
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 22 February 2023
Sec. Clinical Diabetes
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1128069
This article is part of the Research Topic Early Detection and Combination Therapy for Diabetic Peripheral Neuropathy: What does the Future Look Like? View all 6 articles
 Xixi Liu1†
Xixi Liu1† Dong Chen2†
Dong Chen2† Hongmin Fu1
Hongmin Fu1 Xinbang Liu1
Xinbang Liu1 Qiumei Zhang1
Qiumei Zhang1 Jingyun Zhang1
Jingyun Zhang1 Min Ding1
Min Ding1 Juanjuan Wen1
Juanjuan Wen1 Bai Chang2*
Bai Chang2*Background: Early identification and intervention of diabetic peripheral neuropathy is beneficial to improve clinical outcome.
Objective: To establish a risk prediction model for diabetic peripheral neuropathy (DPN) in patients with type 2 diabetes mellitus (T2DM).
Methods: The derivation cohort was from a meta-analysis. Risk factors and the corresponding risk ratio (RR) were extracted. Only risk factors with statistical significance were included in the model and were scored by their weightings. An external cohort were used to validate this model. The outcome was the occurrence of DPN.
Results: A total of 95,604 patients with T2DM from 18 cohorts were included. Age, smoking, body mass index, duration of diabetes, hemoglobin A1c, low HDL-c, high triglyceride, hypertension, diabetic retinopathy, diabetic kidney disease, and cardiovascular disease were enrolled in the final model. The highest score was 52.0. The median follow-up of validation cohort was 4.29 years. The optimal cut-off point was 17.0, with a sensitivity of 0.846 and a specificity of 0.668, respectively. According to the total scores, patients from the validation cohort were divided into low-, moderate-, high- and very high-risk groups. The risk of developing DPN was significantly increased in moderate- (RR 3.3, 95% CI 1.5–7.2, P = 0.020), high- (RR 15.5, 95% CI 7.6–31.6, P < 0.001), and very high-risk groups (RR 45.0, 95% CI 20.5–98.8, P < 0.001) compared with the low-risk group.
Conclusion: A risk prediction model for DPN including 11 common clinical indicators were established. It is a simple and reliable tool for early prevention and intervention of DPN in patients with T2DM.
The number of people with diabetes mellitus (DM) and its comorbidities is growing rapidly worldwide. Diabetic peripheral neuropathy (DPN) is a major complication of diabetes, and at least 50% of patients with diabetes will develop DPN in their lifetime (1, 2). There is a large amount of evidence revealing that DPN is an important factor in the development of diabetic foot ulcer, Charcot neuroarthropathy (3), and even non-traumatic lower-limb amputation (1, 4). Besides, dysfunction of small and large fibers leads to abnormal foot temperature, pain sensation, and proprioception, which ultimately result in repeated foot damage and imbalance, increasing the risk of falls and fractures (5, 6). Furthermore, DPN is a predictor of mortality in patients with diabetes. A 13-year prospective study of patients with diabetes showed that DPN was significantly associated with cardiovascular and all-cause mortality (7). Considering the devastating consequences, DPN has been a public health problem that pose a significant challenge to social, financial and health care systems (8, 9). Unfortunately, there is currently a lack of early diagnosis and effective clinical intervention. DPN is usually insidious and is missed in the onset until it is well-established, at the point it seems to be irreversible (10). So early prevention is critical to tackle this issue.
Hyperglycemia has been recognized as the most important risk factor for DPN in patients with type 2 diabetes mellitus (T2DM). However, the UK prospective diabetes study (UKPDS) (11), the intensified multifactorial intervention in patients with type 2 diabetes (steno-2) study (12) and other large clinical intervention trials (13–15) did not find the benefit of glucose control on the occurrence and development of DPN in patients with T2DM. Antidiabetic treatment alone is insufficient to prevent DPN in individuals with T2DM (16). Besides hyperglycemia, T2DM coexists with other metabolic disorders, such as obesity, dyslipidemia, and hypertension, etc. Recent evidence suggests that these multiple metabolic disorders are involved in DPN onset. Early recognition and comprehensive assessment of these related risk factors allow for an earlier identification of high-risk individuals and an earlier management of DPN. Therefore, a risk prediction model for DPN including related risk factors is developed in this study, and it may be a more effective strategy for preventing DPN.
The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42021246320.
The derivation cohort patients came from a systematic review and meta-analysis. We searched the electronic databases including Pubmed, Embase, and Cochrane Library from inception to May 2020, using the following medical subject heading terms and their keywords: “diabetes mellitus, type 2,” “diabetic neuropathies,” “risk factors,” and “cohort studies.” Ultimately, a total of 95,604 patients with T2DM from 13 prospective cohorts and 5 retrospective cohorts were included. The research subjects were mainly from 19 countries and regions, of which 50% were from Asia, 22.22% were from Europe, 22.22% were from America, and 5.56% were from Oceania. All the 18 cohort studies reported the risk ratio (RR) with 95% confidence interval (CI) of each risk factor, and they were of high quality as assessed by the Newcastle-Ottawa Scale (NOS; provided in Supplementary Table 2). A flow diagram of literature selection process is shown in Figure 1. Details of literature search strategy, inclusion and exclusion criteria, data extraction, publication bias, and quality assessment are shown in Supplementary material.
