- 1Health Field Officer at the International Committee of the Red Cross, Gondar, Ethiopia
- 2Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Internal Medicine, University of Gondar Comprehensive Specialized Hospital, North–West Ethiopia, Gondar, Ethiopia
- 4Department of Clinical Midwifery, School of Midwifery, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Nosocomial infections are major public health problem which affects more than 100 million patients each year globally. This leads to prolonged hospital stays, a high mortality rate, and a vast financial burden to the healthcare system as well as the patients. This study aimed to find out the incidence of nosocomial infections and determinant factors among admitted adult chronic illness patients at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Methods: An institutional-based retrospective follow-up study design was employed among 597 respondents. The secondary data was collected from April 15 to May 15, 2021. A computer-generated random sampling technique was used to select a total of 599 patients using Open-epi software. Structured checklists were used to collect data. For data entry and analysis Epi-Data version 4.6 and STATA 16 were used respectively. To identify statistically significant variables Cox-regressions (univariable and multivariable) were performed. To declare statistically significant variables based on p < 0.05 in the multivariable Cox-regression model, adjusted hazard ratio with 95% CI was used.
Results: A total of 597(99.6%) adult chronic illness patients were included in the study. Of these, 53 (8.88%) participants developed nosocomial infections and the incidence rate of nosocomial infection was 6.6 per 1,000 person-days observation. In this study, not taking antibiotics (AHR = 2.74, 95% CI: 1.49, 5.04), using mechanical ventilation (AHR = 2.67, 95% CI: 1.36, 5.26), being on urinary catheter (AHR = 4.62, 95% CI: 2.22, 9.65), being on intravenous catheter (AHR = 3.42, 95% CI: 1.22, 9.61) and length of hospital stay >20 days (AHR = 2.66, 95% CI: 1.43, 4.94) were significantly associated with nosocomial infections.
Conclusions: The findings have indicated that the incidence of nosocomial infection was low. No taking antibiotics, intravenous insertion, mechanical ventilation, length of hospital stay, and urinary catheterization were the predictors for the development of nosocomial infection. Therefore, we recommend that the healthcare providers need to give emphasis on infection prevention and control in the institution on these factors that have a significant effect on nosocomial infection.
Introduction
Nosocomial infections (NIs) or healthcare associated infections are infections developed during the process of getting health care which occures 48 h after hospital admission without any evidence that the infection was existing at the time of hospital admission (1–3). The types of health care associated infections are catagorised as Catheter-associated urinary tract infections (CAUTI), Central line-associated bloodstream infections (CLABSI), Ventilator-associated pneumonia (VAP), Surgical site infections (SSI), and hospital acquired C.difficile infectons, (HO-CDI). Soft tissue infection, upper respiratory tract infection, central nervous tract infection, and reproductive tract infections may occur as health care associated infections (3).
Globally, nosocomial infections affect more than 100 million patients each year (4). In developed countries, the burden of NIs shows that more than four million patients are affected every year. It mostly affects high-risk admitted patients (3, 5). In a national and multi-center study by the World Health Organization in 2011, reported that the prevalence of nosocomial infections in hospital admitted patients varied from 3.5 to 12% in developed countries of Australia, France, Canada, Germany, Belgium, Finland, and others. A meta-analysis study showed that infections that occur associated with healthcare were 7.6 episodes per 100 patients (4). However, in developing countries, the prevalence of NIs varied from 3 to 15% (6).
In low- and middle-income countries, incidence of NI is as high as 35.2% which ranges from 4.4 up to 88.9%, which affects high-risk populations or those who are admitted to the hospitals. This remains higher in many folds of low and middle-income countries than high income ones (2). In developing countries few studies have focused on NIs, especially in Sub-Saharan Africa. A recent meta-analysis study from 220 studies conducted in developing countries (only 14 studies were from Africa) showed that the incidence rates of nosocomial infections was 7.4 infections per 100 patients (7).
In sub-Saharan Africa the incidence of nosocomial infections ranges from 2 to 49% (8). In Ethiopia, the incidence and prevalence of nosocomial infection are 35.8% (1) and 16.96% respectively (9). In Jimma University hospital wards, the incidence rate of nosocomial infection was 28.15% per 1000 patient days while the overall prevalence was 19.41% (10).
Nosocomial infection has becomes a huge healthcare problem which causes great economic and production loss in the community (11). The impact of NI implies long-term disability and prolonged hospital stay for individuals. In the community and government, it leads to a massive additional financial burden for health systems, increased resistance of microorganisms to antimicrobials, high costs for patients and their families, and increased deaths of patiants. In developed nations, NIs cause 16 million additional days of hospital stay, € 7 billion financial loss, and 37 000 attributable deaths every year (4). But, no clearly defined figures/numbers of nosocomial infections in developing nations due to limited data and low quality of data (12).
In the 21st century, nosocomial infections are more alarming due to the reasons of; hospitals serving a large number of people who are sick and have lower immunity, invasive medical procedures, poor hygiene practice, and routine use of antimicrobial agents (13). Nosocomial infections are still a major public health problem, because of antimicrobial resistance to pathogens (14).
Africa has a less effective infection control program due to: lack of personnel, lack of awareness, poor water supply, poor laboratory backup, ineffective antibiotic policies with the emergence of multiple antibiotic-resistant microbes, poor funding, and non-adherence to safe practices by health workers (15).
Even though there are declarations and interventions for the sustainable development goal of Ethiopia on nosocomial infections through an integration, monitoring, programmed prevention mechanisms, the preventive measures are still low. These preventive mechanisms include:—limiting transmission of organisms between patients, indirect patient care, protecting patients with appropriate use of prophylactic antimicrobials, controlling environmental risks for infection, vaccinations, and nutrition. Other preventive mechanisms also include:- limiting the risk of endogenous infections by minimizing invasive procedures and promoting optimal antimicrobial use, enhancing staff patient care practices, prevention of infection in staff members, and continuing staff education (16). In developing countries, especially in Africa, incidence of nosocomial infection is still high (1). These are due to negligence in useing infection prevention guidelines, financial problems to facilitate the infrastructures including medical equipment, wardrooms as well as personal protective equipment (15).
