
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 25 May 2023
Sec. Public Health Policy
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1144674
 Rachel Greenley1*†
Rachel Greenley1*† Sadie Bell2†
Sadie Bell2† Samuel Rigby3†
Samuel Rigby3† Rosa Legood4
Rosa Legood4 Victoria Kirkby3
Victoria Kirkby3 Martin McKee3
Martin McKee3 the CBIG-SCREEN Consortium
the CBIG-SCREEN ConsortiumBackground: Cervical cancer is a preventable and inequitably distributed disease. Screening plays a vital role in prevention, but many women face barriers to participation. The aims of this scoping review, undertaken to inform the co-design of interventions to equitably increase screening uptake, were to: (1) identify barriers and facilitators to cervical cancer screening for underserved populations, and (2) identify and describe the effectiveness of interventions aimed at improving participation in cervical cancer screening among underserved groups in Europe.
Methods: Qualitative, quantitative, and mixed methods studies focusing on barriers and facilitators to cervical screening participation and interventions to improve uptake undertaken in Europe and published after 2000 were included. Four electronic databases were searched to identify relevant papers. Titles and abstracts were screened, full text reviewed, and key findings extracted. Data were extracted and analyzed according to different health system strata: system-wide (macro), service specific (meso) and individual/community specific (micro). Within these categories, themes were identified, and the population groups impacted were recorded. All findings are presented in accordance with (PRISMA) guidelines.
Results: 33 studies on barriers and facilitators and eight intervention studies met the inclusion criteria. Collectively, the findings of these studies presented a wide array of screening uptake barriers, facilitators, and interventions, predominantly related to screening service and individual/community factors. However, although diverse, certain core themes around information provision, prompts for participation and the need for inclusive spaces were apparent. Implementation of screening programs should focus on: (1) reducing identifiable barriers, (2) increasing public awareness, and (3) providing patient reminders and measures to promote engagement by healthcare providers.
Conclusion: There are many barriers to uptake of cervical cancer screening and this review, nested within a larger study, will inform work to devise a solution alongside groups identified in three European countries.
Cervical cancer remains the fourth most common cancer in women, even though it is preventable (1). In the mid-1980s, when cancer rates across the European Union (EU) were increasing, Europe Against Cancer launched an ambitious program aiming to achieve a 15% reduction in cancer mortality by the year 2000 (2). It included elements of screening and education, with a focus on actions individuals can take to reduce their risk of developing cancer. Initially limited to guidance on good practice, it developed into an action plan to strengthen prevention of cancer and improve early detection and treatment. Yet, despite the priority that the EU has given to preventing cervical cancer, it still kills many women in Europe (1).
Further progress depends on success in fully implementing programs of vaccination against human papillomavirus (HPV) and cervical cancer screening (CCS), both highly effective and core elements of WHO and EU strategies (3–6). Fortunately, all EU member states now have programs in place to provide vaccines to young women, beginning in 2006, and, as of December 2021, young men (except in the three Baltic Republics, Poland, Bulgaria, Romania, and Greece). The programs were first implemented in 2007 (France, Germany, and 3 regions of Spain) and were extended until 2018 (Estonia). Three products are licensed for use in the EU: a bivalent vaccine (Cervarix), a quadri-valent recombinant vaccine, (Gardasil) and a 9-valent vaccine (Gardasil 9) (7). Yet vaccine coverage is often suboptimal, a problem exacerbated by the narrow time window for vaccination to be effective before initiation of sexual activity and the relatively recent implementation of vaccination programs in some countries, leaving many older women unprotected (8). Thus, effective and accessible screening programs will continue to be needed for some time. However, many screening programs also struggle to cover those in need, leaving large numbers of women, and those with cervixes, vulnerable (9).
Concerning the distribution of cervical cancer across European society, data limitations inhibit insights into the scale and nature of inequalities (10). However, a body of evidence does point to large differences in cervical cancer outcomes and care across population groups. One extensive compilation of data, covering 18 countries, found a three-fold difference, increasing to eight-fold in Estonia, in cervical cancer mortality according to education status, adversely affecting those with low educational attainment. The authors of this study noted how the variation in mortality among countries was driven entirely by differences in outcomes for the least educated women (11).
Examination of the distribution of factors known to prevent mortality, namely vaccination and screening, at country level within Europe, provides further insights into cervical cancer inequalities. In Denmark, a country with especially high quality data, vaccine uptake was found to be lower among girls from minority ethnic groups (odds ratio [OR] = 0.49; 95% confidence interval [CI], 0.42–0.57), mothers with only basic education (OR = 0.75; 95% CI, 0.69–0.82), those with low disposable income (OR = 0.67; 95% CI, 0.61–0.73) and mothers who are unemployed (OR = 0.75; 95% CI, 0.69–0.82) or unmarried (OR = 0.70; 95% CI, 0.65–0.76) (12). Meanwhile, in England, girls in all the main minority ethnic groups are about 50% less likely to be vaccinated than their White British counterparts (13). In Île-de-France, the region around Paris, a two-fold variation in vaccine uptake among the eight Departments was observed, with the share of the population that were migrants significant in explaining the difference (14).
Relating to screening uptake, the most useful insights into inequality come from the European Health Interview Survey. As with vaccination, women born outside the EU are about 50% as likely to have been screened. There is also a steep educational gradient (odds ratio [OR] 0.60 for intermediate and 0.27 for low versus higher education), with those in the lowest income quintile having an OR of 0.60 compared with those in the highest. All of these findings were significant (15). Another study using these data highlighted the potential for health system factors to influence these inequalities, finding that differentials were greatly reduced in countries where the screening program was organized rather than ad hoc, and where access to healthcare was good (16).
To equitably reduce the burden of cervical cancer in Europe, and have a realistic hope of achieving the World Health Organization (WHO) 2030 target (17) of cervical cancer elimination, action is urgently needed to identify and respond to barriers to screening uptake affecting those underserved. This review was undertaken to inform the development of interventions to improve cervical cancer screening uptake among underserved women as part of the EU-funded CBIG-SCREEN project which involves working with these women to co-create solutions (18).
To inform these actions, we report the findings of a scoping review of the barriers and facilitators of access to CCS programs and the interventions taken to improve uptake among population groups at risk of being underserved in Europe. They are by virtue of a range of characteristics known to be associated with disadvantage, discrimination, and marginalization, identified through an iterative process of discussion within the research team. To facilitate the use of our findings to inform policy, we structure the different issues into the level of the health system at which they arise and can best be addressed (19) including the health system level (macro level), the screening program level (meso level) and the individual/community level (micro level).
Our first aim was to describe the range of barriers and facilitators to cervical screening reported in the published literature among groups underserved, or at risk of being underserved, by CCS programs or health systems more broadly. Our second aim was to describe interventions that had been developed to improve uptake. We report our findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for scoping reviews.
The criteria for inclusion are papers:
(i) identifying barriers, facilitators and interventions related to uptake of cervical cancer screening;
(ii) targeting underserved populations (see below);
(iii) conducted in Europe;
(iv) reporting primary data; and
(v) published since 2000.
