
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 30 May 2023
Sec. Public Health Education and Promotion
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1078596
This article is part of the Research TopicThe One Health Approach in the Context of Public HealthView all 12 articles
 Samar Khaled Hassan1
Samar Khaled Hassan1 Eman Zmaily Dahmash2*
Eman Zmaily Dahmash2* Thaira Madi1
Thaira Madi1 Omar Tarawneh3
Omar Tarawneh3 Tuqa Jomhawi1
Tuqa Jomhawi1 Worood Alkhob1
Worood Alkhob1 Rola Ghanem4
Rola Ghanem4 Zina Halasa5
Zina Halasa5Objectives: To combat antimicrobial resistance, the World Health Organization (WHO) urged healthcare organizations in Low- and Middle-Income Countries (LMICs) to implement the core elements of the antimicrobial stewardship (AMS) programs. In response, Jordan took action and developed a national antimicrobial resistance action plan (NAP) in 2017 and commenced the AMS program in all healthcare facilities. It is paramount to evaluate the efforts to implement the AMS programs and understand the challenges of implementing a sustainable and effective program, in Low-Middle Income Country (LMIC) contexts. Therefore, the aim of this study was to appraise the compliance of public hospitals in Jordan to the WHO core elements of effective AMS programs after 4 years of commencement.
Methods: A cross-sectional study in public hospitals in Jordan, using the WHO AMS program core elements for LMICs was carried out. The questionnaire comprised 30 questions that covered the program’s six core elements: leadership commitment, accountability and responsibility, AMS actions, education and training, monitoring, and evaluation, and reporting and feedback. A five-point Likert scale was employed for each question.
Results: A total of 27 public hospitals participated, with a response rate of 84.4%. Adherence to core elements ranged from (53%) in the leadership commitment domain to (72%) for AMS procedure application (actions). Based on the mean score, there was no significant difference between hospitals according to location, size, and specialty. The most neglected core elements that emerged as top priority areas were the provision of financial support, collaboration, access, as well as monitoring and evaluation.
Conclusion: The current results revealed significant shortcomings in the AMS program in public hospitals despite 4 years of implementation and policy support. Most of the core elements of the AMS program were below average, which requires hospital leadership commitment, and multifaceted collaborative actions from the concerned stakeholders in Jordan.
Antimicrobial resistance (AMR) is a significant threat to global health. At least 700, 000 deaths occur yearly due to AMR (1). The antimicrobial stewardship (AMS) program is one of the key strategies that has been proposed by the World Health Organization (WHO) Global Action Plan to solve the problem of the inappropriate use of antimicrobials and hence tackle AMR (2). The WHO, through the Global Action Plan on AMR, put five strategic objectives for countries as a guiding principle in developing their own AMR national action plans: The objectives focused on improving awareness and understanding of AMR, strengthening evidence-based knowledge through surveillance and research, reducing the incidence of infection, optimizing the use of antimicrobial medicines, and increase investment in new drugs (3).
In response to this public health threat, Jordan formed, in 2017, a multi-sectoral high-level committee headed by the Ministry of Health (MOH) that launched a 4 years national AMR action plan (NAP) in Jordan (2018–2022) (4). Fulfilling the NAP objectives, Jordan took the first steps in 2018 towards establishing a national AMR surveillance system with the aim of estimating the burden and describing the trend of AMR to inform the national treatment guidelines of prevalent infectious diseases, enhance infection prevention and control (IPC) programs to curb the spread of AMR, as well as to design appropriate AMS programs (5).
Antimicrobial resistance is a serious threat to global health. Jordan, part of the Eastern Mediterranean Region (EMR), has a high prevalence of self-medication with antibiotics; recently reported as 40% (6). In response to the global call, in 2017, Jordan launched the NAP and the implementation commenced. The national AMR surveillance activities started in October 2018. Jordan’s AMR surveillance showed high resistance patterns of pathogens among inpatient and outpatient settings. High prevalence of extended spectrum beta-lactamase (ESBL) producers among Escherichia coli (47% in blood, 40% in urine) and Klebsiella pneumonia (51% in blood, 45% in urine) contributed to treatment failure using third-generation cephalosporins. This practice resulted in an increase in the number of prescriptions of carbapenems by physicians as a last resort in many complicated cases, which resulted in the emergence of carbapenem resistance which is more prominent in Klebsiella pneumonia (7–10).
Antimicrobial stewardship (AMS) is the optimization of the use of antimicrobials with the sole aim of improving patients’ health outcomes and reducing AMR while avoiding unnecessary treatment costs. Designing AMS programs is way far from waving a magic wand to create a ‘one-size fitsall’ program, yet it is a set of different complementary strategies with a common ultimate target; to curb the problem of antibiotic resistance (11). AMS has evolved over the years and is more developed in high-income countries (HICs) compared to low- and middle-income countries (LMICs). Several factors affect the implementation of AMS programs in hospitals. The size and types of care provided, as well as the complexity of antibiotic prescription, are all issues that are considered in designing an effective hospital-based program (12). Taking the special context of LMICs, the WHO has identified in 2019 six core elements to evaluate the AMS programs at the facility level in LMICs, including: (1) Leadership commitment, (2) accountability and responsibility, (3) AMS actions, (4) education and training, (5) monitoring and evaluation, and (6) reporting and feedback (13).
