- 1Peking University Center for Public Health and Epidemic Preparedness and Response, Peking University, Beijing, China
- 2Public Health, Department of Social Medicine, Osaka University Graduate School of Medicine, Osaka, Japan
- 3Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China
- 4Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China
Introduction: Polypharmacy might contribute to a range of adverse outcomes, which could get worse in the elderly with chronic kidney disease (CKD). Evidence on polypharmacy, CKD, and mortality is scarce. We aimed to investigate the prospective association between polypharmacy, CKD and all-cause and cause-specific mortality in adults aged ≥65 years.
Methods: A total of 13,513 adults from the National Health and Nutrition Examination Surveys were included, following up from 1999 to 2018 until December 31, 2019. The simultaneous use of ≥5 medications by one individual was defined as polypharmacy. Survey-weighted Cox proportional hazard models were used to estimate the hazard ratio (HRs) for mortality from all-cause, cardiovascular diseases (CVD), and cancer after adjusting for potential confounding factors.
Results: Among the elderly with CKD, we identified 3,825 total deaths (1,325 CVD and 714 cancer) during a median follow-up of 7.7 years. Participants with polypharmacy had a 27% (HR = 1.27 [1.15, 1.39]) and 39% (HR = 1.39 [1.19, 1.62]) higher risk of all-cause and CVD mortality, respectively, but not for cancer mortality. Compared with the elderly with no polypharmacy and no CKD, the corresponding HRs (95%CIs) for all-cause mortality were 1.04 (0.96, 1.14) for those with no polypharmacy but CKD, 1.24 (1.11, 1.39) for with polypharmacy but no CKD, and 1.34 (1.21, 1.49) for those with both polypharmacy and CKD. A similar pattern was detected for CVD mortality.
Discussion: Polypharmacy was associated with elevated risks of all-cause and CVD mortality among the elderly CKD patients. More evidence-based approaches should be promoted for the appropriate deprescribing in the older adults with CKD.
1. Introduction
Polypharmacy has emerged as an important public health issue in the population, especially among the elderly. There is no consensus as to the number of medications that amounts to polypharmacy, generally, it refers to the simultaneous use of five or more medications by one individual (1). The majority of old people suffered from multimorbidity, contributing to the high utilization of multiple medications or therapy. It was estimated that the prevalence of polypharmacy ranged from 27.0 to 96.5% in the older population in different healthcare settings (2, 3). Polypharmacy might contribute to a range of unfavorable effects and adverse outcomes in older adults, such as adverse drug events, hospitalizations, falls, impaired function, and cognition decline (4, 5). Mortality is one of the common and important measures used for epidemiological surveillance and indicates the progression and severity of a given health event (6). CVD and cancer are among the leading causes of mortality in the U.S. and are responsible for nearly half of all deaths (7). A meta-analysis showed a dose–response relationship between polypharmacy and mortality (8), and another recent meta-analysis revealed that polypharmacy was associated with a 28.0% higher risk of mortality in the elderly (≥65 years) (9). Furthermore, it was indicated by an English aging cohort that polypharmacy had a 2.45 times higher risk of mortality from cardiovascular disease (CVD) in people aged over 50, but cancer mortality was only related to heightened polypharmacy (taking over 10 medications) (10).
Chronic kidney disease (CKD) is a condition characterized by decline in kidney function. The prevalence of CVD and metabolic disease is notably higher among people with CKD, making them vulnerable to multimorbidity and polypharmacy (11–14). Previous research showed the prevalence of polypharmacy ranged from 62.0 to 86.0% in CKD patients (11, 15). Furthermore, the pharmacokinetics and pharmacodynamics might be altered due to kidney dysfunction in the elderly (16, 17), therefore the drug–drug interactions and drug adverse events might be amplificated in the aging CKD population (16, 18, 19). Currently, evidence on polypharmacy and health outcomes in older adults with CKD is scarce. Several cohort studies indicated polypharmacy was associated with an increased risk of kidney failure, fragility fracture, CVD events, and all-cause mortality in patients with CKD (20–22), even regardless of CKD status (20, 22, 23).
In this study, we aimed to investigate the association between polypharmacy, CKD and all-cause mortality, as well as CVD and cancer mortality in adults aged 65 years and old.
2. Methods
2.1. Study population
Data were extracted from the National Health and Nutrition Examination Surveys (NHANES), a nationally representative survey designed to evaluate the health and nutritional status of community-dwelling U.S. adults. NHANES repeats the cycle of surveys every 2 years with a methodology of multistage probability sampling design. The surveys combined self-administrated interviews with physical examinations and laboratory tests. Data were available at.1 NHANES has got approval from the Institutional Review Board of the National Center for Health Statistics. All participants have signed informed consent forms.