These are reported using the PICOS framework in Table 1.
We now consider each factor in turn. A barrier was defined as a factor that obstructs or prevents the uptake of CCS; a facilitator as a factor that supports or promotes uptake of screening; and an intervention was defined as an activity designed to increase screening uptake by members of the population groups that are the subject of the review.
Groups at risk of being underserved were identified in an iterative process of discussion within the research team, many of whom have extensive experience in designing, managing, or evaluating cervical screening programs. The discussion was informed by the concepts of vertical and horizontal equity, seeking groups that do not receive equal cervical screening when they have an equal need or those who do not receive a level of services corresponding to their specific need (20, 21). The groups included were restricted to those who may benefit from cervical screening, i.e., adults with a cervix (including women, trans men, non-binary people, and inter-sex people with a cervix). The discussion concluded that the most appropriate approach was one that combined generic terms, such disadvantage, stigmatization, and discrimination, with more specific terms such as sex work, incarceration, communicable diseases, substance misuse, trafficking and exploitation, and ethnicity. These were then operationalized using synonyms, stems, and Boolean operators in the search strategy (Supplementary Material).
The rationale for limiting our review to Europe is that it will be used to develop interventions there. We recognize that there is an extensive literature on inequalities of access to care from the USA, some of which can offer insights, but much of which is not easily transferable given the very different health system and political contexts. This is less of a problem with literature from other parts of the world but, even then it would be necessary to assess each study to determine its applicability to the European context, especially as, in some cases, the inequalities that will exist in Australasia, Canada, and Latin America will be overlaid by the complex legacy of colonialism. Restricting our review to European studies also avoided the need to scrutinize a large volume of literature addressing issues such as the extremely sparse infrastructure in many low-income countries.
Studies were required to report primary data relevant to the issues set out above. Existing systematic reviews per se were not included, although if identified, reference lists were considered. Beyond this constraint, no studies were excluded based on study design.
Our search included studies published between 2000 – July 2021. This restriction to studies published since 2000 was applied due to shifts in social, legal and health system factors over time which are likely to have impacted experience of barriers and facilitators, such as the establishment of anti-discrimination legislation and health service digitalisation (22).
Our detailed search strategy, which follows from the criteria listed above, is reported in Appendix A. In brief, four electronic databases were searched for relevant documents: Medline (Ovid), EMBASE (Ovid), Global Health (Ovid) and PsychINFO (APA/Ovid) from March to July 2021. Papers in any language were included, although all but one included after initial screening were in English. The full text of the remaining two, in Dutch and one in Estonian, were machine translated. This search was supplemented by a combination of hand searching of reference lists of relevant included studies and forward citation searching. The final search results were exported into EndNote, and duplicates were removed. The search strategy was developed in collaboration with an experienced librarian at the London School of Hygiene and Tropical Medicine and further refined through team discussion.
We extracted data from studies focused on barriers and facilitators covering country of study, the underserved group, and the nature of the barriers/facilitators. With the intervention studies, we extracted information on the nature of the underserved group, country, aim of the study, study design, intervention, and outcomes.
To increase consistency among reviewers (SB, VK, MM), each screened a sample of 95 papers, discussed the results, and amended the screening and data extraction document before beginning screening for this review. Screeners worked in pairs and sequentially evaluated the titles, abstracts and then full text of all publications identified by our searches for potentially relevant publications. Any disagreements on study selection were resolved by consensus and discussion with other reviewers if needed.
Data extraction, allocation to themes, and interpretation of the findings was undertaken by two reviewers (SR and RG) for papers relating to barriers and facilitators. For papers relating to interventions, data were extracted by three reviewers (SB, RL, MM), followed by deductive categorization into micro/meso/macro level phenomena by reviewers (SR and RG).
As this was a scoping review, with very heterogenous studies, a comprehensive critical appraisal of studies was not appropriate.
Unlike a systematic review, where it is important to exclude studies that fail to meet pre-specified quality criteria lest they distort a central measure, this review sought to identify factors that plausibly and convincingly acted as barriers and facilitators and interventions which drew attention to increasing uptake screening. Thus, the quality of the methodology and, therefore, the validity of the findings of individual papers were not assessed.
The barriers, facilitators and interventions acting at each level (macro-, meso-, micro-) were described along with the groups affected.
After removing duplicates, 3,623 unique studies were identified. Screening of titles and abstracts reduced this to 95 studies, with 68 covering barriers/facilitators and 27 reporting interventions (Figure 1). 41 studies remained after full-text screening, with 33 related to barriers and facilitators (Table 2) and eight intervention studies (Table 3). Most studies were conducted in the UK (n = 16), with the remainder in Romania (n = 3), Denmark (n = 2), the Netherlands (n = 2), Norway (n = 2), Sweden (n = 2), Bulgaria (n = 1), Switzerland (n = 1), Estonia (n = 1), Finland (n = 1), France (n = 1), Poland (n = 1), and Portugal (n = 1). One study was conducted in two countries (Bulgaria and Romania).
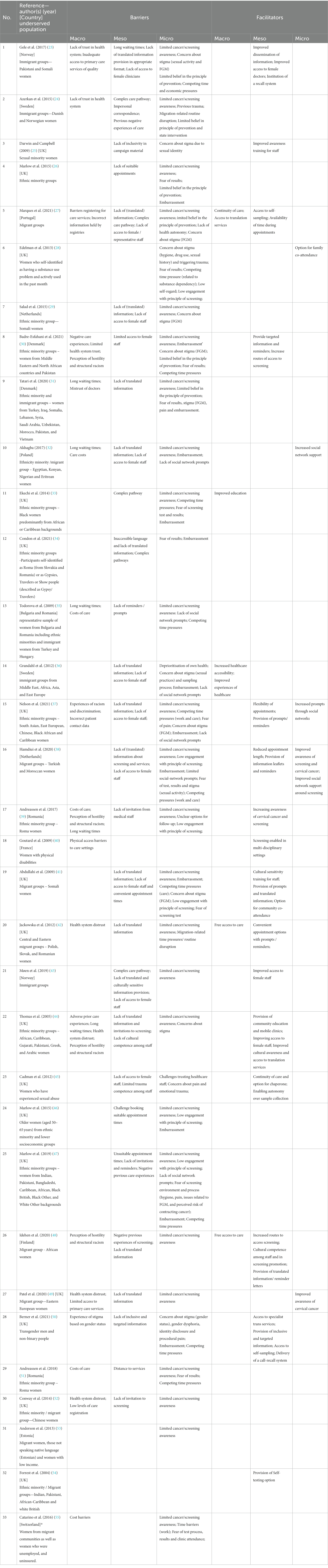
Table 2. Overview of studies reporting barriers and facilitators within micro, meso and macro level framework.
