- 1School of Public Health, China Medical University, Shenyang, Liaoning, China
- 2State Key Laboratory for Infectious Disease Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
Background: The recent increase of group A Streptococcus (GAS) infections in Europe has aroused global concern. We aim to provide molecular biological data for GAS prevention and control in China by analyzing the temporal shift of emm type.
Methods: We collected studies reporting GAS emm types in China from 1990 to 2020 by PRISMA statement and established a summary database including emm types and literature quality assessment. Based on the database we analyzed the geographic distribution of emm types from 1990 to 2020 and assessed the coverage of the known GAS 30-valent vaccine. Outbreak-associated emm types that had been reported over the past 30 years were also included.
Results: 47 high quality studies were included for a systematic analysis of emm type distribution. This generated a database including totally 12,347 GAS isolates and 85 emm types. Shift of dominant emm type was witnessed during the past 30 years in China. In mainland China, dominant types changed from emm3, emm1, emm4, emm12 in 1990s to emm12 and emm1 in 2000s and 2010s. Hong Kong and Taiwan were dominated by emm12, emm4 and emm1, of which emm4 reduced but emm12 increased in 2010s significantly. From 1990 to 2020, newly found emm types were increasingly reported in various regions of China. The reported 30-valent M protein vaccine covered 26 M types prevalent in China, including all dominant types.
Conclusion
Emm12 increased significantly in the past 30 years in China. The prevalent type of Hong Kong and Taiwan is slightly different from that of mainland China by emm4. Our long-term retrospective analysis implicates that the 30-valent M protein vaccine can cover all dominant emm types prevalent in the past 30 years in China, which provide important basic data for future vaccine evaluation.
Introduction
Group A Streptococcus (GAS), also known as Streptococcus pyogenes, is an important gram-positive bacteria and one of the ten leading causes of infectious diseases in the world (1). GAS can cause wide spectrum of infections ranging from mild infections like pharyngitis and superficial skin infections, to serious and more deadly infections like invasive infections and streptococcal toxic shock syndrome (2). The larynx and epithelium of the skin are the primary sites for GAS infection and are also the common sites where GAS is acquired and transported (3). More than 18 million people worldwide are estimated to suffer from severe GAS disease (4). The estimated annual incidence of GAS caused pharyngitis is 0.4 cases per person-year, with more than 423 million children infected with GAS in developing countries (1). Although the most common infection is pharyngitis, invasive disease has increased dramatically in many areas of the world over the past 25 years (5). With the situations that many countries have co-existed with COVID-19, resurgence of group A Streptococcus infections has been noted in the last months in European countries.
