- 1Fondation Mérieux, Lyon, France
- 2Center of Infectiology Christophe Mérieux of Laos, Vientiane, Laos
- 3Fondation Mérieux, Vientiane, Laos
- 4Service Hygiène, Epidémiologie et Prévention, Centre Hospitalier Hôpital Eduard Herriot, Hospices Civils de Lyon, Lyon, France
- 5CIRI, Centre International de Recherche en Infectiologie, Team Public Health, Epidemiology and Evolutionary Ecology of Infectious Diseases (PHE3ID), University Lyon, Inserm, U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, ENS de Lyon, Lyon, France
- 6Communicable Diseases Policy Research Group, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 7Faculty of Public Health, Mahidol University, Bangkok, Thailand
Background: Data on the epidemiology of Streptococcus pneumoniae among influenza-like illness (ILI) cases, particularly in low- and middle-income countries are scarce. This study assessed the prevalence, risk factors and serotype distribution of S. pneumoniae carriage among ILI cases in metropolitan Vientiane, Lao People's Democratic Republic. The 13-valent pneumococcal conjugate vaccine (PCV13) was introduced among infants in October 2013.
Methods: Active ILI surveillance was conducted through weekly phone calls in an open community-based cohort study (April 2015–February 2019), involving 5,690 participants from 1,142 randomly selected households. Participants reporting ILI symptoms provided a nasopharyngeal swab and answered a questionnaire. S. pneumoniae and serotype pneumococcal-positive samples were screened by Multiplex PCR assays. Chi-squared tests and generalized linear mixed models were used to test for variables associated with pneumococcal positivity.
Results: Among 1,621 ILI episodes, 269 (16.6%) tested positive for nasopharyngeal pneumococcal carriage, with the highest prevalence (55.4%) in children under 5 years. Pneumococcal carriage was significantly associated with concurrent detection of Hemophilus influenzae (adjusted odds ratio [aOR]: 6.93; 95% CI: 2.10–22.9) and exposure to household cigarette smoke (aOR: 1.65; 95% CI: 1.07–2.54). PCV13 serotypes accounted for 37.8% of all pneumococcal isolates. Detection of PCV13 serotypes among ILI cases aged under 5 years declined significantly between 2015/16 and 2018/19.
Conclusions: Community-based surveillance of S. pneumoniae among ILI cases complement surveillance at healthcare facilities to provide a more complete picture of pneumococcal carriage. Our findings contribute also to the growing body of evidence on the effects of PCV13 introduction on circulating serotypes and their potential replacement.
1. Introduction
Streptococcus pneumoniae is a major cause of community-acquired pneumonia and meningitis worldwide, particularly in low- and middle-income countries (1). Although all age groups are affected, the highest incidence rates of pneumococcal disease occur in young children and older adults (1, 2). Pneumococcal bacteria also frequently colonize the respiratory tract of asymptomactic carriers. Nasal carriage and related pneumococcal disease have been shown to be influenced by the presence of respiratory viruses, particularly during influenza seasons (3, 4).
Prevention of pneumococcal pneumonia involves the 23-valent pneumococcal polysaccharides vaccines (PPSV23) for adults and pneumococcal conjugate vaccines (PCV) for children. Lao PDR introduced the 13-valent PCV (PCV13) into the national childhood vaccination program in October 2013, using a 3 + 0 schedule at 6, 10 and 14 weeks of age (5). Catch-up vaccination was offered to infants up to 12 months old during the first roll-out. National coverage of the third dose of PCV13 among infants was estimated at 89% in 2020 (6).
Most previous studies on S. pneumoniae burden and serotype distribution in LMICs, including in Lao PDR, have been based on healthcare facility surveillance in infants and young children (5, 7, 8). To better understand S. pnuemoniae epidemiology and transmission, including risk factors for carriage or infection, and the impact of vaccination on circulating serotypes, prospective community-based studies, across all age groups, are also needed.
The longitudinal surveillance study of respiratory infectious agents in metropolitan Vientiane, Lao PDR (LaCoRIS) is a community-based cohort study that monitored the etiology and burden of influenza-like illness among residents of this region from 2015 to 2019. The findings of the first year of surveillance, covering over 20 respiratory pathogens, have been published elsewhere (9), with S. pneumoniae the most comon bacterial agent detected in cases of influenza-like illness (ILI) (9). In this paper, we focus on S. pneumoniae as the main outcome of interest, to report on prevalence, risk factors and serotype distribution of pneumococcal carriage among ILI cases in the LaCoRIS study during the three surveillance years.
2. Methods
Full details on the LaCoRIS study design, including recruitment and surveillance of households in the cohort, are reported elsewhere (9). In brief, participants were recruited from randomly selected households in 25 villages in metropolitan Vientiane. Participant enrolment into the cohort was conducted at two main timepoints. The first enrolment phase began in April 2015, during which a total of 4,855 individuals from 995 households were recruited and followed-up for ~1 year (until 31 May 2016), as reported previously (9). The cohort study then resumed in March 2017 (i.e., approximately a year after the end of the first surveillance period), with a second enrolment phase conducted to initiate a further 2 years of ILI surveillance. For this second phase, all previously recruited households were invited to participate, along with additional, randomly selected households within the same villages, to account for loss of follow-up. Thus, ILI surveillance was conducted among an open cohort over three surveillance years i.e., year 1 (from 28/04/2015 to 31/05/2016), year 2 (from 01/03/2017 to 28/02/2018), and year 3 (from 01/03/2018 to 28/02/2019).
During these surveillance periods, active case finding for ILI was conducted through weekly phone calls to each participating household. Individuals fulfilling the WHO case definition for ILI were visited to administer a questionnaire and collect a nasopharyngeal swab, after obtaining informed written consent (National Ethics Committee 060/NECHR; 1 January 2017). Follow-up questionnaires were administered to ILI cases ~40 days after the sample collection and case investigation, to obtain information on clinical outcomes (e.g., hospitalization or sequelae) that had since occurred in relation to the ILI episode.
