
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 25 July 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1080700
This article is part of the Research Topic Understanding Ebola: a global health challenge, Volume II View all 5 articles
 Reena H. Doshi1*†
Reena H. Doshi1*† Stephanie C. Garbern2†
Stephanie C. Garbern2† Shibani Kulkarni1
Shibani Kulkarni1 Shiromi M. Perera3
Shiromi M. Perera3 Monica K. Fleming1
Monica K. Fleming1 Rigobert Fraterne Muhayangabo4
Rigobert Fraterne Muhayangabo4 Arsene Balene Ombeni4
Arsene Balene Ombeni4 Dieula Delissaint Tchoualeu1
Dieula Delissaint Tchoualeu1 Ruth Kallay1
Ruth Kallay1 Elizabeth Song5
Elizabeth Song5 Jasmine Powell5
Jasmine Powell5 Monique Gainey6
Monique Gainey6 Bailey Glenn7
Bailey Glenn7 Ruffin Mitume Mutumwa4
Ruffin Mitume Mutumwa4 Stephane Hans Bateyi Mustafa8
Stephane Hans Bateyi Mustafa8 Giulia Earle-Richardson9
Giulia Earle-Richardson9 Hongjiang Gao1
Hongjiang Gao1 Neetu Abad1
Neetu Abad1 Gnakub Norbert Soke10
Gnakub Norbert Soke10 David L. Fitter1
David L. Fitter1 Terri B. Hyde1
Terri B. Hyde1 Dimitri Prybylski1
Dimitri Prybylski1 Adam C. Levine3,4
Adam C. Levine3,4 Mohamed F. Jalloh11
Mohamed F. Jalloh11 Eta Ngole Mbong4
Eta Ngole Mbong4Introduction: During the 2018–2020 Ebola virus disease (EVD) outbreak in the eastern part of the Democratic Republic of the Congo (DRC), prevention and control measures, such as Ebola vaccination were challenging by community mistrust. We aimed to understand perceptions regarding Ebola vaccination and identify determinants of Ebola vaccine uptake among HCWs.
Methods: In March 2021, we conducted a cross-sectional survey among 438 HCWs from 100 randomly selected health facilities in three health zones (Butembo, Beni, Mabalako) affected by the 10th EVD outbreak in North Kivu, DRC. HCWs were eligible if they were ≥ 18 years and were working in a health facility during the outbreak. We used survey logistic regression to assess correlates of first-offer uptake (i.e., having received the vaccine the first time it was offered vs. after subsequent offers).
Results: Of the 438 HCWs enrolled in the study, 420 (95.8%) reported that they were eligible and offered an Ebola vaccine. Among those offered vaccination, self-reported uptake of the Ebola vaccine was 99.0% (95% confidence interval (CI) [98.5–99.4]), but first-offer uptake was 70.2% (95% CI [67.1, 73.5]). Nearly all HCWs (94.3%; 95% CI [92.7–95.5]) perceived themselves to be at risk of contracting EVD. The most common concern was that the vaccine would cause side effects (65.7%; 95% CI [61.4–69.7]). In the multivariable analysis, mistrust of the vaccine source or how the vaccine was produced decreased the odds of first-time uptake.
Discussion: Overall uptake of the Ebola vaccine was high among HCWs, but uptake at the first offer was substantially lower, which was associated with mistrust of the vaccine source. Future Ebola vaccination efforts should plan to make repeated vaccination offers to HCWs and address their underlying mistrust in the vaccines, which can, in turn, improve community uptake.
The 10th outbreak (1 August 2018–25 July 2020) of Ebola virus disease (EVD) in the Democratic Republic of the Congo (DRC) affected three provinces and was centered in North Kivu and Ituri. This outbreak became the second largest EVD outbreak globally after the 2014–2016 West Africa EVD outbreak. (1) It was officially declared over after almost 2 years, and 3,481 cases (3,323 confirmed, 158 probable) and 2,299 deaths (2). Unlike previous EVD outbreaks in DRC, this outbreak was unprecedented in terms of the geographical scope, the number of cases, and duration. The region’s long history of armed conflict presented obstacles for response teams to rapidly identify and isolate suspect cases, trace contacts, conduct risk communication and community engagement activities, provide specialized medical care, and administer vaccines (3–5).
Of the confirmed EVD cases during this outbreak in DRC, 5% were health care workers (HCWs) and 44% died (1). Health care workers are at increased risk of EVD given their close physical contact with patients and their bodily fluids (6, 7). Thus, the Strategic Advisory Group of Experts on Immunization (SAGE) recommends Ebola vaccination for HCWs and other contacts during an active EVD outbreak (8, 9). Vaccination reduces the risk of EVD and may prevent nosocomial transmission, especially in the context of poor adherence to infection and prevention control methods (10, 11).
Since 2018, vaccination with the ERVEBO® (rVSVΔG-ZEBOV-GP) vaccine has been an integral part of EVD outbreak response in DRC, administered under a compassionate use/expanded access protocol (12). The vaccine has been shown to be safe and effective against Zaire ebolavirus in Phase 3 trials and was further confirmed through data collected in DRC under the compassionate use protocol. The vaccine has since been approved for use in several countries (13). In 2019, the vaccine was licensed by the European Medicines Agency and the United States Food and Drug Administration, though the use of the licensed product in recent EVD outbreaks had been challenged by supply constraints (14).
Vaccine hesitancy, or the state of indecision and uncertainty about vaccination, has become an increasing public health threat globally (15, 16). Vaccine hesitancy is complex, context-specific, and varies by time, place, and type of vaccine and is influenced by numerous factors including, confidence in the vaccine safety and efficacy; public knowledge and awareness; and religious, cultural, gender, or socioeconomic factors (17, 18). Further, social determinants, such as the ability to take time off from work and travel to vaccination sites, and ability to miss work due to side effects, place additional constraints on individuals who might otherwise accept vaccination. Studies from the 2014–2016 West African EVD outbreak revealed multiple drivers of Ebola vaccine acceptance, including perceived risk of Ebola, altruistic desires to prevent Ebola transmission, trust, or distrust of those offering the vaccines, and fear of side effects (19–26). In North Kivu, dissatisfaction and mistrust, of the government and EVD response teams was widespread (27). Challenges in gaining community confidence in Ebola vaccination and other preventive measures severely undermined response activities (4, 27). Community feedback collected during the outbreak, which included HCWs, found that inadequate knowledge and politicization of EVD, skepticism concerning vaccination efficacy and necessity, perceptions of unfairness around prioritizing contacts for vaccination, safety concerns, and beliefs about international organizations harboring ulterior motives were barriers to Ebola vaccination and undermined vaccine confidence (3, 5, 28).
There is little published data HCWs perceptions and attitudes about Ebola vaccine in DRC. Understanding attitudes among HCWs is not only important because they are at high risk for EVD infection, but also because HCWs can play an important role in encouraging others in the community to be vaccinated. There is evidence that vaccination attitudes among HCWs predict their vaccine uptake and intention to recommend vaccine in both routine health care settings and during health emergencies (27, 29–31).
An improved understanding of the drivers of timely Ebola vaccination uptake among HCWs is needed to better inform new vaccine introduction efforts, especially during emergencies. This survey aimed to understand the perceptions about Ebola vaccination, measure Ebola vaccine uptake, and identify determinants of Ebola vaccine uptake among HCWs, who were offered the vaccine during the 2018–2020 EVD outbreak.
North Kivu Province is in eastern DRC and forms part of the country’s borders with Uganda and Rwanda (Figure 1). The region has more than four decades of conflict and insecurity, the presence of multiple armed groups, large-scale population displacements, poverty, limited access to essential healthcare services, and distrust of both the government and foreigners (4, 5, 32). During the 10th EVD outbreak, vaccination with investigational (unlicensed) doses of the ERVEBO (rVSVΔG-ZEBOV-GP) vaccine was initiated on 8 August 2018, under an “expanded access/compassionate use” protocol, requiring informed consent. Ring vaccination was the primary vaccination strategy, but HCWs were also offered vaccination if they worked in a health facility in active outbreak areas (33, 34). Pregnant and lactating women were initially not eligible for vaccination, but the protocol was revised in June 2019 to include these women due to increasing EVD cases (35).