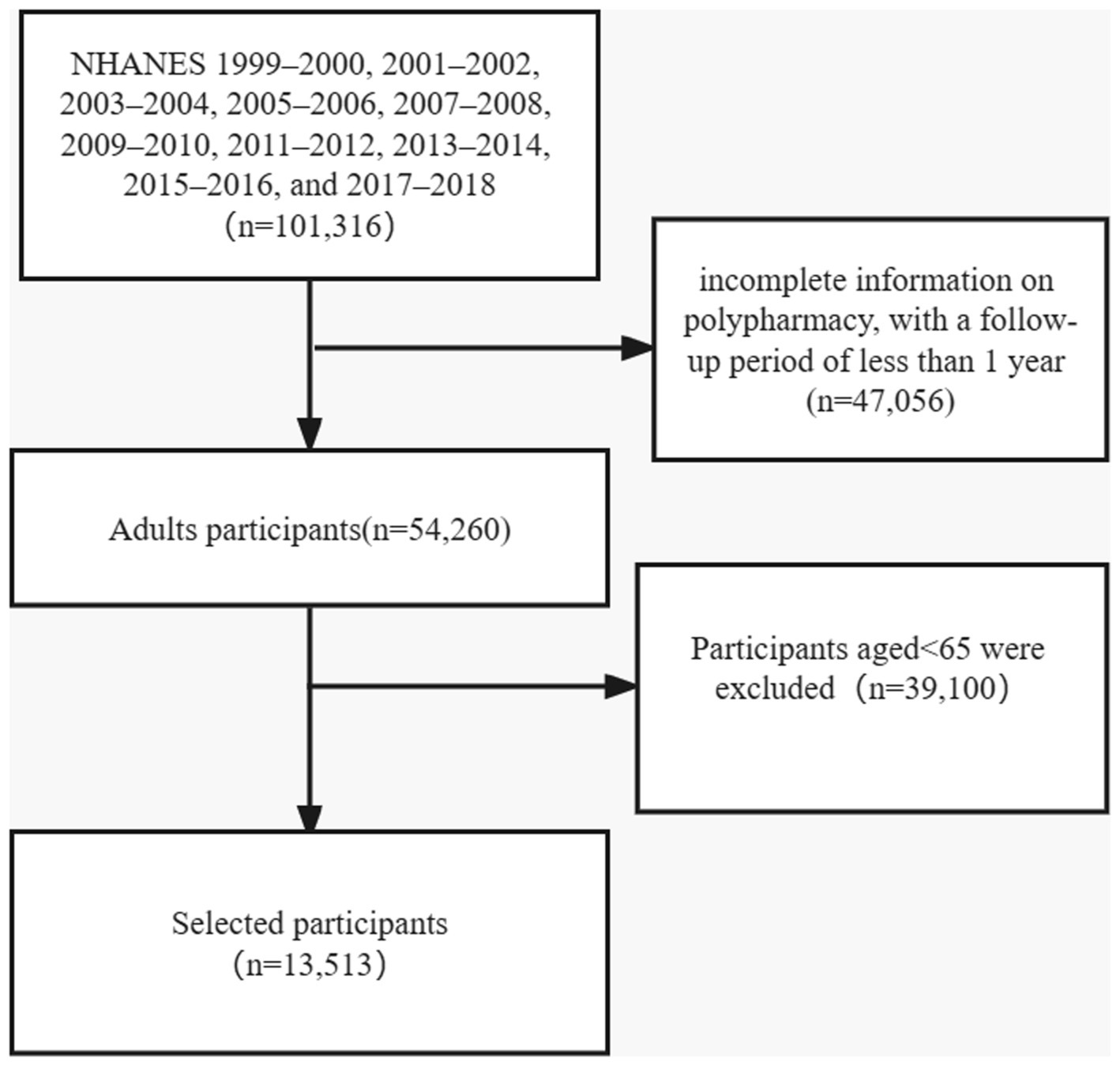
In this present study, a total of 101,316 individuals from ten cycles (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018) was included. Mortality follow-up data were recorded until the date of 31 December 2019. We excluded those younger than 65 years, those with incomplete information on polypharmacy, and with a follow-up period of less than 1 year. Finally, 13,513 individuals were included in the analysis (Figure 1).
2.2. Assessment of polypharmacy
During the in-home interview, participants would be asked, “Have you taken any prescription medications in the past month?” and those who answered “yes” would be further asked to show the containers of all medications they were using. At the same time, the interviewer would record the number and name of the participant’s prescription medications. Simultaneous use of five or more medications was defined as “polypharmacy.”
2.3. Assessment of chronic kidney disease
We calculated the glomerular filtration rate (GFR) estimated according to the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (24): (1) for women, if serum creatinine ≤0.7 mg/dl, GFR = 144 × (serum creatinine (mg/dl)/0.7)−0.329 × (0.993)age; if serum creatinine >0.7 mg/dl, GFR = 144 × (serum creatinine (mg/dl)/0.7)−1.209 × (0.993)age. (2) For men, if serum creatinine ≤0.9 mg/dl, GFR = 141 × (serum creatinine (mg/dl)/0.7)−0.441 × (0.993)age; if serum creatinine >0.9 mg/dl, GFR = 144 × (serum creatinine (mg/dl)/0.7)−0.441 × (0.993)age. The serum creatinine measurements could be obtained from the ten cycles and recommended calibrations in NHANES had been adopted. We defined reduced estimated GFR (eGFR) as <60 ml/min/1.73 m2 and severely reduced eGFR as <30 ml/min/1.73 m2. CKD was defined as reduced eGFR. Albuminuria, defined as a urine albumin-to-creatinine ratio ≥ 30 mg/g, was further added for CKD outcomes (25).
2.4. Covariates
The demographics and socioeconomics included sex, age, race (Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black), and other race - including Multi-Racial, education (high school or below, and college or above), and family income-to-poverty ratio (≤1.0, and > 1.0). Body mass index (BMI) was calculated as weight/(height*height) (kg/m2) (underweight: BMI < 18.5; normal weight: 18.5 ≤ BMI < 25; overweight: 25 ≤ BMI < 30; obesity: BMI ≥ 30). Drinking status was based on the question “Had at least 12 alcohol drinks/1 year?,” with the answer of “yes” or “no.” The medical history included diabetes, hypertension, high cholesterol, heart disease (congestive heart failure, coronary heart disease, angina/angina pectoris, or heart attack), respiratory disease (asthma, emphysema, or chronic bronchitis), and cancer.
2.5. Ascertainment of death
The deidentified and anonymized participant data in NHANES were linked to longitudinal Medicare and mortality data from National Death Index (NDI) until the date of 31 December 2019. The cause of death was based on the International Classification of Diseases, 10th edition (ICD-10). Mortality follow-up data were recorded for participants from the start of the study until to date of death or 31 December 2019, whichever came first. The all-cause mortality and mortality from cardiovascular diseases (I00-I09, I11, I13, I20-I51), and cancer (C00-C97) were evaluated.
2.6. Statistical analysis
The characteristics of the included participants at baseline were presented according to polypharmacy. Continuous variables were shown as mean ± standard deviation. Categorical variables were expressed as numbers (%). Survey-weighted regression models were used to calculate the rate of polypharmacy among participants with CKD. Survey-weighted multivariable Cox proportional hazard models were used to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) of mortality by polypharmacy among all the participants as well as participants with CKD. The proportional hazards assumption tests were conducted by comparing models with and without a multiplicative interaction term between polypharmacy and follow-up duration. We detected no evidence of assumption violations in every model (p > 0.05 for all tests). Model 1 was adjusted for sex and age. Model 2 was further adjusted for race, education, income, BMI, drinking status, diabetes, hypertension, high cholesterol, heart disease, respiratory disease, and cancer. Missing values were treated as separate categories. Furthermore, the joint effect of polypharmacy and CKD on mortality among the older adults were analyzed. We also did sensitivity analyses by dividing polypharmacy as minor polypharmacy (5–9 medications) and major polypharmacy (≥10 medications), to detect the association between polypharmacy and mortality. SAS version 9.4 TS Level 1 M6 software (SAS Institute Inc., Cary, NC, United Sates) was used for all statistical analyses.
