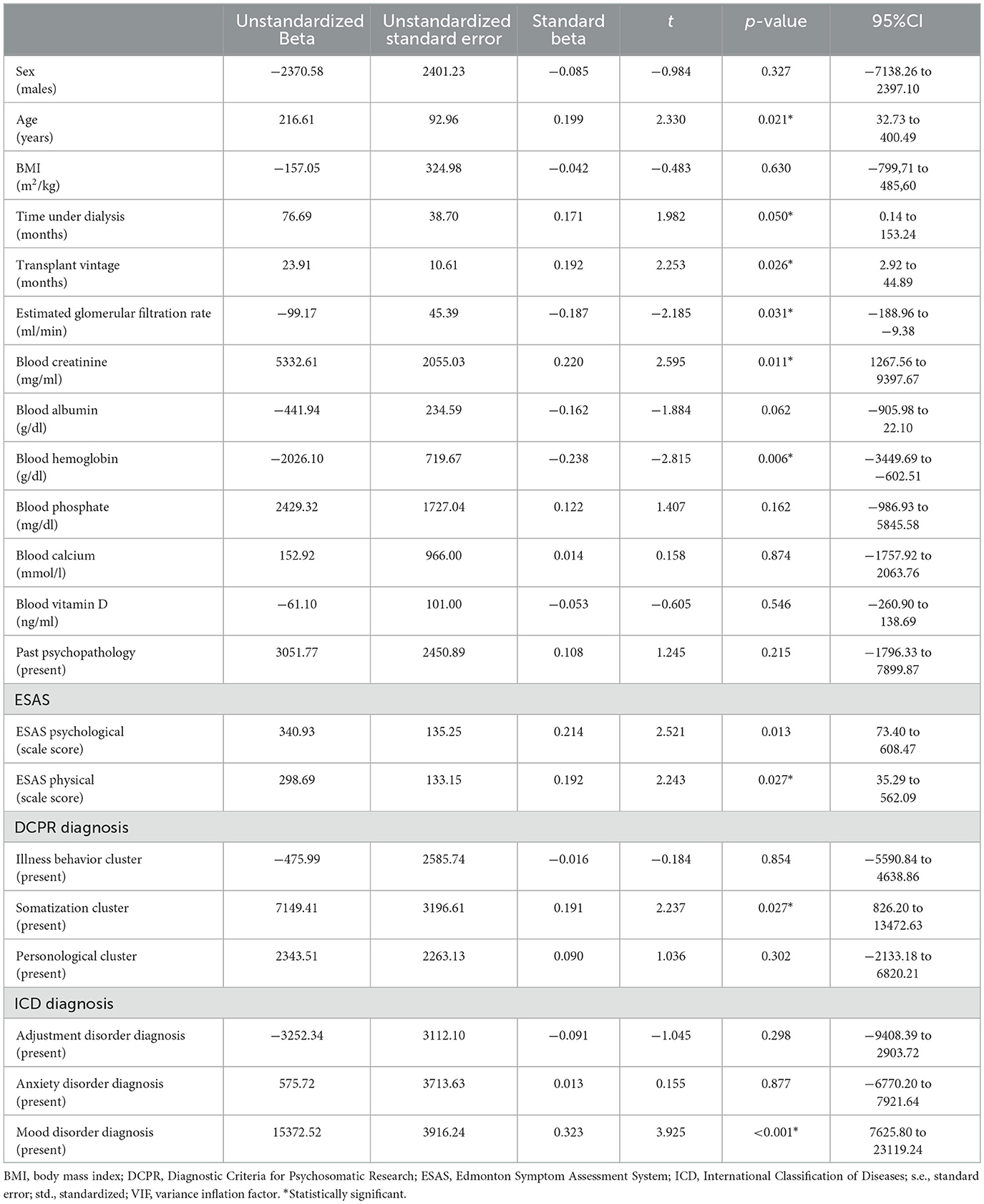
Table 3. Factors associated with total healthcare costs due to hospital admissions and emergency department access in kidney transplant recipients in a univariate linear regression.
