- 1Department of Occupational and Environmental Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, and State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3First Affiliated Hospital, Gannan Medical University, Ganzhou, China
- 4Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou, China
- 5School of Public Health and Health Management, Gannan Medical University, Ganzhou, China
Objective: Previous epidemiological studies have shown that both long-term and short-term exposure to fine particulate matters (PM2.5) were associated with the morbidity and mortality of circulatory system diseases (CSD). However, the impact of PM2.5 on CSD remains inconclusive. This study aimed to investigate the associations between PM2.5 and circulatory system diseases in Ganzhou.
Methods: We conducted this time series study to explore the association between ambient PM2.5 exposure and daily hospital admissions for CSD from 2016 to 2020 in Ganzhou by using generalized additive models (GAMs). Stratified analyses were also performed by gender, age, and season.
Results: Based on 201,799 hospitalized cases, significant and positive associations were found between short-term PM2.5 exposure and hospital admissions for CSD, including total CSD, hypertension, coronary heart disease (CHD), cerebrovascular disease (CEVD), heart failure (HF), and arrhythmia. Each 10 μg/m3 increase in PM2.5 concentrations was associated with a 2.588% (95% confidence interval [CI], 1.161%–4.035%), 2.773% (95% CI, 1.246%–4.324%), 2.865% (95% CI, 0.786%–4.893%), 1.691% (95% CI, 0.239%–3.165%), 4.173% (95% CI, 1.988%–6.404%) and 1.496% (95% CI, 0.030%–2.983%) increment in hospitalizations for total CSD, hypertension, CHD, CEVD, HF, and arrhythmia, respectively. As PM2.5 concentrations rise, the hospitalizations for arrhythmia showed a slow upward trend, while other CSD increased sharply at high PM2.5 levels. In subgroup analyses, the impacts of PM2.5 on hospitalizations for CSD were not materially changed, although the females had higher risks of hypertension, HF, and arrhythmia. The relationships between PM2.5 exposure and hospitalizations for CSD were more significant among individuals aged ≤65 years, except for arrhythmia. PM2.5 had stronger effects on total CSD, hypertension, CEVD, HF, and arrhythmia during cold seasons.
Conclusion: PM2.5 exposure was positively associated with daily hospital admissions for CSD, which might provide informative insight on adverse effects of PM2.5.
1. Introduction
The development of modern industrialization has made air pollution one of the leading public health concerns worldwide (1). According to the latest Global Burden of Disease (GBD) Survey, ambient air pollution could be responsible for 6.7 million deaths in 2019 (2). Accumulating studies have shown that exposure to air pollutants, whether both short- or long-term, may damage human health on multiple levels (3).
PM2.5, named particulate matter with aerodynamic diameter below 2.5 μm, is considered the most sensitive indicator of air quality (4). Ambient PM2.5 mainly comes from natural conditions (volcanic eruptions and dust storms) and human activities such as industrial production, and traffic exhaust emissions (5). PM2.5 has become an environmental problem and has attracted global public health concerns because of its adverse effects on multiple organs (6). Previous studies suggested that PM2.5 exposure might lead to increased risks of respiratory diseases (7), circulatory system diseases (CSD) (8), neurological diseases (9), and metabolic diseases (10). In fact, the impact of PM2.5 on CSD has been extensively reported in previous studies (11–13). However, epidemiologic studies regarding the relationships of short-term exposure to PM2.5 and CSD remain inconclusive. According to a national study containing 379,133 participants, there was a 0.12% (95% CI, 0.001–0.25%) elevation in cardiovascular disease mortality with a 10-μg/m3 increase in PM2.5 levels on the same day (14). However, a study (15) including over 286 million hospitalizations in England and Wales found little evidence of PM2.5 exposure with increased risk of cardiovascular admissions, and even in many cases, PM2.5 was related to decrease risks of cardiovascular hospitalization. More studies are warranted to evaluate the impacts of short-term PM2.5 exposure on CSD risks.
Ganzhou, a city with 9.8402 million population in 2021 and located in southern China, enjoys a typical subtropical monsoon climate. In recent years, great measures were conducted to reduce urban ambient pollution in China and the air quality of Ganzhou has gradually improved in last 10 years. In 2021, the mean concentration of PM2.5 in Ganzhou was 23 μg/m3, 34.3% lower than the that (35 μg/m3) in 168 Chinese cities in the same year. To evaluate potential effects of PM2.5 on the onset of CSD in Ganzhou is also helpful to understand adverse health effects caused by relatively low levels of PM2.5 in China.
In this study, daily concentrations of air pollutants in Ganzhou were collected from China National Environmental Monitoring Center (CNEMC), and hospital admission data for CSD from 2016 to 2020 were extracted from the biggest hospital in Ganzhou. This time-series analysis was conducted to evaluate the relationships of ambient PM2.5 exposure with daily hospitalizations for CSD. The effects of co-exposure to other air pollutants on above relationships were also analyzed.
2. Materials and methods
2.1. Daily hospital admissions data
Daily hospital admissions data of CSD from Jan.1, 2016 to Dec.31, 2020 were extracted from the hospital's admission case registry system in the biggest hospital of Ganzhou. The patient information included gender, age, residential address, date of admission, and principal diagnosis. The CSD in present study were encoded according to the 10th version of the International Classification of Diseases (ICD-10) as follows: total circulatory disease (I00-I99), hypertension (I10-I15), coronary heart disease (CHD, I20-I25), cerebrovascular diseases (CEVD, I60-I69), heart failure (HF, I50), and arrhythmia (I47-I49).
2.2. Ambient air pollutants and meteorological data
The 24-h average concentrations of PM2.5, NO2, PM10, SO2, and CO, and the maximum 8-h mean concentrations of O3 from January 1, 2016 to December 31, 2020 were obtained from the National Real-Time Air Quality Monitoring Data Publishing Platform developed by CNEMC (http://www.cnemc.cn/). Daily average meteorological data including daily average temperature, relative humidity, and wind speed during the 5 years were obtained from the National Meteorological Information Center (http://data.cma.cn/). Present study did not involve/include any personally identifiable information and Institutional Review Board approval was not applicable.
2.3. Statistical analysis
Descriptive statistical analyses were performed to reveal the features of daily circulatory hospital admissions, ambient air pollutants and meteorological factors from January 1, 2016 to December 31, 2020. Spearman's rank correlation test was used to evaluate the bivariate associations between air pollutants and meteorological variables. To address the associations between PM2.5 and hospitalization for CSD, the quasi-Poisson regression method in generalized additive models (GAMs) based on time series was applied. In this model, spline smoothing functions of time trend, daily mean temperature and relative humidity were introduced into the GAMs to exclude the potential confounding effects of long-term time trend and meteorological variables. Besides, the day of the week (DOW) and holiday were also considered as potential confounders. The model finally used was as follows:
Where t is the day of observation; Yt denotes daily count of hospital admissions for CSD at day t; Xt is the daily mean concentration of PM2.5 at day t; β is the regression coefficient which represents the log-relative rate of hospital admissions for CSD with a 10 μg/m3 increase of PM2.5 concentration; DOW means the day of the week; df is degree of freedom whose value is determined based on the Quasi-Akaike information criterion (QAIC); α is the model intercept.
It is universally accepted that ambient air pollutants have persistent and hysteresis effects on health outcomes (16). We considered both single-day lags (lag0–lag14) and multiday lags (lag01–lag014) to evaluate delayed effects of PM2.5. Lag0 refers to the impact of PM2.5 on hospitalizations for CSD on the same day and Lag01 shows the effect of PM2.5 in the current and the previous days. Additionally, stratified analyses were performed to determine whether the associations differed by age (≤65 and >65 years old), gender, season (May-October, warm season; December-April, cold season).