It is paramount to conduct a contextual evaluation of the efforts to implement AMS programs. There are numerous challenges to implementing a sustainable and effective AMS program, in Low-Middle Income Country (LMIC) contexts. It is important to understand these challenges so that the stewardship initiatives can be tailored according to the unique requirements thrown up by these healthcare facilities (14). Consequently, there is an imminent need to gather sufficient data to evaluate the core elements of AMS programs at Jordanian hospitals. Thus, its time to take a more structured approach and evaluate the AMS program core elements in Jordanian hospitals after the release of the national AMR action plan based on WHO directions. Therefore, this research aimed at assessing the implementation status of the AMS program in public hospitals in Jordan. It also aims at identifying the key barriers and challenges to the implementation of AMS interventions.
A cross-sectional study by means of a face-to-face interview was conducted between the 1st of April 2022 and the 30th of April 2022 with participants from the antimicrobial stewardship committees within the small, medium, and large public hospitals in Jordan. While public Hospitals with no established committee for antimicrobial stewardship were excluded. Therefore, 27 out of 32 public hospitals were approached. Private and Military hospitals were excluded due to the lengthy process to obtain approvals and the limited timeframe to conduct the research.
The primary data source was derived from the questionnaires filled in by the focal members of antimicrobial stewardship committees within the participating hospitals. The inclusion criteria comprised the small, medium, and large hospitals, as accredited and non-accredited hospitals within the public healthcare sector. Primary healthcare centers and ambulatory clinics were excluded.
The WHO Questionnaire was used as the research tool. It was distributed to 27 public hospitals that had an AMR committee, and a representative of the committee was interviewed and delegated to answer the questionnaire on behalf of the hospital.
A total of 27 out of the 32 public hospitals were invited to participate in the study, while 5 were excluded due to the lack of an established AMS committee. Three investigators were assigned to contact the management in each hospital through the primary investigator. Each investigator was assigned a group of hospitals and interviewed a representative of the AMS committee in each hospital. In order to unify the interview approach and to exclude any bias, the three investigators met and discussed the questions and agreed upon the description of each question as per the guidance included within the WHO tool.
During the interview, the questionnaire was read and explained to each participant. The explanation was based on the detailed description included within the WHO tool. All participants voluntarily participated in the study and were thus considered exempt from written informed consent, which was written at the beginning of the tool before starting. The study’s aim and objectives were clearly explained at the beginning of the survey questionnaire.
The questionnaire was made up of three sections. The first section focused on participants’ background and demographic information (age, gender, educational level, and job position). The second section entailed questions that described and categorized the characteristics of the hospital (sector, geographic location, hospital size, accreditation status), and the scope of services (general, specialized). The third section dealt with the availability of AMS program core elements. In this section, a five-point Likert scale was employed, and 30 questions were further divided into six focus domains (Supplementary material). The domains are: (1) Leadership commitment, (2) accountability and responsibility, (3) AMS actions, (4) education and training, (5) monitoring and evaluation, and (6) reporting and feedback. The questionnaire was adopted from the WHO practical toolkit for antimicrobial stewardship programs in healthcare facilities in low- and middle-income countries (2019) (13). The tool asked the participants about the degree to which he/she agrees with the level of implementation and availability of each question within the six core elements. The participants’ responses ranged from 1 to 5, and the average score for each area was calculated, as well as the weighted average for each question.
All study participants gave their informed consent for inclusion before they participated in the study. The study protocol was approved by the Ministry of Health in Jordan (IRB approval no. 2232).
Data was analyzed employing SPSS software, version 25. Descriptive statistics were used to describe the participants’ demographic characteristics. Data were reported as mean ± SD for normally distributed variables, while categorical data were reported as percentages (frequencies). Cronbach’s alpha was employed to measure the reliability of the tool with a set of 0.96 as the scale of reliability. Two-way ANOVA was employed to measure significance with a significance level set at 0.05.
A total of 27 healthcare providers representing the AMS committee participated in the study from 27 hospitals. Table 1 details the baseline characteristics of the participants. The majority of participants were females (85.2%) and bachelor’s degree holders (66.7%). When it comes to the experience of participants, 96% of them have more than 5 years of experience. All AMS committee focal points were either pharmacists (44.4%) or clinical pharmacists (55.6%).
Table 2 presents the distribution of participants according to the demographics of their organizations. The majority of hospitals were in the middle region of Jordan (48.1%). Moreover, 19 (70.4%) hospitals were with be capacity ranging from 100 beds to 500 beds. In terms of scope of services, 21 (77.8%) were general hospitals. With regards to accreditation status, the hospitals were almost equally distributed among accredited (51.9%) and non-accredited (48.1%) hospitals.