We then proceeded to apply the stepwise multivariable model to evaluate the independent contribution of each predictor taking into account the covariates and the impact of the other predictors.
The model, concerning total healthcare costs due to hospital admissions and emergency department visits in the 2016-2021 period, extracted four variables as predictors of the outcome according to the predefined threshold for removal, with the other variables excluded for their negligible contribution (Table 4).
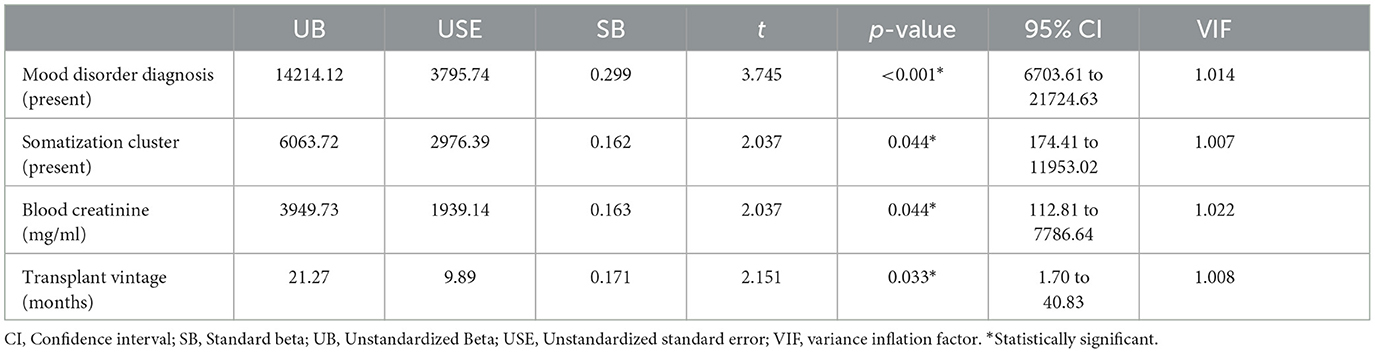
Table 4. Factors associated with total healthcare costs due to hospital admissions and emergency department access in kidney transplant recipients in a stepwise multivariable linear regression model.
In this model, the presence of a mood disorder diagnosis, the presence of the somatization cluster, transplant vintage, and blood creatinine were associated with higher healthcare costs due to hospital use [F(4;128) = 7.88; p < 0.001; R2 = 19.6%; adjusted R2 = 17.1%; f2 = 0.244). Effect size, according to Cohen's f2, was estimated as medium to large. None of the variables in the model had a VIF higher than the suggested cutoff for multicollinearity.
Some diagnostic plots were used for testing the assumptions underlying the linear regression model by taking into account residual errors and fitted values. The model, which focused on total healthcare costs due to hospital use, showed a reasonable adaptation. In the residual vs. fitted plot, the residuals were spread equally around a horizontal line without distinct patterns (and the red line was approximately horizontal near zero), indicating a linear relationship. In the Q-Q plot, the majority of the residuals follow the straight dashed line. In the Scale-Location plot, there was a minor deviation from the homoscedasticity, confirmed by the Breusch–Pagan test (46): BP = 10.22, df = 4, p = 0.037. Just one point (case 19) was identified as influential based on Cook's distance (Supplementary Figure 1). We repeated the analysis by excluding this influential point (Table 5). In the new model, just the presence of a mood disorder diagnosis remained statistically related to healthcare costs due to hospital use, with a decrease in the overall effect size: F(4;127) = 3.06; p = 0.019; R2 = 8.7%; adjusted R2 = 5.9%; f2 = 0.095. According to the diagnostic plots, the model had a good fit, there was no influential point according to Cook's distance, and there was no more deviation from the homoscedasticity (BP = 2.53, df = 4, p = 0.639).
