
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 06 February 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1114223
Background: Under five children are at risk of diarrhea-associated morbidity and mortality. Salmonella and Shigella are major causes of diarrhea in under-five children, especially in developing countries. This study aimed to assess the prevalence, antimicrobial resistance pattern, and associated factors of Salmonella and Shigella among under-five diarrheic children in Debre Markos town public health facilities.
Methods: A cross-sectional study was conducted at public health facilities in Debre Markos town using a consecutive convenient sampling technique. Data on socio-demographic and associated factors were collected using a structured questionnaire. Salmonella serovars and Shigella species were identified using MacConkey, Xylose Lysine Deoxycholate, Salmonella Shigella agar, and biochemical tests. The antimicrobial resistance pattern was determined by using the modified Kirby-Bauer disk diffusion technique.
Results: The overall prevalence of Salmonella and Shigella was 11.7% (26/222; 95% CI = 7.2–17.5%). Isolated Salmonella serovars showed a higher rate of resistance (85.7%, 6/7) for both Ampicillin and Amoxicillin/Clavulanic acid while Shigella isolates showed a higher resistance rate to Amoxicillin/Clavulanic acid (78.9%, 15/19) and Ampicillin (73.7%, 14/19). The overall multidrug resistance (MDR) rate of Salmonella and Shigella isolates was 88.5% (23/26). Parent/guardian educational status ≤ elementary school (AOR = 3.783; 95% CI = 1.28–11.19; P = 0.016), presence of two or more under-five children in the family (AOR = 8.999; 95% CI = 2.93–27.69; P < 0.001), unimproved source of drinking water (AOR = 5.010; 95% CI = 1.56–16.10; P = 0.007), the habit of storing cooked foods for later use (AOR = 3.199; 95% CI = 1.07–9.54; P = 0.037), attendance of the child at social gatherings (AOR = 5.387; 95% CI = 1.78–16.35; P = 0.003), and infrequent child fingernail trimming (every ≥ 2 weeks; AOR = 4.693; 95% CI = 1.47–14.94; P = 0.009) showed statistically significant association with the prevalence of culture-confirmed Salmonella and Shigella isolates.
Conclusion: The prevalence of culture-confirmed Salmonella and Shigella isolates was significantly high in the study area. Salmonella and Shigella isolates exhibited a high rate of MDR pattern. Parent/guardian education level below the elementary school, the presence of two or more under-five children in the family, using unimproved water source, a habit of storing cooked food, and infrequent fingernail trimming were independent predictors of culture-confirmed Salmonella and Shigella. Therefore, besides public health measures, regular surveillance of the prevalence and antimicrobial resistance pattern of Salmonella and Shigella should be routinely practiced in the study setting.
According to World Health Organization (WHO) definition, diarrhea is the passage of three or more abnormal stools (such as loose, bloody, or liquid stool) per day (1). Diarrhea can be mainly caused by protozoa, viruses, and bacteria. Most prevalent diarrheal cases are caused by bacteria (2) and Clostridium botulinum, Campylobacter jejuni, Vibro cholerae, Escherchia coli, Staphylococcus aureus, Salmonella serovars, and Shigella species are the predominant causative agents of food poisoning, enteric fever, and gastroenteritis (3, 4).
Salmonella serovars and Shigella species belong to the family Enterobacteriaceae, and they are among the predominant causative agents of food poisoning, enteric fever, and gastroenteritis. They are responsible for the high prevalence of under-five diarrhea, especially in developing countries owing to lack of access to clean water and poor hygiene (3, 4). Both Salmonella serovars and Shigella species remain to be the foremost cause of acute diarrhea in resource-limited countries and cause serious health problems (5).
Salmonellosis is caused by the genus Salmonella which has two species (Salmonella Enterica and Salmonella Bongori) and over 2,500 serotypes. Salmonella is gram-negative, facultatively anaerobe, rod-shaped, motile, non-lactose fermenting, oxidase-negative, and non-spore-forming bacteria. Salmonella is the leading cause of food-borne infections throughout the world. The route of transmission of Salmonella is feco-oral mainly with contaminated food and water. Once entered, the bacterium attaches to the small intestinal mucosa and invades M cells with subsequent multiplication in the cytoplasmic vacuole. Then, the bacterium will be released into the bloodstream and lymphatic circulation. After 12–72 h of infection, fever, diarrhea, abdominal cramp, and vomiting will appear (6). The infection is more serious in geriatric (above 60 years) and pediatric (under-5 years) patients (7, 8). Salmonella serovars cause diseases ranging from self-limiting gastroenteritis to more severe systemic typhoid fever (8, 9). More than 27 million infections and 200,000 deaths occur each year globally due to Salmonella infection (8).
Shigellosis is caused by the genus Shigella which has four species (Shigella dysenteriae, Shigella sonnei, Shigella boydii, and Shigella flexneri). Genus Shigella contains gram-negative, facultatively anaerobe, rod-shaped, non-motile, non-lactose fermenting, bile salt resistant, oxidase-negative, and non-spore-forming bacteria. The route of transmission for Shigella is also feco-oral mainly through contaminated hands and less commonly through contaminated water and food. As few as 100–200 bacteria can establish shigellosis (8, 10).
Shigella species are acid-tolerant and can reach the intestine by passing through the stomach. They produce Shiga toxin and disrupt protein synthesis in mucosal epithelial cells, causing bacillary dysentery, and watery or bloody diarrhea. Shigella are limited to the human and animal intestinal tract only. Personal and environmental hygiene practices are directly related to both the incidence and spread of the disease (10). Although it is a global problem, shigellosis is more prevalent in developing countries (10, 11).
Different factors may contribute to the transmission and spread of bacterial etiologies of diarrhea in under-five children. These factors could be personal and/or environmental factors, behavioral and hygiene-related factors, socio-demographic factors, or clinical factors (12–20).
Although it is easily preventable, diarrhea is not only the cause of mortality and morbidity in under-five children but also a reason for the loss of a huge amount of money. A systematic review from 2006 to 2018 showed that a weighted mean per episode expenditure was US$ 216.36 and 52.16 for inpatient and outpatient children, respectively in low and middle-income countries (21). A study conducted in Ethiopia on under-five children reported that the respective mean per episode expenditure was 79 and 6 US$ for inpatient and outpatient children in 2013 (22). The highest burden of diarrhea due to Salmonella serovars and Shigella species in under-five children occurs in sub-Saharan Africa (4, 23–25). The burden of Salmonella and Shigella among under-five children had different magnitudes in different parts of Ethiopia. The prevalence of Salmonella serovars and Shigella species was high in Northwest Ethiopia (17.3%) (26).
Antimicrobial resistance is a big global burden associated with the increasing risk of economic burden, mortality, and morbidity in developing countries. These countries are victims of antimicrobial resistance because of unnecessary consumption, compromised drug quality, lack of continuous surveillance, poverty, and antimicrobial misuse in cattle and poultry feed (27). In developing countries, the increasing emergence of antimicrobial-resistant Salmonella serovars and Shigella species to commonly prescribed antimicrobials such as chloramphenicol, ampicillin, tetracycline, and co-trimoxazole has been documented in recent years (18, 20, 28). Therefore, continuous study is important to help solve the problem.
In Ethiopia, the high prevalence of salmonellosis and shigellosis in under-five children is associated with different factors including the lower level of maternal education status, low family economic status, improper disposal of waste, poor handwashing practice, improper disposal of child's feces, low access to clean potable water, and the absence/irregular use of toilet (19, 20). According to data from the central statistics agency, the accessibility of improved drinking water sources and toilet facilities in rural households of Ethiopia was only 57 and 4%, respectively (29). Around 88% of diarrhea-related death is estimated to be due to unsafe water, insufficient hygiene, and inadequate sanitation (30).
Children are highly vulnerable to Salmonella and Shigella infection due to immaturity of the immune system, and high contagiousness of the organisms with low infectious doses. Salmonella serovars and Shigella species that have been frequently isolated in clinical samples showed a varied resistance pattern for commonly prescribed antimicrobials which makes the treatment difficult (19, 20). In Ethiopia, a few studies have been conducted on the prevalence and antimicrobial resistance pattern of Salmonella serovars and Shigella species among under-five diarrheic children, especially in the study area. The main aim of this study was, therefore, to assess the prevalence, antimicrobial resistance pattern, and associated factors of Salmonella and Shigella among under-five diarrheic children in Debre Markos town public health facilities.
The study was conducted in public health facilities in Debre Markos town. Debre Markos is the capital of the East Gojjam zone which is 295 km Northwest of Addis Ababa, the capital city of Ethiopia, and 264 km Southeast of Bahir Dar, the capital of Amhara National Regional State. The geographical location of Debre Markos is 10°17′00′′ to 10°21′30′′ N Latitudes and 37°42′00′′ to 37°45′30′′ E longitudes and it has an elevation of 2,350–2,500 m above sea level (31). The city has 11 administrative kebeles and the population size is estimated to be 111,313 in 2018 (32) of which 51,358 are males. There are four public health centers [Gozamen Health Center (GHC), Debre Markos Health center (DMHC), Wuseta Health Center (WHC), and Hidase Health Center (HHC)] and one comprehensive specialized hospital, Debre Markos comprehensive specialized hospital (DMCSH) in the town.
A cross-sectional study was conducted from June 02 to August 29, 2022.
All under-five diarrheic children seeking medical service in Debre Markos town public health facilities were the source population.
All eligible under-five diarrheic children seeking medical service at public health facilities in Debre Markos town during the study period were the study population.
