- 1Department of Microbial, Cellular and Molecular Biology, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Ethiopian National Tuberculosis Reference Laboratory, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- 3Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
- 4Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates
Background: Homeless individuals are at a high risk of infection with Mycobacterium tuberculosis (M. tuberculosis) as compared to the general population. The number of homeless individuals has been increasing in Addis Ababa City during the last three decades due to the migration of rural inhabitants to the City for better living conditions. The objective of this study was to estimate the prevalence of pulmonary tuberculosis (PTB) and evaluate associated risk factors in homeless individuals in Addis Ababa City.
Methods: A total of 5,600 homeless individuals were screened for PTB symptoms using WHO guideline between February 2019 and December 2020. Sputum samples were cultured from individuals with symptoms of PTB for mycobacterial isolation. Logistic regression analysis was used to identify factors associated with PTB.
Results: The prevalence of bacteriologically confirmed cases was 1.1% (59/5,600) or 10.54 per 1000 population. Multinomial logistic regression analysis showed that being homeless for more than 5 years, body mass index (BMI) < 18.5, smoking cigarette, living in a group of more than five individuals, close contact with chronic coughers, imprisonment and HIV infection were significantly associated with the prevalence of PTB in homeless individuals (P < 0.05).
Conclusion: In conclusion, the result of this study indicated that the prevalence of PTB in homeless individuals was higher than the prevalence of PTB in the general population of Addis Ababa City requiring for the inclusion of the homeless individuals in the TB control program.
Background
Globally, tuberculosis (TB) is the 13th leading cause of death and the second leading infectious killer after COVID-19 (above HIV/AIDS) (1). Despite several efforts have been made in the fight against TB in the past decade, the COVID-19 pandemic overwhelmed health care system has affected TB case notification and treatment outcomes, setting back the achievements made in the fight against TB (2). In 2021, an estimated 10.6 million people fell ill with TB and 1.6 million died from the disease worldwide, an increase of 4.5% from 10.1 million TB cases in 2020. Similarly, the TB incidence rate increased by 3.6% between 2020 and 2021 because of the reduced attention given to TB control as the health sectors were overwhelmed with COVID-19 pandemic (3). The thirty high-burden TB countries accounted for 86% of new TB cases. Over 95% of reported TB cases and deaths are in low-income countries (3).
Ethiopia is one of the 30 countries with a high burden of TB, MDR-TB and TB-HIV co-infection in the world, and in 2021, the incidence of TB was estimate at 119 cases per 100,000 population in Ethiopia (3). Despite extensive global efforts to control TB, the disease is still largely affecting socially marginalized segment of the population, such as the homeless and those in other high-risk settings (4).
Homeless individuals are at a high risk of infection with M. tuberculosis and develop TB compared to the general population. This is not only due to their low-income status but to several risk factors like poverty, overcrowding, malnutrition, HIV infection, smoking, alcoholism, and drug abuse (5). Evidences indicated that TB is the third major cause of illness in homeless individuals, and it is estimated that homeless individuals are 10 to 85 times higher rate risk of developing latent or active TB infection as compared to the general population (6–8). The incidence of smear-positive PTB in homeless individuals in the cities of northern Ethiopian was 2.6% (9). Thus, if the TB control programs do not consider homeless individuals, the incidence of TB in this group will keep on increasing thereby posing a high risk to the general population.
The number of homeless individuals has been increasing in the Addis Ababa City during the last three decades due to the migration of rural inhabitants to the City in search of better living conditions (10). This increase in the number of homeless individuals in Addis Ababa has led to crowded living environments on the streets, which facilitates TB transmission. However, there is limited data on the TB situation in homeless individuals in Ethiopia including in the capital of the country. Therefore, this study conducted to estimate the prevalence of PTB and evaluate associated risk factors in homeless individuals in Addis Ababa, Ethiopia.
Materials and methods
Study setting
A cross-sectional study was conducted between February 2019 and December 2020 in Addis Ababa City, Ethiopia. Addis Ababa is the capital City of Ethiopia. Presently, the size of the its population is estimated to be 3,384,569, with an annual growth rate of 3.8% (11). The City is inhabited by diversified Ethiopian ethnic groups, and only 1% of the population of the City are foreigners. The City is fastest growing City in Africa (12) and constitutes 30% urban population of Ethiopia (11). It has the highest rural-urban migration that accounts for 40% of its population growth. Subsequently, there is an increase in the number of homeless individuals in the City. In order to support the number of homeless individuals, the City Administration established temporary shelters for homeless individuals while this study was being undertaken. Six temporary shelters were established in six sub cities including in Arada, Yeka, Bole, Akaki-Kality, Chirkos and Addis Ketema. These temporary shelters were used for the recruitment of the study participants.
Study participant enrollment
According to the European Typology of Homelessness and Housing Exclusion (ETHOS) (13), homeless are not a separate species of people, but they are by-products of the society on the margins of which they live. These people have no home or shelter to reside in and instead reside on the corners of streets, in parks, or in public places, if not provided a residence by the governmental or non-governmental organizations (14). Homeless individuals who registered for shelter accommodation in Addis Ababa City during the study period, had a history of having been homeless for at least 1 month prior to the study period (15), age 18 years and older, and gave their written informed consent were included in the study.