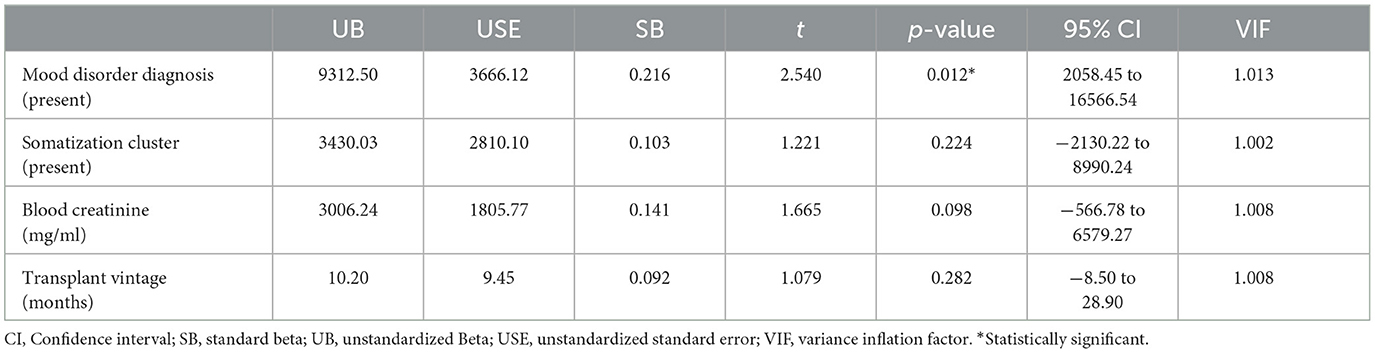
Table 5. Factors associated with total healthcare costs due to hospital admissions and emergency department access in kidney transplant recipients in a stepwise multivariable linear regression model after the exclusion of the influential point.
4. Discussion
In this study, we found that some psychosocial variables and clinical dimensions influenced total healthcare costs, hence total healthcare use, in kidney transplant recipients. In particular, a propensity to somatization and the presence of a mood disorder increased healthcare use costs for emergency department visits and hospital admissions. Furthermore, greater access to the emergency department and a higher chance of admission to the hospital were related to a greater risk of death in KTRs. These findings underline the need to assess psychosocial dimensions, such as somatization and mood disorder as predictors of healthcare use in kidney transplant recipients and possible risk factors for poor outcomes until death, using the DCPR semi-structured interview and MINI6.0 structured interview, respectively.
Mood disorders were also shown to increase total healthcare costs due to emergency department access and hospital admission. Regarding the former, this is in line with the literature, as approximately 50% of frequent emergency department users have a mental health diagnosis (47) and patients with mood disorders have been found to carry a 3-fold risk of frequent emergency department use (48). Besides a possible increase in emergency department use, higher costs might also be the result of the harmful effect of the mood disorder itself, thus raising the total healthcare costs due to hospital admission. Depression, which represents the most common type of mood disorder, specifically represents a risk factor for graft failure and post-transplant mortality (12), and it is associated with poor adherence to immunosuppressive medication (49). Non-compliance to medications can dangerously affect the outcomes of kidney transplantation (50, 51) and, together with alcohol consumption and cigarette smoking (52, 53), is a hallmark of depression (54). The detrimental effect of mood disorders on physical health might be also explained by other mechanisms, such as autonomic dysfunction (55), impaired cellular immune response (56), heightened inflammation (57), and increased platelet aggregation (58). Finally, in patients affected by mood disorders, harm could also come from treatments, as there is evidence that antidepressant medication use, which represents the most prescribed drug for mood disorders, is associated with increased mortality and all-cause graft failure in the year following transplantation (59). Even though this could just represent an association, the consumption of other medications used to treat mood disorders, such as antipsychotics or lithium, represents instead a well-established risk for poorer physical health (60, 61). In our study, the presence of mood disorders remained a significant predictor of increased healthcare costs even when excluding the influential point.
