
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 14 February 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1073824
This article is part of the Research Topic Innovations in Measurement and Evidence for Healthy Aging View all 19 articles
 Jiaofeng Gui1
Jiaofeng Gui1 Yuqing Li1
Yuqing Li1 Haiyang Liu2
Haiyang Liu2 Lei-lei Guo3
Lei-lei Guo3 Jinlong Li4
Jinlong Li4 Yunxiao Lei5
Yunxiao Lei5 Xiaoping Li6
Xiaoping Li6 Lu Sun6
Lu Sun6 Liu Yang7
Liu Yang7 Ting Yuan5
Ting Yuan5 Congzhi Wang7
Congzhi Wang7 Dongmei Zhang8
Dongmei Zhang8 Huanhuan Wei5
Huanhuan Wei5 Jing Li9
Jing Li9 Mingming Liu9
Mingming Liu9 Ying Hua10
Ying Hua10 Lin Zhang7*
Lin Zhang7*Objective: Metabolic syndrome is a common condition among middle-aged and elderly people. Recent studies have reported the association between obesity- and lipid-related indices and metabolic syndrome, but whether those conditions could predict metabolic syndrome is still inconsistent in a few longitudinal studies. In our study, we aimed to predict metabolic syndrome by obesity- and lipid-related indices in middle-aged and elderly Chinese adults.
Method: A national cohort study that consisted of 3,640 adults (≥45 years) was conducted. A total of 13 obesity- and lipid-related indices, including body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR), conicity index (CI), visceral adiposity index (VAI), Chinese visceral adiposity index (CVAI), lipid accumulation product (LAP), a body shape index (ABSI), body roundness index (BRI), and triglyceride glucose index (TyG-index) and its correlation index (TyG-BMI, TyG-WC, and TyG-WHtR), were recorded. Metabolic syndrome (MetS) was defined based on the criteria of the National Cholesterol Education Program Adult Treatment Panel III (2005). Participants were categorized into two groups according to the different sex. Binary logistic regression analyses were used to evaluate the associations between the 13 obesity- and lipid-related indices and MetS. Receiver operating characteristic (ROC) curve studies were used to identify the best predictor of MetS.
Results: A total of 13 obesity- and lipid-related indices were independently associated with MetS risk, even after adjustment for age, sex, educational status, marital status, current residence, history of drinking, history of smoking, taking activities, having regular exercises, and chronic diseases. The ROC analysis revealed that the 12 obesity- and lipid-related indices included in the study were able to discriminate MetS [area under the ROC curves (AUC > 0.6, P < 0.05)] and ABSI was not able to discriminate MetS [area under the ROC curves (AUC < 0.6, P > 0.05)]. The AUC of TyG-BMI was the highest in men, and that of CVAI was the highest in women. The cutoff values for men and women were 187.919 and 86.785, respectively. The AUCs of TyG-BMI, CVAI, TyG-WC, LAP, TyG-WHtR, BMI, WC, WHtR, BRI, VAI, TyG index, CI, and ABSI were 0.755, 0.752, 0.749, 0.745, 0.735, 0.732, 0.730, 0.710, 0.710, 0.674, 0.646, 0.622, and 0.537 for men, respectively. The AUCs of CVAI, LAP, TyG-WC, TyG-WHtR, TyG-BMI, WC, WHtR, BRI, BMI, VAI, TyG-index, CI, and ABSI were 0.687, 0.674, 0.674, 0.663, 0.656, 0.654, 0.645, 0.645, 0.638, 0.632, 0.607, 0.596, and 0.543 for women, respectively. The AUC value for WHtR was equal to that for BRI in predicting MetS. The AUC value for LAP was equal to that for TyG-WC in predicting MetS for women.
Conclusion: Among middle-aged and older adults, all obesity- and lipid-related indices, except ABSI, were able to predict MetS. In addition, in men, TyG-BMI is the best indicator to indicate MetS, and in women, CVAI is considered the best hand to indicate MetS. At the same time, TyG-BMI, TyG-WC, and TyG-WHtR performed better than BMI, WC, and WHtR in predicting MetS in both men and women. Therefore, the lipid-related index outperforms the obesity-related index in predicting MetS. In addition to CVAI, LAP showed a good predictive correlation, even more closely than lipid-related factors in predicting MetS in women. It is worth noting that ABSI performed poorly, was not statistically significant in either men or women, and was not predictive of MetS.