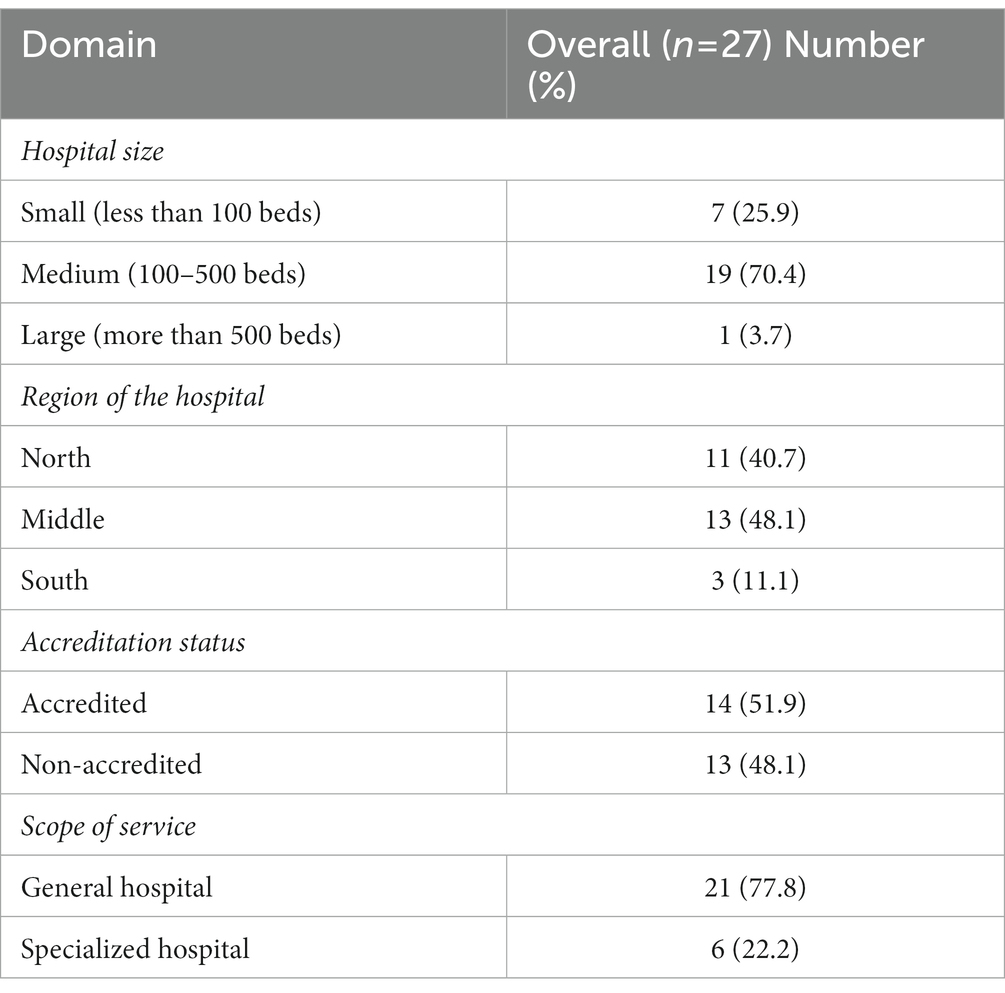
Table 2. Participants’ hospitals characteristics (the distribution was based on participants responses).
The assessment tool consisted of 30 questions based on the WHO’s six core domains of the AMS program. The results of AMS core elements application for all hospitals are showed in Table 3. The cut-off point was estimated by using the quartile percentile; P25 = 2.75, P50 = 3.10, and P75 = 3.60. Therefore, the cut-off point needed to assume a good level of AMS application in the hospital was considered as P50 (3.10). Generally, most of the domains showed low application levels (below 3.10 out of 5); the highest level was in AMS procedure application (actions; 3.61), and the lowest application was in leadership commitment (2.66).
Additionally, the correlation was used to explore the relationship between the availability of AMS core elements and other hospital characteristics such as capacity (large, medium, small), location (north, center, and south), the scope of work (general or specialized), or if the hospital was accredited or not. Generally, the analysis showed that there were no statistical correlations between the availability of core elements for the AMS program and these variables and there was no significant difference among those variables.
Table 4 details the level of application of each question under each core domain. Within the first core domain (leadership Commitment), the highest application within hospitals was for having an AMS action plan (65.86%). While prioritizing the AMS program scored (59.2%) and dedicating financial support scored the lowest (34.8%). For the second core element (Accountability and Responsibility), results showed a score of 74% among participating hospitals for the availability of a multidisciplinary AMS leadership committee with clear terms of reference. However, the presence of clearly defined collaboration between the AMS and IPC programs scored low (51.8%). The availability of AMS actions was the highest-scoring core element among the six AMS areas (72%). Results showed that almost all participating hospitals have a formulary with a list of approved antibiotics. When it comes to Education and Training, the overall compliance was low (57%) among all participating hospitals. Provision of basic training in optimal antibiotic use for healthcare professionals scored only (48.84%). Looking at the availability of the Monitoring and Evaluation core element, results showed that the monitoring of antibiotic susceptibility and resistance rates was scored below average (59.2%). As for the monitoring of the quantity and types of antibiotic use at the unit and/or facility-wide level, it was poorly applied scoring 67.34%. The analysis of the Reporting and Feedback element showed that regular evaluation and sharing of resistance rates were poorly conducted with a score of 56.98%. Developing an antibiogram for key antibiotics attained a low score that did not exceed 59.2%.