To exclude the potential confounding effect of other pollutants, a series of co-pollutant models were developed in addition to the single-pollutant model. Notably, to avoid collinearity, pollutants with correlation coefficients >0.6 were not incorporated into co-pollutant models simultaneously.
In the sensitivity analysis, we adjust the dfs of calendar time to 7 per year to test the stability of the associations, according to relevant studies (17–19). In addition, previous studies have shown that COVID-19 might have impacts on circulatory system (20). In this study, in order to control the impact of COVID-19 on the associations, we also included the occurrence of COVID-19 in the model for sensitivity analysis.
All results were given as percent changes and 95% confidence intervals (CIs) in daily hospitalizations for CSD per 10 μg/m3 increment in PM2.5 levels. All statistical analyses were performed in R version 4.1.2 with the “mgcv” and “tsModel” packages. P-value < 0.05 was considered as statistically significant (2-sided).
3. Results
The descriptive statistics of daily air pollutants, meteorological variables, and hospitalizations for CSD during the 5 years were shown in Table 1. A total of 201,799 hospitalizations for CSD (daily average: 110 hospital admissions) in Ganzhou were included in the analysis. Among all the records, 50.5% were older than 65 years old, and 56.8% were males. As for disease subtypes, hypertension accounted for 47.0% of total CSD, followed by cerebrovascular diseases (20.9%), coronary heart disease (14.2%), heart failure (10.7%) and arrhythmia (7.2%).
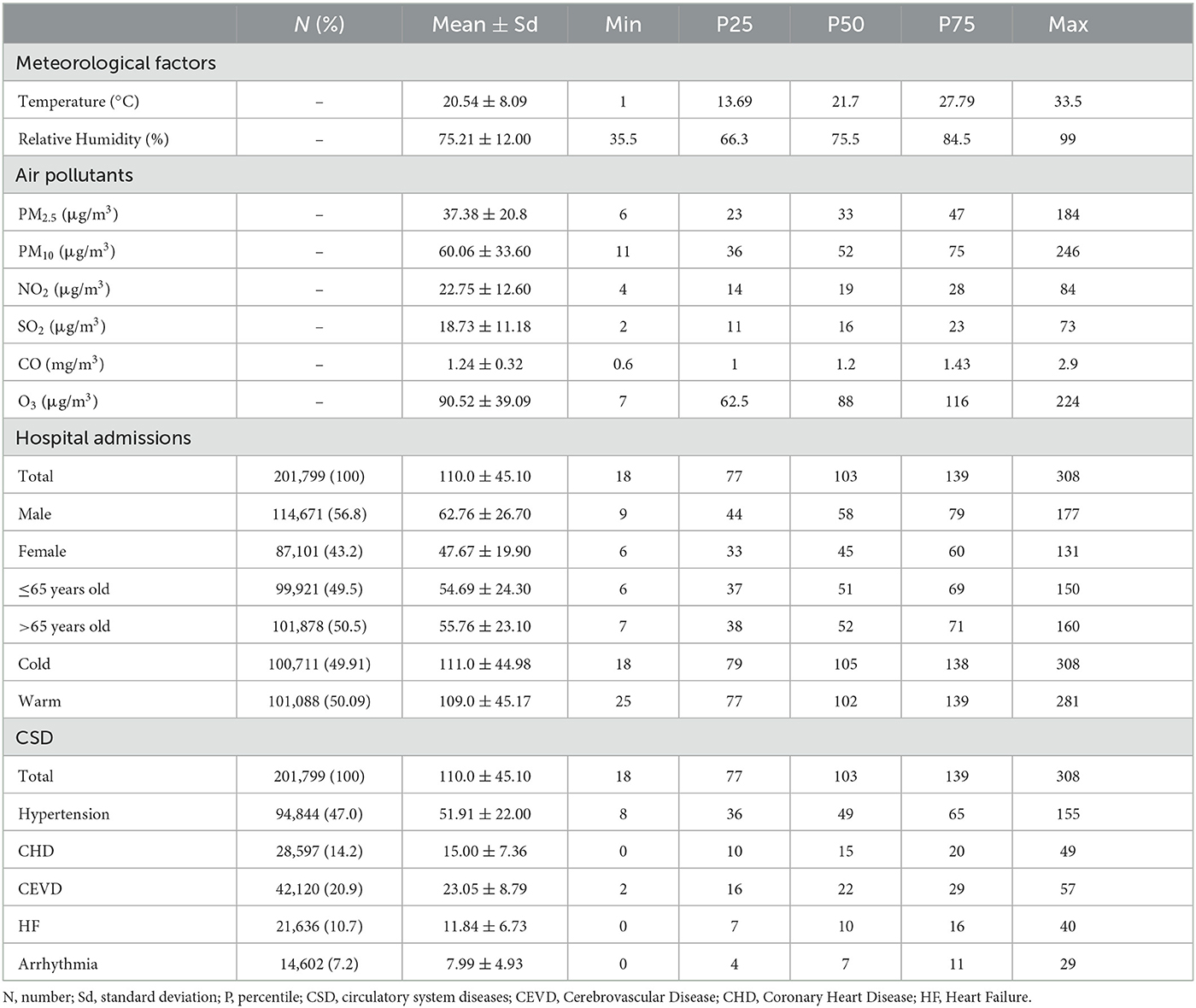
Table 1. Characteristics of meteorological variables, ambient air pollutants, and hospital admissions in Ganzhou (2016–2020).
The average daily concentrations of PM2.5, PM10, NO2, SO2 and CO during research period were 37.38 μg/m3, 60.06 μg/m3, 22.75 μg/m3, 18.73 μg/m3, 1.24 mg/m3, respectively, while the 8-hour average concentration of O3 was 90.52 μg/m3. On average, the daily temperature was 20.54°C and the relative humidity was 75.21 %.
As shown in the time series plots, the levels of PM2.5 in Ganzhou decreased slowly and gradually during the study period (Supplementary Figure 1). The levels of PM10, NO2, SO2 and CO also showed similar trends, except that O3 levels increased. During the same period, hospital admissions for total CSD and five specific subtypes fluctuated and rose steadily (Supplementary Figure 2).
The Spearman's correlation coefficients for exposure variables were given in Figure 1. PM2.5 was highly correlated with PM10 and SO2, and NO2 (r > 0.6 and P < 0.05), moderately correlated with CO and O3 (r = 0.44 and 0.33, respectively, P < 0.05). However, PM2.5 was negatively correlated with relative humidity (r = −0.21, P < 0.05) and temperature (r = −0.22, P < 0.05).
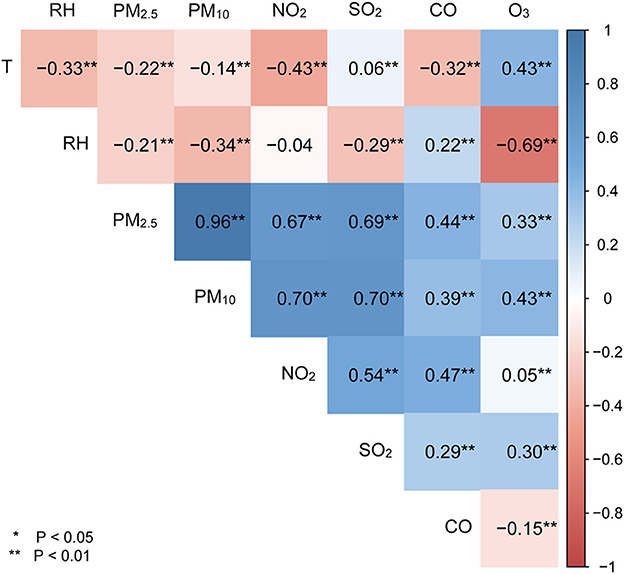
Figure 1. Spearman's rank correlations of meteorological variables with ambient air pollutants. T represents temperature; RH denotes relative humanity; *means P < 0.05, **means P < 0.01.
