- 1Department of Pediatrics, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 2Key Laboratory of Population Health Across Life Cycle, Ministry of Education of the People's Republic of China, Anhui Medical University, Hefei, Anhui, China
- 3Ministry of Education Key Laboratory for Full Life Cycle Population Health, Anhui Medical University, Hefei, Anhui, China
Background: Multiple systematic reviews and meta-analyses have examined the association between neonatal jaundice and autism spectrum disorder (ASD) risk, but their results have been inconsistent. This may be because the included observational studies could not adjust for all potential confounders. Mendelian randomization study can overcome this drawback and explore the causal relationship between the both.
Methods: We used the data of neonatal jaundice, direct bilirubin (DBIL), indirect bilirubin (IBIL), and ASD collected by genome-wide association study (GWAS) to evaluate the effects of neonatal jaundice, DBIL and IBIL on ASD by using a two-sample Mendelian randomized (MR). The inverse variance-weighted method (IVW) was the main method of MR analysis in this study. Weighted median method, MR-Egger regression and mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test were used for sensitivity analysis.
Results: There was no evidence of an effect of neonatal jaundice (OR, 1.002, 95% CI, 0.977–1.027), DBIL (OR, 0.970, 95% CI, 0.884–1.064) and IBIL (OR, 1.074, 95% CI, 0.882–1.308) on ASD risk by IVW test. In the weighted median method, MR-Egger regression and leave-one-out analysis, the results were robust and no heterogeneity or pleiotropy was observed.
Conclusions: We found that neonatal jaundice, DBIL and IBIL were not associated with ASD in this study. However, this paper did not explore the effect of severity and duration of jaundice on ASD in different ethnic populations, which may require further research.
1. Introduction
Autism spectrum disorder (ASD) is a heterogeneous group of neurodevelopmental conditions, characterized by difficulties in social communication and interaction, as well as abnormally limited, repetitive behaviors and interests (1). In recent years, the prevalence of ASD has increased significantly. The prevalence of ASD in children aged 3–17 years in the United States in 2016 was 2.76%. By 2020, the prevalence of ASD had increased to 3.49% (2, 3). A nationwide multicenter population-based study (142,086) conducted in China during 2014–2016, and an estimated prevalence of 0.70% has been reported for children aged 6–12 years (4). The increasing prevalence of ASD is placing enormous pressure on health and social services, families and education (5, 6). Currently, the etiology of ASD is not clear, but it is believed that genetic factors, environmental factors (such as mercury, radiation, diesel waste, etc.), changes in neural connections, perinatal factors (such as age of parents, maternal medication, infection, etc.) and postpartum factors (such as meningitis, low birth weight, etc.) are associated with the pathogenesis of ASD (6, 7).
Neonatal jaundice is a common disease in the neonatal period. Jaundice occurs in about 60% of term infants and 80% of preterm infants (8). Bilirubin is produced from heme and, to a certain extent, is neuroprotective because of its free radical scavenging effect. Unconjugated bilirubin is a fat-soluble molecule that easily crosses the blood-brain barrier, and when it reaches a high level, it may cause a range of neurological symptoms (9, 10). Bilirubin deposition may cause neuropathological changes in the cerebellum and hippocampus (11–13), which have also been found in ASD patients (14, 15). Therefore, it is possible that there is an association between neonatal jaundice and ASD (16).
Jenabi et al., in a systematic review including 21 studies, reported that neonatal jaundice was associated with ASD in children (odds ratio (OR), 1.35, 95% confidence interval (CI), 1.02–1.68, risk ratio (RR), 1.39, 95% CI, 1.05–1.74) (17). However, Kujabi et al., based on the quality assessment of the literature, did not support the association between neonatal jaundice and ASD in 6 studies with low risk of bias (RR, 1.09, 95% CI, 0.99–1.20, OR, 1.29, 95% CI, 0.95–1.76) (18). In a systematic review including 13 observational studies, Amin et al. reported that neonatal jaundice diagnosed based on serum total bilirubin concentration was associated with an increased risk of ASD (OR, 1.43, 95% CI 1.22–1.67), but unconjugated bilirubin may be a better predictor of neurotoxicity in preterm infants than total bilirubin (16). These system reviews were mainly analyzed on the basis of observational studies, so it is difficult to avoid the interference of confounding factors. For example, hospitalized newborns are more likely to be diagnosed with jaundice, whereas non-hospitalized newborns are at much lower risk (18). Therefore, investigating the causal association of neonatal jaundice with ASD should adjust for all covariates that influence neonatal hospitalization and ASD occurrence. Including woman's first child, parental age, neonatal comorbidity, complicated delivery, child's sex, Apgar score, gestational age, birth weight and year, etc. (18). Most clinical epidemiological research methods, such as case-control studies and cohort studies, have difficulty in controlling all potential confounding factors (19), while mendelian randomization (MR) studies can solve this problem.