Metabolic syndrome (MetS) is a common condition among middle-aged and elderly people. The prevalence was 19.2% in men and 27.0% in women in China (1). The MetS consists of five major components, namely, central obesity, elevated triglyceride (TG) levels, low high-density lipoprotein cholesterol (HDL-C) levels, elevated BP, and elevated fasting plasma glucose (FPG) levels (2). Central obesity (3) and insulin resistance are considered the main pathogenesis of MetS, while chronic inflammation (4, 5) and stress response (6) also play important roles. Various pathological damages, such as glucose and lipid dysfunction in the liver, free fatty acid (FFAs) release from adipose tissues, impaired glucose uptake in muscle, and defective insulin secretion in the pancreas, can lead to central obesity or insulin resistance. At the same time, chronic inflammation and stress reaction also play an important role in this process. Figure 1 shows the main physiological changes of MetS.
Several studies (7, 8) have shown that MetS causes different degrees of damage to the cardiovascular system, digestive system, and endocrine system. Obesity can result in a significantly increased risk of death from other conditions such as cardiovascular disease, digestive tract diseases, endocrine diseases, or cancer (9). A cross-sectional analysis of 59 studies in the Middle East in 2017 found that the comprehensive estimated prevalence of MetS was 25%, and it was also a significant cause of stroke, coronary heart disease, and cardiovascular diseases (10). Gluvic et al. (11) found that for patients with cardiovascular disease, the mortality rate of patients with MetS is much higher than that of patients without MetS. Meanwhile, MetS may lead to cancer (12).
A meta-analysis of global data from 28 million subjects showed that the worldwide prevalence of MetS ranged from 12.5 to 31.4%, with the Eastern Mediterranean region and the Americas showing significantly higher prevalence than other regions (13). Among Europeans between the ages of 30 and 89 years, the incidence of MetS in men is 41%, while that in women is 38% lower than that in men (14). Adult white men were more likely to have abdominal obesity than other races, whereas white women lacked the opposite, which may explain this phenomenon (15). In the Asia Pacific region, the Philippines reported a minimum prevalence of 11.9%, while Pakistan reported a MetS prevalence of 49.0% (16). Possible factors that affect the large difference in the incidence of MetS in the Asia-Pacific region include smoking, alcohol consumption, lack of physical activity, and unhealthy diet (17). We also observed an increasing trend in the prevalence of MetS in China (13.7% in 2000–2001 and 21.3% in 2009) (18, 19). A cross-sectional study of 8,040 community residents in Jiangsu Province, China, showed that the prevalence of MetS was 35.2% in 2014 (20).
Middle-aged and older adults often suffer from a variety of chronic diseases. At the same time, their body immunity was low. Therefore, MetS was more likely to cause more serious consequences or even death. Consequently, it was necessary to find a practical index for predicting MetS. Mounting evidence demonstrates that obesity- and lipid-related indices could be associated with MetS. The current meta-analysis attempts to estimate the strength of the relationship between obesity- and lipid-related indices and MetS. A recent meta-analysis conducted with 155 patients by Ecder and Sasak (21) found that waist-to-height ratio (WHtR) in women [area under the ROC curve (AUC) = 0.815, 95% CI = 0.687–0.942] and waist circumference (WC) in men (AUC = 0.826, 95% CI = 0.741–0.911) prove to be better than other anthropometric measures for predicting MetS in renal transplant recipients. Another meta-analysis performed for the 232 participants by Khan et al. (22) found that WHtR and AVI performed best in predicting MetS. In addition, a number of meta-analysis studies (23–25) also proved the association between obesity- and lipid-related indices and MetS. Although the meta-analysis used cohort and cross-sectional studies to confirm the potential association between obesity- and lipid-related indicators and MetS, the strength of the prediction of obesity- and lipid-related indicators for MetS was not analyzed in the same field. In addition, most meta-analyses included only Western participants, and some studies considered other ethnic groups, such as Asians. Therefore, further studies in middle-aged and older adults in China are needed to determine the strength of the association between obesity- and lipid-related indices in predicting MetS in Chinese participants.
To fill these gaps, we used longitudinal data from a nationally representative sample of participants of community residents in China who were ≥45 years old and explored to examine the relationship between obesity- and lipid-related indices and incidence of MetS. In addition, this study explored the stability of the association between components of obesity- and lipid-related indicators and MetS by controlling for potential confounders.