The FTD® Respiratory pathogens 21 Plus technology was used for real-time Polymerase Chain Reaction (qPCR) testing of swabs, as detailed previously (9). Detection of S. pneumoniae with this assay targets the lyt A gene, a largely conserved gene encoding the major autolytic enzyme of pneumococcus. This approach is considered a gold standard among culture-independent S. pneumoniae assays, with reported sensitivity and specificity of 100 and 99.5%, respectively (10), with a detection limit of one plasmid copy per 10μl reaction (11). S. pneumoniae-positive samples were then screened for 40 different serotypes using a multiplex qPCR assay, following published protocols (12). This assay has shown high sensitivity for the included serotypes, able to detect < 100 colony forming units (CFU) per reaction for the majority (91%) of strains tested (12).
All statistical analyses were conducted in R version 4.2.2. Incidence of ILI and 95% confidence intervals (CI) were estimated using complex survey analysis methods, adjusting for the demographic structure (age and sex) of the Vientiane urban population and clustering in the survey design, as detailed previously (9, 13). The prevalence of pneumococcal detection among ILI cases, and the proportional distribution of serotype categories among pneumococcal-positive ILI cases, were calculated and assessed for associations with age-group, surveillance year, and climatic season (wet vs. dry months) using chi-squared tests. Binomial generalized linear mixed models (GLMMs), adjusting for random effects at household-level, were used to further investigate risk factors for pneumococcal positivity among ILI cases using the “lme4” R package (14). Bivariable GLMM analyses tested for associations with each independent variable separately, while multivariable GLMMs included age group, sex, and any covariates which showed associations with P < 0.05 in the bivariable models. We were unable to adjust for individual level random effects in these GLMMs as the majority of study subjects with ILI experienced only a single episode throughout the surveillance period (Models specifying two nested levels of random effects, i.e., for both households and individuals, displayed poor convergence and singularity issues, indicating that these were overly complex for the dataset). However, to assess whether the results of our GLMM analyses may have been influenced by individual-level random effects which could not be adjusted for in the models, we also repeated these analyses with a subset of the data which included only a single ILI episode per subject. Simpson's Diversity Indices with 95% Cis were calculated using the “simboot” package (15) to assess for differences in diversity of serotypes between age groups and surveillance years, following Løchen et al. (16).
3. Results
Throughout the study period, a total of 1,142 households (5,690 individuals) were recruited. Of 995 households (4,885 individuals) enrolled in the first surveillance period (2015/16) (9), 936 households (94.1%) enrolled for the second phase (2017–19), along with an additional 147 new households (765 individuals). The mean duration of ILI surveillance for each household was 148 weeks (range 51–141 weeks), with a total follow-up size of 16,104 person-years across all households and surveillance years.
3.1. Incidence of ILI
During the three surveillance years, 1,621 ILI episodes were reported among 1,116 (19.6%) individuals from 613 (53.6%) households. The mean age of ILI cases was 37 years (range 1–100), with 74 (4.6%) and 316 (19.5%) of cases aged < 5 years and 5–15 years, respectively. Over half (970; 59.8%) of the ILI cases were females. The estimated incidence of ILI, adjusted for participant follow-up time and the demographic structure of the Vientiane population, was 10.0 (95% CI: 9.1–11.0) episodes per 100 person-years.
3.2. Prevalence and risk factors for pneumococcal carriage
Among 1,621 ILI episodes, 269 (16.6%) tested positive for presence of nasopharyngeal S. pneumoniae. The highest prevalence was observed in children under 5 years (55.4%), followed by those aged 5–15 years (39.2%), with relatively low prevalence observed in cases aged over 16 years (8.4%) ( 256.6, P < 0.001) (Table 1, Figure 1A). In addition to strong associations with age, pneumococcal carriage was significantly associated with concurrent nasopharyngeal detection of Hemophilus influenzae (adjusted odds ratio [aOR]: 6.93; 95% CI: 2.10–22.9) and exposure to household cigarette smoke (aOR: 1.65; 95% CI: 1.07–2.54) in both bivariable and multivariable GLMM analyses (Table 1). No significant associations were observed with variables such as surveillance year, season, sex, socio-economic status, or respiratory virus co-infections. Household “crowding” (number of persons per sleeping room), and the number of children within the household, were significantly associated with pneumococcal carriage in bivariable analyses, but not after adjusting for other covariates. GLMM analyses conducted on a subset of the data, which excluded data from repeat ILI episodes within the same individuals, identified very similar bivariable and multivariable associations (Supplementary Table 1). This suggests that the presence of any individual-level random effects, which we were unable to adjust for in the GLMMs (see Methods), did not substantially bias or otherwise influence the associations shown in Table 1.