The population groups most commonly featuring in studies of barriers and facilitators were immigrant and migrant groups (n = 16) and ethnic minority women (n = 16). Less frequently featured populations included LGBTQI+ groups (n = 2), people who inject drugs (PWID) (n = 1), women with physical disabilities (n = 1), and women who have experienced sexual abuse (n = 1). Two studies addressed groups characterized by more than one factor. One study focused on women with lower school education, migrant women, and older women. Most studies focused on the perspectives of groups at risk of being underserved only (n = 26). Three studies included the perspectives of both healthcare professionals and underserved groups, and two included healthcare professionals or community workers only. Most studies focused on barriers rather than facilitators.
Interventions studied predominantly focused on psychological capability barriers (e.g., increasing understanding around cervical cancer and CCS) and physical opportunity barriers (e.g., sending invites, removing financial barriers, and offering HPV self-testing). Measures of effectiveness were mostly screening completion (recorded or self-reported) rather than treatment outcomes or survival rates. They comprised one non-randomized controlled trial, one before-and-and-after study, one retrospective cohort study and five randomized controlled trials.
The findings relating to barriers, facilitators, and interventions, presented according to the system level, are as follows.
There were many structural barriers to accessing any form of healthcare, with implications for screening, related to healthcare financing, health system bureaucracy, and trust in the health system.
Financial barriers, including lack of insurance coverage and concern about out-of-pocket costs, including ‘under the table’ costs, featured in four papers, all affecting ethnic minority groups in Central and Eastern Europe (32, 35, 39, 51). Two studies identified removal of financial barriers as a facilitator, in ethnic minority and migrant groups in the UK, and in Finland (42, 48).
Bureaucracy-related barriers were identified across four studies including migrant and ethnic minority women, three of which were based in the UK (27, 37, 49, 52). These barriers included challenges registering with primary care services (27, 49, 52), difficulties obtaining paperwork required to access services (27), and incorrect information on registers that prevented invitations being sent and engagement with screening services (27, 37).
A third barrier at this level was lack of trust. This was reported in studies of migrant women, those in ethnic minority groups and transgender men. The studies were from Norway, Sweden, Romania, and Finland, with two from Denmark, and four from the UK. Mistrust predominantly manifested as reputational concern about service quality or personal of experiences using the service, often involving discrimination (23, 24, 30, 31, 37, 39, 42, 44, 48–50, 52, 64). Conversely, previous positive health system experiences and perceptions of the system valuing women’s health were identified as facilitators in Sweden (36).
Only one intervention study was identified at the macro level. Contrary to reports (highlighted above) that removing cost barriers could facilitate access, this study, which randomized women living in a low-income urban area in Sweden to free screening or paying the standard fee (SEK 100 [€11]), found no significant difference in participation according to care fees (RR 0.93; CI 0.85–1.02) (60). However, the study design applied may have meant women did not realize the fee had been abolished for them. Additionally, as the authors noted, their finding conflicted with that from a Finnish study (65) on breast cancer screening finding that price was indeed a barrier to uptake.
At the screening service (meso) level, factors impacting engagement with screening were identified along the screening process. Five core themes were identified: information provision, prompts to participate in screening, screening pathway navigation, screening access options and staff interactions.
Nineteen studies described problems with information provided, including lack of cultural relevance or inclusivity, or failure to translate material (23–25, 27, 29, 31, 32, 34, 36–38, 41–45, 48–50, 63). While these problems affected many groups, migrant populations and those in ethnic and sexual minority groups appeared particularly affected. It follows that provision of targeted (30, 50) and accessible information (38) disseminated in appropriate formats and languages (23, 27, 41, 48) were highlighted as facilitators, with examples of good practice from Denmark, Finland, Romania, Sweden, Netherlands, Norway, and the UK.
A failure to provide prompts or invitations was a frequently cited barrier to participation (35, 37, 44, 47, 50, 52). In some cases this was due to inaccurate contact information held by screening services, an issue whose solution lies at the macro level where registry data are managed (37, 44). The use of call/recall systems (23, 50), reminder letters or phone calls (37, 38, 41, 42, 48) and proactive encouragement from healthcare professionals (41, 48) all acted as facilitators.
Those who decide to undergo screening may need help to navigate the system. This was often difficult, especially for migrant and ethnic minority groups across five studies in multiple country settings: Norway, Romania, and England. Specific navigation barriers included long waiting times for screening (23) and difficulty in booking suitable appointments (33, 34, 39, 41, 47). Additionally, complex pathway designs (24, 27, 33, 34, 63) created barriers for some migrant and ethnic minority groups (24, 27, 33, 34, 63). Distance to services was also a barrier facing Roma women in Romania (51).
Concerning routes of access, multiple studies identified screening care integration with other services (30, 40, 48), including enabling access through midwifery and multi-disciplinary care environments, as a facilitator of care.
Several studies identified flexibility in screening clinics as a facilitator, including extending appointment times and booking options (37, 42), providing mobile clinics (44), and enabling people within a community to attend together (41). The benefit of flexibility extended to how screening was undertaken, with options of chaperones, control over how clinicians perform the test, and self-testing all identified as facilitators (27, 45, 50, 54).
The final meso-level theme for barriers and facilitators related to interactions with screening staff. Several studies, not specific to any particular group or geographic area, highlighted uptake barriers relating to previous traumatic and negative care experiences (24, 28), embarrassment about the private nature of the examination (26, 30–34, 36–38, 41, 47, 49, 50) and perceptions of discrimination and stigma (23, 25, 27–30, 36–38, 41, 44, 50).
Some of these barriers also crossed to the micro level as they touched on issues such as access to female staff (44, 63), staff from similar backgrounds (48), and staff able to translate into the users’ languages (27, 41, 48). Some studies also highlighted how training could facilitate uptake by those identifying as trans or affected by female genital mutilation (25, 41, 44, 48, 50).
Interventions at the meso-level included information provision, prompts to participate in screening, choice of ways to access screening, and support to navigate the screening pathway.
Two studies examined the effectiveness of invitations, with one simultaneously studying the impact of information provision. The latter was performed in Germany (58) and randomized participants to receive an invitation, an invitation with a brochure, or no invitation at all (control). The addition of the invitation increased uptake from 85% to 92%, but the further addition of the brochure had little effect. The invitation letters were most effective with women who had never been screened, with older women, those with low educational attainment and migrants.
The second study, based in the Netherlands (63), compared the impact of invitations from GPs with those from the local public health department. Invitations from GPs increased participation by 7.9% (95% CI: 7.5–8.3), and more so among migrants from Morocco, Turkey, Surinam, and the Netherlands Antilles/Aruba, and those on low incomes, in urban areas and in the lowest age group.
Only two studies were found that intervened to address cultural relevance, lack of inclusivity, or failure to translate material. One, from Norway (56), used a community-based intervention among women from Pakistani and Somali communities with practical information presented verbally in Urdu or Somali. Screening uptake was almost five percentage points higher in the intervention group (from 45.9% to 50.8%), which reduced to 3% (95% CI 2%–6%) after adjusting for confounders. This study benefitted from the ability to link the subjects to registry data rather than relying on self-report, which tends to overestimate screening and varies with ethnicity. One controversial limitation that was noted was the non-involvement of men, recognizing the role they play in women’s decisions in certain cultures, with the authors speculating that including them might have had additional benefits.
