
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 03 November 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1113024
This article is part of the Research TopicVector-borne diseases and consequences on human health: a multidisciplinary approachView all 12 articles
Used as a communicative tool for risk management, risk maps provide a service to the public, conveying information that can raise risk awareness and encourage mitigation. Several studies have utilized risk maps to determine risks associated with the distribution of Borrelia burgdorferi, the causal agent of Lyme disease in North America and Europe, as this zoonotic disease can lead to severe symptoms. This literature review focused on the use of risk maps to model distributions of B. burgdorferi and its vector, the blacklegged tick (Ixodes scapularis), in North America to compare variables used to predict these spatial models. Data were compiled from the existing literature to determine which ecological, environmental, and anthropic (i.e., human focused) variables past research has considered influential to the risk level for Lyme disease. The frequency of these variables was examined and analyzed via a non-metric multidimensional scaling analysis to compare different map elements that may categorize the risk models performed. Environmental variables were found to be the most frequently used in risk spatial models, particularly temperature. It was found that there was a significantly dissimilar distribution of variables used within map elements across studies: Map Type, Map Distributions, and Map Scale. Within these map elements, few anthropic variables were considered, particularly in studies that modeled future risk, despite the objective of these models directly or indirectly focusing on public health intervention. Without including human-related factors considering these variables within risk map models, it is difficult to determine how reliable these risk maps truly are. Future researchers may be persuaded to improve disease risk models by taking this into consideration.
Tick borne diseases are caused by pathogens transmitted by infected ticks to an uninfected host. As the climate warms, it becomes possible for ticks to have increased abundance, survival, and feeding activity, and to expand their geographic distribution northwards (1, 2). Human activities and their impacts on natural habitats are further altering the distribution of these disease vectors, and human-wildlife contacts are increasing through means of socio-demographics (globalization, urbanization, etc...) and public health systems [vector control and other health interventions (3)]. Due to the constant changes in the environment, it is challenging to assess the risks associated with these infections without predictive modeling. Many current and future scenario predictive risk maps have been developed to monitor zoonotic infections for public health interventions (4–7). Epidemiologic risk maps are effective visualization tools used to identify geographical areas of high risk for disease transmission and potential future high-risk regions. Used as a communicative tool for risk management, these maps provide a service to the public, conveying information that can raise risk awareness and encourage mitigation strategies (8–10). Since climate and land use changes are constantly altering the dynamics between vector and host, continuous monitoring of the emergence and expansion of the disease vector is required. This is true of the spread of Lyme disease and other tick-borne diseases (2, 3).
In eastern North America, Lyme disease is typically caused by an infection of the spirochaete Borrelia burgdorferi via blacklegged ticks [Ixodes scapularis (11)]. In humans, the infection can result in a multisystem illness that substantially affects the individual's quality of life, if left untreated (12, 13). As a common and widespread disease (14–16) that can lead to severe conditions (17), effective communication tools for risk management of this infection are necessary.
Risk maps for Lyme disease in North America tend to focus on the impact climate change has on the distribution of blacklegged ticks (18–22), since these ectoparasites are vectors for B. burgdorferi (23) and their geographic range is increasing (1, 24). The geographic range of blacklegged ticks is heavily dependent on environmental variables such as temperature and precipitation (25–28). The geographical ranges of their hosts also play a major role (25). Blacklegged ticks require a single host for each life stage (29, 30), and will migrate with these hosts, such as small mammals, birds, and ruminants (31, 32).
Different variables have been considered to affect Lyme disease distribution. These include ecological variables related to blacklegged ticks [tick density, dispersion (33–35)] and their small mammal reservoir and migratory avian hosts (36, 37), as well as environmental variables such as temperature, humidity, and forest fragmentation (20, 38, 39). Recent risk map publications have adopted “One Health” approaches, which incorporate sociological, ecological, and biological knowledge into their research (40, 41). This approach aims to examine and integrate human-related or anthropic variables that may influence human health or risk (22, 42) including those variables beyond human demographics.
For instance, one may expect that human exposure and the risk of becoming infected by Lyme disease is also dependent on individual human behavior (e.g., knowledge, activity). As such, outdoor workers have been found to be more at risk for zoonotic diseases than those who are outdoors recreationally, due to their degree of exposure to the environment (43), and those with immune deficiencies may be more at risk for severe symptoms (44). Studies have included surveys of a population to gauge their knowledge on their risks to Lyme disease or tick infections (22, 42, 45, 46). Socio-economic status and ethnicity have also been found to play a role in Lyme disease risk (42, 47, 48). Knowing that not all individuals are equally at risk for being infected with diseases, including Lyme disease, it should be expected that studies in which Lyme disease risk maps are developed would include variables associated with human characteristics and behaviors. For these studies to be relevant to public health, variables associated with humans (i.e., social, economic, risk perception) should be taken into consideration, as these factors directly affect the risk posed to the public.
Here, we reviewed the literature to identify the variables past research has considered influential to the distribution of Lyme disease via blacklegged ticks in North America. Variables that researchers routinely included in risk models were examined, and those human variables that were often disregarded but may be informative were highlighted. By calling attention to the lack of human variables found in previous risk maps, future researchers may be persuaded to enhance models by including anthropic factors to improve disease risk prediction.
We focused on past studies that are comparable due to similarities in geography [same continent, overlapping tick populations (49, 50)], disease vector, and spirochaete strain (B. burgdorferi). For this reason, we focused on one tick vector (black-legged tick) which is endemic to eastern North America (17). On March 31, 2023, a descriptive literature review was conducted following methods by Paré and Kitsiou (51) using Google Scholar, PubMed, and CrossRef with the inclusion criteria terms: “Ixodes scapularis,” “blacklegged ticks,” “risk map” (exact phrase), “Lyme disease,” “risk assessment,” and “B. burgdorferi.” This literature review concentrated on eastern North American (across Canada, the United States, and Mexico) risk assessments of Lyme disease transmitted by blacklegged ticks only. Geographical scale varied across studies, with some focusing on areas at the municipality, provincial/state, regional, or national scale. However, it should be noted that there are several risk assessments for Lyme disease concentrated in Europe and western North America where other tick species and spirochaete vectors can transmit Lyme disease (17, 20, 52). Only studies in which analyses included at least one risk map in their results was considered for this review, as we were specifically interested in comparing studies that produced risk map models to evaluate risk. We performed a systematic review following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (53). The initial search consisted of 145 studies published between 2000 and 2022 with the above criteria. Twenty studies were excluded, as they did not include a risk map in their results. Twelve more studies were excluded since their results were based outside of North America, another two studies were excluded as they focused on different vectors or bacteria and diseases and one article was removed from the literature review as it was retired and no longer considered relevant. Finally, 49 government reports, reviews, and theses were removed, leaving a total of 61 peer-reviewed articles meeting our criteria to be included in analyses (Figure 1; Supplementary Table 1).