Participants in this national cohort study were Chinese community residents aged older than 45 years who participated in the China Health and Retirement Longitudinal Study (CHARLS) survey. CHARLS is a nationally representative longitudinal survey of middle-aged and elderly Chinese adults and their spouses. The CHARLS began in 2011 with a cohort of 17,596 participants between 45 and 101 (Waves1) years and collected data in 2013 (Waves2) and 2015 (Waves3). Participants will undergo face-to-face, computer-assisted personal interviews (CAPIs), and structured questionnaires every 2 years. The study used data from participants in Waves1 and Waves3. We excluded individuals who met any of the following criteria at baseline: (1) participants with MetS, (2) one of the 13 indices missing, and (3) age/sex/educational level/marital status/current smoking/alcohol drinking/exercise/chronic diseases/live place/activities missing. In addition, we excluded participants with no follow-up data. The numbers of individuals who completed both the baseline and follow-up surveys were 3,640 for the long term (2011–2015). Figure 2 shows a flow diagram of the study individuals, follow-up, and missed follow-up.
The NCEP ATP III (2005) (26, 27) proposed the definition and diagnostic criteria of MetS. According to the standard Chinese definition (28, 29), components of MetS are divided into five categories:
(1) The waist circumference (WC) of central obesity is defined as ≥80 cm for women and ≥90 cm for men.
(2) Elevated TG levels: TG levels of ≥150 mg/dl.
(3) Low HDL-C levels: HDL-C levels of <40 mg/dl for men and <50 mg/dl for women.
(4) Elevated BP: systolic blood pressure (SBP) of ≥130 mmHg and/or diastolic blood pressure (DBP) of ≥85 mmHg or using antihypertensive therapy.
(5) Elevated FPG levels: FPG levels of ≥100 mg/dl or using antidiabetic medications or self-reported medical history of diabetes.
When three of the five listed characteristics are present, a diagnosis of MetS can be made.
In this study, we classified the participants into two groups according to gender. According to the International Diabetes Federation (IDF) (30), age, obesity, and a lack of exercise are all risk factors for MetS. Yang et al.'s (31) research shows that there is a strong link between MetS and age and place of residence. At the same time, smoking and drinking alcohol have also been confirmed to lead to MetS (32, 33). According to the previous standards (34, 35), we classified 14 chronic diseases, which were divided into three categories according to the number of chronic diseases: 0, 1–2, and 3–14. Referring to our previous research (34, 36–42), we formulated the basis for choosing the 13 obesity- and lipid-related indices. These bases included age, education, marriage, residence, drinking, smoking, activities, exercise, and chronic diseases. The classification of nine covariates is shown as follows:
(1) Age: (1) below 45–54 years, (2) 55–64 years, (3) 65–74 years, and (4) above 75 years.
(2) Education level: (1) illiterate, (2) less than elementary school, (3) high school, and (4) above vocational school.
(3) Marital status: (1) single (divorced, never married, widowed, or separated) and (2) married.
(4) Current residence: (1) rural and (2) urban.
(5) Current smoking: (1) current smokers, (2) former smokers, and (3) never smokers.
(6) Alcohol drinking: (1) never drinker, (2) less than once a month, and (3) more than once a month.
(7) Taking activities: (1) yes and (2) no.
(8) Having regular exercises: (1) no physical exercise, (2) less than regular physical exercise, and (3) regular physical exercise.
(9) Chronic diseases: (1) 0, (2) 1–3, and (3) 4–16.
At the end of exhalation and before inhalation, the circumference of the midpoint line is measured between the lowest point of the rib and the upper edge of the iliac crest with a soft ruler, which is called WC (43). It is worth noting that the remaining 12 indicators need to be calculated. BMI is calculated as the body mass (kg) divided by the square of the body height (m) (44). WHtR is defined as the WC (m) divided by the height (m) (45). The visceral adiposity index (VAI), Chinese visceral adiposity index (CVAI), lipid accumulation product (LAP), and triglyceride glucose index (TyG-index) are calculated to obtain TG and HDL through invasive examination. VAI is calculated by four indices, namely, WC, BMI, TG, and HDL (46). It is worth noting that the figures in the calculation formulas of VAI for men and women are slightly different, but they are all completed by these four same measurement indices. A body shape index (ABSI) is determined by a comprehensive calculation of WC, BMI, and height (47). Body roundness index (BRI) is calculated using two basic metrics, i.e., WC and height (48). LAP is calculated slightly differently by subtracting a number (males: 65 cm, females: 58 cm) from WC and multiplying by TG (49). The measurement method of CI is completed by WC, weight, and height (50). CVAI is calculated based on VAI to develop a more suitable measurement of the Chinese people (51). The calculation result with TG and glucose is defined as the TyG index (52). At the same time, TyG-BMI, TyG-WC, and TyG-WHtR are obtained by multiplying TyG with BMI, WC, and WHtR (53–55). The 12 indices except WC are calculated as follows:
(1) BMI = Weight/Height2
(2) WHtR = WC/Height
(3) Males: VAI
Females: VAI
(4) ABSI
(5) BRI
(6) Males: LAP = [WC (cm) – 65] × TG (mmol/L)
Females: LAP = [WC (cm) – 58] × TG (mmol/L)
(7) CI
(8) Males: CVAI = −267.93 + 0.68 × age + 0.03 × BMI (kg/m2) + 4.00 × WC (cm) + 22.00 × Log10TG (mmol/L) – 16.32 × HDL-C (mmol/L)
Females: CVAI = −187.32 + 1.71 × age + 4.32 × BMI (kg/m2) + 1.12 × WC (cm) + 39.76 × Log10TG (mmol/L) – 11.66 × HDL-C (mmol/L)
(9) TyG index = Ln [(TG (mg/dl) × glucose (mg/dl)/2)]
(10) TyG-BMI = TyG × BMI
(11) TyG-WC = TyG × WC
(12) TyG-WHtR = TyG × WHtR.