Positive linear exposure-response relationships between PM2.5 concentrations and daily hospitalizations for total and cause-specific CSD were observed (Figure 2). Hospitalizations for total CSD, hypertension, CHD, CEVD, and HF increased rapidly at high levels of PM2.5, except for arrhythmia, which showed a slowly linear rise. In single-pollutant models, significantly positive associations were observed between PM2.5 levels and hospital admissions for studied CSD in both single-day (lag1–lag14) and cumulative-day (lag01–lag014) lag structures (Table 2). The largest single day effect of PM2.5 was at lag6 for total CSD, hypertension, CHD, lag4 for CEVD, and lag1 for HF and arrhythmia. The greatest cumulative day effect for total CSD, hypertension, CHD and HF were observed at lag014. The effect of PM2.5 on hospitalizations for CEVD peaked at lag011. Every 10 μg/m3 increment of ambient PM2.5 concentrations was associated with a 2.588% [95% confidence interval (CI), 1.161–4.035%], 2.773% (95% CI, 1.246–4.324%), 2.865% (95% CI, 0.786–4.893%), 1.691% (95% CI, 0.239–3.165%), 4.173% (95% CI, 1.988–6.404%) and 1.496% (95% CI, 0.030–2.983%) increment in hospitalizations for total CSD, hypertension, CHD, CEVD, HF, and arrhythmia, respectively.
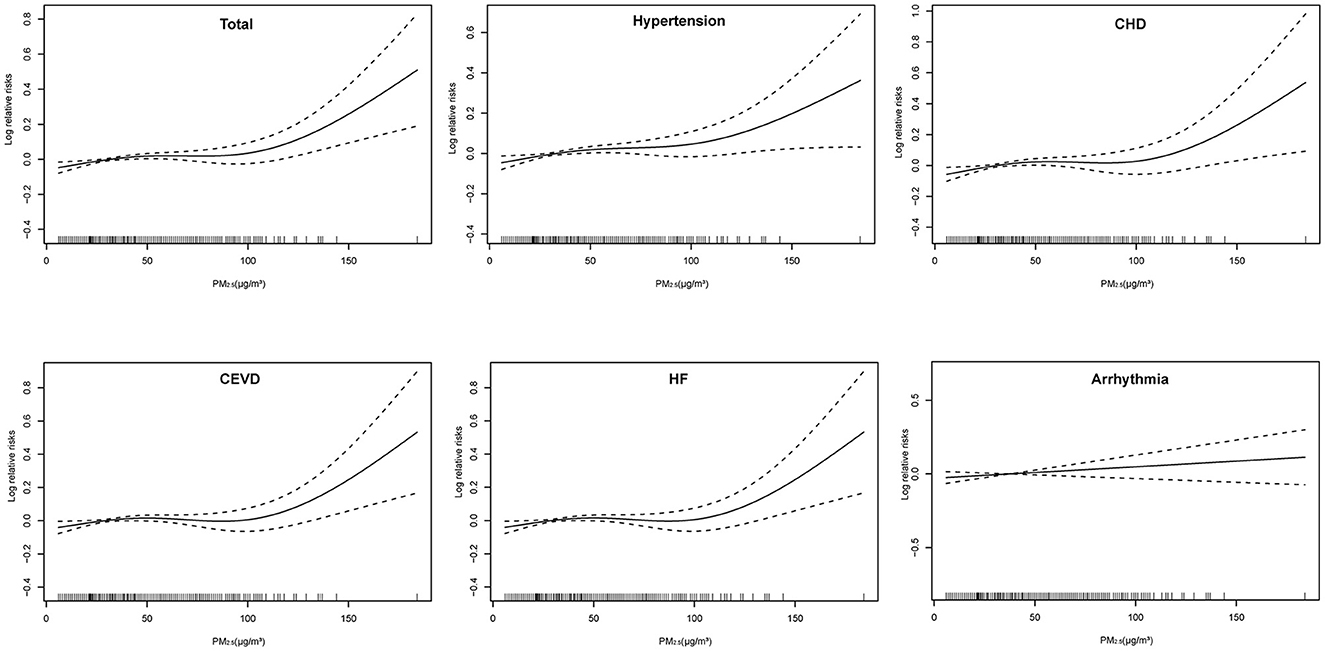
Figure 2. Exposure-response relationships of PM2.5 concentrations with daily hospital admissions for total and cause-specific circulatory system diseases. CEVD, Cerebrovascular Disease; CHD, Coronary Heart Disease; HF, Heart Failure. The concentration response curve is the solid line and the 95% CI is presented by the dotted line.
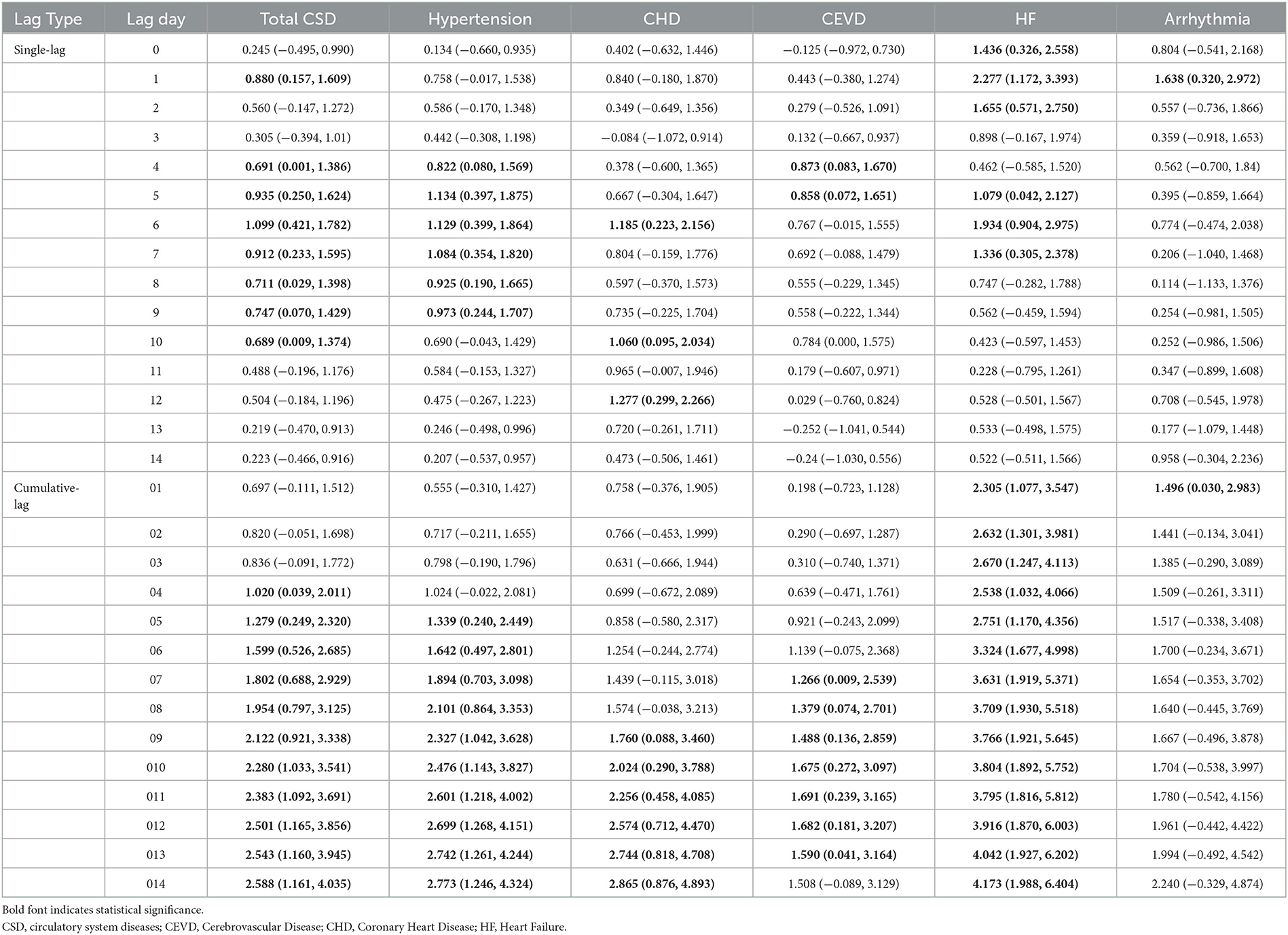
Table 2. Percent changes and 95% confidence intervals (CIs) of daily hospital admissions for total and cause-specific circulatory system diseases by each 10 μg/m3 increase of PM2.5 concentrations at different lag structures in the single-pollutant model.