In Ethiopia, there are little evidences on the incidence and determinants of nosocomial infections. In addition, there are limited studies in the areas regarding incidence and determinants of nosocomial infections among hospital admitted adult chronic illness patients and with this method of analysis.
Therefore, this study aims to determine the incidence and determinants of nosocomial infections in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Methods
Study area
The study was conducted at the University of Gondar Comprehensive Specialized Hospital, Amhara regional state, Northwest Ethiopia. The University of Gondar Comprehensive Specialized Hospital is found in Gondar city, which is found 738 kilometers (km) from the Northwest of Addis Ababa, the capital city of Ethiopia. It gives services to more than seven million persons in the catchment area (17). The hospital has specializations in pediatrics, internal medicine, surgery, gynecology, and other health related specializations and it serves more than five million people in inpatient and outpatient departments (18). The health service units include maternity clinics, outpatient clinics, adult in-patients, emergency wards, community clinics, pediatrics in-patients, radiology, dermatology, pathology, ophthalmology, dentistry, pharmacy and medical laboratory (19). The hospital has about 700 beds in 27 wards for inpatient, emergency, and outpatient departments (17). It is staffed by about 1,040 health care professionals (20).
Study design and period
A retrospective follow-up study was conducted among adult chronic illness patients admitted in the medical wards from January 01, 2016 to December 31, 2020.
Participants
All adult chronic illness patients who were admitted to the University of Gondar Comprehensive Specialized Hospital were the source populations, while all adult chronic illness patients who were admitted at the University of Gondar Comprehensive Specialized Hospital between 2016–2020 were the study populations.
Sample size calculation and sampling procedures
The sample size was calculated by using the formula for survival analysis by considering the following statistical assumptions:
N = required sample size, = 1.96, the corresponding Z-score for the 95% CI, m = events of nosocomial infection = 448, Pm = Proportion events of nosocomial infection = 0.86 taken from the previous study conducted in Debre-Markos Referral Hospital, North-west Ethiopia (21), HR = Hazard ratio = 1.32, Zβ = the critical value of the standard normal distributed variable at 20% of β, which is the probability of type two error (0.8416),
By considering non-response rate 15% (22), the calculated maximum sample size was (521*0.15) = 78; then 521 + 78 = 599.
A computer-generated random sampling technique was used to select a total of 599 patients using Open-epi software.
Inclusion and exclusion
All admitted adult chronic illness patients between January 01, 2016 to December 31, 2020 and who have no new signs and symptoms of infection before 48 h of admission were included. Adult chronic illness patients who had missing admission dates and discharge dates were excluded.
Operational definitions
Nosocomial infections
Infections acquired in the hospital by a patient during a hospital stay.
Incidence of nosocomial infection
The number of NIs occurring among admitted adult chronic illness patients from 2016 to 2020.
Chronic illness
An illness of patients who have at least one of the following (hypertensive, or type-I and type-II DM or cardiac, or COPD, or asthma, or renal, or liver disease).
Event
Development of nosocomial infections following admission until discharge.
Censored
Patients have not developed nosocomial infections from admission to the time of discharge or who were lost before developing any nosocomial infection.
Length of hospital stay
The time of a patient that stays admitted to the hospital.
Invasive medical devices
Medical devices like urinary catheter or IV-catheter or chest tube or NG-tube or mechanical/nasal ventilator.
Study variables
The outcome variable of this study was time to nosocomial infections (event, censored), while others like socio-demographic, behavioral factors, clinical and healthcare-related factors were the explanatory variables.
Data collection tools and procedures
Data extraction formats were used to collect the data that was prepared in English. The checklist/formats were adapted from different literatures. Data were collected by BSc nurses and followed by the supervisor who managed the overall data collection process. A one-day training was given to the supervisor and data collectors about data collection tools, the purpose of the study, data collection techniques, collection of the data, and ethical issues. The supervisor assessed the completeness and consistency of data daily. All detailed data like socio-demographic, behavioral, and clinical, and health-related variables were reviewed from the charts of hospital-admitted adult chronic illness patients.
Statistical analysis
The data entry was performed using Epi-Data version 4.6 and then exported into STATA 16 for analysis. Descriptive statistics like mean, median, frequency, and percentage were used to present variables using texts, tables, and graphs. A p-value of ≤ 0.05 was used as statistically significant. To identify the determinants of nosocomial infection, Cox proportional hazard model was used. To identify statistically significant variables, Cox regression (Bivariable and multivariable) was performed using a p value ≤ 0.2 in the univariable Cox regression analysis to identify candidate variables for multivariable Cox regression. To declare statistically significant variables, an adjusted hazard ratio with 95% CI was used, based on p value < 0.05 in the multivariable Cox regression analysis. The goodness of fit of the model was tested by using the Cox-Snell residuals together with Nelson Aalen's cumulative hazard function.
Quality assurance mechanisms
To assure the quality of the data, the tool was prepared first by language experts in English. Data collectors and the supervisor were trained in the data collection process for 1 day. A pretest was conducted from 5% of the total sample size in the hospital which was selected for actual data collection. Appropriate modifications were made to the tool accordingly. Data collection was closely monitored by investigators and the supervisor. Moreover, the data quality was assured by using statistical parameters to assess the consistency and completeness of the data.
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board (IRB) of the University of Gondar on behalf of the ethical review committee of the Department of Epidemiology and Biostatistics. A formal letter of cooperation from the University of Gondar was delivered to the hospital and permission was obtained from the hospital administration. Wavering was taken from the referral hospital after detailed information was provided about the objective of the study and the data collection was started and the study was started after complete consent is obtained.
Results
Respondents' sociodemographic characteristics
Of the overall sample required (N = 599), 597 participants were included in the study, giving a response rate of 99.6%. The mean age of the participants was 51.7 years with SD ± 18.6. Among all respondents, of all, 305 (51.09%) were males and 418 (70.02%) were married. Regarding educational status, 344 (57.62%) were unable to read and write. Moreover, 127 (21.27%) were housewives by occupation (Table 1).