3. Results
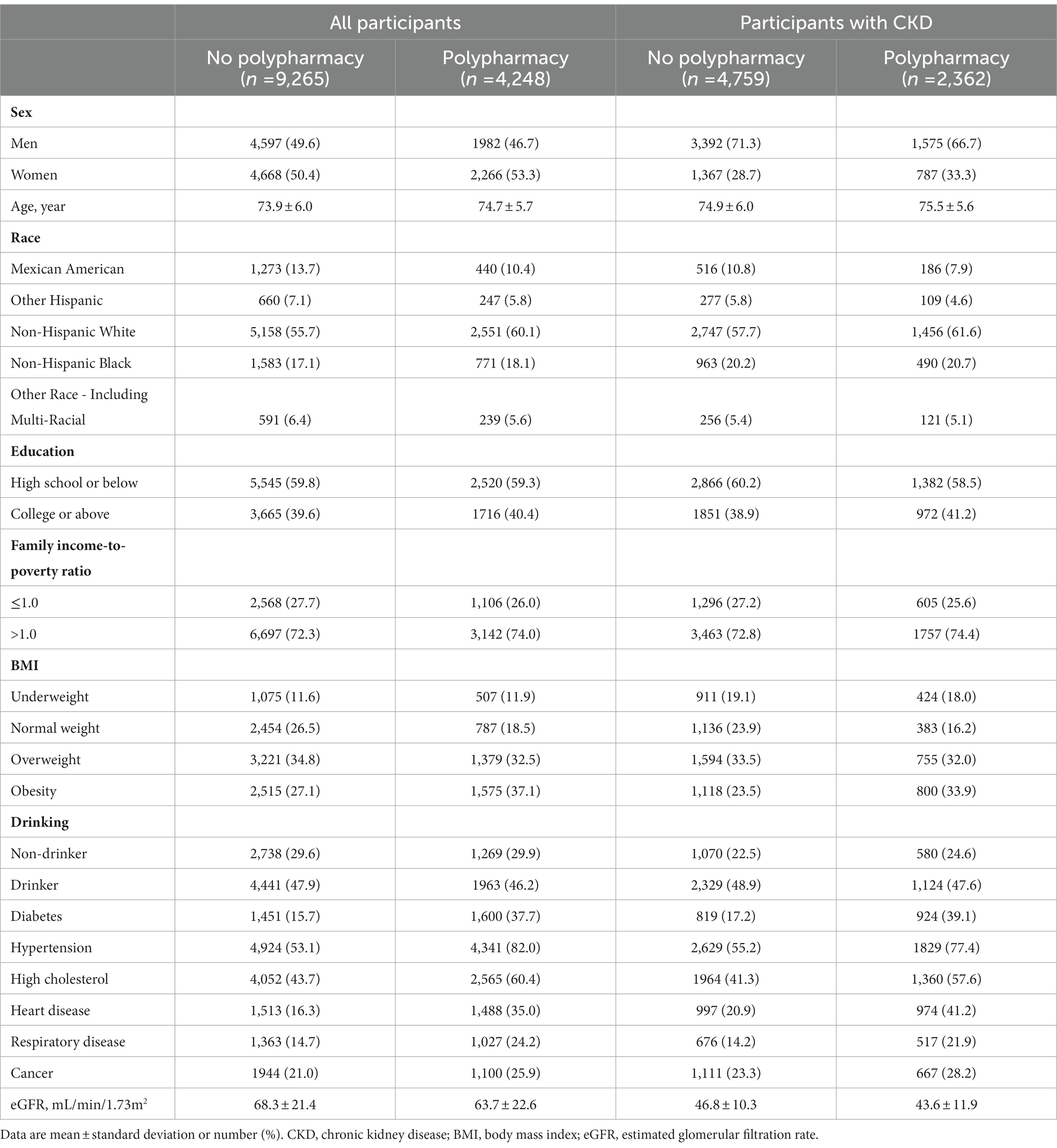
Table 1 shows the characteristics of the included participants aged 65 or older at baseline according to polypharmacy. Older adults with polypharmacy were more likely to be slightly older, to be women, to be non-Hispanic White, and to have obesity, hypertension, diabetes, high cholesterol, heart disease, respiratory disease, and cancer., and a lower level of eGFR. A similar pattern was observed among older adults with CKD.
Among all the elderly participants, we identified 6,049 total deaths, including 2067 CVD deaths and 1,134 cancer deaths during a median follow-up of 7.9 years. As shown in Table 2, after adjusting for age and sex (model 1), compared with the no polypharmacy group, the elderly with polypharmacy had a 46% (HR, 95%CI = 1.46 [1.36, 1.56]) and 77% (HR, 95%CI = 1.77 [1.58, 1.99]) increase in the risk of all-cause mortality and CVD mortality (i.e., 1.46 times and 1.77 times more likely to experience all-cause death and CVD death), respectively. After further adjustment for race, education, income, BMI, drinking status, diabetes, hypertension, high cholesterol, heart disease, respiratory disease, and cancer (model 2), it showed a 27% (HR = 1.27 [1.17, 1.37]) and 39% (HR = 1.39 [1.22, 1.58]) higher risk of mortality from all-cause and CVD, respectively, among the elderly with polypharmacy, in comparison to those with no polypharmacy. However, the association was non-significant for cancer mortality in the two models (HR = 1.14 [0.97, 1.33] and HR = 1.07 [0.91, 1.27], respectively). Sensitivity analyses showed similar results of minor polypharmacy and major polypharmacy for mortality from all-cause, CVD, and cancer (Supplementary Table S1).