These homeless individuals were screened for PTB using the WHO guideline TB symptom screening standard document (16) between February 2019 and December 2020 by trained health professionals. The symptoms include cough ≥ 2 weeks, fever ≥ 2 weeks, night sweats ≥ 2 weeks, appetite loss ≥ 2 weeks, chest pain ≥ 2 weeks and weight loss ≥ 2 weeks. Each participant completed a questionnaire with the help of trained health professionals. The questionnaire included demographic data (age, gender, total duration of homelessness, previous residence, educational background, and marital status), TB risk behaviors (cigarette smoking, alcohol consumption, chewing khat [a plant contain amphetamine-like stimulants that has legally been used for centuries in East Africa and the Southern Arabian Peninsula. Fresh leaves of this plant contain more than 40 types of alkaloid compounds, of which cathinone and cathine are known stimulants. The stimulants cause excitement, loss of appetite and immune modulation on the users (17), drug abuse, HIV status, and malnutrition] and TB specific information (history and symptoms of TB, imprisonment, history of contact with chronically coughing individuals and history of contact with TB patients) (18). All symptoms were recorded as reported by the participants and assessed by another qualified health professional.
Case definitions
Presumptive TB cases: patients with cough ≥2 weeks, fever ≥ 2 weeks, night sweats, hemoptysis or weight loss (19). Pulmonary tuberculosis (PTB): a participant with pulmonary TB confirmed by Xpert, smear microscopy or clinically diagnosed according to the national (19) and WHO (20) TB guidelines. Bacteriologically confirmed TB case: a patient from whom at least one sputum was positive for M. tuberculosis by GeneXpert, smear microscopy or culture (19, 20). Clinically diagnosed TB case: a participant who did not meet the criteria for a bacteriologically confirmed case, but was diagnosed with TB by an experienced clinician and received a full course of TB treatment (19, 20).
Sputum sample collection
A total of 5,600 homeless individuals were screened for PTB symptoms using WHO TB symptoms screening guidelines (21). Of these, 641 were screening positive and provided sputum samples for bacteriological analysis. The participants were properly advised by trained health worker on how to produce a good sputum sample. Two sputum samples were collected from each participant (21). The first sample was collected on the spot and sent to the health facilities for Gene-Xpert MTB/RIF assay [a fully automated molecular diagnostic test for TB that simultaneously detects MTBC and resistance to rifampin (RIF) in < 2 h] (22), while the second sample was collected the next day in the early morning (morning sputum) and transported to the Aklilu Lemma Institute of Pathobiology (ALIPB) TB Laboratory, Addis Ababa University in a cold box and stored at 2–8°C until processed for mycobacteria isolation as previously described (23).
Mycobacterial isolation and identification
Sputum samples were digested and decontaminated by the modified Petroff method (24). The digested sputum samples were inoculated onto solid Lowenstein Jensen (LJ) medium. Bacterial growth was checked for contamination and fast growers in the first week. Contaminated cultures were recorded as contaminated. The cultures were observed on weekly basis and if there was no growth at the end of 8 weeks, the result was considered negative (25). The sputum samples with growth of M. tuberculosis were confirmed based on microscopic examination of acid-fast bacilli in a culture smear (26).
Anthropometric measurements
The weight and height of each study participant were measured by trained health professionals in order to estimate body mass index (BMI) of the study participants. A digital scale was used to measure the weight of each study participant, and weight was measured to the nearest 0.1 kg, while height was measured with the vertical measuring rod to the nearest 0.1 cm (27, 28).
Rapid HIV test
The HIV status of study participants was determined through the provision of pre-test counseling by trained health professionals. Briefly, a whole blood sample was collected by finger prick. The presence of antibodies against HIV-1 and HIV-2 was determined by using HIV antibody colloidal gold (1 + 2) rapid diagnostic kits (KHB, Shanghi Kehua Bio-engineering Co Ltd, China) as a screening test, followed by HIV½ STAT -PAK® (Chembio Diagnostics, USA), when the KBH result was reactive. When the STAT-PAK® result was discordant with KBH, a third test, Unigold TM HIV (Trinity Biotech, Ireland), was also used as a tiebreaker to determine the test result following the manufacturer's instructions. Finally, post-test counseling was provided to all participants (29).
Quality control
The questionnaire was prepared in English and translated into the local language (Amharic) and then back-translated into English by an expert fluent in both languages to maintain consistency. The questionnaire was pretested among 30 (5%) randomly selected homeless individuals before the actual data collection. The project supervisor and principal investigator strictly followed the data collection process day to day. Reagents and culture media were checked for sterility and performance characteristics in each batch of newly prepared reagent lots.