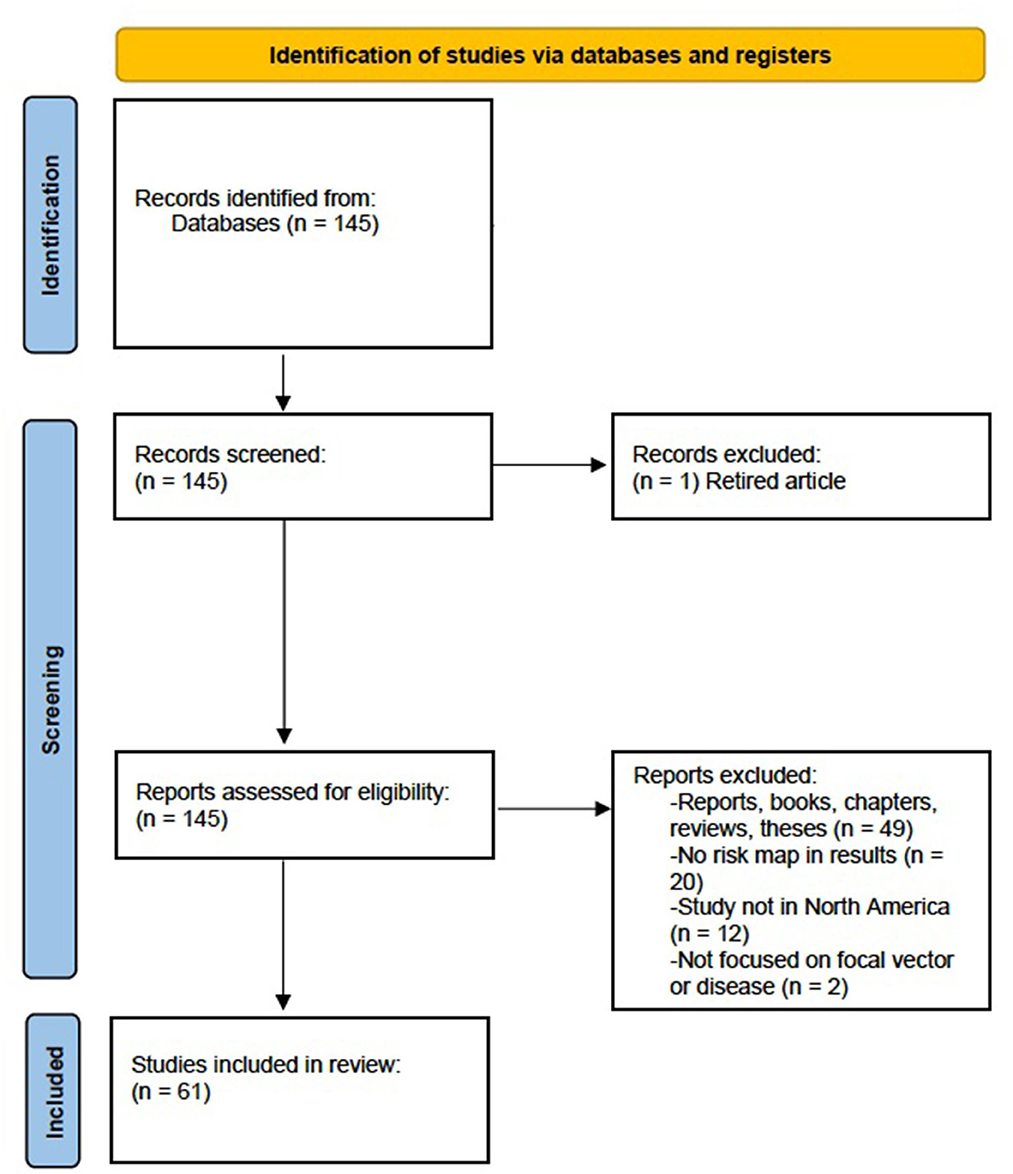
Figure 1. Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow diagram for systematic review which included searches of databases for risk maps related to Lyme disease and blacklegged tick (Ixodes scapularis) distributions.
Once the literature review was completed, data on the models within the articles were collected. This included collecting information on the number of ecological, environmental, and anthropic variables used in the studies to develop risk map models. Frequency counts for the number of each ecological, environmental, and anthropic variable in a risk map model were recorded. Ecological variables included variables that influence or dictate the relationship between an organism and its environment (i.e., tick occurrence, B. burgdorferi prevalence). Environmental variables included natural resource factors that define an ecosystem or habitat (i.e., temperature, humidity, land cover). Anthropic variables were defined as variables related to human beings (i.e., population density, sex, age). As Lyme disease is primarily transmitted via tick vectors (11), these observations do not provide any information on how the disease circulates within a human population. Further, certain predictor variables were simplified to allow comparisons more easily. For example, forest cover and vegetation index were categorized together, as were vapor pressure and humidity, elevation and altitude, human population size and density, and tick abundance and density (Supplementary Table 2).
Additional elements that characterized these risk models were collected and recorded, including the year of publication (Supplementary Table 3), the study's focal location (Country), Map Type (predictive vs. surveillance), the period of the study (year), the focal Scale of study (local, regional, national), the distribution of the study considered– vector (tick) vs. host (human or otherwise) vs. vector and host (both considered)—the Tick Surveillance methodology used for the model (passive vs. active vs. no tick surveillance), and the Tick Life Stage the tick data was based on (immature ticks vs. adult vs. all stages vs. no tick data; Table 1; Supplementary Table 4). Map Type (predictive vs. surveillance) was also included, whereby “predictive” maps referred to future predictive map models, as they predict future scenarios, while current predictive maps will be referred to as “surveillance” models, as they pertain to current risks.