In total, 2,608 patients with T2DM who were admitted to Tianjin Medical University Metabolic Diseases Hospital at least twice (baseline from September 2010 to September 2020) were considered for our study. We further selected patients aged 35–79 years old, without DPN at baseline, and with a follow-up of more than 12 months for inclusion in the validation cohort. Exclusion criteria included the presence of acute complications, serious infection, myocardial infarction, stroke, and cancer. We excluded 81 patients aged <35 or >79 years old, 715 patients with a follow-up for <12 months, 942 patients with DPN at baseline, 358 patients with acute diabetic complications or serious infection, and 50 patients with incomplete data. Finally, 462 patients were selected as the retrospective validation cohort. The flowchart is shown in Figure 2.
The outcome was the occurrence of DPN. DPN was diagnosed by a combination of symptoms, signs and nerve conduction function consistent with the guideline provided by the 2010 Toronto Consensus (17).
Smoking was defined as a total number of ≥100 cigarettes in their lifetime (18). Low high-density lipoprotein (HDL-c) was defined as HDL-c <1.3 mmol/L. High triglyceride (HTG) was defined as TG ≥ 1.7 mmol/L. Hypertension (HTN) was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg. Diabetic retinopathy (DR) was confirmed by ophthalmoscopy. Diabetic kidney disease (DKD) was identified clinically by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and/or urinary albumin-to-creatinine ratio (UACR) ≥ 30 mg/g caused by diabetes mellitus for ≥3 months (19). Cardiovascular diseases (CVD) included angina, previous myocardial infarction, or electrocardiographic manifestations of coronary ischemia.
The RR value and 95% CI of each risk factor were extracted from the included cohorts, and then pooled to screen out risk factors according to the heterogeneity across studies. Heterogeneity test was analyzed by Q-test, and measured by I2-value. When there was statistically significant heterogeneity (P-value < 0.10 or I2-value > 50%), the pooled RR and 95% CI were generated by a random effects model, otherwise by a fixed effects model. Subgroup analyses were performed according to the magnitude of the increase in continuous variables. Continuous variables included age (years, increment by 1 vs. 5–10), BMI (kg/m2, increment by 1–5), and duration of DM (years, increment by 1 vs. 5–10). Sensitivity analyses were conducted to evaluate the robustness of the results after a single study was omitted. Publication biases were determined using Begg's and Egger's linear regression tests, and the latter one prevailed if the two results were inconsistent. All tests were considered statistically significant at two-tailed P-value < 0.05, except for heterogeneity test and publication bias were at P-value < 0.1. Statistical analyses were performed with Stata software (version 12.0 StataCorp, College Station, TX).
We developed a risk score system, which is simple and convenient for clinical practice. First, all the risk factors with appropriate stratifications from the above systematic review and meta-analysis were incorporated into the model. We selected appropriate RR value and 95% CI to calculate the corresponding β-coefficient (β-coefficient), which represents the multiple increase in the risk of an individual developing a certain disease when each risk variable increases by one level. Second, multiplying β-coefficient by 10, and then rounding it to one decimal place (20), we further obtained the respective score of each risk factor. At last, all risk factors were stratified and assigned scores to construct a risk prediction model for DPN according to meta-analysis and clinical practice guidelines. The total score was calculated by adding up the score of each risk factor (21). For individuals, the higher the cumulative score, the higher risk of DPN in the future.
Continuous variables with normal distributions were expressed as mean ± standard deviation, and those with skewed distributions were described as median (interquartile range). Categorical variables were represented by frequency (percentage). A receiver operating characteristic (ROC) curve was performed based on the total scores. The sensitivity, specificity, the area under ROC curve (AUC) values, and optimal cut-off point were calculated. The AUC means prediction accuracy, with the value ranging from 0.5 to 1.0. The higher the AUC value, the better the prediction accuracy. The optimal cut-off point with higher sensitivity and a certain specificity was determined according to the Youden index. According to the optimal cumulative score, patients were segmented into four risk groups, including low-, moderate-, high-, and very high-risk. Kaplan–Meier curves were conducted to evaluate the cumulative risk of morbidity in different groups. Statistical analyses were performed using the SPSS 26.0 (IBMCorp, Armonk, NY, USA) and Stata software version 12.0 (StataCorp, College Station, TX).
We roughly analyzed the baseline data of participants from the included cohorts. A total of 95,604 patients with T2DM were included in the derivation cohort, with age between 35 and 79 years old, male accounting for 49.6%, and duration of DM ranging 1–19 years. The follow-up was 1–13 years, equivalent to 95,604 to 1,242,853 person-years. Among the patients, the mean body mass index (BMI) ranged 24.9–31.0 kg/m2, mean hemoglobin A1c (HbA1c) ranged 7.0–8.7% (53.0–71.6 mmol/mol), mean SBP ranged 135–143 mmHg, mean DBP ranged 77–87 mmHg, mean HDL-c ranged 1.30–3.40 mmol/L, and mean TG ranged 1.37–9.91 mmol/L. 6.6–80.2% of participants were smokers. 23.4–88.1% were with hyperlipidemia, 41.5–77.8% were with hypertension, 12.0–32.1% were with DR, 14.6–43.4% were with DKD, and 6.0–44.6% were with CVD. Across the studies, 54.2–88.9% received oral antidiabetic drugs (OAD), 1.0–47.8% received insulin injection, 7.8–80.0% received stains, and 18.5–82.8% received angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB). During follow-up, 19,399 DPN events were observed, with an estimated incidence of 20.3%. There were 24 risk factors available from these studies, including age, gender, marital status, smoking, height, BMI, waist circumference, duration of DM, fasting plasma glucose (FPG), HbA1c, total cholesterol (TC), TG, HDL-c, low density lipoprotein (LDL-c), SBP, DBP, C-reactive protein, eGFR, hypertension, DR, DKD, CVD, insulin, and statins. Baseline characteristics and risk factors of the 18 cohorts are provided in Supplementary Tables 1, 3.