All diarrheic under-five children ≥6 months of age were included in the study while children who took antimicrobials in the past 2 weeks before specimen collection and those treated for the current diarrhea attack were excluded from the study.
The sample size was calculated by using a single population proportion formula based on the assumption of a 95% confidence interval (Zα/2 = 1.96), 5% margin of error, and a prevalence of 17.45% from a previous study in Arba Minch, Ethiopia (18). Accordingly,
p = previous prevalence of Salmonella and Shigella.
d = margin of error.
n = (1.96)2* (0.1745 * 0.0.8255) ÷ 0.0025.
n = 222.
Finally, a total of 222 study participants were enrolled by using a consecutive convenient sampling technique. Study participants were allocated proportionally for all public health facilities in the study area based on 2021/2022 (2014 E.C) fiscal year second quarter under-five children's diarrhea report. The 2014 E.C second quarter diarrhea report of DMCSH, DMHC, HHC, WHC, and GHC was 617, 381, 294, 239, and 189, respectively. So, 79, 49, 38, 31, and 25 samples were taken from DMCSH, DMHC, HHC, WHC, and GHC, respectively (Figure 1).
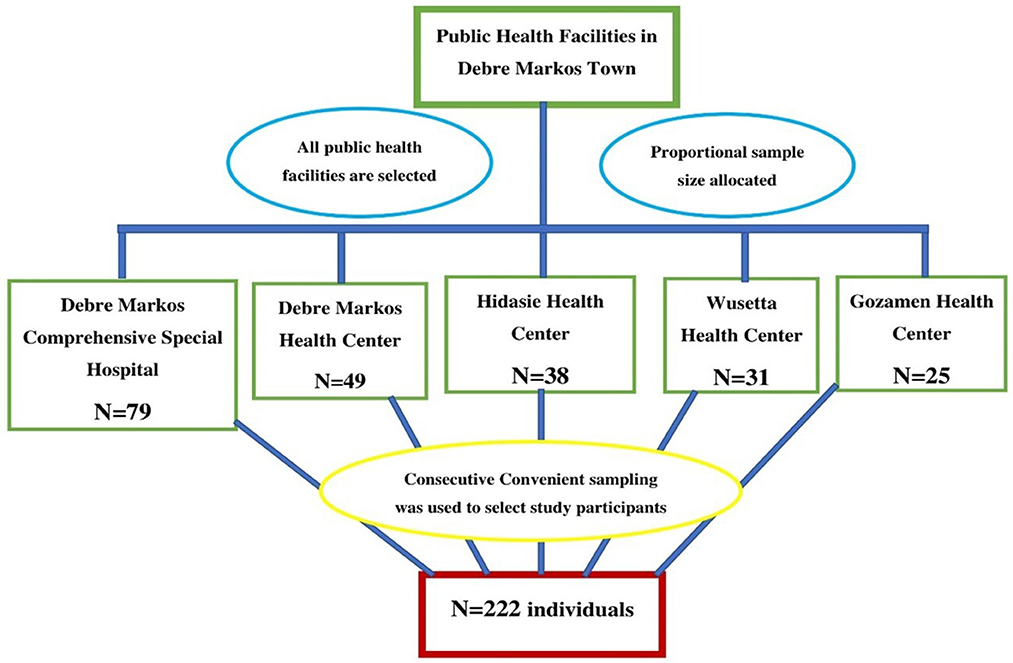
Figure 1. Proportional allocation of study participants among public health facilities in Debre Markos town, 2022.
Data on socio-demographic, clinical, and hygiene-related factors were collected through face-to-face interviews with a structured questionnaire adapted from Ethiopian demographic health survey and other previous studies (29, 33). Parents/guardians were interviewed in the Amharic language (local language) by trained nurses. The nutritional status of the study participants was assessed by measuring their mid-upper arm circumference (MUAC).
Stool specimens were collected, handled, and properly stored by trained laboratory professionals. A clean and properly labeled stool specimen container was given to parents/guardians after explaining how to collect the stool specimen. A single stool specimen about 1 gm or 1 ml (one teaspoonful in case of loose stools) was collected from each study participant by using a screwcap container (6). Then, it was placed into a Cary-Blair transport medium and was transported to the DMCSH microbiology laboratory for analysis within 3 h of collection.
By using a sterile wire loop, the stool sample was inoculated into MacConkey agar (Oxoid Ltd) and incubated at 37°C aerobically for 24 h. Then, non-lactose fermenting colorless/pale colonies were inoculated into Xylose Lysine Deoxycholate (XLD) agar (Oxoid Ltd) and Salmonella Shigella (SS) agar (Oxoid Ltd) and incubated aerobically at 37°C for 24 h. Finally, pink to red colonies on XLD agar and colorless colonies on SS agar with or without black centers were further analyzed with biochemical tests for the identification of Salmonella serovars and Shigella species (6).
Biochemical tests were performed on pink to red colonies from XLD agar and/or colorless colonies from SS agar with or without black centers for the identification of the Salmonella serovars and Shigella species. Bacteria were identified by using triple sugar iron agar (TSI; H2S and gas production, carbohydrate fermentation), motility test, indole test, citrate utilization test, urea hydrolysis test, lysine decarboxylation (LDC), mannitol fermentation, and oxidase tests.
The antimicrobial resistance pattern of bacteria was determined by using the modified Kirby–Bauer disk diffusion technique on Mueller–Hinton agar (MHA; Oxoid Ltd) according to the Clinical and Laboratory Standards Institute (CLSI 2021) guideline (34). The inoculum was prepared by suspending 4–5 isolated pure colonies in 5 ml of nutrient broth and incubated at 37°C for 30 min. The turbidity of the suspension was adjusted with 0.5 McFarland's standard. A sterile cotton swab was used to inoculate the organism from the broth into the MHA plates uniformly. Antimicrobial disks; Ampicillin (10 μg), Amoxicillin-clavulanic acid (20/10 μg), Azithromycin (15 μg), Ceftazidime (30 μg), Ceftriaxone (30 μg), Chloramphenicol (30 μg), Ciprofloxacin (5 μg), Doxycycline (30 μg), Tetracycline (30 μg), and Trimethoprim-sulfamethoxazole (1.25/23.75 μg; Oxoid Ltd) were tested for both Salmonella serovars and Shigella species (34). Antimicrobial disks were placed and incubated at 35–37°C overnight. The diameter of the zone of inhibition was measured and interpreted based on CLSI 2021 guidelines (34). The result of each antimicrobial test was interpreted as sensitive, intermediate, or resistant (34). Isolates were reported as MDR when resistance to at least one antimicrobial agent in three or more antimicrobial categories was observed (35).
The questionnaire was first prepared in English language and translated into the local language (Amharic). Then, it was translated back into the English language to assure consistency. The questionnaire was validated through conducting a pre-test on 10% of the total sample size (23 clients) at Lumamie primary hospital and modifications were made accordingly.
Data collectors (nurses) and laboratory professionals were trained for 1 day and Standard Operating Procedures (SOPs), as well as manufacturer's instructions, were strictly followed during specimen collection, transportation, and processing. The collected stool specimens were transported with maximum care using a cold chain and transport medium and tested within 3 h. Stool specimens with insufficient volume or contaminated with urine, soil, or water were rejected. The dry hot oven sterilization method was applied to test tubes, cotton swabs, and Petri dishes. The sterility of culture media and biochemical tests for each batch was checked by incubating 5% of the prepared media without inoculation for 24 h at 37°C.
E. coli ATCC® 25922, P. aeruginosa ATCC® 27853e, S. typhimurium ATCC 14028 (34), and S. flexnerii ATCC 12022 (36) were reference strains used to check the performance of the culture media, biochemical tests, and antimicrobial disks. Moreover, quality control strains were tested for growth, resistance pattern, and biochemical tests parallel with stool samples to assess the validity of the test procedure. The expiry date of all reagents, supplies and antimicrobial disks was also checked.
Standard laboratory data management policies and protocols were followed during result interpretation, documentation, and reporting. The results were recorded carefully using a laboratory result registration sheet. Generally, close supervision was undertaken seriously, and questionnaires were checked for completeness during and after data collection. Each data was checked for completeness before processing.
Cleaned and coded data were entered into EpiData version 4.6 and exported to SPSS version 25.0 for statistical analysis. Descriptive statistics were presented using tables, graphs, and percentages. Binary logistic regression models were performed to determine the presence of significant association between predictors and the dependent variable. Predictor variables that had a significant association in bivariable logistic regression (P ≤ 0.25) were fitted to multivariable backward stepwise logistic regression for final analysis and variables with P-value ≤ 0.05 were considered statistically significant. The model fitness was checked using Hosmer and Lemeshow test. The strength of association was measured using an odds ratio at 95% CI. The antimicrobial resistance pattern of Salmonella and Shigella isolates was analyzed using WHONET software version 22.8.23. MUAC measurement value was used to classify the nutritional status of the participants. Participants were classified as under-nutrition when their MUAC was ≤ 12.5 cm (37).
Ethical clearance was obtained from Debre Markos University College of Health sciences before the actual data collection with letter number HSC/R/C/Ser/PG/Co/195/11/14 and Amhara public health institute with a letter numbered 03/1434. A supporting letter was written to the zonal health department from the college with letter numbered 196/11/14. Written permission to conduct the study was obtained from East Gojjam Zone health office and Debre Markos town health office. Then, a support letter was written to health institutions to obtain permission for the data collection process. Written informed consent was obtained from the parents/guardians of the study participants. Consent was taken from parents/guardians because our study participants were too young to volunteer to participate as well as to decide to withdraw from the study. Participation was only based on voluntarism and participants' parents/guardian were informed that they have the right to withdraw participants from the study at any time. Issues of confidentiality and anonymity were also maintained. The result of each study participant was reported to their respective clinician immediately for appropriate intervention.