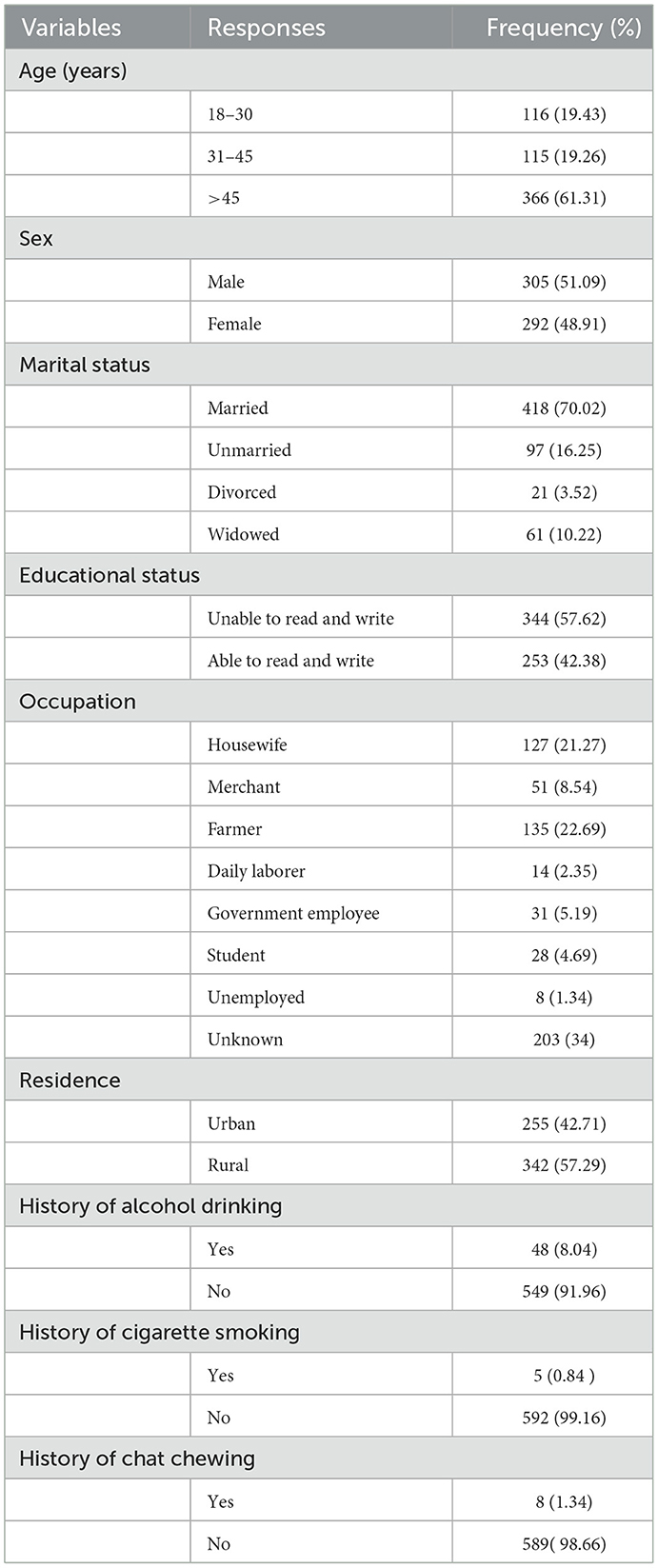
Table 1. Socio-demographic and baseline behavioral characteristics among adult chronic illness patients admitted at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2016–2020 (N = 597).
Clinical and health-related characteristics of chronic illness patients
Of all, 26 (4.36%) and 82 (13.74%) had type I and type II diabetes mellitus, respectively. Of all, 236 (39.53%) had cardiac disease. Fifty-five (9.21%) of the study subjects had chronic renal disease. More than a quarter of the study participants, 174 (29.15%) had hypertension. Among the total participants, 47(7.85%), 32(5.36%), and 28 (4.67%), were admitted to the hospital due to stroke, shock, and severe pneumonia respectively. Among the total admitted patients, 423 (70.85%) had an intravenous catheter during admission and 524 (87.48%) received antimicrobial medication. Concerning invasive medical devices, 49 (8.21%) were applied to intervention. From the total, 53 nosocomial infection patients, 3 (5.66%), 48 (90.57%), and 2 (3.77%) were died, improved, and lost to follow up respectively (Table 2).