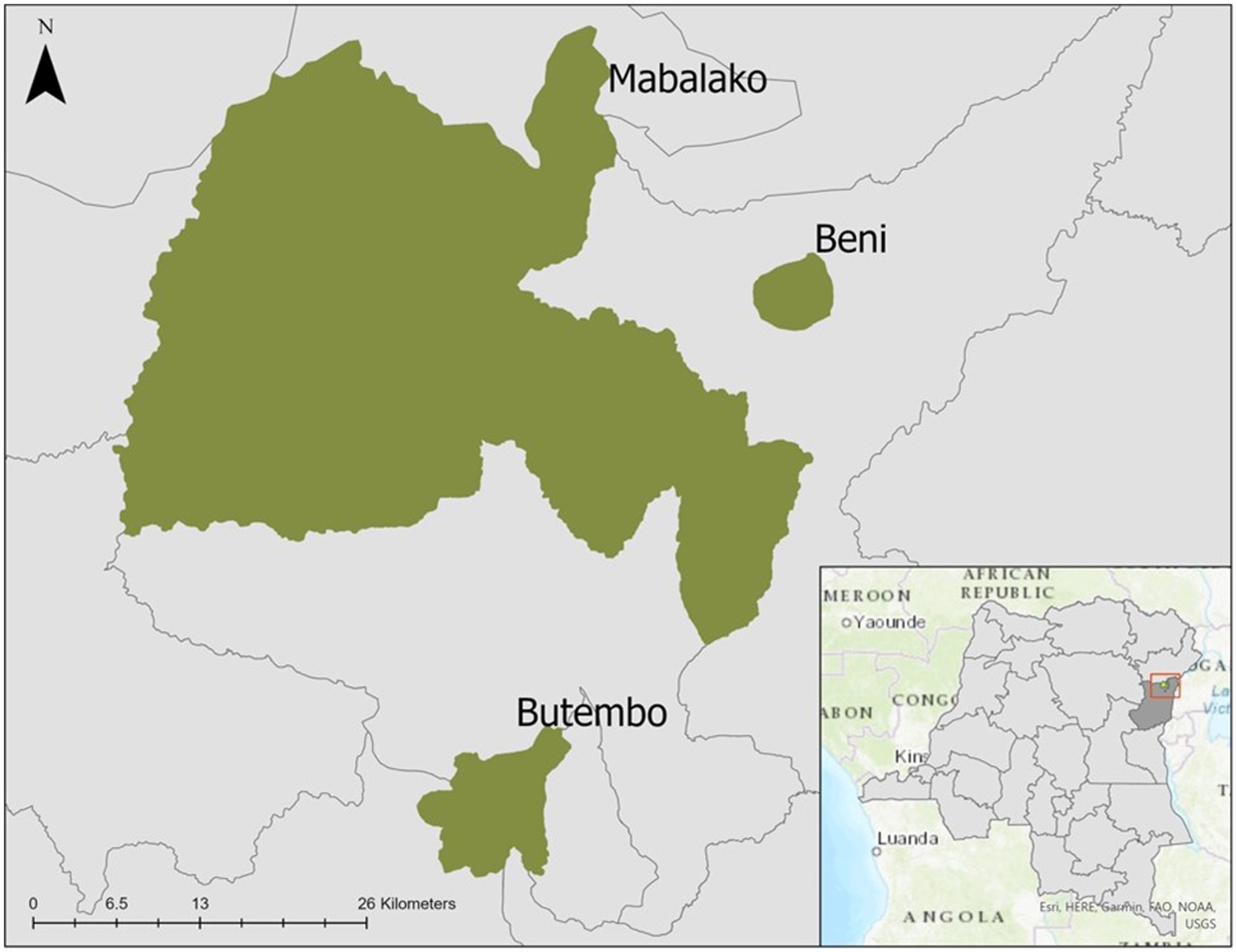
Figure 1. Map of selected health zones in North Kivu (Beni, Butembo, Mabalako), Democratic Republic of the Congo, 2021.
In March 2021, we conducted a cross-sectional survey among HCWs in three health zones in North Kivu Province, DRC. Butembo, Beni and Malabako health zones were selected due to their high EVD cases counts and reports of community resistance to response activities during the 10th outbreak in DRC. We used a stratified one stage cluster design assuming a design effect of 2, halfwidth of 95% confidence interval (CI) equal to 7.5%. A random sample of 100 health facilities was chosen from a sampling frame of 187 facilities (81 in Butembo, 79 in Beni, and 27 in Mabalako) that were operational during the outbreak and provided health services to the local communities. The sampling frame included primary health facilities (e.g., health posts, dispensaries, health centers) generally run by community HCW and nurses, and secondary health facilities (e.g., hospitals, referral medical centers where the population might receive more specialized care). Both public and private health facilities were included in the sample.
HCW were defined as anyone working at a health facility at the time of data collection and included both clinical and support roles (including administrative, cleaners, hygienists). A line list of all staff at each selected facility was created and HCWs were invited to participate if they indicated they had been working in any health facility during the outbreak (August 2018–July 2020) and were at least 18 years of age. A second visit to the health facility was made to enroll any absent the first day. In total, 445 HCWs were screened and 440 were eligible; two HCWs declined to participate.
A structured questionnaire was developed that consisted of the following topic areas: survey eligibility, participant demographics, and knowledge, attitudes and beliefs about the Ebola vaccine and the vaccination program. These questions were mapped to five areas that were expected to influence vaccine uptake: perceived risk, communications (including rumors, misinformation, community engagement), societal factors (including religion, community leaders, government, security concerns), health systems (e.g., vaccination teams, geography, logistics) and vaccine specific factors (e.g., safety and efficacy). The topic areas were selected based on review of the literature, review of community feedback produced during the outbreak, and knowledge of the region. To assess general vaccine confidence, we utilized a series of six questions that had been psychometrically validated in the context of childhood vaccination in Sierra Leone (36). A draft survey was pilot tested in a selected community near the training site (the pilot data were not included in the final analysis).
Face to face interviews were conducted in March 2021 at the health facilities. Trained multi-lingual interviewers were recruited locally and received a four-day training. Interviewers worked in pairs (male and female) and interviewed participants who were of the same sex as themselves. Participation was anonymous, voluntary, and uncompensated. Interviewers administered the survey in French for francophone participants and performed on-the-spot translations in Swahili, as necessary per training guidance. Responses from the participants were recorded by interviewers on mobile devices pre-programmed with KoBoCollect (Kobo, Inc.) All data were uploaded daily with nightly back-ups to the KoBo Toolbox server. Data collection teams were required to follow COVID-19 mitigation measures, including wearing a mask, using hand sanitizer, and social distancing where possible.
To describe Ebola vaccine uptake and perceptions about Ebola vaccination, we calculated frequencies of survey responses related to vaccine uptake, attitudes, beliefs and vaccine concerns. Vaccine safety, efficacy, and trust were measured on a 5-point bipolar Likert scale in the questionnaire (ranging from strongly agree to strongly disagree). Point estimates and 95% were calculated using STATA’s “svy: tabulate procedure.”
Logistic regression was used to examine correlates associated with HCW acceptance or delay of the vaccine. The outcome variable was HCW self-reporting accepting the Ebola vaccine the first time it was offered vs. those who accepted at the second or later offers. We selected variables for inclusion in the model based on expert consensus and taking into account the constructs in the Health Belief Model (37). The “svyset” command in STATA was used to specify clustering at the health facility level, applying sampling weights to account for the cluster survey design. Stratification of health facilities as primary or secondary facilities were included in the survey sampling design.
The sample was limited to 415 participants that were eligible for the Ebola vaccine, offered vaccination, recalled the number of times they were offered the vaccine, and took the vaccine at the first offer or on subsequent offers. To identify factors significantly associated with vaccine uptake, three composite vaccine confidence variables (safety, efficacy, and trust) were created by first dichotomizing the 5-point Likert scale by classifying “strongly agree,” and “agree” as “yes” and “neither agree or disagree,” “disagree,” and “strongly disagree” as “no.” For general vaccine confidence, we computed a composite score, using six items (Supplementary Table 1) that have been psychometrically validated in the context of childhood vaccination in Sierra Leone (36). Each question had a scale of 0–4 corresponding to low-high vaccine acceptance and the total composite score was then categorized as low (<25th percentile), medium (25–75th percentile), or high vaccine (>75th percentile). The following variables were included in the model: sex, age, highest education level attained, religion’s influence on health decisions, perceived risk of contracting Ebola during the outbreak, perception that Ebola can be prevented with a vaccine and the perception of the Ebola vaccine as having severe side effects, trust in vaccine source/how it was produced and general vaccine acceptance. We conducted a sensitivity analysis, substituting the variable of the current perceived risk of Ebola (“I think I am now at risk of contracting Ebola”) as a binary variable (strongly agree and agree coded as yes and neutral, disagree, and strongly disagree as no) instead of the recalled perceived risk of contracting Ebola during the outbreak in regression analysis. All analyses were performed using Stata version 16 (Stata Corp; College Station, United States).