The second study, from the United Kingdom (57), assessed the incremental benefit of offering one-to-one counseling to women with moderate and severe learning disabilities by a learning disability nurse. This achieved a six-percent increase in uptake, from 16% to 22% among women attending screening for the first time. To deliver the intervention, nurses were given a health education pack with pictures adapted for women with varying levels of learning ability and a decision-making pathway (offering guidance on consent) to support nurses. Although multiple women were prompted to participate in screening through this program, the uptake achieved was still very low, which the authors attributed to a range of factors, including the low risk that these women faced because of low sexual activity and problems cooperating with the procedure, in some case because of their physical disabilities.
One cluster randomized controlled trial in Norway (61) evaluated a package of measures delivered in general practice aimed at increasing migrant women’s participation in CCS. It had three parts (1): a 10- to 15-min targeted educational session for healthcare providers held during the lunch break at the general practice (2), a mouse pad to remind GPs of the intervention in their day-to-day work, and (3) a poster for waiting rooms with the message, “You can prevent cervical cancer with a simple test. Make an appointment with your doctor today!” in Somali, Polish, English, and Urdu. The proportion of immigrant women screened increased by 2.6% in the intervention group versus 0.6% in the control group. After adjustment for screening status at baseline, women in the intervention group were more likely to have participated in CCS (OR, 1.24 [95% CI: 1.11–1.38]). This was unchanged after adjustment for women’s characteristics and was reduced, but still significant, after further adjustment for GP characteristics (OR, 1.19 [95% CI, 1.06–1.34]). In subgroup analyses, the intervention increased participation most by women who had not previously been screened at baseline (OR, 1.35 [95% CI: 1.16–1.56]) and those from Poland, Pakistan, and Somalia (OR, 1.74 [95% CI: 1.17–2.61]), after adjusting for baseline screening status.
A study in France (62) evaluated the impact of offering self-sampling CCS tests in a community disadvantaged due to factors such as low-income, low levels of education, and experience of homelessness and sub-standard housing. All women eligible and included in the study were offered an individual sexual and reproductive health consultation covering HPV infection, cervical cancer, and gynecological care and screening access information. The intervention group were provided with self-sampling HPV test kits, while the control group were referred for Pap-smear tests. Those randomized to self-sampling could take their sample at the clinic or at home, receiving a referral if a non-negative result was returned. Those provided self-sampling tests had over twice the likelihood of completing screening tests compared to those in the control group (hazard ratio = 2.48 [95%CI: 1.99–3.08]).
A randomized trial in the south of France (59) was undertaken among non-attenders to a first invitation for a Pap-smear. This group was sent either a mailed invitation and kit for HPV self-sampling (HPVHR test) or a 2nd invitation (reminder) to Pap-smears. The outcomes were participation and detection of cervical dysplasia. Self-sampling markedly increased participation, from 2.0% with Pap-smears (198/9,901) versus 18.3% with HPVHR tests (1,613/8,829). The main limitation was that, despite increased screening uptake and intensive follow-up, most women with positive HPV screening tests did not accept the subsequent invitation to have a Pap-smear followed by a colposcopy examination if indicated.
At the individual and community (micro) level, barriers and facilitators related to themes of a lack of awareness about screening, fear of screening, competing pressures, and attitudes to screening and preventive care in general.
Limited awareness of cervical cancer or CCS was a common theme, featuring in 22 papers, often attributed to the suboptimal provision of information, something that crosses the micro and meso levels.
Many barriers related to awareness involved a lack of understanding about cervical cancer and associated risk factors (24, 30, 32, 33, 38, 39, 41, 47–49), and a lack of awareness of cervical screening and available screening services (23, 26, 27, 29, 31, 32, 35, 37, 38, 41–44, 47–50, 52, 63). These affected many groups, in particular migrant and ethnic minority groups, older women, transgender men, people who inject drugs (PWID) and those in socioeconomically deprived groups.
Measures to increase awareness of cervical cancer were identified as facilitators in several studies among ethnic minority groups (38, 39, 49).
Fear of screening also affected several groups and was reported in 24 papers. It took several forms, including aversion to the physical process of sample collection, concern about social interactions around screening (including stigma and embarrassment), and fear of receiving an adverse screening result.
Fear of pain was the main barrier related to the process of collecting a sample, featuring in seven papers and affecting several groups (23, 31, 33, 37, 45, 47, 50). However, an additional concern, reported only by migrant women, was the potential for screening to affect their virginity, with the associated stigma (36).
Concerns around the social experience of screening were also common, with embarrassment and fear of stigma featuring in 18 papers (23, 25, 27–32, 34, 36–38, 41, 44, 46, 47, 49, 50). Sub-themes of perceived or experienced stigma or dysphoria related to history of female genital mutilation (23, 27, 29, 30, 37, 47), gender status (25, 50), substance misuse history and sexual history also featured (28). While some studies described concern about health professionals engaging in stigmatizing behavior, others highlighted concern that participation in screening was itself stigmatized as it implied prior sexual activity (23, 36, 44). Others expressed fear of recalling trauma during previous sexual abuse (24, 28, 45).
Fear of what screening might reveal featured in 10 studies from many countries and population groups (24, 26, 28, 30, 31, 33, 34, 38, 47, 51), highlighting the importance of considering downstream care and support and related perceptions.
Other micro themes included competing pressures, the impact of screening perceptions within social networks, and beliefs about screening and prevention in principle.
Many, from a wide array of groups, cited competing priorities as a barrier (23, 24, 26, 28, 30, 33, 35, 37, 38, 41, 42, 47, 50, 51). Childcare and work both featured as competing priorities in three studies involving women from ethnic minority groups (37, 38, 41). Some PWID described pressures arising from substance use (28). Some migrant groups faced difficulties associated with the complex challenges arising from the migration process (24, 42). Meanwhile, many simply deprioritized screening owing to feelings of low self-worth (28, 36, 45, 66).
Many women described how social networks could support attendance (28, 32, 37, 38) with one study finding that PWID may benefit from having an option to be joined by family members when attending screening (28). However, particularly in migrant and ethnic minority groups, social networks could be a barrier to participation, especially where discussion of sexual health was ‘taboo’ (27, 36–38, 47).
Concerning beliefs relating to screening and prevention, many, especially those in migrant and ethnic minority groups, held a fatalistic belief about cancer or disengaged from the idea of prevention, seeing screening results as something that should not be sought (23, 27, 30, 31, 38, 39, 41, 47).
We did not identify any interventions at the micro level.
A first step in understanding the reasons behind the mismatch between intentions and outcomes of health policy is to appreciate the reality faced by people who are constrained by poverty, geographical isolation, or other disadvantages.