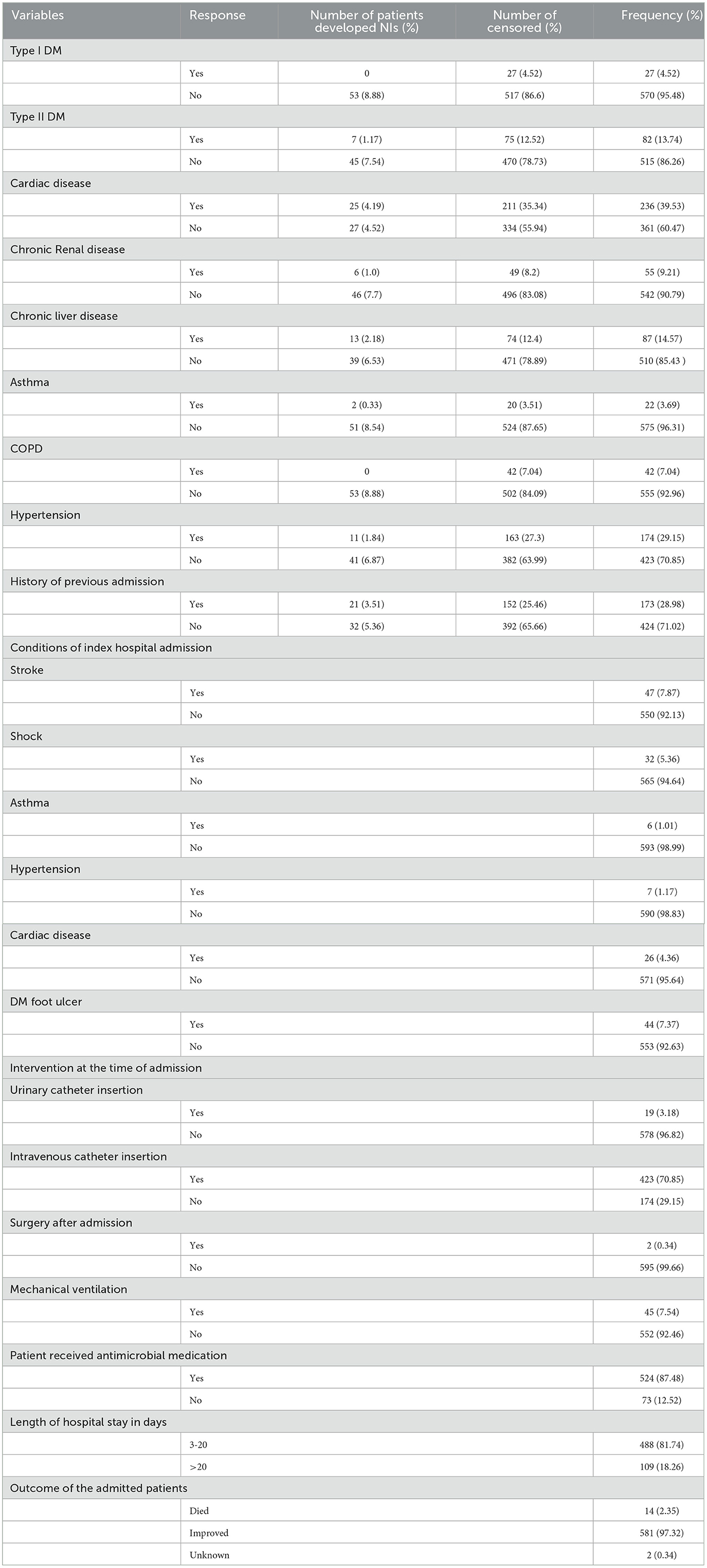
Table 2. Clinical factors among adult chronic illness patients admitted at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2016–2020 (N = 597).
Incidence of nosocomial infection
The overall incidence rate of nosocomial infection was 6.6 cases (95% CI: 5.1, 8.7) per 1,000 person-days observation with the prevalence of 8.88%. Study subjects were followed for a total of 7,984 person-days observation with a median time of 10 days (IQR = 7–16 days).
Types of nosocomial infection in respect to chronic illness
In this study the most common types of nosocomial infection were respiratory tract infection or hospital acquired pneumonia 30 (56.6%) and blood stream infection 13 (24.53%). An estimated 13.21, 5.66, and 1.88% of the participants were developed urinary tract infection, surgical site infection and skin infection respectively (Figure 1).
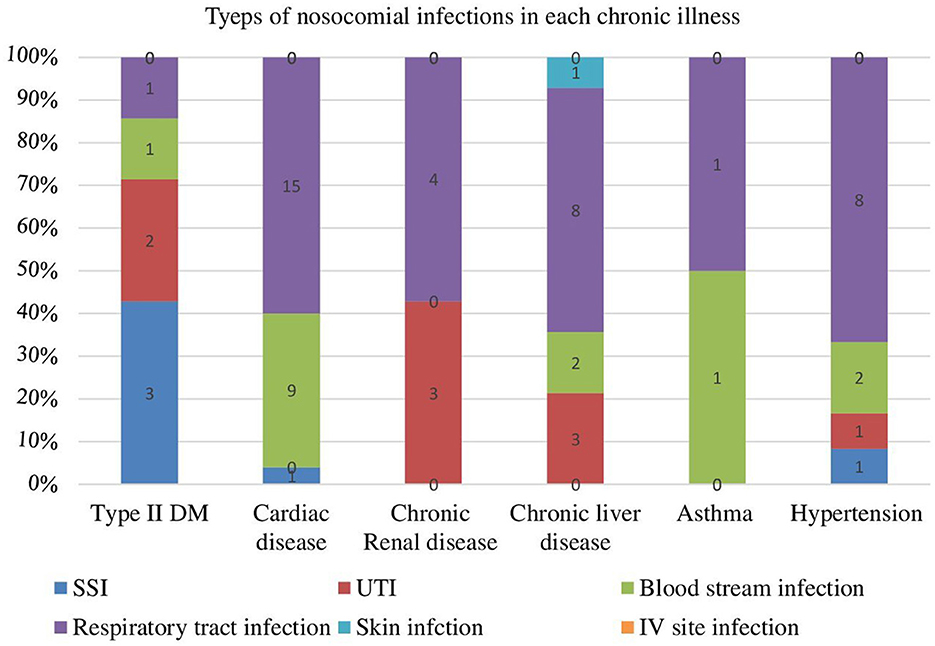
Figure 1. Types of nosocomial infection among chronic illness patients at University of Gondar Comprehensive Specialized Hospital Northwest Ethiopia from 2016–2020 (N = 53).
Factors associated with the development of nosocomial infection
From the multi-variable Cox-regression analysis, 5 predictor variables: length of hospital stay (>20 days), urinary catheter insertion, intravenous catheter insertion, mechanical ventilation, and no use of antibiotics during admission were found associated with the occurrence of nosocomial infection at 95% CI with P-value ≤ 0.05.
In our study, the hazard of developing NIs among hospital admitted adult chronic illness patients who had a urinary catheter was 4.62 times (AHR = 4.62, 95% CI: 2.22, 9.65) higher compared to non-urinary catheterized patients. The hazard of developing NIs among hospital admitted adult chronic illness patients who have an intravenous catheter was 3.42 times (AHR = 3.42, 95% CI: 1.22, 9.61) higher compared to the patients with no intravenous catheter. The hazard of developing NIs among hospital admitted adult chronic illness patients who have mechanical ventilation during admission was 2.67 times (AHR = 2.67, 95% CI: 1.36, 5.26) higher than the patients with no mechanical ventilation. The hazard of developing NIs among hospital admitted adult chronic illness patients who did not take antibiotics during admission was 2.74 times (AHR = 2.74, 95% CI: 1.49, 5.04) higher compared to patients who took. The hazard of developing NIs among hospital admitted adult chronic illness patients who had >20 days of hospital stay was 2.66 times (AHR = 2.66, 95% CI: 1.43, 4.94) higher compared to patients who stayed < 20+ days of admission in the hospital (Table 3).