Data analysis
The data captured in the questionnaires were pooled, error-checked, cleaned, and entered into Microsoft Excel version 2013 for storage. Later the data were transferred to SPSS version 26 statistical software and analyzed. Descriptive statistics were used to summarize socio-demographic, behavioral, environmental factors and the morbidity history of the patients. BMI was used to assess nutritional status. BMI was calculated as = weight (in kilograms)/height (in meters). A BMI value of < 18.5 kg/m2 was considered underweight (27). Cross-tabulation was performed between the potential risk factors and prevalence of PTB for the determination of chi-square and p-values. Binomial and multinomial logistic regression analyses were performed to identify potential risk factors associated with PTB. In the multivariable logistic regression analysis, adjusted Odds Ratio (AOR) and 95% confidence interval (CI) were determined. P-value < 0.05 was considered statistically significant.
Ethical consideration
Ethical clearance prior to data collection was obtained from the Addis Ababa University, College of Natural and Computational Sciences Institutional Review Board (IRB) and Addis Ababa City Administration Health Bureau. Then, an official permission letter was obtained from Addis Ababa City Administration, Labor and Social Affairs Bureau. Written informed consent was obtained from all study participants after providing adequate information on the possible benefits and risks of the study in the local language (Amharic). Those participants who tested positive for TB and/or HIV infection were connected to health facilities in temporary shelters for treatment and follow-up. It is noteworthy that the decision to decline screening did not affect shelter access. Patient disease status was kept confidential through the use of anonymous personal identifiers.
Results
Socio-demographic characteristics of the study participants
The sociodemographic characteristics of the study participants is presented in Table 1. Majority of the participants (80.3%) were male and their mean ± SD age was 27.8 ± 9.5 years. Significant proportion of the participants addicted to smoking (82.1%) and alcohol (78.0%). Addictions to khat and drug were observed only in 1.1% and 1.0% (31/2,980) culture positive PTB cases.
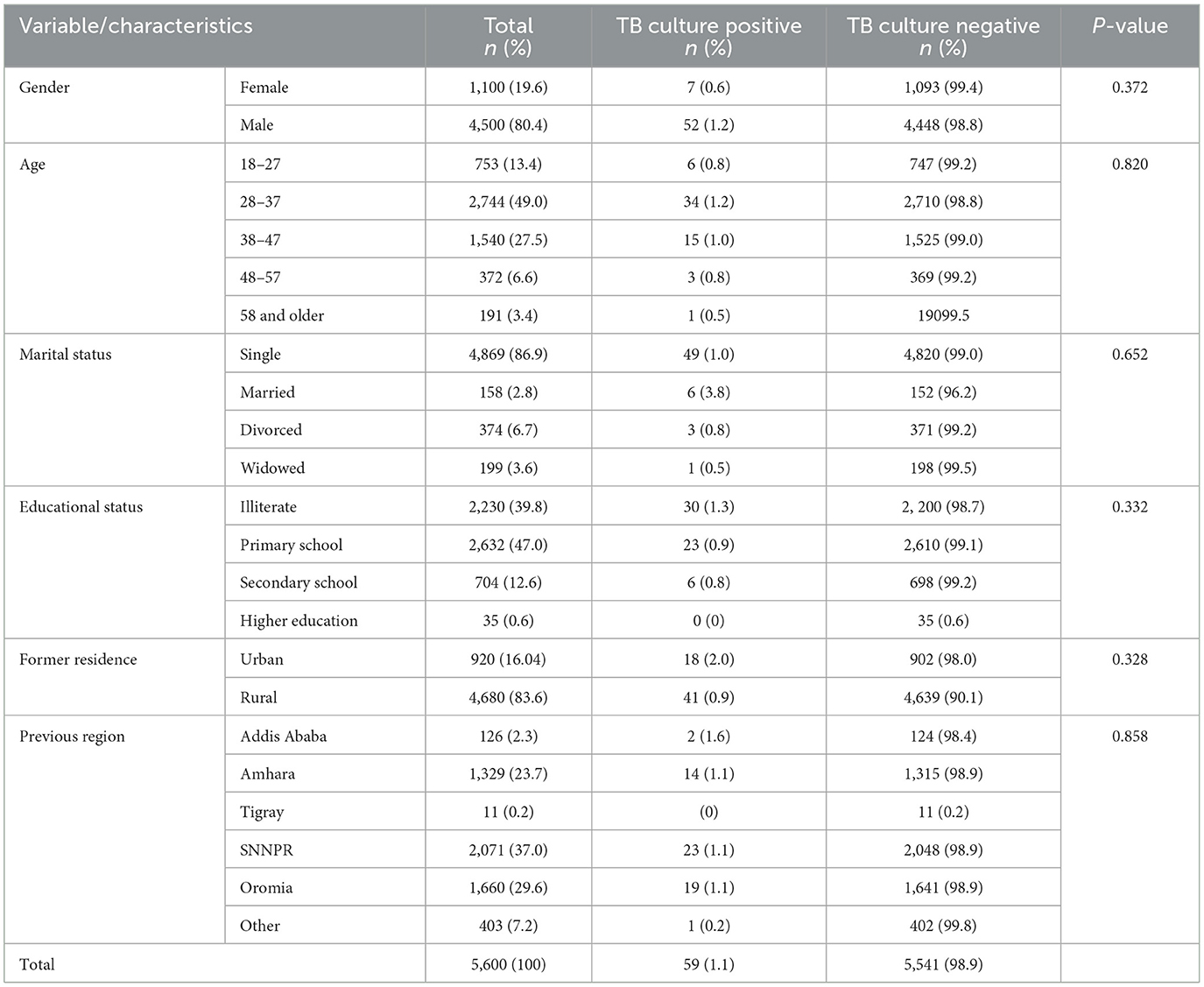
Table 1. Socio-demographic characteristics of homeless individuals (n = 5, 600) with bacteriological confirmed PTB prevalence, Addis Ababa, Ethiopia, 2021.