This review shows how women, and those with cervixes, eligible for CCS face a wide array of barriers to participation, ranging from limited awareness of screening services to a lack of culturally acceptable services which can be afforded and easily reached without fear of stigma. Positively, there are many ways to facilitate uptake, including interventions with proven effectiveness. The latter reduce barriers to participation by providing information on what to do, especially when it is in a language that is understood and is culturally appropriate, reducing financial, temporal, and geographic barriers and by introducing easier ways to obtain samples, in particular self-sampling. Collectively, these findings offer many ideas that can help reduce the inequitable burden of cervical cancer in Europe.
There are many reasons why disadvantaged women are at greater risk of both developing cervical cancer and experiencing its progression, too often to preventable death. These groups start at higher risk, particularly from infection with HPV and HIV or tobacco use, compounded by low uptake of HPV vaccination and screening. Most European countries have implemented organized cervical screening programs, either with Pap smear or HPV testing, although there is still much to do to implement solutions which address barriers across the care pathway. Underserved women find themselves having to overcome multiple barriers to obtain screening, from finding where the services are, to making an appointment, paying out-of-pocket expenses, and experiencing unwelcoming care settings. Some women are especially likely to face psychological and emotional barriers and report economic, social, and healthcare system barriers. For example, migrant women from countries where girls face barriers to education may find it difficult to navigate a foreign healthcare system. Few healthcare systems proactively seek to meet the needs of minorities, and although civil society organizations may compensate for this, there is only so much that they can do.
This review builds on previous literature highlighting the complex interplay of factors influencing the uptake of cervical screening in the general population (67–69). The studies we have reviewed point to some general points. There is a diversity of barriers and facilitators which require a range of interventions if they are to be overcome. Interventions evidenced within this scoping review addressed the need to: (i) increase access/participation, (ii) provide culturally appropriate information, and (iii) improve the experience of being screened.
It goes without saying that universal access to easily accessible screening services is important and seems intuitive, and consistent with economic theory, that removal of financial barriers would be important, so the findings of the Swedish study should be interpreted with caution and may not be generalisable to other settings where there are also organizational barriers, where the fee is higher, or where levels of income support are lower (24).
Screening should also be organized as a comprehensive system that goes all the way from maintaining accurate population registers to ensuring follow-up of those found to have positive results (67). Good communication between providers and service users is imperative. Invitations to screening need to be timely, culturally sensitive, and accessible (focusing on screening/cancer-specific information). Perhaps the best evidence relates to self-sampling for HPV, shown in many studies to be effective (59, 62, 70–72), especially for some populations disadvantaged by traditional programs. However, there is scarce evidence on how interventions can be integrated into existing healthcare systems or can enhance women’s ability to make informed decisions. It is clear from this review that diverse efforts sensitive to a wide array of barriers are needed to meet the WHO 2030 targets for eliminating cervical cancer as a public health problem and to reduce inequalities in its incidence and mortality.
The review is, to our knowledge, the first to examine the entire range of barriers and facilitators to participation in CCS and potential interventions to increase uptake across identifiable population groups in Europe, with a focus on those at risk of being underserved by CCS programs.
The review focused on Europe because it is feeding directly into an EU project (CBIG-SCREEN) but this clearly limits generalisability to populations in other parts of the world. Another potential limitation is the use of a priori definitions of underserved populations. This was necessary to develop a search strategy capable of identifying relevant literature. However, it risks inadvertently excluding studies on underserved groups not recognized as being so. It is important to acknowledge that, even when insights into the barriers and facilitators faced by certain groups are obtained, these are not necessarily generalizable to the same groups elsewhere. People are not defined by a single characteristic and many of those that disadvantage individuals interact, either to exacerbate or mitigate their situation, a phenomenon termed intersectionality. The challenge of generalisability is compounded by the imbalanced geographical distribution of studies, with most studies of migrant and ethnic minority women coming from the United Kingdom. Consequently, a cautious approach is required when applying insights obtained from this review to settings other than where the research was undertaken. To aid generalisability, funders should prioritize future research in this field in other settings, as well as including possibilities to probe intersectionality.
The use of macro, meso, and micro levels allowed us to take a structured approach to synthesis and analysis that aligned with wider approaches to cervical screening policy and interventions in Europe (i.e., through CBIG-SCREEN). However, the distinction between categories was occasionally blurred. Awareness is one such example, as it transcends micro and meso levels. Undeniably, providing information on services has a role in improving awareness of cervical screening and its indications; however, cervical screening services cannot be held solely accountable for low levels of health literacy.
As this is a scoping review, the quality of the papers was not appraised in detail. Additionally, the heterogeneity and sparsity of the intervention studies precluded a meta-analysis. We must also consider that none of the authors falls into any of the underserved groups, something that may, sub-consciously, have influenced our interpretation of the results, although care was taken to challenge positionality and address reflexivity during the analysis and write-up.
Although screening is a highly effective means of preventing invasive cervical cancer, it remains inaccessible to many women in Europe, for a wide range of reasons. Yet there is much that can be done to change this situation by removing financial, cultural, and other barriers and by providing services that respond to the needs and expectations of those groups that are disadvantaged. This project, CBIG-SCREEN, is now taking forward the findings of this review to engage with these several of these groups in three European countries to co-create models of care that they will feel a sense of ownership over.
RG led the review. SB initiated the review protocol. RG and SR revised the review protocol and search strategy, participated in the writing, reviewing and revising the final versions of the report and coordinated the process of getting it to publication standards. SB, RG, and SR conducted all the screening, extraction, and analysis of texts and created the tables and figures. RL acted as second screener. VK led the design and refined the initial search strategy and study protocol and was responsible for conducting the electronic database searches and removing duplicate articles. MM conceived and scoped the review, provided expert advice, made critical comments that helped the interpretation of results, and revised reviewed the data extraction sheet, reviewed, and revised the report. All authors contributed to the article and approved the submitted version.
CBIG-SCREEN has received funding from the EU’s Horizon 2020 research and innovation program under grant agreement No 964049. The work was supported by European Commission’s Horizon 2020 program as part of the CBIG-SCREEN research project; however, the views expressed do not necessarily reflect the European Commission’s official policies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the CBIG-SCREEN Consortium members:
Institut national de la santé et de la recherche médicale (INSERM), France.
Marc Bardou.
RHR
B. S. Andersen, Pia Kirkegaard, Rikke Buus Bøje, Mette Tranberg.
London School of Hygiene & Tropical Medicine, United Kingdom.
Rosa Legood, Anna Foss, Li Sun, Martin McKee, Sadie Bell, Rachel Greenley, Samuel Rigby, Victoria Kirkby.
Azienda Unita Sanitaria Locale di Reggio Emilia, Italy.
Paolo Giorgi Rossi, Luca Ghirottoo, Letizia Bartolini, Noemi Auzzi, Paola Mantellini, Giusy Iorio, Laura Bonvicini.
Instituto de Saude Publica da Universidade do Porto, Portugal.