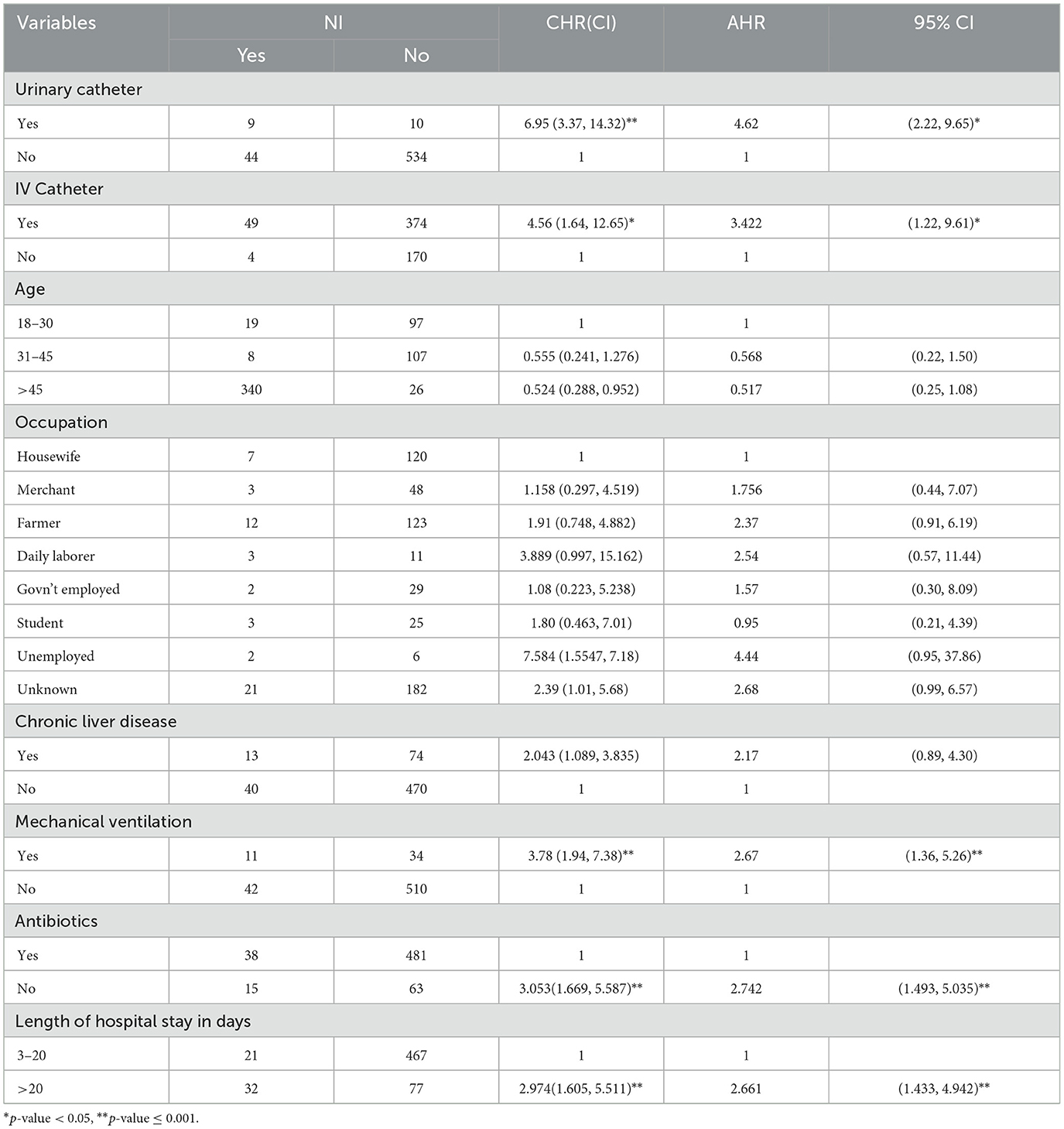
Table 3. Factors affecting the occurrence of nosocomial infection among adult chronic illness patients admitted at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2016–2020 (N = 597).
Discussion
This study assessed the incidence of nosocomial infection and examined the socio-demographic, behavioral, and clinical determinants of NIs among adult hospital admitted chronic illness patients at the University of Gondar comprehensive and specialized hospital. In this study, the overall incidence rate of NIs was 6.6 (95% CI: 5.1, 8.7) per 1,000 person-days of observation. This finding is in-line with the study conducted in Sab-Saharan Africa (8) and Southeast Asia, Taiwan (23).
This finding was higher than studies conducted in low-income countries like Nepal (24) and Gabon (25). Moreover, the finding was higher than studies conducted in high-income countries of Southeast Asia (South Korea and Japan) (23) and Trinidad-Tobago (26). This higher incidence of NIs also could be due to poor infrastructure and maybe poor adherence to aseptic measures that facilitate the transmission and occurrence of nosocomial infections.
On the other hand, this finding was lower than a study conducted in Ethiopia (10), a review done in Africa (27), Kenya (28), Cameron (29), and Northern India (30). The difference could be due to study time, which currently is high laboratory access and different infection prevention infrastructures that have a high quality of detection and prevention of Nis, respectively. The level of development of health system affects the occurrence of NIs due to the presence or absence of equipments, as well as NIs control measurements (31). The other reason may be due to differences in study participants; in those studies the participants were in different wards, such as ICU, emergency wards, and surgical wards, which may be a higher risk of developing NIs (27).