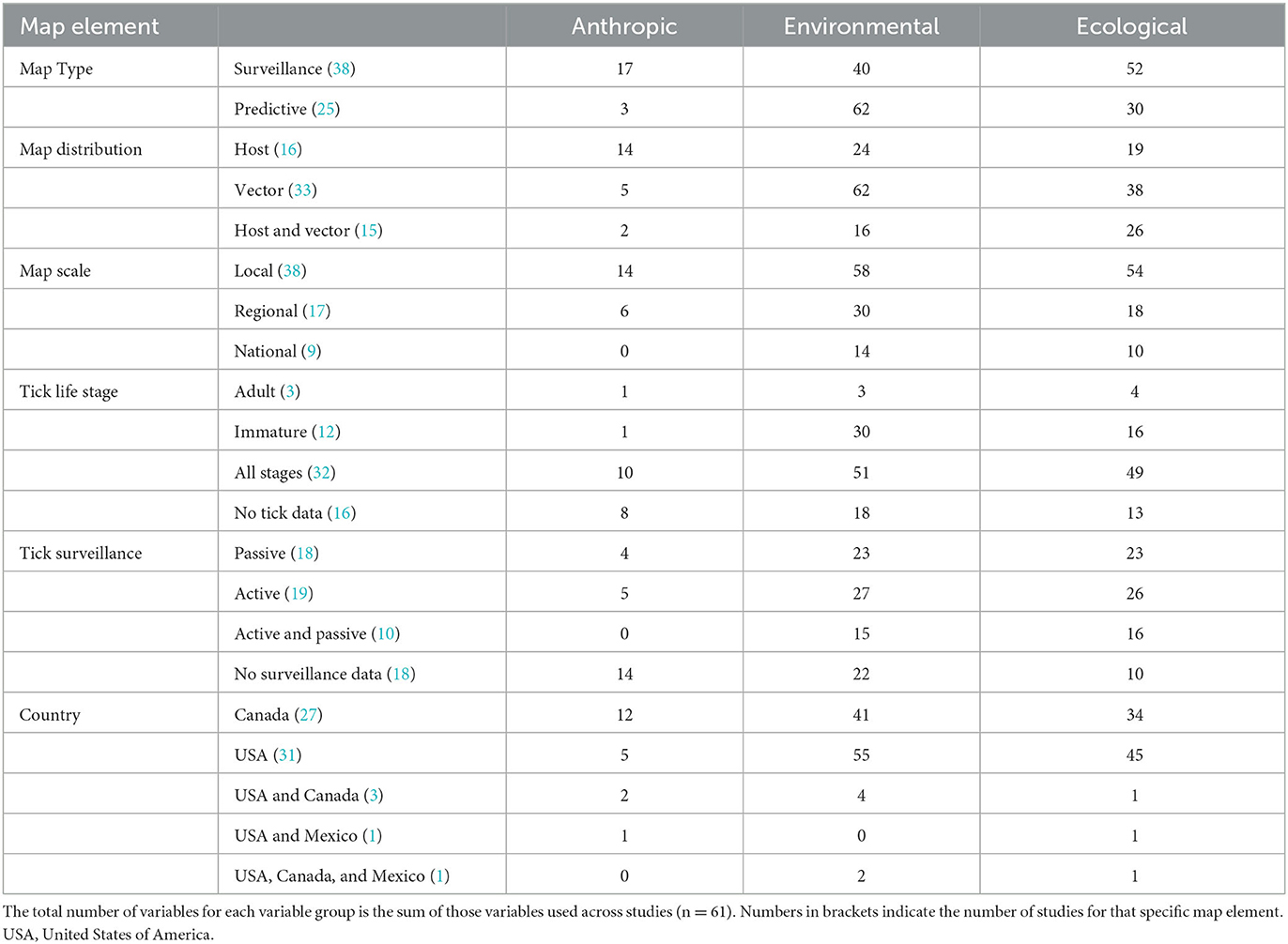
Table 1. The cumulative number of anthropic, environmental, and ecological variables used across map elements in the reviewed studies.
Most risk map models are predictive as they use modeling to create these maps. However, some models are used to predict risks associated with potential future geographical ranges and distributions of Lyme disease (18, 38, 39, 54, 55), while others are used to predict the current risk or prevalence (56–59). In addition, Map Scale was considered and categorized by whether the study focused their spatial scale by country (national), province or state (regional), or a smaller unit (e.g., census division; local).
A non-metric multidimensional scaling (NMDS) analysis was performed in R [(60); version 1.4.1717] using the “vegan” package (version 2.6-2) to determine any significant differences in the frequency of ecological, environmental, and anthropic variables used across studies (n = 61), depending on their map elements: Map Type, Map Scale, Map Distribution, Year, Tick Life Stage, Map Surveillance method, and Country. Here, the NMDS, which is commonly used as an ordination for community ecology (61), was performed where “sites” were the individual studies, and “environmental data” were the map elements that influenced the abundance of “species” (ecological, environmental, and anthropic variables).
Linear models were then used to determine if groups of variables (ecological, environmental, and anthropic) differed in frequency across the different map elements identified by the NMDS. A binomial linear regression was conducted with Map Type as the response variable (surveillance vs. predictive) and the number of ecological, environmental, and anthropic variables used in the studies as predictors. An ordinal linear regression with the “logit” function and “equidistant” threshold was conducted (62) with Map Distribution (as a factor) as the response variable (host vs. vector. vs. host and vector), using the package “ordinal” [version 2019.10 (63)]. Post-hoc tests were conducted, and box plots were used to visualize variation within those map elements that were identified as significant by the NMDS.
Comparatively, certain map elements were more frequently used across the studies assessed in this literature review. For Map Type, there were more predictive maps than surveillance. For Map Distribution, there were more vector (blacklegged tick) distributions considered than human or both human and tick distributions. Maps at the local scale were most common across studies. All stages for Tick Life Stage were most frequently considered vs. specific life stages. Both active and passive data for Tick Surveillance was most frequently included in risk map models and most studies in this assessment were based in the United States (Table 1). The most common variable included in Lyme disease or blacklegged tick risk maps was temperature (n = 28 studies). Of the 10 most frequently used variables in these map models, six were environmental variables, four were ecological, and none were anthropic (Figure 2). The results of the NMDS analysis suggested that there were differences in the frequency of ecological, environmental, and anthropic variables used across Map Type and Map Distribution (Table 2; Figure 3; Supplementary Table 5).
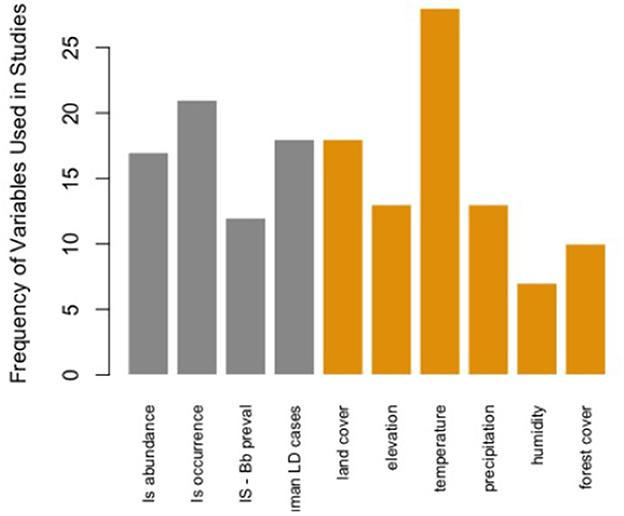
Figure 2. Frequency of the top 10 variables used within the 61 Lyme disease risk map models in North American studies published between 2000 and 2022. Is abundance, Ixodes scapularis abundance; Is occurrence, Ixodes scapularis presence/absence; Is—Bb preval = Borrelia burgdorferi prevalence in Ixodes scapularis; LD, Lyme disease.
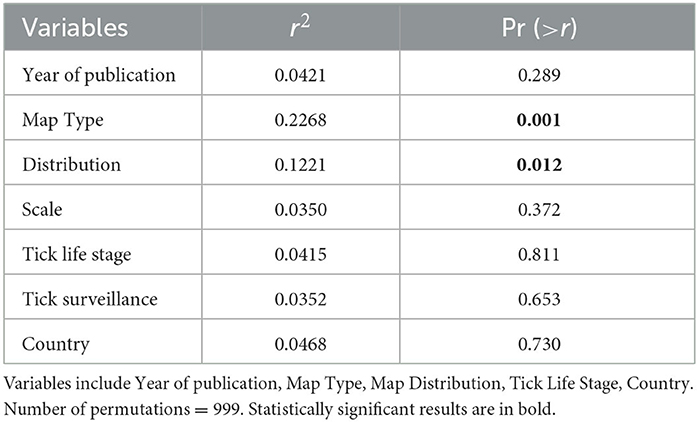
Table 2. Non-metric multidimensional scaling (NMDS) goodness of fit results of map elements classifying studies based on the frequency of ecological, environmental, and anthropic variables incorporated in each risk map model (n = 61).