The University of Kinshasa School of Public Health Ethics Committee approved the survey (protocol approval #203-2020). The assessment was determined to be a non-research public health activity by CDC. The purpose of the assessment was explained to all participants with additional details provided on an information sheet in the local language. Verbal informed consent was obtained and documented electronically on the data collection tool by trained research staff, due to low literacy rates and the need to limit physical contact during the pandemic.
Of the 438 participating HCW, the median age was 35 years (IQR 29–42) with females comprising 53.7% of the sample. Nearly all respondents (90.1%) had at least some secondary school education. The most reported occupations were nurse (47.7%), hygienist (17.4%), and administrator (10.5%). Overall, 40.2% of HCWs participated in the outbreak response in DRC (Table 1).
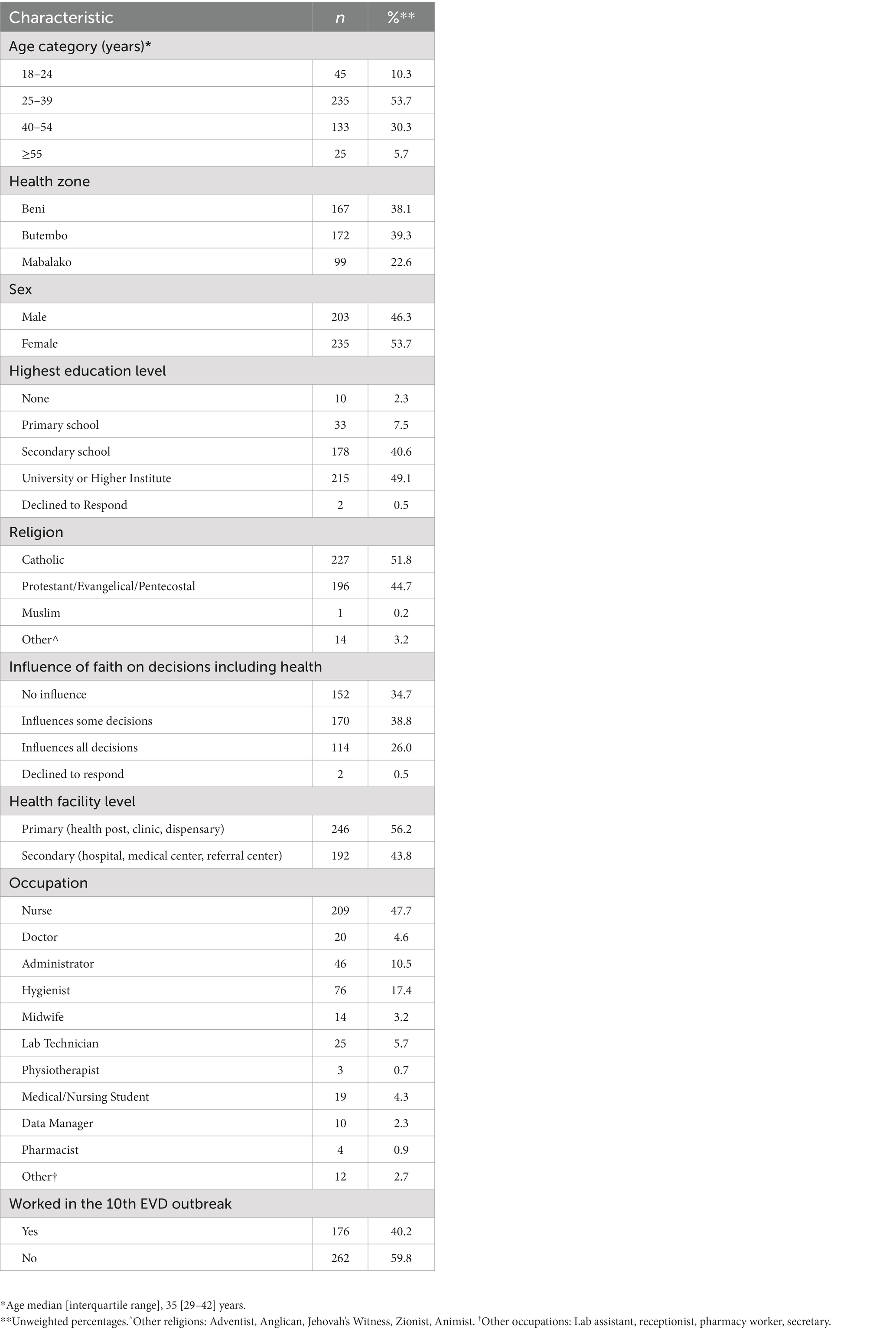
Table 1. Characteristics of surveyed health care workers (N = 438), North Kivu, Democratic Republic of the Congo, 2021.
Nearly all HCWs, 94.3% [95% Confidence Interval: 92.7%–95.5%] perceived themselves to be at some risk of contracting EVD during the tenth outbreak. Approximately half 51.8% [95% CI: 48.8%–54.8] reported they had been in contact with someone with EVD during the outbreak and 2.7% [95% CI: 2.0–3.7] reported they had been diagnosed with EVD.
The majority of HCWs felt that Ebola is a potentially fatal disease, with 76.9% ([95% CI: 74.5–79.2]) strongly agreeing and 22.2% ([95% CI: 19.9–24.5]) agreeing (Table 2). Most respondents had favorable beliefs regarding the vaccines’ efficacy with 56.6%; ([95% CI: 53.5–59.7]) strongly agreeing and 36.8% (95% CI: [33.8–39.8]) agreeing that the vaccine is needed to prevent disease spread during outbreaks, and 44.1% (95% CI: [40.8–47.4]) strongly agreeing and 41.8% (95% CI: [39.0–44.6]) agreeing that the vaccine reduces disease severity. Some HCWs did not have confidence in the vaccine with 5.7% (95% CI: [4.7–7.0]) strongly agreeing and 21.0% (95% CI: 18.9%–23.3%) agreeing that they did not trust the Ebola vaccine source or how it was produced (Table 2). Among all respondents, 64.6% (95% CI: [61.4–67.7]) felt that the Ebola vaccine should be mandatory for HCWs (data not shown in table).
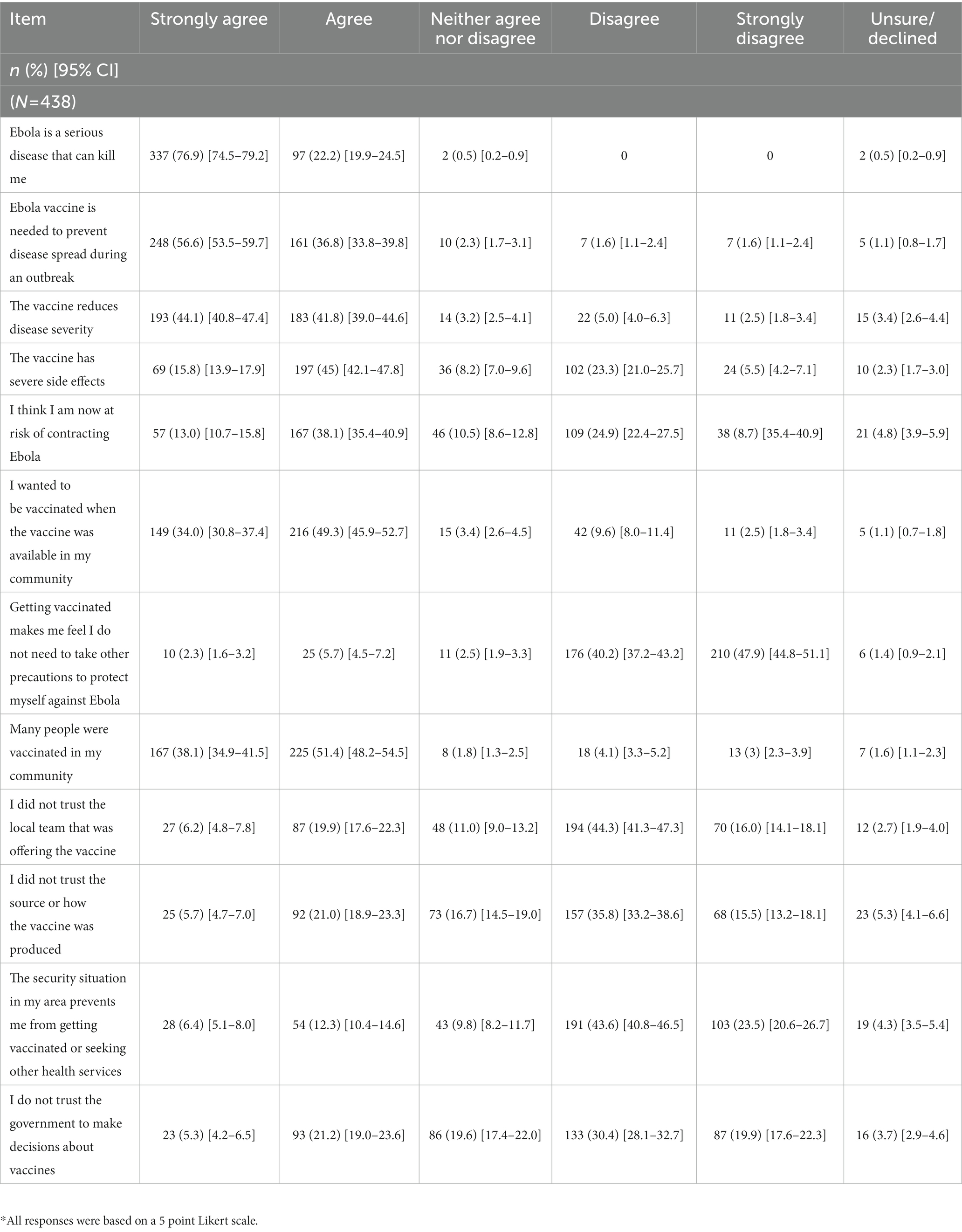
Table 2. Perceptions of Ebola virus disease and the Ebola vaccine among surveyed health care workers, North Kivu, Democratic Republic of the Congo, 2021.