All data were analyzed using the SPSS software, version 25.0 (IBM SPSS, Armonk, NY, USA). The chi-square test was used to classify variables, and the t-test was used to determine the significant differences between continuous variables. Using these two methods, we tested the degree of correlation between covariates and 13 obesity- and lipid-related indices and gender in Table 1. In Table 2, the degree of association between these indices and the presence or absence of MetS was examined. The receiver operating characteristic (ROC) curve was drawn and AUC was calculated to check the ability of these indices to identify the MetS. The cutoff points were selected using the Youden index (sensitivity + specificity – 1). The likelihood ratio was determined, and the effect size (ES) and odds ratio (OR) for each metric were calculated to determine the predictor for each metric. The p-value obtained by the statistical significance test was generally considered statistically significant when P < 0.05 and extremely significant when P < 0.001. According to optimal cutoff values of the 13 obesity- and lipid-related indices, they were divided into two categorical variables, and OR and 95% CI of each obesity- and lipid-related indices with MetS components were recalculated. After adjusting for age, education level, marital status, current residence, current smoking, alcohol drinking, taking activities, doing regular exercise, and chronic diseases, OR and 95% CI of each obesity- and lipid-related indices with MetS components were calculated.
Table 1 shows the baseline characteristics of participants according to gender differences. The number of participants was 3,640; 54.07% were men and 45.93% were women. Notably, 10.27% were single; 94.59% were living in rural areas; 8.30% were former smokers and 36.13% were current smokers; 8.27% were drinking less than once a month and 29.12% were drinking more than once a month; 48.13% were taking activities; 50.05% had 1–2 chronic diseases and 14.56% had 3–14 chronic diseases. Meanwhile, the current residence, taking activities, doing regular exercises, and chronic disease counts were not statistically significant between the male and female subgroups (P > 0.05).
Table 2 shows the baseline characteristics of the study participants with and without MetS by sex. A total of 3,640 participants were classified into two groups according to sex. A total of 1,968 men, robust with MetS (12.86%) and without MetS (87.14%) at baseline, were included in the national cohort analysis. A total of 1,672 women, robust with MetS (25.00%) and without MetS (75.00%) at baseline, were included in the national cohort analysis. There was no significant difference in ABSI between subgroups of patients with and without MetS, whether in men or women (P > 0.05). At the same time, the remaining 12 indicators differed between subgroups of patients with and without MetS (P < 0.05).
Table 3 shows ROC analysis and AUC results of obesity- and lipid-related indices. We observed the predictive value of obesity- and lipid-related indicators for MetS by sex using the ROC. The ROC curves of each indicator in the prediction of MetS risk in men and women are shown in Figures 3, 4, respectively. In men, the largest AUC was observed for the TyG-BMI index (AUC = 0.755, Std. Error = 0.016, 95% CI = 0.723–0.787, and optimal cutoff value = 187.919). The prediabetes predictive values were similar to the CVAI (AUC = 0.752, Std. Error = 0.016, 95% CI = 0.721–0.784, and optimal cutoff value = 85.284), TyG-WC (AUC = 0.749, Std. Error = 0.016, 95% CI = 0.717–0.781, and optimal cutoff value = 705.405), and LAP (AUC = 0.745, Std. Error = 0.016, 95% CI = 0.713–0.777, and optimal cutoff value = 16.896) indices. In contrast, the AUC of ABSI did not reach statistical significance (P = 0.055). In women, the largest AUC was observed for the CVAI index (AUC = 0.687, Std. Error = 0.015, 95% CI = 0.658–0.716, and optimal cutoff value = 86.785). The prediabetes predictive values were similar to the LAP (AUC = 0.674, Std. Error = 0.015, 95% CI = 0.644–0.703, and optimal cutoff value = 23.723), TyG-WC (AUC = 0.674, Std. Error = 0.015, 95% CI = 0.645–0.703, and optimal cutoff value = 644.630), and TyG-WHtR (AUC = 0.663, Std. Error = 0.015, 95% CI = 0.633–0.692, and optimal cutoff value = 4.265) indices. As in men, the AUC of ABSI in women was not statistically significant (P = 0.009).