The risk of hospital admissions seems to be higher in males for total CSD, CHD, and CEVD, and in females for hypertension, HF, and arrhythmia (Supplementary Figure 3 and Figure 3).
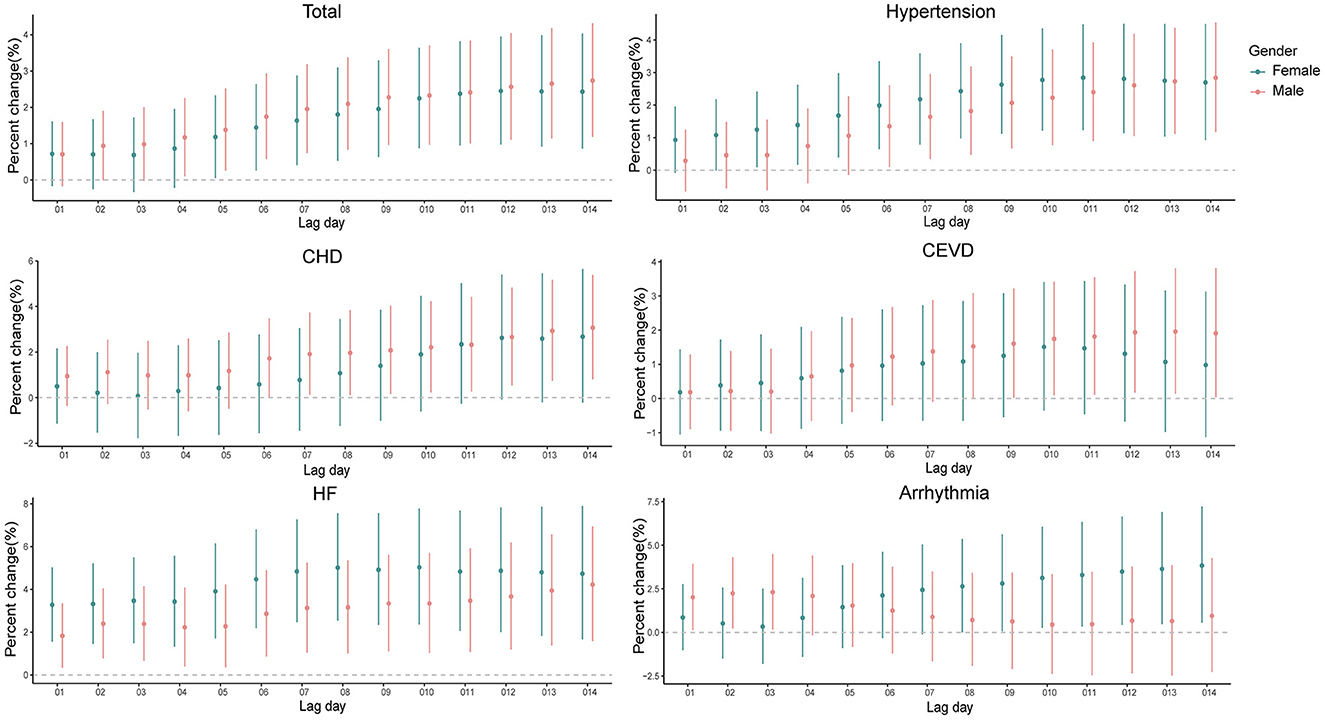
Figure 3. Percent changes and 95% confidence intervals (CIs) of daily hospital admissions for total and cause-specific circulatory system diseases by each 10 μg/m3 increase of PM2.5 concentrations stratified by gender at the cumulative-day lag models. HBP, hypertension; CHD, Coronary Heart Disease; CEVD, Cerebrovascular Disease; HF, Heart Failure.
When analyses were stratified by age (≤65 and >65 years old), the results were not materially changed. In the elderly (>65 years old), significantly positive associations were observed of PM2.5 exposure with all the outcomes of interest in present study (Supplementary Figure 4 and Figure 4), slightly different from those in the younger (≤65 years old). As for the young people, PM2.5 levels were significantly associated with hospitalizations for CSD, except for arrhythmia.
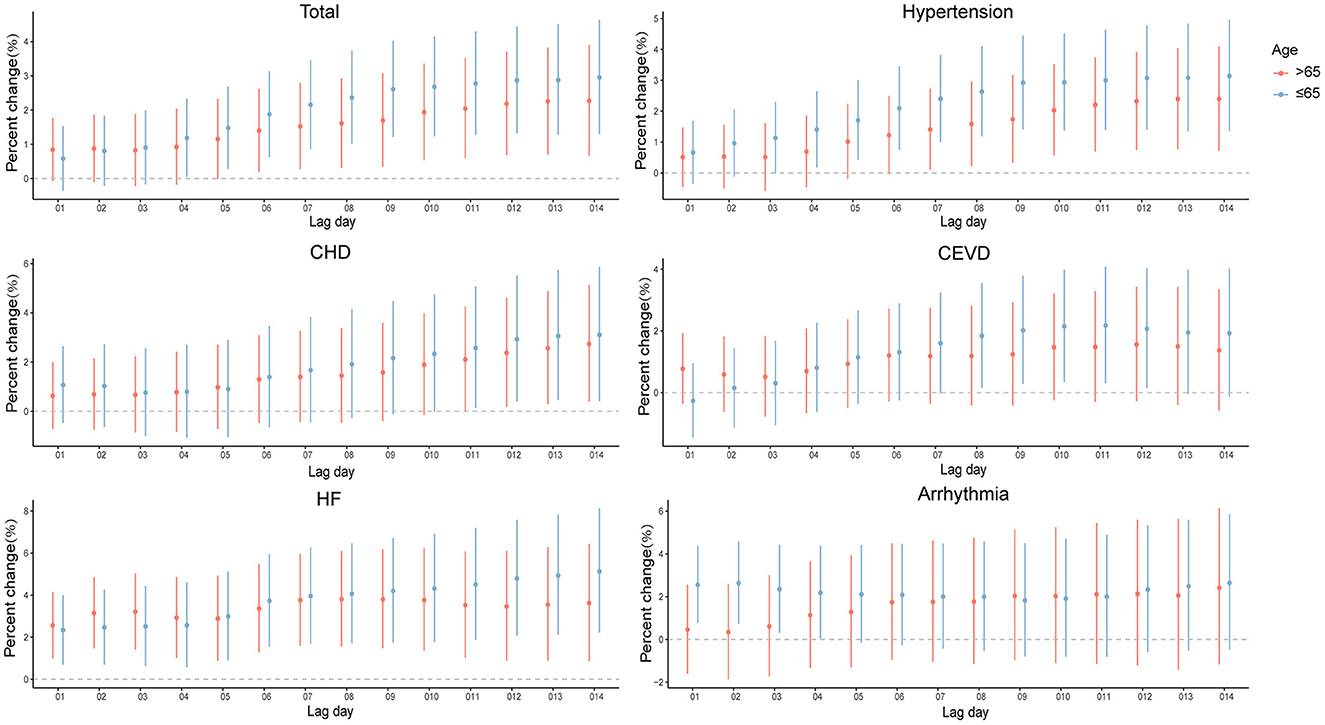
Figure 4. Percent changes and 95% confidence intervals (CIs) of daily hospital admissions for total and cause-specific circulatory system diseases by each 10 μg/m3 increase of PM2.5 concentrations stratified by age in the cumulative-day lag models. HBP, hypertension; CHD, Coronary Heart Disease; CEVD, Cerebrovascular Disease; HF, Heart Failure.