Regarding somatization, it was found to be a predictor of higher costs. Compared with the general population, this tendency to experience and communicate somatic distress in response to psychosocial stress (and to seek medical help for it) has been associated with a higher hospital length of stay, higher inpatient costs, and more specialist visits (62). Patients with these conditions often present with vague and difficult-to-identify symptoms, leading to detrimental economic effects (63). In fact, the annual medical costs for “somatizers” have indeed been found to be 2.3 times that for a “non-somatizer', with three times as many hospitalizations (62). Furthermore, KTRs affected by this cluster of syndromes might be more exposed to iatrogenic harm (64–67), leading to a further increase in hospital stays, examinations, and costs.
Furthermore, some limitations of our study should be also mentioned. First, the lack of data regarding the costs of ambulatory care and their changes on the basis of psychosocial clinically significant conditions in KTRs. However, it is complex to quantify the economic burden of this activity as it requires the systematic quantification of the additional costs, which is not always comparable, due to the multiple medical and surgical procedures. Second, the absence of a control group with chronic kidney disease in other settings. Third, some demographics, such as the socioeconomic status (68) (a combined measure of education, income, and occupation) of KTRs, biochemical (69, 70), and ultrasound (71, 72) data were not available to better characterize the population. Additionally, the therapeutic protocols to treat chronic kidney rejection, including steroid dosage, and the economic contribution of physical activity levels, both modifiable risk factors of mental health (73–75), were not evaluated. Finally, no healthcare cost before the kidney transplant was collected.
5. Conclusion
This study demonstrated that higher healthcare costs for hospital admissions and emergency department access were strongly predicted by the DCPR diagnosis of somatization cluster and the ICD diagnosis of mood disorder, respectively. In addition, these healthcare costs were associated with a higher risk of poor outcomes until death in kidney transplant recipients. Further studies of cost analysis, cost-effectiveness, cost-benefit, and cost minimization analysis should be conducted to estimate the economic advantages of early diagnosis and treatment of psychosocial syndromes in kidney transplant recipients. Indeed, the healthcare allocation strategy, a pressing question in the transplantation community, should be rethought to invest accurately the resources that are even more limited; therefore, a comprehensive systematic economic analysis of the physical, physiological, and social aspects is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee AVEC. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: YB. Investigation: LZ and YB. Formal analysis: AP. Data curation: NN and FG. Writing and editing—original draft: YB and LZ. Writing—review and editing: PE and FB. Supervision: RC and AS. Validation: LG. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1158387/full#supplementary-material
References
1. Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. CJASN. (2008) 3:471–80. doi: 10.2215/CJN.05021107
2. Lentine KL, Smith JM, Hart A, Miller J, Skeans MA, Larkin L, et al. OPTN/SRTR 2020 annual data report: kidney. Am J Transpl. (2022) 22:21–136. doi: 10.1111/ajt.16982
3. Overbeck I, Bartels M, Decker O, Harms J, Hauss J, Fangmann J. Changes in quality of life after renal transplantation. Transplant Proc. (2005) 37:1618–21. doi: 10.1016/j.transproceed.2004.09.019
4. Provenzano M, Andreucci M, De Nicola L, Garofalo C, Battaglia Y, Borrelli S, et al. The role of prognostic and predictive biomarkers for assessing cardiovascular risk in chronic kidney disease patients. Biomed Res Int. (2020) 2020:128 doi: 10.1155/2020/2314128
5. Rosselli D, Rueda JD, Diaz C. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. J Kid Dis Transpl. (2015) 26:733. doi: 10.4103/1319-2442.160175
6. Jensen CE, Sørensen P, Petersen KD. In Denmark kidney transplantation is more cost-effective than dialysis. Dan Med J. (2014) 61:A4796.