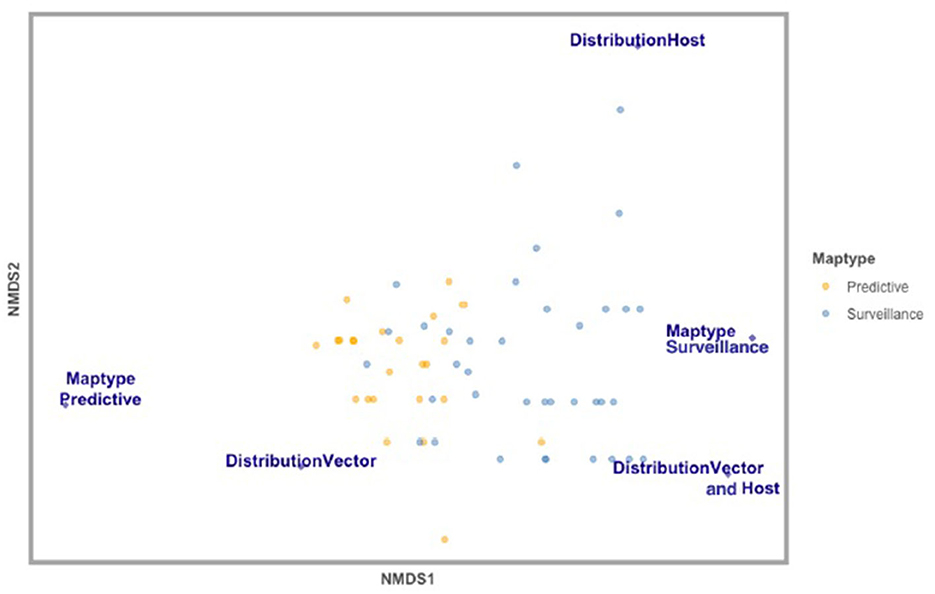
Figure 3. Non-metric multidimensional scaling (NMDS) analysis of map elements classifying studies based on the number of ecological, environmental, and anthropic variables incorporated in each study included in this review (n = 61). Variables displayed are significantly distinct variables across studies—Map Type (surveillance vs. predictive), Map Distribution (vector vs. host vs. vector and host), and Map Scale (local, regional, national). Studies that developed Predictive risk maps are in yellow, and studies that developed Surveillance risk maps are in blue.
When comparing Map Types (surveillance vs. predictive), the difference in use of environmental variables was statistically significant (p < 0.0173), with predictive maps using more of these types of variables. For both types of risk maps, anthropic variables were rarely used (Table 3; Figure 4A). Comparing across Map Distributions, studies that included host distributions in risk models considered more anthropic variables than studies that included vector distributions (p < 0.0152). Meanwhile studies that included both host and vector distributions tended to include more ecological variables (p < 0.0291; Table 4; Figure 4B). There was no significant difference across Year of publication, Surveillance Type used, Map Scale, Country of study origin, or Tick Life Stage focused on in studies (Table 2).
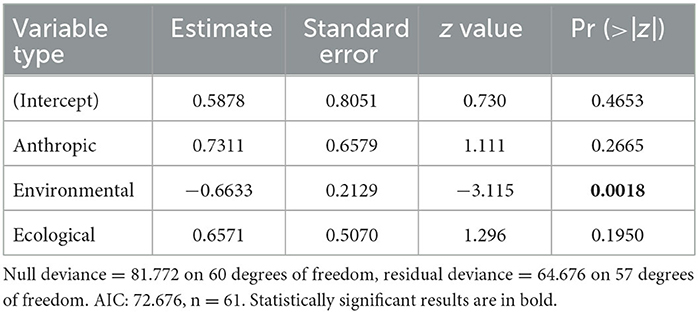
Table 3. General linear model results for Map Type ~ anthropic + environmental + ecological, where family = “binomial” and the independent variables are counts (the number of variables in each category).
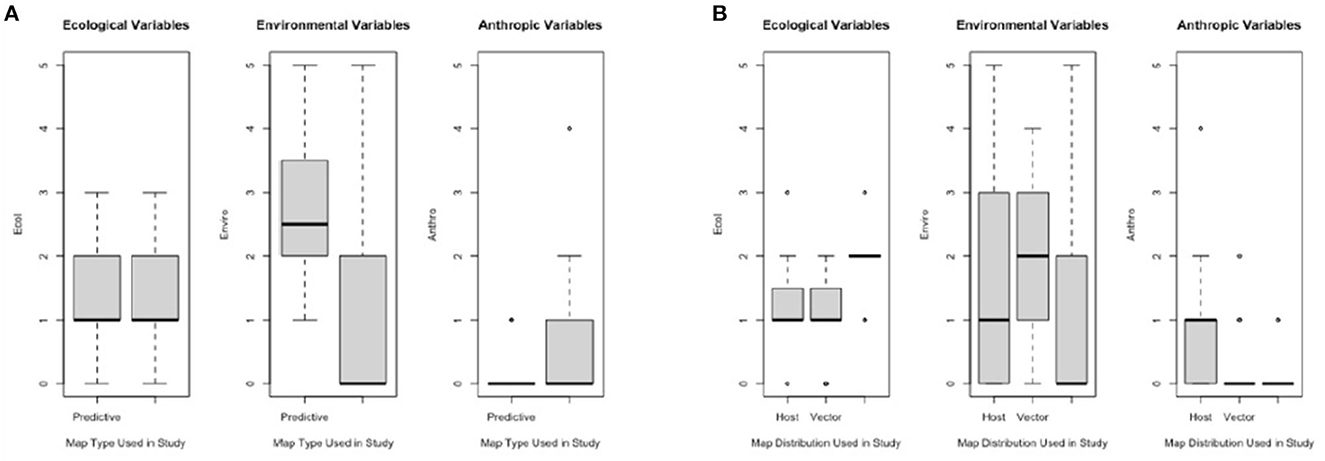
Figure 4. Frequency of ecological, environmental, and anthropic variables used across studies (n = 61) for varying map elements: (A) Map Types—predictive (future scenario; n = 24) and surveillance (current distribution; n = 37), and (B) Map Distributions—host (n = 15), vector (n = 15), vector (n = 32), or both vector and host (V & H; n = 14).
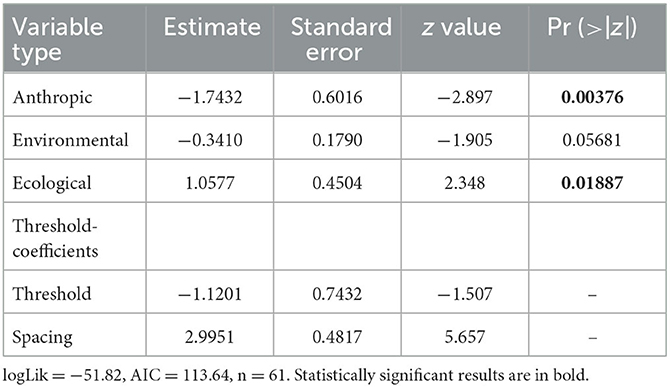
Table 4. Cumulative link model results for map distribution ~ anthropic + environmental + ecological, where link = “logit,” threshold = “equidistant” and the independent variables are counts (the number of variables in each category).