This study explored the current status of the implementation of the AMS program in public hospitals in Jordan. Overall, 84.4% of the public-hospital (27 out of 32) established an AMS committee, which was the target of this study. Using the WHO’s core elements, we found that compliance ranged from 52 to 72%, and hospitals reported having an AMS program that implemented all the core elements defined by the WHO in 2018 in response to the national action plan (4). Four years after starting the implementation of the NAP, the results of the national AMR surveillance program reflect alarmingly rising levels of multidrug-resistant pathogens (15). The findings of the surveillance reports clearly revealed the scale of the problem, yet the effectiveness of solutions and interventions that have been put in place 4 years ago is not clearly demonstrated.
In 2017, the implementation of the AMS program core elements, as aligned by the CDC, was evaluated in Jordanian Hospitals. Ababneh et al. conducted a cross-sectional study in 41 Jordanian hospitals regarding adherence to the CDC criteria for the AMS program. Among the enrolled hospitals in the study, 17.1% had an infectious diseases specialist as the head of AMS, whereas 73.2% had clinical pharmacists involved as AMS leaders (16). In comparison, the results of the current study revealed interesting findings pertinent to the structure of the AMS committee. All public hospitals had either a pharmacist or clinical pharmacist leading the AMS program. Despite variations among countries in terms of AMS committee structure, the consensus is that pharmacists/ clinical pharmacists are a fundamental part of the committee (17–19). Their role can entail developing and managing guidelines, education, monitoring compliance with antimicrobial use and auditing outcome of use (18, 20, 21). However, the role of pharmacists in Jordan was explored due to the shortage of medical professional expertise. Several studies reported that pharmacists expressed difficulties conveying their opinions and recommendations on antibiotic therapy to physicians despite frequent communications (22). Thereover, it is vital to empower pharmacists to lead AMS interventions and drive change.
The findings in this study showed an alarming result when it relates to leadership commitment, particularly in terms of the provision of financial support with a total score of 34.8%. In fact, AMS programs in hospitals need significant funding support, trained human resource, and political will (23–25). A robust level of implementation of stewardship measures in a hospital requires a committed team of experts; and the support of microbiology laboratories and hospital information systems (26). In the context of Jordanian hospitals, very little effort has been taken to look at the feasibility of implementing AMS interventions (27). The interventions that are feasible and effective in low-resource settings, may be different from those which has succeeded in larger hospitals situated in high-income countries (14). Similar findings were reported as a key challenge to effective AMS programs (28, 29) even in high-income countries like the United States (30). A study in Indonesia reported that less than 50% of hospitals allocated funds to support AMS programs (28). A multinational group of experts among EU and US hospitals agreed on core indicators to assess the AMS programs, financial support to the provision of salary funding for dedicated support of AMS activities was considered a core indicator (30). Similar results were also reported in the study of Ababneh et al. (16) where none of the assessed facilities presented a financial report endorsing ASP responsibilities.
Two encouraging findings of this study are associated with the two key questions in the action domain, which received the highest mean score: the availability of a formulary with a list of approved antibiotics (85.1%) and the availability of an approved list of restricted antibiotics (76.96%). However, the lack of adequate audit (68.82%) prevents the feasible evaluation of the effectiveness of these processes. Unfortunately, the antibiotic restriction may not stop the possible overuse of existing broad-spectrum antimicrobials (31). The availability of structure indicators is vital in AMS programs, however, process and outcome indicators of the program were not encouraging (16, 32). Particularly if that was not accompanied by continuous education and training (32, 33).
In this study, education and training scored below average (57%). Literature also supports that investment in education and training can substantially improve the outcome (23–25, 30, 34). The next logical step is to translate commitment and education into actions. Several studies reported that the main source of the misuse of antimicrobials is a lack of knowledge among healthcare professionals (11, 13, 34). A cross-sectional study in Jordan in 2021, focused on identifying the perceptions and practices among Jordanian healthcare practitioners toward AMS programs. The findings revealed a positive perception towards the program, while practices pertinent to this element were suboptimal. Further, the results showed that longer years of practice, postgraduate studies, and practice in academia sectors yielded higher perception scores (p value = 0.0335, 0.0328, and 0.0007 respectively) (35). Therefore, it is now vital that both academia and the MOH cooperatively focus on integrating antimicrobial resistance and good practices in all healthcare professional curricula and in-service educational sessions (34). Such educational sessions need also to include community awareness. A study by Alzoubi and coworkers (36), reported a low average knowledge about the use of antibiotics among 1,091 Jordanian patients attending outpatient clinics. Only 20.1% of the participants stated that antibiotics were used for bacterial infections. Moreover, several studies revealed that the prevalence of self-medication with antibiotics in Jordan remains high (37, 38), this in turn counteracts efforts of AMS in hospitals and leads to them being ineffective. Enforcement of legislation may need to be pursued to enable successful AMS programs in Jordan.