MR is a method that uses genetic variants as instrumental variables to assess causal relationships between exposure or risk factors and clinical outcomes of interest (20). MR is similar to randomized controlled trials (RCTs) because segregation of genetic variants is randomly assigned, and is independent of environmental factors. Thus, known and unknown confounders were equally distributed across the groups (21). Moreover, the data of MR study can be derived from Genome-wide association studies (GWAS). Therefore, MR study has the advantages of convenience, rapidity, large sample size, avoiding confounding to the greatest extent and avoiding reverse causality (22, 23). In this study, we investigated the causal effects of neonatal jaundice, direct bilirubin (DBIL) and indirect bilirubin (IBIL) on ASD by using a two-sample MR.
2. Methods
2.1. Study design and data sources
In this study, two-sample MR was used to evaluate the causal relationship between neonatal jaundice, DBIL, IBIL and the risk of ASD. Effective instrument variables [in this study are single nucleotide polymorphisins (SNPs)] need to satisfy three core assumptions: (i) assumption of relevance: SNPs are correlated with exposure, (ii) assumption of independence: SNPs are not related to confounders, (iii) assumption of exclusivity: SNPs are related to outcomes only by exposure (24).
GWAS is a research method to study the correlation between genetic mutations and phenotypes (25). The GWAS databases aggregate genotype and phenotype associations from genome-wide association studies. We obtained the latest and largest sample size GWAS summary data for exposures from IEU OpenGWAS project (mrcieu.ac.uk). The exposures data's that we acquired included neonatal jaundice (133 cases and 218,608 controls), DBIL (6,961 cases and 6,961 controls) and IBIL (6,972 cases and 6,972 controls). The study population for neonatal jaundice data was European, while the study population for DBIL and IBIL was the South Asian. Symptoms of jaundice can be observed in neonates with total serum bilirubin levels above 5.0 mg/dl (26). However, the bilirubin threshold for intervention is affected by factors such as gestational age, postnatal age, ABO/Rh hemolytic disease, and glucose-6-phosphate dehydrogenase (G6PD) deficiency (27). In general, the bilirubin threshold requiring clinical intervention was reached when the total serum bilirubin level in term infants exceeded 5 mg/dl on the 1st day after birth, 10 mg/dl on the 2nd day, or 13 mg/dl on the third day and beyond (28). Hepatobiliary injury is indicated when direct bilirubin levels exceed 1.0 mg/dL, or represent more than 20% of total bilirubin (26). GWAS summary data for result (in this study is ASD) were also obtained from IEU OpenGWAS project (mrcieu.ac.uk). The GWAS data of ASD included 4,949 experimental and 5,314 control subjects, with a European population.
2.2. Instrumental variable selection criteria
SNPs were initially selected from relevant GWAS databases using the following criteria: (i) p-value of <5 × 10−8, ii) a linkage disequilibrium (LD) r2 of <0.001, within a 10,000 kb window. According to this standard, 3 SNPs of DBIL and 4 SNPs of IBIL were screened out, and no SNPs meeting the standard for neonatal jaundice were found. For better study, we relaxed the p-value of the SNP associated with neonatal jaundice to 5 × 10−6, and adjusted the p-value of the SNP associated with DBIL and IBIL to 5 × 10−7. Previous studies have used similar thresholds for instrumental variables (29, 30). F statistic can be used to assess the instrument strength for each of the exposed SNPs, which was calculated by the formula β2/σ2 (β indicates the association between SNP and exposure) (29). Typically, F statistic >10 is considered to be strongly associated with exposure (31).
Afterwards, the information of SNPs which were selected through the above process in the outcome was extracted and discarded if it were not available in the outcome. A SNP (rs6750992) of IBIL lacked σ (means variance) and was abandoned when MR analysis was performed. Next, we combined the exposure and outcome data which were used for the MR analysis.
2.3. MR analysis
The inverse variance-weighted method (IVW) was the main method of MR analysis in this study, and its basic assumption is that all selected instrumental variables are valid, that is, the three core assumptions of MR need to be satisfied. If the SNPs have horizontal pleiotropy, they violate the exclusivity assumption and may cause a large bias in the IVW results (32).
