- 1Postgraduate School, University of Harbin Sport, Harbin, China
- 2Xiangya Hospital, Central South University, Changsha, China
- 3Norman Bethune Health Science Center, Jilin University, Changchun, China
- 4Department of International Culture Education, Chodang University, Muan-gun, Republic of Korea
- 5Centre for Active Living and Learning, University of Newcastle, Callaghan, NSW, Australia
- 6College of Human and Social Futures, University of Newcastle, Callaghan, NSW, Australia
- 7Winter Olympic School, Harbin Sport University, Harbin, China
Objective: This study aims to evaluate the intervention effect of concurrent training on children with malignant tumors to provide evidence for prescribing exercise for children with malignant tumors.
Methods: Twelve databases were searched from inception to October 15, 2022. Two researchers independently screened the literature, evaluated the quality, extracted the data, and performed the meta-analysis using R.
Result: A total of nine randomized controlled trials involving 371 children were included in this study. The meta-analysis revealed that muscle strength was significantly greater in the exercise group compared to the usual care group [SMD = 0.26, 95% CI (0.04, 0.48), P = 0.023], with subgroup analysis showing no significant difference in upper limb [SMD = 0.13, 95% CI (−0.17, 0.43), P = 0.318] and a considerable difference in lower limb strength [SMD = 0.41, 95% CI (0.08, 0.74), P = 0.015]. Physical activity [SMD = 0.57, 95% CI (0.03, 1.1), P = 0.038], timed up and down stairs test [SMD = −1.22, 95% CI (−2.04, −0.4), P = 0.004], 6-min walking ability [SMD = 0.75, 95% CI (0.38, 1.11), P < 0.01], quality of life [SMD = 0.28, 95% CI (0.02, 0.53), P = 0.033], and cancer-related fatigue [SMD = −0.53, 95% CI (−0.86, −0.19), P = 0.002] were significantly better than the usual care group. There were no significant differences in peak oxygen uptake [SMD = 0.13, 95% CI (−0.18, 0.44), P = 0.397], depression [SMD = 0.06, 95% CI (−0.38, 0.5), P = 0.791], and withdrawal rates [RR = 0.59, 95% CI (0.21, 1.63), P = 0.308] between the two groups.
Conclusion: Concurrent training could improve physical performance for children with malignancy but had no significant effect on mental health. Because the quality level of evidence is mostly very low, future high-quality randomized controlled trials are required to confirm these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=364140, identifier CRD42022308176.
Introduction
Pediatric cancer is a significant cause of the global burden of childhood disease, with annual increases in incidence rates (1–3). Globally, an estimated 300,000 children and adolescents between 0 and 19 are diagnosed with cancer yearly (4), of which more than 175,000 (70%) are children under the age of 15. Today, tremendous advancements in cancer treatment have improved the survival rates of children and adolescents with cancer, with 5-year survival rates in developed countries exceeding 80% (5). Although the prognosis for children with cancer has improved significantly recently, the rate of improvement has slowed as the intensity of treatment for many pediatric cancers may have reached limitations (6).
The prevalence of severe chronic health conditions among long-term survivors of pediatric cancers is three times higher than in matched controls (7), with approximately two-thirds of pediatric cancer patients experiencing at least one chronic or long-term side effect after treatment (8). Side effects are health complications caused by cancer or cancer treatments (surgery, chemotherapy, radiation therapy, and stem cell transplantation) that do not disappear or recur months or years after treatment is completed, negatively impacting the quality of life and overall health (9). These effects include decreased muscle and soft tissue function (e.g., muscle weakness, radiation fibrosis syndrome, and soft tissue contractures), pain (10), peripheral neuropathy (11), fatigue, weakness (12), anxiety (13), range of motion limitations, and impairments such as balance and gait deficits (14), all of which can negatively affect a child's athletic performance (15) and quality of life (16, 17). Consequently, there is a growing awareness of the necessity for planned survivorship care, including medical follow-up and monitoring of the long-term effects of cancer treatment (18).
The benefits of aerobic and resistance training alone are well documented, but studies of concurrent exercise in pediatric populations are limited. Concurrent training improves the physical fitness and athletic performance of adolescents more than unimodal training (19) and refers to a combination of resistance and aerobic training to gain strength, muscle hypertrophy, explosive power, and endurance-enhancing training in the same training phase (20). Indeed, compared to single aerobic or resistance exercise, concurrent training is more effective in improving patients' cardiopulmonary function and muscle strength and has been used to rehabilitate adult patients (21) with chronic kidney disease (22), obesity (23), and cancer (24). However, due to anthropometric, physiological, and biomechanical differences, the characteristics of neuromuscular adaptation to concurrent training in children may differ from those of relatively older populations. The concurrent training program is complex, and the interaction of variables (e.g., training mode and intensity, training content, subject characteristics, training muscle groups, etc.) may induce different adaptations to train. Therefore, this study involved a meta-analysis to systematically evaluate the effects of concurrent training on exercise performance and quality of life in children with cancer to provide evidence for clinical healthcare professionals to prescribe exercise interventions in children with malignant tumors.
Methods
This meta-analysis was performed and reported in accordance with PRISMA guidelines (The details are shown in Supplementary Appendix A) and registered with the PROSPERO (CRD42022308176).
Literature search
We performed a systematic search for relevant articles written in English identified by title and abstract in OVID, PubMed, Web of Science, Embase, Cochrane Library, Scopus, SPORT Discus, CINAHL, SinoMed, Wanfang, CNKI, VIP from inception of the database to October 15, 2022. The search strategy was a combination of subject terms and free words (The details are shown in Supplementary Appendix B). In addition to the electronic literature search, additional searches were completed by examining reference lists of relevant articles to identify studies not retrieved from the databases.
Study selection
First, duplicates were removed from the retrieved literature, then the titles and abstracts of the remaining articles were independently screened by two authors based on the inclusion and exclusion criteria. Manual screening of the references was also performed to identify further relevant studies. Finally, the full text of the conforming literature was obtained to decide whether to include it or not. Any differences in literature selection were discussed by the two authors to reach a consensus; otherwise, the decision was made by the third author. The inclusion criteria were as follows: (1) randomized controlled trials; (2) studies comparing concurrent training AND usual care as the control group to treat patients with pediatric cancer;(3) studies in which any of the following clinical outcomes were reported cancer-related fatigue, quality of life, 6-min walking ability (6WMT), Peak oxygen uptake (VO2peak), muscle strength, physical activity, timed up and down stair test, or depression. The exclusion criteria were: (1) non-clinical studies such as animal studies; (2) non-English and Chinese literature; (3) the full text of the literature was not available.
Data extraction
The two authors extracted the data from the included studies, including: (1) the basic study such as title, first author, year of publication, cancer type, and disease stage; (2) experimental information such as study type, intervention method of the exercise group and control group; (3) patient information such as the number of patients in each group, gender ratio, age; (4) outcomes mentioned above.
Quality assessment
The risk of bias in the RCTs was assessed in the domains of the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result using the revised Cochrane risk of bias tool for randomized trials (RoB2). Any disagreement was negotiated between the two authors to reach a consensus, otherwise was decided by the third author. Each domain was rated as “low,” “some concerns,” or “high” and the overall ROB for each trial was based on the highest risk attributed to any one domain. The quality of the evidence for our outcomes was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework.