Among the 438 enrolled respondents, 420 (95.9%; 95% CI: [94.7–96.8]) reported that they were eligible for and offered the Ebola vaccine. Of the 420 respondents who were offered vaccination, 99.0% (95% CI: [98.5–99.4]) self-reported that they took the vaccine (Table 3). Among those who took the Ebola vaccine (n = 416), uptake at the first offer was 70.2% (95% CI: [67.1–73.5]) compared to 12.3% (95% CI: [10.4–14.4]) at the second offer, 7% (95% CI: 5.6–8.7) at the third offer, and 10.3% (95% CI: [8.6–12.4]) at the fourth offer or later (Table 3).
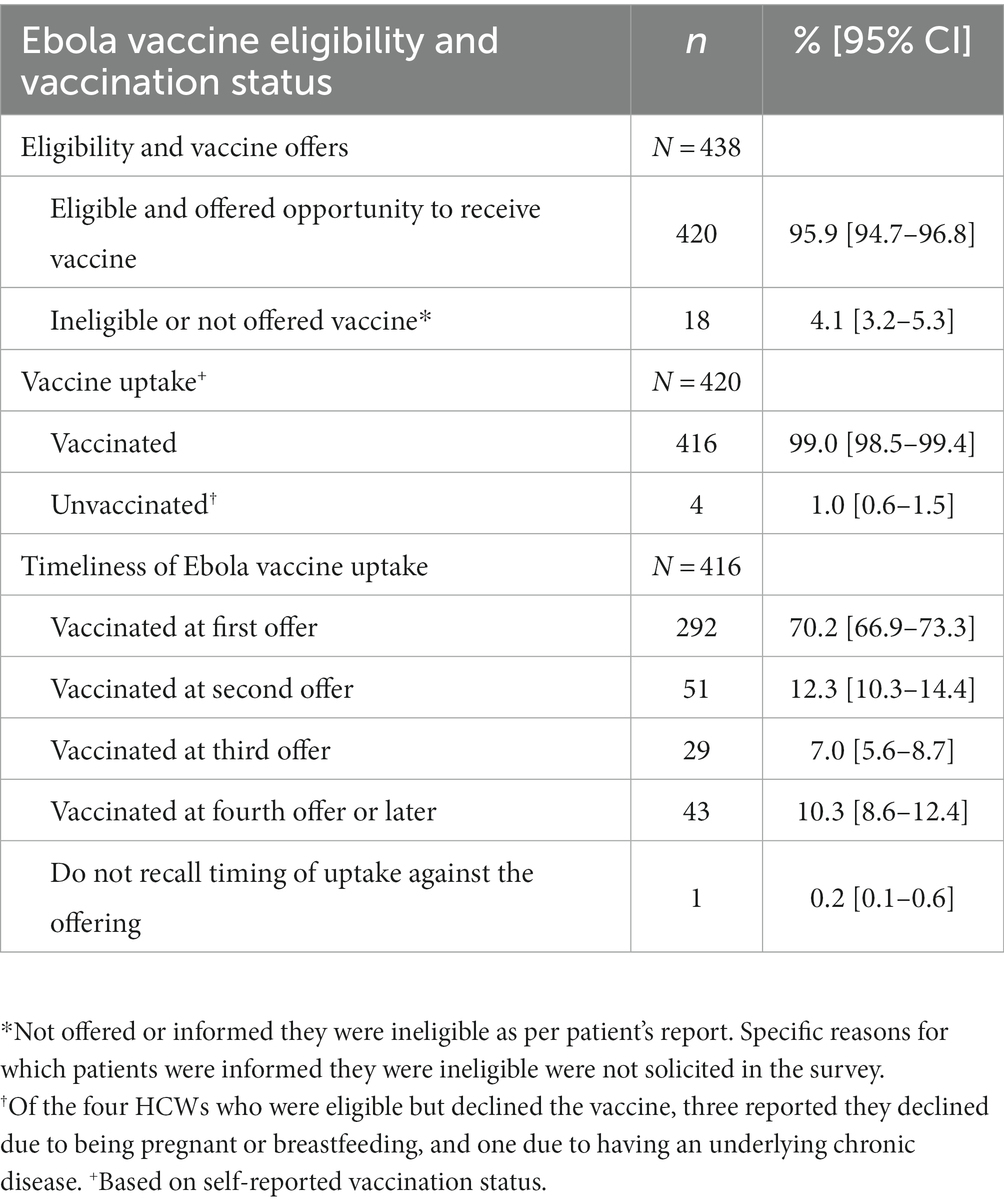
Table 3. Ebola vaccination status and the number of offers prior to vaccine receipt among vaccinated healthcare workers, North Kivu, Democratic Republic of the Congo, 2021.
Nearly half (48.3%; [95% CI: 45.7–51]) of the vaccinated participants (n = 416) reported they had concerns about the vaccine when they received it (Table 4). The most common concerns were that the vaccine would cause side effects (65.7%; [95% CI: 61.4–69.7]), or death (48.5%; [95% CI:44.6–52]), cause EVD (29.4%; [95% CI: 26.4–32.5%]), and cause infertility (33.3%; [95% CI: 29.3–37.4]). Fewer were concerned that the vaccine was not effective at preventing EVD (17.9%; [95% CI: 14.8–21.5]) or had a lack of trust in the vaccine manufacturer (12.9%; [95% CI 10.7–15.6]) and the process used to make the vaccine (4.5%; [95% CI: 3.3–6.0]). In an unadjusted analysis (data not shown), HCWs with concerns about the vaccine had lower odds of first-offer vaccine acceptance (OR 0.26; [95% CI: 0.14–0.50]) vs. those without concerns.