Weighted median method and MR-Egger regression were used to carry out sensitivity analysis. The weighted median method is to make estimates of causal effects under the assumption that less than 50% of the SNPs are null (33). MR-Egger regression can also give consistent causal effect estimates when all SNPS are considered as invalid instrumental variables. And it can be used to test for pleiotropy bias (34).
Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test was also a way to assess sensitivity. MR-PRESSO can identify outliers for horizontal pleiotropy in MR summary data, remove outliers to correct horizontal pleiotropy, and compare the differences between the MR analysis results before and after correction (35). In this study, we also conducted the simple mode, weighted mode, Cochrane's Q statistic and leave-one-out analysis. All the above analyses were performed in Rstudio software using the R packages “TwosampleMR” and “MR-PRESSO”.
2.4. Statistical power
We calculated the statistical power on https://shiny.cnsgenomics.com/mRnd/ (36). The power calculation required the total value of the R2 (the proportion of variance explaining the association between the SNP and the exposure). We calculate it by the following formula: 2*EAF*(1-EAF)*β2 (37).
3. Results
3.1. Overview
After selection, 8 SNPs of neonatal jaundice, 5 SNPs of DBIL and 6 SNPs of IBIL could be used for MR analysis. In this study, the F statistics of the selected SNPs were all >10. Final analysis data are shown in Table 1.
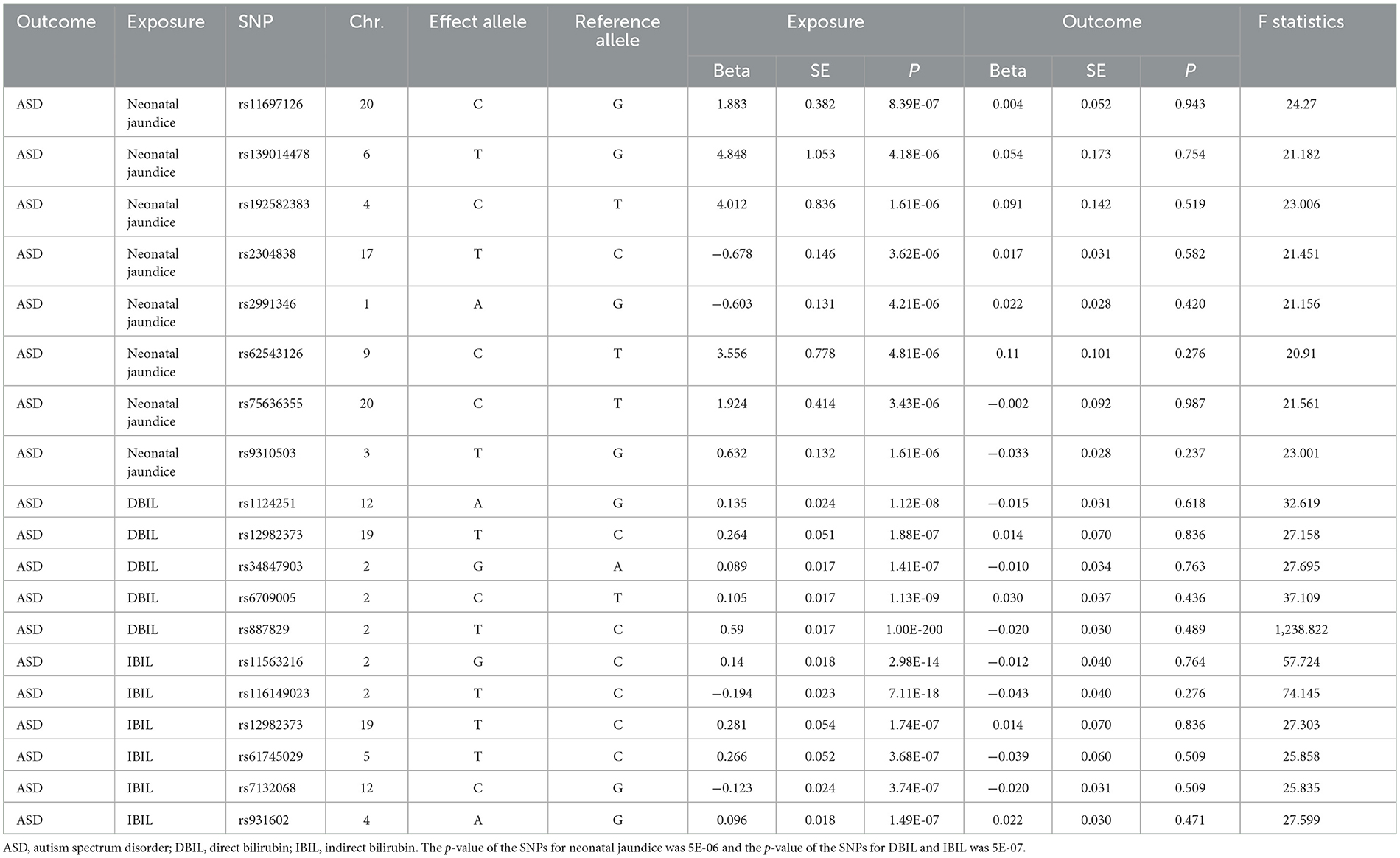
Table 1. Causal effects of neonatal jaundice, DBIL, and IBIL on ASD in Mendelian randomization analysis.