Statistical analysis
A meta-analysis of studies reporting results of physical capacity-related endpoints as “mean” (plus standard deviation (SD) or standard error of the mean) values before and after the exercise intervention was performed. For these continuous variables, pooled standardized mean differences of endpoint data across interventions (last evaluation at the end of the follow-up minus baseline) were computed. I2 was used to test for heterogeneity between studies, and the P-value was calculated. According to Cochrane's handbook, when I2 is more than 50%, the fixed effect model is used; otherwise, the random effect model. All data analyses were performed using the meta package in R (4.1.3) (25). However, due to the limited number of included studies, we did not test for publication bias.
GRADE quality of meta evidence
The (GRADE) guidance tools were used to assess the quality of evidence (26). The GRADE system assesses five domains: study risk of bias, publication bias, indirectness, imprecision, and inconsistency, and assigns grading levels of high, moderate, low, and very low for each outcome.
Results
Literature search
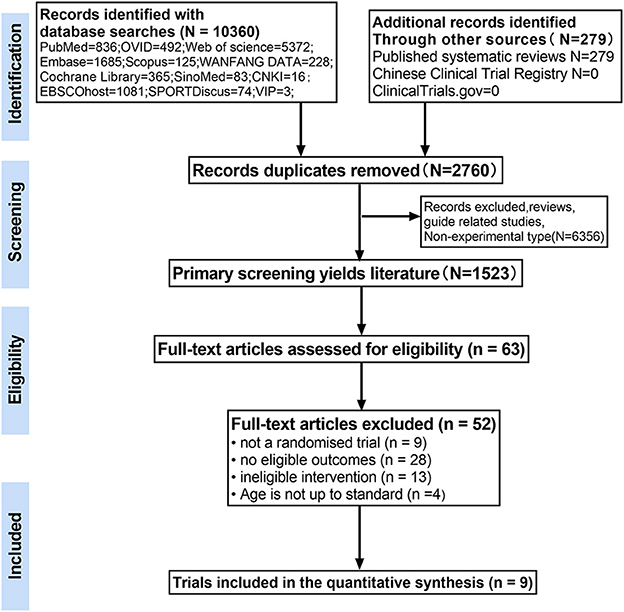
A total of 10,639 relevant articles were obtained for the initial review, and 63 potentially eligible articles were selected for full-text review. Of these, 54 articles were excluded for reasons including non-randomized controlled studies (n = 9), studies that included subjects who did not meet the inclusion criteria (n = 4), studies with interventions that did not meet the inclusion criteria (n = 13), and studies that did not have outcomes of interest (n = 28). A total of nine papers met the inclusion criteria and were used for further analysis (the flow chart of study selection is shown in Figure 1).
Study characteristics
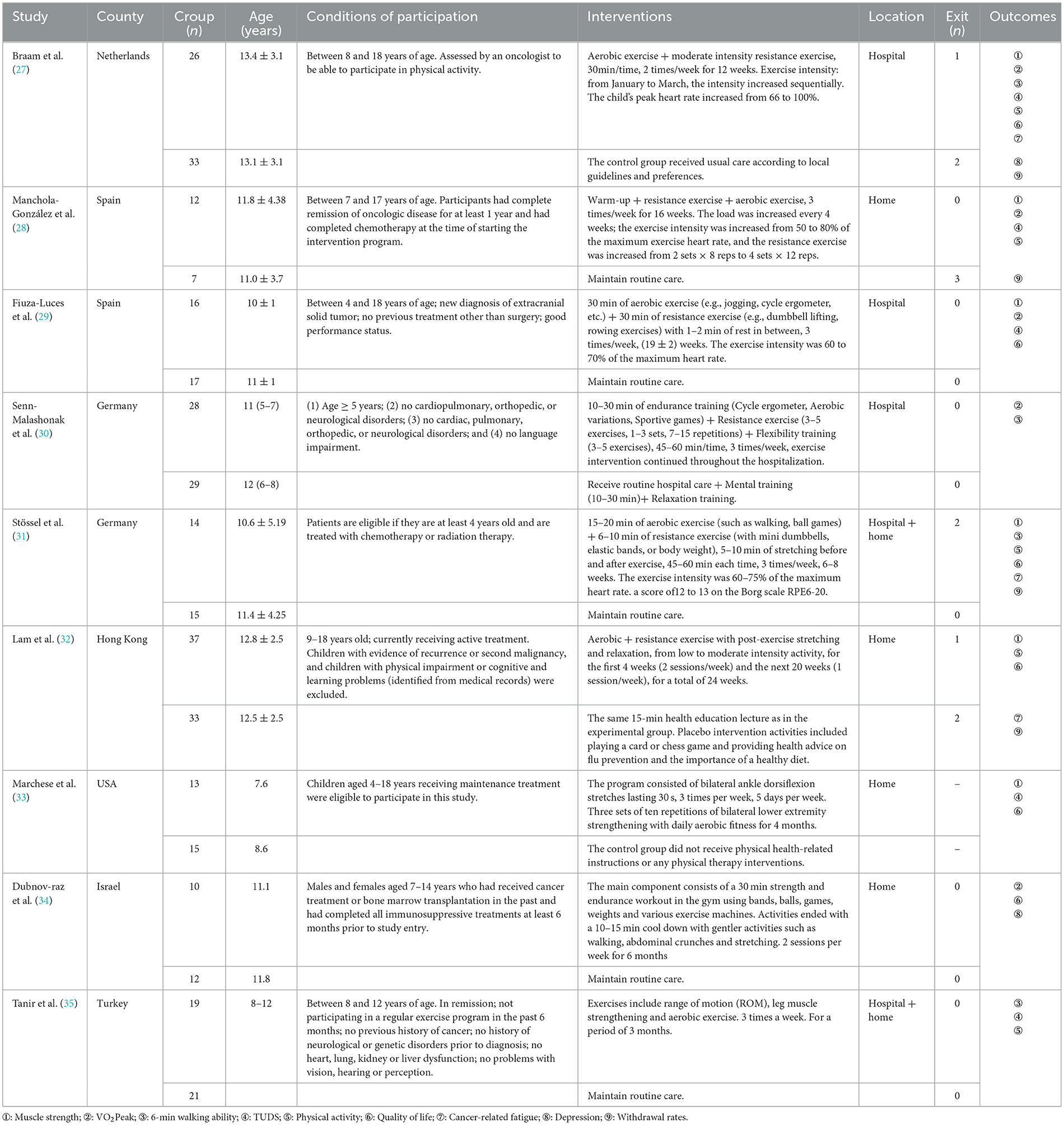
A total of nine trials involving 371 children with malignancies during or after treatment were analyzed in the meta-analysis. Only one of these studies was conducted in a developing country, and the others were conducted in developed nations or regions. The age of the study participants ranged from 4 to 18 years. The form of the concurrent training interventions varied among the studies, and the duration of the interventions for children with malignancy ranged from 6 to 24 weeks. The duration of one study was the entire hospitalization period, one study had an intervention duration of 6–8 weeks, and the remaining seven studies had a duration of 12–24 weeks. Six studies involved three interventions per week, and three studies had two interventions weekly. Nine studies had a duration of 60 mins or less per intervention. VO2 Peak, muscle strength, physical activity, walking ability, up and down stairs test, cancer-related fatigue, depression, and quality of life were used as outcome indicators. Data were pooled from the above studies and included in the meta-analysis. Detailed characteristics of the included studies are displayed in Table 1.