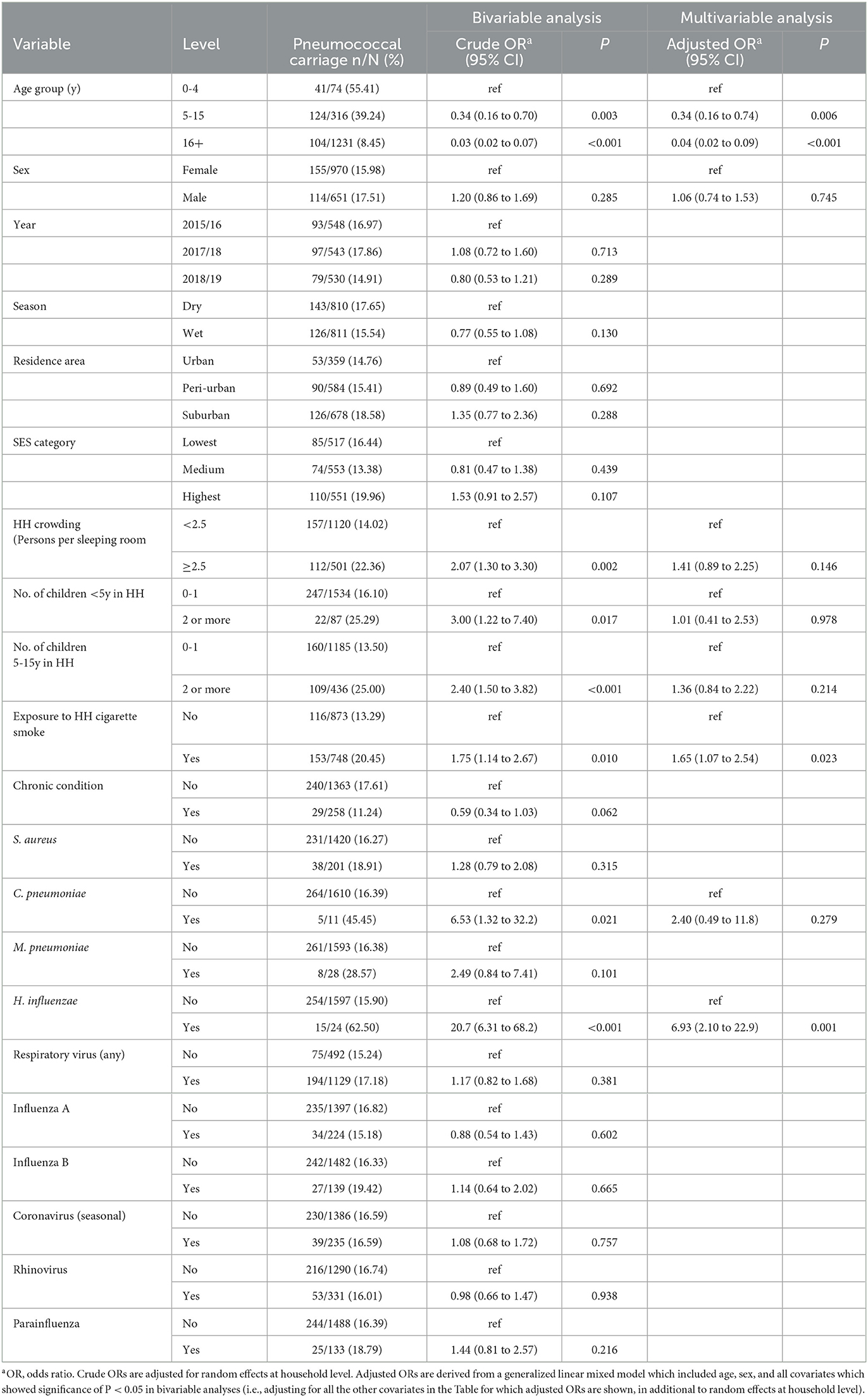
Table 1. Bivariable and multivariable analyses for associations with nasopharyngeal pneumococcal detection among influenza-like illness cases (n = 1,621).
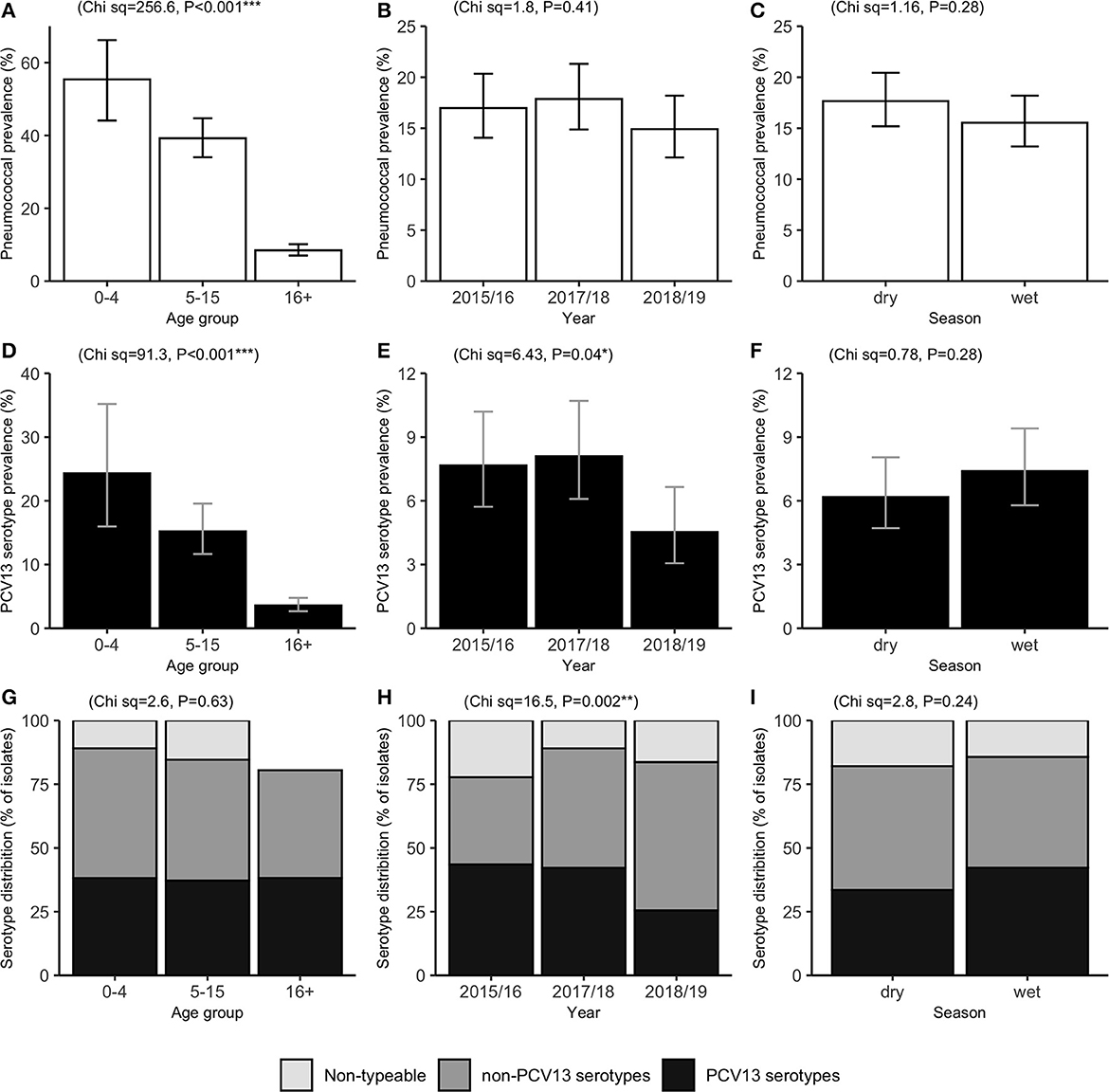
Figure 1. Prevalence of pneumococcal carriage (A–C) and PCV13 serotype carriage (D–F), and the percentage of pneumococcal isolates by serotype category (G–I). Results are shown among influenza-like illness cases (n = 1,621), by age group (left), surveillance period (middle), and season (right). PCV13: 13-valent pneumococcal conjugate vaccine. Error bars represent exact 95% confidence intervals. Chi-squared (Ch sq) statistics and P-values for association with x-axis levels are shown at the top of each plot.