The impacts of PM2.5 on hospitalizations for CSD in cold seasons were stronger than those in warm seasons (Supplementary Figure 5 and Figure 5). In cold seasons, positive associations were observed in at least one exposure lag structure in present study, except for CHD. In warm seasons, hospitalizations for CHD and HF increased 2.618% (95% CI, 0.725–4.546%) and 2.769% (95% CI, 0.493–5.097%) per 10 μg/m3 elevation in PM2.5 levels, while there were no significant increases observed for total CSD, hypertension, CEVD, and arrhythmia.
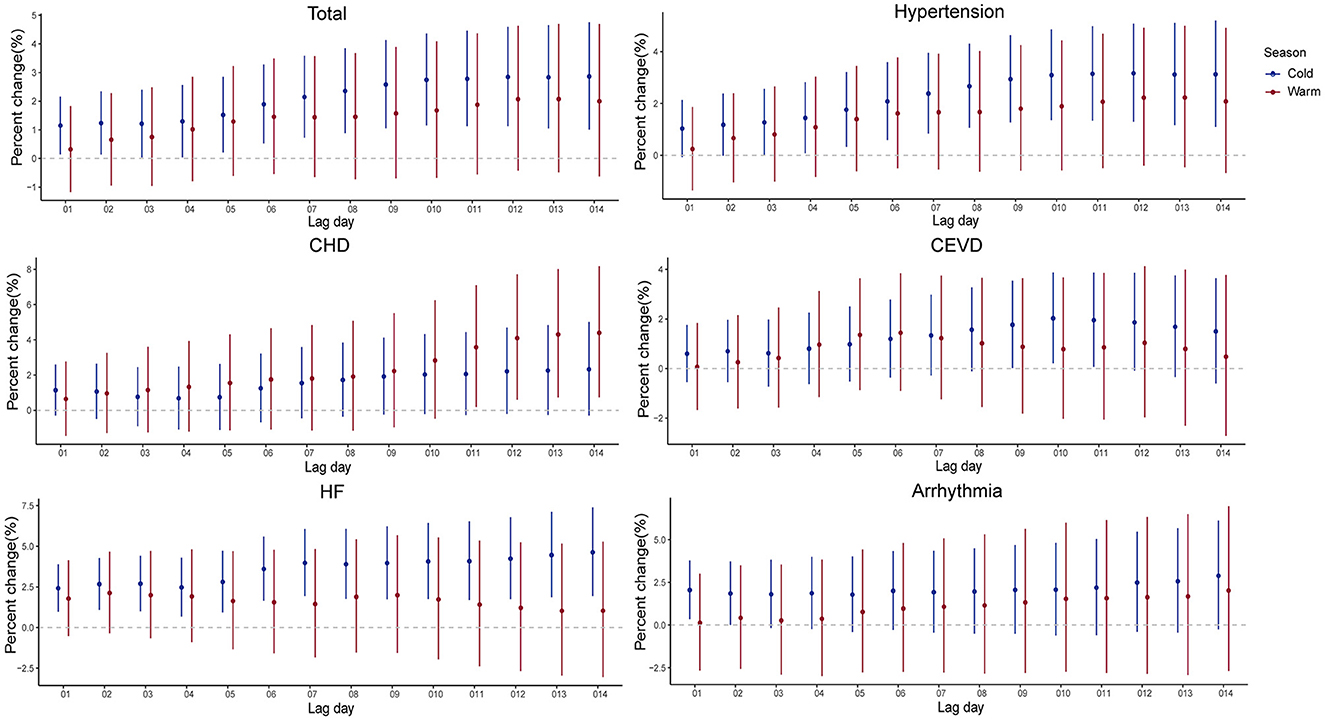
Figure 5. Percent changes and 95% confidence intervals (CIs) of daily hospital admissions for total and cause-specific circulatory system diseases by each 10 μg/m3 increase of PM2.5 concentrations stratified by season in the cumulative-day lag models. HBP, hypertension; CHD, Coronary Heart Disease; CEVD, Cerebrovascular Disease; HF, Heart Failure.
The results of co-pollutant models were shown in Table 3. The associations remained largely unchanged when additionally adjusted for CO or/and O3, and were similar with additionally adjusted for all major air pollutants. Moreover, the results were similar when we changed the df for secular time (Supplementary Tables 1, 2 and Supplementary Figures 6–11) and adjusted the effect of COVID-19 (Supplementary Table 3), which illustrated the robustness of our findings.
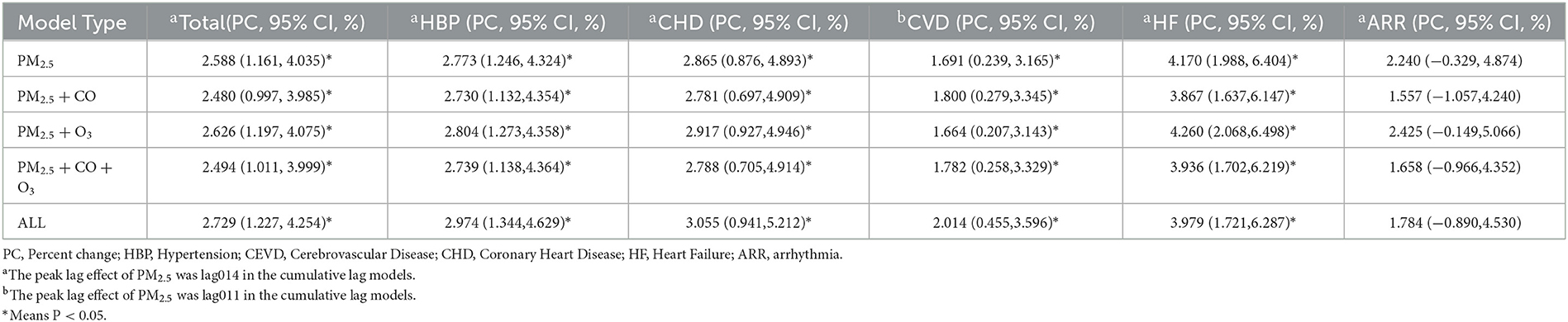
Table 3. Percent changes and 95% CIs of hospital admissions associated with every 10 μg/m3 increase of PM2.5 under co-pollutant models.
4. Discussion
In this study, we found that short-term exposure to PM2.5 was positively associated with hospitalizations for CSD, including total CSD, hypertension, CHD, CEVD, HF and arrhythmia, with significant lag effects. When analyses were stratified by age, gender, and season, there were no material changes of our findings, although the risk of hospitalizations seems to be higher among young people and cold seasons. Our results kept robust in the co-exposure models.