✓ Infrequent fingernail trimming: trimming fingernail in ≥2 weeks.
✓ Attendance at social gatherings: attending social gatherings within a week before the clinical onset of diarrhea.
✓ Unimproved source of drinking water: water not treated with chemicals before consumption.
✓ Poor house floor: when the house floor is not covered with plastic shed or ceramic.
A total of 222 study participants were enrolled in the study of which 95.5% (212/222) were urban dwellers and 52.7% (117/222) were males. The age of study participants ranged from 6 to 59 months with a median of 33 months (IQR = 30.25). About 75.7% (168/222) of parents/guardians were married and the family monthly income ranged from 1,500 to 12,500 Ethiopian Birr with a median of 8,000 Birr (IQR = 3,500; Table 1).
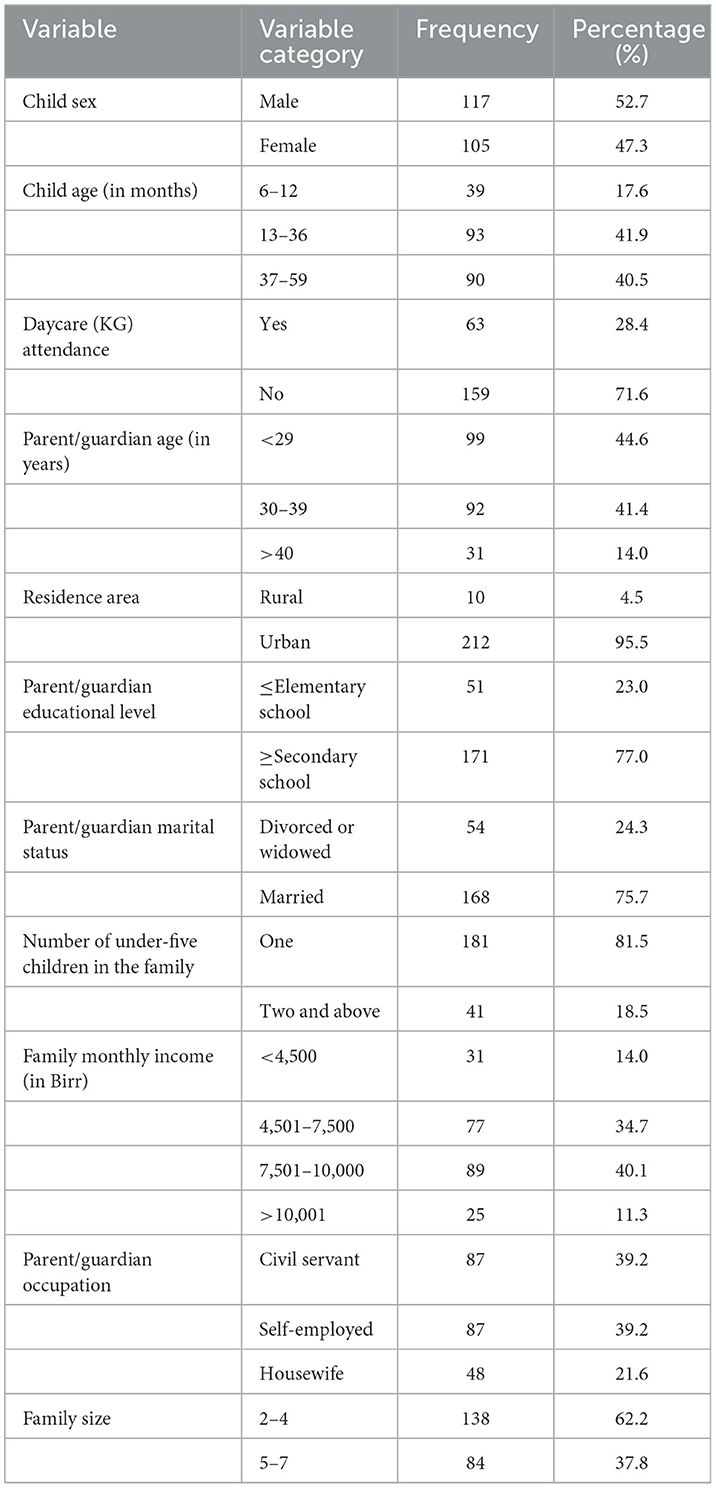
Table 1. Socio-demographic characteristics of the study participants in public health facilities in Debre Markos town, 2022.
Less than half (44.1%, 98/222) of the study participants were under-nutrition. The frequency of diarrhea per day ranged from 3 to 12 with a mean of 4.91 (SD ± 1.257). Family history of diarrhea in the past week was reported in 20.3% (45/222) of the study participants. Details are described in Table 2.
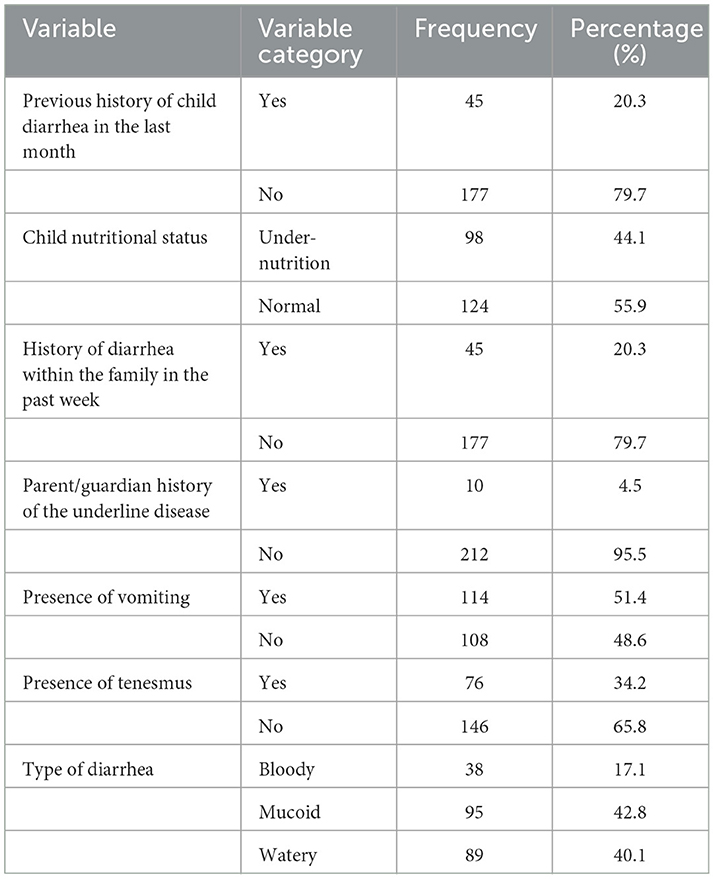
Table 2. Clinical characteristics of the study participants in public health facilities in Debre Markos town, 2022.
Most of the study participants (93.7%, 208/222) had good house floor material. All respondents had a latrine and about half of them (51.4%, 114/222) had a handwashing facility near the latrine. One-third (33.3%, 74/222) of the study participants had a history of attendance at social gatherings within 7 days before disease onset. Around one-fifth (18.9%, 42/222) of the respondents had a travel history. The source of drinking water for most study participants (72.1%, 160/222) was improved (Table 3).
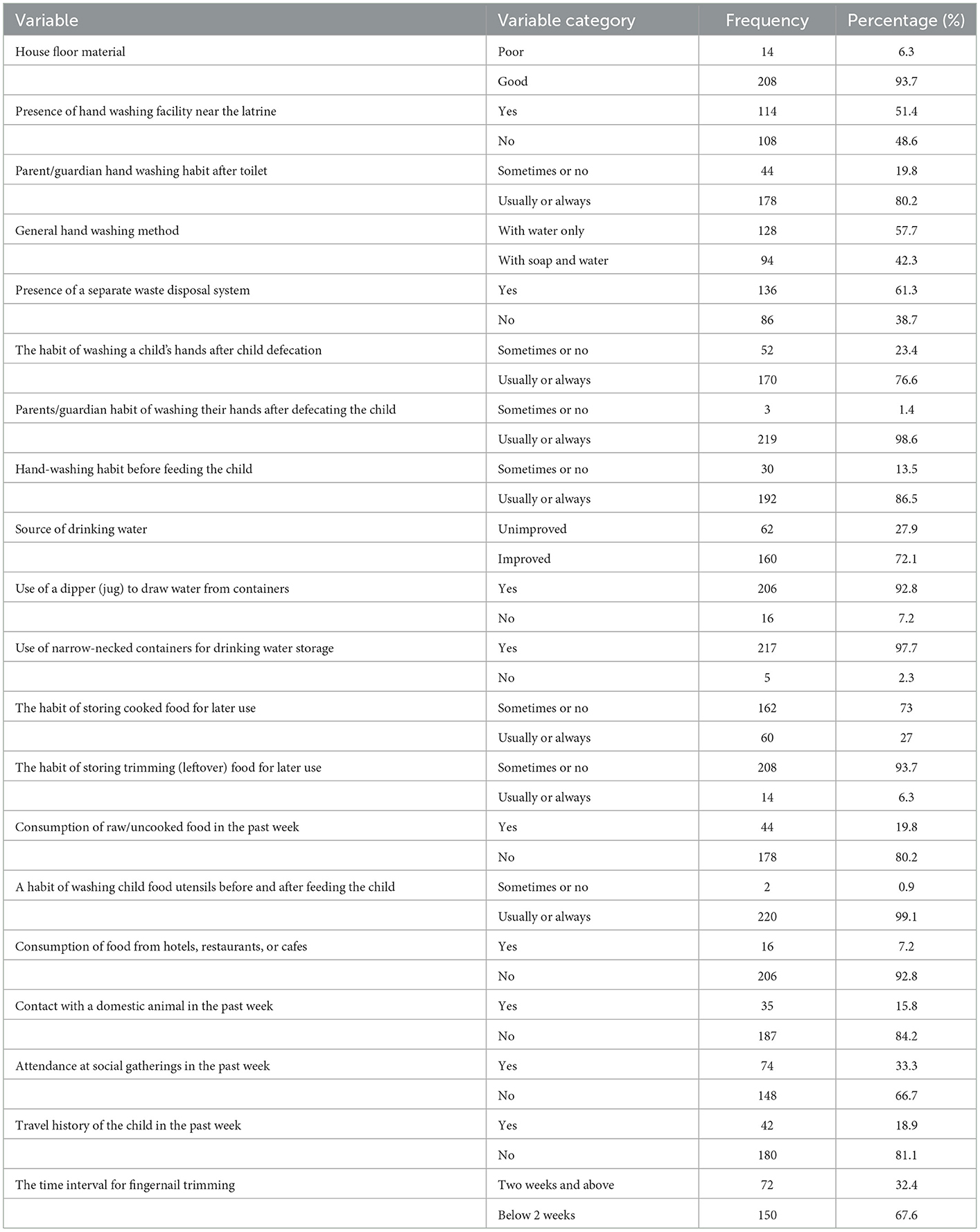
Table 3. Hygiene-related and behavioral characteristics of the study participants in public health facilities in Debre Markos town, 2022.