Nuno Lunet, João Firmino-Machado, Margarida Teixeira, Ana Fernandes, Mariana Amorim, Inês Baía.
Tartu Ulikool, Estonia.
Anneli Uusküla, Anna Tisler.
Universitatea Babes Bolyai, Romania.
Aadriana Baban, Diana Tăut, Nicoleta Jiboc.
Institute of Oncology Cluj-Napoca, Romania.
Florian Nicula, Alexandra Tolnai.
European Institute of Women’s Health, Ireland.
Rebecca Moore, Vanessa Moore.
International Agency for Research on Cancer, France.
Partha Basu, Isabel Mosquera Metcalfe, Keitly Mensah, Eric Lucas.
Ecole d’économie de Paris, France.
Lise Rochaix, Camilla Fiorina.
Health Psychology Research Center, Bulgaria.
Irina Todorova, Yulia Panayotova, Tatyana Kotzeva.
European Cancer Leagues, Switzerland.
David Ritchie, Helena Ros Comesana, Meritxel Mallafré-Larrosa, Ginevra Papi.
Inserm Transfert SA, France.
Christiane Dascher-Nadel.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1144674/full#supplementary-material
1. Arbyn, M, Weiderpass, E, Bruni, L, de Sanjosé, S, Saraiya, M, Ferlay, J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. Boyle, P, Veronesi, U, Tubiana, M, Alexander, F, da Silva, FC, Denis, L, et al. European school of oncology advisory report to the European Commission for the “Europe against Cancer Programme” European code against cancer. Eur J Cancer. (1995) 31:1395–405. doi: 10.1016/0959-8049(95)00334-F
3. Falcaro, M, Castañon, A, Ndlela, B, Checchi, M, Soldan, K, Lopez-Bernal, J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. (2021) 398:2084–92. doi: 10.1016/S0140-6736(21)02178-4
4. Ronco, G, Dillner, J, Elfstrom, KM, Tunesi, S, Snijders, PJ, Arbyn, M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. (2014) 383:524–32. doi: 10.1016/S0140-6736(13)62218-7
5. European Commission. (2021). Europe’s beating cancer plan: European Commission Brussels. Available at: https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (Accessed March 28, 2023).
7. Colzani, E, Johansen, K, Johnson, H, and Celentano, LP. Human papillomavirus vaccination in the European Union/European economic area and globally: a moral dilemma. Eur Secur. (2021) 26:2001659. doi: 10.2807/1560-7917.ES.2021.26.50.2001659
8. Bruni, L, Saura-Lázaro, A, Montoliu, A, Brotons, M, Alemany, L, Diallo, MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
9. Ponti, A, Anttila, A, Ronco, G, and Senore, C. Cancer screening in the European Union (2017) In:. Report on the implementation of the council recommendation on cancer screening France. Lyon, France: IARC (2017)
10. Van Poppel, H, Battisti, NML, Lawler, M, Kolarova, T, Daly, J, Rizvi, K, et al. European Cancer Organisation's inequalities network: putting Cancer inequalities on the European policy map. JCO Glob Oncol. (2022) 8:e2200233. doi: 10.1200/GO.22.00233
11. Vaccarella, S, Georges, D, Bray, F, Ginsburg, O, Charvat, H, Martikainen, P, et al. Socioeconomic inequalities in cancer mortality between and within countries in Europe: a population-based study. The Lancet Regional Health–Europe. (2023) 25:100551. doi: 10.1016/j.lanepe.2022.100551
12. Slåttelid Schreiber, SM, Juul, KE, Dehlendorff, C, and Kjær, SK. Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish childhood immunization program. J Adolesc Health. (2015) 56:402–7. doi: 10.1016/j.jadohealth.2014.12.008
13. Fisher, H, Audrey, S, Mytton, JA, Hickman, M, and Trotter, C. Examining inequalities in the uptake of the school-based HPV vaccination programme in England: a retrospective cohort study. J Public Health (Oxf). (2014) 36:36–45. doi: 10.1093/pubmed/fdt042
14. Héquet, D, and Rouzier, R. Determinants of geographic inequalities in HPV vaccination in the most populated region of France. PLoS One. (2017) 12:e0172906. doi: 10.1371/journal.pone.0172906
15. Bozhar, H, McKee, M, Spadea, T, Veerus, P, Heinävaara, S, Anttila, A, et al. Socio-economic inequality of utilization of cancer testing in Europe: a cross-sectional study. Prev Med Rep. (2022) 26:101733. doi: 10.1016/j.pmedr.2022.101733
16. De Prez, V, Jolidon, V, Willems, B, Cullati, S, Burton-Jeangros, C, and Bracke, P. Cervical cancer screening programs and their context-dependent effect on inequalities in screening uptake: a dynamic interplay between public health policy and welfare state redistribution. Int J Equity Health. (2021) 20:1–14. doi: 10.1186/s12939-021-01548-6
17. World Health Organization. WHO report on cancer: Setting priorities, investing wisely and providing care for all. Geneva: WHO (2020).
18. CBIG-SCREEN (2023). CBIG-SCREEN Project. Available at: https://cbig-screen.eu/ (Accessed March 28, 2023).
19. Sheikh, K, Gilson, L, Agyepong, IA, Hanson, K, Ssengooba, F, and Bennett, S. Building the field of health policy and systems research: framing the questions. PLoS Med. (2011) 8:e1001073. doi: 10.1371/journal.pmed.1001073
20. Lairson, DR, Hindson, P, and Hauquitz, A. Equity of health care in Australia. Soc Sci Med. (1995) 41:475–82. doi: 10.1016/0277-9536(94)00352-T
21. Kreng, VB, and Yang, C-T. The equality of resource allocation in health care under the National Health Insurance System in Taiwan. Health Policy. (2011) 100:203–10. doi: 10.1016/j.healthpol.2010.08.003
22. McCrudden, C, and Prechal, S. The concepts of equality and non-discrimination in Europe: A practical approach. Brussels: European Commission, Directorate-General for Employment, Social Affairs and Equal Opportunities, Unit G (2009). 2 p.