Quality assessment
The risk of bias assessment for the included studies is illustrated in Figure 2.
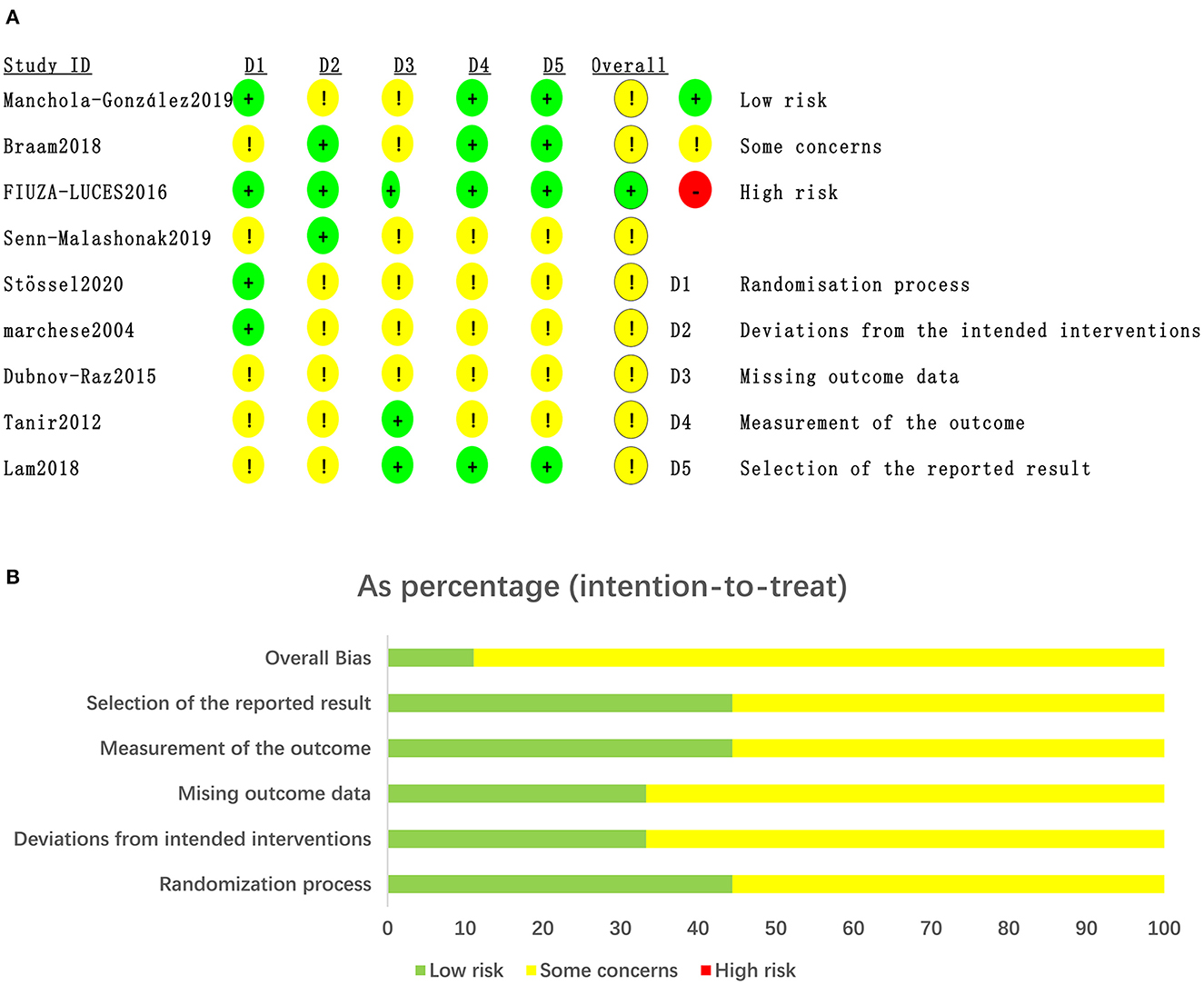
Figure 2. Quality of studies included in the meta-analysis. (A) Risk of bias graph and summary for the including 11RCTs. (B) The distribution of the methodological quality of included 11RCTs.
Muscle strength
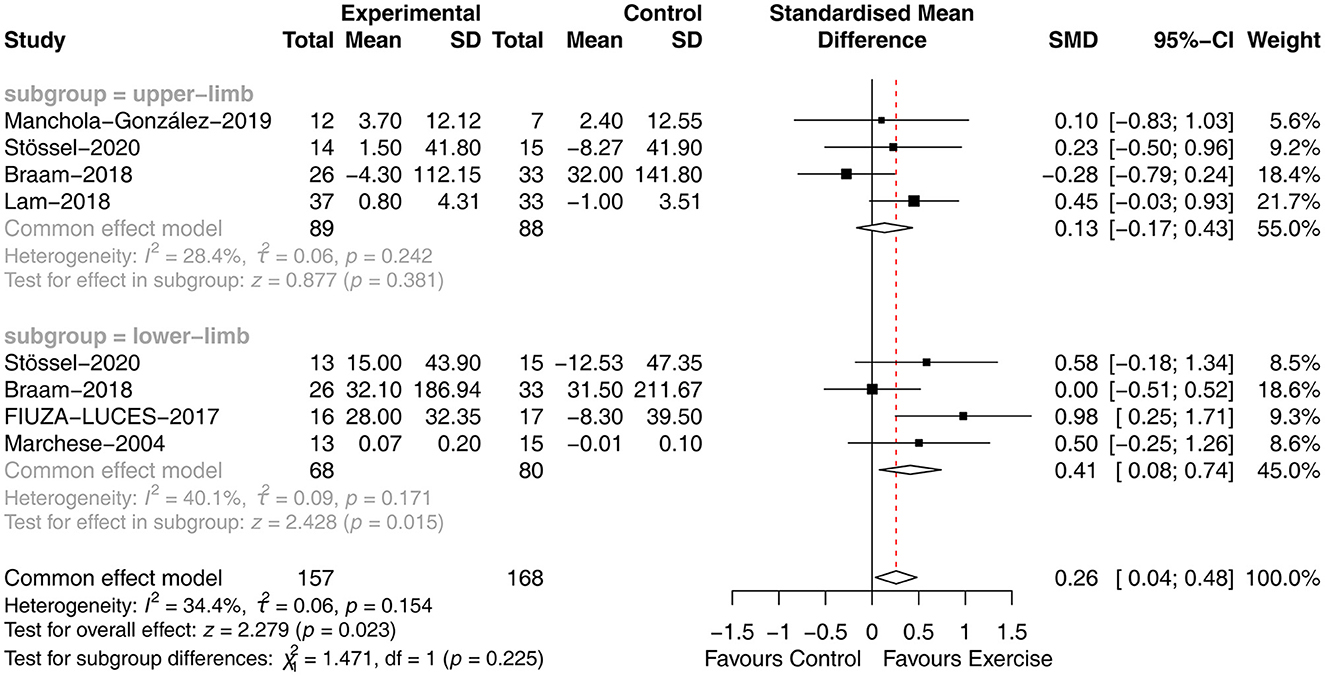
Six studies (27, 29, 31–33, 36) analyzed the effect of concurrent training on muscle strength in children with malignancy. Due to the different measurement tools used, the SMD was used. The heterogeneity between studies was small (P = 0.154, I2 = 34.4%), and a fixed-effects model was used for analysis, showing that muscle strength was significantly improved in the exercise group compared to the usual care group [SMD = 0.26, 95% CI (0.04, 0.48), P = 0.023]. Four studies tested the upper extremity strength of children, and four studies tested the lower extremity strength of children. Subgroup analysis was performed using a fixed effects model, with less heterogeneity between studies for upper extremity strength (P = 0.242, I2 = 28.4%) and lower extremity strength (P = 0.171, I2 = 40.1%). However, subgroup analysis revealed differences in the effect of concurrent training on the impact of upper [SMD = 0.13, 95% CI (−0.17, 0.43), P = 0.318] and lower [SMD = 0.41, 95% CI (0.08, 0.74), P = 0.015] extremity strength in children with malignancy (Figure 3).