3.2. Casual estimates of neonatal jaundice, DBIL and IBIL on ASD
When p-value < 5 × 10−6, no significant association was found between neonatal jaundice and ASD by IVW test (OR, 1.002, 95% CI, 0.977–1.027, p = 0.885). Similarly, no significant relationship was found between DBIL and ASD (OR, 0.970, 95% CI, 0.884–1.064, p = 0.514) when p-value < 5 × 10−7, and the relationship between IBIL and ASD [OR = 1.074, 95% CI = (0.882, 1.308), p = 0.475] was the same result. The above results are presented in Table 2.
3.3. Sensitive, heterogeneity and pleiotropy analysis
MR-PRESSO and MR-Egger intercept were used to test horizontal pleiotropy. If abnormal SNPs are found by MR-PRESSO method, they need to be eliminated and re-analyzed by MR. No abnormal SNPs were identified in this study. The results of MR-Egger intercept are shown in Table 2. All p-values were >0.05, therefore, there was no horizontal pleiotropy in the causal estimates of neonatal jaundice, DBIL and IBIL on ASD. Heterogeneity was tested using the Cochran's Q test. The p-value of Q based on IVW method and MR-Egger method is >0.05 in this study (the specific results are shown in Table 2), which indicates that there is no heterogeneity in the above three causal studies.