3.3. Prevalence and distribution of pneumococcal serotypes
Serotyping data was successfully obtained from 215 (79.9%) of the pneumococcal-positive cases; the remainder were classified as carrying non-typeable (NT) isolates. Carriage of at least one vaccine serotype (VT), defined as any serotype included in the PCV13 vaccine, was detected in 110 cases, corresponding to 6.8% of all ILI episodes and 40.9% of pneumococcal-positive ILI episodes. The percentage and cumulative percentage of pneumococcal isolates by each serotype are shown in Figure 2A. VT isolates accounted for 126 (37.8%) of all pneumococcal isolates (including NT isolates) detected across ILI specimens. The most commonly detected VT serotypes were 6AB, 19F, 3 and Sg18, while no isolates of serotype 5 were detected. Of the 28 non-vaccine serotypes (NVTs) included in the serotyping PCR assay, 24 were detected in at least one ILI episode, with the most common NVTs being 11A, 35B, 15A, and 20. Simpson's diversity indices did not indicate any significant differences in serotype diversity between age groups or surveillance years (data not shown).
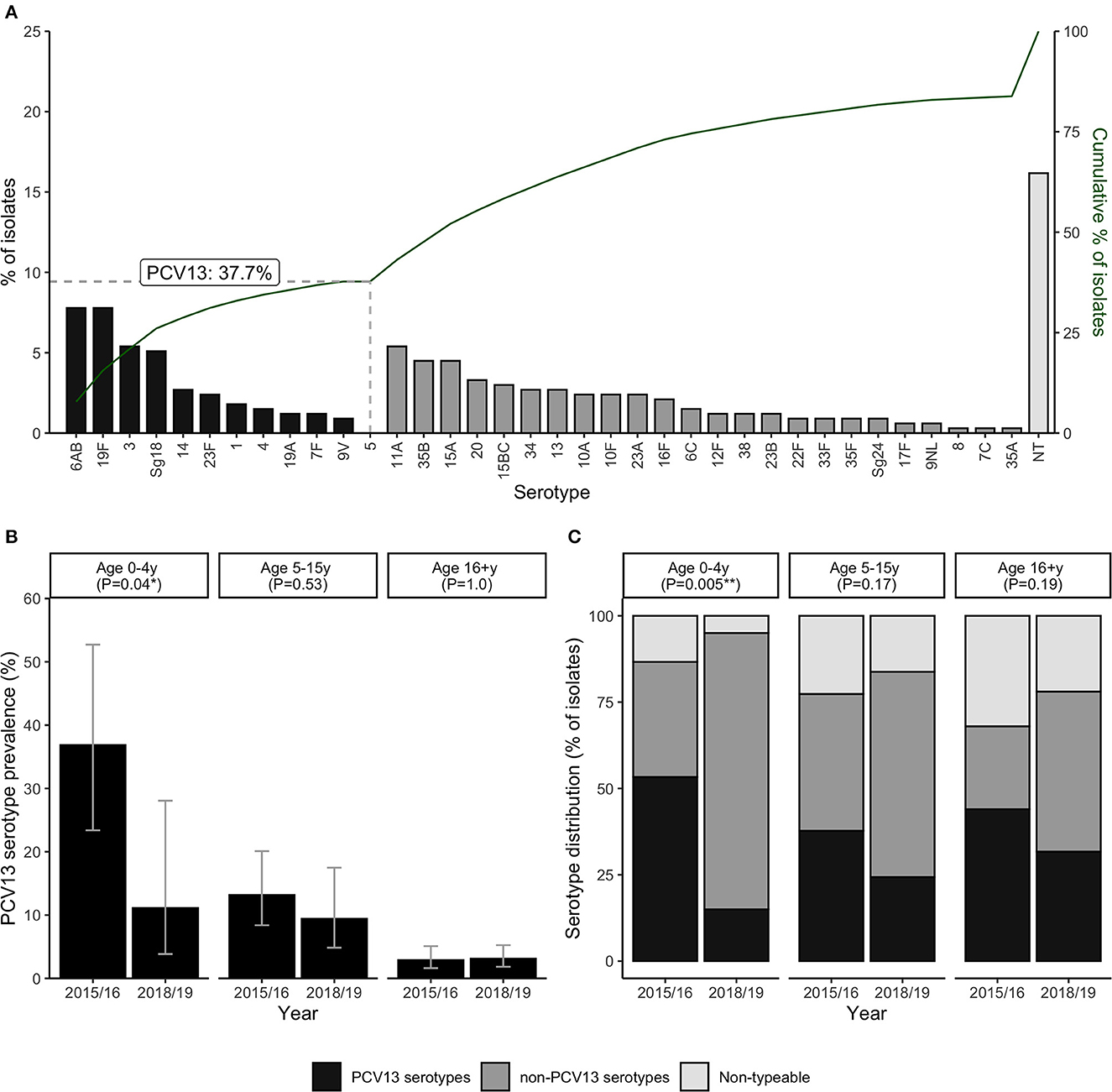
Figure 2. Carriage of pneumococcal vaccine (PCV13) and non-vaccine serotypes among ILI cases (n = 1,621). Panels show the percentage distribution and cumulative distribution of pneumococcal isolates by individual serotype (A); PCV13 serotype carriage by age group and surveillance year (B); and the percentage of pneumococcal isolates in each serotype category by age group and surveillance year (C). P-values in panels B, C are derived from Chi-squared tests for differences between surveillance years. Error bars in panel B represent exact 95% confidence intervals.