A total of 462 patients with T2DM were enrolled, including 315 males (68.2%). The median follow-up time was 4.29 years, and 249 patients (162 males and 87 females) developed DPN at the end of follow-up. The incidence was 53.8%. Among all patients at baseline, the mean age was 52.4 ± 12.2 years old, duration of DM was 6.0 (3.0–11.0) years, mean BMI was 27.49 ± 4.43 kg/m2, mean HbA1c was 8.52 ± 1.88% (69.5 ± 20.5 mmol/mol), mean SBP was 136 ± 66 mmHg, mean DBP was 81 ± 11 mmHg, mean HDL-c was 1.19 ± 0.28 mmol/L, and TG was 1.63 (1.15, 2.56) mmol/L. Two hundred (43.3%) participants were smokers. Two hundred and thirty-six (51.1%) had hypertension, 109 (23.6%) had DR, 120 (26.0%) had DKD, and 224 (48.5%) had CVD. Four hundred and twenty-seven (92.4%) patients received OAD, 269 (58.2%) received insulin, 183 (39.6%) received statins, and 186 (40.3%) received ACEI or ARB. Baseline data of the validation cohort are shown in Supplementary Table 5.
Of the 24 risk factors identified from the above meta-analysis, 11 risk factors were involved in DPN onset. The risk stratification methods were carefully selected by subgroup or sensitivity analyses, which were most reasonable considering the feasibility and convenience of clinical practice. These 11 risk factors included in the final model were as follows: age incremented by 1 year (RR 1.02, 95% CI 1.01–1.03, P = 0.001; β-coefficient 0.020, score 0.2), smoking (RR 1.43, 95% CI 1.29–1.59, P < 0.001; β-coefficient 0.358, score 3.0), BMI incremented by 1–5 kg/m2 (RR 1.18, 95% CI 1.02–1.37, P = 0.030; β-coefficient 0.166, score 1.5), duration of diabetes incremented by 5–10 years (RR 1.39, 95% CI 1.21–1.60, P < 0.001; β-coefficient 0.329, score 3.0), HbA1c incremented by 1% (RR 1.14, 95% CI 1.08–1.19, P < 0.001; β-coefficient 0.131, score 1.5), low HDL-c (RR 1.34, 95% CI 1.13–1.59, P = 0.001; β-coefficient 0.293, score 3.0), high triglyceride (HTG; RR 1.34, 95% CI 1.19–1.51, P < 0.001; β-coefficient 0.293, score 3.0), hypertension (RR 1.35, 95% CI 1.08–1.68, P = 0.008; β-coefficient 0.300, score 3.0), DR (RR 2.05, 95% CI 1.25–3.37, P = 0.005; β-coefficient 0.718, score 7.0), DKD (RR 1.91, 95% CI 1.32–2.77, P = 0.001; β-coefficient 0.647, score 6.5), and CVD (RR 1.66, 95% CI 1.33–2.08, P < 0.001; β-coefficient 0.507, score 5.0). A forest plot of heterogeneity test of 11 risk factors is presented in Figure 3A, and subgroup and sensitivity analyses are shown in Figure 3B. These risk factors, risk stratification, RRs, 95% CIs, β-coefficients, and risk scores are shown in Supplementary Table 6.