VO2Peak
Five papers (27, 29, 30, 34, 36) analyzed the effect of concurrent training on VO2Peak in children with malignancy, and SMD was chosen for the pooling of effect sizes because of the different measurement tools used. The heterogeneity between studies was small (P = 0.32, I2 = 14.9%), and a fixed-effects model was used for analysis, showing no statistically significant difference between the exercise and usual care groups in VO2Peak [SMD = 0.13, 95% CI (−0.18, 0.44), P = 0.397] (Figure 4A).
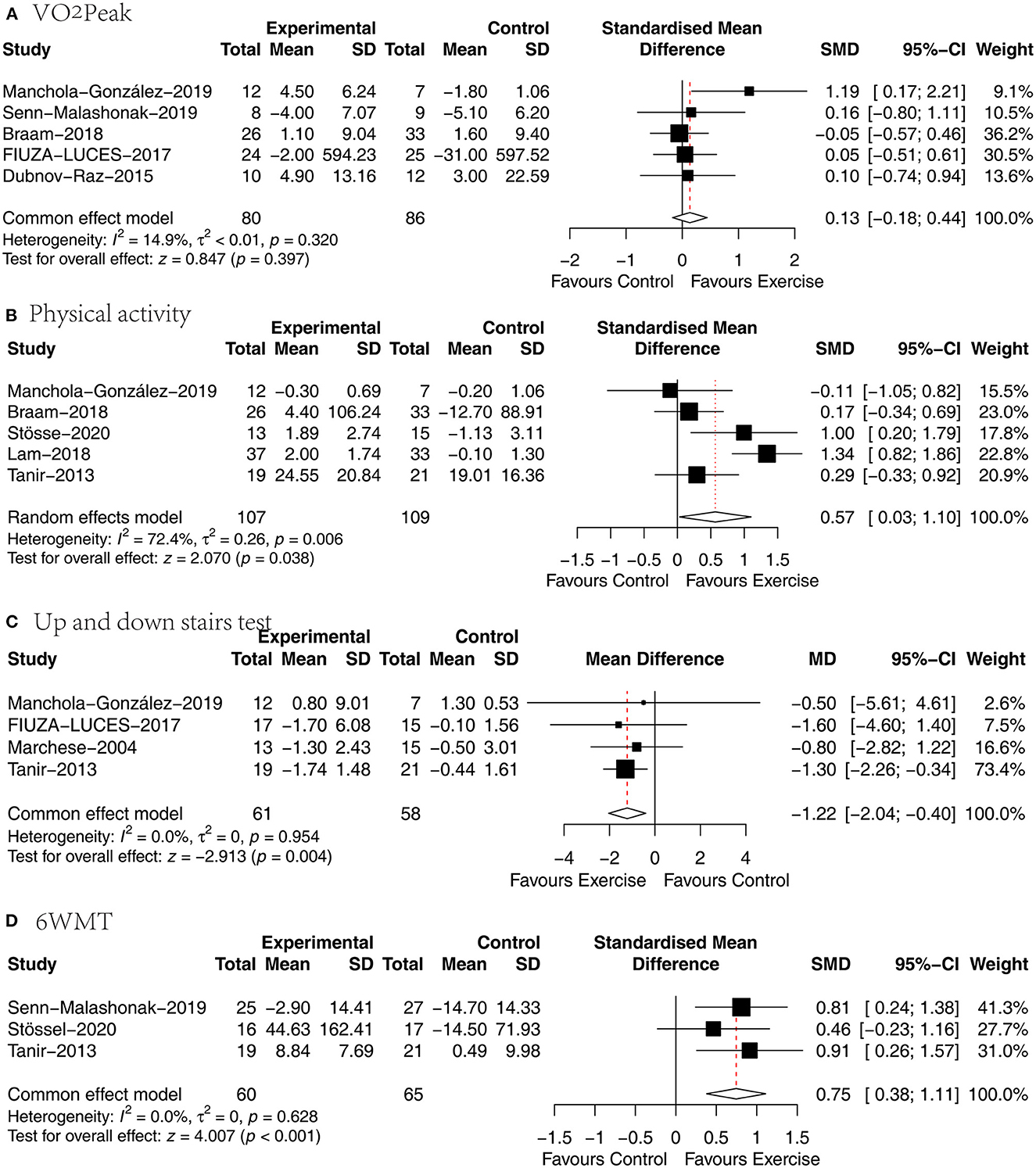
Figure 4. Results of meta-analysis of (A) VO2Peak, (B) physical activity, (C) up and down stairs test, (D) 6WMT.
Physical activity
Five articles (27, 31, 32, 35, 36) analyzed the effect of concurrent training on physical activity in children with malignancy, and5 the SMD was selected for effect size pooling because of the different physical activity measurement tools used. Heterogeneity between studies was significant (P = 0.006, I2 = 72.4%), and a random effects model was used for analysis, revealing a statistically significant difference in physical activity between the exercise and usual care groups [SMD = 0.57, 95% CI (0.03, 1.1), P = 0.038] (Figure 4B).
Up and down stairs test (TUDS)
Four papers (29, 33, 35, 36) analyzed the effect of concurrent training on time spent walking up and down stairs in children with malignancy, and the WMD was selected to pool effect sizes because of the consistency of the measurement tools used. The heterogeneity between studies was small (P = 0.954, I2 = 0.0%), and a fixed-effects model was used for analysis, showing that the time spent walking up and down stairs was significantly shorter in the exercise group than in the usual care group [SMD = −1.22, 95% CI (−2.04, −0.4), P = 0.004] (Figure 4C).
6WMT
Three papers (30, 31, 35) analyzed the effect of concurrent training on walking distance in children with malignancy, and the SMD was chosen for the combination of effect sizes because of the different measurement times used. There was no significant heterogeneity between studies (P = 0.628, I2 = 0%), and a fixed-effects model was used for the analysis revealing that the exercise group walked significantly farther than the usual care group [SMD = 0.75, 95% CI (0.38, 1.11), P < 0.01] (Figure 4D).