The overall prevalence of Salmonella and Shigella among the study participants was 11.7% (26/222; 95% CI = 7.2, 17.5%). Among these, the prevalence of Salmonella serovars, S. dysenteriae, and other Shigella species was 3.15% (7/222), 3.60% (8/222), and 4.95% (11/222), respectively.
The frequency of Salmonella and Shigella was high among females and age group of 13–36 months as shown in Figure 2.
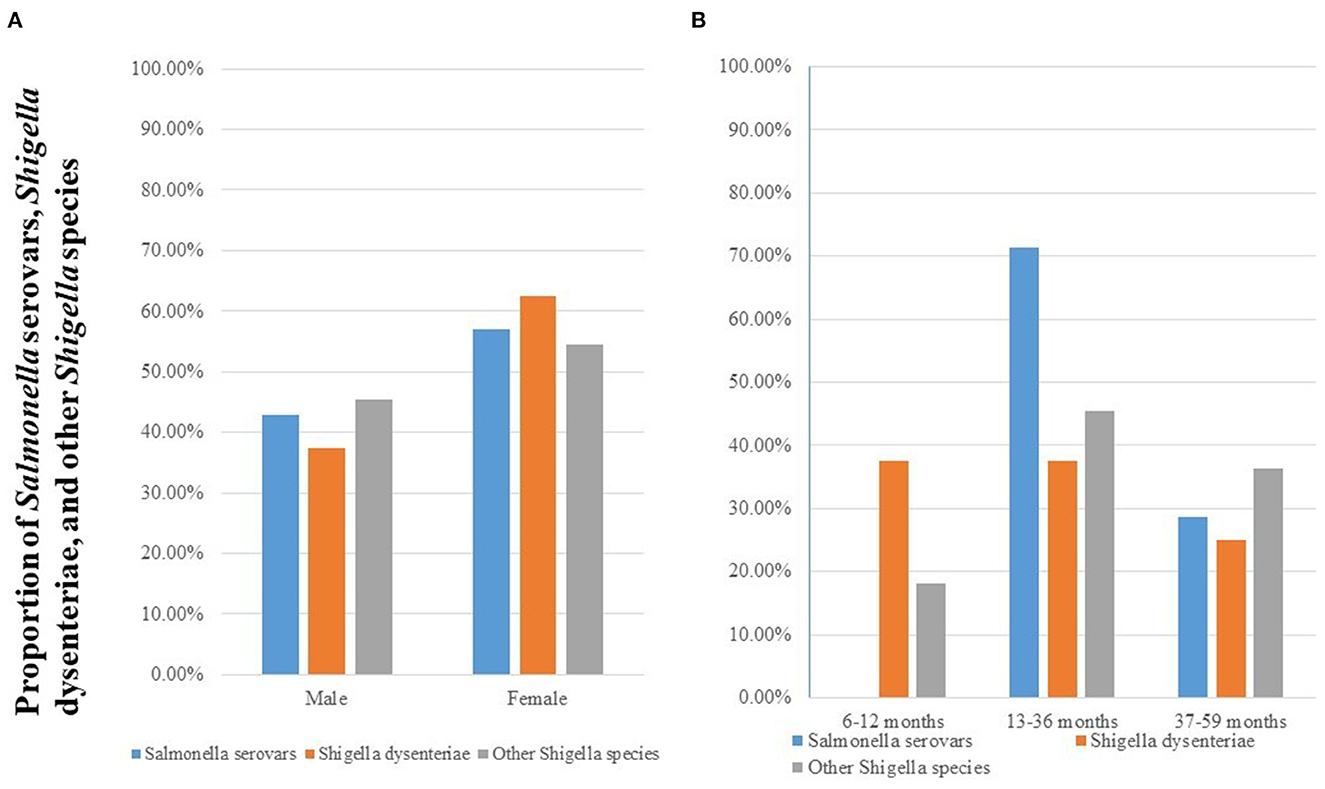
Figure 2. Prevalence of Salmonella serovars, S. dysenteriae, and other Shigella species stratified by sex (A) and age group (B) among diarrheic under-five children in public health facilities in Debre Markos town, Northwest Ethiopia, 2022.
Both Salmonella serovars and Shigella species showed different resistance patterns against the tested antimicrobial disks. Among the tested Salmonella serovars, a higher rate of susceptibility was observed for Ciprofloxacin and Ceftriaxone (71.4%, 5/7 for each). In contrast, these species showed a higher rate of resistance (85.7%, 6/7) for both Ampicillin and Amoxicillin/Clavulanic acid. S. dysenteriae isolates were highly susceptible (87.5%, 7/8) to Ceftazidime, Ciprofloxacin, and ceftriaxone. However, they were highly resistant to Ampicillin (87.5%, 7/8) and Amoxicillin/Clavulanic acid (75%, 6/8). Other Shigella species showed a higher rate of susceptibility for Ciprofloxacin (90.9%, 10/11) and Ceftriaxone (81.8%, 9/11) whereas they exhibited a higher resistance rate for Amoxicillin/Clavulanic acid and Doxycycline (81.8%, 9/11 for each). All Shigella species showed a higher resistance pattern to Amoxicillin/Clavulanic acid (78.9%, 15/19) and Ampicillin (73.7%, 14/19) as shown in Table 4.
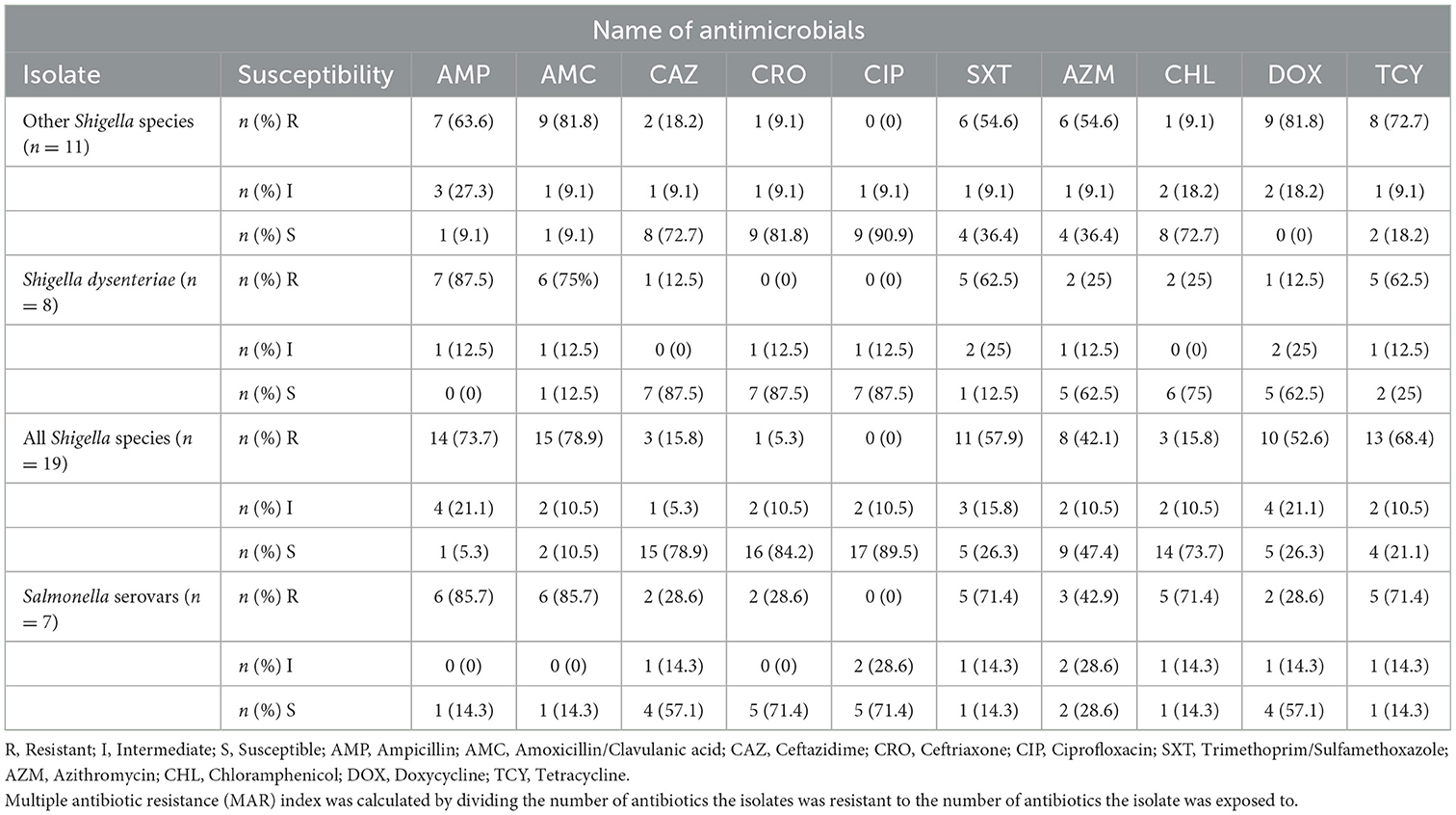
Table 4. Antimicrobial resistance pattern of Salmonella serovars, S. dysenteriae, other Shigella species, and all Shigella species isolated from under-five diarrheic children in public health facilities in Debre Markos town, Northwest Ethiopia, 2022.