The percentage distributions of isolates by serotype category (VT, NVT, and NT) were very similar across age groups ( 2.6, P = 0.63) (Figure 1). However, there was evidence of a temporal change in VT carriage ( = 6.4, P = 0.04), which fell from 7.6 to 8.1% in Years 2015/16 and 2017/18, to 4.5% in 2018/19. This trend was also observed in the distribution of isolates by serotype category ( 16.5, P = 0.002), with VT accounting for over 40% of isolates in 2015/16 and 2017/18, and only 25.5% in 2018/19 (Figure 1).
Further analyses were carried out to investigate whether this decrease in prevalence of VT detection over the study period was age-group specific (Figures 2B, C). Data from the second surveillance year (2017/18) were excluded from these age-stratified analyses, due to the low sample size of children under 5y during this period (only 9 ILI cases, of which 3 were pneumococcal-positive). Notably, the decrease in VT detection between 2015/16 and 2018/9 was much more pronounced, and only statistically significant, in children under 5y. In this age group, prevalence of VT detection fell from 36.8% (14/38) to 11.1% (3/27) ( 1.2, P = 0.04) (Figure 2B), and the percentage of isolates identified as VT serotypes fell from 55.3% to 15.0% ( 10.5, P = 0.005) (Figure 2C).
3.4. Serotype co-detection
More than one serotype was detected in 52 (19.3%) of the 269 pneumococcal-positive cases (Figure 3). Two serotypes were detected in the majority (41/52; 78.8%) of cases with serotype co-detection, while three serotypes were detected in nine cases (17.3%), and four in two cases (3.8%). Among pneumococcal-positive cases, prevalence of serotype co-detection was highest in cases under 5y (11/41; 26.8%), followed by 5–15y (24/124; 19.4%) and cases >15y (17/104; 16.3%), although this trend was not significant ( =2.07, P = 0.35).
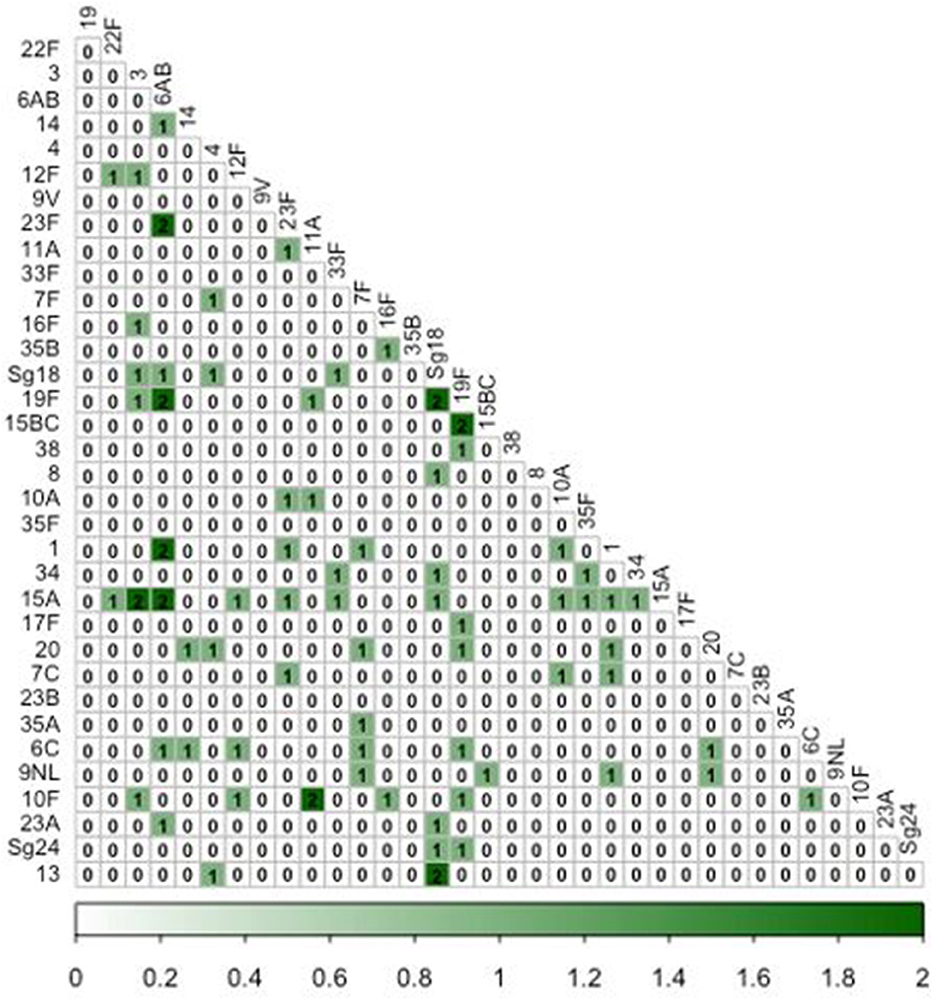
Figure 3. Co-occurrence matrix of pneumococcal serotypes among influenza-like illness cases. Numbers within cells represent the number of ILI episodes in which each pairwise combination of serotypes was detected.