Quality of life
Six papers (27, 29, 31–34) analyzed the effect of concurrent training on health-related quality of life in children with malignancy, and the SMD was selected for the pooling of effect sizes because of the different quality-of-life measurement scales used. There was no heterogeneity between studies (P = 0.906, I2 = 0%), and a fixed effects model was used for analysis showing a statistically significant difference between the two groups in terms of health-related quality of life [SMD = 0.28, 95% CI (0.02, 0.53), P = 0.033] (Figure 5A).
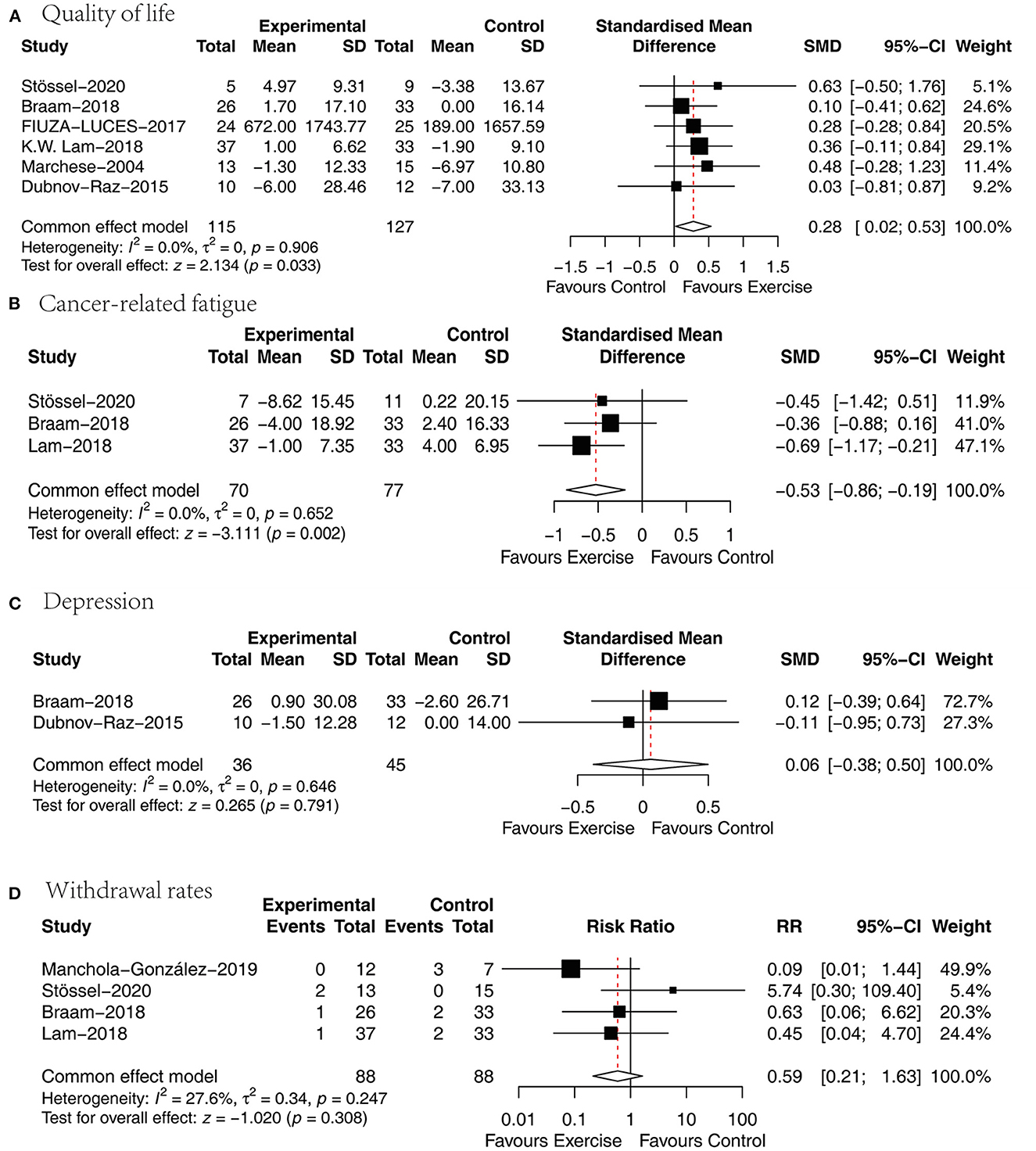
Figure 5. Results of meta-analysis of (A) quality of life, (B) cancer-related fatigue, (C) depression, (D) withdrawal rates.
Cancer-related fatigue
Three papers (27, 31, 32) analyzed the effect of concurrent training on cancer-related fatigue in children with malignancy, and the SMD was chosen for the combination of effect sizes because different cancer-caused fatigue measurement tools were used. One article used a cancer-related fatigue measurement tool that was assigned in the opposite direction to the rest of the studies, so this study data were processed according to the Cochrane Handbook so that they were assigned in the same direction. The heterogeneity between studies was small (P = 0.652, I2 = 0%), and a fixed-effects model was used for analysis showing that the cancer-related fatigue scores were significantly lower in the exercise group than in the usual care group [SMD = −0.53, 95% CI (−0.86, −0.19), P = 0.002] (Figure 5B).
Depression
Two papers (27, 34) analyzed the effect of concurrent training on the degree of depression in children with malignancy, and the SMD was chosen for pooling effect sizes because of the different depression measurement tools. There was a small heterogeneity between studies (P = 0.646, I2 = 0%), and a fixed effects model was used for the analysis revealing an insignificant difference between the two groups in terms of depression [SMD = 0.06, 95% CI (−0.38, 0.5), P = 0.791] (Figure 5C).
Withdrawal rates
Four papers (27, 31, 32, 36) presented active dropouts in the exercise group with those in the usual care group. There was a small heterogeneity between studies (P = 0.247, I2 = 27.6%), and a fixed-effects model was used for analysis showing that the difference was not statistically significant when comparing the two groups in terms of withdrawal rates [MD = 0.59, 95% CI (0.21, 1.63), P = 0.308] (Figure 5D).