The weighted median method and MR-Egger regression were used to test the sensitivity in the paper, and the results were consistent with the results of IVW. The results obtained by simple mode and weighted mode methods were also consistent with those of the above three methods. This illustrates the robustness of the results of this study. The robustness was also verified by leave-one-out analysis. Scatter plots of the causal estimates of neonatal jaundice, DBIL and IBIL on ASD and the leave-one-out plot are shown in Figures 1, 2.
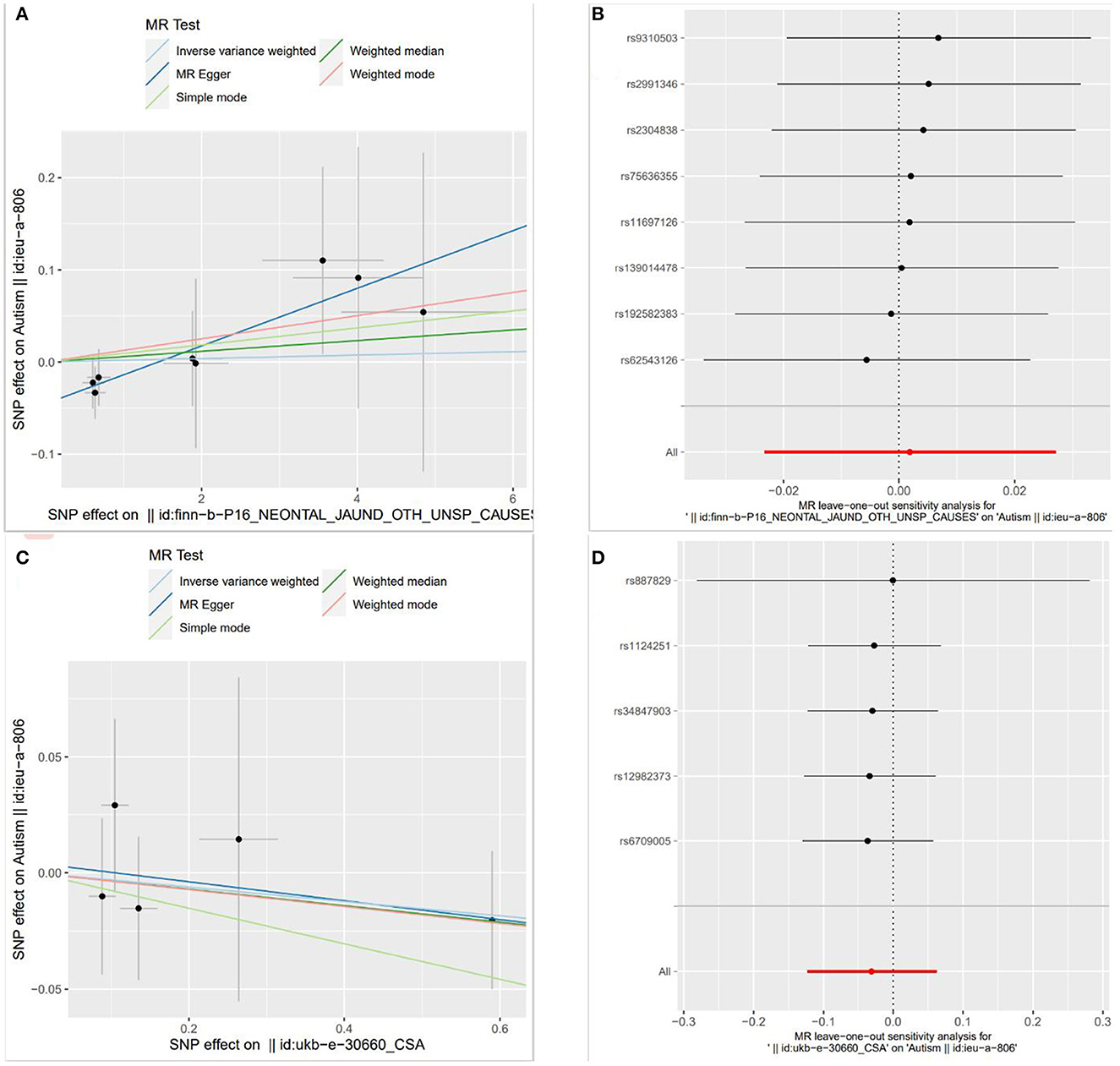
Figure 1. Scatter plots and leave-one-out plot of the causal estimates of neonatal jaundice and direct bilirubin (DBIL) on autism spectrum disorder (ASD) [Scatter plots of MR analysis were calculated by IVW, MR-egger, weighted median, single mode and weighted mode methods. The slopes indicate the causality of each method. (A) Illustrates the relationship between neonatal jaundice and ASD, and (C) illustrates the relationship between DBIL and ASD; Leave-one-out analyses demonstrated the robustness of the associations of neonatal jaundice (B) and DBIL (D) with ASD].
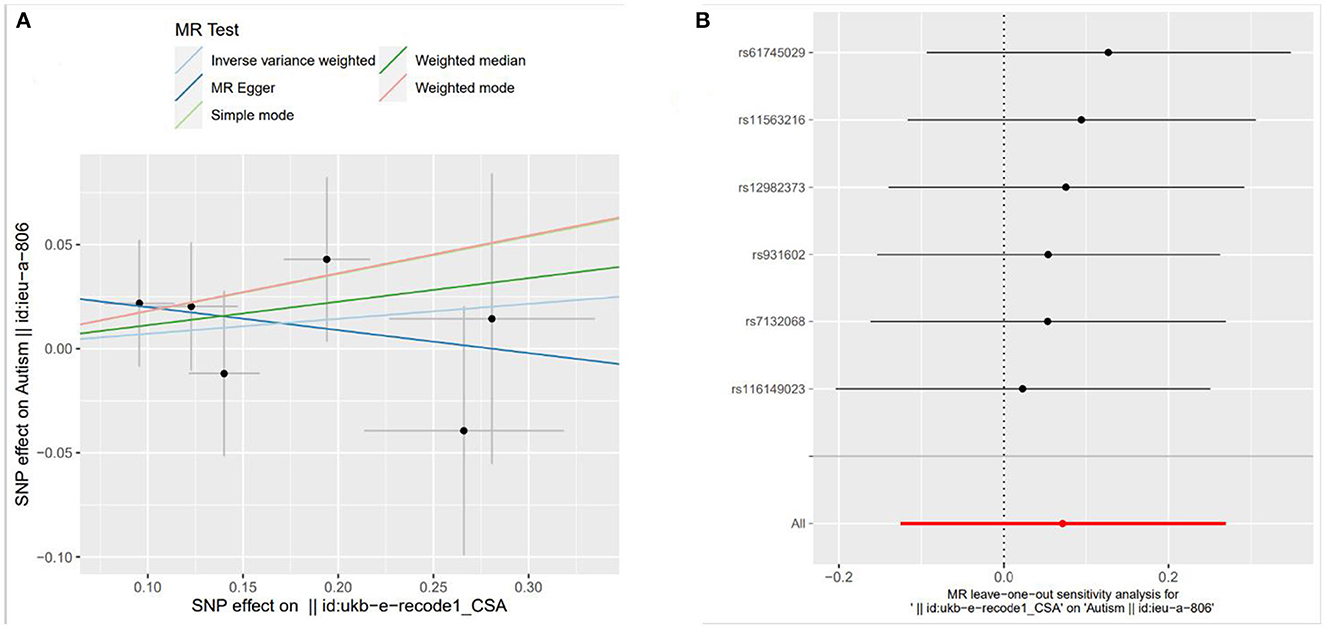
Figure 2. Scatter plots and leave-one-out plot of the causal estimates of indirect bilirubin (IBIL) on autism spectrum disorder [Scatter plots of MR analysis were calculated by IVW, MR-egger, weighted median, single mode and weighted mode methods. The slopes indicate the causality of each method. (A) Illustrates the relationship between IBIL and ASD; Leave-one-out analyses demonstrated the robustness of the associations of IBIL (B) with ASD].