Prevalence of PTB among homeless individuals
GeneXpert and LJ culture were performed for 641 sputa samples of which 59 sputa were positive for PTB in both tests. The prevalence of bacteriological confirmed PTB in homeless individuals was 1.1% (59/5,600) or 10.54 per 1,000 population. The highest (1.53%) prevalence of PTB was recorded in Yeka subcity, while the lowest (0.39%) was in Bole sub city (Figure 1). Co-infection with HIV was observed in 32.2% (19/59) of bacteriologically confirmed TB cases (Table 1). The 582 homeless individuals with symptoms suggestive of TB who were bacteriologically negative were treated with antibiotics for pneumonia for 2 weeks and all were recovered.
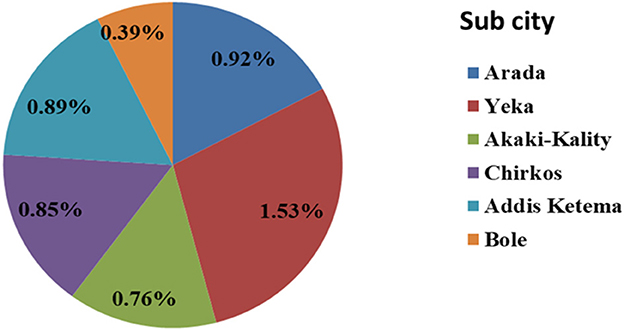
Figure 1. Prevalence of bacteriological confirmed PTB among homeless individuals in sub cities of Addis Ababa, Addis Ababa, Ethiopia, 2021.
Factors associated with the occurrence of PTB among homeless individuals
All variables significantly associated with the prevalence of bacteriological confirmed PTB in the binary logistic regression analysis remained significant in the multivariable logistic regression analysis except alcohol consumption and drug abuse. Accordingly, being homeless for more than 5 years [AOR = 3.99, 95% CI: 1.31, 11.59], BMI < 18.5 [AOR = 3.20, 95% CI : 1.68, 6.18], smoking cigarette[AOR = 5.71, 95% CI: 1.29, 25.28], living or sleeping in a restricted area with more than five individuals [AOR = 7.82 , 95% CI: 1.02, 60.16], close contact with chronically coughers [AOR = 3.53, 95% CI: 1.14, 10.91], history of imprisonment (AOR = 2.26, 95% CI: 1.08,5.89) and HIV infection [AOR = 2.34 , 95% CI: 1.17, 4.71] were significantly associated with the prevalence of PTB (P < 0.05) (Table 2).
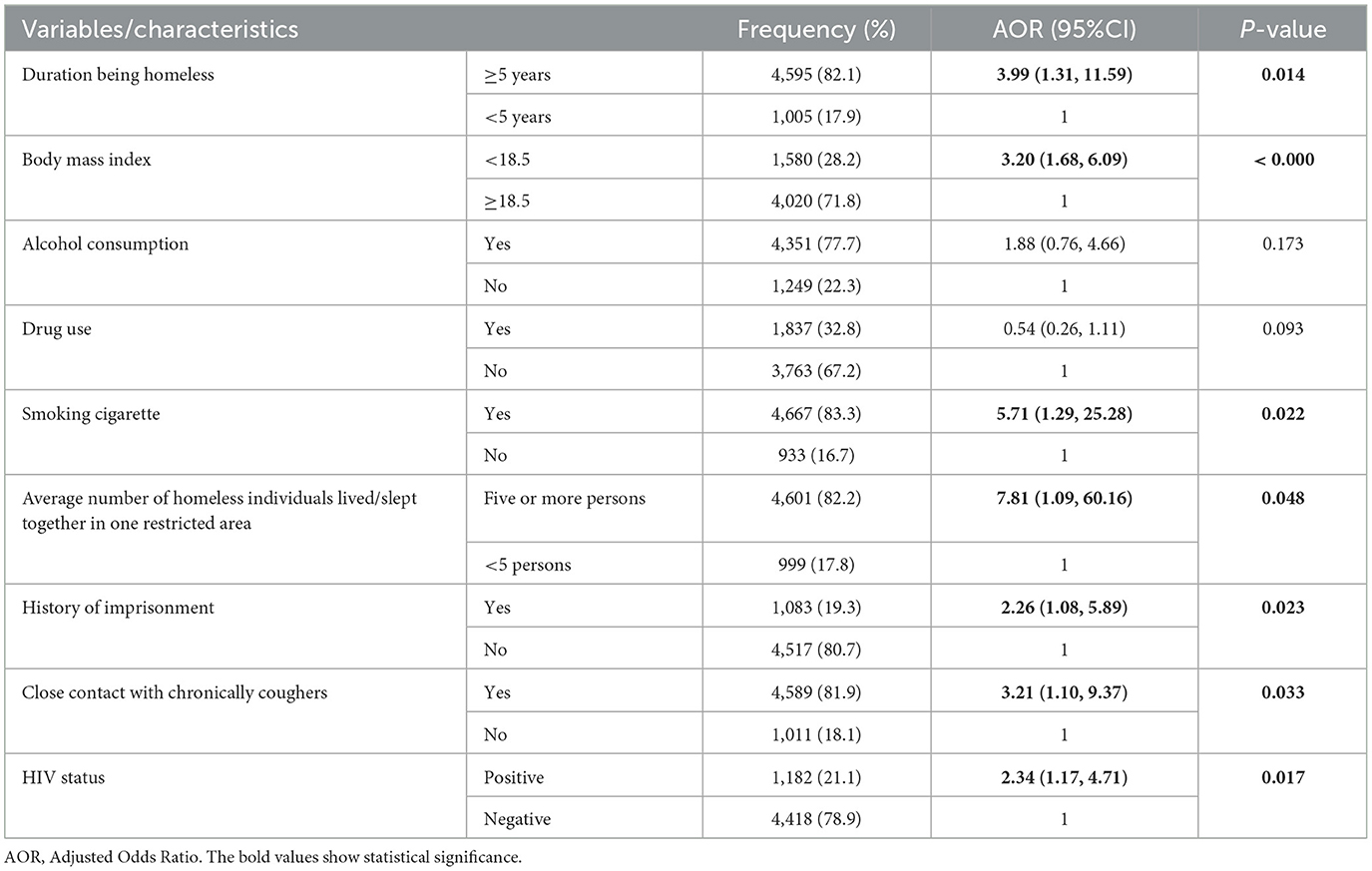
Table 2. Factors associated with bacteriological confirmed PTB among homeless individuals (n = 5,600), Addis Ababa, Ethiopia, 2021.
Discussion
The current cross-sectional study was conducted in Addis Ababa, Ethiopia, to investigate the prevalence of PTB and evaluate associated risk factors in homeless individuals. This study is the first study to be conducted in Addis Ababa, the capital of Ethiopia, where the number of homeless individuals is estimated to be high due to the highest rural-urban migration.