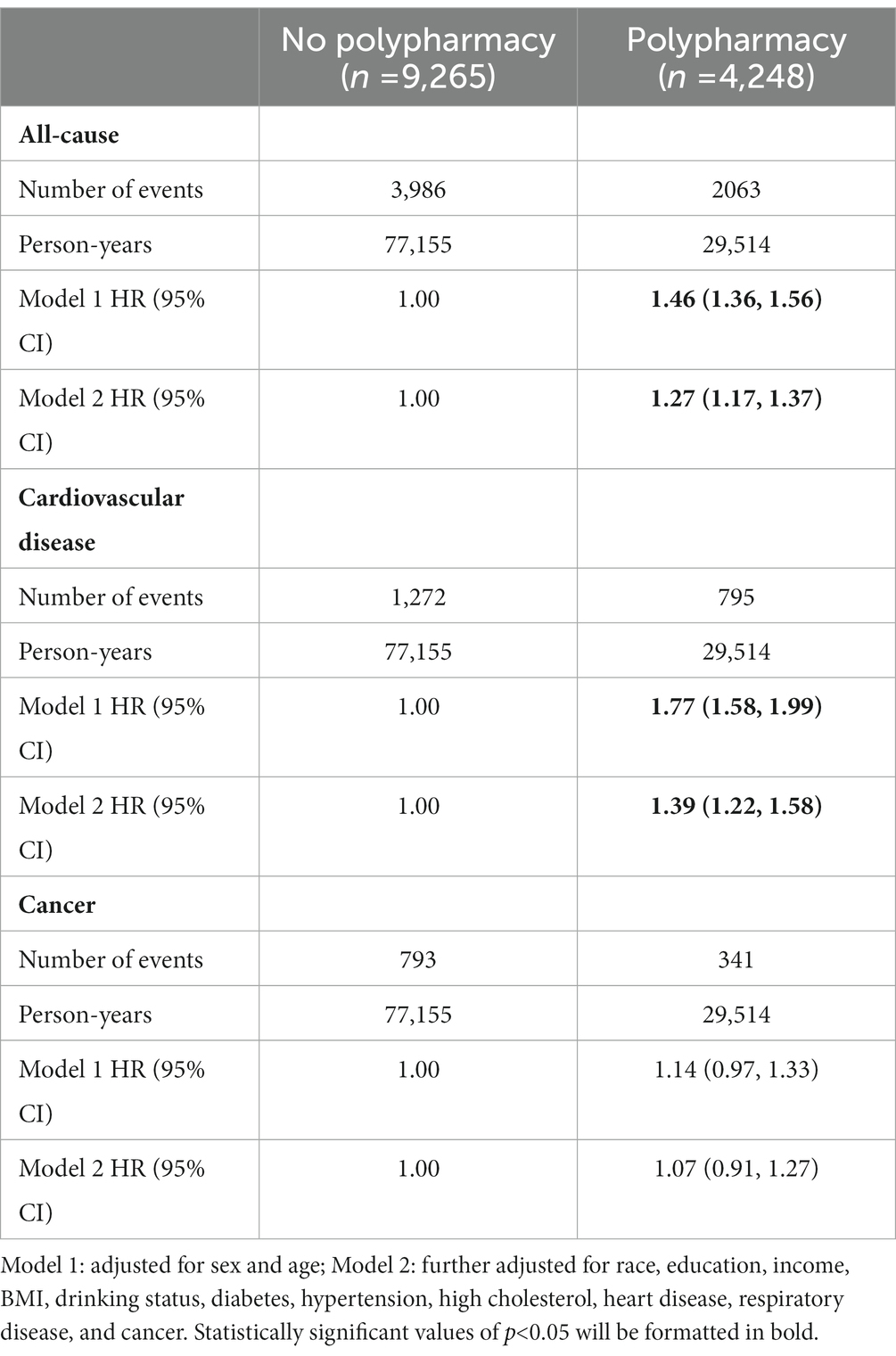
Table 2. Hazard ratios and 95% confidence intervals (HRs, 95% CIs) for mortality from cardiovascular disease, cancer, and all-cause according to polypharmacy among older adults.
In Table 3, among the elderly with reduced eGFR, we identified 3,825 total deaths, including 1,325 CVD deaths and 714 cancer deaths during a median follow-up of 7.7 years. In model 1, compared with the no polypharmacy group, participants with polypharmacy had 44% (HR = 1.44 [1.32, 1.57]), 77% (HR = 1.77 [1.53, 2.03]), and 21% (HR = 1.21 [1.01, 1.45]) higher risk of mortality from all-cause, CVD, and cancer, respectively. In model 2, the associations were still significant for all-cause and CVD mortality, the elderly with polypharmacy had a 27% (HR = 1.27 [1.15, 1.39]) and 39% (HR = 1.39 [1.19, 1.62]) higher risk of all-cause and CVD mortality, respectively. However, the association was not significant for cancer mortality (HR = 1.18 [0.98, 1.43]). Sensitivity analyses showed similar patterns for mortality from cardiovascular disease, cancer, and all-cause according to polypharmacy (Supplementary Table S2). Among the elderly with severely reduced eGFR (eGFR <30 ml/min/1.73m2), similar patterns of associations of polypharmacy with mortality from all-cause, CVD, and cancer were observed. After further adjustment for covariates, in comparison to those with no polypharmacy, the elderly with polypharmacy had a 24% (HR = 1.24 [1.07, 1.43]) and 54% (HR = 1.54 [1.21, 1.95]) higher risk of all-cause and CVD mortality, respectively, but not for cancer mortality (HR = 0.91 [0.68, 1.23]). Among the elderly with albuminuria, the association between polypharmacy and mortality showed similar results (Table 3). In the multivariable models, compared with no polypharmacy group, the elderly with polypharmacy had a 31% (HR = 1.31 [1.14, 1.50]) and 49% (HR = 1.49 [1.18, 1.90]) higher risk of all-cause and CVD mortality, respectively.