Of the culture-confirmed Salmonella serovars, 42.9% (3/7) were resistant to three classes of antimicrobials while nearly one-fourth (28.6% 2/7) were resistant to seven drug classes. All isolates of Salmonella serovars were MDR. Three-fourth [75% (6/8), 95% CI: 69.3–80.7] of culture-confirmed S. dysenteriae isolates and 90.9% (9/11; 95% CI: 87.1–94.7) of culture-confirmed other Shigella species were MDR. Moreover, 84.2% (16/19; 95% CI: 67.8–99.9) of culture-confirmed Shigella isolates were MDR. Overall, the rate of MDR among Salmonella and Shigella isolates was 88.5% (23/26; 95% CI: 84.3–92.7; Table 5).
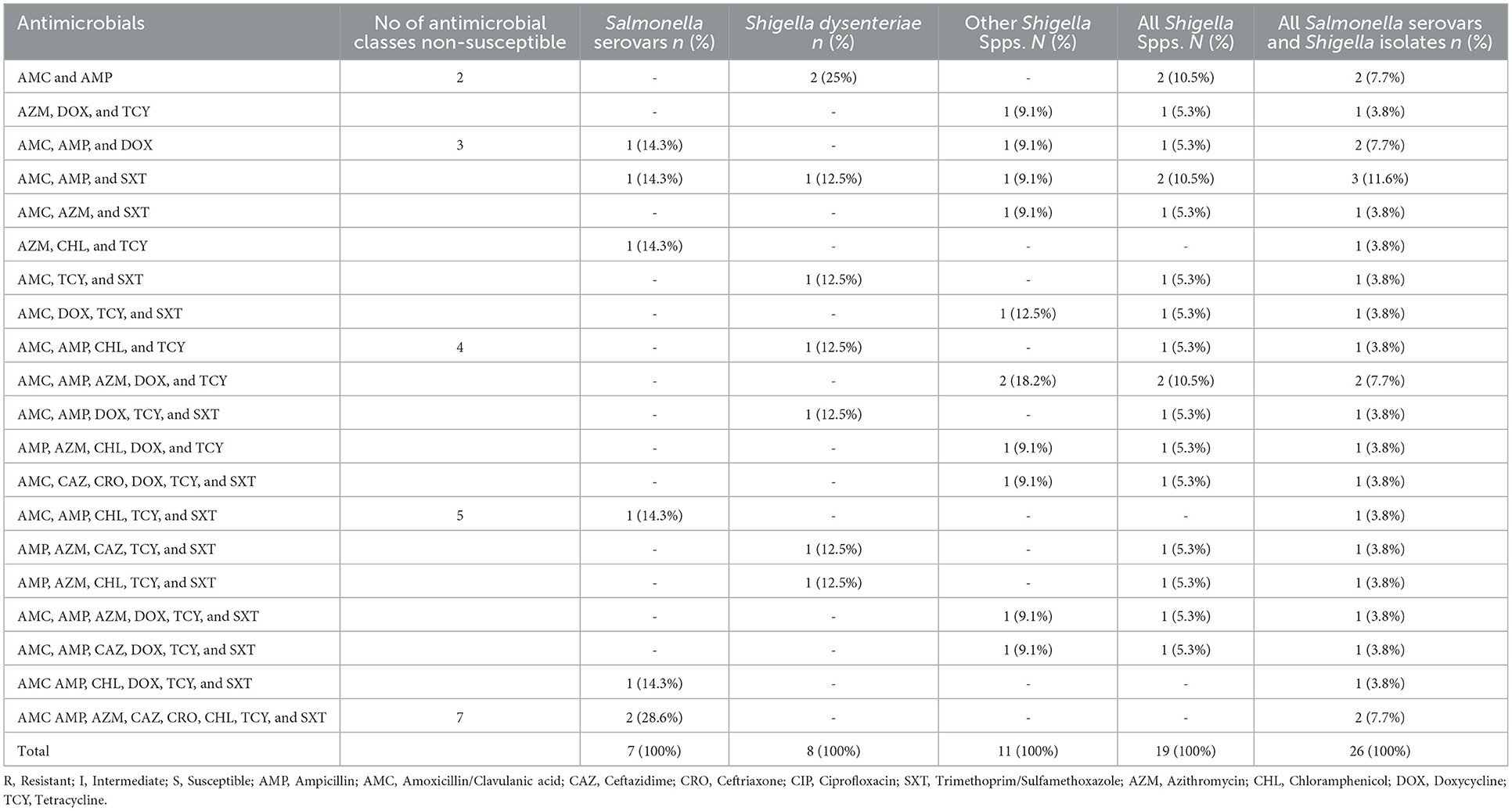
Table 5. MDR (resistant drugs combination) pattern of Salmonella and Shigella isolates from under-five diarrheic children in public health facilities in Debre Markos town, Northwest Ethiopia, 2022.
After adjusting for confounding factors, parents'/guardians' educational level ≤ elementary school, presence of two or more under-five children in the family, unimproved source of drinking water, a frequent habit of storing cooked food for later use, attendance of the child at social gatherings within 1 week before infection, and infrequent child fingernail trimming (≥2 weeks) were significant predictors of Salmonella and Shigella among under-five diarrheic children (P ≤ 0.05; Table 6).
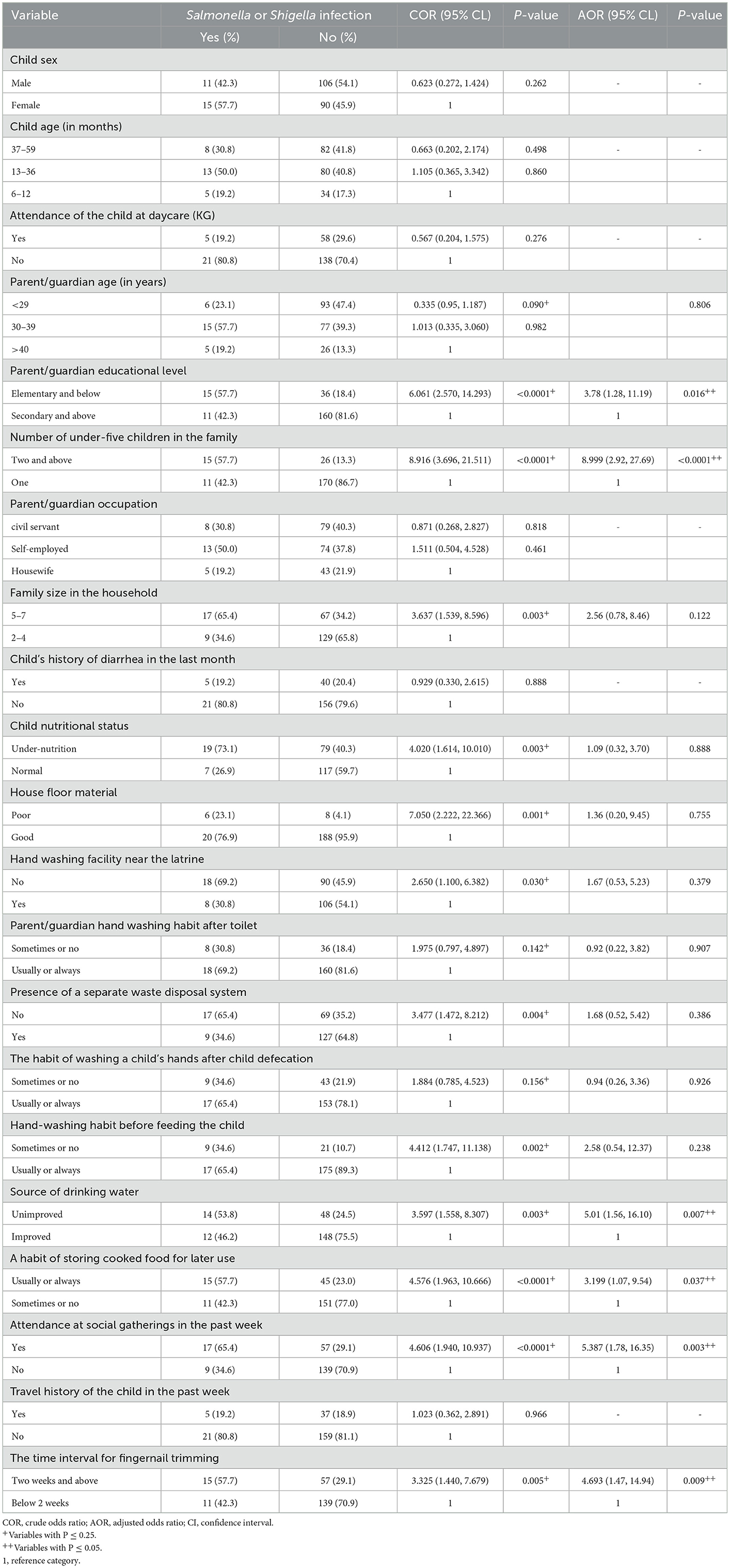
Table 6. Bivariable and multivariable logistic regression analysis of factors associated with Salmonella and Shigella infection among under-five children at public health facilities in Debre Markos town, 2022.