M protein is a major virulence determinant and protective antigen of GAS. Emm, the gene encoding M protein, is the basis for sequence typing that used to differentiate strain serotype. Emm typing is based on sequence diversity of the 5′ region of emm (6–9). Sequence diversity of M protein has generated over 260 different emm types currently reported in the database from US CDC. Emm genotyping can provide basic information for possible vaccine implementation (10) and long-term surveillance of emm types is very important for detection of emerging types. It is always a challenge to develop a safe vaccine against group A Streptococcus infection (11). Recently a recombinant 30-valent M protein vaccine has passed Phase I clinical trial and could be potentially safe and effective for prevention of GAS infection (12). However, the molecular epidemiological data for GAS circulating in various geographies of the world is insufficient, which could limit further evaluation and utilization of a vaccine. In a study reported on global emm type distribution, little data was available from China and it is also in need to update the molecular data (13).
China has always attached great importance on the management and prevention of group A Streptococcus diseases. Research on GAS molecular epidemiology began in the early 1990s (14). Many studies from different geographies of China have reported collections of large number of GAS isolates and emm genotypes (15–19), there is a tremendous need to make a systematic review and summarization on these studies. In this study we collected and reviewed all reports on emm genotyping of China GAS isolates during the past 30 years. We aim to provide comprehensive emm data for GAS prevention and control, as well as to assess the coverage of 30-valent M protein vaccine for China.
Materials and Methods
Data sources
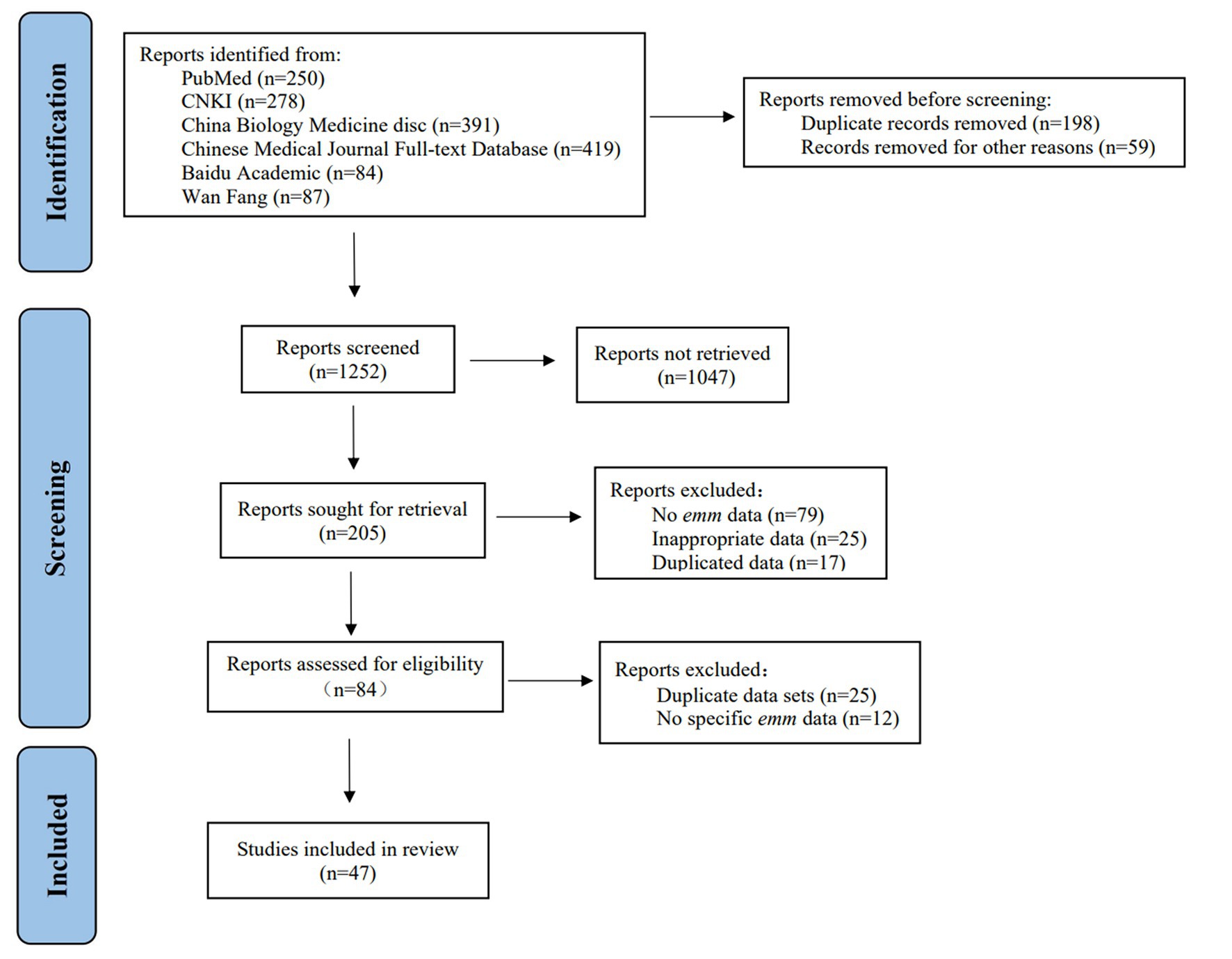
Studies describing the molecular epidemiology of GAS based on emm or M typing were searched by using a systematic approach consistent with the PRISMA statement (20). The search process was shown in Figure 1. We searched for publications involved in GAS genotyping and molecular epidemiology published from January 1, 1990 to June 30, 2021, with the search updated on May 26, 2022. The language was limited to Chinese and English.
Chinese databases (CNKI, Wan Fang, China Biology Medicine disc, Chinese Medical Journal Full-text Database, Baidu Academic) and English database (PubMed) were searched. Search terms included “genotype,” “genotyping,” “group A streptococci,” “group A Streptococcus,” “Streptococcus pyogenes,” “GAS,” “emm,” “molecular typing,” “molecular epidemiology,” etc. (Supplementary Appendix S1).
Study quality assessment
We established a database that dedicated to aggregated data on results about emm genotype of GAS. The contents of the database include study time, region, population, emm typing method, etc., in order to evaluate the data and its eligibility for inclusion. Regarding data extraction and citation, two researchers screened the literature (Supplementary Appendix S1, S2).
In order to make an overall estimation of all GAS genotypes in China, we included studies of mainland, Taiwan, and Hong Kong. Macau was excluded since there were few reported cases of GAS. When there was duplicate data (for example, multiple studies reporting data from the same region or at the same time, or using the same strains), we included only the most recent study and remove repeated reports.
Definition
To evaluate the time and regional distribution characteristics of China GAS emm types, the regions were divided into mainland, Hong Kong and Taiwan, and the time was divided into three periods: the 1990s, 2000s and 2010s.
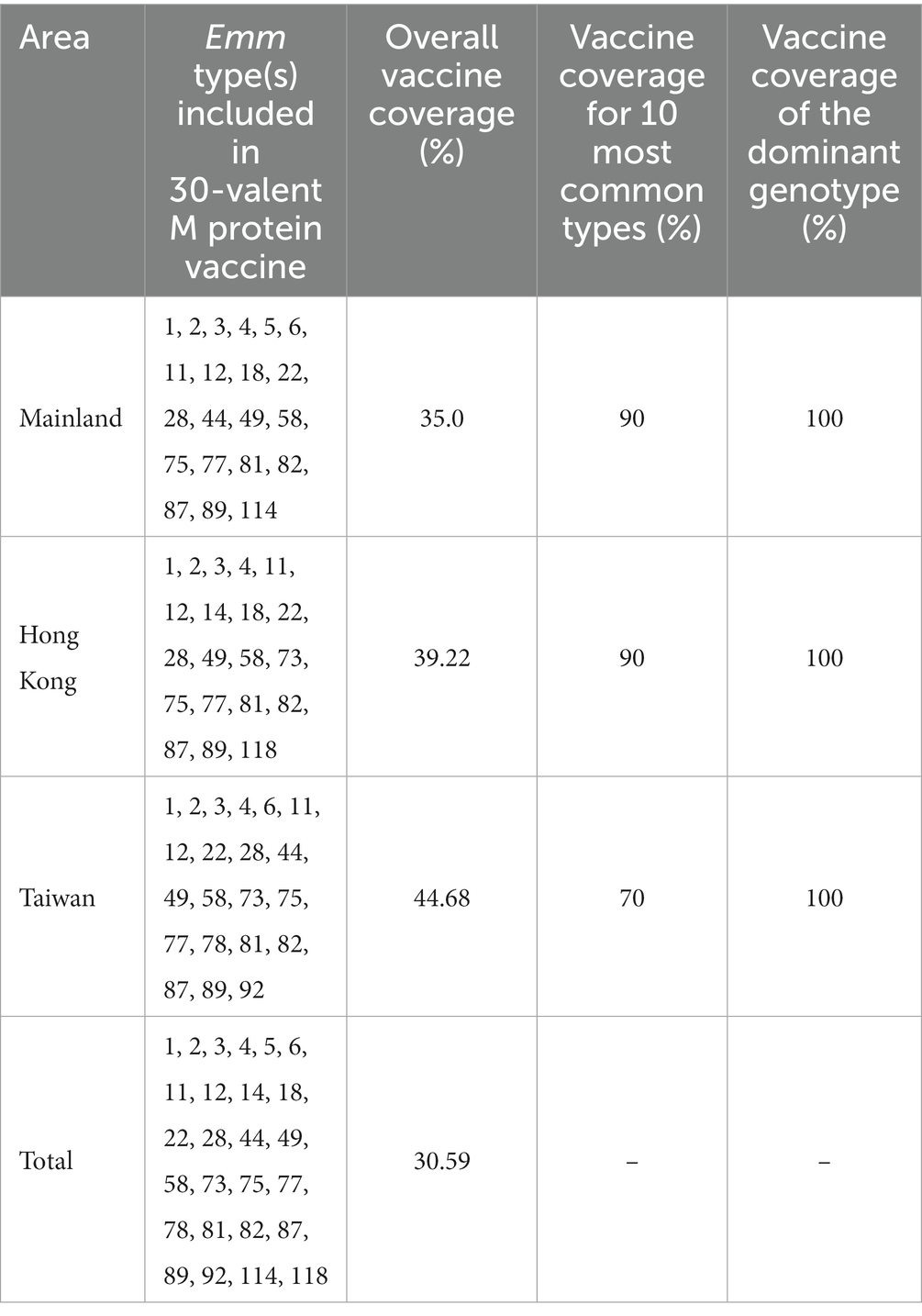