GRADE evidence of outcomes
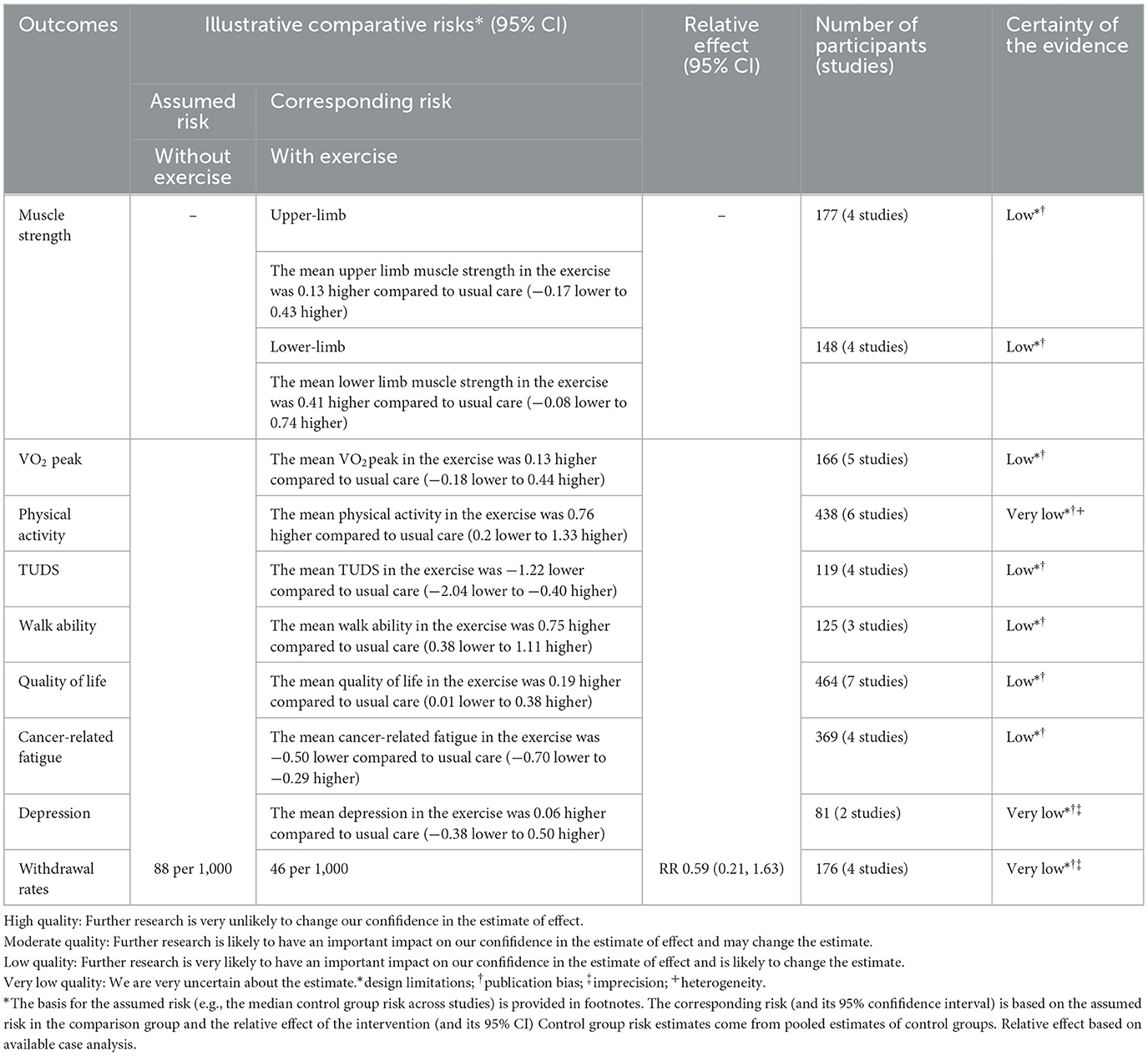
The GRADE system to assess the quality of evidence of the included studies revealed low to very low quality for each outcome. This may be due to poor or missing randomization methods, allocation sequence concealment, and risk of bias due to blinding. In addition, inconsistencies may arise in the occurrence and progression of childhood malignancies. Also, the study sample sizes were inadequate, likely introducing imprecision and publication bias. Detailed information on the quality of evidence for this meta-analysis is presented in Table 2.
Discussion
Many children with malignancies experience serious health problems during and after treatment. In rehabilitation medicine, it is commonly acknowledged that exercise rehabilitation has a good effect on a patient's functional level and physical health (37). Concurrent training improves athletic performance in athletes, but this is the first study to our knowledge to examine the impact of concurrent training on physical function and quality of life in children with malignancy. This systematic review and meta-analysis of nine randomized controlled trials included 371 children with malignancies demonstrated that compared to usual care, concurrent training enhanced muscle strength, increased mobility, shortened time to and from stairs, improved walking ability, and effectively reduced fatigue in children with cancer but had no significant effect on peak oxygen uptake and depression.
The 6MWT, as a measure of the body's dynamic endurance, reflects an individual's fitness level in terms of motor endurance by measuring 6WMT (28). Currently, the 6MWT is generally used to assess the cardiopulmonary function of patients, to guide patient treatment, to reflect patient prognosis and treatment outcome, and to predict morbidity and mortality. The present meta-analysis revealed that concurrent training prolonged the 6WMT in children with malignancy, which is consistent with the findings of Morales et al. This may be because concurrent training includes aerobic exercise, a form of endurance exercise that promotes metabolism and improves cardiopulmonary function in children, thereby increasing the 6WMT. This study demonstrated that concurrent training could effectively enhance the muscle strength of the lower limbs of children. The therapy for pediatric cancer and a prolonged sedentary lifestyle leads to muscle atrophy and impacts bone health (38). Concurrent training includes resistance exercise, which effectively increases muscle strength and can stimulate skeletal muscle, increase muscle blood supply, and increase the cross-sectional area of skeletal muscle type I and type II muscle fibers, thereby preventing the reduction of muscle volume and improving muscle strength in children with the disease. The present study depicted that the concurrent training reduced the completion time and significantly improved the children's ability to walk up and down stairs, which is consistent with the physical activity outcome and may be due to the huge development of the children's muscles and endurance. Despite the excellent performance in the physical function test, no significant changes were found in some of the physical function test indicators. Concurrent training had no significant impact on peak oxygen uptake in children with malignancy, which is consistent with the findings of Braam et al. (27), Morales et al. (39) suggested that long-term monitoring of this outcome should be performed to further clarify the effectiveness of exercise.
Cancer-related fatigue (CRF) is a painful, persistent, subjective feeling of fatigue and is associated with the tumor or tumor treatment and interferes with daily life (40). Almost all patients experience CRF during oncology treatment (41), and guidelines for managing CRF have been published in the United States, Canada, the United Kingdom, and China (42, 43). These guidelines highlight exercise as a cost-effective, non-pharmacological treatment with few adverse effects. This study confirms that concurrent training has a significant ameliorative effect on fatigue in children with malignancy. The mechanism of action may be closely related to the role of regulating HPA axis dysfunction by inhibiting the release of ACTH and promoting the release of Cor. Quality of life is a common indicator of cancer treatment and is widely used to measure the health status of children with malignant tumors. This indicator is measured by the PedsQLTM scale, and this study found that concurrent training was more effective than usual care. In addition, there was no statistically significant effect of concurrent training on depression in children with malignancy, which is inconsistent with the previous studies conducted in healthy children. An RCT of children demonstrated that a 9-month physical activity intervention improved the structure and function of brain networks associated with cognitive function and had a beneficial effect on mental health (44). Exercise increases brain-derived neurological factor (BDNF) (45) in the central nervous system and increases brain dopamine, 5-hydroxytryptamine, and norepinephrine concentrations, thereby improving anxiety and depressive symptoms (46). The reasons for the lack of significant effect of concurrent training on depressive symptoms in children with malignancy may be multifaceted. The short and low frequency of the included studies with intervention periods of 12 weeks and 6 months and a frequency of 2 sessions/week failed to significantly improve depressive symptoms. Data from several large cohort studies have depicted an association between lower levels of cardiorespiratory fitness and depressive symptoms (47–49). The results of this study indicate that concurrent training did not improve VO2Peak in children with malignancy, which may have contributed to the failure to alleviate depressive symptoms. Hayek noted that exercise intolerance is associated with emotional distress among pediatric cancer survivors and may lead to diminished interest in exercise in children, which in turn leads to reduced training effectiveness (50). There was no significant difference in the drop-out rate between the concurrent training group and the usual care group, indicating that the concurrent training was appropriate and feasible.