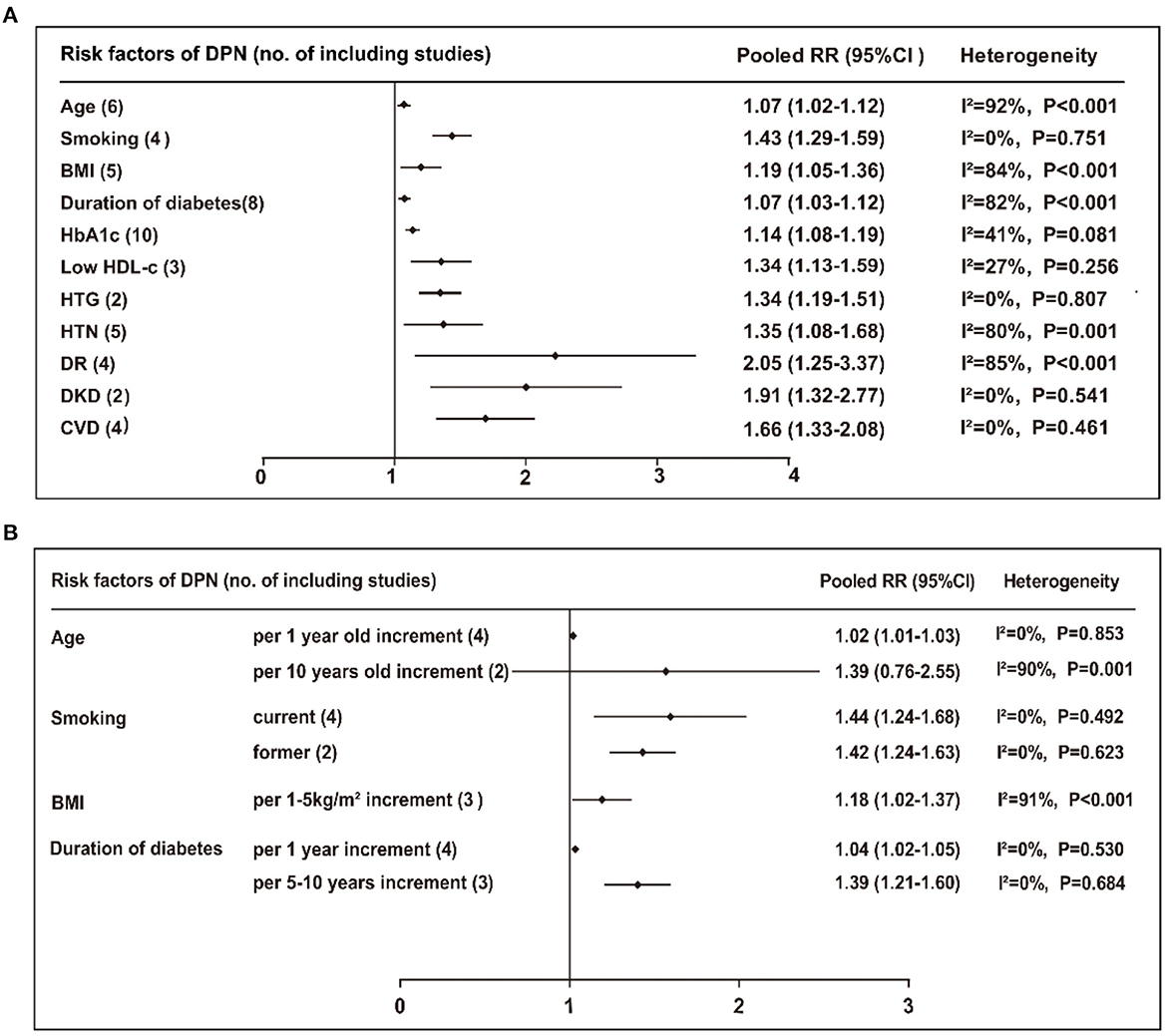
Figure 3. (A) Pooled RR (95% CI) and heterogeneity test of the risk factors for developing DPN. (B) Subgroup or sensitivity analyses of the risk factors for DPN. BMI, body mass index; HbA1c, Hemoglobin A1c; HDL-c, high density lipoprotein cholesterol; HTG, high triglyceride; HTN hypertension; DR, diabetic retinopathy; DKD, diabetic kidney disease; CVD, cardiovascular disease.
According to the stratifications and scores of the above risk factors, a simple DPN risk prediction model was developed as follows: age (years, 35–49 = 0, 50–59 = 2.0, 60–69 = 4.0, 70–79 = 6.0), smoking (no = 0, yes = 3.5), BMI (kg/m2, <24.00 = 0, 24.00–27.99 = 1.5, ≥28.00 = 3.0), duration of DM (years, <5.0 = 0, 5.0–9.9 = 2.5, 10.0–19.9 = 5.0, ≥20.0 = 7.5), HbA1c (%, <7.0 = 0, 7.0–7.9 = 1.5, 8.0–8.9 = 3.0, ≥9.0 = 4.5), HDL-c (mmol/L, ≥1.30 = 0, <1.30 = 3.0), TG (mmol/L, <1.70 = 0, ≥1.70 = 3.0), hypertension (no = 0, yes = 3.0), DR (no = 0, yes = 7.0), DKD (no = 0, yes = 6.5), and CVD (no = 0, yes = 5.0; shown in Table 1).
In the validation cohort, the AUC value of this model was 0.831 (95% CI 0.794–0.868, P < 0.001). The ROC curve is provided in Figure 4A. The purpose of constructing this model was to early identify the high-risk population of DPN, so the sensitivity should be as high as possible on the premise of ensuring a certain degree of specificity when selecting the cut-off point. Therefore, a score of 17.0 was finally selected as the best predictive cut-off point, with a higher sensitivity of 0.846, a specificity of 0.668, and a maximum Youden index value of 0.531. Sensitivity, specificity and Youden indexes of different cut-off scores are shown in Supplementary Table 7. Based on the frequencies of the total risk scores, 462 patients were divided into four groups: low- (n = 97, 0–12.5 scores), moderate- (n = 81, 13.0–16.5 scores), high- (n = 149, 17.0–24.5 scores), and very high-risk (n = 135, 25.0–52.0 scores), and the corresponding numbers of patients who developed DPN at the end of the follow-up were 11 (11.3%), 24 (29.6%), 99 (66.4%), and 115 (85.2%), respectively. The risk of developing DPN was significantly increased in moderate- (RR 3.3, 95% CI 1.5–7.2, P = 0.020), high- (RR 15.5, 95% CI 7.6–31.6, P < 0.001), and very high-risk groups (RR 45.0, 95% CI 20.5–98.8, P < 0.001) compared with the low-risk group. The Kaplan-Meier curves for these four groups are shown in Figure 4B. The cumulative risk for each group was provided in Table 2.