7. Yang F, Liao M, Wang P, Yang Z, Liu Y. The cost-effectiveness of kidney replacement therapy modalities: a systematic review of full economic evaluations. Appl Health Econ Health Policy. (2021) 19:163–80. doi: 10.1007/s40258-020-00614-4
8. Abdi F, Alinia C, Taghizadeh Afshari A, Yusefzadeh H. Cost–benefit analysis of kidney transplant in patients with chronic kidney disease: a case study in Iran. Cost Eff Res Alloc. (2022) 20:37. doi: 10.1186/s12962-022-00372-1
9. Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. (2014) 27:19–27. doi: 10.1111/tri.12217
10. Rao NN, Coates PT. Cardiovascular disease after kidney transplant. Semin Nephrol. (2018) 38:291–7. doi: 10.1016/j.semnephrol.2018.02.008
11. Cheung CY, Tang SCW. An update on cancer after kidney transplantation. Nephrol Dial Transplant. (2019) 34:914–20. doi: 10.1093/ndt/gfy262
12. Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, DeVito Dabbs AJ, Posluszny DM, et al. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: a systematic review and meta-analysis. Transplantation. (2015) 100:988–1003. doi: 10.1097/TP.0000000000000901
13. Dew MA, DiMartini AF. Transplantation Oxford Handbook of Health Psychology. Oxford: Oxford University Press. (2011).
14. Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. (2002) 70:771–83. doi: 10.1037/0022-006X.70.3.771
15. Fukunishi I, Sugawara Y, Takayama T, Makuuchi M, Kawarasaki H, Surman OS. Psychiatric disorders before and after living-related transplantation. Psychosomatics. (2001) 42:337–43. doi: 10.1176/appi.psy.42.4.337
16. Boostani H, Ghorbani A, Heydarazadzadeh M. The comparison of general health status between hemodialysis and kidney transplant patients in university hospitals of Ahvaz, Iran. J Renal Inj Prev. (2014) 3:27–30. doi: 10.12861/jrip.2014.09
17. Müller HH, Englbrecht M, Wiesener MS, Titze S, Heller K, Groemer TW, et al. Depression, anxiety, resilience and coping pre and post kidney transplantation – initial findings from the psychiatric impairments in kidney transplantation (PI-KT)-study. PLoS ONE. (2015) 10:1–15. doi: 10.1371/journal.pone.0140706
18. Chilcot J, Spencer BWJ, Maple H, Mamode N. Depression and kidney transplantation. Transplantation. (2014) 97:7. doi: 10.1097/01.TP.0000438212.72960.ae
19. Arapaslan B, Soykan A, Soykan C, Kumbasar H. Cross-sectional assessment of psychiatric disorders in renal transplantation patients in Turkey: a preliminary study. Transplant Proc. (2004) 36:1419–21. doi: 10.1016/j.transproceed.2004.04.087
20. Novak M, Zsolt Molnar M, Szeifert L, Zsofia Kovacs A, Panna Vamos E, Zoller R, et al. Depressive symptoms and mortality in patients after kidney transplantation: a prospective prevalent cohort study. Psychosom Med. (2010) 72:6. doi: 10.1097/PSY.0b013e3181dbbb7d
21. Shah VS, Ananth A, Sohal GK, Bertges-Yost W, Eshelman A, Parasuraman RK, et al. Quality of life and psychosocial factors in renal transplant recipients. Transplant Proc. (2006) 38:1283–5. doi: 10.1016/j.transproceed.2006.03.027
22. Battaglia Y, Martino E, Piazza G, Cojocaru E, Massarenti S, Peron L, et al. Abnormal illness behavior, alexithymia, demoralization, and other clinically relevant psychosocial syndromes in kidney transplant recipients: a comparative study of the diagnostic criteria for psychosomatic research system versus icd-10 psychiatric Nosolo. Psychother Psychosomatic. (2018) 47:375–6. doi: 10.1159/000490000
23. Fava GA. Beyond the biopsychosocial model: Psychological characterization of medical illness. J Psychosom Res. (1996) 40:117–20. doi: 10.1016/0022-3999(95)00522-6
24. Fava G, Mangelli L, Ruini C. Assessment of psychological distress in the setting of medical disease. Psychother Psychosom. (2001) 70:171–5. doi: 10.1159/000056249