Though previous studies have reported relationships of PM2.5 with the risks of CSD, the results were largely inconsistent. Most studies found positive relationships of PM2.5 with increased risk of CSD. A multi-country time series study including 30 countries reported that every 10-μg/m3 elevation in PM2.5 concentrations was significantly relevant to a 0.12%, 0.42%, and 0.17% increase in cardiovascular diseases (CVD), acute myocardial infarction (AMI), and CHD on the same day (14). Furthermore, another study conducted in Beijing found that increasing PM2.5 levels were associated with hospitalizations for total CVD, CHD and atrial fibrillation (AF) (21). However, a few studies argued that there were no significantly associations of PM2.5 exposure with the risks of CSD (12, 15). Notably, the case data in above studies mainly came from government departments, which means that the data might be incomplete. Moreover, the confounding effect of time was not considered. Here, this time-series study based on more complete data were conducted and significantly positive associations of PM2.5 exposure with hospitalizations for CSD were observed, which was keeping with most studies.
The delayed effect of PM2.5 was also inconsistent in previous studies. Some studies across different countries showed that the impacts of PM2.5 on CSD mortality were peaked on the current day (22) and lag03 (13, 23, 24). A study in Kraków, Poland reported the delayed effects of PM2.5 levels on the risk of Myocardial Infarction (MI) admissions were observed at lag4 and lag6 (11). In our study, the earliest positive association between PM2.5 and hospitalizations for total CSD was at lag1 and peaked at lag6, as well as lag04 and lag014 in the cumulative-day lag model, which were longer than the hysteresis of other studies. The potential underlying reasons are as follows. First of all, the air quality of Ganzhou city is relatively good, and the average levels of PM2.5 from 2016 to 2020 was 37.38 μg/m3, which was significantly lower than that in other studies (21, 25). According to previous toxicological studies, PM2.5 exposure could lead to chronic systemic inflammation (26, 27), oxidative stress (28), stress hormone secretion (29–31) and vascular endothelial disfunction (32, 33), thereby causing to cardiovascular system damage. It takes several days from PM2.5 exposure to symptoms and hospitalization. Therefore, we speculated that exposure to higher levels of PM2.5 might have an acute effect on circulatory system health, while relatively low levels of PM2.5 tended to have delayed effects. Further studies are definitely needed to verify the hypothesis. Besides, in present study, hypertension and CHD accounted for relatively higher proportion of 47.0% and 14.2% in total CSD, respectively (Table 1). Worthy of note was that patients with hypertension and CHD are more inclined to self-medicate rather than to be hospitalized until their conditions worsen, which might be responsible for longer days' lag and underestimations of the impacts of PM2.5.
In this study, the cumulative-day lag model generally has higher estimates than the single-day lag model, with the greatest effects observed at lag 014. Similar results have been observed in other studies (34–36). The health effects of air pollutants usually last for several days, therefore, a cumulative lag model might be more accurate than a single-day lag model in assessing the health effects of air pollutants.
Our results regarding the positive associations of PM2.5 with CSD were correspondent with current mainstream understanding of the damage effects of PM2.5 on the circulatory system. Previous toxicological studies (28) have shown that PM2.5 is inhaled into lung through respiration, causing lung inflammation. The particles and inflammatory mediators released by alveolar macrophages could also enter the blood circulation system directly through the capillaries in the lungs and cause systemic inflammation and vascular endothelial dysfunction (37). These responses may underlie PM-induced circulatory system damage. PM2.5 could also activate the hypothalamic–pituitary–adrenal (HPA) axis, triggering an increase of stress hormones release, thereby causing vasoconstriction, increased blood pressure and a series of pathological reactions (29). However, full details regarding the biological mechanisms remain largely unclear and warrant further study.
The associations between PM2.5 and hospitalizations for CSD were not materially changed in different gender subgroups. The impacts of PM2.5 on the hospitalizations for arrhythmia seemed to be stronger in females compared with males, consistent with some studies (35–37), which might be attributable to more vulnerable biological systems of females.
In age subgroups, we found that younger people (≤65 years old) showed greater sensitivity to PM2.5 exposure in total CSD, hypertension, CHD, CEVD, and heart failure. In addition to air pollution, numerous factors such as occupational exposure (38), lifestyles (39), and even social status (40) and psychological factors (8) could also affect the health of circulatory system. Compared with the elderly, younger people tend to spend more time outdoors and are more vulnerable to harmful ambient hazards and occupational factors, such as industrial dust, chemicals, and noise (41), which can explain why younger people are more susceptible to PM2.5. Overall, more researches are warranted to probe the potential modifiers in age and sex on associations of PM2.5 exposure with the risks of CSD.
In our study, the impacts of PM2.5 on the CSD, except for CHD, dominated during the cold seasons. The differences in PM2.5 levels and compositions might be accounted for the seasonal variations in the relationships of PM2.5 exposure with hospitalizations for CSD. The mean levels of PM2.5 during cold seasons from 2016 to 2020 in Ganzhou is 43.62 μg/m3, significantly higher than that in warm seasons (31.22 μg/m3), shown in Supplementary Table 2. Furthermore, some constituents in PM2.5, including polycyclic aromatic hydrocarbons (PAHs) and metals, which were acknowledged to cause damage to the circulatory system (42, 43), increased significantly compared with warm seasons according to related studies (44, 45).
Profound elucidation for the exposure-response relationship is essential for public health policy formulation regarding the limit for PM2.5. In this study, the exposure-response curves were approximately linear with relatively steeper increases at higher concentrations of PM2.5 (>110 mg/m3 for HF, >100 mg/m3 for other circulatory system diseases). A series of previous studies also reported similar linear exposure-response relationships (21, 36, 46–50). For example, a study in Beijing found that the hospitalizations for ischemic stroke had a stable increase at lower concentrations (<100 μg/m3) and a steeper increment at higher concentrations of PM2.5 (46).
Notably, the average 24h concentration of PM2.5 in Ganzhou from 2016 to 2020 was 37 μg/m3, lower than current National Ambient Air Quality Standard (NAAQS) for PM2.5 (75 μg/m3) (51). However, significantly positive associations of PM2.5 and hospitalizations for CSD were still observed. Our findings were consistent with some studies. In a study of 200 Chinese cities (52), which included 58.52 million hospital admissions, the positive relationships of PM2.5 with hospitalizations were observed when the daily levels met the current NAAQS (75 μg/m3). Furthermore, a recent analysis of Europe (53) also revealed that long-term low levels of PM2.5 exposure was related to the morbidity of stroke and CHD. Additionally, a study in USA (54) also reported the deleterious effects of PM2.5 at levels below the specified limits. From the perspective of public health, our study suggests that more stringent PM2.5 standard limits than current NAAQS should be established to minimize the harmful effects of ambient PM2.5.
In the co-pollutant models, after adjusting CO or/and O3, and other major pollutants, the hospitalizations for CSD per 10-μg/m3 elevation of PM2.5 still significantly increased, indicating that the impacts of PM2.5 on the risks of CSD were robust, in keeping with most studies (21, 23, 55).
There are several strengths in the current study. Firstly, this study estimated the associations between PM2.5 levels and the risk of hospitalizations for CSD in Ganzhou for the first time. Besides, hospital admission data was selected as the effect indicator, which was more sensitive than mortality and has great public health implications.