Although there is a plethora of literature dedicated to identifying factors that can influence an individual's risk for Lyme disease via tick vectors directly and indirectly (48, 64, 65), risk maps that demonstrate the spatial breadth of these risks are less common. The results of this review have shown that within this subset of risk maps, there is no standardized risk score, or variable being used across studies. Temperature was the most common variable used in risk map models, however, it was included in less than half of the risk maps considered. There is extensive research on the relationship between blacklegged ticks and temperature, as it affects a tick's development, survival, and host-seeking behavior (19, 66, 67). These are significant factors that influence tick abundance and distribution, and therefore influence the distribution and incidence of Lyme disease (19, 68). In general, the most common variables included in Lyme disease (or blacklegged tick) risk map models were environmental and ecological, while anthropic were lacking. It should be noted that certain studies, such as Slatculescu et al. (69) considered many anthropic variables, including population density, walkability scores in an urban setting, median income, and drew conclusions about the contribution of an individual's variability on their Lyme disease risk, however, they did not express these results spatially in a risk map model. Similarly, several other ecological variables often studied and considered influential to Lyme disease and/or blacklegged tick distributions were rarely included in these risk map models. For instance, blacklegged tick distributions are affected by reservoir and migratory host distributions (36, 70) while Lyme disease distributions can be influenced by the genetic diversity of B. burgdorferi strains (49, 71). Without considering these variables within risk map models, it is difficult to determine how reliable these risk maps truly are.
Depending on the Map Type and Map Distribution used, studies significantly differed in the number of ecological, environmental, and anthropic variables used to produce risk maps. Studies that utilized different Map Types also significantly varied in their usage of environmental variables, where studies that produced predictive maps used this group of variables more often. Interestingly, predictive map studies also used anthropic variables less often. This suggests that when research focuses on future scenarios, they are reliant on how the environment may change, but do not consider human behavior. Although it is difficult to assess future trends in human behavior, it is still possible to include anthropic variables such as socio-economic status and demographic information (22) to gain a better understanding of the patterns in human attributes that could influence their risk for Lyme disease.
Across Map Distribution types, studies that included host distributions in their risk maps had more anthropic variables than other distributions (vector only or host and vector distributions). Risk maps that included vector only or both host and vector distributions tended to include more ecological variables. As most host distributions in these risk map models were human distributions, it is logical for human attributed variables to be included in these models. Meanwhile, research that incorporated both host and vector distributions, or only vector distributions may be more likely to include variables from the environment, as they are less directly focused on Lyme disease risk for humans specifically. Rather, they use the result of their risk map model that included disease vector distributions to indirectly make conclusions for public health risks (18, 19, 42).
This review was limited to research that included risk maps as results within a study, excluding studies that included maps within introductions and methods for context. In some cases, a single variable (e.g., tick distribution) was used as a proxy for Lyme disease distribution and correlated with environmental or human-related data [e.g., forest cover, urban development; (36, 72)]. Other studies considered several variables, but these distribution models were not applied to develop risk maps (38, 73). It should also be noted that despite only 20 incidences of human-related variables being included in these studies' models overall (Table 5), more recent research has begun to consider general public influence within their studies by including citizen science or “Google trends” to determine blacklegged tick and Lyme disease distributions (37, 89, 90). Other human demographic data can be acquired through public government agencies such as population densities and household incomes (69, 74–76). More personal and individualistic information can be acquired via questionnaire surveys (22, 42, 45, 46).
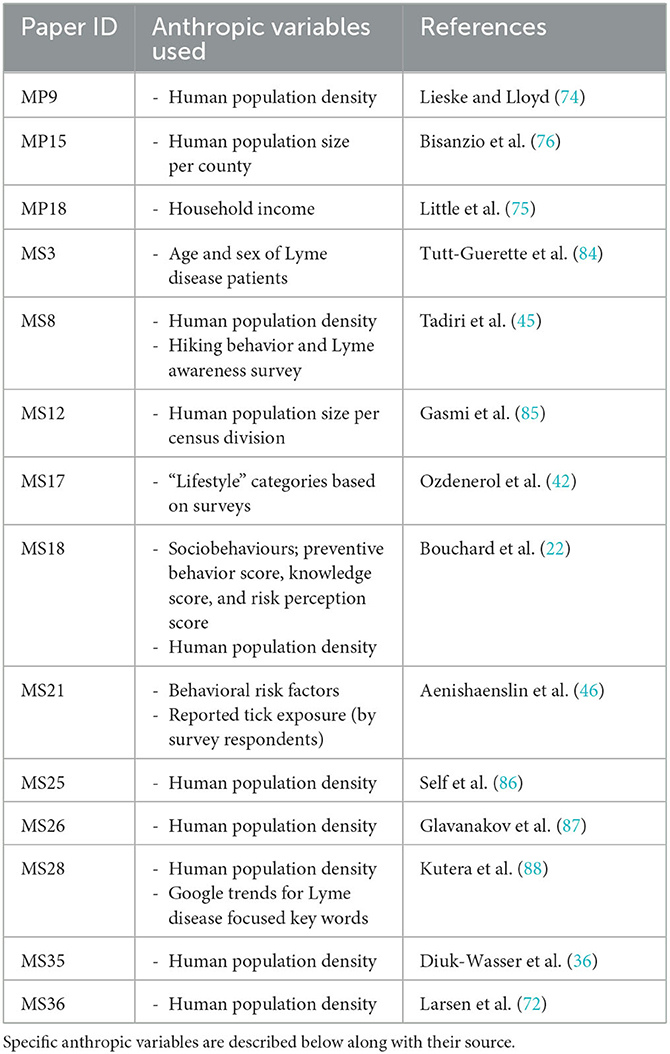
Table 5. List of peer reviewed articles included in the literature review that incorporated anthropic variables in their risk assessments where a map was produced as a result.
There is a clear understanding that environmental factors heavily influence the distribution of diseases and their vectors (21, 52, 77, 78). However, very few studies consider that human related factors may also influence these distributions (22, 69). Further, human population growth, urbanization, and travel can affect vector-borne distributions (3), particularly when human movement or land development influences animal movement, effectively altering the host dynamics of tick vectors (79, 80). This is concerning, as the risks related to Lyme disease directly affect human health, and human behavior can affect infection rates (46, 69, 81, 82). Predictive Map Type risk maps especially ignore how human-related factors may influence Lyme disease risks for humans, and this may be due to studies focusing on B. burgdorferi distribution rather than infection rates.