25. Achille MA, Ouellette A, Fournier S, Vachon M, Hébert MJ. Impact of stress, distress and feelings of indebtedness on adherence to immunosuppressants following kidney transplantation. Clin Transplant. (2006) 20:301–6. doi: 10.1111/j.1399-0012.2005.00478.x
26. Noohi S, Khaghani-Zadeh M, Javadipour M, Assari S, Najafi M, Ebrahiminia M, et al. Anxiety and depression are correlated with higher morbidity after kidney transplantation. Transplant Proc. (2007) 39:1074–8. doi: 10.1016/j.transproceed.2007.04.002
27. Fiebiger W, Mitterbauer C, Oberbauer R. Health-related quality of life outcomes after kidney transplantation. Health Qual Life Outcomes. (2004) 2:2. doi: 10.1186/1477-7525-2-2
28. Matas AJ, Halbert RJ, Barr ML, Helderman JH, Hricik DE, Pirsch JD, et al. Life satisfaction and adverse effects in renal transplant recipients: a longitudinal analysis. Clin Transplant. (2002) 16:113–21. doi: 10.1034/j.1399-0012.2002.1o126.x
29. Baines LS, Joseph JT, Jindal RM. Prospective randomized study of individual and group psychotherapy versus controls in recipients of renal transplants. Kidney Int. (2004) 65:1937–42. doi: 10.1111/j.1523-1755.2004.00594.x
30. Abbey S, Farrow S. Group therapy and organ transplantation. Int J Group Psychother. (1998) 48:163–85. doi: 10.1080/00207284.1998.11491535
31. Sambucini D, Ciacchella C, Pellicano GR, Zingaretti G, Pierro L, Aceto P, et al. Psychosocial treatment on psychological symptoms, adherence, and physiological function on transplanted patients: a systematic review and metanalysis. J Psychosom Res. (2022) 154:110717. doi: 10.1016/j.jpsychores.2022.110717
32. Kalra G, Desousa A. Psychiatric aspects of organ transplantation. Int J Organ Transplant Med. (2011) 2:9–19.
33. Battaglia Y, Zerbinati L, Piazza G, Martino E, Provenzano M, Esposito P, et al. Screening performance of edmonton symptom assessment system in kidney transplant recipients. J Clin Med. (2020) 9:4. doi: 10.3390/jcm9040995
34. World Medical Association. Declaration of helsinki ethical principles for medical research involving human subjects. JAMA. (2000) 284:3043–5. doi: 10.1001/jama.284.23.3043
35. Sheehan D, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 33:quiz 34-57. doi: 10.1037/t18597-000
36. Porcelli P, Guidi J. The clinical utility of the diagnostic criteria for psychosomatic research: a review of studies. Psychother Psychosom. (2015) 84:265–72. doi: 10.1159/000430788
37. Battaglia Y, Zerbinati L, Piazza G, Martino E, Massarenti S, Provenzano M, et al. The use of demoralization scale in Italian kidney transplant recipients. J Clin Med. (2020) 9:7. doi: 10.3390/jcm9072119
38. Battaglia Y, Zerbinati L, Murri MB, Provenzano M, Esposito P, Andreucci M, et al. Exploring the level of post traumatic growth in kidney transplant recipients via network analysis. J Clin Med. (2021) 10: 20. doi: 10.3390/jcm10204747
39. BattagliaY, Martino E, Piazza G, Cojocaru E, Massarenti S, Peron L, et al. Abnormal illness behavior, alexithymia, demoralization, and other clinically relevant psychosocial syndromes in kidney transplant recipients: A comparative study of the diagnostic criteria for psychosomatic research system versus ICD-10 Psychiatric Nosolo. Psychother Psychosom. (2018) 87:6.
40. Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. (2006) 69:1621–5. doi: 10.1038/sj.ki.5000184
41. Dano S, Pokarowski M, Liao B, Tang E, Ekundayo O, Li V, et al. Evaluating symptom burden in kidney transplant recipients: validation of the revised edmonton symptom assessment system for kidney transplant recipients – a single-center, cross-sectional study. Transpl Int. (2020) 33:423–36. doi: 10.1111/tri.13572
42. Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Supp Care Cancer. (2006) 14:30–7. doi: 10.1007/s00520-005-0834-3
43. Soper DS. A-Priori Sample Size Calculator for Multiple Regression. (2023). Available online at: https://www.danielsoper.com/statcalc (accessed January 20, 2023).