Another prominent challenge that was identified in this study was the lack of collaboration. This was evident from the answers to the following question “There is a clearly defined collaboration between the AMS and IPC programs” (score 51.8%). Effective AMS programs required a multifaceted collaboration within the organization and even outside it (39). Collaboration between IPC and AMS committees can enhance performance between the two parties, produce synergistic actions and mitigate any impediments (25, 40). Collaboration between the two committees has it rational as both serve a common purpose and they share similar expertise. The earlier study in Jordan (16) reported that the personnel who were most collaborative with the AMS team were clinicians (51.2%) and the least collaborative were microbiologists (17.1%). Therefore, this lack of collaboration will impact the effectiveness of the AMS program.
Access to IT services, laboratory, and imaging services to support AMS activities were scored low, 61.42, and 59.20%, respectively. Delays in service provision are critical for effective clinical outcome. Several reports identified the importance of timely intervention and that the reduction in the use of antibiotics is not the key attribute, reduction of inappropriate use and timely access to effective treatment is the key indicator for effective AMS programs (24, 41). Limited access imparts timeliness to the provision of care. Effective AMS program requires regular updates on relevant information that may include resistant bacteria, the incidence of antibiotic-resistant bacteria which requires easy access to IT and laboratory services (42). The IT services need to be integrated within the AMS program due to their role in the provision of support for decisions concerning antibiotic prescriptions, offering facilities for the collection and reporting of antibiotic use, as well as providing information and protocols that can be directly linked to AMS program or clinical guidelines, hence, possibly improving the rational use of antimicrobials (28, 43). An example of the use of technology that supported AMS programs is the implementation of automatic stop order (ASO), which is considered one of the technological tools by which identified medications are re-evaluated and reviewed on a consistent basis to ensure preventing unreasonable and prolonged use of drugs. With ASO, stop dates are automatically applied to an antimicrobial order when the duration of therapy is not specified. The goal is to ensure that antimicrobials are continued no longer than necessary. ASO encourages reassessment of the duration of therapy based on the patient’s response to treatment, and prescriber review of laboratory, microbiology and diagnostic imaging results after the specified length of time (44). In Jordan, the use of ASO is in its infancy.
Monitoring and evaluation (overall score of 60%), as well as reporting and feedback (overall score 59%) were also scored low in this study. Jordan public hospitals are still in the early stages of implementation and a lot of efforts need to be made to comply with such aspects. In comparison with other countries, A study in acute care hospitals in the United States reported that 79.3% of surveyed hospitals complied with monitoring of prescribing and antibiotic resistance patterns (30). Several countries employed pharmacists and clinical pharmacists to lead the monitoring and evaluation processes of the AMS programs (17–19). In a study in India, pharmacist-led model over 1 year resulted in an increase in prescribed antibiotic appropriateness from 56 to 80% and compliance to recommendations increased from 54 to 70% (21). In reference to that, as pharmacists/clinical pharmacists are leading the AMS program in Jordan, it is proposed that they are empowered to lead the monitoring and evaluation processes.
The WHO core elements for the assessment of the effectiveness of AMS programs needs to be employed to conduct periodic cross-sectional audits for the public and private sector in Jordan. This will enable tracking the progress of the implementation of the program across the country and identifying gaps within the practice. Jordan has made commitments to curb antimicrobial resistance using the NAP (4). Nevertheless, the findings of this study raise apprehensions over the implementation gaps in rendering the political commitment of the MOH into favorable actions. Consequently, the findings of this study set as a reminder to accelerate the implementation of NAP that entails AMS programs in various healthcare settings in Jordan and other LMICs. There is a need to expand training and professional education on AMR, MoH could consider focusing efforts on physicians. A recent study found that one-third of physicians reported no knowledge of any initiatives on antibiotic awareness and resistance and nearly 90% were unaware or unsure of the existence of a NAP on AMR (45). Additionally, IPC compliance and effectiveness needs to be regularly assessed in health facilities. Further, sufficient and consistent financing is key to the sustainable implementation of AMR actions in Jordan, therefore budget allocation for the implementation of AMS program is needed within each hospital.
Additionally, it is important to further explore barriers and facilitators of implementing interventions to improve antimicrobial stewardship in Jordan through a qualitative study to investigate the real implementation status of AMS program. A study reported that in 2019, approximately 59% of the antibiotics consumed nationally were from the WHO’s AWaRe classification of antibiotics (46, 47). Therefore, future study needs to consider evaluating the class of antibiotic usage.