3.4. Statistical power
The statistical power of MR analysis for the causal effect of neonatal jaundice, DBIL and IBIL on ASD was 0.05, 0.10, and 0.49, respectively.
4. Discussion
We investigated the causal association of neonatal jaundice, DBIL and IBIL with ASD using a mendelian randomization study approach. We found genetic proof that neonatal jaundice, DBIL and IBIL had no significant causal relationship with ASD.
Neonatal jaundice is usually a mild disease (38) when bilirubin level is in a safe range. Bilirubin increases in newborns, especially during the 1st week of life, due to the immature liver enzyme system, increased red blood cell breakdown and the presence of enterohepatic circulation. Neurotoxic effects that occur when bilirubin levels are above age-appropriate thresholds may lead to kernicterus and may manifest clinically in changes in muscle tone, abnormal hearing, and abnormalities in speech and language (39, 40). Kernicterus often affects the globus pallidus, but can also involve the subthalamic nucleus, brain stem, hippocampus and cerebellum. The resulting pathological changes in the brain include cerebellar damage (reduced number of Purkinje cells) and impaired pathways between cerebellar and cortical neurons, which is similar to the pathogenesis of ASD (7, 39). High values of bilirubin, especially extremely high values of bilirubin, may increase the worry of family members, and if neonatal bilirubin is related to ASD, this may increase the worry and burden of family members. The results of this paper may provide some reassurance to them.
Result of this paper is consistent with the results of the literature review by Kujabi et al., who rigorously evaluated the included articles (18). However, it is inconsistent with the results of the literature review by Amin et al. and Jenabi et al. (16, 17). But many of the included articles did not control for confounding factors, some articles did not clearly define jaundice and some information on bilirubin levels was retrieved from parents, which may have led to various biases (16, 17). In this article, we only considered the presence or absence of jaundice and do not account for the severity or duration of jaundice or geographic differences in race (41), which may have contributed to our lack of positive findings. Amin et al. and Jenabi et al. found a link between increased severity of jaundice and a possible increased risk of ASD (16, 17), suggesting that more severe jaundice is associated with a greater likelihood of developing ASD. Without stratification of jaundice by severity, including mild jaundice cases in the analysis may have attenuated the effect estimates between jaundice and ASD. In the course of the study, we did not consider this point. The duration of jaundice, which can worsen over time in the absence of intervention, is also an important factor, and this may increase ASD risk (41). Ethnic and geographic differences are also important factors. The prevalence of neonatal jaundice and kernicterus was different in different nationalities and regions. For example, black infants have a higher prevalence of kernicterus (42). Other groups, such as persons of African, Asian, Mediterranean, or Middle Eastern ancestry, may have an increased risk of neonatal hyperbilirubinemia because of G6PD deficiency (43). A higher threshold of concern for the evaluation of jaundice in such neonates may lead to an increased incidence of neurologic impairment. And infants in low-income and middle-income countries are more likely to develop severe jaundice due to lower levels of diagnosis, lower levels of care, delayed or inappropriate treatment (39, 44). However, differences in study populations may increase or decrease the effect estimates between neonatal jaundice and ASD (41). The GWAS databases we used were only European or South Asian, which may not be so representative. In addition, jaundice is highly prevalent in the neonatal period, and the GWAS database for neonatal jaundice used in our study had only 133 cases compared with 218,606 controls. Therefore, there may be an information deficit in the GWAS data we used, which may underestimate the effect of neonatal jaundice on ASD.