44. Johnston R, Jones K, Manley D. Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual Quant. (2018) 52:1957–76. doi: 10.1007/s11135-017-0584-6
45. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edn. London: Routledge. (1988).
46. Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. (1979) 47:1287. doi: 10.2307/1911963
47. Hunt KA, Weber EJ, Showstack JA, Colby DC, Callaham ML. Characteristics of frequent users of emergency departments. Ann Emerg Med. (2006) 48:1–8. doi: 10.1016/j.annemergmed.2005.12.030
48. Fehlmann CA, Miron-Celis M, Chen Y, Perry J, Eagles D. Association between mood disorders and frequent emergency department use: a cross-sectional study. CJEM. (2022) 24:55–60. doi: 10.1007/s43678-021-00204-w
49. Cukor D, Newville H, Jindal RM. Depression and immunosuppressive medication adherence in kidney transplant patients. Gen Hosp Psychiatry. (2008) 30:386–7. doi: 10.1016/j.genhosppsych.2007.12.003
50. Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am. J Transpl. (2012) 12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x
51. Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transpl. (2009) 9:2597–606. doi: 10.1111/j.1600-6143.2009.02798.x
52. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. (2004) 61:1107–15. doi: 10.1001/archpsyc.61.11.1107
53. Hartka E, Johnstone B, Leino EV, Motoyoshi M, Temple MT, Fillmore KM, et al. meta-analysis of depressive symptomatology and alcohol consumption over time. Br J Addict. (1991) 86:1283–98. doi: 10.1111/j.1360-0443.1991.tb01704.x
54. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. (2000) 160:2101–7. doi: 10.1001/archinte.160.14.2101
55. Kinder LS, Kamarck TW, Baum A, Orchard TJ. Depressive symptomatology and coronary heart disease in Type I diabetes mellitus: a study of possible mechanisms. Health Psychol. (2002) 21:542–52. doi: 10.1037/0278-6133.21.6.542
56. Fortes C, Farchi S, Forastiere F, Agabiti N, Pacifici R, Zuccaro P, et al. Depressive symptoms lead to impaired cellular immune response. Psychother Psychosom. (2003) 72:253–60. doi: 10.1159/000071896
57. Penninx BWJH, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. (2003) 54:566–72. doi: 10.1016/S0006-3223(02)01811-5
58. Lederbogen F, Gilles M, Maras A, Hamann B, Colla M, Heuser I, et al. Increased platelet aggregability in major depression? Psychiatry Res. (2001) 102:255–61. doi: 10.1016/S0165-1781(01)00259-1
59. Lentine KL, Naik AS, Ouseph R, Zhang Z, Axelrod DA, Segev DL, et al. Antidepressant medication use before and after kidney transplant: implications for outcomes - a retrospective study. Transpl Int. (2018) 31:20–31. doi: 10.1111/tri.13006
60. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. (2016) 4:27. doi: 10.1186/s40345-016-0068-y
61. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. (2018) 17:341–56. doi: 10.1002/wps.20567
62. Barsky A, Orav E, Bates D. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry. (2005) 62:903–10. doi: 10.1001/archpsyc.62.8.903
63. Konnopka A, Schaefert R, Heinrich S, Kaufmann C, Luppa M, Herzog W, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. (2012) 81:265–75. doi: 10.1159/000337349
64. Viarasilpa T, Panyavachiraporn N, Osman G, Akioyamen NO, Wasade VS, Barkley G, et al. Intubation for psychogenic non-epileptic attacks: frequency, risk factors, and impact on outcome. Seizure - Eur J Epilepsy. (2020) 76:17–21. doi: 10.1016/j.seizure.2019.12.025