Regarding the trial locations, the concurrent training interventions for children with malignant tumors were mostly conducted in developed regions and countries, while the interventions for children with malignant tumors in developing countries still faced many obstacles. For example, the primary caregivers of children with malignant tumors often questioned the safety of exercise due to conventional beliefs; there was an absence of systematic exercise intervention programs for children with malignant tumors and a lack of awareness of the importance of exercise for children with malignant tumors among the children's families and healthcare professionals. Therefore, it is recommended to provide education and guidance on exercise intervention for children and their primary caregivers, as well as to increase multidisciplinary teamwork to design a safe and scientific exercise intervention program for children with malignant tumors. Currently, research on exercise interventions for children with malignancy in developing countries is still in its infancy, so pilot studies with small samples should be conducted to investigate the feasibility and application of exercise in children with malignancy.
Finally, this study has many limitations. First, due to the specificity of the disease, there are differences in the frequency, duration, and assessment tools of exercise interventions for children with malignancy, as well as large national and regional differences in the included literature and possible differences in the ethnicity and physical quality of the children, all of which contribute to clinical heterogeneity. Second, the monitoring effect of concurrent training is not clearly stated, as the way the child performs exercise at home and afterward may affect the results. Third, given the small number of included studies, the analysis is limited, and the results may not represent the entire population. Fourth, most of the included literature does not mention allocation concealment methods and blinding, which may lead to methodological heterogeneity. Finally, the lack of adherence due to exercise modality may also impact the experimental results. For example, the negative attitudes and self-evaluations regarding the concurrent training of children in the intervention group may have influenced the accuracy of the results.
Conclusion
Current evidence suggests that concurrent training could improve cancer-related fatigue, quality of life, muscle strength, and physical capacity in children with malignancy but has no significant effect on VO2Peak and Mental health. Because the quality level of evidence is mostly very low. High-quality, large multicenter randomized controlled trials are needed in the future to provide more reliable evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WZ, MF, and MZ: research idea and study design. WZ, MF, HC, and HS: data acquisition. WZ and MF: data analysis/interpretation. WZ, MF, HC, JY, XH, DL, and MZ: supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
This research was supported by the key project of the National Innovation Program (s202110632099).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1127255/full#supplementary-material
References
1. Collaborators GCC. The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol. (2019) 20:1211–25. doi: 10.1016/s1470-2045(19)30339-0
2. Fadhil I, Soliman R, Jaffar S, Al Madhi S, Saab R, Belgaumi A, et al. Estimated incidence, prevalence, mortality, and registration of childhood cancer (ages 0–14 years) in the WHO Eastern Mediterranean region: an analysis of GLOBOCAN 2020 data. Lancet Child Adolescent Health. (2022) 6:466–73. doi: 10.1016/S2352-4642(22)00122-5
3. Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. (2017) 18:719–31. doi: 10.1016/s1470-2045(17)30186-9
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
5. Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2016. Natl Cancer Inst. (2019) 1:16.
6. Butler E, Ludwig K, Pacenta HL, Klesse LJ, Watt TC, Laetsch TW. Recent progress in the treatment of cancer in children. CA Cancer J Clin. (2021) 71:315–32. doi: 10.3322/caac.21665
7. McEachron TA, Helman LJ. Recent advances in pediatric cancer research. Cancer Res. (2021) 81:5783–99. doi: 10.1158/0008-5472.CAN-21-1191
8. Skinner R. Long-term effects of cancer therapy in children–functional effects, late mortality and long-term follow-up. Paediat Child Health. (2012) 22:248–52. doi: 10.1016/j.paed.2012.02.008
9. Green DM. The 11th international conference on long-term complications of treatment of children and adolescents for cancer. Forward Pediatr Blood Cancer. (2012) 58:111. doi: 10.1002/pbc.23378
10. Zheng XQ, Wu YH, Huang JF, Wu AM. Neurophysiological mechanisms of cancer-induced bone pain. J Adv Res. (2022) 35:117–27. doi: 10.1016/j.jare.2021.06.006
11. Rodwin RL, Kairalla JA, Hibbitts E, Devidas M, Whitley MK, Mohrmann CE, et al. Persistence of chemotherapy-induced peripheral neuropathy despite vincristine reduction in childhood B-acute lymphoblastic Leukemia. J Natl Cancer Inst. (2022) 114:1167–75. doi: 10.1093/jnci/djac095
12. van Deuren S, Boonstra A, van Dulmen-den Broeder E, Blijlevens N, Knoop H, Loonen J. Severe fatigue after treatment for childhood cancer. Cochrane Database Syst Rev. (2020) 3:Cd012681. doi: 10.1002/14651858.CD012681.pub2
13. Grossoehme DH, Friebert S, Baker JN, Tweddle M, Needle J, Chrastek J, et al. Association of religious and spiritual factors with patient-reported outcomes of anxiety, depressive symptoms, fatigue, and pain interference among adolescents and young adults with cancer. JAMA Netw Open. (2020) 3:e206696. doi: 10.1001/jamanetworkopen.2020.6696
14. Van Cleve L, Muñoz CE, Savedra M, Riggs M, Bossert E, Grant M, et al. Symptoms in children with advanced cancer: child and nurse reports. Cancer Nurs. (2012) 35:115–25. doi: 10.1097/NCC.0b013e31821aedba
15. Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol. (2013) 31:2799–805. doi: 10.1200/JCO.2012.47.8081
16. Hudson MM, Armenian SH, Armstrong GT, Chow EJ, Henderson TO. Optimization of health and extension of lifespan through childhood cancer survivorship research. J Clin Oncol Off J Am Soc Clin Oncol. (2018) 36:2133–4. doi: 10.1200/JCO.2018.79.0477
17. Morales JS, Valenzuela PL, Rincón-Castanedo C, Takken T, Fiuza-Luces C, Santos-Lozano A, et al. Exercise training in childhood cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. (2018) 70:154–67. doi: 10.1016/j.ctrv.2018.08.012
18. Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. (2014) 61:1282–8. doi: 10.1002/pbc.25029
19. Gäbler M, Prieske O, Hortobágyi T, Granacher U. The effects of concurrent strength and endurance training on physical fitness and athletic performance in youth: a systematic review and meta-analysis. Front Physiol. (2018) 9:1057. doi: 10.3389/fphys.2018.01057
20. Wilson JM, Marin PJ, Rhea MR, Wilson SM, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. (2012) 26:2293–307. doi: 10.1519/JSC.0b013e31823a3e2d
21. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. (2018) 20:124. doi: 10.1186/s13058-018-1051-6