3.5. Serotype recurrence among individuals with multiple ILI episodes
Throughout the surveillance period, 42 individuals experienced more than one pneumococcal-positive ILI episode (with a median 2, and range of 2 to 4 episodes across these individuals). However, repeat detections of the same serotype were observed in only nine (21.4%) of these individuals. The serotypes involved in these repeat detections were 6AB (n = 3 individuals), 11A (n = 2), 6C, 19A, 15A and 13. The median time interval between ILI episodes with a recurring serotype was 4.5 months (range: 1 to 36 months). It is unknown whether these events represented serotype persistence, or independent acquisitions at different time points.
3.6. Hospitalizations
Based on follow-up questionnaires (administered ~40 days after each ILI case investigation and sample collection), hospital admissions among ILI episodes were low (1.2%), and not significantly associated with pneumococcal positivity. Only three (1.1%) of the pneumococcal-positive ILI episodes were hospitalized; these cases were aged 4, 12, and 14 years, with serotypes 11A, 15A, and Sg18, respectively. The significance of S. pneumoniae detection in these cases is unclear; all three samples were also positive for at least one respiratory virus (influenza A/H3N2 in two cases, one of whom was also positive for seasonal coronavirus 229, while the third case was positive for respiratory syncytial virus). Their hospital stays were brief (~24 h), and all had fully recovered at the time of the follow-up visit. Official clinical diagnoses were not recalled by these participants or their relatives.
4. Discussion
Few studies have reported on pneumococcal detection and serotypes among ILI cases, particularly among cases identified through community-based surveillance as opposed to surveillance at healthcare facilities. In this study, we describe the prevalence and risk factors of nasopharyngeal pneumococcal detection among subjects with ILI in Lao PDR. In addition to higher prevalence in younger age groups, pneumococcal positivity was significantly associated with passive smoking and concurrent detection of Hemophilus influenzae. Detection of PCV13 serotypes was observed to decrease over the study period, although this trend was only significant in ILI cases under 5 y.
The inverse relationship between age and pneumococcal carriage has been widely reported elsewhere, including in studies among healthy children in Lao PDR (7) and other countries in Southeast Asia (17).
While the high prevalence of pneumococcal carriage in young children could implicate S. pneumoniae as an important etiological pathogen of ILI in this population, it is important to acknowledge that the clinical significance of pneumococcal detection among our study participants is unclear. S. pneumoniae bacteria commonly colonize the nasopharynx without leading to invasive disease, and ILI symptoms can be caused by a diverse range of viral and bacterial pathogens. We cannot provide evidence of a temporal association between ILI occurrence and pneumococcal acquisition/infection during the study period because only a single nasopharyngeal specimen was collected during the acute phase of ILI. Furthermore, we did not collect nasopharyngeal swabs in study participants without ILI. Thus, we refer to pneumococcal-positivity among ILI cases in our study as “carriage”, rather than pneumococcal infection or disease. While it is possible that some cases may have represented bacterial pneumonia initially presenting as ILI, our follow-up data on hospitalizations do not provide evidence support this. Hospitalizations among ILI cases were low, and not associated with S. pneumoniae detection. Moreover, all three pneumococcal-positive cases who were briefly admitted were also positive for at least one respiratory virus.
Consistently with other studies (18, 19), we identified passive smoking as a risk factor for pneumococcal detection. Exposure to cigarette smoke has been shown to impair nasal mucociliary clearance and increase pneumococcal adherence to respiratory epithelia (20–22), which may explain the associations observed in our study. Carriage of Hemophilus influenzae was also significantly associated with increased risk of pneumococcal positivity. A synergistic association between S. pneumoniae and H. influenzae colonization has been reported among Australian Aboriginal (23) and Italian children (24). The latter study also found that children vaccinated with PCV were even more likely to be colonized by H. influenzae. Commensal coexistence of these bacteria within a biofilm in the human nasopharynx has been suggested as a mechanism enabling their persistence in the host (25).
Similar to a study among hospitalized children with acute respiratory infection in Lao PDR between 2013 and 2019 (5), VT strains were found in 41% of pneumococcal-positive ILI cases, and accounted for 38% of all pneumococcal isolates detected. The significant decrease in VT detection between 2015/16 and 2018/19 among ILI cases aged < 5y was likely due to the impact of vaccination; all ILI cases in this age group that occurred in 2015/16 were born prior to October 2013 (i.e., before PCV13 introduction in Lao PDR), while the ILI cases aged < 5y in 2018/19 were born during or after this month, and therefore more likely to have been vaccinated. However, the relative contributions of direct vs. indirect effects of PCV13 on VT carriage in young children cannot be ascertained through our data. The cohort study was designed to investigate the etiology and epidemiology of ILI in the community, rather than PCV13 effectiveness in young children, and data on vaccination status of study participants were not available. Nonetheless, the temporal decrease in the proportion of VT isolates also seen among older age-groups (who would not have been vaccinated), while not statistically significant, may point to an emerging indirect (herd) effect due to decreasing circulation of PCV13 serotypes. Cross-sectional carriage surveys pre- and post-PCV have similarly reported evidence of the indirect effect of PCV13 in Lao PDR (5, 8). We also note that, despite the temporal decline in VT detection, we did not observe a significant change in overall pneumococcal prevalence during the study period. This may represent preliminary evidence of serotype replacement, although longer term studies are needed to further support this.