65. Fink P. Surgery and medical treatment in persistent somatizing patients. J Psychosom Res. (1992) 36:439–47. doi: 10.1016/0022-3999(92)90004-L
66. Flynn TW, Smith B, Chou R. Appropriate use of diagnostic imaging in low back pain: a reminder that unnecessary imaging may do as much harm as good. J Orthop Sports Phys Ther. (2011) 41:838–46. doi: 10.2519/jospt.2011.3618
67. Warren JW, Morozov V, Howard FM, Wesselmann U, Gallicchio L, Langenberg P, et al. Before the onset of interstitial cystitis/bladder pain syndrome, the presence of multiple non-bladder syndromes is strongly associated with a history of multiple surgeries. J Psychosom Res. (2014) 76:75–9. doi: 10.1016/j.jpsychores.2013.10.013
68. Isaacs R. Ethical implications of ethnic disparities in chronic kidney disease and kidney transplantation. Adv Ren Replace Ther. (2004) 11:55–8. doi: 10.1053/j.arrt.2003.10.008
69. Russo D, Battaglia Y. Clinical Significance of FGF-23 in Patients with CKD. Int J Nephrol. (2011) 2011:1–5. doi: 10.4061/2011/364890
70. Esposito P, Picciotto D, Battaglia Y, Costigliolo F, Viazzi F, Verzola D. Myostatin: Basic biology to clinical application. Adv Clin Chem. (2022) 106:1–18. doi: 10.1016/bs.acc.2021.09.006
71. Battaglia Y, Fiorini F, Gisonni P, Imbriaco M, Lentini P, Zeiler M, et al. Ultrasonographic assessment of atherosclerotic renal artery stenosis in elderly patients with chronic kidney disease: an italian cohort study. Diagnostics. (2022) 12:6. doi: 10.3390/diagnostics12061454
72. Tirtayasa PMW, Duarsa GWK, Situmorang GR, Yudiana IW, Santosa KB, Oka AAG, et al. Association between Early Resistive Index Measurement and Early Graft Function and Long-term graft survival after kidney transplantation: an evidence-based clinical review. Acta Med Indones. (2019) 51:77–85.
73. Papini CB, Campos L de, Nakamura PM, Brito BTG de, Kokubun E. Cost-analysis and cost-effectiveness of physical activity interventions in Brazilian primary health care: a randomised feasibility study. Cien Saude Colet. (2021) 26:5711–26. doi: 10.1590/1413-812320212611.27142020
74. Aucella F, Gesuete A, Battaglia Y. A “nephrological” approach to physical activity. Kidney Blood Press Res. (2014) 39:2–3. doi: 10.1159/000355796
75. Aucella F, Battaglia Y, Bellizzi V, Bolignano D, Capitanini A, Cupisti A. Physical excercise programs in CKD: lights, shades and perspectives: a position paper of the “Physical exercise in CKD study group” of the Italian society of nephrology. J Nephrol. (2015) 28:2. doi: 10.1007/s40620-014-0169-6
Keywords: psychiatric diagnosis, ICD, DCPR, mood, somatization, distress, hospital admission, emergency access
Citation: Zerbinati L, Guerzoni F, Napoli N, Preti A, Esposito P, Caruso R, Bulighin F, Storari A, Grassi L and Battaglia Y (2023) Psychosocial determinants of healthcare use costs in kidney transplant recipients. Front. Public Health 11:1158387. doi: 10.3389/fpubh.2023.1158387
Received: 03 February 2023; Accepted: 11 May 2023;
Published: 02 June 2023.
Edited by:
Carlo Alfieri, University of Milan, ItalyReviewed by:
Ciro Esposito, University of Pavia, ItalyRoberto Cacciola, Policlinico Tor Vergata, Italy
Copyright © 2023 Zerbinati, Guerzoni, Napoli, Preti, Esposito, Caruso, Bulighin, Storari, Grassi and Battaglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuri Battaglia, eXVyaS5iYXR0YWdsaWFAdW5pdnIuaXQ=
 Luigi Zerbinati
Luigi Zerbinati Franco Guerzoni2
Franco Guerzoni2 Pasquale Esposito
Pasquale Esposito Francesca Bulighin
Francesca Bulighin Luigi Grassi
Luigi Grassi Yuri Battaglia
Yuri Battaglia