Overall, it was found that risk maps that focused on Lyme disease and blacklegged tick distributions differed across the types of variables used according to the study goal and intended use of the map. Because of these inconsistencies, it is difficult to compare and validate these models to accurately forecast Lyme disease risks geographically or temporally. At the same time, geospatial data related to anthropic factors can be difficult to acquire. Our results bring attention to the fact that there is no consistent “risk” variable or assessment across studies, likely because these studies tend to vary in specific objectives, despite the general intent of public health intervention. For this reason, risk maps should be scrutinized more thoroughly. As our knowledge on blacklegged ticks and B. burgdorferi increases, we must continually re-assess how risk models have predicted their geographic distributions over time. Differences in tick exposure patterns and Lyme disease risk is likely across regions and can depend on the scale and socioeconomic factors included in the assessment (69). Future studies should consider improvements for forecasting these risks, as well as exploring risk assessments beyond comparison of blacklegged ticks and Lyme disease. Expanding the scope to other tick-borne diseases or co-infections of other bacteria including Babesia and Anaplasma sp. (16, 83) may demonstrate further patterns with spatial risk models.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
EF: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, visualization, and project administration. MV: resources, writing—review and editing, project administration, and funding acquisition. VM: conceptualization, resources, writing—review and editing, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
This project was funded through a Research Grant from the Institut Robert Sauvé en Santé du Travail (IRSST) Competitive Research Grant 2019-0001. VM was supported by funds from a NSERC Discovery Grant RGPIN-2017-03839.
We thank G. Joanisse and B. Chalghaf as well as the Institut Robert Sauvé en Santé du Travail (IRSST) for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1113024/full#supplementary-material
1. Sonenshine DE. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Public Health. (2018) 15:478. doi: 10.3390/ijerph15030478
2. Rocklöv J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. (2020) 21:479–83. doi: 10.1038/s41590-020-0648-y
3. Chala B, Hamde F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front Public Health. (2021) 9:715759. doi: 10.3389/fpubh.2021.715759
4. Fichet-Calvet E, Rogers DJ. Risk maps of lassa fever in West Africa. PLoS Negl Trop Dis. (2009) 3:e388. doi: 10.1371/journal.pntd.0000388
5. Zhao X, Thanapongtharm W, Lawawirojwong S, Wei C, Tang Y, Zhou Y, et al. Malaria risk map using spatial multi-criteria decision analysis along yunnan border during the pre-elimination period. Am J Trop Med Hyg. (2020) 103:793–809. doi: 10.4269/ajtmh.19-0854
6. García-Carrasco JM, Muñoz AR, Olivero J, Segura M, Real R. Mapping the risk for west nile virus transmission, Africa. Emerg Infect Dis. (2022) 28:777–85. doi: 10.3201/eid2804.211103
7. Minigan JN, Hager HA, Peregrine AS, Newman JA. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis. (2018) 9:354–62. doi: 10.1016/j.ttbdis.2017.11.012
8. Dransch D, Rotzoll H, Poser K. The contribution of maps to the challenges of risk communication to the public. Int J Digit Earth. (2010) 3:292–311. doi: 10.1080/17538941003774668
9. Waller LA, Carlin BP. Disease mapping. Chapman Hall CRC Handb Mod Stat Methods. (2010) 2010:217–43. doi: 10.1201/9781420072884-c14
10. Jordan S, Jorgensen L, Mitterhofer H. Performing risk and the project: risk maps as mediating instruments. Manag Account Res. (2013) 24:156–74. doi: 10.1016/j.mar.2013.04.009
11. Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. (2001) 33:676–85. doi: 10.1086/322681
12. Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. (2006) 43:1089–134. doi: 10.1086/508667
13. Aucott JN, Yang T, Yoon I, Powell D, Geller SA, Rebman AW, et al. Risk of post-treatment Lyme disease in patients with ideally-treated early Lyme disease: a prospective cohort study. Int J Infect Dis. (2022) 116:230–7. doi: 10.1016/j.ijid.2022.01.033
14. Gray JS, Kirstein F, Robertson JN, Stein J, Kahl O. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in a recreational park in south-western Ireland. Exp Appl Acarol. (1999) 23:717–29.
15. Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. (2004) 113:1093–101. doi: 10.1172/JCI21681
16. Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease-United States, 2008–2015. MMWR Surveill Summ. (2017) 66:1–12. doi: 10.15585/mmwr.ss6622a1
17. Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, et al. Vital Signs: trends in reported vectorborne disease case-United States and territories, 2004-2016. NMWR. (2018) 67:496–501. doi: 10.15585/mmwr.mm6717e1
18. Leighton PA, Koffl JK, Pelcat Y, Lindsay LR, Ogden NH. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J App Ecol. (2012) 49:457–64. doi: 10.1111/j.1365-2664.2012.02112.x
19. Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J Med Entomol. (2015) 53:250–61. doi: 10.1093/jme/tjv199
20. Li S, Gilbert L, Harrison PA, Rounsevell MDA. Modelling the seasonality of lyme disease risk and the potential impacts of a warming climate within the heterogenous landscapes of Scotland. J R Soc Interface. (2016) 13:2016140. doi: 10.1098/rsif.2016.0140
21. Ginsberg HS, Albert M, Acevedo L, Dyer MC, Arsnoe IM, Tsao JL, et al. Environmental factors affecting survival of immature Ixodes scapularis and implications for geographical distribution of Lyme disease: the climate/behavior hypothesis. PLoS ONE. (2017) 12:e0168723. doi: 10.1371/journal.pone.0168723
22. Bouchard C, Aenishaenslin C, Rees EE, Koffi JK, Pelcat Y, Ripoche M, et al. Integrated social-behavioral and ecological risk maps to prioritize local public health responses to Lyme disease. Environ Health Perspect. (2018) 15:1048. doi: 10.1289/EHP1943
23. Ostfeld R, Miller M, Hazler K. Causes and consequences of tick (Ixodes scapularis) burdens on white-footed mice (Peromyscus leucopus). J Mamm. (1996) 77:166–273. doi: 10.2307/1382727
24. Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H. The emergence of Lyme disease in Canada. CMAJ. (2009) 180:1221–4. doi: 10.1503/cmaj.080148
25. Simon JA, Marrotte RR, Desrosiers N, Fiset J, Gaitan J, Gonzalez A, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol Appl. (2014) 7:750–64. doi: 10.1111/eva.12165
26. Burtis JC, Sullivan P, Levi T, Oggenfuss K, Fahey TJ, Ostfeld RS, et al. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasit Vectors. (2016) 9:606. doi: 10.1186/s13071-016-1894-6
27. Clow K, Weese M, Rousseau JS, Jardine JCM. Microbiota of field-collected Ixodes scapularis and Dermacentor variabilis from eastern and southern Ontario, Canada. Ticks Tick Borne Dis. (2018) 9:235–44. doi: 10.1016/j.ttbdis.2017.09.009
28. McClure M, Diuk-Wasser MA. Climate impacts on blacklegged tick host-seeking behavior. Int J Parasit. (2019) 49:37–47. doi: 10.1016/j.ijpara.2018.08.005
29. Keirans J, Hutcheson JH, Durden L, Klompen JSH. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. (1996) 33:297–318. doi: 10.1093/jmedent/33.3.297