Children whose parent/guardian had below primary school educational level have about four times more likely to get Salmonella and Shigella infection compared to their counterparts (AOR = 3.78; 95% CI = 1.28–11.19; P = 0.016). It is about nine times (AOR = 8.999; 95% CI = 2.93–27.69; P = 0.000) more likely that a child who had been living in a family with two or more under-five children was infected with Salmonella and Shigella. Children who drank unimproved water were five times more likely to get infected with Salmonella and Shigella than those who drank improved water (AOR = 5.010; 95% CI = 1.56–16.098; P = 0.007).
Children with parents/guardians with the habit of storing cooked food for later use were about 3.2 times in odds of getting an infection with Salmonella and Shigella compared to their counterparts (AOR = 3.199; 95% CI = 1.07–9.54; P = 0.037). Children who had attended social gatherings had about five times chance of harboring Salmonella and Shigella (AOR = 5.387; 95% CI = 1.78–16.35; P = 0.003). Besides, infrequent child fingernail trimming was another independent predictor of Salmonella and Shigella infection (AOR = 4.693; 95% CI = 1.47–14.94; P = 0.009; Table 6).
Enterobacteriaceae are among the predominant causative agents of gastroenteritis and Salmonella serovars and Shigella species are among the foremost agents. In this study, the overall prevalence of culture-confirmed Salmonella and Shigella infection among under-five diarrheic children was 11.7% (26/222; 95% CI: 7.2–15.5). This result is in line with studies conducted in different parts of Ethiopia; Dire Dawa 9.2% (13), Hawassa 9.5% (12), Bale Robe 11.2% (19), and Hosanna 9.5% (17). It is also in line with studies conducted in different countries like Kenya 12.5% (38), Zambia 12.4% (39), and Bangladesh 8.5% (40). Moreover, it is also in agreement with a systematic review and meta-analysis done in Sub-Saharan Africa 14.1% (23). However, it is lower than studies conducted in Arba Minch-Ethiopia 17.45% (18), Zanzibar 28.84% (41), India 36.4% (42), Iran 15.9% (43), and another study in Iran 25.9% (44). The reason for this difference could be due to the difference in the number of study participants enrolled. The number of study participants enrolled in previous studies was higher compared to our study. Moreover, the duration of the study period may also be another factor since these studies were conducted over a relatively longer study period (up to 4 years). The result of this study is higher than studies reported from Ambo-Ethiopia 3.8% (16) to Sudan 6% (45). This discrepancy could be due to the difference in study time which is 2014 for a study from Ambo-Ethiopia and 2013 for the study from Sudan. Moreover, seasonal variation may also be another factor. The study conducted in Ambo was conducted in winter while this study was conducted in summer.
The prevalence of culture-confirmed Salmonella serovars was 3.2% (95% CI: 0.9–5.6%) which is comparable with studies from different parts of Ethiopia; Debre Berhan 3.1% (46), Addis Ababa 3.95% (11), Dire Dawa 3.6% (13), Hawassa 2.5% (12) and different countries like Sudan 2% (45), and Bangladesh 4.9% (40). It is also comparable with a meta-analysis done in Sub-Saharan Africa 4.5% (23) and the global prevalence of 1.9% (47). This finding is lower than results reported from studies conducted in other parts of Ethiopia; Bahir Dar 7.8% (26), Bale Robe 6.9% (19), Arba Minch 12.6% (18), and different countries such as Zambia 8% (39), India 20.5% (42), Iran 13.1% (44), and another study in Iran 7% (43). These differences could be associated with differences in geographical location, and study populations study participants in Iran and India were selected from admission rooms in selected hospitals. This finding is slightly higher than reports from different areas of Ethiopia; Ambo 1.3% (16) and Hosanna 1% (17) which could be due to difference in study time as studies from Ambo and Hosanna were conducted in 2013 and 2017, respectively.
The prevalence of culture-confirmed Shigella species was 8.6% (95% CI: 4.9–12.3%) which is comparable with reports from different areas of Ethiopia; Bahir Dar 9.5% (26), Addis Ababa 9.1% (11), Dire Dawa 5.6% (13), Hawassa 7% (12), and Hosanna 8.3% (17). It is also in agreement with studies in different countries; Kenya 7.4% (48), another study from Kenya 5% (38), Mexico 6% (49), and Iran 8.9% (43). Moreover, it is also in line with a meta-analysis done in Sub-Saharan Africa 9.6% (23). Our finding is lower than studies reported from Zanzibar 16.93% (41), India 15.9% (42), Iran 26.17% (50), and another study from Iran 12.8% (44). This variation could be due to differences in the study setting and sampling technique. In these studies, outpatients and admitted patients were selected from different hospitals. A systematic random sampling technique was used in these studies to select participants. Moreover, the number of participants enrolled was relatively larger compared to our study. In contrast to this study, a lower rate of isolation was reported from parts of Ethiopia; Debre Berhan 1.8% (46), Ambo 2.5% (16), Bale Robe 4.3% (19), Arba Minch 4.85% (18) and different countries; Sudan 4% (45), Zambia 4.4% (39), and Bangladesh 3.6% (40). The reason for this difference could be seasonal variations and variations in socio-demographic factors. Studies in Ambo, Bale Robe, Arba Minch, and Debre Berhan were conducted in the winter season, but our study was conducted in the summer.
The antimicrobial resistance rate of culture-confirmed Salmonella serovars and Shigella species for different antimicrobials has been increasing around the world, especially in developing countries. In our study, 10 antimicrobials from eight different drug classes were tested for isolates showing different rates of resistance. A high level of resistance of Salmonella isolates was observed for ampicillin [%R = 85.7% (6/7), 95% CI: 42–99.2%]. This is in line with previous studies conducted in different parts of Ethiopia; Bahir Dar 93.9% (26), Addis Ababa 80% (11), Ambo 88.9% (16), and Dire Dawa 71.4% (13). However, it is lower than studies conducted in Debre Markos 100% (51), Alamura 100% (20), Hosanna 100% (17), and Arba Minch 100% (18). This could be due to differences in the study population. A study in Debre Markos was done among food handlers and a study conducted in Alamura was carried out on under 14 children. Salmonella isolates demonstrated a high rate of resistance to Chloramphenicol [71.4% (5/7), 95% CI: 30.3–94.9]. This is in line with a study from Arba Minch 43% (18) but higher than studies from other parts of Ethiopia; Dire Dawa 14.3% (13), Bale Robe 24.1% (19), and Hosanna 0% (17). This could be due to the increasing habit of using antimicrobials in cattle production in the country.
Our study revealed that isolated Shigella species had shown a high rate of resistance to amoxicillin/clavulanic acid 78.9% (15/19; 95% CI: 60.55–97.25). This is in line with a study conducted in Addis Ababa 91.4% (11). In contrast, it is higher than reports from Gondar 10.3% (28), Alamura 55.6% (20), and French Guiana 12% (52). In our study, the resistance rate of Shigella species for ampicillin was 73.7% (14/19; 95% CI: 53.9–93.5). This finding is comparable with reports from Ethiopia; Gondar 76% (28), Hawassa 63.6% (12), Alamura 81.8% (20), Hosanna 82.4% (17), Ambo 88.9% (16), Dire Dawa 90.9% (13), and also different countries such as French Guiana 58% (52), Iran 67.8% (44), and another study in Iran 61.5% (53). Moreover, it is also in line with the result of pooled Ethiopian studies 83.1% (54). On the contrary, it is lower than reports from Debre Markos 100% (51), Addis Ababa 95.7% (11), and Iran 96.2% (43). This variation could be due to differences in study time, study population, and socio-demographic factors.
In our study, 100% (7/7) of Salmonella isolates were MDR. This is higher than reports from different parts of Ethiopia; Bale Robe (31.03%) (19), Arba Minch (37.5%) (18), Hosanna (50%) (17), Ambo (66.7%) (16), and in Bangladesh (26.67%) (40). Moreover, 84.2% (16/19; 95% CI: 67.8–99.9) of culture-confirmed Shigella isolates were MDR. This result is in line with reports from Ambo-Ethiopia (83.3%) (16), Iran (92%) (55), and Bangladesh (93%) (40). Howe ever, this is higher than reports from different parts of Ethiopia; Gondar (37.9%) (28), Arba Minch (37.5%) (18), Hosanna (64.7%) (17), and in Nigeria (58.3%) (43). This discrepancy could be due to the widespread nature of resistant strains, the emergence of new resistant strains, and the increasing habit of using antimicrobials in cattle and poultry production. Moreover, an increasing habit of antimicrobial misuse with self-prescription may also have a contribution.