To the best of our knowledge, this is the first study in Jordan that assess the AMS program after 4 years of the implementation of the NAP in 2017 using the WHO core elements for the AMS program for LMIC. There is a limited number of publications in LMIC assessing the effectiveness of the AMS program. However, this study had a few limitations. First, the use of self-reported data is subject to bias. Some of the participants may have overestimated or underestimated their responses to the questionnaire, impacting the accuracy of the findings. Second, the military and private hospitals were not included and therefore, the generalizability of the results is not feasible. Third, no pre-implementation data were available to attribute the observed results to the AMS program. Despite that, in terms of impact, the study described specific gaps in the AMS program in public hospitals in Jordan. These results are similarly useful for other hospitals in Jordan (private or military) as well as other LMICs. An additional limitation is pertinent to the limited number of studies that evaluated AMS programs in LMICs using the WHO core elements, particularly in the middle east, which limited our ability to compare our findings with similar programs. Owing to the study design (questionnaire with closed-end answers), we might have missed some information that could be captured using open end questions.
The current study elaborated on the level of implementation of the AMS program in public hospitals in Jordan, using the WHO core elements for the evaluation of the AMS program at the facility level in LMICs. Overall, the level of implementation of the program was not optimal and there is still much more that needs to be done. Key gaps were identified pertinent to the provision of financial support, lack of training, poor collaboration, and inadequate access to IT and diagnostic services. A well-structured monitoring, and evaluation processes as well as feedback provision were below the average. Leadership and key stakeholders’ commitment and support underpin the success of the AMS program. Overall, the results provided a baseline to monitor progress toward the national AMR action plan (NAP) in Jordan.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
All study participants gave their informed consent for inclusion before they participated in the study. The study protocol was approved by the Ministry of Health in Jordan (IRB approval no. 2232).
SH: conceived the study and project management. ED: led writing, reviewing and editing the manuscript, and validation of results. TM: conceptualization and project supervision. OT: implementation of the research and statistical analysis. TJ and WA: data collection and implementation of the research. RG: study design and implementation of the research. ZH: implementation of the research. All authors contributed to the article and approved the submitted version.
The study was supported by the Healthcare Accreditation Council, Amman, Jordan.
The authors would like to thank Jordan’s Ministry of Health for their support in approving the study and accessing public hospitals. Moreover, the authors would like to thank the WHO country office for their guidance and support in providing the WHO data collection tool.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1078596/full#supplementary-material
1. O’Neill, J . Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Government of the United Kingdom (2016).
2. World Health Assembly . Resolution 68.7: Global Action Plan on Antimicrobial Resistance [internet]. Sixty-eighth World Health Assembly. (2015). Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R7-en.pdf
3. World Health organization (WHO) . Global Action Plan on Antimicrobial Resistance [internet]. Geneva: WHO (2016).
4. Ministry of Health . National Action Plan to Combat Antimicrobial Resistance in Hashemite Kingdom of Jordan (2018–2022) [internet]. Amman: MOH (2018).
5. World Health Organization . Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020 [internet]. Geneva: World Health Organization (2020).
6. Nusair, MB, Al-azzam, S, Alhamad, H, and Momani, MY. The prevalence and patterns of self-medication with antibiotics in Jordan: a community-based study. Int J Clin Pract. (2021) 75:e13665. doi: 10.1111/ijcp.13665
7. Ny, S, Edquist, P, Dumpis, U, Gröndahl-Yli-Hannuksela, K, Hermes, J, Kling, A-M, et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J Glob Antimicrob Resist. (2019) 17:25–34. doi: 10.1016/j.jgar.2018.11.004
8. Ghulam, ND, and Hamimah, H. Emergence of extensive drug-resistant Acinetobacter baumannii in north of Jordan. African J Microbiol Res. (2011) 5:1070–4. doi: 10.5897/AJMR11.098
9. Organization WH . Implementation Manual to Prevent and Control the Spread of Carbapenem-resistant Organisms at the National and Health Care Facility Level: Interim Practical Manual Supporting Implementation of the Guidelines for the Prevention and Control of Carbapenem-res. Geneva: World Health Organization (2019).
10. Hayajneh, WA, Hajj, A, Hulliel, F, Sarkis, DK, Irani-Hakimeh, N, Kazan, L, et al. Susceptibility trends and molecular characterization of gram-negative bacilli associated with urinary tract and intra-abdominal infections in Jordan and Lebanon: SMART 2011–2013. Int J Infect Dis. (2015) 35:56–61. doi: 10.1016/j.ijid.2015.04.011
11. Nathwani, D, Varghese, D, Stephens, J, Ansari, W, Martin, S, and Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. (2019) 8:1–13. doi: 10.1186/s13756-019-0471-0
12. Li, DX, and Cosgrove, SE. “Antimicrobial stewardship: efficacy and implementation of strategies to address antimicrobial overuse and resistance,” in Antimicrobial Stewardship. eds. C. Pulcini, Ö. Ergönül, F. Can, and B. Beović (London, United Kingdom: Elsevier), (2017). 13–28.