A 30-valent GAS vaccine based on M protein had been reported, including emm1, 2, 3, 4, 5, 6, 11, 12, 14, 18, 19, 22, 24, 28, 29, 44, 49, 58, 73, 75, 77, 78, 81, 82, 83, 87, 89, 92, 114, 118 (12). Vaccine coverage was defined as the percentage of the number of emm genotypes covered by the reported 30-valent GAS vaccine in a given region to the total number of emm genotypes in the region. Vaccine coverage for 10 most common types was the extent to which the 30-valent vaccine can cover the 10 most common GAS emm genotypes in a region. It was calculated as the number of emm genotypes included in the 30-valent vaccine among the 10 most common emm genotypes in each region divided by 10 and then multiplied by 100%. Vaccine coverage of the dominant genotype referred to the percentage of the ratio of the number of dominant genotypes to the number of dominant genotypes covered by the 30-valent vaccine. If the number of strains of a certain emm type accounted for more than 10% of all strains, it was regarded as a dominant genotype.
GAS outbreak study was defined as the first reported outbreak study on the emm genotype of GAS in a certain region. GAS case study was defined as the newly discovered or first reported case study on the emm genotype of GAS in a certain region.
Statistical analysis
By sorting the percentages of different emm genotypes of GAS strains in the total number of GAS strains in the designated area, we compared the distribution of emm types in different regions of China. We also compared the distribution of emm types in different time periods by the same method. Excel 2019 was used to establish the database and conduct data analysis.
Results
Diversity of GAS emm types in China
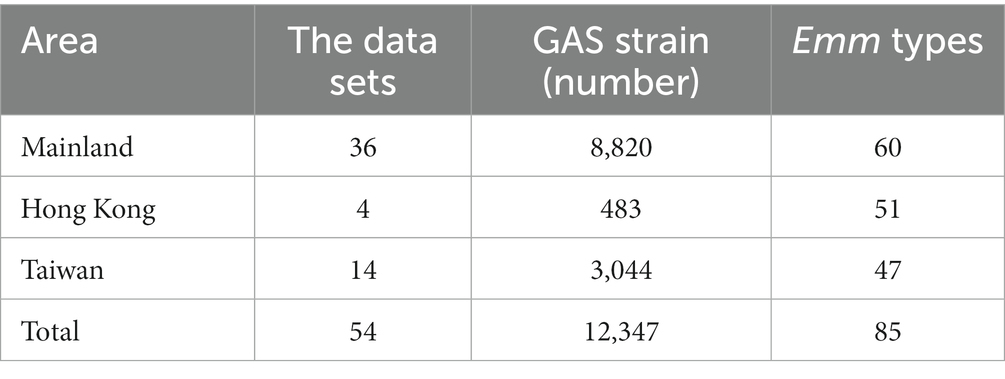
We retrieved 1,509 documents in specific databases according to the search method and search terms. We removed duplicate literatures. After preliminary screening of titles and abstracts, 1,304 literatures did not meet the research content were removed and the remaining 205 full-text literatures were kept for further analysis. Furthermore, we excluded 158 articles by quality assessment on the 205 published datasets (Figure 1). Finally, 47 published high-quality articles were included for qualitative analysis (Supplementary Appendix S1), including 9 articles on GAS outbreaks and case studies about emm genotypes in China. Totally 54 datasets were included in our database, including 36 datasets for mainland, 14 datasets for Taiwan, and 4 datasets for Hong Kong (Table 1).
We pooled 12,347 GAS strains related to 85 emm genotypes (Table 1). The most popular emm genotypes are emm12 (50.08%), emm1 (30.47%), emm4 (6.31%), emm22 (1.98%), emm6 (1.91%), accounting for 90.75% of all strains (Supplementary Appendix S2).
Temporal distribution of emm types
In the mainland, compared with the period of 1990s, strains of emm1, emm12 and emm22 increased significantly in the 2000s, whereas emm3, emm4 and emm6 decreased significantly. Compared with the 2000s, emm1 showed little change in the 2010s, whereas emm12 significantly increased. In Hong Kong, emm1, emm2, emm4 and emm22 increased in the 2000s compared with the period of 1990s, but emm58 decreased significantly. Emm12 did not change much. Compared with the 2000s, emm2 and emm4 GAS strains decreased significantly in the 2010s, but emm12 increased significantly. In Taiwan, compared with the period of 1990s, emm1, emm6 and emm12 increased significantly in the 2000s, whereas emm4 decreased significantly. Moreover, emm12 had been increasing over the past 30 years, while emm4 had been decreasing (Figure 2).