However, several limitations should also be considered. First, using outdoor air pollution measured at outdoor fixed sited monitors as a proxy for individual exposure levels might lead to the misestimation of the exposure assessment. Secondly, our case data were only collected from one hospital in Ganzhou, which was inevitably to the ecological fallacy and the extrapolation of our research results were limited, to a certain extent. In addition, confounding factors such as smoking, alcohol consumption, occupation and education levels were not considered in the analysis due to lack of information. Finally, we were unable to evaluate the long-term influence of PM2.5 on the CSD under the time-series analysis design. Therefore, more well-designed studies are needed to explore the short-term and long-term impacts of PM2.5 exposure on the incidence of CSD in depth.
5. Conclusion
In this study, we found significantly positive associations of relatively low PM2.5 exposure with daily hospitalizations for total and cause-specific CSD in Ganzhou. And the associations varied in age, gender, and season subgroups. Our findings provide substantial insight regarding the effects of PM2.5 exposure on CSD, which may provide evidence of stricter limits on PM2.5 concentrations and help local policymakers to formulate or promulgate prevention policies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XY contributed to conception, the design of the study, data analysis, and wrote the first draft of the manuscript. XC contributed to data analysis and the design of the study. YG, DW, WQ, CZ, and MZ contributed to manuscript revision. WC and XZ contributed to conception, the design of the study, funding acquisition, and project administration. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Doctoral Fund of Gannan Medical University (No. QD201901) and Science and Technology Project Founded by the Education Department of Jiangxi Province (No. GJJ190786).
Acknowledgments
We sincerely appreciated all participants recruited in the study and the support from the study team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1134516/full#supplementary-material
References
1. Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. (2008) 151:362–7. doi: 10.1016/j.envpol.2007.06.012
2. Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Ahmadian Heris J, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. Bmj. (2022) 378:e069679. doi: 10.1136/bmj-2021-069679
3. Hankey S, Marshall JD. Urban form, air pollution, and health. Curr Environ Health Rep. (2017) 4:491–503. doi: 10.1007/s40572-017-0167-7
4. van den Elshout S, Léger K, Heich H. CAQI Common air quality index–update with PM(2.5) and sensitivity analysis. Sci Total Environ. (2014) 488–9:461–8. doi: 10.1016/j.scitotenv.2013.10.060
5. Liang CS, Duan FK, He KB, Ma YL. Review on recent progress in observations, source identifications and countermeasures of PM2.5. Environ Int. (2016) 86:150–70. doi: 10.1016/j.envint.2015.10.016
6. Feng S, Gao D, Liao F, Zhou F, Wang X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Safety. (2016) 128:67–74. doi: 10.1016/j.ecoenv.2016.01.030
7. Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis. (2016) 8:E69–74. doi: 10.3978/j.issn.2072-1439.2016.01.19
8. Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. (2018) 15:215–29. doi: 10.1038/nrcardio.2017.189
9. Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. Long-term PM2.5 exposure and neurological hospital admissions in the Northeastern United States. Environ Health Perspect. (2016) 124:23–9. doi: 10.1289/ehp.1408973
10. Rajagopalan S, Park B, Palanivel R, Vinayachandran V, Deiuliis JA, Gangwar RS, et al. Metabolic effects of air pollution exposure and reversibility. J Clin Invest. (2020) 130:6034–40. doi: 10.1172/JCI137315
11. Konduracka E, Niewiara Ł, Guzik B, Kotynia M, Szolc P, Gajos G, et al. Effect of short-term fluctuations in outdoor air pollution on the number of hospital admissions due to acute myocardial infarction among inhabitants of Kraków, Poland. Pol Arch Intern Med. (2019) 129:88–96. doi: 10.20452/pamw.4424
12. Moein N, Garakyaraghi M, Shafie D, Rabiei K, Hosseini SM, Jafari-Koshki T, et al. The association between particulate matter 2.5 and hospitalization and mortality rates of heart failure: The CAPACITY study. ARYA Atheroscler. (2019) 15:253–9. doi: 10.22122/arya.v15i6.1825
13. Sicard P, Khaniabadi YO, Perez S, Gualtieri M, De Marco A. Effect of O(3), PM(10) and PM(2.5) on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ Sci Pollut Res Int. (2019) 26:32645–65. doi: 10.1007/s11356-019-06445-8
14. Chen C, Zhu P, Lan L, Zhou L, Liu R, Sun Q, et al. Short-term exposures to PM(2.5) and cause-specific mortality of cardiovascular health in China. Environ Res. (2018) 161:188–94. doi: 10.1016/j.envres.2017.10.046
15. Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart. (2014) 100:1093–8. doi: 10.1136/heartjnl-2013-304963
16. Loxham M, Davies DE, Holgate ST. The health effects of fine particulate air pollution. Bmj. (2019) 367:l6609. doi: 10.1136/bmj.l6609
17. Tian Y, Liu H, Zhao Z, Xiang X, Li M, Juan J, et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. (2018) 15:e1002668. doi: 10.1371/journal.pmed.1002668
18. Wang L, Liu C, Meng X, Niu Y, Lin Z, Liu Y, et al. Associations between short-term exposure to ambient sulfur dioxide and increased cause-specific mortality in 272 Chinese cities. Environ Int. (2018) 117:33–9. doi: 10.1016/j.envint.2018.04.019
19. Yin P, Chen R, Wang L, Meng X, Liu C, Niu Y, et al. Ambient ozone pollution and daily mortality: a nationwide study in 272 Chinese cities. Environ Health Perspect. (2017) 125:117006. doi: 10.1289/EHP1849
20. Liu F, Liu F, Wang L. COVID-19 and cardiovascular diseases. J Mol Cell Biol. (2021) 13:161–7. doi: 10.1093/jmcb/mjaa064
21. Amsalu E, Wang T, Li H, Liu Y, Wang A, Liu X, et al. Acute effects of fine particulate matter (PM(2.5)) on hospital admissions for cardiovascular disease in Beijing, China: a time-series study. Environ Health. (2019) 18:70. doi: 10.1186/s12940-019-0506-2
22. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. (2015) 350:h1295. doi: 10.1136/bmj.h1295
23. Zhou X, Wang C, Chen P, Chen Y, Yin L, Du W, et al. Time series analysis of short-term effects of particulate matter pollution on the circulatory system disease mortality risk in Lishui District, China. Environ Sci Pollut Res Int. (2022) 29:17520–9. doi: 10.1007/s11356-021-17095-0
24. Kim H, Bell ML, Lee JT. Multi-dimensional community characteristics in linking particulate matter pollution and cause-specific mortality: 72 communities of South Korea. Environ Res. (2021) 196:110989. doi: 10.1016/j.envres.2021.110989
25. Guan M, Sun C, Tang D, Kang H, Chen F. A time-series analysis on the association between fine particulate matter and daily mortality - Shijiazhuang City, Hebei Province, China, 2015-2020. China CDC Wkly. (2022) 4:226–31.