In this study, children whose parents/guardians' educational level ≤ elementary school had higher odds of getting infection with Salmonella and Shigella. This may be due to a low level of knowledge, attitude, and practice as compared to parents/guardians with secondary school and above educational level. The presence of two or more under-five children in a family had increased the risk of getting Salmonella and Shigella infections in 9-folds. This result is supported by a study conducted in Cameroon (56). This may be due to the inability of parents/guardians to provide appropriate care for those children because of cost and time shortages.
In our study, the habit of storing cooked food for later use had a significant association with the prevalence of Salmonella and Shigella which is supported by a study conducted in Alamura (southern Ethiopia) (20). This could be because bacteria can multiply in significant numbers and cause disease upon storage in ambient temperatures. Similarly, infrequent fingernail trimming (≥2 weeks) had increased the odds of getting an infection with Salmonella and Shigella by 4.7 times. This result is in agreement with a study done in Arba Minch (18) entailinguntrimmed fingers can easily be contaminated with soil and other wastes carrying the indicated bacteria.
This study confirmed that participants who had unimproved sources of drinking water have higher odds (five times) of getting infected with Salmonella and Shigella comparable with a study conducted in Dire Dawa (13). It is known that contaminated water and food are responsible for the transmission of diarrheal diseases. Other factors associated with a high rate of Salmonella and Shigella in under-five diarrheic children was the attendance of the child at social gatherings within 1 week before disease onset. This increased the risk of infection by 5.4 times. This may be because attending and eating at these ceremonies may increase the risk of contamination and infection.
In this study, we couldn't identify Salmonella serovars and all Shigella species and also failed to include molecular techniques which could hamper the anticipated prevalence of the bacterial isolates. Having these limitations, this study provides a glimpse into the prevention and control of drug resistance and diarrhea caused by Salmonella and Shigella in the study area.
In this study, the prevalence of Salmonella and Shigella infection was significantly high compared to the global prevalence. Most Shigella isolates were highly resistant to amoxicillin/clavulanic acid, doxycycline, and tetracycline whereas most Salmonella isolates were highly resistant to ampicillin, amoxicillin/clavulanic acid, trimethoprim-sulfamethoxazole, chloramphenicol, and tetracycline. Both Salmonella and Shigella isolates exhibited a high rate of MDR pattern. Ceftazidime, ceftriaxone, ciprofloxacin, and chloramphenicol could be possible antimicrobial choices in case of Shigella infection while ceftazidime and ciprofloxacin could be antimicrobials of choice for treating Salmonella infection. Our study also revealed that low parent/guardian educational level, presence of two or more under-five children in the family, consumption of unimproved water, the habit of storing cooked foods for later use, attendance of the child at social gatherings, and infrequent fingernail trimming were independent predisposing factors for the higher rate of Salmonella and Shigella infection. Culture-based bacterial detection and antimicrobial sensitivity test-based antimicrobial prescription should be routinely practiced. Public health measures should be in place to improve the hygiene of the community, improve sources of drinking water, create awareness in the community, and improve environmental and personal hygiene. Large-scale longitudinal studies are also recommended.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical clearance was obtained from Debre Markos University College of Health Sciences before the actual data collection with letter number HSC/R/C/Ser/PG/Co/195/11/14 and Amhara Public Health Institute with a letter numbered 03/1434. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
MD conceived and designed the study, performed the data collection and analysis, and wrote the manuscript. HM and GM supervised and assisted in the designing, data analysis and interpretation, and critical editing and revision of this manuscript. All authors read and approved the final manuscript.
We would like to acknowledge Debre Markos University and Debre Markos Comprehensive Specialized Hospital for their support in administrative issues. We also acknowledge all health staff of public health facilities in Debre Markos town for their support in data collection. Besides, we duly acknowledge all study participants for their voluntary participation in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. Diarrhoeal Disease. Geneva: World Health Organization (2017). Available online at: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed Febrarury 23, 2022).
2. Zhang S-X, Zhou Y-M, Tian L-G, Chen J-X, Tinoco-Torres R, Serrano E, et al. Antibiotic resistance and molecular characterization of diarrheagenic Escherichia coli and non-typhoidal Salmonellastrains isolated from infections in Southwest China. Infect Dis Poverty. (2018) 7:1–11. doi: 10.1186/s40249-018-0427-2
3. Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. (2017) 17:909–48. doi: 10.1016/S1473-3099(17)30276-1
4. Kotloff KL. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am. (2017) 64:799–814. doi: 10.1016/j.pcl.2017.03.006
5. Zhu X-H, Tian L, Cheng Z-J, Liu W-Y, Li S, Yu W-T, et al. Viral and bacterial etiology of acute diarrhea among children under 5 years of age in Wuhan, China. Chin Med J. (2016) 129:1939–44. doi: 10.4103/0366-6999.187852
6. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part-2. 2nd ed. New York, NY: Cambridge University Press (2006). doi: 10.1017/CBO9780511543470
7. Coburn B, Grassl GA, Finlay B. Salmonella, the host and disease: A brief review. Immunol Cell Biol. (2007) 85:112–8. doi: 10.1038/sj.icb.7100007
8. Murray PR, Rosenthal KS, Pfaller MA. Medical Microbiology. 8th ed. Chatswood, NSW: Elsevier Inc. (2016).
9. Brooks GF, Carroll KC, Butel JS, Morse SA, Meitzner TA. Jawetz, Melnick, & Adelberg's Medical Microbiology. 26th ed. New York, NY: McGraw-Hill Companies (2013).
10. Ray CG, Ryan KJ. Sherris Medical Microbiology. 6th ed. New York, NY: McGraw-Hill Education (2014).
11. Mamuye Y, Metaferia G, Birhanu A, Desta K, Fantaw S. Isolation and antibiotic susceptibility patterns of Shigella and Salmonella among under 5 children with acute diarrhoea: A cross-sectional study at selected public health facilities in Addis Ababa, Ethiopia. Clin Microbiol. (2015) 4:1–7. doi: 10.4172/2327-5073.1000186
12. Getamesay M, Getenet B, Ahmed Z. Prevalence of Shigella, Salmonella, and Campylobacter species and their susceptibility patters among under five children with diarrhea in Hawassa Town, South Ethiopia. Ethiop J Health Sci. (2014) 24:101–8. doi: 10.4314/ejhs.v24i2.1
13. Mekonnen M, Geda B, Teklemariam Z, Weldegebreal F, Balakrishnan S. Prevalence of childhood diarrhea and associated risk factors in Dire Dawa, eastern Ethiopia. J Pub Health. (2018) 26:29–37. doi: 10.1007/s10389-017-0843-y
14. Teferi SC. Prevalence, antibiotic susceptibility profile, and associated risk factors of Salmonella isolate among diarrheal patients visiting dessie referral hospital, Northeast Ethiopia. Int J Microbiol. (2020) 2020:8834107. doi: 10.1155/2020/8834107
15. Terfassa A, Jida M. Prevalence and antibiotics susceptibility pattern of Salmonella and Shigella species among diarrheal patients attending Nekemte Referral Hospital, Oromia, Ethiopia. Int J Microbiol. (2018) 2018:9214689. doi: 10.1155/2018/9214689
16. Tosisa W, Mihret A, Ararsa A, Eguale T, Abebe T. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Pediatr. (2020) 20:1–8. doi: 10.1186/s12887-020-1970-0
17. Abebe W, Earsido A, Taye S, Assefa M, Eyasu A, Godebo G. Prevalence and antibiotic susceptibility patterns of Shigella and Salmonella among children aged below 5 years with Diarrhoea attending Nigist Eleni Mohammed memorial hospital, South Ethiopia. BMC Pediatr. (2018) 18:1–6. doi: 10.1186/s12887-018-1221-9
18. Ameya G, Tsalla T, Getu F, Getu E. Antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella infections among under five children in Arba Minch, South Ethiopia. Ann Clin Microbiol Antimicrobs. (2018) 17:1–7. doi: 10.1186/s12941-018-0253-1
19. Assefa A, Girma M. Prevalence and antimicrobial susceptibility patterns of Salmonella and Shigella isolates among children aged below 5 years with diarrhea attending Robe General Hospital and Goba Referral Hospital, South East Ethiopia. Trop Dis Travel Med Vaccines. (2019) 5:1–11. doi: 10.1186/s40794-019-0096-6
20. Hayamo M, Alemayehu T, Tadesse B, Mitiku E, Bedawi Z. Magnitude, risk factors and antimicrobial susceptibility pattern of Shigella and Salmonella, among children with diarrhea in Southern Ethiopia: A cross-sectional Study. SAGE Open Med. (2021) 9:20503121211009729. doi: 10.1177/20503121211009729
21. Baral R, Nonvignon J, Debellut F, Agyemang SA, Clark A, Pecenka C. Cost of illness for childhood diarrhea in low-and middle-income countries: A systematic review of evidence and modelled estimates. BMC Public Health. (2020) 20:1–13. doi: 10.1186/s12889-020-08595-8
22. Memirie ST, Metaferia ZS, Norheim OF, Levin CE, Verguet S, Johansson KA. Household expenditures on pneumonia and diarrhoea treatment in Ethiopia: A facility-based study. BMJ Glob Health. (2017) 2:e000166. doi: 10.1136/bmjgh-2016-000166
23. Oppong T, Yang H, Amponsem-Boateng C, Kyere ED, Abdulai T, Duan G, et al. Enteric pathogens associated with gastroenteritis among children under 5 years in sub-Saharan Africa: A systematic review and meta-analysis. Epidemiol Infect. (2020) 148:e64. doi: 10.1017/S0950268820000618
24. Charyeva Z, Cannon M, Oguntunde O, Garba AM, Sambisa W, Bassi AP, et al. Reducing the burden of diarrhea among children under 5 years old: Lessons learned from oral rehydration therapy corner program implementation in Northern Nigeria. J Health Popul Nutr. (2015) 34:1–8. doi: 10.1186/s41043-015-0005-1
25. Ablaku B, Sabo Z, Ani A. Prevalence of Enteropathogens causing diarrhea in chilhren (0–5 years) in Keffi local government area of Nasarawa state, Nigeria. Trends Food Sci Technol. (2018) 3:83–6.