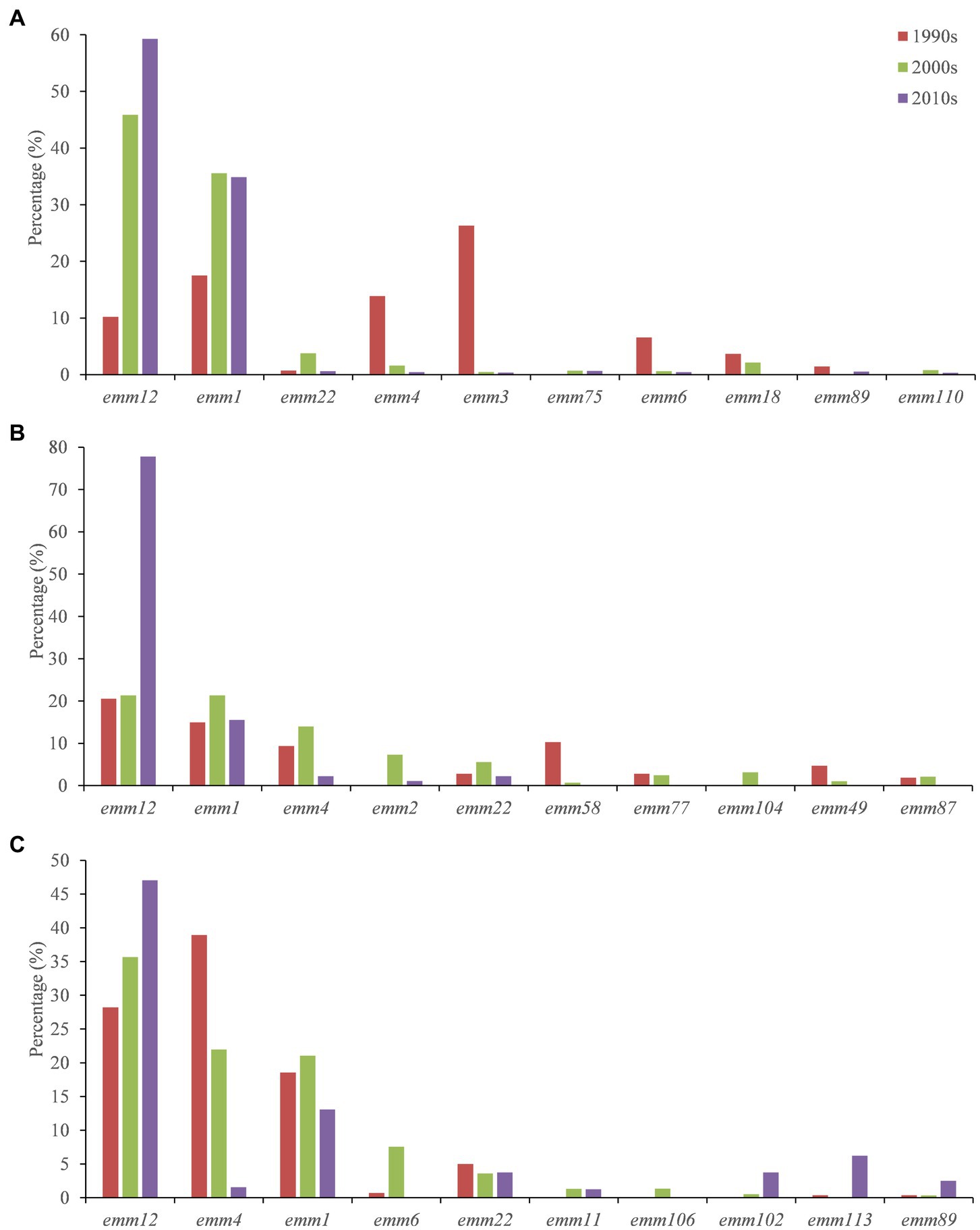
Figure 2. Temporal distribution of emm types of GAS strains in mainland (A), Hong Kong (B) and Taiwan (C).
Regional distribution of emm types
The distribution of GAS emm genotypes in different regions of China was different (Supplementary Appendix S2). We aggregated 8,820 strains and contained 60 emm genotypes in mainland region (Table 1). Emm12 (55.87%) and emm1 (34.73%) were the dominant genotypes, followed by emm22 (1.25%). Figure 3A listed 10 common emm genotypes of GAS strains in the mainland, which accounted for 96.11% of the GAS strains in the mainland. The remaining 50 emm genotypes accounted for 2.63% of the GAS strains in the mainland.
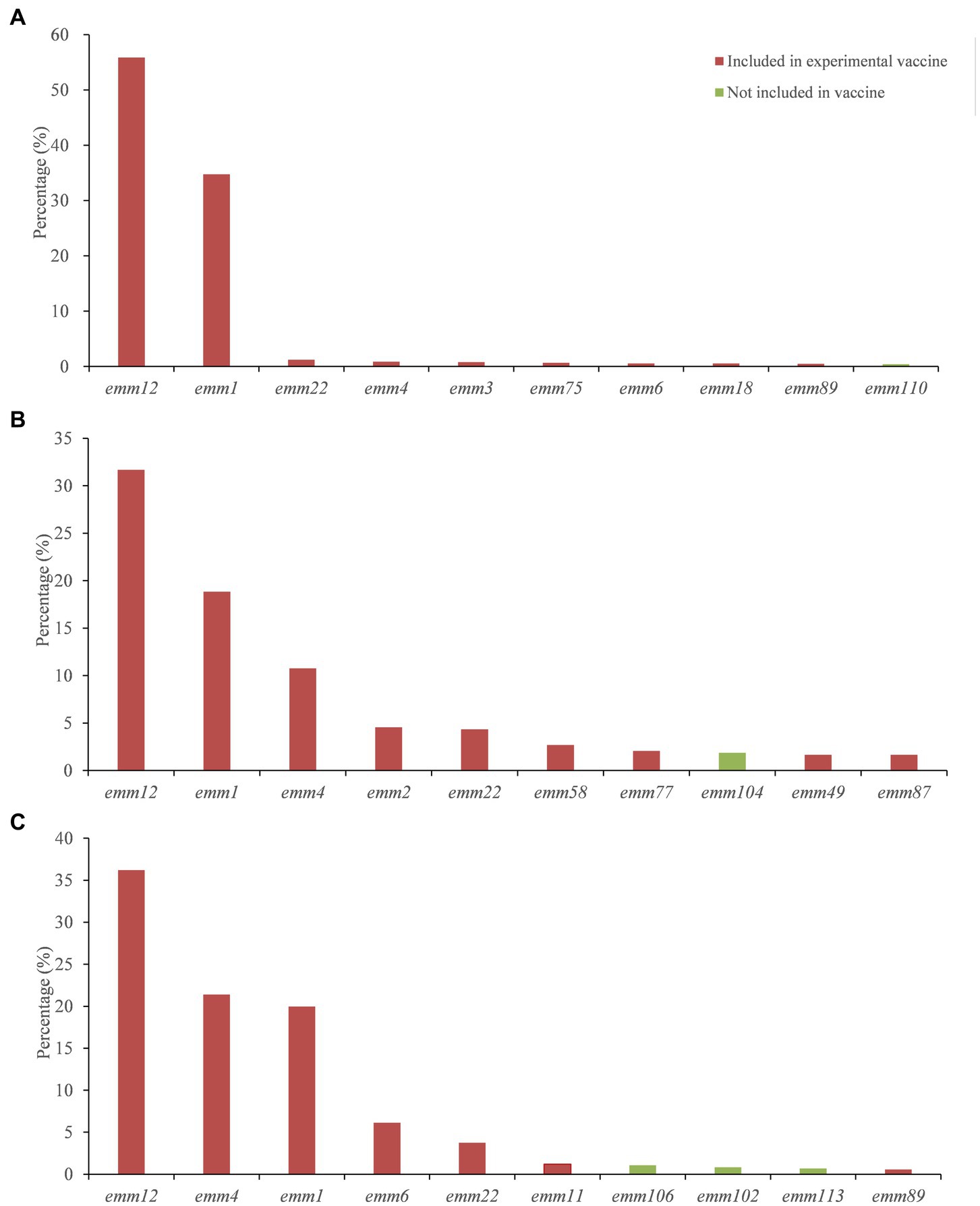
Figure 3. Emm types among the top 10 covered by the 30-valent M protein vaccine in mainland (A), Hong Kong (B) and Taiwan (C).