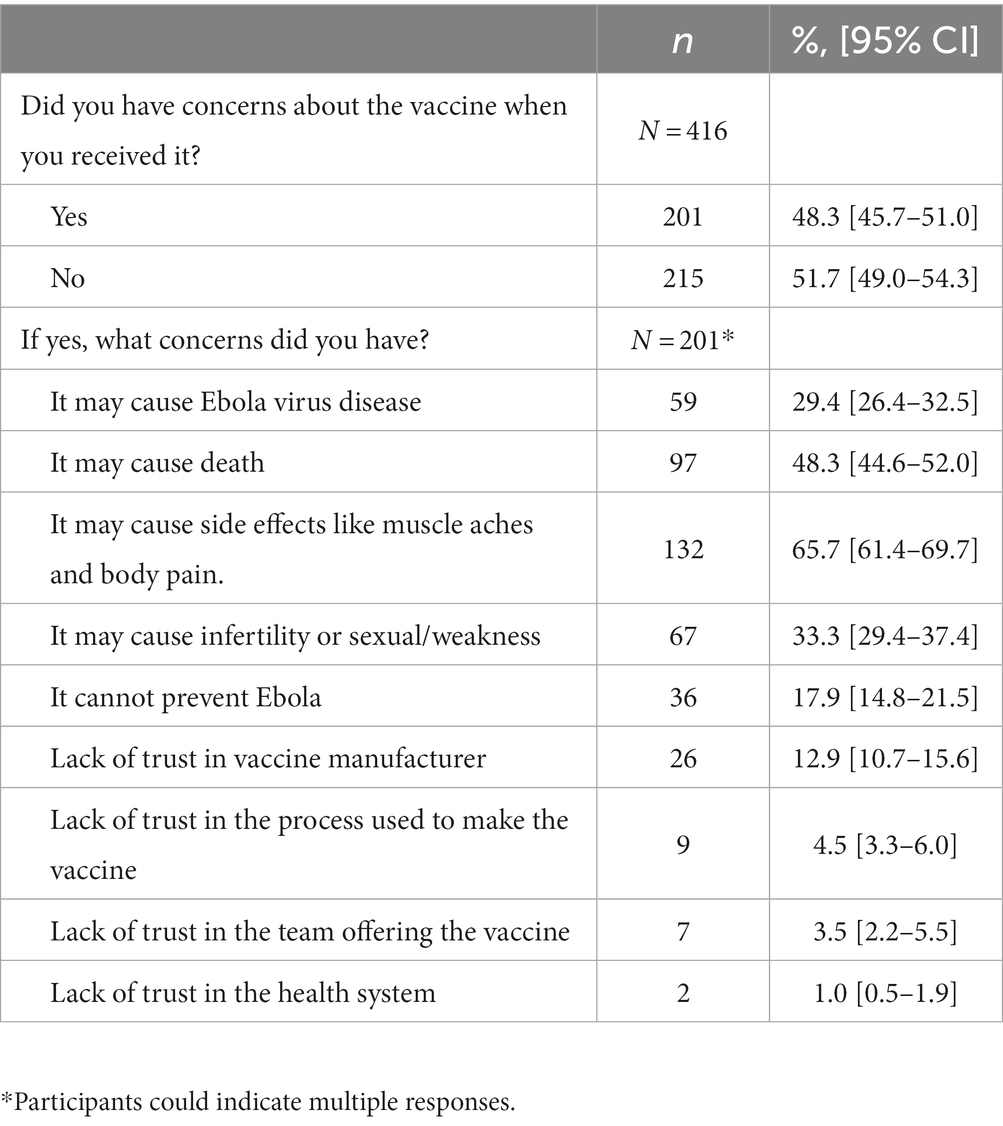
Table 4. Ebola vaccine concerns at the time of vaccination among health care workers (N = 416), North Kivu, Democratic Republic of the Congo, 2021.
Participants had overall positive attitudes toward vaccines in general with 88.6% (95% CI: [86.8–90.2]) reporting they “very much” or “somewhat” agreed that vaccines are good and 81.7%; (95% CI: [79.5–83.8]) “very much” or “somewhat” agreed that vaccines are safe (Supplementary Table 1). However, only 10.3% (95% [CI: 8.5–12.3]) reported that the community spoke positively about vaccines in general, while 24.9% (95% [CI: 22.5–27.5]) reported the community spoke negatively.
In the multivariable analysis, older age (adjusted Odds Ratio (aOR) 1.14 [95% CI: 1.02–1.28]), having been an Ebola response team member (aOR 1.41 [95% CI: 1.06–1.87]), perception that Ebola can be prevented with a vaccine (aOR 1.92 [95% CI: 1.47–2.51]) and having high general vaccine confidence (aOR 2.33 [95% CI: 1.70–3.21]) were all associated with a higher odds of first-offer vaccine uptake. Participants who expressed mistrust of the vaccine source or how the vaccine was produced were found to have lower odds of first-offer vaccine uptake (aOR 0.38 [95% CI: 0.30–0.47]). Those with perceived risk of contracting EVD during the outbreak (aOR 0.43 [95% CI: 0.22–0.87]) were negatively associated with first-offer vaccine uptake in the primary analysis, however, in sensitivity analyses, current perceived risk of contracting EVD was found to have no association with first-offer vs. later offer uptake (aOR 0.86 [95% CI: 0.67–1.10]). All other covariates in the model, including sex, educational level, religion’s influence, and belief that the Ebola vaccine has severe side effects, were not significantly associated with first-offer uptake (Table 5).
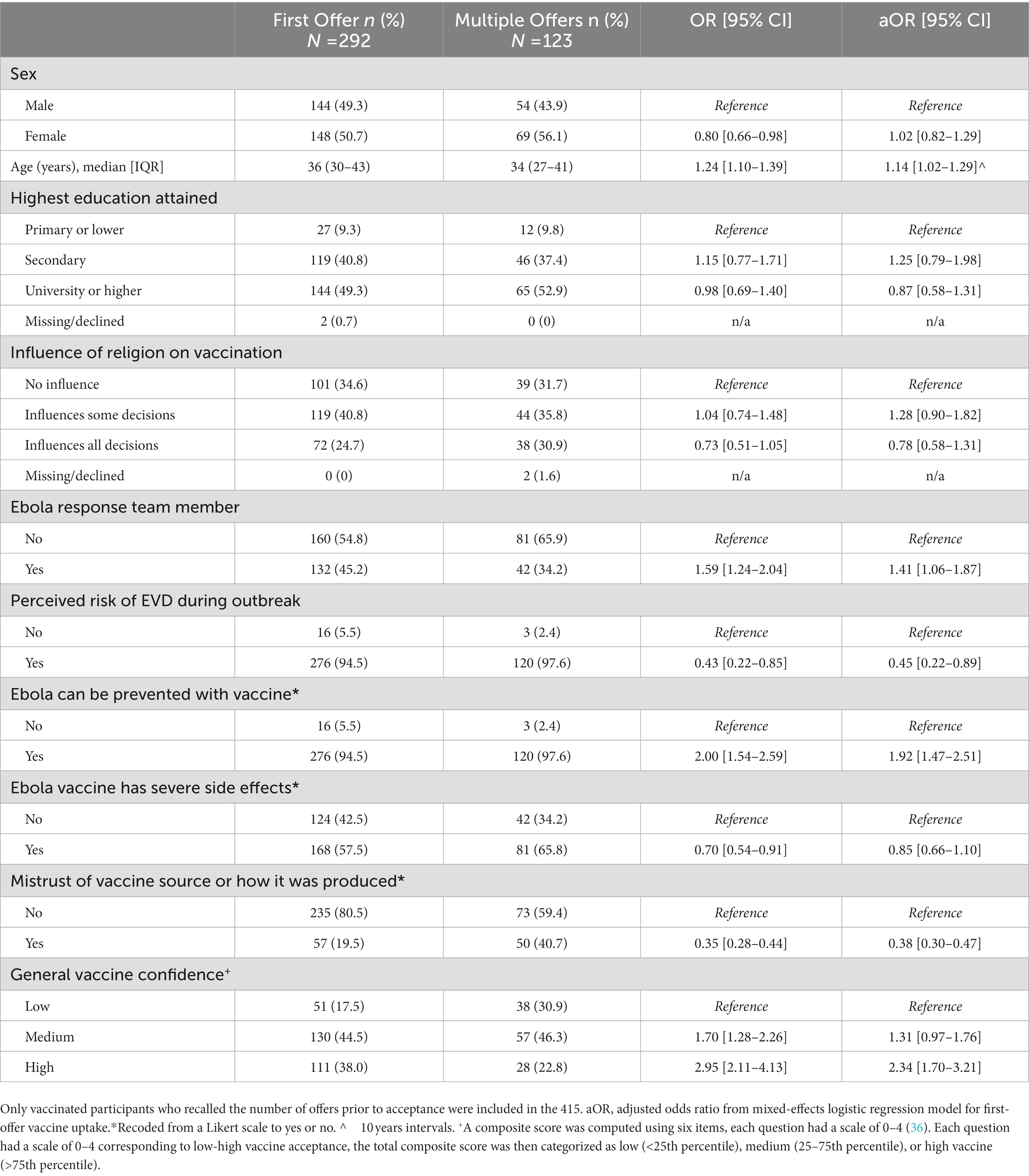
Table 5. Correlates of Ebola vaccine uptake at the first offer vs. subsequent offers among vaccinated health care workers, North Kivu, Democratic Republic of the Congo, 2021 (N = 415).