This study revealed that patients who had urinary catheters were 4.62 times at higher hazard of developing NIs compared with non-catheterized chronic patients. This result is consistent with other studies done in Morocco (32), Egypt (31, 33), Poland (34), China (35), Pune India (36), and Northern India (30). This may be due to microorganisms causing NIs deriving from the patient's perineal flora or the hands of healthcare personnel during catheter insertion or manipulation of the collection system by direct inoculation, or by ascending organisms from the perineum by capillary action in the thin mucous film contiguous to the external catheter surface. Reflux of microorganisms gaining access to the catheter lumen causes intraluminal contamination from contamination of urine in the collection bag or the failure of closed drainage (37).
In this study, patients who had intravenous catheters were 3.42 times at higher hazard of developing NIs compared with non-catheterized chronic patients. The study was supported by different studies conducted in Morocco (32), Egypt (31, 33), Poland (34), Switzerland (38), and Northern India (30). This may be due to bacterial dissemination along with the tube into the patient's body. An intravenous catheter may become blocked, leak fluid into the skin, and cause infection. Skin colonization is a significant source of central venous catheter colonization and infection (39).
Patients who had mechanical ventilation during admission were 2.67 times at higher hazard of developing NIs compared with chronic patients without mechanical ventilation. This was supported by a study done in Adama (40), Jimma (10), Egypt (33), Brazil (41), China (35), Pune India (36), and Northern India (30). Patients who receive mechanical ventilation have a markedly increased risk of developing NIs. Both nasotracheal and orotracheal tubes bypass natural host defenses which permit leakage of bacteria and secretions around the cuff into the trachea that leads to damage the ciliated epithelium of the trachea and decrease bacterial clearance (42). This is due to the colonization of pathogens into the oropharynx and its contiguous structures, like dental plaque, sinuses, gastric reservoir and trachea, and aspiration of the contaminated secretions into the lower airway causing NIs (43).
In this study, patients who did not take antibiotics during admission were 2.74 times at higher hazard of developing NIs compared with chronic patients who took. The finding was supported by the study conducted in Italy (44). The reason may be due to antibiotic prophylaxis being used to prevent infections by preventing and eradicating carriage of aerobic, pathogenic micro-organisms from the oropharynx, stomach, and gut (44). On the contrary, a study which was conducted in Bahir Dar (45) and the finding observed in Morocco (32) stated that patients who received antibiotics prophylaxis were more at risk for the occurrence of NIs than those not received prophylaxis. This may be due to the entrance of drug-resistant bacteria or pathogens. Broad-spectrum containing antimicrobials prescribed to patients increased the risk of hospital-acquired drug-resistant infections (46).
Lastly, patients who stayed >20 days admitted in the hospital were 2.66 times at higher hazard of developing NIs compared with those chronic patients who stayed < 20 days. The finding was supported by other studies done in Africa (6), Morocco (32), Tunisia (47), Scotland (48), Pune India (36), Egypt (31), Bahir Dar (45), Jimma (10), Tanzania (49), and Wolaita-Sodo University teaching hospital (50). This is due to the long duration of hospital stay makes patients to be exposed to environment pathogens which increases cross-contamination and increases the patient's susceptibility to hospital-acquired infections. In addition, an increase in duration of stay among those who had underlying morbidity and those who required invasive procudgers (51).
Limitations of the study
To conduct the study secondary data was used for this reason important variables like nursing care given to the patients, patient-to-bed ratio, hygiene, and sanitation couldn't be assessed.
In this study, isolating organisms laboratory culture was not used which is a guide to confirm the results of Nis. This could have affected our results.
Conclusions
The incidence rate of nosocomial infection among chronic illness patients in the University of Gondar Comprehensive Specialized Hospital was low. Patients who did not take antibiotics during admission, intravenous insertion, mechanical/nasal ventilation, length of hospital stay (>20 days), and urinary catheterization were significant predictors for the development of nosocomial infection among adult chronic illness patients.
Data availability statement
The datasets presented in this article are not readily available because we generate the data in reasonable request. Requests to access the datasets should be directed to ZT, emV3YXNpZTdAZ21haWwuY29t.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IBR) of the University of Gondar on behalf of the ethical review committee of the Department of Epidemiology and Biostatistics. The Ethics Committee waived the requirement of written informed consent for participation.
Author contributions
ZT, YA, TA, and ET wrote the proposal, participated in data collection, analyzed the data, drafted the paper and prepared the manuscript, approved the proposal with few revisions, participated in data analysis, and revised subsequent drafts of the paper. GT wrote the proposal, participated in data collection, and approved the proposal with few revisions. All the authors read and approved the final manuscript.
Acknowledgments
We are very indebted to the University of Gondar Comprehensive Specialized Hospital for permitting us to conduct the study and providing the necessary preliminary information while conducting this study. We do wish to extend our gratitude to the supervisor and data collectors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHR, Adjusted Hazard Ratio; AOR, Adjusted Odd Ratio; BPH, Benign Prostate Hyperplasia; CAUTI, Catheter-Associated Urinary Tract Infections; CI, Confidence Interval; CLABSI, Central Line-Associated Bloodstream Infections; COPD, Chronic Obstructed Pulmonary Disease; CVC, Central Venous Catheter; DM, Diabetes Mellitus; ECG, Eco Cardio Gram; FHRH, Felege Hiwot Referral Hospital; HR, Hazard Ratio; HTN, Hypertension; IRB, Institutional Review Board; ICU, Intensive Care Unit; IQR, Interquartile Range; OPD, Out-Patient Diagnosis; OR, Odd Ratio; PI, Principal Investigator; NIs, Nosocomial Infections; RC, Research Committee; RR, Relative Risk; SPSS, Statistical Package for Social Science; SSI, Surgical Site Infections; UK, United Kingdom; URTI, Upper Respiratory Tract Infections; UTI, Urinary Tract Infection; UoGCSH, University of Gondar Comprehensive Specialized Hospital; UTI, Urinary Tract Infection; VAP, Ventilator-Associated Pneumonia; WHO, World Health Organization.