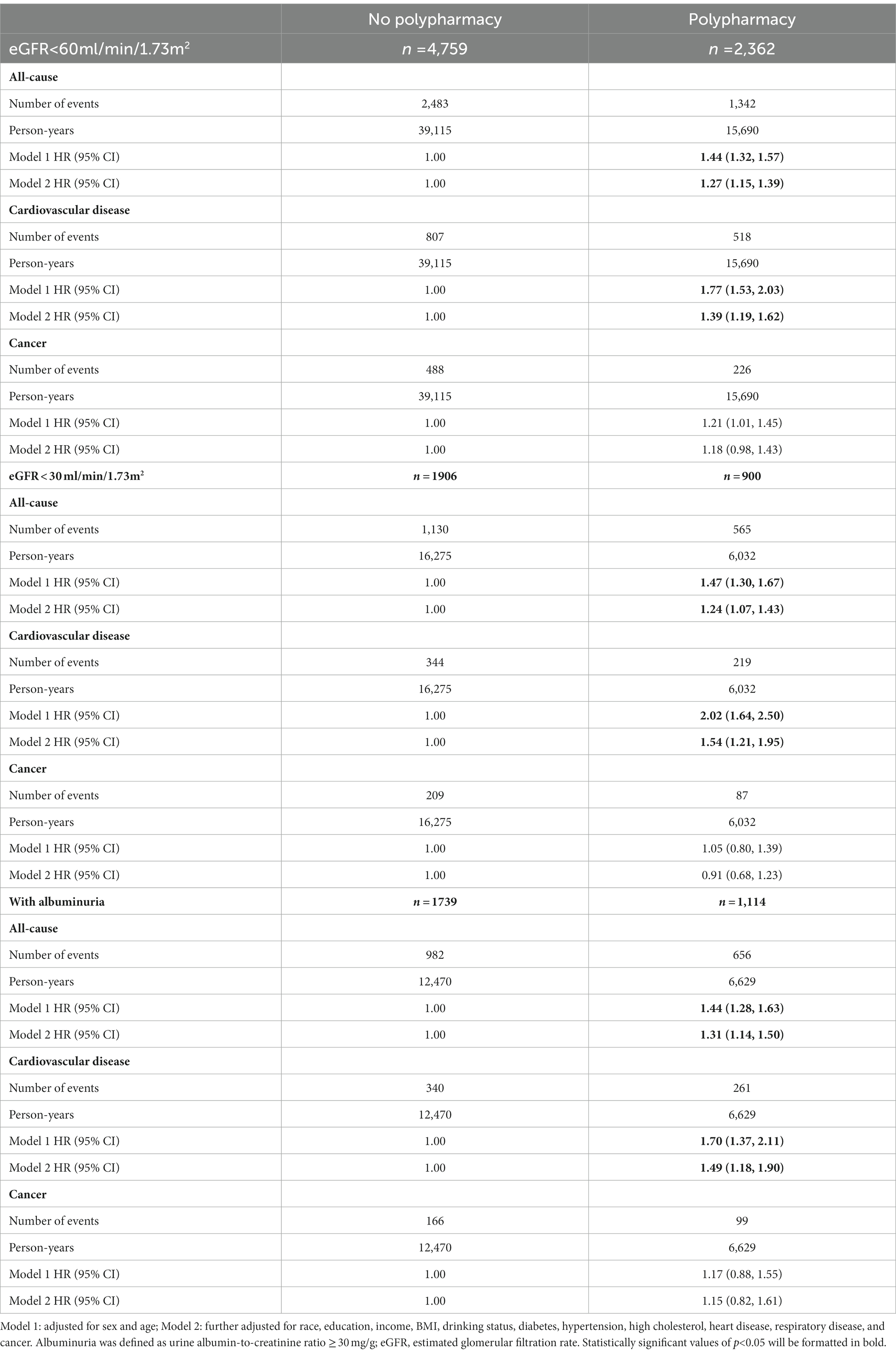
Table 3. Hazard ratios and 95% confidence intervals (HRs, 95% CIs) for mortality from cardiovascular disease, cancer, and all-cause according to polypharmacy and kidney function among participants with chronic kidney disease.
We then conducted the joint-association analyses of polypharmacy and CKD on mortality from all-cause, CVD, and cancer in the elderly (Figure 2). For all-cause mortality, compared with the elderly with no polypharmacy and no CKD, the corresponding HRs (95%CIs) were 1.04 (0.96, 1.14) for those with no polypharmacy but CKD, 1.24 (1.11, 1.39) for with polypharmacy but no CKD, and 1.34 (1.21, 1.49) for those with both polypharmacy and CKD (Figure 2A). For CVD mortality, a similar pattern was detected where the corresponding HRs (95%CIs) were 1.03 (0.90, 1.19), 1.34 (1.11, 1.61), and 1.47 (1.23, 1.76), respectively (Figure 2B). However, we did not observe any significant association with cancer mortality (Figure 2C).
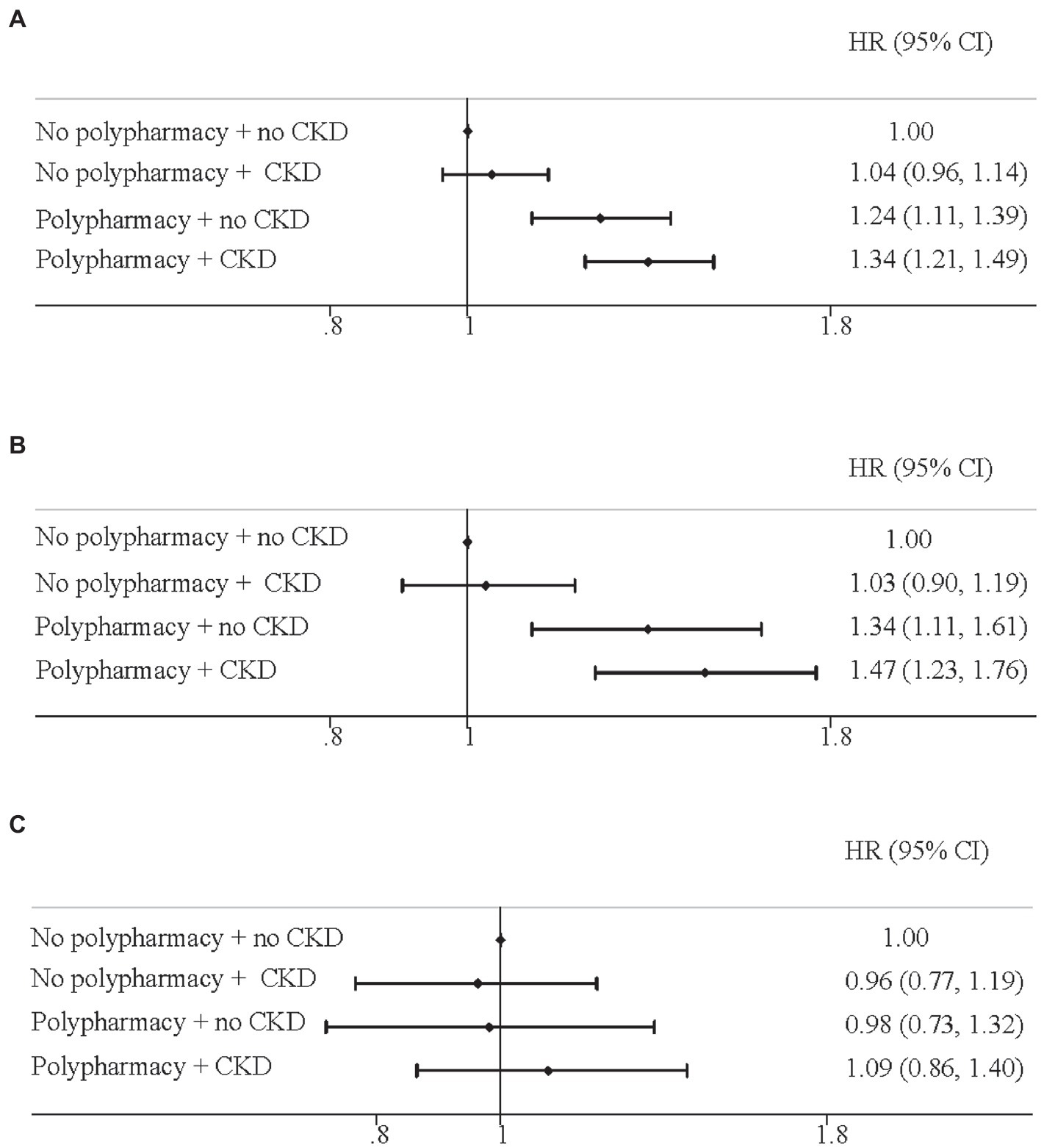
Figure 2. Joint effect of polypharmacy and chronic kidney disease on mortality for (A) all-cause; (B) cardiovascular disease; (C) cancer. Hazard ratios and 95% confidence intervals (HRs, 95% CIs) were adjusted for age, sex. Race, education, income, body mass index, drinking status, diabetes, hypertension, high cholesterol, heart disease, respiratory disease, and cancer.