Our findings revealed high uptake of Ebola vaccine among HCWs in three health zones heavily affected by the 10th EVD outbreak in DRC. While the majority of HCWs considered EVD to be a serious disease that could kill them, almost a third of the eligible HCWs delayed vaccination. Being a response worker, the perception that Ebola could be prevented with a vaccine and having higher general confidence in vaccines were all associated with higher odds of accepting the vaccine on first offer, while mistrust in the vaccine source was negatively associated with vaccine uptake at the first offer. Taken together, our findings imply that we must address underlying trust in the vaccine and that uptake is enhanced with multiple vaccination offers. Vaccine outreach activities with strong risk communication and community engagement efforts that improve the timeliness of EVD vaccination among HCWs may have the dual benefit of protecting the frontline workforce and increasing the likelihood that HCWs may encourage others to take the vaccine. Early efforts to target this group can build vaccine confidence, which is important in controlling outbreaks of vaccine preventable diseases.
The high level of vaccine acceptance in this HCW population is consistent with prior research and might be explained by several reasons (24, 38). HCWs likely had multiple opportunities to get vaccinated given that Beni, Butembo, and Mabalako were all frequent epicenters during the outbreak and vaccination teams were likely present in the communities at multiple points during the 22-month outbreak. Many HCWs were eligible and likely offered vaccination multiple times, especially if they were working in a facility at the time of the outbreak, or if they were part of a ring (i.e., a contact or contact of contact of an EVD case). Strong encouragement from response workers or the health facilities and potentially the fear of job loss, might also explain the high vaccination rate.
Despite high acceptance, mixed perceptions regarding the vaccine were common, with nearly half of respondents reporting that they had concerns about the vaccine, including vaccine side effects, vaccine safety, and vaccine effectiveness. Fear of side effects and lack of confidence in vaccine effectiveness has been associated with lower vaccine acceptance (39) and many of the vaccine side effects overlap with EVD symptoms, which may have increased fear (40). Many of these concerns were communicated to individuals during the informed consent process, however only those that agreed to take the vaccine were consented.
While almost all HCWs in this sample were eventually vaccinated, almost 30% indicated that they did not take the Ebola vaccine the first time it was offered, with about 10% only accepting after 4 or more offers. Circulating rumors and misinformation on social media and throughout the local community may have contributed to the concerns, including that the experimental drugs and vaccines were brought to exterminate the local population, that the vaccine might cause death or EVD, or lead to infertility or sexual dysfunction (3, 41). We did not identify differences by age and sex, nor religion, which contrasts with past work suggesting that vaccine uptake may be closely linked to religious beliefs and perceptions from community leaders (42, 43). In the multivariable analysis, only mistrust of vaccine source was associated with delayed vaccine acceptance. Before 2018, the vaccine had not been widely deployed for use in an outbreak, and during the 10th outbreak, the vaccine was unlicensed and investigational doses were being offered under a compassionate use/expanded access protocol. This involved multiple steps, including a lengthy written informed consent process. Vaccination staff reviewed the consent form with each participant, which included information on previous safety evaluations. In addition, staff described the side effects of the vaccine and were provided with paracetamol post vaccination. In response to increasing case counts and geographic spread, the protocol was revised to include pregnant women (after the first trimester) and lactating women (9). Additionally, a fractional dose was offered to preserve vaccine supply (9) and a second vaccine, offered as a two dose regimen, was given in areas near the active outbreak (44). During the West African EVD outbreak in 2014–2016, HCWs expressed concerns about the experimental status of the vaccine (19–21, 24). Despite necessary, these changes mid-outbreak may have led to confusion and distrust in the vaccine and the process (3).
It is important to note that this EVD outbreak occurred in an active conflict zone, where low institutional trust has been documented (27). In 2019, community resistance, including attacks on response teams, HCWs, and Ebola treatment centers escalated as armed group activities also increased (45). The EVD response work was frequently undermined by misinformation from government authorities and the rapid mobilization of staff and resources for the EVD outbreak contradicted the government’s failure to protect the community from continuous violence (27, 46). Prior work from the region suggested that low levels of trust in government institutions and dissatisfaction with perceived inadequacies in the response effort, including violation of cultural burial practices have been linked to reducing adherence to EVD preventive behaviors (27).
While the Ebola vaccine acceptance was essentially ubiquitous across this group, our findings underscore the importance of efforts to engage the “moveable middle” i.e., the group that has vaccine concerns, but who is open to changing vaccination intent with additional information (47). The importance of this group has also been seen with the COVID-19 vaccine (48). With any new vaccine introduction, clear communication on potential vaccine side effects, and its safety and efficacy are crucial (13, 34, 49). Additionally, HCWs are prioritized for the Ebola vaccination owing to their nature of work; therefore, addressing vaccine confidence issues in HCWs early on can also contribute to better peer-to-peer support for improving social norms around vaccination. HCWs also act as ambassadors for trusted health information and vaccine confidence in the local community. Thus, the development of targeted strategies that empower HCWs with knowledge and ways to combat misinformation may lead to improved community uptake of the vaccine (50). Notably, general vaccine acceptance for RI was high among this sample of HCWs. While general vaccine acceptance is often correlated with acceptance for other vaccines, newer adult vaccines such as EVD or even COVID-19 may take more time to build confidence and may result in delayed acceptance (51).
More than half of the HCWs indicated that had they contact with an EVD case. HCWs serve as frontline workers during an EVD outbreak and are at higher risk for occupational exposure and transmission (6, 42). Additionally, more than half felt that Ebola vaccination should be mandatory for HCWs. There are an estimated 236,000 HCWs in 13 countries with a history of EVD outbreaks, thus preemptive vaccination for HCWs has been proposed as a strategy to avert another large-scale outbreak (52, 53). However, during the March 2021 meeting, SAGE reiterated that widespread preventive use of either Ebola vaccine was not recommended in the absence of an outbreak due to current supply constraints (54). A Gavi-funded global emergency stockpile of 500,000 doses of the Ebola vaccine is currently in progress and doses are accessible to countries in the event of an outbreak. The high vaccination acceptance among this sample of HCWs shed light on the effectiveness of prophylactic vaccination, which may be reconsidered once supply increases (8, 33). Modeling of vaccination scenarios during outbreaks suggests that prophylactic vaccination of HCWs might contribute to reductions in the outbreak size (11, 55).
Our results are subject to several limitations. While we randomly sampled from a list of health facilities in three health zones, we acknowledge that our sampling frame may not have been comprehensive of all health facilities in these zones, specifically informal facilities. Further, HCWs are a very specific group; thus, caution is required when extrapolating these results to other categories or the local communities. We were unable to quantify levels of mistrust and how it may have changed overtime. Due to the COVID-19 pandemic, data collection was delayed by 9 months. During this delay, two subsequent EVD (11th and 12th) outbreaks occurred in DRC and may have influenced our results in ways that we cannot discern. Additionally, there is a possibility of misclassification due to recall inaccuracies, particularly for questions on exposure during the outbreak and number of times the vaccine was offered. Although the survey tool was translated, piloted, and adapted to the country context, and interviewers received special training, some questions and responses may have been misinterpreted or mistranslated by the interviewers.
This work highlights the complex nature of vaccine hesitancy among HCWs in a complex environment. It also provides important information on the effectiveness of a prophylactic vaccination program for HCWs. While overall uptake of the Ebola vaccine was very high among HCWs, we found that delayed uptake was relatively common, and associated with mistrust of the vaccine source. The final decision to receive the vaccine was likely a combination of contextual, individual, community, and vaccine-specific factors, including the length and severity of the outbreak. Future Ebola vaccination efforts in this setting or other similar settings should plan to address underlying mistrust of the vaccines and to offer vaccination repeatedly. Regularly utilizing data from the novel Integrated Outbreak Analytics (IOA) approach other behavioral surveys can provide actional social science evidence to improve response activities (56). Interventions aimed at increasing trust in the vaccine by disseminating accurate vaccine information and addressing rumors may aid in increasing Ebola vaccine uptake during future outbreaks.
Preliminary results from this work were presented at the American Society for Tropical Medicine and Hygiene 2021 virtual annual conference.
The datasets presented in this article are not readily available because they are the property of the DRC Ministry of Health. Requests to access the datasets should be directed to the authors.
The studies involving human participants were reviewed and approved by Kinshasa School of Public Health. The Ethics Committee waived the requirement of written informed consent for participation.