Similar to our results, serotypes 6A/B, and 19F have been found as the most prevalent PCV13 serotypes among 12–23-month-old Laotian children both before and after PCV13 introduction (8). The most common NVT serotypes in our study were 11A, 35B, 15A and 20. Serotypes 15A and 11A have been identified as among the top 10 serotypes causing invasive pneumococcal disease post-vaccination in France and Norway, respectively (16). Further investigation is warranted on the need for broader pneumococcal vaccines in Lao PDR, including the PPSV23, PCV15 or PCV20 which have been introduced, for example in older age groups, in other countries.
Two key limitations of our study are that, as discussed above, we are unable to draw strong conclusions regarding clinical significance of pneumococcal carriage in our study population, or the precise role of PCV13 introduction on changes in serotype distributions. Indeed, these reflect challenges associated with S. pneumoniae surveillance in ILI cases more generally. The use of serological assays (26, 27), alongside nasopharyngeal qPCR testing, could help elucidate the clinical significance of pneumococcal detection in future ILI studies. Nonetheless, our study illustrates how samples and data from community-based ILI surveillance can still be leveraged, for example to identify risk factors, circulating serotypes, and emerging trends in which may indicate serotype replacement. Another limitation is that serotype data could not be obtained for 21.1% of pneumococcal-positive swabs. S. pneumoniae screening was based on PCR detection of the lyt A gene, an important virulence marker which is thought to play a role in nasopharyngeal colonization (28). Realtime PCR targeting of lyt A is considered one of the gold standards among culture-independent S. pneumoniae assays, showing high diagnostic sensitivity and specificity (10, 11, 28). However, lyt A positivity has also been reported for some non-encapsulated pneumococci, as well as a small number of non-pneumococcal isolates (e.g., Streptococcus pseudopneumoniae carrying lyt A homologs) (10). Thus, while it seems likely that most of our “non-typeable” samples represent encapsulated S. pneumoniae serotypes (i.e., which were not included in the multiplex typing PCR), it is possible that some were due to non-encapsulated strains or even non-pneumococci, which are of lower clinical and vaccine importance. We also acknowledge the possibility that variation in detection limits of the serotyping assay for different strains could have had some influence on the observed serotype and NT frequencies (12).
Conclusion
In conclusion, we found pneumococcal carriage among ILI cases in Vientiane to be associated with younger age, passive smoking, and concurrent H. influenzae colonization. Our findings contribute also to the growing body of evidence from low- and middle-income countries indicating the effect of PCV13 on serotype distributions. Analysis of S. pneumoniae carriage data among ILI cases detected through community-based surveillance, such as the LaCORIS study, can complement data from surveillance at health facilities, to provide a more complete picture of pneumococcal burden and circulating serotypes across all age-groups, including associations with other respiratory pathogens. Such evidence can help inform future studies aiming to evaluate the impact of vaccination on community transmission, and identify which serotypes may be important to consider in future vaccines.
Data availability statement
The datasets presented in this article are not readily available because it is confidential information that will require the authorization of several study partners including the Funder, MoH, and Ethics Committee. Requests to access the datasets and R scripts should be directed to the corresponding author VSP.
Ethics statement
The studies involving human participants were reviewed and approved by the Lao National Ethics Committee for Health Research in Lao PDR (060/NECHR; 1 January 2017). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
VSP designed the study. IK, PP, and BF coordinated and supervised data collection. JR analyzed the data. MS-E, JR, and VSP drafted the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was funded by the Naval Medical Research Unit Two. The funders had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1124016/full#supplementary-material
References
1. Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
2. Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. (2014) 20:45–51. doi: 10.1111/1469-0691.12461
3. Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis. (2014) 58:1369–76. doi: 10.1093/cid/ciu148s
4. Shrestha S, Foxman B, Weinberger DM, Steiner C, Viboud C, Rohani P. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. (2013) 5:982. doi: 10.1126/scitranslmed.3005982
5. Chan J, Lai JYR, Nguyen CD, Vilivong K, Dunne EM, Dubot-Pérès A, et al. Indirect effects of 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in children hospitalised with acute respiratory infection despite heterogeneous vaccine coverage: an observational study in Lao People's Democratic Republic. BMJ Glob Health. (2021) 6:e005187. doi: 10.1136/bmjgh-2021-005187
6. WHO/UNICEF. Pneumococcal Vaccination Coverage. WHO Immun Data Portal. (2023). Available online at: https://immunizationdata.who.int/pages/coverage/pcv.html (Accessed March 27, 2023).