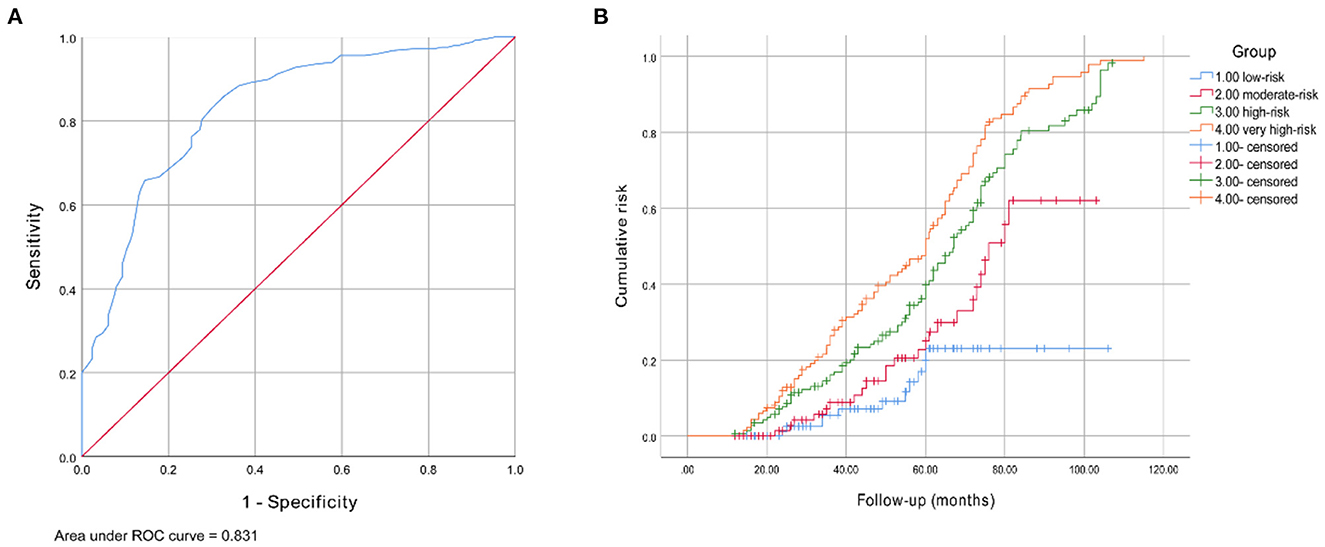
Figure 4. (A) Receiver operating characteristic curve for the DPN risk prediction model. The AUC and 95% CI were 0.831 (0.794–0.868). (B) Kaplan-Meier curve of DPN end point for four risk group: moderate- (RR 3.3, 95% CI 1.5–7.2, P = 0.020), high- (RR 15.5, 95% CI 7.6–31.6, P < 0.001), and very high-risk groups (RR 45.0, 95% CI 20.5–98.8, P < 0.001).
Recent evidence suggests that multiple metabolic disorders are all involved in DPN onset, but the results of different studies were not entirely consistent. Given that high-quality meta-analysis is at the top of the evidence-based medicine level pyramid, and it helps to establish a more robust prediction model than a single study. Meta-analysis was applied to integrate relevant cohort studies (22–39). We screened out the risk factors of DPN from a meta-analysis, and constructed a simple risk prediction model for DPN by quantitatively evaluating risk factors. This model provided quantitative standards for early identifying high-risk groups, thus we can develop comprehensive and individualized prevention and intervention strategies.
We included 18 cohort studies with a total of 95,604 patients, and screened out 11 risk factors of DPN, including age, smoking, BMI, duration of DM, HbA1c, HDL-c, TG, hypertension, DR, DKD, and CVD. Except age and duration of DM were non-modifiable, the remaining factors can be modifiable by lifestyle optimization and drug intervention. We recommended that patients at low-risk improve self-monitoring and conduct regular risk assessment; High-risk groups, based on risk assessment, actively improve lifestyle, such as quitting smoking, optimizing diet structure, participating in moderate physical exercise, and weight control, etc. They should optimize the basic controlling of blood glucose, lipids and blood pressure, and further intervene in diabetic complication under the guidance of professional doctors. It should be noted that once a patient is classified as more than low risk, neurological examination for DPN should be performed, especially the sensitive tests for small fiber neuropathy, to monitor the stage and progression of DPN. Through systematic clinical prevention strategies, we can reduce the incidence of DPN, improve life quality of patients, and save costs of clinical management.