Over 80.3% of the homeless individuals were males, which is consistent with the observation of previous similar studies in Brazil (30) and Ethiopia (9). Furthermore, majority of the homeless individuals in the present study were young and TB was observed in this young group of individuals, which is also in agreement with the reports of earlier studies (31, 32). This observation could be due to the socio-economic crisis of low-income countries in which parents in the urban and rural areas could not satisfy the basic needs and demands of their children that leads to migration of the children to the street for search of their needs. This leads to a significant increase in the number of young homeless individuals and high prevalence of TB in this young group (9). Regarding the gender difference, there are different reasons which contribute to higher percentage of males in homeless individuals in Addis Ababa. One reason sociocultural influence in which restricts the females leave their homes to live on the street. Even though the females face significant socioeconomic problems in their parents' homes, it is not easy for female youths to leave their home and to live on the street. The other contributing factor is that female youths have better chances of employment as the majority of them are absorbed as maids in individuals' homes either in the Ethiopia or abroad in the Middle East countries. In addition, most of the cafeterias, bars, and cafes prefer to recruit female youths than male youths.
According to the result of this study, the prevalence of bacteriological confirmed PTB in the homeless individuals was higher than the national prevalence of TB in the general population of Ethiopia (33). Similarly, several studies (32, 34–36) showed that the prevalence of TB in homeless individuals could be 10 times higher than its prevalence in the general population. For example, earlier studies reported prevalence values, which are similar a prevalence value from Marseille (5), Iran (37) and Japan (34). But other studies reported lower prevalence values than the prevalence recorded in this study from London (38), USA (39), India (40) and even from Ethiopia (9). Comparative evaluation of prevalence values of TB in Ethiopia indicated that the current prevalence was nine times higher than that of the general population of Ethiopia (3, 41) and four times higher than that of the general population of the Addis Ababa City (42) which was 0.67% (43). This could be due to the fact that homeless individuals are neglected section of the population for basic medical care and even have less chance of obtaining basic human needs, such as finding shelter and food, over getting medical care (44). With regard to previous origin of the homeless individuals there was no significant variation in prevalence of bacteriological confirmed PTB. Based on the current residence, majority of the bacteriological confirmed PTB cases were found in the Yeka sub city. This may be due to enrollment of large number of homeless individuals in this sub city.