A total of 483 strains were collected in Hong Kong, including 51 emm genotypes (Table 1). Emm12 (31.68%), emm1 (18.84%) and emm4 (10.77%) were the dominant genotypes, accounting for 61.29% of the emm genotypes of the GAS strains included in Hong Kong. Figure 3B listed 10 common emm genotypes of GAS strains in Hong Kong, which accounted for 80.13% of all GAS strains. The remaining 41 emm genotypes accounted for 18.01% of all GAS strains in Hong Kong.
In Taiwan, 3,044 strains were collected, including 47 emm genotypes (Table 1). Emm12 (36.2%), emm4 (21.39%) and emm1 (19.97%) were the dominant genotypes, accounting for 77.56% of the emm genotypes of the GAS strains included in Taiwan. Figure 3C listed 10 common emm genotypes of GAS strains in Taiwan, which accounted for 91.78% of GAS strains in Taiwan. The remaining 37 emm genotypes accounted for 4.7% of all GAS strains in Taiwan.
Assessment of 30-valent experimental vaccine coverage
The 30-valent M protein vaccine covered 26 M types prevalent in China (21, 20, and 21 emm genotypes in mainland, Hong Kong and Taiwan, respectively). Table 2 summarized the coverage of international 30-valent GAS M protein vaccine of the Chinese GAS strain emm genotypes by region. Overall, the vaccine coverage in mainland, Taiwan and Hong Kong was low, however vaccine coverage for the 10 most common types and the dominant genotypes were very high (Figure 3).
Emm types involved in outbreaks and case studies
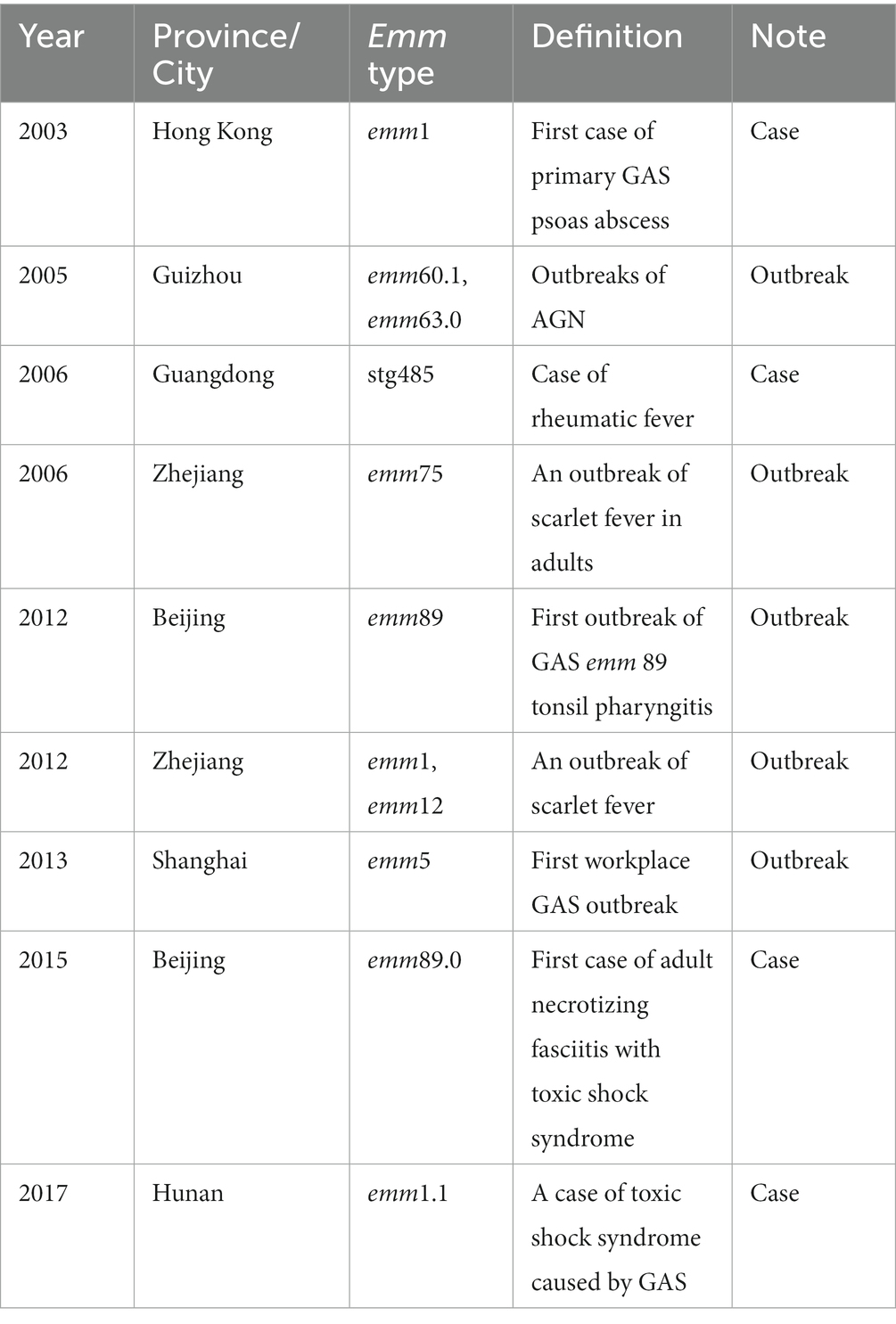
We also summarized GAS emm genotypes from outbreaks and case studies in China from 1990 to 2020. The results were shown in Table 3. In the past 30 years, GAS outbreaks caused by different emm types had occurred in various regions of China. In 2005, acute glomerulonephritis (AGN) outbreaks occurred in two counties in Guizhou Province, which were caused by GAS emm60.1 and emm63.0 (21). In 2006, an outbreak of adult scarlet fever caused by the GAS emm75 genotype occurred in Zhejiang Province (22). In 2012, the first outbreak of tonsillopharyngitis caused by GAS emm89 genotype occurred in Beijing (23), in the same year an outbreak of scarlet fever caused by the emm1 and emm12 genotypes occurred in Zhejiang Province (24). In 2013, an outbreak of GAS caused by the emm5 genotype occurred in Shanghai Province, which was the first GAS outbreak in the workplace (25).
In the past 30 years in China, there were not only several new GAS genotypes that were unreported previously, but also more and more unusual GAS infections were reported. In 2003, the first case of primary GAS psoas abscess caused by emm1 was found in Hong Kong (26). In 2006, a patient with rheumatic fever caused by GAS emm stg485 was found in Guangdong Province (27). In 2015, Chinese first case of adult necrotizing fasciitis with toxic shock syndrome caused by emm89.0 was reported in Beijing (28). A case of toxic shock syndrome caused by GAS emm1.1 occurred in Hunan Province in 2017 (29). The findings of these reports emphasize the importance of surveillance of invasive GAS infection in China.
Discussion