22. Uchiyama K, Adachi K, Muraoka K, Nakayama T, Oshida T, Yasuda M, et al. Home-based aerobic exercise and resistance training for severe chronic kidney disease: a randomized controlled trial. J Cachexia Sarcopenia Muscle. (2021) 12:1789–802. doi: 10.1002/jcsm.12775
23. Yarizadeh H, Eftekhar R, Anjom-Shoae J, Speakman JR, Djafarian K. The effect of aerobic and resistance training and combined exercise modalities on subcutaneous abdominal fat: a systematic review and meta-analysis of randomized clinical trials. Adv Nutr. (2021) 12:179–96. doi: 10.1093/advances/nmaa090
24. Scott JM, Thomas SM, Herndon JE, Douglas PS Yu AF, Rusch V, et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial. J Cachexia Sarcopenia Muscle. (2021) 12:1456–65. doi: 10.1002/jcsm.12828
25. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
26. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
27. Braam KI, van Dijk-Lokkart EM, Kaspers GJL, Takken T, Huisman J, Buffart LM, et al. Effects of a combined physical and psychosocial training for children with cancer: a randomized controlled trial. BMC Cancer. (2018) 18:1289. doi: 10.1186/s12885-018-5181-0
28. Chan WLS, Pin TW. Reliability, validity and minimal detectable change of 2-min walk test, 6-min walk test and 10-meter walk test in frail older adults with dementia. Exp Gerontol. (2019) 115:9–18. doi: 10.1016/j.exger.2018.11.001
29. Fiuza-Luces C, Padilla JR, Soares-Miranda L, Santana-Sosa E, Quiroga JV, Santos-Lozano A, et al. Exercise intervention in pediatric patients with solid tumors: the physical activity in pediatric cancer trial. Med Sci Sports Exerc. (2017) 49:223–30. doi: 10.1249/MSS.0000000000001094
30. Senn-Malashonak A, Wallek S, Schmidt K, Rosenhagen A, Vogt L, Bader P, et al. Psychophysical effects of an exercise therapy during pediatric stem cell transplantation: a randomized controlled trial. Bone Marrow Transpl. (2019) 54:1827–35. doi: 10.1038/s41409-019-0535-z
31. Stössel S, Neu MA, Wingerter A, Bloch W, Zimmer P, Paret C, et al. Benefits of exercise training for children and adolescents undergoing cancer treatment: results from the randomized controlled MUCKI trial. Front Pediatr. (2020) 8:243. doi: 10.3389/fped.2020.00243
32. Lam KKW Li WHC, Chung OK, Ho KY, Chiu SY, Lam HS, Chan GCF. An integrated experiential training programme with coaching to promote physical activity, and reduce fatigue among children with cancer: a randomised controlled trial. Patient Educ Couns. (2018) 101:1947–56. doi: 10.1016/j.pec.2018.07.008
33. Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. (2004) 42:127–33. doi: 10.1002/pbc.10481
34. Dubnov-Raz G, Azar M, Reuveny R, Katz U, Weintraub M, Constantini NW. Changes in fitness are associated with changes in body composition and bone health in children after cancer. Acta Paediat. (2015) 104:1055–61. doi: 10.1111/apa.13052
35. Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabil Nurs. (2013) 38:48–59. doi: 10.1002/rnj.58
36. Manchola-González JD, Bagur-Calafat C, Girabent-Farrés M, Serra-Grima JR, Pérez R, Garnacho-Castaño MV, et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. (2020) 28:3171–8. doi: 10.1007/s00520-019-05131-2
37. Zang W, Fang M, He H, Mu L, Zheng X, Shu H, et al. Comparative efficacy of exercise modalities for cardiopulmonary function in hemodialysis patients: a systematic review and network meta-analysis. Front Public Health. (2022) 10:704. doi: 10.3389/fpubh.2022.1040704
38. Kelly PM, Pottenger E. Bone health issues in the pediatric oncology patient. Semin Oncol Nurs. (2022) 38:151275. doi: 10.1016/j.soncn.2022.151275
39. Morales JS, Santana-Sosa E, Santos-Lozano A, Baño-Rodrigo A, Valenzuela PL, Rincón-Castanedo C, et al. Inhospital exercise benefits in childhood cancer: a prospective cohort study. Scand J Med Sci Sports. (2020) 30:126–34. doi: 10.1111/sms.13545
40. Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol Off J Am Soc Clin Oncol. (2014) 32:1840–50. doi: 10.1200/JCO.2013.53.4495
41. Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. (2003) 362:640–50. doi: 10.1016/S0140-6736(03)14186-4
42. Snowden JA, Greenfield DM, Bird JM, Boland E, Bowcock S, Fisher A, et al. Guidelines for screening and management of late and long-term consequences of myeloma and its treatment. Br J Haematol. (2017) 176:888–907. doi: 10.1111/bjh.14514
43. Carlson RW, Larsen JK, McClure J, Fitzgerald CL, Venook AP, Benson AB, et al. International adaptations of NCCN clinical practice guidelines in oncology. J Natl Compreh Cancer Netw. (2014) 12:643–8. doi: 10.6004/jnccn.2014.0068
44. Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, et al. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. (2014) 134:e1063–71. doi: 10.1542/peds.2013-3219
45. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. (2007) 30:464–72. doi: 10.1016/j.tins.2007.06.011
46. Young SN. How to increase serotonin in the human brain without drugs. J Psychiatry Neurosci JPN. (2007) 32:394–9. doi: 10.1503/jpn.070169
47. Wilson CL, Chemaitilly W, Jones KE, Kaste SC, Srivastava DK, Ojha RP, et al. Modifiable factors associated with aging phenotypes among adult survivors of childhood acute lymphoblastic Leukemia. J Clin Oncol Off J Am Soc Clin Oncol. (2016) 34:2509–15. doi: 10.1200/JCO.2015.64.9525
48. Dishman RK, Sui X, Church TS, Hand GA, Trivedi MH, Blair SN. Decline in cardiorespiratory fitness and odds of incident depression. Am J Prev Med. (2012) 43:361–8. doi: 10.1016/j.amepre.2012.06.011
49. Willis BL, Leonard D, Barlow CE, Martin SB, DeFina LF, Trivedi MH. Association of midlife cardiorespiratory fitness with incident depression and cardiovascular death after depression in later life. JAMA Psychiatry. (2018) 75:911–7. doi: 10.1001/jamapsychiatry.2018.1467
50. Hayek S, Brinkman TM, Plana JC, Joshi VM, Leupker RV, Durand JB, et al. Association of exercise intolerance with emotional distress, attainment of social roles, and health-related quality of life among adult survivors of childhood cancer. JAMA Oncol. (2020) 6:1194–202. doi: 10.1001/jamaoncol.2020.2054
Keywords: pediatric, physical performance, quality of life, concurrent training, exercises (physical-skill)
Citation: Zang W, Fang M, Chen H, Huang X, Li D, Yan J, Shu H and Zhao M (2023) Effect of concurrent training on physical performance and quality of life in children with malignancy: A systematic review and meta-analysis. Front. Public Health 11:1127255. doi: 10.3389/fpubh.2023.1127255
Received: 19 December 2022; Accepted: 28 February 2023;
Published: 17 March 2023.
Edited by:
Henrique Pereira Neiva, University of Beira Interior, PortugalReviewed by:
David Hollar, Mercer University School of Medicine, United StatesWei Xia, Wuhan Medical Center for Women and Children, China
Copyright © 2023 Zang, Fang, Chen, Huang, Li, Yan, Shu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyuan Zhao, emhhb21pbmd5dWFuQGhyYmlwZS5lZHUuY24=
 Wanli Zang
Wanli Zang Mingqing Fang
Mingqing Fang Haohao Chen1
Haohao Chen1 Xinmeng Huang
Xinmeng Huang Heng Shu
Heng Shu Mingyuan Zhao
Mingyuan Zhao