References
1. Endalafer N, Gebre-Selassie S, Kotiso B. Nosocomial bacterial infections in a tertiary hospital in Ethiopia. J Infect Prev. (2011) 12:38–43. doi: 10.1177/1757177410376680
2. Wang L, Zhou K-H, Chen W, Yu Y, Feng S-F. Epidemiology and risk factors for nosocomial infection in the respiratory intensive care unit of a teaching hospital in China: A prospective surveillance during 2013 and 2015. BMC Infect Dis. (2019) 19:1–9. doi: 10.1186/s12879-019-3772-2
4. WHO. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. Geneva: WHO (2011).
5. Tikhomirov E. WHO programme for the control of hospital infections. Chemioterapia. (1987) 6:148–51.
6. Fraser JL, Mwatondo A, Alimi YH, Varma JK, Vilas VJDR. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: a review. Int J Infect Dis. (2021) 103:469–77. doi: 10.1016/j.ijid.2020.12.030
7. Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. (2011) 377:228–41. doi: 10.1016/S0140-6736(10)61458-4
8. Mbim EN, Mboto CI, Agbo BE. A review of nosocomial infections in Sub-Saharan Africa. Microbiol Res J Int. (2016) 15:1–11. doi: 10.9734/BMRJ/2016/25895
9. Alemu AY, Endalamaw A, Bayih WA. The burden of healthcare-associated infection in Ethiopia: a systematic review and meta-analysis. Trop Med Health. (2020) 48:77. doi: 10.1186/s41182-020-00263-2
10. Ali S, Birhane M, Bekele S, Kibru G, Teshager L, Yilma Y, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. (2018) 7:2. doi: 10.1186/s13756-017-0298-5
11. Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. (2015) 5:509–14. doi: 10.1016/j.apjtb.2015.05.001
12. WHO. Health Care-Associated Infections Fact Sheet. Geneva: World Health Organization (2015). p. 4.
13. Revelas A. Healthcare–associated infections: a public health problem. Nigerian Med J. (2012) 53:59. doi: 10.4103/0300-1652.103543
14. Sah M, Mishra S, Ohora H, Kirikae T, Sherchan J, Rijal B, et al. Nosocomial Bacterial Infection and Antimicrobial Resistant Pattern in a Tertiary Care Hospital in Nepal. J Institute Med. (2014) 36.
15. Samuel S, Kayode O, Musa O, Nwigwe G, Aboderin A, Salami T, et al. Nosocomial infections and the challenges of control in developing countries. Afr J Clin Exp Microbiol. (2010) 11. doi: 10.4314/ajcem.v11i2.53916
16. WHO. Prevention of Hospital-Acquired Infections: A Practical Guide. Geneva, Switzerland: World Health Organization (2002).
17. Getahun KA, Redia AS, Aragaw TJ. Evaluation of medicine-use pattern using World Health Organization's core drug-use indicators and completeness of prescription at University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia: cross-sectional study. Integrated Pharmacy Res Practice. (2020) 9:219. doi: 10.2147/IPRP.S261320
18. Wondimeneh Y, Muluye D, Alemu A, Atinafu A, Yitayew G, Gebrecherkos T, et al. Urinary tract infection among obstetric fistula patients at Gondar University Hospital, Northwest Ethiopia. BMC Women's health. (2014) 14:1–6. doi: 10.1186/1472-6874-14-12
19. Google. sitefinder.tghn.org/sites/university-of-gondar-hospital/. (2013). Available online at: www.sitefinder.tghn.org/sites/university-of-gondar-hospital/
20. Gedif G, Sisay Y, Alebel A, Belay YA. Level of job satisfaction and associated factors among health care professionals working at University of Gondar Referral Hospital, Northwest Ethiopia: a cross-sectional study. BMC Res Notes. (2018) 11:1–7. doi: 10.1186/s13104-018-3918-0
21. Ketema DB, Wagnew F, Assemie MA, Ferede A, Alamneh AA, Leshargie CT, et al. Incidence and predictors of surgical site infection following cesarean section in North-west Ethiopia: a prospective cohort study. BMC Infect Dis. (2020) 20:1–11. doi: 10.1186/s12879-020-05640-0
22. Morton SM, Bandara DK, Robinson EM, Carr PEA. In the 21st century, what is an acceptable response rate? : Wiley Online Library. (2012). doi: 10.1111/j.1753-6405.2012.00854.x
23. Ling ML, Apisarnthanarak A, Madriaga G. The burden of healthcare-associated infections in Southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. (2015) 60:1690–9. doi: 10.1093/cid/civ095