In consistent with the observation of this study, previous studies conducted on homeless individuals in Colombia (45, 46), Poland (47), USA (48), Rome (49) and South Korea (50) reported higher prevalence of TB in homeless individuals than in the general populations of their respective cities. Similarly, a systematic review and meta-analysis of the prevalence of TB in the homeless individuals recorded a range prevalence values that overlaps with the prevalence value recorded by the present study (51, 52). Such higher prevalence of TB in homeless individuals could linked to risk factors, such as poverty, overcrowding, malnutrition, HIV-AIDS, smoking, alcoholism, and drug abuse (1).
The significant association of the various risk factors with the prevalence of bacteriological confirmed PTB could be explained by overcrowding and delayed diagnosis (53). Low BMI and smoking cigarettes were significantly associated with bacteriological confirmed PTB and this observation agrees with studies conducted in Korea (50) and Rome (54). Previous studies indicated that increase in susceptibility to new infection or reactivation of latent TB infection is associated with malnutrition and smoking (36, 49, 54). Similarly, the association between TB-HIV co-infection has been well documented in the USA (28) and Canada (38) which could also be due to increased susceptibility to new infection and or reactivation of latent TB infection because of immunosuppression by HIV.
Limitations of the study
A chest X-ray was not performed for screening participants with suspected TB; this may have underestimated the true prevalence of PTB. Extrapulomnary tuberculosis was not included in the study due to time and logistic limitations.
Conclusion
The prevalence of bacteriological confirmed PTB among homeless individuals in Addis Ababa, Ethiopia was nine times higher than its prevalence in the general population of Ethiopia and four times higher than in the general population of Addis Ababa City. This finding suggests that homeless congregations may be hotspots for TB transmission. Therefore, the TB control program should incorporate homeless individuals in the TB prevention and control program
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Addis Ababa University, College of Natural and Computational Sciences Institutional Review Board (IRB) and Addis Ababa City Administration Health Bureau. The patients/participants provided their written informed consent to participate in this study.
Author contributions
TS participated in the conception, design, acquisition of the data, statistical analysis, interpretation of the data, and drafting the manuscript. SM participated in data acquisition and critical edition of the manuscript. BG and MG participated in the analysis, interpretation of the data, and edition of the manuscript. BP and GA contributed in the design, guiding the data collection, interpretation of the result, and edition of the manuscript. All authors approved the manuscript for publication and agreed to be accountable for all aspects of the work done.
Funding
The study was funded by the Addis Ababa University.
Acknowledgments
This study was conducted under Department of Microbial, Cellular, and Molecular Biology, Faculty of Natural and Computational Sciences, Addis Ababa University. The authors wish to thank the homeless individuals for their cooperation and willingness to participate in the study. We thank the data collectors involved in this study. We would also be grateful to the Addis Ababa City Administration Labor and Social Affairs Bureau and Health Bureau.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global tuberculosis report 2020–reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113:S7–12. doi: 10.1016/j.ijid.2021.02.107
4. Dememew ZG, Jerene D, Datiko DG, Hiruy N, Tadesse A, Moile T, et al. The yield of community-based tuberculosis and HIV among key populations in hotspot settings of Ethiopia: a cross-sectional implementation study. PLoS One. (2020) 15:e0233730. doi: 10.1371/journal.pone.0233730
5. Amiri FB, Doosti-Irani A, Sedaghat A, Fahimfar N, Mostafavi E. Knowledge, attitude, and practices regarding HIV and TB among homeless people in Tehran, Iran. Int J Health Policy Manag. (2018) 6:549. doi: 10.15171/ijhpm.2017.129
6. Scholze AR, Alves JD, Berra TZ, Ramos ACV, Pieri FM, Pillon SC, et al. Tuberculosis among people living on the street and using alcohol, tobacco, and illegal drugs: analysis of territories in extreme vulnerability and trends in Southern Brazil. Int J Environ Res Public Health. (2022) 19:7721. doi: 10.3390/ijerph19137721
7. Tankimovich M. Barriers to and interventions for improved tuberculosis detection and treatment among homeless and immigrant populations: a literature review. J Commun Health Nurs. (2013) 30:83–95. doi: 10.1080/07370016.2013.778723
8. Ranzani OT, Carvalho CR, Waldman EA, Rodrigues LC. The impact of being homeless on the unsuccessful outcome of treatment of pulmonary TB in São Paulo State, Brazil. BMC Med. (2016) 14:1–3. doi: 10.1186/s12916-016-0584-8
9. Semunigus T, Tessema B, Eshetie S, Moges F. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in Dessie and Debre Birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob. (2016) 15:1–8. doi: 10.1186/s12941-016-0165-x
10. Merga M, Anteab K, Sintayehu M, Bayu H. Challenges in decision making among homeless pregnant teens in Addis Ababa, Ethiopia: a descriptive phenomenological study. J Preg Child Health. (2015) 2:140.
11. Haile K, Umer H, Fanta T, Birhanu A, Fejo E, Tilahun Y, et al. Pathways through homelessness among women in Addis Ababa, Ethiopia: a qualitative study. PLoS ONE. (2020) 15:e0238571. doi: 10.1371/journal.pone.0238571
12. Adam AG. Understanding competing and conflicting interests for peri-urban land in Ethiopia's era of urbanization. Environ Urban. (2020) 32:55–68. doi: 10.1177/0956247819890215
13. Amore K, Baker M, Howden-Chapman P. The ETHOS definition and classification of homelessness: an analysis. Eur J Homelessness. (2011) 5:19–37.