26. Guan L, Geng X, Stone C, Cosky EEP Ji Y, Du H, et al. PM(2.5) exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ Toxicol. (2019) 34:530–8. doi: 10.1002/tox.22707
27. Yue W, Tong L, Liu X, Weng X, Chen X, Wang D, et al. Short term Pm2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox Biol. (2019) 22:101161. doi: 10.1016/j.redox.2019.101161
28. Zhao T, Qi W, Yang P, Yang L, Shi Y, Zhou L, et al. Mechanisms of cardiovascular toxicity induced by PM(2.5): a review. Environ Sci Pollut Res Int. (2021) 28:65033–51. doi: 10.1007/s11356-021-16735-9
29. Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. (2017) 136:618–27. doi: 10.1161/CIRCULATIONAHA.116.026796
30. Nääv Å, Erlandsson L, Isaxon C, Åsander Frostner E, Ehinger J, Sporre MK, et al. Urban PM2.5 Induces Cellular Toxicity, Hormone Dysregulation, Oxidative Damage, Inflammation, and Mitochondrial Interference in the HRT8 Trophoblast Cell Line. Front Endocrinol. (2020) 11:75. doi: 10.3389/fendo.2020.00075
31. Liu C, Yang J, Guan L, Zhu Y, Geng X. Filtered air intervention reduces inflammation and hypothalamus-pituitary-adrenal axis activation in adult male and female rats after PM 2.5 exposure. Environ Sci Pollut Res Int. (2020) 27:35341–8. doi: 10.1007/s11356-020-09564-9
32. Münzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, et al. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J. (2018) 39:3543–50. doi: 10.1093/eurheartj/ehy481
33. Liang S, Zhang J, Ning R, Du Z, Liu J, Batibawa JW, et al. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part Fibre Toxicol. (2020) 17:61. doi: 10.1186/s12989-020-00391-x
34. Qiu X, Wei Y, Wang Y, Di Q, Sofer T, Awad YA, et al. Inverse probability weighted distributed lag effects of short-term exposure to PM(2.5) and ozone on CVD hospitalizations in New England Medicare participants - Exploring the causal effects. Environ Res. (2020) 182:109095. doi: 10.1016/j.envres.2019.109095
35. Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou-Nielsen O. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J. (2010) 31:2034–40. doi: 10.1093/eurheartj/ehq188
36. Ren Z, Liu X, Liu T, Chen D, Jiao K, Wang X, et al. Effect of ambient fine particulates (PM(2.5)) on hospital admissions for respiratory and cardiovascular diseases in Wuhan, China. Respir Res. (2021) 22:128. doi: 10.1186/s12931-021-01731-x
37. Xie W, You J, Zhi C, Li L. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells J Appl Toxicol. (2021) 41:713–23. doi: 10.1002/jat.4138
38. Davis-Lameloise N, Philpot B, Janus ED, Versace VL, Laatikainen T, Vartiainen EA, et al. Occupational differences, cardiovascular risk factors and lifestyle habits in South Eastern rural Australia. BMC Public Health. (2013) 13:1090. doi: 10.1186/1471-2458-13-1090
39. Mechanick JI, Zhao S, Garvey WT. The adipokine-cardiovascular-lifestyle network: translation to clinical practice. J Am Coll Cardiol. (2016) 68:1785–803. doi: 10.1016/j.jacc.2016.06.072
40. Piedra LM, Andrade FCD, Hernandez R, Perreira KM, Gallo LC, González HM, et al. Association of subjective social status with life's simple 7s cardiovascular health index among hispanic/latino people: results from the HCHS/SOL. J Am Heart Assoc. (2021) 10:e012704. doi: 10.1161/JAHA.119.012704
41. Scarselli A, Corfiati M, Di Marzio D, Marinaccio A, Iavicoli S. Gender differences in occupational exposure to carcinogens among Italian workers. BMC Public Health. (2018) 18:413. doi: 10.1186/s12889-018-5332-x
42. Nickel NP, Yuan K, Dorfmuller P, Provencher S, Lai YC, Bonnet S, et al. Beyond the lungs: systemic manifestations of pulmonary arterial hypertension. Am J Respir Crit Care Med. (2020) 201:148–57. doi: 10.1164/rccm.201903-0656CI
43. Chowdhury R, Ramond A, O'Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. (2018) 362:k3310. doi: 10.1136/bmj.k3310
44. Ma WL, Sun DZ, Shen WG, Yang M, Qi H, Liu LY, et al. Atmospheric concentrations, sources and gas-particle partitioning of PAHs in Beijing after the 29th Olympic Games. Environ Pollut. (2011) 159:1794–801. doi: 10.1016/j.envpol.2011.03.025
45. Trusz A, Ghazal H, Piekarska K. Seasonal variability of chemical composition and mutagenic effect of organic PM2.5 pollutants collected in the urban area of Wrocław (Poland). Sci Total Environ. (2020) 733:138911. doi: 10.1016/j.scitotenv.2020.138911
46. Tian Y, Xiang X, Wu Y, Cao Y, Song J, Sun K, et al. Fine particulate air pollution and first hospital admissions for ischemic stroke in Beijing, China. Sci Rep. (2017) 7:3897. doi: 10.1038/s41598-017-04312-5
47. Lee S, Lee W, Kim D, Kim E, Myung W, Kim SY, et al. Short-term PM(2.5) exposure and emergency hospital admissions for mental disease. Environ Res. (2019) 171:313–20. doi: 10.1016/j.envres.2019.01.036
48. Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. (2006) 295:1127–34. doi: 10.1001/jama.295.10.1127
49. Li M, Wu Y, Tian YH, Cao YY, Song J, Huang Z, et al. Association between PM(2.5) and daily hospital admissions for heart failure: a time-series analysis in Beijing. Int J Environ Res Public Health. (2018) 15:2217. doi: 10.3390/ijerph15102217
50. Yao C, Wang Y, Williams C, Xu C, Kartsonaki C, Lin Y, et al. The association between high particulate matter pollution and daily cause-specific hospital admissions: a time-series study in Yichang, China. Environ Sci Pollut Res Int. (2020) 27:5240–50. doi: 10.1007/s11356-019-06734-2
51. Wu R, Song X, Bai Y, Chen J, Zhao Q, Liu S, et al. Are current Chinese national ambient air quality standards on 24-hour averages for particulate matter sufficient to protect public health? J Environ Sci. (2018) 71:67–75. doi: 10.1016/j.jes.2018.01.017
52. Tian Y, Liu H, Liang T, Xiang X, Li M, Juan J, et al. Fine particulate air pollution and adult hospital admissions in 200 Chinese cities: a time-series analysis. Int J Epidemiol. (2019) 48:1142–51. doi: 10.1093/ije/dyz106
53. Wolf K, Hoffmann B, Andersen ZJ, Atkinson RW, Bauwelinck M, Bellander T, et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. (2021) 5:e620–e32. doi: 10.1016/S2542-5196(21)00195-9
54. Makar M, Antonelli J, Di Q, Cutler D, Schwartz J, Dominici F. Estimating the causal effect of low levels of fine particulate matter on hospitalization. Epidemiology. (2017) 28:627–34. doi: 10.1097/EDE.0000000000000690
Keywords: circulatory system diseases, PM2.5, hospital admissions, air pollution, generalized additive model (GAM), time series study
Citation: You X, Cao X, Guo Y, Wang D, Qiu W, Zhou C, Zhou M, Chen W and Zhang X (2023) Associations between short-term PM2.5 exposure and daily hospital admissions for circulatory system diseases in Ganzhou, China: A time series study. Front. Public Health 11:1134516. doi: 10.3389/fpubh.2023.1134516
Received: 30 December 2022; Accepted: 20 February 2023;
Published: 09 March 2023.
Edited by:
Juliana Jalaludin, Universiti Putra Malaysia, MalaysiaReviewed by:
Zhijing Lin, Anhui Medical University, ChinaArthit Phosri, Mahidol University, Thailand
Copyright © 2023 You, Cao, Guo, Wang, Qiu, Zhou, Zhou, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Chen, d2NoZW5AbWFpbHMudGptdS5lZHUuY24=; Xiaokang Zhang, emhhbmd4aWFva2FqdUAxNjMuY29t
†These authors have contributed equally to this work
 Xiaojie You
Xiaojie You Xiuyu Cao
Xiuyu Cao You Guo
You Guo Dongming Wang
Dongming Wang Weihong Qiu
Weihong Qiu Chuanfei Zhou
Chuanfei Zhou Min Zhou
Min Zhou Weihong Chen
Weihong Chen Xiaokang Zhang
Xiaokang Zhang