30. Halsey S, Allan B, Miller J. The role of Ixodes scapularis, Borrelia burgdorferi and wildlife hosts in Lyme disease prevalence: a quantitative review. Ticks Tick Borne Dis. (2018) 9:1103–14. doi: 10.1016/j.ttbdis.2018.04.006
31. Khatchikian C, Prusinski M, Stone M, Backenson PB, Wang I, Foley E, et al. Recent and rapid population growth and range expansion of the Lyme disease tick vector, Ixodes scapularis, in North America. Evolution. (2015) 69:1678–89. doi: 10.1111/evo.12690
32. Kada S, McCoy K, Boulinier T. Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents. Parasit Vectors. (2017) 10:375. doi: 10.1186/s13071-017-2261-y
33. Ogden NH, Bigras-Poulin M, O'Callaghan CJ, Barker IK, Lindsay LR, Maarouf A, et al. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol. (2005) 35:375–89. doi: 10.1016/j.ijpara.2004.12.013
34. Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, et al. Geographic variation in the relationship between human lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg. (2012) 86:1062–71. doi: 10.4269/ajtmh.2012.11-0630
35. Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J, et al. Estimated effects of projected climate change on the basic reproductive number of the lyme disease vector Ixodes scapularis. Environ Health Perspect. (2014) 631–8. doi: 10.1289/ehp.1307799
36. Diuk-Wasser MA, VanAcker MC, Fernandez MP. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J Med Entomol. (2021) 58:1546–64. doi: 10.1093/jme/tjaa209
37. Cull B. Monitoring trends in distribution and seasonality of medically important ticks in North America using online crowdsourced records from iNaturalist. Insects. (2022) 13:404. doi: 10.3390/insects13050404
38. Ogden NH, St-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron DF, et al. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int J Health Geogr. (2008) 7:1–5. doi: 10.1186/1476-072X-7-24
39. Kotchi SO, Bouchard C, Brazeau S, Ogden NH. Earth observation-informed risk maps of the Lyme disease vector Ixodes scapularis in Central and Eastern Canada. Remote Sens. (2021) 3:524–44. doi: 10.3390/rs13030524
40. Kalani TJ, South A, Talmadge C, Leibler J, Whittier C, Rosenbaum M, et al. One map: using geospatial analysis to understand lead exposure across humans, animals, and the environment in an urban US city. One Health. (2021) 13:100341. doi: 10.1016/j.onehlt.2021.100341
41. Bouchard C, Dumas A, Baron G, Bowser N, Leighton PA, Lindsay LR, et al. Integrated human behavior and tick risk maps to prioritize Lyme disease interventions using a 'One Health' approach. Tick Tick Borne Dis. (2023) 14:102083. doi: 10.1016/j.ttbdis.2022.102083
42. Ozdenerol E, Bingham-Byrne RM, Seboly JD. The effects of lifestyle on the risk of lyme disease in the united states: evaluation of market segmentation systems in prevention and control strategies. Int J Environ Res Public Health. (2021) 18:12883. doi: 10.3390/ijerph182412883
43. Piacentino JD, Schwartz BS. Occupational risk of Lyme disease: an epidemiological review. Occup Environ Med. (2002) 59:75–84. doi: 10.1136/oem.59.2.75
44. Gutierrez-Hoffmann MG, O'Meally RN, Cole RN, Tiniakou E, Darrah E, Soloski MJ, et al. Borrelia burgdorferi-induced changes in the class II self-immunopeptidome displayed on HLA-DR molecules expressed by dendritic cells. Front Med. (2020) 7:568. doi: 10.3389/fmed.2020.00568
45. Tadiri C, Ainsworth N, De Bono N, Gavin S, Li J, Milbers K, et al. Assessment of human health risk for Lyme disease in a peri urban park in southern Quebec. McGill Sci Undergrad Res J. (2011) 6:56–61. doi: 10.26443/msurj.v6i1.96
46. Aenishaenslin C, Charland K, Bowser N, Perez-Trejo E, Baron G, Milord F, et al. Behavioral risk factors associated with reported tick exposure in a Lyme disease high incidence region in Canada. BMC Public Health. (2022) 22:807. doi: 10.1186/s12889-022-13222-9
47. Fix AD, Peña CA, Strickland T. Racial differences in reported Lyme disease incidence. Am J Epidemiol. (2000) 152:756–9. doi: 10.1093/aje/152.8.756
48. Dong Y, Zhou G, Cao W, Xu X, Zhang Y, Ji Z, et al. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: a systematic review and meta-analysis. BMJ Global Health. (2022) 7:e007744. doi: 10.1136/bmjgh-2021-007744
49. Xu G, Wielstra B, Rich SM. Northern and southern blacklegged (deer) ticks are genetically distinct with different histories and Lyme spirochete infection rates. Sci Rep. (2020) 10:10289. doi: 10.1038/s41598-020-67259-0
50. Ginsberg HS, Hickling GJ, Burke RL, Ogden NH, Beati L, LeBrun RA, et al. Why Lyme disease is common in the northern US, but rare in the south: the roles of host choice, host-seeking behavior, and tick density. PLoS Biol. (2021) 19:e3001396. doi: 10.1371/journal.pbio.3001396
51. Paré G, Kitsiou S. Chapter 9: Methods for literature reviews. In:Lau F, Kuziemsky C, , editors. Handbook of eHealth Evaluation: An Evidence-based Approach. Victoria, BC: University of Victoria (2017). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK481583/ (accessed August 1, 2023).
52. Kjaer LJ, Soleng A, Edgar KS, Lindstedt HEH, Paulsen KM, Andreassen AK, et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Euro Surveill. (2019) 24:1800101. doi: 10.2807/1560-7917.ES.2019.24.9.1800101
53. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
54. Clow KM, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM. Distribution of ticks and the risk of lyme disease and other tick-borne pathogens of public health significance in Ontario, Canada. Vector Borne Zoonotic Dis. (2016) 16:215–22. doi: 10.1089/vbz.2015.1890
55. Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE. (2017) 12: e0189393. doi: 10.1371/journal.pone.0189393
56. Koffi JK, Leighton PA, Pelcat Y, Trudel L, Lindsay LR, Milord F, et al. Passive surveillance for I. scapularis ticks: enhanced analysis for early detection of emerging Lyme disease risk. J Med Entomol. (2012) 49:400–9. doi: 10.1603/ME11210
57. Tokarska-Rodak M, Plewik D, Michalski AJ, Kolodziej M, Melgies A, Panczuk A, et al. Serological surveillance of vector-borne and zoonotic diseases among hunters in eastern Poland. J Vector Borne Dis. (2016) 53:355–61.
58. Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, et al. Surveillance for Lyme disease in Canada: 2009-2015. Can Commun Dis Rep. (2017) 43:194–9. doi: 10.14745/ccdr.v43i10a01
59. Chilton NB, Curry PS, Lindsay LR, Rochon K, Lysyk TJ, Dergousoff SJ, et al. Passive and active surveillance for Ixodes scapularis (Acari: Ixodidae) in Saskatchewan, Canada. J Med Entomol. (2020) 57:156–63. doi: 10.1093/jme/tjz155
60. R Studio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC (2021). Available online at: http://www.rstudio.com/ (accessed August 12, 2023).
61. Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. R package version 2.5-7. (2020). Available online at: https://CRAN.R-project.org/package=vegan (accessed August 12, 2023).
62. Guisan A, Harrell FE. Ordinal response regression models in ecology. J Veg Sci. (2000) 11:617–26. doi: 10.2307/3236568
63. Christensen RHB. Ordinal - Regression Models for Ordinal Data. R package version 2019.12-10. (2019). Available online at: https://CRAN.R-project.org/package=ordinal (accessed August 12, 2023).