4. Discussion
In this prospective study, among the elderly with CKD, in comparison to those with no polypharmacy, individuals with polypharmacy had 1.27 times and 1.39 times the risk of all-cause and CVD mortality, respectively, but not for cancer mortality. The older adults with polypharmacy and CKD had the highest risk of mortality. A previous cohort comprised of U.S. adults aged ≥45 years with a 5-year follow-up showed that only major polypharmacy (≥ 8 medications) was associated with increased mortality (HR = 1.24 [1.06, 1.45]), but not for minor polypharmacy (6–7 medications) (HR = 1.15 [0.98, 1.36]) in those with CKD, and there was no evidence that this association was modified by CKD status (22). Another Japanese cohort consisting of 1,117 participants reported that both polypharmacy (5–9 medications) and hyperpolypharmacy (≥10 medications) were associated with kidney failure (HR = 2.28 [1.00, 5.21] and HR = 2.83 [1.21, 6.66], respectively), but only hyperpolypharmacy was related to all-cause mortality (HR = 2.80 [1.41–5.54]) (21). However, our sensitivity analysis suggested even minor polypharmacy significantly increased mortality in the elderly CKD patients. A review concluded that medication deprescribing interventions could offer small reductions in mortality (OR = 0.74 [0.58, 0.95]), though no significant effect on hospitalizations, falls, or health-related quality of life among community-dwelling people aged ≥65 years (26).
To our knowledge, we firstly reported the adverse impacts of polypharmacy on cardiovascular mortality in the older adults with CKD. People with CKD could release a high level of cytokines, hormones, and enzymes, as well as alter hemodynamics due to kidney insufficiency or injury (27–29). An international prescribing pattern survey showed approximately 39% of the participants most frequently prescribed cardiovascular medications and 91% were experiencing polypharmacy (13). It is controversial that polypharmacy was shown to be efficacious or safe (30–32). Polypharmacy might exert an additive effect on the cardiac damage caused by CKD, thus leading to the deterioration of CVD outcomes (31, 33, 34).
The highest mortality was observed in the elderly with both polypharmacy and CKD. The amount of medication taken may reflect the number of medical conditions they have and severity of the diseases. CKD patients who had comorbidities (e.g., diabetes, atrial fibrillation, hypertension) had increased mortality rates (35). Furthermore, it has been reported that polypharmacy is associated with a broad range of adverse outcomes, including bleeding, hospitalizations, falls, fractures, frailty, and poor health-related quality of life (4, 36). Also, polypharmacy might be associated with kidney impairment (37, 38). For example, a Switzerland study reported every additional prescribed drug was related to a decrease in eGFR by 0.39 ml/min per 1.73 m2 over 24 months (38). Kidney dysfunction could further exacerbate multimorbidity, hence higher levels of comorbidities and chronic inflammation could result in worse clinical consequences (34, 39–41). Interestingly, a study found while people with CKD had higher absolute risk of death, there was no difference in the relative risk associated with polypharmacy by CKD status. Polypharmacy-related adverse events could be mitigated if drug management was undertaken by a nephrologist in patients with much advanced CKD (23). Overall, our study indicated the importance of recommendations or guidelines for lowering medication burden to achieve appropriate and safe medication management in the elderly with CKD. So far, several trials have been attempted to improve the health outcomes intended for older patients receiving polypharmacy. For example, intervention on clinical geriatric assessment combined with a thorough medications review, as well as a meeting between the geriatrician and the family physician could contribute to positive impacts on health-related quality of life in home-dwelling older patients receiving polypharmacy (42, 43).
The present study was strengthened by using nationally representative population and a prospective cohort design. Several limitations should be addressed. First, we assessed the CKD by using serum creatinine according to a single measurement, which could lead to the misclassification of CKD. However, the misclassification was inclined to be non-differential. Second, we quantified the prescription by using the term of polypharmacy to indicate the overall medication burden by one individual, but did not further analyze the specific type of medication. Third, the outcomes included all-cause mortality and mortality from CVD and cancer, while other causes, such as sepsis, cerebrovascular diseases, influenza and pneumonia were not considered. Although CVD and cancer are among the leading causes of mortality in the U.S. and are responsible for approximately half of all deaths (7). Furthermore, the covariates for diseases, such as diabetes and hypertension were self-reported, though previous studies have supported the high validity of clinical records in NHANES (44–46). Lastly, it is possible that residual confounding existed due to some unmeasured variables (e.g., dietary factors).