In the risk factors for DPN from our meta-analysis, DR, DKD, and CVD were the most powerful, and they usually share common factors and are often co-morbid. Our study suggested that both DR and DKD were increased the risk of DPN by nearly 2 times, and CVD increased the risk by ~1.7 times. Duration of DM, smoking, low HDL-c, high TG and hypertension were the relatively moderate risk factors. Previous cross-sectional and cohort studies shown that the incidence of DPN reached 50% after 8 years of diagnosis in diabetic patients (34). We indicated that the risk of DPN increased by 39% for every 10-year increment in the duration of DM. Smoking is an independent risk factor for DPN in diabetic patients (40). Consistent with previous researches, our study reported the risk increased by about 40%, whether quitting smoking or not. Smoking was defined as a total number of ≥ 100 cigarettes in their lifetime (18). Dyslipidemia is closely correlated with the progression of DPN, especially high TG and low HDL-c levels (34, 37). As TG ≥ 1.70 mmol/L or HDL-c <1.30 mmol/L, the risk for DPN increased by 34% in this study. We found no statistical significance when it came to LDL-c, and there is still a lack of research data. Considering that patients with T2DM are prone to comorbid dyslipidemia, the widespread use of statins at baseline may affect the results. Hypertension plays a crucial role in the occurrence and development of DPN. Our result reported that hypertension increased the risk of DPN by 35%, but no correlation was found between SBP, DBP, and DPN. The relationship between blood pressure and DPN risk still needs to be investigated in large-scale prospective studies. Age, HbA1c, and BMI were also major risk factors of DPN. Prospective researches have shown that age was independently related with DPN. For every 10 years increment in age, the risk of DPN was increased by 20% in this study. According to study population included in our meta-analysis, the results were mainly applicable to patients with T2DM aged 35–79 years old. Patients with type 1 diabetes mellitus tend to be younger, and there is currently a lack of data on DPN risk in patients with early-onset (age <35 years old) T2DM. Further prospective studies with large samples are needed. With HbA1c incremented by 1%, the risk of DPN increased by 14% in this study. We did not find a correlation between FPG and DPN. FPG is the blood glucose level at a certain time point, while HbA1c reflects the average level in the recent 2–3 months. Therefore, HbA1c could better reflect blood glucose control. BMI is the most common measure of obesity and plays a crucial role in initiation of DPN. Obesity is often associated with insulin resistance and dyslipidemia, which are components of cardiovascular disease risk factors, and their interaction will also increase the risk of DPN (41). When BMI incremented by 1–5kg/m2, the risk of DPN increased by 18% in our study. The difficulty lied in the excessive stratification of a certain risk factor and the occurrence of extreme value, which led to its excessive weight. In order to avoid this influence, appropriate results and stratification methods were selected for some risk factors. For example, considering the weight of BMI is too high, we converted it into categorical variable. According to Chinese standards for BMI (42), the population was divided into three groups: normal (<24.00 kg/m2), overweight (24.00–27.99 kg/m2), and obese (≥28.00 kg/m2). We defined BMI < 24.00 kg/m2 as the normal group with a 0 score, BMI ranged 24.00–27.99 kg/m2 as the overweight group with a score of 1.5, and BMI ≥28.00 kg/m2 as the obese group with a score of 3.0.
Some investigators have established prediction models for DPN (43–46). However, those models were mostly constructed based on cross-sectional studies, small sample cohort studies or post-hoc analysis of randomized controlled trials. A prior model developed by Basu et al. (44) used complex computer algorithms, which is not convenient for clinical promotion. Hence, based on meta-analysis from 18 cohort studies with 95,604 patients with T2DM, we established a simple and robust risk prediction model for DPN that consisting of lifestyle and clinical data, including age, smoking, BMI, duration of DM, HbA1c, low HDL-c, high TG, hypertension, DR, DKD and CVD.
Furthermore, patients with T2DM from China were included as an external cohort to verify the predictive performance of this model. AUC of our model was 0.831, indicating good predictive performance. Since this model aimed at early identification of high-risk population of DPN, the sensitivity should be improved as far as possible on the premise of ensuring a certain degree of specificity when selecting cut-off point. Finally, a score of 17.0 with a higher sensitivity of 0.846 and a specificity of 0.668 was selected as the optimal cut-off point. Sensitivity represents the true positive rate, our model achieved a high sensitivity, indicating that the authenticity of positive prediction is relatively high, which is in line with the purpose of the prediction model. Specificity stands for the true negative rate. There is a shortcoming that the true negative rate is slightly low. The reason may be that some indicators which may affect the occurrence and development of DPN were not included due to the meta-analysis, such as C-reactive protein, erythrocyte sedimentation rate, fibrinogen, and D-dimer, etc. Few related cohort researches were available in this meta-analysis, so we failed to include them in our model. Further prospective studies on these indicators are still needed, and we expect to include them to improve the model's specificity. Patients with a cumulative score of ≥17.0 were at high-risk of DPN onset. According to the total scores of participants, we further divided them into four groups, namely, low-, moderate-, high- and very high-risk groups. Compared with the low-risk group, the moderate-, high- and very high-risk groups had 3.3-, 15.5-, and 45.0-fold increases in the rate of developing DPN, respectively. Through the application of this model to assess the risk factors and make targeted measures, it is expected to transform high-risk individuals into lower-risk groups, so as to achieve dynamic management and ultimately reduce the occurrence of DPN.
Nevertheless, the study presented here faces some limitations. First, heterogeneity among literatures is inevitable because of differences in study design and diversities in race and sex compositions of the included studies. Although subgroup analysis and sensitivity analysis were further conducted to minimize heterogeneity, the causes for heterogeneity of some factors were still not explicit. Second, the number of included researches on some risk factors is small. Some other clinical indicators, such as C-reactive protein, erythrocyte sedimentation rate, fibrinogen and D-dimer, may involve in the progression of DPN, but few related cohort studies were retrievable, so we failed to include them in our model. All these confounding factors may bias the results. We expect more prospective studies on these factors to be explored and included them to update our model in the future. Third, participants in the derivation cohort were from several countries and regions, while the validation cohort was only consisted of Chinese patients. Therefore, multicenter external cohorts remained to verify the predictive performance of this model.
Based on meta-analysis, we developed a simple and reliable risk prediction model for DPN in combined with lifestyle and clinical data, including age, smoking, BMI, duration of DM, HbA1c, low HDL-c, high TG, hypertension, DR, DKD, and CVD. This model is important for early prevention and individual intervention of DPN.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tianjin Medical University Metabolic Diseases Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XixL and DC conducted the research, collected the data, statistical analysis, and wrote the manuscript. HF, XinL, QZ, JZ, MD, and JW contributed to the discussion, performed study quality assessment, and reviewed the manuscript. BC contributed to concept, design, and manuscript revision. All authors contributed to the article and approved the submitted version.