13. World Health organization (WHO) . Antimicrobial Stewardship Programmes in Health-care Facilities in Low-and middle-income Countries: A WHO Practical Toolkit. Geneva: World Health Organization (2019).
14. Cox, JA, Vlieghe, E, Mendelson, M, Wertheim, H, Ndegwa, L, Villegas, MV, et al. Antibiotic stewardship in low-and middle-income countries: the same but different? Clin Microbiol Infect. (2017) 23:812–8. doi: 10.1016/j.cmi.2017.07.010
15. Al-Orphaly, M, Hadi, HA, Eltayeb, FK, Al-Hail, H, Samuel, BG, Sultan, AA, et al. Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa region. Msphere. (2021) 6:e00202–21. doi: 10.1128/mSphere.00202-21
16. Ababneh, MA, Issa, N, and Alkhatatbeh, M. Evaluation of Core elements of antimicrobial stewardship programs in Jordanian hospitals. Jordan. J Pharm Sci. (2017) 10:127–34. doi: 10.12816/0040700
17. Garau, J, and Bassetti, M. Role of pharmacists in antimicrobial stewardship programmes. Pharm World Sci. (2018) 40:948–52. Available from:. doi: 10.1007/s11096-018-0675-z
18. Gilchrist, M, Wade, P, Ashiru-Oredope, D, Howard, P, Sneddon, J, Whitney, L, et al. Antimicrobial stewardship from policy to practice: experiences from UK antimicrobial pharmacists. Infect Dis Ther. (2015) 4:51–64. doi: 10.1007/s40121-015-0080-z
19. Dustin, WC . Pharmacist-driven antimicrobial stewardship program in an institution without infectious diseases physician support. Am J Health Syst Pharm. (2015) 72:466–8. doi: 10.2146/ajhp140381
20. Wickens, HJ, and Jacklin, A. Impact of the hospital pharmacy initiative for promoting prudent use of antibiotics in hospitals in England. J Antimicrob Chemother. (2006) 58:1230–7. doi: 10.1093/jac/dkl405
21. Nampoothiri, V, Sudhir, AS, Joseph, MV, Mohamed, Z, Menon, V, Charani, E, et al. Mapping the implementation of a clinical pharmacist-driven antimicrobial stewardship Programme at a tertiary Care Centre in South India. Antibiotics (Basel). (2021) 10:202–229. doi: 10.3390/antibiotics10020220
22. Wong, LH, Tay, E, Heng, ST, Guo, H, Kwa, ALH, Ng, TM, et al. Hospital pharmacists and antimicrobial stewardship: a qualitative analysis. Antibiotics. (2021) 10:1441. doi: 10.3390/antibiotics10121441
23. Resman, F . Antimicrobial stewardship programs; a two-part narrative review of step-wise design and issues of controversy. Part II Ten Quest reflecting Knowl gaps issues Controv F Antimicrob Steward. Ther Adv Infect Dis. (2020) 7:2049936120945083. doi: 10.1177/2049936120945083
24. Harbarth, S, Balkhy, HH, Goossens, H, Jarlier, V, Kluytmans, J, Laxminarayan, R, et al. Antimicrobial resistance: One world, one fight! Antimicrob Resist Infect Control [internet]. (2015) 4:49. doi: 10.1186/s13756-015-0091-2
25. Assi, M, Abbas, S, Nori, P, Doll, M, Godbout, E, Bearman, G, et al. Infection prevention and antimicrobial stewardship program collaboration during the COVID-19 pandemic: a window of opportunity. Curr Infect Dis Rep. (2021) 23:15. doi: 10.1007/s11908-021-00759-w
26. MacDougall, C, and Polk, RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. (2005) 18:638–56. doi: 10.1128/CMR.18.4.638-656.2005
27. Van Dijck, C, Vlieghe, E, and Cox, JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ. (2018) 96:266. doi: 10.2471/BLT.17.203448
28. Herawati, F, Ananta, SC, Parwitha, IAA, Ressandy, SS, Rahmatin, NL, Rachmadini, NA, et al. Interview-based cross-sectional needs assessment to advance the implementation of an effective antibiotic stewardship program in Indonesian hospitals. Heal Policy OPEN [Internet]. (2020) 1:100002. doi: 10.1016/j.hpopen.2019.100002
29. Quirós, RE, Bardossy, AC, Angeleri, P, Zurita, J, Aleman Espinoza, WR, Carneiro, M, et al. Antimicrobial stewardship programs in adult intensive care units in Latin America: implementation, assessments, and impact on outcomes. Infect Control. (2022) 43:181–90. doi: 10.1017/ice.2021.80
30. Pollack, LA, van Santen, KL, Weiner, LM, Dudeck, MA, Edwards, JR, and Srinivasan, A. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network Annual Hospital Survey. Rev Infect Dis. (2016) 63:443–9. doi: 10.1093/cid/ciw323