5. Conclusions and implications
Polypharmacy might contribute to a range of adverse outcomes, which could get worse in the elderly with CKD. Since evidence on polypharmacy, CKD, and mortality was scarce, our study highlighted that polypharmacy was associated with higher risks of all-cause and CVD mortality among the older adults with CKD. The older adults with both polypharmacy and CKD had the highest risk of mortality. Our study also indicated the importance of recommendations or guidelines for lowering medication burden to achieve appropriate and safe medication management in the elderly with CKD. More evidence-based approaches, such as clinical geriatric assessment and collaborative medication reviews by physicians should be promoted for the appropriate deprescribing in the older adults with CKD.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving human participants were reviewed and approved by NHANES has got approval from the Institutional Review Board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XW: conceptualization, formal analysis, data curation, and writing−original draft preparation. J−YD: conceptualization, formal analysis, and writing−reviewing and editing. CY: data curation and writing−reviewing and editing. JJ: writing−reviewing and editing. YH: validation, and writing−reviewing and editing. YH: supervision, validation, and writing−reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National key research and development program of China (grant no. 2022YFC3600804).
Acknowledgments
The authors would like to thank the NHANES research group. The developers and funders of NHANES had no roles in data analysis and interpretation or manuscript writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1116583/full#supplementary-material
Footnotes
References
1. Nwadiugwu, MC. Frailty and the risk of polypharmacy in the older person: enabling and preventative approaches. J Aging Res. (2020) 2020:6759521–6. doi: 10.1155/2020/6759521
2. Pazan, F, and Wehling, M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med. (2021) 12:443–52. doi: 10.1007/s41999-021-00479-3
3. Midao, L, Giardini, A, Menditto, E, Kardas, P, and Costa, E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch Gerontol Geriatr. (2018) 78:213–20. doi: 10.1016/j.archger.2018.06.018
4. Fried, TR, O’Leary, J, Towle, V, Goldstein, MK, Trentalange, M, and Martin, DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. (2014) 62:2261–72. doi: 10.1111/jgs.13153
5. Turgeon, J, Michaud, V, and Steffen, L. The dangers of polypharmacy in elderly patients. JAMA Intern Med. (2017) 177:1544. doi: 10.1001/jamainternmed.2017.4790
6. Hernandez, JBR, and Kim, PY. Epidemiology Morbidity and Mortality. StatPearls, StatPearls Publishing (2022).
7. Xu, J, Murphy, SL, Kochanek, KD, and Bastian, BA. Deaths: final data for 2013. Natl Vital Stat Rep. (2016) 64:1–119.
8. Leelakanok, N, Holcombe, AL, Lund, BC, Gu, X, and Schweizer, ML. Association between polypharmacy and death: a systematic review and meta-analysis. J Am Pharm Assoc (2003);57:729–738.e10. doi: 10.1016/j.japh.2017.06.002.
9. Li, Y, Zhang, X, Yang, L, Yang, Y, Qiao, G, Lu, C, et al. Association between polypharmacy and mortality in the older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2022) 100:104630. doi: 10.1016/j.archger.2022.104630
10. Huang, YT, Steptoe, A, Wei, L, and Zaninotto, P. Dose-response relationships between polypharmacy and all-cause and cause-specific mortality among older people. J Gerontol A Biol Sci Med Sci. (2022) 77:1002–8. doi: 10.1093/gerona/glab155
11. Schmidt, IM, Hubner, S, Nadal, J, Titze, S, Schmid, M, Barthlein, B, et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German chronic kidney disease study. Clin Kidney J. (2019) 12:663–72. doi: 10.1093/ckj/sfz046
12. Liabeuf, S, and Laville, M. Drug prescription in patients with chronic kidney disease: a true challenge. Nephrol Dial Transplant. (2021) 36:385–6. doi: 10.1093/ndt/gfaa164
13. Hayward, S, Hole, B, Denholm, R, Duncan, P, Morris, JE, Fraser, SDS, et al. Investigators ES. International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: results from the European quality study. Nephrol Dial Transplant. (2021) 36:503–11. doi: 10.1093/ndt/gfaa064
14. Parker, K, and Wong, J. Is polypharmacy an increasing burden in chronic kidney disease? The German experience. Clin Kidney J. (2019) 12:659–62. doi: 10.1093/ckj/sfz072
15. Laville, SM, Metzger, M, Stengel, B, Jacquelinet, C, Combe, C, Fouque, D, et al. Chronic kidney disease-renal E, information network study C. evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol. (2018) 84:2811–23. doi: 10.1111/bcp.13738
16. Laville, SM, Gras-Champel, V, Moragny, J, Metzger, M, Jacquelinet, C, Combe, C, et al. Chronic kidney disease-renal E, information network study G. adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol. (2020) 15:1090–102. doi: 10.2215/CJN.01030120
17. Santos-Diaz, G, Perez-Pico, AM, Suarez-Santisteban, MA, Garcia-Bernalt, V, Mayordomo, R, and Dorado, P. Prevalence of potential drug-drug interaction risk among chronic kidney disease patients in a Spanish hospital. Pharmaceutics. (2020) 12:713. doi: 10.3390/pharmaceutics12080713
18. Sommer, J, Seeling, A, and Rupprecht, H. Adverse drug events in patients with chronic kidney disease associated with multiple drug interactions and polypharmacy. Drugs Aging. (2020) 37:359–72. doi: 10.1007/s40266-020-00747-0
19. Gallieni, M, and Cancarini, G. Drugs in the elderly with chronic kidney disease: beware of potentially inappropriate medications. Nephrol Dial Transplant. (2015) 30:342–4. doi: 10.1093/ndt/gfu191
20. Wakasugi, M, Yokoseki, A, Wada, M, Momotsu, T, Sato, K, Kawashima, H, et al. Polypharmacy, chronic kidney disease, and incident fragility fracture: a prospective cohort study. J Bone Miner Metab. (2022) 40:157–66. doi: 10.1007/s00774-021-01272-9