By using qualitative analysis on all included literatures and excluding strains that could not be typed, totally 85 emm types were found for China GAS strains in the past 30 years, which indicated that emm genotypes of China GAS were diverse. The most prevalent genotype was emm12, accounting for 50.08% of those included in this study, followed by emm1 (30.47%) and emm4 (6.31%). This is different from the situation in Europe and North America, where emm1 is the dominant type, occasionally replaced by other emm types (30). More importantly, the emergent M1UK clone in Europe accounted for the majority of increasing scarlet fever and invasive infection cases in the past years (31), which suggests that genomic epidemiologic surveillance is in urgent need in China to screen the transmission of such virulent clones on time.
In the past 30 years, not only some new GAS genotypes and new types of infections that were never reported previously appeared in various regions of China, but also many GAS outbreaks caused by different emm genotypes had occurred. These results suggest that there are potentially other unknown emm genotypes that were not identified in China. Therefore, it is necessary to perform long-term molecular epidemiological monitoring of GAS genotypes. Especially under the context of COVID-19 epidemic, introduction of novel emm types could probably trigger more GAS outbreaks hence increase medical burden.
There were different emm genotypes of GAS prevalent in different times and regions in China during the past 30 years. In particular, the molecular epidemiology and shift of GAS emm pattern in mainland China, Taiwan and Hong Kong were not exactly consistent. In mainland China during the past 30 years, emm1 and emm12 had been the dominant types since 2000s, with the percentage of emm1 increasing from 17.52% in the 1990s to 35.55% in the 2000s, and the percentage of emm12 increasing from 10.22% in the 1990s to 45.89% in the 2000s (Supplementary Appendix S1). Lu et al. collected 140 S. pyogenes from infected patients in 10 tertiary general hospitals from 7 cities/provinces in China during 2009 and 2016, and found that emm 12 and emm1 had a relatively high proportion among all isolates (32). Compared with the period of 2000s, the percentage of emm1 GAS strains in the 2010s did not change too much from 35.55% to 34.86%, but emm12 increased significantly from 45.89% to 59.28% (Supplementary Appendix S1). There are regional differences between various cities or provinces of mainland. Although emm12 and emm1 were the dominant GAS types in Beijing, emm12 ratio decreased from 76.4% in 2011 to 46.49% in 2020–2021 and emm1 ratio increased from 17.1% (2011) to 25.44% (2020–2021), respectively (16, 18). Emm12 was also the predominant circulating type (91.4%) in Shanghai in 2011 (15). Meanwhile, emm12 took up to 60.3% in fluoroquinolone non-susceptible GAS during 2011 to 2016 in Shanghai (17). The outbreak and resurgence of group A streptococcal disease such as scarlet fever is probably due to the emergence of a newly found macrolide resistant emm12 clone in 2011 (15, 17–19, 33). You et al. conducted genomic studies on emm12 strains and found several mobile genetic elements that are associated with the scarlet fever outbreak in recent years. Such as ICE-emm12 which encoding macrolide and tetracycline resistance genes, and the prophage φHKU.vir encoding virulence factors SSA, SpeC and Spd1 (34). Chen et al. found that emm1 GAS strains in Shanghai during 2011–2015 harbored a superantigen profile similar to emm12 isolates, except for the SpeA gene (35). These studies could partially explain the increase of emm1 and emm12 GAS strains in the mainland over the last 30 years though more detail studies are needed.