This work was funded by the National Natural Science Foundation of China (81973614), Tianjin Key Medical Discipline (Specialty) Construction Project (grant number: TJYXZDXK-032A), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (2018KJ047), and the Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (grant number: 2019ZDKF03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1128069/full#supplementary-material
Systematic review and meta-analysis to identify the risk factors of diabetic peripheral neuropathy onset in patients with type 2 diabetes mellitus is available in the Supplementary material.
1. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/S2213-8587(19)30081-6
2. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Prim. (2019) 5:42. doi: 10.1038/s41572-019-0092-1
3. Boulton AJ. The pathway to foot ulceration in diabetes. Med Clin North Am. (2013) 97:775–90. doi: 10.1016/j.mcna.2013.03.007
4. Boulton AJM, Armstrong DG, Kirsner RS, Attinger CE, Lavery LA, Lipsky BA, et al. Diagnosis and Management of Diabetic Foot Complications. Arlington: American Diabetes Association (2018). doi: 10.2337/db20182-1
5. Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabet Care. (2002) 25:1983–6. doi: 10.2337/diacare.25.11.1983
6. Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture. (2012) 35:662–8. doi: 10.1016/j.gaitpost.2011.12.021
7. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in US adults: A prospective cohort study. Ann Intern Med. (2021) 174:167–74. doi: 10.7326/M20-1340
8. Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. (2013) 42:747–87. doi: 10.1016/j.ecl.2013.06.001
9. Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. (2015) 29:212–7. doi: 10.1016/j.jdiacomp.2014.10.013
10. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. (2017) 8:646–55. doi: 10.1111/jdi.12650
11. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. (1999) 48:643–8. doi: 10.1046/j.1365-2125.1999.00092.x
12. Gæde P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. (2016) 59:2298–307. doi: 10.1007/s00125-016-4065-6
13. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: Lessons from recent large clinical trials. Curr Diab Rep. (2014) 14:528. doi: 10.1007/s11892-014-0528-7
14. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. (2012) 11:521–34. doi: 10.1016/S1474-4422(12)70065-0
15. Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: The ADDITION-Denmark study. Diabetes Care. (2011) 34:2244–9. doi: 10.2337/dc11-0903
16. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochr Datab Systemat Rev. (2012) 6:Cd007543. doi: 10.1002/14651858.CD007543.pub2
17. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10-1303
18. Haig C, Carrick D, Carberry J, Mangion K, Maznyczka A, Wetherall K, et al. Current smoking and prognosis after acute ST-segment elevation myocardial infarction: New pathophysiological insights. JACC Cardiovasc Imaging. (2019) 12:993–1003. doi: 10.1016/j.jcmg.2018.05.022
19. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/CJN.11491116
20. Chen D, Wang M, Shang X, Liu X, Liu X, Ge T, et al. Development and validation of an incidence risk prediction model for early foot ulcer in diabetes based on a high evidence systematic review and meta-analysis. Diabetes Res Clin Pract. (2021) 180:109040. doi: 10.1016/j.diabres.2021.109040
21. Glümer C, Carstensen B, Sandbaek A, Lauritzen T, Jørgensen T, Borch-Johnsen K, et al. Danish diabetes risk score for targeted screening: The Inter99 study. Diabetes Care. (2004) 27:727–33. doi: 10.2337/diacare.27.3.727
22. Pek SLT, Lim SC, Ang K, Kwan PY, Tang WE, Sum CF, et al. Endothelin-1 predicts incident diabetic peripheral neuropathy in Type 2 Diabetes: A cohort study. Eur J Endocrinol. (2020) 182:429–38. doi: 10.1530/EJE-19-0523
23. Teliti M, Cogni G, Sacchi L, Dagliati A, Marini S, Tibollo V, et al. Risk factors for the development of micro-vascular complications of type 2 diabetes in a single-centre cohort of patients. Diabet Vasc Dis Res. (2018) 15:424–32. doi: 10.1177/1479164118780808
24. Mohammadian-Hafshejani A, Majdzadeh R, Mansournia N, Mansournia MA. Risk factors of peripheral neuropathy in patients with type 2 diabetes in Isfahan: Results of a cohort study in Iran. Biomed Res Ther. (2018) 5:2926–36. doi: 10.15419/bmrat.v5i12.512
25. Fawwad A, Mustafa N, Zafar AB, Khalid M. Incidence of microvascular complications of type 2 diabetes: A 12 year longitudinal study from Karachi-Pakistan. Pak J Med Sci. (2018) 34:1058–63. doi: 10.12669/pjms.345.15224
26. Aryan Z, Ghajar A, Faghihi-Kashani S, Afarideh M, Nakhjavani M, Esteghamati A. Baseline high-sensitivity C-reactive protein predicts macrovascular and microvascular complications of type 2 diabetes: A population-based study. Ann Nutr Metab. (2018) 72:287–95. doi: 10.1159/000488537
27. Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care. (2018) 41:1068–75. doi: 10.2337/dc17-2062