21. Kimura, H, Tanaka, K, Saito, H, Iwasaki, T, Oda, A, Watanabe, S, et al. Association of Polypharmacy with kidney disease progression in adults with CKD. Clin J Am Soc Nephrol. (2021) 16:1797–804. doi: 10.2215/CJN.03940321
22. Cashion, W, McClellan, W, Judd, S, Goyal, A, Kleinbaum, D, Goodman, M, et al. Polypharmacy and mortality association by chronic kidney disease status: the REasons for geographic and racial differences in stroke study. Pharmacol Res Perspect. (2021) 9:e00823. doi: 10.1002/prp2.823
23. Secora, A, Alexander, GC, Ballew, SH, Coresh, J, and Grams, ME. Kidney function, polypharmacy, and potentially inappropriate medication use in a community-based cohort of older adults. Drugs Aging. (2018) 35:735–50. doi: 10.1007/s40266-018-0563-1
24. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
25. Chen, TK, Knicely, DH, and Grams, ME. Chronic kidney disease diagnosis and management: a review. JAMA. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
26. Bloomfield, HE, Greer, N, Linsky, AM, Bolduc, J, Naidl, T, Vardeny, O, et al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med. (2020) 35:3323–32. doi: 10.1007/s11606-020-06089-2
27. Schefold, JC, Filippatos, G, Hasenfuss, G, Anker, SD, and von Haehling, S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. (2016) 12:610–23. doi: 10.1038/nrneph.2016.113
28. Nasrallah, R, Hassouneh, R, and Hebert, RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol. (2016) 27:666–76. doi: 10.1681/ASN.2015050528
29. Agharazii, M, St-Louis, R, Gautier-Bastien, A, Ung, RV, Mokas, S, Lariviere, R, et al. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens. (2015) 28:746–55. doi: 10.1093/ajh/hpu225
30. Volpe, M, Chin, D, and Paneni, F. The challenge of polypharmacy in cardiovascular medicine. Fundam Clin Pharmacol. (2010) 24:9–17. doi: 10.1111/j.1472-8206.2009.00757.x
31. Page, RL 2nd, O’Bryant, CL, Cheng, D, Dow, TJ, Ky, B, Stein, CM, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. (2016) 134:e32–69. doi: 10.1161/CIR.0000000000000426
32. Abolbashari, M, Macaulay, TE, Whayne, TF, Mukherjee, D, and Saha, S. Polypharmacy in cardiovascular medicine: problems and promises! Cardiovasc Hematol Agents Med Chem. (2017) 15:31–9. doi: 10.2174/1871525715666170529093442
33. Beezer, J, Al Hatrushi, M, Husband, A, Kurdi, A, and Forsyth, P. Polypharmacy definition and prevalence in heart failure: a systematic review. Heart Fail Rev. (2022) 27:465–92. doi: 10.1007/s10741-021-10135-4
34. Jankowski, J, Floege, J, Fliser, D, Bohm, M, and Marx, N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation (2021);143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686.
35. Page, AT, Falster, MO, Litchfield, M, Pearson, SA, and Etherton-Beer, C. Polypharmacy among older Australians, 2006-2017: a population-based study. Med J Aust. (2019) 211:71–5. doi: 10.5694/mja2.50244
36. Leiss, W, Mean, M, Limacher, A, Righini, M, Jaeger, K, Beer, HJ, et al. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J Gen Intern Med. (2015) 30:17–24. doi: 10.1007/s11606-014-2993-8
37. Kang, H, and Hong, SH. Risk of kidney dysfunction from polypharmacy among older patients: a nested case-control study of the south Korean senior cohort. Sci Rep. (2019) 9:10440. doi: 10.1038/s41598-019-46849-7
38. Ernst, R, Fischer, K, Rezende, DG, Costa Molino, C, Orav, EJ, Theiler, R, et al. Polypharmacy and kidney function in community-dwelling adults age 60 years and older: a prospective observational study. J Am Med Dir Assoc. (2020) 21:254–259 e1. doi: 10.1016/j.jamda.2019.07.007
39. Salimi, S, Shardell, MD, Seliger, SL, Bandinelli, S, Guralnik, JM, and Ferrucci, L. Inflammation and trajectory of renal function in community-dwelling older adults. J Am Geriatr Soc. (2018) 66:804–11. doi: 10.1111/jgs.15268
40. Mallappallil, M, Friedman, EA, Delano, BG, McFarlane, SI, and Salifu, MO. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond). (2014) 11:525–35. doi: 10.2217/cpr.14.46
41. De Lima, JJG, Gowdak, LHW, David-Neto, E, and Bortolotto, LA. Diabetes, cardiovascular disease, and cardiovascular risk in patients with chronic kidney disease. High Blood Press Cardiovasc Prev. (2021) 28:159–65. doi: 10.1007/s40292-021-00434-0
42. Romskaug, R, Skovlund, E, Straand, J, Molden, E, Kersten, H, Pitkala, KH, et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician for improving health-related quality of life in home-dwelling older patients receiving polypharmacy: a cluster randomized clinical trial. JAMA Intern Med. (2020) 180:181–9. doi: 10.1001/jamainternmed.2019.5096
43. Mahlknecht, A, Wiedermann, CJ, Sandri, M, Engl, A, Valentini, M, Vogele, A, et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial. BMC Geriatr. (2021) 21:659. doi: 10.1186/s12877-021-02612-0
44. Yoon, SS, Dillon, CF, Illoh, K, and Carroll, M. Trends in the prevalence of coronary heart disease in the U.S.: National Health and nutrition examination survey, 2001-2012. Am J Prev Med. (2016) 51:437–45. doi: 10.1016/j.amepre.2016.02.023
45. Cheng, YJ, Kanaya, AM, Araneta, MRG, Saydah, SH, Kahn, HS, Gregg, EW, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. (2019) 322:2389–98. doi: 10.1001/jama.2019.19365
Keywords: NHANES, chronic kidney disease, mortality, elderly, cohort, polypharmacy
Citation: Wang X, Yang C, Jiang J, Hu Y, Hao Y and Dong J-Y (2023) Polypharmacy, chronic kidney disease, and mortality among older adults: A prospective study of National Health and nutrition examination survey, 1999–2018. Front. Public Health. 11:1116583. doi: 10.3389/fpubh.2023.1116583
Edited by:
Shuang Liang, People’s Liberation Army General Hospital, ChinaReviewed by:
Lakshmi Kannan, University of Pikeville Kentucky College of Osteopathic Medicine, United StatesMartin Nwadiugwu, Tulane University, United States
Copyright © 2023 Wang, Yang, Jiang, Hu, Hao and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuantao Hao, aGFveXRAYmptdS5lZHUuY24=; Jia-Yi Dong, ZG9uZ2p5QG1haWwzLnN5c3UuZWR1LmNu
 Xiaowen Wang
Xiaowen Wang Chao Yang
Chao Yang Jie Jiang
Jie Jiang Yonghua Hu4
Yonghua Hu4 Yuantao Hao
Yuantao Hao Jia-Yi Dong
Jia-Yi Dong