14. WHO. Working With Street Children: A Training Package on Substance Abuse, Sexual and Reproductive Health Including HIV/AIDS and STDs. Geneva: World Health Organization (2000).
15. Aldridge RW, Story A, Hwang SW, Nordentoft M, Luchenski SA, Hartwell G, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. The Lancet. (2018) 391:241–50. doi: 10.1016/S0140-6736(17)31869-X
16. Maher D, Grzemska M, Coninx R, Reyes H. Guidelines for the Control of Tuberculosis in Prisons. Geneva: World Health Organization (1998).
17. Soboka, M., Tolessa, O., Tesfaye, M., Adorjan, K., Krahl, W., Tesfaye, E. Magnitude and predictors of khat use among patients with tuberculosis in Southwest Ethiopia: A longitudinal study. PloS ONE. (2020) 15:e0236154 doi: 10.1371/journal.pone.0236154
19. Mussie KM, Gradmann C, Manyazewal T. Bridging the gap between policy and practice: a qualitative analysis of providers' field experiences tinkering with directly observed therapy in patients with drug-resistant tuberculosis in Addis Ababa, Ethiopia. BMJ Open. (2020) 10:e035272. doi: 10.1136/bmjopen-2019-035272
20. WHO. Definitions and Reporting Framework for Tuberculosis−2013 Revision: Updated December 2014 and January 2020. Geneva: World Health Organization (2014).
21. Mehay A, Raj T, Altass L, Newton A, O'Moore E, Railton C, et al. Frater A. An audit of tuberculosis health services in prisons and immigration removal centres. J Public Health. (2017) 39:387–94. doi: 10.1093/pubmed/fdw033
22. Piatek AS, Van Cleeff M, Alexander H, Coggin WL, Rehr M, Van Kampen S. GeneXpert for TB diagnosis: planned and purposeful implementation. Global Health: Sci Practice. (2013) 1:18–23. doi: 10.9745/GHSP-D-12-00004
24. Kent PT. Public Health Mycobacteriology: A Guide for the Level III Laboratory. US Department of Health and Human Services, Public Health Service. Washington, DC: CDC. (1985).
25. Narvaiz I, Kim KS, Frieden T, Laszlo A, Luelmo F, Norval PY, et al. Laboratory Services in Tuberculosis Control. Geneva: World Health Organization (1998).
26. Lagier JC, Edouard S, Pagnier I, Mediannikov O, Drancourt M, Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev. (2015) 28:208–36. doi: 10.1128/CMR.00110-14
27. Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the national health examination and nutrition examination survey (NHANES) 1999–2002. J Gen Intern Med. (2007) 22:1018–23. doi: 10.1007/s11606-007-0192-6
28. Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LÅ, Rasmussen KM. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin. (2008) 87:1852–9. doi: 10.1093/ajcn/87.6.1852
29. Constantine NT. and Zink, H. HIV testing technologies after two decades of evolution. Indian J Med Res. (2005) 121:519–38.
30. Santos ACED, Brunfentrinker C, Pena LDS, Saraiva SDS, Boing AF. Analysis and comparison of tuberculosis treatment outcomes in the homeless population and in the general population of Brazil. J Brasileiro de Pneumol. (2021) 47:178. doi: 10.36416/1806-3756/e20200178
31. Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. (2012) 18:458–65. doi: 10.3201/eid1803.111107
32. Bamrah S, Yelk Woodruff RS, Powell K, Ghosh S, Kammerer JS, Haddad MB. Tuberculosis among the homeless, United States, 1994–2010. I J Tuberc Lung Dis. (2013) 17: 1414–9. doi: 10.5588/ijtld.13.0270
33. Kebede AH, Alebachew Z, Tsegaye F, Lemma E, Abebe A, Agonafir M, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010-2011. Int J Tuberc Lung Dis. (2014) 18:635–9. doi: 10.5588/ijtld.13.0417
34. Tabuchi T, Takatorige T, Hirayama Y, Nakata N, Harihara S, Shimouchi A, et al. Tuberculosis infection among homeless persons and caregivers in a high-tuberculosis-prevalence area in Japan: a cross-sectional study. BMC Infect Dis. (2011) 11:1–8. doi: 10.1186/1471-2334-11-22
35. Johnston D, McInerney P, Miot J, A. profile of the health of homeless at an inner city primary health care clinic in South Africa. J Health Care Poor Underserved. (2019) 30:1455–66. doi: 10.1353/hpu.2019.0093
36. Story A, Murad S, Roberts W, Verheyen M, Hayward AC. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. (2007) 62:667–71. doi: 10.1136/thx.2006.065409