There are some limitations of this study. First, because the original screening criteria of p < 5 × 10−8 were set, which reduced the number of SNPs available for analysis, we relaxed the screening criteria, which may have introduced instrumental bias. Although the F-statistics showed that the SNPs in the final analysis were all strong instrumental variables. Second, the study populations of GWAS data for DBIL and IBIL are not consistent with those of GWAS data for ASD, which may lead to population heterogeneity bias. Moreover, the sample size of GWAS data on neonatal jaundice is relatively small. Third, although evaluation by the MR-Egger intercept, the MR-PRESSO method, and leave-one-out analysis did not reveal significant pleiotropy, this possibility cannot be excluded. Fourth, the statistical power of our study was low and did not reach the target power (0.80). This may be related to the insufficient sample size, so a more in-depth study is needed. In addition, the duration and severity of jaundice and the correlation with age of onset, severity of autism, and predictive parameters were not taken into account in this study, which also requires further investigation. Finally, the GWAS data we used is relatively limited in population, and the generalization of the results to other populations may require larger and more comprehensive studies.
5. Conclusion
In conclusion, in this study, we did not find a causal relationship between neonatal jaundice and ASD, nor did DBIL and IBIL. Data from a larger sample may be needed to verify the causal relationship between neonatal jaundice and ASD. Further exploration of the impact of severity and duration of jaundice on ASD in different ethnic populations is also needed.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
L-wC participated in conceptualizing and writing the draft. YZ and D-dX were responsible for methodology and statistical analysis. YW participated in writing review and editing. While HG was mainly responsible for funding acquisition, writing review, and editing. All authors agreed to the final version as submitted.
Funding
This paper was funded by the National Natural Science Foundation of China (No. 82103856), the Provincial Natural Science Foundation of Anhui (No. 2108085QH359), and funds of the MOE Key Laboratory of Population Health Across Life Cycle (No. JK20204).
Acknowledgments
We are grateful to the investigators who made their raw data publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lai M, Lombardo MV, Chakrabarti B, Baron-Cohen S. Subgrouping the Autism “Spectrum”: Reflections on DSM-5. PLoS Biol. (2013) 11:1544. doi: 10.1371/journal.pbio.1001544
2. Xu G, Strathearn L, Liu B, Bao W. Prevalence of Autism Spectrum Disorder Among US Children and Adolescents, 2014–2016. JAMA. (2018) 319:81–82. doi: 10.1001/jama.2017.17812
3. Li Q, Li Y, Liu B, Chen Q, Xing X, Xu G, et al. Prevalence of Autism Spectrum Disorder Among Children and Adolescents in the United States From 2019 to 2020. JAMA Pediatr. (2022) 176:943–945. doi: 10.1001/jamapediatrics.2022.1846
4. Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/s12264-020-00530-6
5. Rogge N, Janssen J. The economic costs of autism spectrum disorder: a literature review. J Autism Dev Disord. (2019) 49:2873–900. doi: 10.1007/s10803-019-04014-z
6. Styles M, Alsharshani D, Samara M, Alsharshani M, Khattab A, Qoronfleh MW, et al. Risk factors, diagnosis, prognosis and treatment of autism. Front Biosci. (2020) 25:1682–717. doi: 10.2741/4873
7. Bhat S, Acharya UR, Adeli H, Bairy GM, Adeli A. Autism: cause factors, early diagnosis and therapies. Rev Neurosci. (2014) 25:841–50. doi: 10.1515/revneuro-2014-0056
8. Burke BL, Robbins JM, Bird TM, Hobbs CA, Nesmith C, Tilford JM. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics. (2009) 123:524–32. doi: 10.1542/peds.2007-2915
9. Mancuso C. Bilirubin and brain: A pharmacological approach. Neuropharmacology. (2017) 118:113–23. doi: 10.1016/j.neuropharm.2017.03.013
10. Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. (2002) 140:396–403. doi: 10.1067/mpd.2002.123098
11. Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatr Neurol. (2003) 29:410–21. doi: 10.1016/j.pediatrneurol.2003.09.011
12. Hansen TW, Bratlid D. Bilirubin and brain toxicity. Acta Paediatr Scand. (1986) 75:513–22. doi: 10.1111/j.1651-2227.1986.tb10242.x
13. Ahdab-Barmada M, Moossy J. The neuropathology of kernicterus in the premature neonate: diagnostic problems. J Neuropathol Exp Neurol. (1984) 43:45–56. doi: 10.1097/00005072-198401000-00004
14. Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. (2004) 127:2572–83. doi: 10.1093/brain/awh287
15. Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. (2005) 23:183–7. doi: 10.1016/j.ijdevneu.2004.09.006
16. Amin SB, Smith T, Wang H. Is neonatal jaundice associated with autism spectrum disorders: a systematic review. J Autism Dev Disord. (2011) 41:1455–63. doi: 10.1007/s10803-010-1169-6
17. Jenabi E, Bashirian S, Khazaei S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clinical and Experimental Pediatrics. (2020) 63:8–13. doi: 10.3345/kjp.2019.00815
18. Kujabi ML, Petersen JP, Pedersen MV, Parner ET, Henriksen TB. Neonatal jaundice and autism spectrum disorder: a systematic review and meta-analysis. Pediatr Res. (2021) 90:934–49. doi: 10.1038/s41390-020-01272-x
19. Sessler DI, Imrey PB. Clinical research methodology 2: observational clinical research. Anesth Analg. (2015) 121:1043–51. doi: 10.1213/ANE.0000000000000861
20. Evans DM, Davey SG. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
21. Gupta V, Walia GK, Sachdeva MP. ‘Mendelian randomization': an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
22. Sekula P, Del GMF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
23. Davey SG, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
24. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
25. Dehghan A. Genome-wide association studies. Methods Mol Biol. (2018) 1793:37–49. doi: 10.1007/978-1-4939-7868-7_4
26. Anderson NB, Calkins KL. Neonatal indirect hyperbilirubinemia. Neoreviews. (2020) 21:e749–60. doi: 10.1542/neo.21-11-e749
27. Olusanya BO, Kaplan M, Hansen T. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. (2018) 2:610–20. doi: 10.1016/S2352-4642(18)30139-1
28. Mishra S, Agarwal R, Deorari AK, Paul VK. Jaundice in the newborns Congress. Indian J Pediatr. (2008) 75:157–163. doi: 10.1007/s12098-008-0024-7
29. Chen X, Yao T, Cai J, Fu X, Li H, Wu J. Systemic inflammatory regulators and 7 major psychiatric disorders: A two-sample Mendelian randomization study. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 116:110534. doi: 10.1016/j.pnpbp.2022.110534
30. Dardani C, Riglin L, Leppert B, Sanderson E, Rai D, Howe LD, et al. Is genetic liability to ADHD and ASD causally linked to educational attainment? Int J Epidemiol. (2022) 50:2011–23. doi: 10.1093/ije/dyab107
31. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
32. Bowden J, Del GMF, Minelli C, Davey SG, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
33. Bowden J, Davey SG, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
34. Bowden J, Davey SG, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
35. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
36. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
37. Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. (2020) 11:597. doi: 10.1038/s41467-020-14389-8
38. Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med (Lond). (2017) 78:699–704. doi: 10.12968/hmed.2017.78.12.699
39. Cayabyab R, Ramanathan R. High unbound bilirubin for age: a neurotoxin with major effects on the developing brain. Pediatr Res. (2019) 85:183–90. doi: 10.1038/s41390-018-0224-4
40. Bhutani VK, Johnson-Hamerman L. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med. (2015) 20:6–13. doi: 10.1016/j.siny.2014.12.008
41. Wilde VK. Neonatal jaundice and autism: precautionary principle invocation overdue. Cureus. (2022) 14:e22512. doi: 10.7759/cureus.22512
42. Okolie F, South-Paul JE, Watchko JF. Combating the hidden health disparity of kernicterus in black infants: a review. JAMA Pediatr. (2020) 174:1199–205. doi: 10.1001/jamapediatrics.2020.1767
Keywords: autism spectrum disorder, neonatal jaundice, indirect bilirubin (IBIL), direct bilirubin, Mendelian randomization (MR)
Citation: Chen L-w, Zhang Y, Xu D-d, Wang Y and Gao H (2023) Causal relationships of neonatal jaundice, direct bilirubin and indirect bilirubin with autism spectrum disorder: A two-sample Mendelian randomization analysis. Front. Public Health 11:1137383. doi: 10.3389/fpubh.2023.1137383
Received: 04 January 2023; Accepted: 28 March 2023;
Published: 13 April 2023.
Edited by:
Roopali Rajput, Jamia Hamdard University, IndiaReviewed by:
Jitender Sharma, University of Delhi, IndiaAmbika Gupta, Apollo Hospitals, India
Jesper Padkær Petersen, Aarhus University Hospital, Denmark
Copyright © 2023 Chen, Zhang, Xu, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wang, dy55YW5nMTI2QDEyNi5jb20=; Hui Gao, Z2gyMDE5MDEzMEAxNjMuY29t
 Li-wen Chen
Li-wen Chen Yi Zhang2
Yi Zhang2 Dou-dou Xu
Dou-dou Xu Yang Wang
Yang Wang Hui Gao
Hui Gao