RD and MF: study conceptualization. MF, RD, MJ, DP, GE-R, SK, DT, NA, AL, and EM: study design. EM, RiM, AO, SH, and RuM: data collection. SG, SP, ES, JP, MG, BG, SK, MJ, and HG: data analysis and interpretation. SG, RD, SP, SK, and MJ: article drafting. GS, DF, TH, RK, AL, HG, and MJ: critical revision. All authors contributed to the article and approved the submitted version.
Funding for this work was provided through grants from the U.S. Centers for Disease Control and Prevention (Federal Identifier: NU2GGH002058) to International Medical Corps. This work was also supported in part by the National Institute of Allergy and Infectious Diseases R25AI140490.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or International Medical Corps or any institutions that the authors are affiliated with.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1080700/full#supplementary-material
1. World Health Organization. Ebola virus disease – Democratic Republic of the Congo, Disease Outbreak News. Geneva: WHO (2020).
2. World Health Organization. Ebola: North Kivu/Ituri, Democratic Republic of the Congo, august 2018–June 2020. Geneva: WHO (2020).
3. Muzembo, BA, Ntontolo, NP, Ngatu, NR, Khatiwada, J, Ngombe, KL, Numbi, OL, et al. Local perspectives on Ebola during its tenth outbreak in DR Congo: a nationwide qualitative study. PLoS One. (2020) 15:e0241120. doi: 10.1371/journal.pone.0241120
4. Claude, KM, Underschultz, J, and Hawkes, MT. Ebola virus epidemic in war-torn eastern DR Congo. Lancet. (2018) 392:1399–401. doi: 10.1016/S0140-6736(18)32419-X
5. Masumbuko Claude, K, Underschultz, J, and Hawkes, MT. Social resistance drives persistent transmission of Ebola virus disease in eastern Democratic Republic of Congo: a mixed-methods study. PLoS One. (2019) 14:e0223104. doi: 10.1371/journal.pone.0223104
6. Kilmarx, PH, Clarke, KR, Dietz, PM, Hamel, MJ, Husain, F, McFadden, JD, et al. Ebola virus disease in health care workers--Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. (2014) 63:1168–71.
7. Matanock, A, Arwady, MA, Ayscue, P, Forrester, JD, Gaddis, B, Hunter, JC, et al. Ebola virus disease cases among health care workers not working in Ebola treatment units--Liberia, June-august, 2014. MMWR Morb Mortal Wkly Rep. (2014) 63:1077–81.
8. WHO. 197 Meeting of the meeting report Strategic Advisory Group of Experts on Immunization, 22-24.2021. Conclusions and recommendations. Wkly Epidemiol Rec. (2021) 96:197–216.
9. World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, 2019—Conclusions and recommendations. Wkly Epidemiol Rec. (2019) 94:261–80.
10. Hakiziman, B. Conceptualizing Ebola virus disease (EDV) infection prevention and control (IPC) program in the context of Democratic Republic of the Cono (DRC) during the 2018-2020 EVD outbreak: pilot study. Int J Fam Med Prim Care. (2020) 1:1005.
11. Rupani, N, Ngole, ME, Lee, JA, Aluisio, AR, Gainey, M, Perera, SM, et al. Effect of recombinant vesicular stomatitis virus-Zaire Ebola virus vaccination on Ebola virus disease illness and death, Democratic Republic of the Congo. Emerg Infect Dis. (2022) 28:1180–8. doi: 10.3201/eid2806.212223
12. Mbala-Kingebeni, P, Villabona-Arenas, CJ, Vidal, N, Likofata, J, Nsio-Mbeta, J, Makiala-Mandanda, S, et al. Rapid confirmation of the Zaire Ebola virus in the outbreak of the Equateur Province in the Democratic Republic of Congo: implications for public health interventions. Clin Infect Dis. (2019) 68:330–3. doi: 10.1093/cid/ciy527
13. Henao-Restrepo, AM, Camacho, A, Longini, IM, Watson, CH, Edmunds, WJ, Egger, M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola ca Suffit!). Lancet. (2017) 389:505–18. doi: 10.1016/S0140-6736(16)32621-6
16. Larson, HJ, Gakidou, E, and Murray, CJL. The vaccine-hesitant moment. N Engl J Med. (2022) 387:58–65. doi: 10.1056/NEJMra2106441
17. Larson, HJ, Jarrett, C, Eckersberger, E, Smith, DM, and Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. (2014) 32:2150–9. doi: 10.1016/j.vaccine.2014.01.081
18. Eskola, J, Duclos, P, Schuster, M, and MacDonald, NE, SW.G.o.V. Hesitancy. How to deal with vaccine hesitancy? Vaccine. (2015) 33:4215–7. doi: 10.1016/j.vaccine.2015.04.043
19. Irwin, KL, Jalloh, MF, Corker, J, Alpha Mahmoud, B, Robinson, SJ, Li, W, et al. Guinean household survey of Ebola virus disease project, attitudes about vaccines to prevent Ebola virus disease in Guinea at the end of a large Ebola epidemic: results of a national household survey. Vaccine. (2017) 35:6915–23. doi: 10.1016/j.vaccine.2017.06.026
20. Jalloh, MF, Robinson, SJ, Corker, J, Li, W, Irwin, K, Barry, AM, et al. Knowledge, attitudes, and practices related to Ebola virus disease at the end of a National Epidemic – Guinea. MMWR Morb Mortal Wkly Rep. (2015) 66:1109–15. doi: 10.15585/mmwr.mm6641a4
21. Jalloh, MF, Sengeh, P, Monasch, R, Jalloh, MB, DeLuca, N, Dyson, M, et al. National survey of Ebola-related knowledge, attitudes and practices before the outbreak peak in Sierra Leone: august 2014. BMJ Glob Health. (2017) 2:e000285. doi: 10.1136/bmjgh-2017-000285
22. Kpanake, L, Sorum, PC, and Mullet, E. Willingness to get vaccinated against Ebola: a mapping of Guinean people positions. Hum Vaccin Immunother. (2018) 14:2391–6. doi: 10.1080/21645515.2018.1480236
23. Grantz, KH, Claudot, P, Kambala, M, Kouyate, M, Soumah, A, Boum, Y, et al. Factors influencing participation in an Ebola vaccine trial among front-line workers in Guinea. Vaccine. (2019) 37:7165–70. doi: 10.1016/j.vaccine.2019.09.094
24. Jalloh, MF, Wallace, AS, Bunnell, RE, Carter, RJ, Redd, JT, Nur, SA, et al. Ebola vaccine? Family first! Evidence from using a brief measure on Ebola vaccine demand in a national household survey during the outbreak in Sierra Leone. Vaccine. (2020) 38:3854–61. doi: 10.1016/j.vaccine.2020.03.044
25. Enria, L, Lees, S, Smout, E, Mooney, T, Tengbeh, AF, Leigh, B, et al. Power, fairness and trust: understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health. (2016) 16:1140. doi: 10.1186/s12889-016-3799-x
26. Kennedy, SB, Neaton, JD, Lane, HC, Kieh, MW, Massaquoi, MB, Touchette, NA, et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: design, procedures, and challenges. Clin Trials. (2016) 13:49–56. doi: 10.1177/1740774515621037
27. Vinck, P, Pham, PN, Bindu, KK, Bedford, J, and Nilles, EJ. Institutional trust and misinformation in the response to the 2018-19 Ebola outbreak in north Kivu, DR Congo: a population-based survey. Lancet Infect Dis. (2019) 19:529–36. doi: 10.1016/S1473-3099(19)30063-5
28. Earle-Richardson, G, Erlach, E, Walz, V, Baggio, O, Kurnit, M, Camara, CA, et al. New mixed methods approach for monitoring community perceptions of Ebola and response efforts in the Democratic Republic of the Congo. Glob Health. (2021) 9:332–43. doi: 10.9745/GHSP-D-21-00144
29. Wiley, KE, Massey, PD, Cooper, SC, Wood, N, Quinn, HE, and Leask, J. Pregnant women's intention to take up a post-partum pertussis vaccine, and their willingness to take up the vaccine while pregnant: a cross sectional survey. Vaccine. (2013) 31:3972–8. doi: 10.1016/j.vaccine.2013.06.015
30. Paterson, P, Meurice, F, Stanberry, LR, Glismann, S, Rosenthal, SL, and Larson, HJ. Vaccine hesitancy and healthcare providers. Vaccine. (2016) 34:6700–6. doi: 10.1016/j.vaccine.2016.10.042
31. Nguyen, KH, Yankey, D, Lu, P-j, Kriss, JL, Brewer, NT, Razzaghi, H, et al. Report of health care provider recommendation for COVID-19 vaccination among adults, by recipient COVID-19 vaccination status and attitudes—United States, April–September 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1723–30. doi: 10.15585/mmwr.mm7050a1
32. Claude, KM, Serge, MS, Alexis, KK, and Hawkes, MT. Prevention of COVID-19 in internally displaced persons camps in war-torn north Kivu, Democratic Republic of the Congo: a mixed-methods study. Glob Health Sci Pract. (2020) 8:638–53. doi: 10.9745/GHSP-D-20-00272
33. World Health Organization, Global Ebola vaccine implementation team (GEVIT) practical guidance on the use of the Ebola vaccine in an outbreak response, Available at: https://www.afro.who.int/news/ebola-jj-vaccination-campaign-launched-jointly-rwanda-and-democratic-republic-congo (2016).