26. Admassu M, Yemane G, Kibret M, Abera B, Nibret E, Adal M. Prevalence and antibiogram of Shigella and Salmonella spp. from under five children with acute diarrhea in Bahir Dar Town. Ethiop J Sci Technol. (2015) 8:27–35. doi: 10.4314/ejst.v8i1.3
27. Chowdhury MMH, Kubra K, Islam MT, Rahman MM, Mehedy ME. Indiscriminate uses of antibiotics as a threat to public health demand implementation of effective drug practices and enhancement of public awareness in Bangladesh. Eur J Sci. (2015) 133:187–95.
28. Alemu A, Geta M, Taye S, Eshetie S, Engda T. Prevalence, associated risk factors and antimicrobial susceptibility patterns of Shigella infections among diarrheic pediatric population attending at Gondar town healthcare institutions, Northwest Ethiopia. Trop Dis Travel Med Vac. (2019) 5:1–8. doi: 10.1186/s40794-019-0079-7
29. CSA. Ethiopia Demographic and Health Survey 2016. Addis Ababa; Rockville, MD: CSA and ICF (2016). p. i-516.
30. CDC. Diarrhea: Common Illness, Global Killer: Center for Disease Control (USA). (2017). p. 4. Available online at: https://www.cdc.gov/healthywater/pdf/global/programs/globaldiarrhea508c.pdf (accessed September 18, 2022).
31. Andarge A. Monitoring the urban growth of Debre Markos Town (1984-2012), Ethiopia: Using satellite images and GPS. J Geogr Reg Plan. (2017) 10:69–76. doi: 10.5897/JGRP2016.0533
32. Mekuriaw T, Gokcekus H. The impact of urban expansion on physical environment in Debre Markos Town, Ethiopia. Civ Environ Res. (2019) 11:16–26. doi: 10.7176/CER/11-5-03
33. EPHI. Ethiopia Mini Demographic and Health Survey 2019. Rockville, MD: CSA and ICF (2021). p. i-181.
34. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. Malvern, PA: CLSI Supplement M100: Clinical and Laboratory Standards Institute (2021). p. i-352.
35. Kwon KH, Hwang SY, Moon BY, Park YK, Shin S, Hwang C-Y, et al. Occurrence of antimicrobial resistance and virulence genes, and distribution of enterococcal clonal complex 17 from animals and human beings in Korea. J Vet Diagn Invest. (2012) 24:924–31. doi: 10.1177/1040638712455634
36. Daligault H, Davenport K, Minogue T, Bishop-Lilly K, Broomall S, Bruce D, et al. Genome assembly of Shigella flexneri ATCC 12022, a quality control reference strain. Genome Announc. (2014) 2:e01052–14. doi: 10.1128/genomeA.01052-14
37. FMoH. National Guideline for the Management of Acute Malnutrition in Ethiopia. Addis Ababa: Government of Ethiopia, Federal Ministry of Health (2019). p. 1-156.
38. Kibet S, Matiru V, Kibaba P, Mucheke A, Louis H. Prevalence and antibiotic susceptibility patterns of Shigella and Salmonella causing Diarrhoea in children below 5 years at Thika level five district hospital. World News Nat Sci. (2017) 11:28–36.
39. Chiyangi H, Muma JB, Malama S, Manyahi J, Abade A, Kwenda G, et al. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0–59 months at the University Teaching Hospital, Lusaka, Zambia: A prospective cross sectional study. BMC Infect Dis. (2017) 17:1–9. doi: 10.1186/s12879-017-2232-0
40. Sharif N, Nobel NU, Sakib N, Liza SM, Khan ST, Billah B, et al. Molecular and epidemiologic analysis of diarrheal pathogens in children with acute gastroenteritis in Bangladesh during 2014–2019. Pediatr Infect Dis J. (2020) 39:580–5. doi: 10.1097/INF.0000000000002637
41. Omar M, Kigadye E, Massomo SM, Mchenga S, Simai B. Prevalence of pathogenic enteric bacteria associated with diarrhoea in children of under 5 years and their sensitivity to antibiotics in Unguja island, Zanzibar. Eur J Health Sci. (2019) 4:48–59. doi: 10.47672/ejhs.421
42. Chaudhary K, Kumari A. Prevalence and isolation of bacteria associated with diarrhoea among children attending hospitals in Kasauli. World J Pharm Pharm Sci. (2020) 8:755–61.
43. Farahani NN, Jazi FM, Nikmanesh B, Asadolahi P, Kalani BS, Amirmozafari N. Prevalence and antibiotic susceptibility patterns of Salmonella and Shigella species isolated from pediatric diarrhea in Tehran. Arch Pediatr Infect Dis. (2018) 6:e57328. doi: 10.5812/pedinfect.57328
44. Mahmoudi S, Pourakbari B, Moradzadeh M, Eshaghi H, Ramezani A, Ashtiani MTH, et al. Prevalence and antimicrobial susceptibility of Salmonella and Shigella spp. among children with gastroenteritis in an Iranian referral hospital. Microb Pathog. (2017) 109:45–8. doi: 10.1016/j.micpath.2017.05.023
45. Saeed A, Abd H, Sandstrom G. Microbial aetiology of acute diarrhoea in children under 5 years of age in Khartoum, Sudan. J Med Microbiol. (2015) 64:432–7. doi: 10.1099/jmm.0.000043
46. Zelelie TZ, Gebreyes DS, Tilahun AT, Craddock HA, Gishen NZ. Enteropathogens in under-five children with diarrhea in health facilities of Debre Berhan Town, North Shoa, Ethiopia. Ethiop J Health Sci. (2019) 29:203–14. doi: 10.4314/2Fejhs.v29i2.7
47. Leopold SJ, van Leth F, Tarekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: A systematic review. J Antimicrob Chemother. (2014) 69:2337–53. doi: 10.1093/jac/dku176
48. Mbuthia OW, Mathenge SG, Oyaro MO, Ng'ayo MO. Etiology and pathogenicity of bacterial isolates: A cross sectional study among diarrheal children below 5 years in central regions of Kenya. Pan Afr Med J. (2018) 31:88–102. doi: 10.11604/pamj.2018.31.88.15644
49. Huchin C, Briceño MA, Mendoza T, Martínez AP, Ramírez MA, Torres JC. Prevalence and drug-resistance patterns of enterotoxigenic Escherichia coli and Shigella species among children with diarrhea in Merida City, Mexico. Int J Biosci Med. (2017) 6:22–33. doi: 10.4236/jbm.2018.61004
50. Jafari-Sales A, Shariat A. Antibiotic resistance pattern in Shigella species isolated from children with acute diarrhea in Tabriz city, Iran. J Health Sci. (2021) 4:219–22. doi: 10.32322/jhsm.859497
51. Mengist A, Mengistu G, Reta A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. Int J Infect Dis. (2018) 75:74–9. doi: 10.1016/j.ijid.2018.08.008
52. Parisot M, Parez N, Boukhari R, Breurec S, Jolivet A. Shigella infection in children under 5 years old in western French Guiana. Epidemiol Infect. (2018) 146:980–4. doi: 10.1017/S0950268818000912
53. Avakh Majalan P, Hajizade A, Nazarian S, Pourmand MR, Amiri Siyavoshani K. Investigating the prevalence of Shigella species and their antibiotic resistance pattern in children with acute diarrhea referred to selected hospitals in Tehran, Iran. J Appl Biotechnol Rep. (2018) 5:70–4. doi: 10.29252/JABR.05.02.06
54. Hussen S, Mulatu G, Yohannes Kassa Z. Prevalence of Shigella species and its drug resistance pattern in Ethiopia: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. (2019) 18:1–11. doi: 10.1186/s12941-019-0321-1
55. Abbasi E, Abtahi H, van Belkum A, Ghaznavi-Rad E. Multidrug-resistant Shigella infection in pediatric patients with diarrhea from central Iran. Infect Drug Resist. (2019) 12:1535–44. doi: 10.2147/IDR.S203654
Keywords: prevalence, antimicrobial resistance pattern, Salmonella, Shigella, under-five children, Debre Markos, Ethiopia
Citation: Dessale M, Mengistu G and Mengist HM (2023) Prevalence, antimicrobial resistance pattern, and associated factors of Salmonella and Shigella among under five diarrheic children attending public health facilities in Debre Markos town, Northwest Ethiopia. Front. Public Health 11:1114223. doi: 10.3389/fpubh.2023.1114223
Received: 02 December 2022; Accepted: 20 January 2023;
Published: 06 February 2023.
Edited by:
Tsegaye Alemayehu, Hawassa University, EthiopiaReviewed by:
Heba Ahmed, Zagazig University, EgyptCopyright © 2023 Dessale, Mengistu and Mengist. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hylemariam Mihiretie Mengist,  aHlsZW1hcmlhbUBnbWFpbC5jb20=
aHlsZW1hcmlhbUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.