24. Moen I. Risk Factors for Healthcare Associated Infections after Elective General Surgery in Nepal. (2018).
25. Scherbaum M, Kösters K, Mürbeth RE, Ngoa UA, Kremsner PG, Lell B, et al. Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect Dis. (2014) 14:1–8. doi: 10.1186/1471-2334-14-124
26. Elliott C, Vaillant A. Nosocomial infections at three tertiary hospitals in trinidad and Tobago. Biomed Sci J. (2020) 1:14. doi: 10.31579/2692-9406/001
27. Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. (2011) 89:757–65. doi: 10.2471/BLT.11.088179
28. Ndegwa L. Hospital-acquired infections surveillance in three Kenyan Hospitals, 2010–2012. Open Forum Infect Dis. (2015) 2:1729. doi: 10.1093/ofid/ofv133.1279
29. Nouetchognou JS, Ateudjieu J, Jemea B, Mesumbe EN, Mbanya D. Surveillance of nosocomial infections in the Yaounde University Teaching Hospital, Cameroon. BMC Res Notes. (2016) 9:1–8. doi: 10.1186/s13104-016-2310-1
30. Habibi S, Wig N, Agarwal S, Sharma SK, Lodha R, Pandey RM, et al. Epidemiology of nosocomial infections in medicine intensive care unit at a tertiary care hospital in northern India. Trop Doct. (2008) 38:233–5. doi: 10.1258/td.2008.070395
31. Hassan R, El-Gilany A-H, El-Mashad N, Azim DA. An overview of healthcare-associated infections in a tertiary care hospital in Egypt. Infect Prevent Practice. (2020) 2:100059. doi: 10.1016/j.infpip.2020.100059
32. Razine R, Azzouzi A, Barkat A, Khoudri I, Hassouni F, Chefchaouni AC, et al. Prevalence of hospital-acquired infections in the university medical center of Rabat, Morocco. Int Arch Med. (2012) 5:1–8. doi: 10.1186/1755-7682-5-26
33. Fakhr AE, Fathy FM. Bacterial pattern and risk factors of hospital acquired infections in a tertiary care hospital, Egypt. Egypt J Med Microbiol. (2018) 27:9–16. doi: 10.12816/0054166
34. Deptuła A, Trejnowska E, Ozorowski T, Hryniewicz W. Risk factors for healthcare-associated infection in light of two years of experience with the ECDC point prevalence survey of healthcare-associated infection and antimicrobial use in Poland. J Hospital Infect. (2015) 90:310–5. doi: 10.1016/j.jhin.2015.03.005
35. Zhang Y, Zhang J, Wei D, Yang Z, Wang Y, Yao Z. Annual surveys for point-prevalence of healthcare-associated infection in a tertiary hospital in Beijing, China, 2012–2014. BMC Infect Dis. (2016) 16:1–7. doi: 10.1186/s12879-016-1504-4
36. Nair V, Sahni A, Sharma D, Grover N, Shankar S, Chakravarty A, et al. Point prevalence & risk factor assessment for hospital-acquired infections in a tertiary care hospital in Pune, India. Indian J Med Res. (2017) 145:824. doi: 10.4103/ijmr.IJMR_1167_15
37. Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. (2001) 7:342. doi: 10.3201/eid0702.010240
38. Pittet D, Harbarth S, Ruef C, Francioli P, Sudre P, Petignat C, et al. Prevalence and risk factors for nosocomial infections in four university hospitals in Switzerland. Infect Control Hospital Epidemiol. (1999) 20:37–42. doi: 10.1086/501554
39. Luft D, Schmoor C, Wilson C, Widmer AF, Bertz H, Frei R, et al. Central venous catheter-associated bloodstream infection and colonisation of insertion site and catheter tip. What are the rates and risk factors in haematology patients? Ann Hematol. (2010) 89:1265–75. doi: 10.1007/s00277-010-1005-2
40. Chernet AZ, Dasta K, Belachew F, Zewdu B, Melese M, Ali MM. Burden of healthcare-associated infections and associated risk factors at Adama Hospital Medical College, Adama, Oromia, Ethiopia. Drug Healthc Patient Saf. (2020) 12:177. doi: 10.2147/DHPS.S251827
41. Rodríguez-Acelas AL, de Abreu Almeida M, Engelman B, Cañon-Montañez Cañon-Montañez W. Risk factors for health care–associated infection in hospitalized adults: systematic review and meta-analysis. Am J Infect Control. (2017) 45:e149–e56. doi: 10.1016/j.ajic.2017.08.016
42. Nseir S, Di Pompeo C, Pronnier P, Beague S, Onimus T, Saulnier F, et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respirat J. (2002) 20:1483–9. doi: 10.1183/09031936.02.00012902
43. Vincent J-L, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin M-H, et al. The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA. (1995) 274:639–44. doi: 10.1001/jama.274.8.639
44. Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L. Antibiotic prophylaxis to prevent nosocomial in patients in intensive care units: evidence that struggle to convince practising clinicians. Internal Emergency Med. (2006)1:160–62. doi: 10.1007/BF02936546
45. Melaku S, Gebre-Selassie S, Damtie M, Alamrew K. Hospital acquired infections among surgical, gynaecology and obstetrics patients in Felege-Hiwot referral hospital, Bahir Dar, northwest Ethiopia. Ethiop Med J. (2012)50:135–44.
46. Chan M-C, Chiu S-K, Hsueh P-R, Wang N-C, Wang C-C, Fang C-T. Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: a case-control study. PLoS ONE. (2014) 9:e85973. doi: 10.1371/journal.pone.0085973
47. Ketata N, Ayed HB, Hmida MB, Trigui M, Jemaa MB, Yaich S, et al. Point prevalence survey of health-care associated infections and their risk factors in the tertiary-care referral hospitals of Southern Tunisia. Infect Dis Health. (2021) 26:284–91. doi: 10.1016/j.idh.2021.06.004
48. Stewart S, Robertson C, Kennedy S, Kavanagh K, Haahr L, Manoukian S, et al. Personalized infection prevention and control: identifying patients at risk of healthcare-associated infection. J Hospital Infect. (2021) 114:32–42. doi: 10.1016/j.jhin.2021.03.032
49. Gosling R, Mbatia R, Savage A, Mulligan J-A, Reyburn H. Prevalence of hospital-acquired infections in a tertiary referral hospital in northern Tanzania. Ann Trop Med Parasitol. (2003) 97:69–73. doi: 10.1179/000349803125002724
50. Awoke N, Arba A, Girma A. Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PLoS ONE. (2019) 14:e0226140. doi: 10.1371/journal.pone.0226140
Keywords: chronic illness, nosocomial infection, incidence rate, determinants, University of Gondar Comprehensive Specialized Hospital
Citation: Taye ZW, Abebil YA, Akalu TY, Tessema GM and Taye EB (2023) Incidence and determinants of nosocomial infection among hospital admitted adult chronic disease patients in University of Gondar Comprehensive Specialized Hospital, North–West Ethiopia, 2016–2020. Front. Public Health 11:1087407. doi: 10.3389/fpubh.2023.1087407
Received: 02 November 2022; Accepted: 07 February 2023;
Published: 24 February 2023.
Edited by:
Guadalupe Miranda-Novales, Mexican Social Security Institute (IMSS), MexicoReviewed by:
Fortino Solórzano-Santos, Federico Gómez Children's Hospital, MexicoElham Aboualigalehdari, Medical University of Ilam, Iran
Copyright © 2023 Taye, Abebil, Akalu, Tessema and Taye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eden Bishaw Taye,  ZWRiaXNoYXcxNkBnbWFpbC5jb20=
ZWRiaXNoYXcxNkBnbWFpbC5jb20=
 Zewdu Wasie Taye1
Zewdu Wasie Taye1 Eden Bishaw Taye
Eden Bishaw Taye