28. Yang CP, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, et al. Variability of fasting plasma glucose increased risks of diabetic polyneuropathy in T2DM. Neurology. (2017) 88:944–51. doi: 10.1212/WNL.0000000000003682
29. Yang CP, Lin CC, Li CI, Liu CS, Lin WY, Hwang KL, et al. Cardiovascular risk factors increase the risks of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus. Medicine. (2015) 94:e1783. doi: 10.1097/MD.0000000000001783
30. Cardoso CRL, Moran CBM, Marinho FS, Ferreira MT, Salles GF. Increased aortic stiffness predicts future development and progression of peripheral neuropathy in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetologia. (2015) 58:2161–8. doi: 10.1007/s00125-015-3658-9
31. Ybarra-Muñoz J, Jurado-Campos J, Garcia-Gil M, Zabaleta-Del-Olmo E, Mir-Coll T, Zabalegui A, et al. Cardiovascular disease predicts diabetic peripheral polyneuropathy in subjects with type 2 diabetes: A 10-year prospective study. Eur J Cardiovasc Nurs. (2014) 15:248–54. doi: 10.1177/1474515114565215
32. Cardoso CR, Salles GF. Predictors of development and progression of microvascular complications in a cohort of Brazilian type 2 diabetic patients. J Diabetes Complications. (2008) 22:164–70. doi: 10.1016/j.jdiacomp.2007.02.004
33. Abougalambou SSI, Abougalambou AS. Explorative study on diabetes neuropathy among type II diabetic patients in Universiti Sains Malaysia Hospital. Diabet Metab Syndr. (2012) 6:167–72. doi: 10.1016/j.dsx.2012.09.002
34. Davis TME, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: The Fremantle Diabetes Study. Diabetologia. (2008) 51:562–6. doi: 10.1007/s00125-007-0919-2
35. Sands ML, Shetterly SM, Franklin GM, Hamman RF. Incidence of distal symmetric (sensory) neuropathy in NIDDM. The San Luis Valley diabetes study. Diabet Care. (1997) 20:322–9. doi: 10.2337/diacare.20.3.322
36. Chuengsamarn S, Rattanamongkolgul S, Sittithumcharee G, Jirawatnotai S. Association of serum high-sensitivity C-reactive protein with metabolic control and diabetic chronic vascular complications in patients with type 2 diabetes. Diabetes Metab Syndr. (2017) 11:103–8. doi: 10.1016/j.dsx.2016.08.012
37. Cho YN, Lee KO, Jeong J, Park HJ, Kim SM, Shin HY, et al. The role of insulin resistance in diabetic neuropathy in Koreans with type 2 diabetes mellitus: A 6-year follow-up study. Yonsei Med J. (2014) 55:700–8. doi: 10.3349/ymj.2014.55.3.700
38. Christensen DH, Knudsen ST, Gylfadottir SS, Christensen LB, Nielsen JS, Beck-Nielsen H, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: A nationwide study of 5,249 patients in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) Cohort. Diabet Care. (2020). doi: 10.2337/dc20-1234/suppl.12006117
39. Al Sayah F, Soprovich A, Qiu W, Edwards AL, Johnson JA. Diabetic foot disease, self-care and clinical monitoring in adults with type 2 diabetes: The Alberta's Caring for Diabetes (ABCD) Cohort Study. Can J Diabet. (2015) 39(Suppl.3):S120–6. doi: 10.1016/j.jcjd.2015.05.006
40. Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: A systematic review and meta-analysis. J Gen Intern Med. (2015) 30:1193–203. doi: 10.1007/s11606-015-3354-y
41. Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001-2004. Diabet Care. (2011) 34:1642–7. doi: 10.2337/dc10-2150
42. Ji CY, Chen TJ. Empirical changes in the prevalence of overweight and obesity among Chinese students from 1985 to 2010 and corresponding preventive strategies. Biomed Environ Sci. (2013) 26:1–12. doi: 10.3967/0895-3988.2013.01.001
43. Lagani V, Chiarugi F, Thomson S, Fursse J, Lakasing E, Jones RW, et al. Development and validation of risk assessment models for diabetes-related complications based on the DCCT/EDIC data. J Diabet Complicat. (2015) 29:479–87. doi: 10.1016/j.jdiacomp.2015.03.001
44. Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabet Endocrinol. (2017) 5:788–98. doi: 10.1016/S2213-8587(17)30221-8
45. Bongaerts BW, Ziegler D, Shaw JE, Heier M, Kowall B, Herder C, et al. A clinical screening score for diabetic polyneuropathy: KORA F4 and AusDiab studies. J Diabet Complicat. (2015) 29:44–9. doi: 10.1016/j.jdiacomp.2014.09.014
Keywords: type 2 diabetes mellitus, diabetic peripheral neuropathy, risk factors, prediction model, cohort study, meta-analysis
Citation: Liu X, Chen D, Fu H, Liu X, Zhang Q, Zhang J, Ding M, Wen J and Chang B (2023) Development and validation of a risk prediction model for early diabetic peripheral neuropathy based on a systematic review and meta-analysis. Front. Public Health 11:1128069. doi: 10.3389/fpubh.2023.1128069
Received: 20 December 2022; Accepted: 06 February 2023;
Published: 22 February 2023.
Edited by:
Mark Yorek, The University of Iowa, United StatesReviewed by:
Uazman Alam, University of Liverpool, United KingdomCopyright © 2023 Liu, Chen, Fu, Liu, Zhang, Zhang, Ding, Wen and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bai Chang,  Y2hhbmdiYWkxOTcyQDEyNi5jb20=
Y2hhbmdiYWkxOTcyQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.