34. World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: An example of integration of research into epidemic response. Geneva: WHO (2019).
35. World Health Organization. SAGE interim recommendations on vaccination against Ebola virus disease (EVD) WHO (2019).
36. Jalloh, MF, Sengeh, P, Ibrahim, N, Kulkarni, S, Sesay, T, Eboh, V, et al. Association of community engagement with vaccination confidence and uptake: a cross-sectional survey in Sierra Leone, 2019. J Glob Health. (2022) 12:04006. doi: 10.7189/jogh.12.04006
37. Strecher, VJ, and Rosenstock, IM. The health belief model. In: Cambridge handbook of psychology, health and medicine. Eds. Baum A, Newman S, Weinman J, West R, and McManus C. (Cambridge: Cambridge University Press), 113–7 (1997).
38. Kasereka, MC, Sawatzky, J, and Hawkes, MT. Ebola epidemic in war-torn Democratic Republic of Congo 2018: Acceptability and patient satisfaction of the recombinant vesicular stomatitis virus–Zaire ebolavirus vaccine. Vaccine. (2019) 37:2174–8. doi: 10.1016/j.vaccine.2019.03.004
39. Bono, SA, Faria de Moura Villela, E, Siau, CS, Chen, WS, Pengpid, S, Hasan, MT, et al. Factors affecting COVID-19 vaccine acceptance: an international survey among Low- and middle-income countries. Vaccines. (2021) 9:515. doi: 10.3390/vaccines9050515
40. Bache, BE, Grobusch, MP, and Agnandji, ST. Safety, immunogenicity and risk-benefit analysis of rVSV-DeltaG-ZEBOV-GP (V920) Ebola vaccine in phase I-III clinical trials across regions. Future Microbiol. (2020) 15:85–106. doi: 10.2217/fmb-2019-0237
41. Spinney, L. Fighting Ebola is hard in Congo, fake news makes it harder. Science. (2019) 18:213–214. doi: 10.1126/science.aaw6657
42. Andrada, C., Ebola vaccine and the crucial role of healthcare workers, outbreak Observatory, (2018).
43. Pugliese-Garcia, M, Heyerdahl, LW, Mwamba, C, Nkwemu, S, Chilengi, R, Demolis, R, et al. Factors influencing vaccine acceptance and hesitancy in three informal settlements in Lusaka, Zambia. Vaccine. (2018) 36:5617–24. doi: 10.1016/j.vaccine.2018.07.042
44. World Health Organization, Ebola J&J Vaccination Campaign launched jointly by Rwanda and Democratic Republic of Congo, Available at: https://www.afro.who.int/news/ebola-jj-vaccination-campaign-launched-jointly-rwanda-and-democratic-republic-congo (2019).
45. Ilunga Kalenga, O, Moeti, M, Sparrow, A, Nguyen, VK, Lucey, D, and Ghebreyesus, TA. The ongoing Ebola epidemic in the Democratic Republic of Congo, 2018-2019. N Engl J Med. (2019) 381:373–83. doi: 10.1056/NEJMsr1904253
46. Murphy, R. UN peacekeeping in the Democratic Republic of the Congo and the protection of civilians. J Conflict Secur Law. (2016) 21:209–46. doi: 10.1093/jcsl/krv030
47. Grindrod, K, Waite, N, Constantinescu, C, Watson, KE, and Tsuyuki, RT. COVID-19 vaccine hesitancy: pharmacists must be proactive and move the middle. Can Pharm J. (2021) 154:133–5. doi: 10.1177/17151635211005763
48. Hyland, P, Vallieres, F, Hartman, TK, McKay, R, Butter, S, Bentall, RP, et al. Detecting and describing stability and change in COVID-19 vaccine receptibility in the United Kingdom and Ireland. PLoS One. (2021) 16:e0258871. doi: 10.1371/journal.pone.0258871
49. Metzger, WG, and Vivas-Martinez, S. Questionable efficacy of the rVSV-ZEBOV Ebola vaccine. Lancet. (2018) 391:1021. doi: 10.1016/S0140-6736(18)30560-9
50. Tafuri, S, Gallone, MS, Cappelli, MG, Martinelli, D, Prato, R, and Germinario, C. Addressing the anti-vaccination movement and the role of HCWs. Vaccine. (2014) 32:4860–5. doi: 10.1016/j.vaccine.2013.11.006
51. Gargano, LM, Thacker, N, Choudhury, P, Weiss, PS, Pazol, K, Bahl, S, et al. Predictors of administration and attitudes about pneumococcal, Haemophilus influenzae type b and rotavirus vaccines among pediatricians in India: a national survey. Vaccine. (2012) 30:3541–5. doi: 10.1016/j.vaccine.2012.03.064
52. Skrip, LA, and Galvani, AP. Next steps for Ebola vaccination: deployment in non-epidemic, High-Risk Settings. PLoS Negl Trop Dis. (2016) 10:e0004802. doi: 10.1371/journal.pntd.0004802
53. Walldorf, JA, Cloessner, EA, Hyde, TB, MacNeil, A, and Taskforce, CDCEEV. Considerations for use of Ebola vaccine during an emergency response. Vaccine. (2019) 37:7190–200. doi: 10.1016/j.vaccine.2017.08.058
54. Strategic advisory Group of Experts on immunization (SAGE). COVID-19 vaccines technical documents. Geneva: World Health Organization (2021).
55. Potluri, R, Kumar, A, Maheshwari, V, Smith, C, Oriol Mathieu, V, Luhn, K, et al. Impact of prophylactic vaccination strategies on Ebola virus transmission: a modeling analysis. PLoS One. (2020) 15:e0230406. doi: 10.1371/journal.pone.0230406
56. Carter, SE, Ahuka-Mundeke, S, Pfaffmann Zambruni, J, Navarro Colorado, C, van Kleef, E, Lissouba, P, et al. How to improve outbreak response: a case study of integrated outbreak analytics from Ebola in eastern Democratic Republic of the Congo. BMJ Glob Health. (2021) 6:6736. doi: 10.1136/bmjgh-2021-006736
Keywords: Ebola, Ebola vaccine, vaccine hesitancy, vaccine acceptance, Democratic Republic of the Congo, Ebola virus disease (EVD), vaccines
Citation: Doshi RH, Garbern SC, Kulkarni S, Perera SM, Fleming MK, Muhayangabo RF, Ombeni AB, Tchoualeu DD, Kallay R, Song E, Powell J, Gainey M, Glenn B, Mutumwa RM, Hans Bateyi Mustafa S, Earle-Richardson G, Gao H, Abad N, Soke GN, Fitter DL, Hyde TB, Prybylski D, Levine AC, Jalloh MF and Mbong EN (2023) Ebola vaccine uptake and attitudes among healthcare workers in North Kivu, Democratic Republic of the Congo, 2021. Front. Public Health. 11:1080700. doi: 10.3389/fpubh.2023.1080700
Received: 26 October 2022; Accepted: 19 June 2023;
Published: 25 July 2023.
Edited by:
Eilish Cleary, Indigenous Services Canada (ISC), CanadaReviewed by:
Setia Pramana, Politeknik Statistika STIS, IndonesiaCopyright © 2023 Doshi, Garbern, Kulkarni, Perera, Fleming, Muhayangabo, Ombeni, Tchoualeu, Kallay, Song, Powell, Gainey, Glenn, Mutumwa, Hans Bateyi Mustafa, Earle-Richardson, Gao, Abad, Soke, Fitter, Hyde, Prybylski, Levine, Jalloh and Mbong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reena H. Doshi, aHFvM0BjZGMuZ292
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.