64. Fischhoff IR, Keesing F, Ostfeld RS. Risk factors for bites and diseases associated with black-legged ticks: a meta-analysis. Am J Epidemiol. (2019) 188:1742–50. doi: 10.1093/aje/kwz130
65. Sharareh N, Behler RP, Roome AB, Shepherd J, Garruto RM, Sabounchi NS, et al. Risk factors of Lyme disease: an intersection of environmental ecology and systems science. Healthcare. (2019) 7:66. doi: 10.3390/healthcare7020066
66. Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. (2006) 43:166–76. doi: 10.1093/jmedent/43.2.166
67. Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdis Perspect Infect Dis. (2009) 2009:1–12. doi: 10.1155/2009/593232
68. Endo A, Amarasekare P. Predicting the spread of vector-borne diseases in a warming world. Front Ecol Evol. (2022) 10:758277. doi: 10.3389/fevo.2022.758277
69. Slatculescu AM, Duguay C, Ogden NH, Sander B, Desjardins M, William Cameron D, et al. Spatiotemporal trends and socioecological factors associated with Lyme disease in eastern Ontario, Canada from 2010-2017. BMC Public Health. (2022) 22:736. doi: 10.1186/s12889-022-13167-z
70. Talbot B, Slatculescu A, Thickstun CR, Koffi JK, Leighton PA, McKay R, et al. Landscape determinants of density of blacklegged ticks, vectors of Lyme disease, at the northern edge of their distribution in Canada. Sci Rep. (2019) 9:16652. doi: 10.1038/s41598-019-50858-x
71. Feria-Arroyo TP, Castro-Arellano I, Gordillo-Perez G, Cavazos AL, Vargas-Sandoval M, Grover A, et al. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texs-Mexico transboundary region. Parasit Vectors. (2014) 7:199. doi: 10.1186/1756-3305-7-199
72. Larsen AE, MacDonald AJ, Plantinga AJ. Lyme disease risk influences human settlement in the wildland-urban interface: evidence from a longitudinal analysis of counties in the Northeastern United States. Am J Trop Med Hyg. (2014) 91:747–55. doi: 10.4269/ajtmh.14-0181
73. Baldwin H, Landesman WJ, Borgmann-Winter B, Allen D. A geographic information system approach to map tick exposure risk at a scale for public health intervention. J Med Entomol. (2022) 59:162–72. doi: 10.1093/jme/tjab169
74. Lieske DJ, Lloyd VK. Combining public participatory surveillance and occupancy modelling to predict the distributional response of Ixodes scapularis to climate change. Ticks Tick Borne Dis. (2018) 9:695–706. doi: 10.1016/j.ttbdis.2018.01.018
75. Little EAH, Anderson JF, StaXord III KC, Eisen L, Eisen RJ, Molaei G. Predicting spatiotemporal patterns of Lyme disease incidence from passively collected surveillance data for Borrelia burgdorferi sensu lato-infected Ixodes scapularis ticks. Ticks Tick Borne Dis. (2019) 10:970–80. doi: 10.1016/j.ttbdis.2019.04.010
76. Bisanzio D, Fernandez MP, Martello E, Reithinger R, Diuk-Wasser MA. Current and future spatiotemporal patterns of Lyme disease reporting in the Northeastern United States. JAMA Network Open. (2020) 3:e200319. doi: 10.1001/jamanetworkopen.2020.0319
77. Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, Folsom O'Keefe C, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS ONE. (2014) 9:e84758. doi: 10.1371/journal.pone.0084758
78. Eisen L, Eisen RJ. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme Disease cases. J Med Entomol. (2018) 53:1050–62. doi: 10.1093/jme/tjw092
79. Fèvre EM, Bronsvoort BM, Hamilton KA, Cleaveland S. Animal movements and the spread of infectious diseases. Trends Microbiol. (2006) 14:125–31. doi: 10.1016/j.tim.2006.01.004
80. Muehlenbein MP. Human-wildlife contact and emerging infectious diseases. Hum.-Environ Interact. (2012) 1:79–94. doi: 10.1007/978-94-007-4780-7_4
81. MacDonald AJ, Larsen AE, Plantinga AJ. Missing the people for the trees: identifying coupled natural–human system feedbacks driving the ecology of Lyme disease. J Appl Ecol. (2019) 56:354–64. doi: 10.1111/1365-2664.13289
82. Telford S. Human Behaviour Trumps Entomological Risk. CABI Climate Change Series. Wallingford: CABI International. doi: 10.1079/9781789249637.0040
83. Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit Vectors. (2016) 9:265. doi: 10.1186/s13071-016-1529-y
84. Tutt-Guerette M, Yuan M, Szaroz D, McKinnon B, Kestens Y, Guillot C, et al. Modelling spatiotemporal patterns of Lyme disease emergence in Quebec. Int J Environ Res Public Health. (2021) 18:9669. doi: 10.3390/ijerph18189669
85. Gasmi S, Ogden NH, Ripoche M, Leighton PA, Lindsay RL, Nelder MP, et al. Detection of municipalities at-risk of Lyme disease using passive surveillance of Ixodes scapularis as an early signal: a province specific indicator in Canada. PLoS ONE. (2019) 14:e0212637. doi: 10.1371/journal.pone.0212637
86. Self SCW, Liu Y, Nordone SK, Yabsley MJ, Walden HS, Lund RB, et al. Canine vector-borne disease: mapping and the accuracy of forecasting using big data from the veterinary community. Anim Health Res Rev. (2019) 20:47–60. doi: 10.1017/S1466252319000045
87. Glavanakov S, White DJ, Caraco T, Lapenis A, Robinson GR, Szymanski BK, et al. Lyme disease in New York State: spatial pattern at a regional scale. AM J Trop Med Hyg. (2001) 65:538–45. doi: 10.4269/ajtmh.2001.65.538
88. Kutera M, Berke O, Sobkowich K. Spatial epidemiological analysis of Lyme disease in southern Ontario utilizing Google Trends searches. EHR. (2022) 64:105–10. doi: 10.5864/d2021-025
89. Porter WT, Motyka PJ, Wachara J, Barrand ZA, Hmood Z, McLaughlin M, et al. Citizen science informs human-tick exposure in the Northeastern United States. Int J Health Geogr. (2019) 18: 9. doi: 10.1186/s12942-019-0173-0
Keywords: blacklegged ticks, data synthesis, human-related, Lyme disease, risk assessment, risk map
Citation: Fellin E, Varin M and Millien V (2023) Risky business: human-related data is lacking from Lyme disease risk models. Front. Public Health 11:1113024. doi: 10.3389/fpubh.2023.1113024
Received: 01 February 2023; Accepted: 09 October 2023;
Published: 03 November 2023.
Edited by:
Miguel Angel Garcia Bereguiain, University of the Americas, EcuadorReviewed by:
Vett Lloyd, Mount Allison University, CanadaCopyright © 2023 Fellin, Varin and Millien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erica Fellin, ZXJpY2EuZmVsbGluQG1haWwubWNnaWxsLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.