37. Habtamu D, Adamu A. Assessment of sexual and reproductive health status of street children in Addis Ababa. J Sex Transm Dis. (2013) 2013:1–20. doi: 10.1155/2013/524076
38. Moss AR, Hahn JA, Tulsky JP, Daley CL, Small PM, Hopewell PC. Tuberculosis in the homeless: a prospective study. Am J Respir Crit. (2000) 162:460–4. doi: 10.1164/ajrccm.162.2.9910055
40. Dolla C, Padmapriyadarsini C, Pradeep Menon A, Muniyandi M, Adinarayanan S, Sekar G, et al. Tuberculosis among the homeless in Chennai city, South India. T Roy Soc Trop Med H. (2017) 111:479–81. doi: 10.1093/trstmh/trx081
41. Alene KA, Clements AC. Spatial clustering of notified tuberculosis in Ethiopia: a nationwide study. PLoS ONE. (2019) 14:e0221027. doi: 10.1371/journal.pone.0221027
42. Omerov P, Craftman ÅG, Mattsson E, Klarare A. Homeless persons' experiences of health-and social care: a systematic integrative review. Health Soc Care Commun. (2020) 28:1–1. doi: 10.1111/hsc.12857
43. Alene KA, Elagali A, Barth DD, Rumisha SF, Amratia P, Weiss DJ, et al. Spatial co-distribution of HIV, tuberculosis and malaria in Ethiopia. BMJ Global Health. (2022) 7:e007599. doi: 10.1136/bmjgh-2021-007599
44. Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. (2013) 2013:1–11. doi: 10.1155/2013/828939
45. Haddad MB, Wilson TW, Ijaz K, Marks SM, Moore M. Tuberculosis and homelessness in the United States, 1994-2003. JAMA. (2005) 293:2762–6. doi: 10.1001/jama.293.22.2762
46. Hernández Sarmiento JM, Correa N, Correa M, Franco JG, Alvarez M, Ramírez C, et al. Tuberculosis among homeless population from Medellín, Colombia: associated mental disorders and socio-demographic characteristics. J Immigr Minor Health. (2013) 15:693–9. doi: 10.1007/s10903-013-9776-x
47. Romaszko J, Buciński A, Kuchta R, Bednarski K, Zakrzewska M. The incidence of pulmonary tuberculosis among the homeless in north-eastern Poland. Open Med. (2013) 8:283–5. doi: 10.2478/s11536-012-0114-9
48. Notaro SJ, Khan M, Kim C, Nasaruddin M, Desai K. Analysis of the health status of the homeless clients utilizing a free clinic. J Commun Health. (2013) 38:172–7. doi: 10.1007/s10900-012-9598-0
49. Laurenti P, Bruno S, Quaranta G, La Torre G, Cairo AG, Nardella P, et al. Tuberculosis in sheltered homeless population of Rome: an integrated model of recruitment for risk management. Sci World J. (2012) 2012:1–7. doi: 10.1100/2012/396302
50. Jia X, Yang L, Dong M, Chen S, Lv L, Cao D, et al. The bioinformatics analysis of comparative genomics of Mycobacterium tuberculosis complex (MTBC) provides insight into dissimilarities between intraspecific groups differing in host association, virulence, and epitope diversity. Front Cell Infect Microbiol. (2017) 7:88. doi: 10.3389/fcimb.2017.00088
51. Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12:859–70. doi: 10.1016/S1473-3099(12)70177-9
52. Alecrim TFDA, Mitano F, Reis AAD, Roos CM, Palha PF, Protti-Zanatta ST. Experience of health professionals in care of the homeless population with tuberculosis. Rev Escola Enfermagem da USP. (2016) 50:808–15. doi: 10.1590/s0080-623420160000600014
53. Kim HJ, Lee CH, Shin S, Lee JH, Kim YW, Chung HS, et al. The impact of nutritional deficit on mortality of in-patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. (2010) 14:79–85.
Keywords: Addis Ababa City, homeless individuals, prevalence, pulmonary tuberculosis, risk factor
Citation: Shamebo T, Mekesha S, Getahun M, Gumi B, Petros B and Ameni G (2023) Prevalence of pulmonary tuberculosis in homeless individuals in the Addis Ababa City, Ethiopia. Front. Public Health 11:1128525. doi: 10.3389/fpubh.2023.1128525
Received: 20 December 2022; Accepted: 27 March 2023;
Published: 06 April 2023.
Edited by:
Raquel Duarte Bessa De Melo, University of Porto, PortugalReviewed by:
Prakash Doke, Bharati Vidyapeeth Deemed University, IndiaJerzy Romaszko, University of Warmia and Mazury in Olsztyn, Poland
Copyright © 2023 Shamebo, Mekesha, Getahun, Gumi, Petros and Ameni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gobena Ameni, Z29iZW5hLmFtZW5pQHVhZXUuYWMuYWU=; Z29iZW5hLmFtZW5pQGFhdS5lZHUuZXQ=
 Tsegaye Shamebo
Tsegaye Shamebo Sindew Mekesha2
Sindew Mekesha2 Muluwork Getahun
Muluwork Getahun Balako Gumi
Balako Gumi Gobena Ameni
Gobena Ameni