7. Dunne EM, Choummanivong M, Neal EFG, Stanhope K, Nguyen CD, Xeuatvongsa A, et al. Factors associated with pneumococcal carriage and density in infants and young children in Laos PDR. PLoS ONE. (2019) 14:e0224392. doi: 10.1371/journal.pone.0224392
8. Satzke C, Dunne EM, Choummanivong M, Ortika BD, Neal EFG, Pell CL, et al. Pneumococcal carriage in vaccine-eligible children and unvaccinated infants in Lao PDR two years following the introduction of the 13-valent pneumococcal conjugate vaccine. Vaccine. (2019) 37:296–305. doi: 10.1016/j.vaccine.2018.10.077
9. Rudge JW, Inthalaphone N, Pavlicek R, Paboriboune P, Flaissier B, Monidarin C, et al. Epidemiology and aetiology of influenza-like illness among households in metropolitan Vientiane, Lao PDR: A prospective, community-based cohort study. PLoS One. (2019) 14:e0214207. doi: 10.1371/journal.pone.0214207
10. Tavares DA, Handem S, Carvalho RJ, Paulo AC, de Lencastre H, Hinds J, et al. Identification of Streptococcus pneumoniae by a real-time PCR assay targeting SP2020. Sci Rep. (2019) 9:3285. doi: 10.1038/s41598-019-39791-1
11. Fast Track Diagnostics. FTD Respiratory pathogens 21 plus - Mikrogen. Manual. (2012) https://www.yumpu.com/en/document/view/4862693/ftd-respiratory-pathogens-21-mikrogen [Accessed March 23, 2023]
12. Messaoudi M, Milenkov M, Albrich WC, van der Linden MPG, Bénet T, Chou M, et al. The relevance of a novel quantitative assay to detect up to 40 major streptococcus pneumoniae serotypes directly in clinical nasopharyngeal and blood specimens. PLoS ONE. (2016) 11:e0151428. doi: 10.1371/journal.pone.0151428
13. Lumley T. Analysis of complex survey samples. J Stat Softw. (2004) 9:1–19. doi: 10.18637/jss.v009.i08
14. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using LME4. J Stat Softw. (2015) 67:8–21. doi: 10.18637/jss.v067.i01
15. Scherer R, Pallmann P. Simboot: Simultaneous Inference for Diversity Indices. (2017) Available online at: https://CRAN.R-project.org/package=simboot (accessed March 15, 2023).
16. Løchen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. (2020) 10:18977. doi: 10.1038/s41598-020-75691-5
17. Nguyen HAT, Fujii H, Vu HTT, Parry CM, Dang AD, Ariyoshi K, et al. An alarmingly high nasal carriage rate of Streptococcus pneumoniae serotype 19F non-susceptible to multiple beta-lactam antimicrobials among Vietnamese children. BMC Infect Dis. (2019) 19:241. doi: 10.1186/s12879-019-3861-2
18. Farida H, Severin JA, Gasem MH, Keuter M, Wahyono H, van den Broek P, et al. Nasopharyngeal carriage of streptococcus pneumonia in pneumonia-prone age groups in semarang, java Island, Indonesia. PLoS ONE. (2014) 9:e87431. doi: 10.1371/journal.pone.0087431
19. Bakhshaee M, Naderi HR, Ghazvini K, Sotoudeh K, Amali A, Ashtiani SJ. Passive smoking and nasopharyngeal colonization by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in daycare children. Eur Arch Otorhinolaryngol. (2012) 269:1127–32. doi: 10.1007/s00405-011-1811-8
20. Prasetyo A, Sadhana U, Budiman J. Nasal mucociliary clearance in smokers: a systematic review. Int Arch Otorhinolaryngol. (2021) 25:e160–9. doi: 10.1055/s-0040-1702965
21. Piatti G, Gazzola T, Allegra L. Bacterial adherence in smokers and non-smokers. Pharmacol Res. (1997) 36:481–4. doi: 10.1006/phrs.1997.0255
22. Jiang C, Chen Q, Xie M. Smoking increases the risk of infectious diseases: a narrative review. Tob Induc Dis. (2020) 18:123845. doi: 10.18332/tid/123845
23. Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. (2010) 10:304. doi: 10.1186/1471-2334-10-304
24. Camilli R, Vescio MF, Giufrè M, Daprai L, Garlaschi ML, Cerquetti M, et al. Carriage of Haemophilus influenzae is associated with pneumococcal vaccination in Italian children. Vaccine. (2015) 33:4559–64. doi: 10.1016/j.vaccine.2015.07.009
25. Tikhomirova A, Kidd SP. Haemophilus influenzae and Streptococcus pneumoniae : living together in a biofilm. Pathog Dis. (2013) 69:114–26. doi: 10.1111/2049-632X.12073
26. Borges IC, Andrade DC, Vilas-Boas A-L, Fontoura M-SH, Laitinen H, Ekström N, et al. Detection of antibody responses against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis proteins in children with community-acquired pneumonia: effects of combining pneumococcal antigens, pre-existing antibody levels, sampling interval, age, and duration of illness. Eur J Clin Microbiol Infect Dis. (2015) 34:1551–7. doi: 10.1007/s10096-015-2385-y
27. Kyu S, Ramonell RP, Kuruvilla M, Kraft CS, Wang YF, Falsey AR, et al. Diagnosis of Streptococcus pneumoniae infection using circulating antibody secreting cells. PLoS ONE. (2021) 16:e0259644. doi: 10.1371/journal.pone.0259644
Keywords: Streptococcus pneumoniae, Lao PDR, influenza-like illness, cohort study, surveillance, pneumococcal vaccine, serotypes
Citation: Sanchez Picot V, Keovichith I, Paboriboune P, Flaissier B, Saadatian-Elahi M and Rudge JW (2023) Epidemiology and serotype distribution of Streptococcus pneumoniae carriage among influenza-like illness cases in metropolitan Vientiane, Lao PDR: a community-based cohort study. Front. Public Health 11:1124016. doi: 10.3389/fpubh.2023.1124016
Received: 14 December 2022; Accepted: 31 March 2023;
Published: 20 April 2023.
Edited by:
Nuno Sepulveda, Warsaw University of Technology, PolandReviewed by:
Oana Sandulescu, Carol Davila University of Medicine and Pharmacy, RomaniaJose Maria Marimon, Biodonostia Health Research Institute Donostia-San Sebastian, Spain
Copyright © 2023 Sanchez Picot, Keovichith, Paboriboune, Flaissier, Saadatian-Elahi and Rudge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Sanchez Picot, dmFsZW50aW5hLnBpY290QGZvbmRhdGlvbi1tZXJpZXV4Lm9yZw==
 Valentina Sanchez Picot
Valentina Sanchez Picot Inthalaphone Keovichith2
Inthalaphone Keovichith2 Mitra Saadatian-Elahi
Mitra Saadatian-Elahi James W. Rudge
James W. Rudge