23. Gele, AA, Musse, FK, Shrestha, M, and Qureshi, S. Barriers and facilitators to contraceptive use among Somali immigrant women in Oslo: a qualitative study. PLoS One. (2020) 15:e0229916. doi: 10.1371/journal.pone.0229916
24. Azerkan, F, Widmark, C, Sparén, P, Weiderpass, E, Tillgren, P, and Faxelid, E. When life got in the way: how Danish and Norwegian immigrant women in Sweden reason about cervical screening and why they postpone attendance. PLoS One. (2015) 10:e0107624. doi: 10.1371/journal.pone.0107624
25. Darwin, Z, and Campbell, C. Understandings of cervical screening in sexual minority women: a Q-methodological study. Fem Psychol. (2009) 19:534–54. doi: 10.1177/0959353509342919
26. Marlow, LA, Waller, J, and Wardle, J. Barriers to cervical cancer screening among ethnic minority women: a qualitative study. J Fam Plann Reprod Health Care. (2015) 41:248–54. doi: 10.1136/jfprhc-2014-101082
27. Marques, P, Gama, A, Santos, M, Heleno, B, Vermandere, H, and Dias, S. Understanding cervical Cancer screening barriers among migrant women: a qualitative study with healthcare and Community Workers in Portugal. Int J Environ Res Public Health. (2021) 18:7248. doi: 10.3390/ijerph18147248
28. Edelman, NL, Patel, H, Glasper, A, and Bogen-Johnston, L. Understanding barriers to sexual health service access among substance-misusing women on the south east coast of England. J Fam Plann Reprod Health Care. (2013) 39:258–63. doi: 10.1136/jfprhc-2012-100507
29. Salad, J, Verdonk, P, de Boer, F, and Abma, TA. “A Somali girl is Muslim and does not have premarital sex. Is vaccination really necessary?” a qualitative study into the perceptions of Somali women in the Netherlands about the prevention of cervical cancer. Int J Equity Health. (2015) 14:1–13. doi: 10.1186/s12939-015-0198-3
30. Badre-Esfahani, S, Petersen, LK, Tatari, CR, Blaakær, J, Andersen, B, and Seibæk, L. Perceptions of cervical cancer prevention among a group of ethnic minority women in Denmark—a qualitative study. PLoS One. (2021) 16:e0250816. doi: 10.1371/journal.pone.0250816
31. Tatari, CR, Andersen, B, Brogaard, T, Badre-Esfahani, SK, Jaafar, N, and Kirkegaard, P. Perceptions about cancer and barriers towards cancer screening among ethnic minority women in a deprived area in Denmark–a qualitative study. BMC Public Health. (2020) 20:1–10. doi: 10.1186/s12889-020-09037-1
32. Akhagba, OM. Migrant women’s knowledge and perceived sociocultural barriers to cervical cancer screening programme: a qualitative study of African women in Poland. Health Psychol Rep. (2017) 5:263–71. doi: 10.5114/hpr.2017.65238
33. Ekechi, C, Olaitan, A, Ellis, R, Koris, J, Amajuoyi, A, and Marlow, LA. Knowledge of cervical cancer and attendance at cervical cancer screening: a survey of black women in London. BMC Public Health. (2014) 14:1–9. doi: 10.1186/1471-2458-14-1096
34. Condon, L, Curejova, J, Morgan, DL, Miles, G, and Fenlon, D. Knowledge and experience of cancer prevention and screening among gypsies, Roma and Travellers: a participatory qualitative study. BMC Public Health. (2021) 21:360. doi: 10.1186/s12889-021-10390-y
35. Todorova, I, Baban, A, Alexandrova-Karamanova, A, and Bradley, J. Inequalities in cervical cancer screening in Eastern Europe: perspectives from Bulgaria and Romania. Int J Public Health. (2009) 54:222–32. doi: 10.1007/s00038-009-8040-6
36. Grandahl, M, Tydén, T, Gottvall, M, Westerling, R, and Oscarsson, M. Immigrant women's experiences and views on the prevention of cervical cancer: a qualitative study. Health Expect. (2015) 18:344–54. doi: 10.1111/hex.12034
37. Nelson, M, Patton, A, Robb, K, Weller, D, Sheikh, A, Ragupathy, K, et al. Experiences of cervical screening participation and non-participation in women from minority ethnic populations in Scotland. Health Expect. (2021) 24:1459–72. doi: 10.1111/hex.13287
38. Hamdiui, N, Marchena, E, Stein, ML, van Steenbergen, JE, Crutzen, R, van Keulen, HM, et al. Decision-making, barriers, and facilitators regarding cervical cancer screening participation among Turkish and Moroccan women in the Netherlands: a focus group study. Ethn Health. (2021) 27:1147–65. doi: 10.1080/13557858.2020.1863921
39. Andreassen, T, Weiderpass, E, Nicula, F, Suteu, O, Itu, A, Bumbu, M, et al. Controversies about cervical cancer screening: a qualitative study of Roma women's (non) participation in cervical cancer screening in Romania. Soc Sci Med. (2017) 183:48–55. doi: 10.1016/j.socscimed.2017.04.040
40. Goutard, S, Baron, C, Bouton, C, Penisson-Besnier, I, Fossé, G, Aube-Nathier, A-C, et al. Contraception and screening for cervical and breast cancer in neuromuscular disease: a retrospective study of 50 patients monitored at a clinical reference Centre. Ann Phys Rehabil Med. (2009) 52:538–45. doi: 10.1016/j.rehab.2009.06.003
41. Abdullahi, A, Copping, J, Kessel, A, Luck, M, and Bonell, C. Cervical screening: perceptions and barriers to uptake among Somali women in Camden. Public Health. (2009) 123:680–5. doi: 10.1016/j.puhe.2009.09.011
42. Jackowska, M, Von Wagner, C, Wardle, J, Juszczyk, D, Luszczynska, A, and Waller, J. Cervical screening among migrant women: a qualitative study of polish, Slovak and Romanian women in London, UK. J Fam Plann Reprod Health Care. (2012) 38:229–38. doi: 10.1136/jfprhc-2011-100144
43. Møen, KA. Cervical cancer screening among immigrants in Norway: challenges, possibilities and the effect of an intervention. Bergen Open Res Arch. (2019) 2022.
44. Thomas, VN, Saleem, T, and Abraham, R. Barriers to effective uptake of cancer screening among black and minority ethnic groups. Int J Palliat Nurs. (2005) 11:562–71. doi: 10.12968/ijpn.2005.11.11.20096
45. Cadman, L, Waller, J, Ashdown-Barr, L, and Szarewski, A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. (2012) 38:214–20. doi: 10.1136/jfprhc-2012-100378
46. Marlow, LA, Wardle, J, and Waller, J. Understanding cervical screening non-attendance among ethnic minority women in England. Br J Cancer. (2015) 113:833–9. doi: 10.1038/bjc.2015.248
47. Marlow, L, McBride, E, Varnes, L, and Waller, J. Barriers to cervical screening among older women from hard-to-reach groups: a qualitative study in England. BMC Womens Health. (2019) 19:38. doi: 10.1186/s12905-019-0736-z
48. Idehen, EE, Pietilä, A-M, and Kangasniemi, M. Barriers and facilitators to cervical screening among migrant women of African origin: a qualitative study in Finland. Int J Environ Res Public Health. (2020) 17:7473. doi: 10.3390/ijerph17207473
49. Patel, H, Sherman, SM, Tincello, D, and Moss, EL. Awareness of and attitudes towards cervical cancer prevention among migrant eastern European women in England. J Med Screen. (2020) 27:40–7. doi: 10.1177/0969141319869957
50. Berner, AM, Connolly, DJ, Pinnell, I, Wolton, A, MacNaughton, A, Challen, C, et al. Attitudes of transgender men and non-binary people to cervical screening: a cross-sectional mixed-methods study in the UK. Br J Gen Pract. (2021) 71:e614–25. doi: 10.3399/BJGP.2020.0905
51. Andreassen, T, Melnic, A, Figueiredo, R, Moen, K, Şuteu, O, Nicula, F, et al. Attendance to cervical cancer screening among Roma and non-Roma women living in North-Western region of Romania. Int J Public Health. (2018) 63:609–19. doi: 10.1007/s00038-018-1107-5
52. Conway, A, Clamp, AR, Hasan, J, Goonetilleke, D, Shore, K, Wong, L, et al. Accessing cancer services in N orth W Est E ngland: the C hinese population. Eur J Cancer Care. (2014) 23:570–81. doi: 10.1111/ecc.12171
53. Anderson, E, Toompere, K, Reile, R, and Uusküla, A. Naiste teadlikkus emakakaelavähki ennetavatest meetmetest Eestis 2011. aastal. Eesti Arst. (2013) 92:195–203.