In Taiwan and Hong Kong, the constitution of prevalent emm types in the past thirty years is different. Ten most common emm genotypes in Taiwan are emm12, emm4, emm1, emm6, emm22, emm11, emm106, emm102, emm113, emm89. Chiang-Ni et al. collected 677 isolates from 1994 to 2008 in a hospital in southern Taiwan. They found that forty-four different emm types, the five most frequent types were emm12, emm4, emm1, emm11 and emm81, which were responsible for 61.4% of all isolates (36). In Hong Kong, the 10 most common types were emm12, 1, 4, 2, 22, 58, 77, 104, 49, 87, which were different from the mainland and Taiwan except for the most dominant types emm12, emm1, and emm4. The reasons for emm pattern difference and diversity between mainland, Taiwan and Hong Kong remain unclear. More comprehensive surveillance data is needed for further clarification. Our findings also have implications for vaccine development and evaluation. M protein can elicit bactericidal antibodies following GAS infection that persist in human serum for extended periods of time. There is a 30-valent M protein vaccine reported safe and immunogenic for humans, and has passed Phase I clinical trials (12). Our results showed that the 30-valent vaccine covered 26 GAS emm genotypes in China. Although the overall genotype coverage in China was low, the coverage of 10 most common types and the dominant genotypes were very high.
There are some limitations for our study. Firstly, our data did not cover all provinces of China, especially those western provinces where little data is available, this could potentially affect the diversity of emm types found in this study, which emphasize the importance of sampling and GAS genotyping in these areas. Secondly, most of the included studies were conducted during 2000s and 2010s. Few data are available in 1990s. Therefore, the qualitative comparison results obtained in this study need to be interpreted with caution.
In conclusion, according to the available data from various regions in China, the dominant emm types between mainland China, Hong Kong and Taiwan are similar, but shifted in different time period. Emm12 and emm1 are the two most important types that need to be highly concerned in future surveillance. The experimental 30-valent M protein vaccine provides good coverage on dominant emm genotypes in China, which could be useful for the prevention and control of GAS infection in China.
Author contributions
CX collected data and made systematic review. CX and YY write the manuscript. JZ and FZ help review data and revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Surveillance and epidemic response of bacterial and fungal infectious diseases (08025) and Project for Novel Detection Techniques of Bacterial Pathogens (32073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1157289/full#supplementary-material
References
1. Carapetis, JR, Steer, AC, Mulholland, EK, and Weber, M. The global burden of group A streptococcal diseases. Lancet Infect Dis. (2005) 5:685–94. doi: 10.1016/s1473-3099(05)70267-x
2. Sherwood, E, Vergnano, S, Kakuchi, I, Bruce, MG, Chaurasia, S, David, S, et al. Invasive group A streptococcal disease in pregnant women and young children: a systematic review and meta-analysis. Lancet Infect Dis. (2022) 22:1076–88. doi: 10.1016/s1473-3099(21)00672-1
3. Bessen, DE, McShan, WM, Nguyen, SV, Shetty, A, Agrawal, S, and Tettelin, H. Molecular epidemiology and genomics of group A Streptococcus. Infect Genet Evol. (2015) 33:393–418. doi: 10.1016/j.meegid.2014.10.011
4. Ralph, AP, and Carapetis, JR. Group A streptococcal diseases and their global burden. Curr Top Microbiol Immunol. (2013) 368:1–27. doi: 10.1007/82_2012_280
5. Wang, B, and Cleary, PP. Intracellular invasion by Streptococcus pyogenes: invasins, host receptors, and relevance to human disease. Microbiol Spectr. (2019) 7. doi: 10.1128/microbiolspec.GPP3-0049-2018
6. Aranha, MP, Penfound, TA, Salehi, S, Botteaux, A, Smeesters, P, Dale, JB, et al. Design of broadly cross-reactive M protein-based group A streptococcal vaccines. J Immunol. (2021) 207:1138–49. doi: 10.4049/jimmunol.2100286
8. Frost, HR, Laho, D, Sanderson-Smith, ML, Licciardi, P, Donath, S, Curtis, N, et al. Immune cross-opsonization within emm clusters following group A Streptococcus skin infection: broadening the scope of type-specific immunity. Clin Infect Dis. (2017) 65:1523–31. doi: 10.1093/cid/cix599
9. McMillan, DJ, Drèze, PA, Vu, T, Bessen, DE, Guglielmini, J, Steer, AC, et al. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect. (2013) 19:E222–9. doi: 10.1111/1469-0691.12134
10. Koutouzi, F, Tsakris, A, Chatzichristou, P, Koutouzis, E, Daikos, GL, Kirikou, E, et al. Streptococcus pyogenes emm types and clusters during a 7-year period (2007 to 2013) in pharyngeal and nonpharyngeal pediatric isolates. J Clin Microbiol. (2015) 53:2015–21. doi: 10.1128/JCM.00301-15
11. McNeil, SA, Halperin, SA, Langley, JM, Smith, B, Warren, A, Sharratt, GP, et al. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. (2005) 41:1114–22. doi: 10.1086/444458
12. Pastural, E, McNeil, SA, MacKinnon-Cameron, D, Ye, L, Langley, JM, Stewart, R, et al. Safety and immunogenicity of a 30-valent M protein-based group A streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study. Vaccine. (2020) 38:1384–92. doi: 10.1016/j.vaccine.2019.12.005
13. Steer, AC, Law, I, Matatolu, L, Beall, BW, and Carapetis, JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. (2009) 9:611–6. doi: 10.1016/S1473-3099(09)70178-1
14. Ma, Y, Yang, Y, Huang, M, Wang, Y, Chen, Y, Deng, L, et al. Characterization of emm types and superantigens of Streptococcus pyogenes isolates from children during two sampling periods. Epidemiol Infect. (2009) 137:1414–9. doi: 10.1017/s0950268809002118
15. Chen, M, Yao, W, Wang, X, Li, Y, Chen, M, Wang, G, et al. Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr Infect Dis J. (2012) 31:e158–62. doi: 10.1097/INF.0b013e31825874f3
16. Li, H, Zhou, L, Zhao, Y, Ma, L, Zhang, H, Liu, Y, et al. Epidemiological analysis of group A Streptococcus infection diseases among children in Beijing, China under COVID-19 pandemic. BMC Pediatr. (2023) 23:76. doi: 10.1186/s12887-023-03885-7
17. Shen, Y, Cai, J, Davies, MR, Zhang, C, Gao, K, Qiao, D, et al. Identification and characterization of fluoroquinolone non-susceptible Streptococcus pyogenes clones harboring tetracycline and macrolide resistance in Shanghai, China. Front Microbiol. (2018) 9:542. doi: 10.3389/fmicb.2018.00542
18. Yang, P, Peng, X, Zhang, D, Wu, S, Liu, Y, Cui, S, et al. Characteristics of group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg Infect Dis. (2013) 19:909–15. doi: 10.3201/eid1906.121020
19. Yu, D, Liang, Y, Lu, Q, Meng, Q, Wang, W, Huang, L, et al. Molecular characteristics of Streptococcus pyogenes isolated from Chinese children with different diseases. Front Microbiol. (2021) 12:722225. doi: 10.3389/fmicb.2021.722225
20. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
21. Zheng, MH, Jiao, ZQ, Zhang, LJ, Yu, SJ, Tang, GP, Yan, XM, et al. Genetic analysis of group A Streptococcus isolates recovered during acute glomerulonephritis outbreaks in Guizhou Province of China. J Clin Microbiol. (2009) 47:715–20. doi: 10.1128/jcm.00747-08
22. Dong, H, Song, Q, Lin, H, Jin, C, and Xu, G (2007). “Study of genotypic characteristics and virulent genes of group A Streptococcus isolates recovered during a scarlet fever outbreak”, in Zhejiang Medical Virology, Medical Microbiology and Immunology Academic Annual Meeting, Zhejiang, China, 2007, 110–114.