31. Paskovaty, A, Pflomm, JM, Myke, N, and Seo, SK. A multidisciplinary approach to antimicrobial stewardship: evolution into the 21st century. Int J Antimicrob Agents. (2005) 25:1–10. doi: 10.1016/j.ijantimicag.2004.09.001
32. Alawi, MM, and Darwesh, BM. A stepwise introduction of a successful antimicrobial stewardship program: experience from a tertiary care university hospital in Western, Saudi Arabia. Saudi Med J. (2016) 37:1350. doi: 10.15537/smj.2016.12.15739
33. Saleem, Z, Godman, B, Azhar, F, Kalungia, AC, Fadare, J, Opanga, S, et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): a narrative review and the implications. Expert Rev Anti Infect Ther [Internet]. (2022) 20:71–93. doi: 10.1080/14787210.2021.1935238
34. Pulcini, C, and Gyssens, IC. How to educate prescribers in antimicrobial stewardship practices. Virulence. (2013) 4:192–202. doi: 10.4161/viru.23706
35. Khasawneh, RA, Ababneh, MA, and Al-Azzam, SI. Antimicrobial stewardship programs: perceptions and practices among Jordanian healthcare practitioners. J Pharm Heal Serv Res. (2021) 12:235–41. doi: 10.1093/jphsr/rmaa034
36. Alzoubi, K, Al Azzam, S, Alhusban, A, Mukattash, T, Al Zubaidy, S, Alomari, N, et al. An audit on the knowledge, beliefs and attitudes about the uses and side-effects of antibiotics among outpatients attending 2 teaching hospitals in Jordan. EMHJ-Eastern Mediterr Heal Journal. (2013) 19:478–84. doi: 10.26719/2013.19.5.478
37. Almaaytah, A, Mukattash, TL, and Hajaj, J. Dispensing of non-prescribed antibiotics in Jordan. Patient Prefer Adherence. (2015) 9:1389. doi: 10.2147/PPA.S91649
38. Saleh, D, Abu-Farha, R, Mukattash, TL, Barakat, M, and Alefishat, E. Views of community pharmacists on antimicrobial resistance and antimicrobial stewardship in Jordan: a qualitative study. Antibiotics. (2021) 10:384. doi: 10.3390/antibiotics10040384
39. Fishman, N . Antimicrobial stewardship. Am J Infect Control. (2006) 34:S55–63. doi: 10.1016/j.ajic.2006.05.237
40. Abbas, S, and Stevens, MP. The role of the hospital epidemiologist in antibiotic stewardship. Med Clin. (2018) 102:873–82. doi: 10.1016/j.mcna.2018.05.002
41. Laxminarayan, R, Matsoso, P, Pant, S, Brower, C, Røttingen, J-A, Klugman, K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. (2016) 387:168–75. doi: 10.1016/S0140-6736(15)00474-2
42. Hwang, S, and Kwon, KT. Core elements for successful implementation of antimicrobial stewardship programs. Infect Chemother. (2021) 53:421. doi: 10.3947/ic.2021.0093
43. Stenehjem, E, Hyun, DY, Septimus, E, Yu, KC, Meyer, M, Raj, D, et al. Antibiotic stewardship in small hospitals: barriers and potential solutions antibiotic stewardship in small hospitals. Clin Infect Dis. (2020) 70:1781–7. doi: 10.1093/cid/ciz910
45. Karasneh, RA, Al-Azzam, SI, Ababneh, MA, Basheti, IA, Al-Azzeh, O, Al Sharie, S, et al. Exploring information available to and used by physicians on antibiotic use and antibiotic resistance in Jordan. Antibiotics. (2021) 10:963. doi: 10.3390/antibiotics10080963
46. Al-Azzam, S, Mhaidat, NM, Banat, HA, Alfaour, M, Ahmad, DS, Muller, A, et al. An assessment of the impact of coronavirus disease (COVID-19) pandemic on national antimicrobial consumption in Jordan. Antibiotics. (2021) 10:690. doi: 10.3390/antibiotics10060690
Keywords: antimicrobial resistance, antimicrobial stewardship program, WHO, low- and middle-income countries (LMICs), Jordan
Citation: Hassan SK, Dahmash EZ, Madi T, Tarawneh O, Jomhawi T, Alkhob W, Ghanem R and Halasa Z (2023) Four years after the implementation of antimicrobial stewardship program in Jordan: evaluation of program’s core elements. Front. Public Health. 11:1078596. doi: 10.3389/fpubh.2023.1078596
Received: 25 October 2022; Accepted: 09 May 2023;
Published: 30 May 2023.
Edited by:
Shahzad Ali, University of Veterinary and Animal Sciences, PakistanReviewed by:
Faris El-Dahiyat, Al Ain University, United Arab EmiratesCopyright © 2023 Hassan, Dahmash, Madi, Tarawneh, Jomhawi, Alkhob, Ghanem and Halasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eman Zmaily Dahmash, ZS5kYWhtYXNoQGtpbmdzdG9uLmFjLnVr,
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.