54. Forrest, S, McCaffery, K, Waller, J, Desai, M, Szarewski, A, Cadman, L, et al. Attitudes to self-sampling for HPV among Indian, Pakistani, African-Caribbean and white British women in Manchester. UK J Med Screen. (2004) 11:85–8. doi: 10.1258/096914104774061065
55. Catarino, RR, Vassilakos, PP, Royannez-Drevard, II, Guillot, CC, Alzuphar, SS, Fehlmann, AA, et al. Barriers to cervical cancer screening in Geneva (DEPIST study). J. Low. Genit. Tract Dis. (2016) 20:135–8.
56. Qureshi, SA, Igland, J, Møen, K, Gele, A, Kumar, B, and Diaz, E. Effect of a community-based intervention to increase participation in cervical cancer screening among Pakistani and Somali women in Norway. BMC Public Health. (2021) 21:1–10. doi: 10.1186/s12889-021-11319-1
57. Biswas, M, Whalley, H, Foster, J, Friedman, E, and Deacon, R. Women with learning disability and uptake of screening: audit of screening uptake before and after one to one counselling. J Public Health. (2005) 27:344–7. doi: 10.1093/pubmed/fdi055
58. Radde, K, Gottschalk, A, Bussas, U, Schülein, S, Schriefer, D, Seifert, U, et al. Invitation to cervical cancer screening does increase participation in Germany: results from the MARZY study. Int J Cancer. (2016) 139:1018–30. doi: 10.1002/ijc.30146
59. Sancho-Garnier, H, Tamalet, C, Halfon, P, Leandri, F, Retraite, LL, Djoufelkit, K, et al. HPV self-sampling or the pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int J Cancer. (2013) 133:2681–7. doi: 10.1002/ijc.28283
60. Alfonzo, E, Andersson Ellström, A, Nemes, S, and Strander, B. Effect of fee on cervical cancer screening attendance—ScreenFee, a Swedish population-based randomised trial. PLoS One. (2016) 11:e0150888. doi: 10.1371/journal.pone.0150888
61. Møen, KA, Kumar, B, Igland, J, and Diaz, E. Effect of an intervention in general practice to increase the participation of immigrants in cervical Cancer screening: a cluster randomized clinical trial. JAMA Netw Open. (2020) 3. doi: 10.1001/jamanetworkopen.2020.1903
62. Reques, L, Rolland, C, Lallemand, A, Lahmidi, N, Aranda-Fernández, E, Lazzarino, A, et al. Comparison of cervical cancer screening by self-sampling papillomavirus test versus pap-smear in underprivileged women in France. BMC Womens Health. (2021) 21:1–14. doi: 10.1186/s12905-021-01356-8
63. De Nooijer, D, De Waart, F, van Leeuwen, A, and Spijker, W. Participation in the Dutch national screening programme for uterine cervic cancer higher after invitation by a general practitioner, especially in groups with a traditional low level of attendance. Ned Tijdschr Geneeskd. (2005) 149:2339–2343.
64. Ice, UV. Knowledge, attitudes, and beliefs regarding cervical cancer and screening and perceived barriers to cervical cancer screening programs among Thai immigrant women living in Germany. Albuquerque, NM: The University of New Mexico (2012).
65. Immonen-Räihä, P, Kauhava, L, Parvinen, I, Helenius, H, and Klemi, P. Customer fee and participation in breast-cancer screening. Lancet. (2001) 358:1425. doi: 10.1016/S0140-6736(01)06520-5
66. Vorsters, A, Cornelissen, T, Leuridan, E, Bogers, J, Vanden Broeck, D, Benoy, I, et al. Prevalence of high-risk human papillomavirus and abnormal pap smears in female sex workers compared to the general population in Antwerp, Belgium. BMC Public Health. (2016) 16:477. doi: 10.1186/s12889-016-3099-5
67. Priaulx, J, de Koning, HJ, de Kok, I, Széles, G, and McKee, M. Identifying the barriers to effective breast, cervical and colorectal cancer screening in thirty one European countries using the barriers to effective screening tool (BEST). Health Policy. (2018) 122:1190–7. doi: 10.1016/j.healthpol.2018.08.004
68. Jansen, EEL, Zielonke, N, Gini, A, Anttila, A, Segnan, N, Vokó, Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. (2020) 127:207–23. doi: 10.1016/j.ejca.2019.12.013
69. Priaulx, J, Turnbull, E, Heijnsdijk, E, Csanádi, M, Senore, C, de Koning, HJ, et al. The influence of health systems on breast, cervical and colorectal cancer screening: an overview of systematic reviews using health systems and implementation research frameworks. J Health Serv Res Policy. (2020) 25:49–58. doi: 10.1177/1355819619842314
70. Verdoodt, F, Jentschke, M, Hillemanns, P, Racey, C, Snijders, P, and Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer. (2015) 51:2375–85. doi: 10.1016/j.ejca.2015.07.006
71. Gupta, S, Palmer, C, Bik, EM, Cardenas, JP, Nunez, H, Kraal, L, et al. Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health. (2018) 6:77. doi: 10.3389/fpubh.2018.00077
Keywords: cervical cancer screening, vulnerable and underserved populations, barriers, facilitators, Europe
Citation: Greenley R, Bell S, Rigby S, Legood R, Kirkby V, McKee M and the CBIG-SCREEN Consortium (2023) Factors influencing the participation of groups identified as underserved in cervical cancer screening in Europe: a scoping review of the literature. Front. Public Health. 11:1144674. doi: 10.3389/fpubh.2023.1144674
Received: 14 January 2023; Accepted: 28 April 2023;
Published: 25 May 2023.
Edited by:
Andrew Scott LaJoie, University of Louisville, United StatesReviewed by:
Naomi Lee, Northern Arizona University, United StatesCopyright © 2023 Greenley, Bell, Rigby, Legood, Kirkby, McKee, and the CBIG-SCREEN Consortium. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Greenley, cmFjaGVsLmdyZWVubGV5MUBsc2h0bS5hYy51aw==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.