23. Liu, YM, Zhao, JZ, Li, BB, Yang, JY, Dong, XG, Zhang, JJ, et al. A report on the first outbreak of a single clone group A Streptococcus (emm-type 89) tonsillopharyngitis in China. J Microbiol Immunol Infect. (2014) 47:542–5. doi: 10.1016/j.jmii.2013.08.011
24. Xu, Y, Luo, Y, Sun, Y, and Chen, Y. Etiological detection and molecular characteristics analysis of the outbreaks of scarlet fever. Chin J Health Lab Tec. (2016) 26:553–5. doi: CNKI:SUN:ZWJZ.0.2016-04-035
25. Chen, M, Wang, W, Tu, L, Zheng, Y, Pan, H, Wang, G, et al. An emm5 group A streptococcal outbreak among workers in a factory manufacturing telephone accessories. Front Microbiol. (2017) 8:1156. doi: 10.3389/fmicb.2017.01156
26. Lau, SK, Woo, PC, Yim, TC, To, APC, and Yuen, KY. Molecular characterization of a strain of group A Streptococcus isolated from a patient with a psoas abscess. J Clin Microbiol. (2003) 41:4888–91. doi: 10.1128/jcm.41.10.4888-4891.2003
27. Huang, J, Yu, B, and Gu, J. A strain of group A β-hemolytic Streptococcus isolated from the throat of a patient with rheumatic fever. The J Pract Med. (2006) 9:1058. doi: 10.3969/j.issn.1006-5725.2006.09.060
28. Wang, P, Yang, Q, Zhou, X, Zhao, X, Wang, Y, Wang, H, et al. Clinical analysis of adults with toxic shock syndrome induced by Streptococcus pyogenes. Chin J Nosocomiol. (2016) 26:2251–2253+2259. doi: 10.11816/cn.ni.2016-160887
29. Ning, X, Zhu, H, Qian, C, Deng, L, and Xie, X. Analysis of toxic shock syndrome caused by Streptococcus pyogenes. Lab Med Clin. (2017) 14:3444–6. doi: 10.3969/j.issn.1672-9455.2017.23.008
30. Gherardi, G, Vitali, LA, and Creti, R. Prevalent emm types among invasive GAS in Europe and North America since year 2000. Front Public Health. (2018) 6:59. doi: 10.3389/fpubh.2018.00059
31. Lynskey, NN, Jauneikaite, E, Li, HK, Zhi, X, Turner, CE, Mosavie, M, et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis. (2019) 19:1209–18. doi: 10.1016/s1473-3099(19)30446-3
32. Lu, B, Fang, Y, Fan, Y, Chen, X, Wang, J, Zeng, J, et al. High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front Microbiol. (2017) 8:1052. doi: 10.3389/fmicb.2017.01052
33. You, YH, Song, YY, Yan, XM, Wang, HB, Zhang, MH, Tao, XX, et al. Molecular epidemiological characteristics of Streptococcus pyogenes strains involved in an outbreak of scarlet fever in China, 2011. Biomed Environ Sci. (2013) 26:877–85. doi: 10.3967/bes2013.016
34. You, Y, Davies, MR, Protani, M, McIntyre, L, Walker, MJ, and Zhang, J. Scarlet fever epidemic in China caused by Streptococcus pyogenes serotype M12: epidemiologic and molecular analysis. EBioMedicine. (2018) 28:128–35. doi: 10.1016/j.ebiom.2018.01.010
35. Chen, M, Cai, J, Davies, MR, Li, Y, Zhang, C, Yao, W, et al. Increase of emm1 isolates among group A Streptococcus strains causing scarlet fever in Shanghai, China. Int J Infect Dis. (2020) 98:305–14. doi: 10.1016/j.ijid.2020.06.053
Keywords: group A Streptococcus, emm type, prevalent type, China, vaccine coverage
Citation: Xiang C, Zhang J, Zhao F and You Y (2023) Emm type distribution of group A Streptococcus in China during 1990 and 2020: a systematic review and implications for vaccine coverage. Front. Public Health. 11:1157289. doi: 10.3389/fpubh.2023.1157289
Edited by:
Kai Zhou, First Affiliated Hospital of Southern University of Science and Technology, ChinaCopyright © 2023 Xiang, Zhang, Zhao and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Zhao, emhhb2ZlaUBpY2RjLmNu; Yuanhai You, eW91eXVhbmhhaUBpY2RjLmNu
 Caixin Xiang
Caixin Xiang Jianzhong Zhang
Jianzhong Zhang Fei Zhao
Fei Zhao Yuanhai You
Yuanhai You