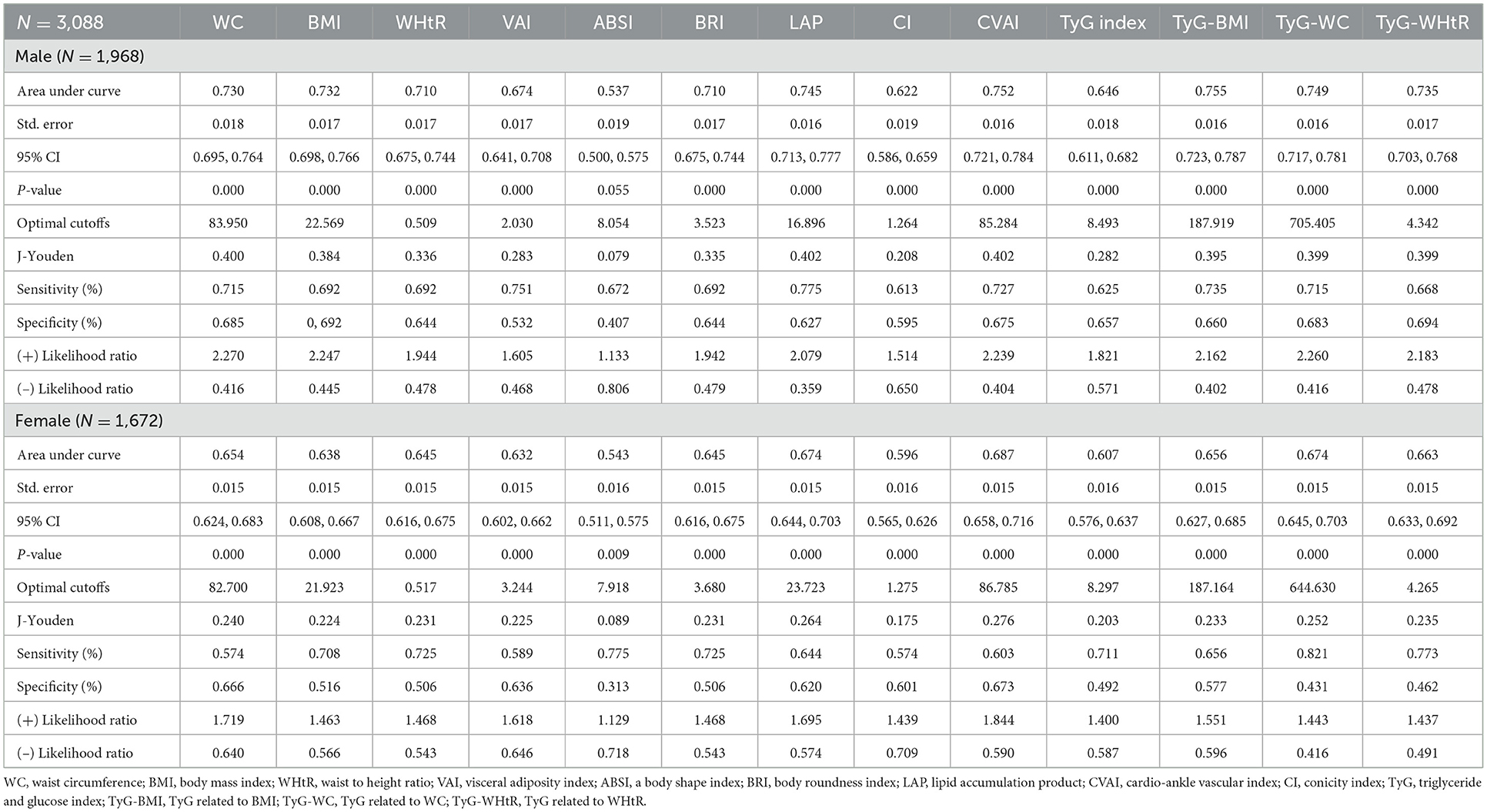
Table 3. Cutoff value between area under curve, sensitivity, and specificity for obesity- and lipid-related indices to detect metabolic syndrome (CHARLS2011–2015) by sex.
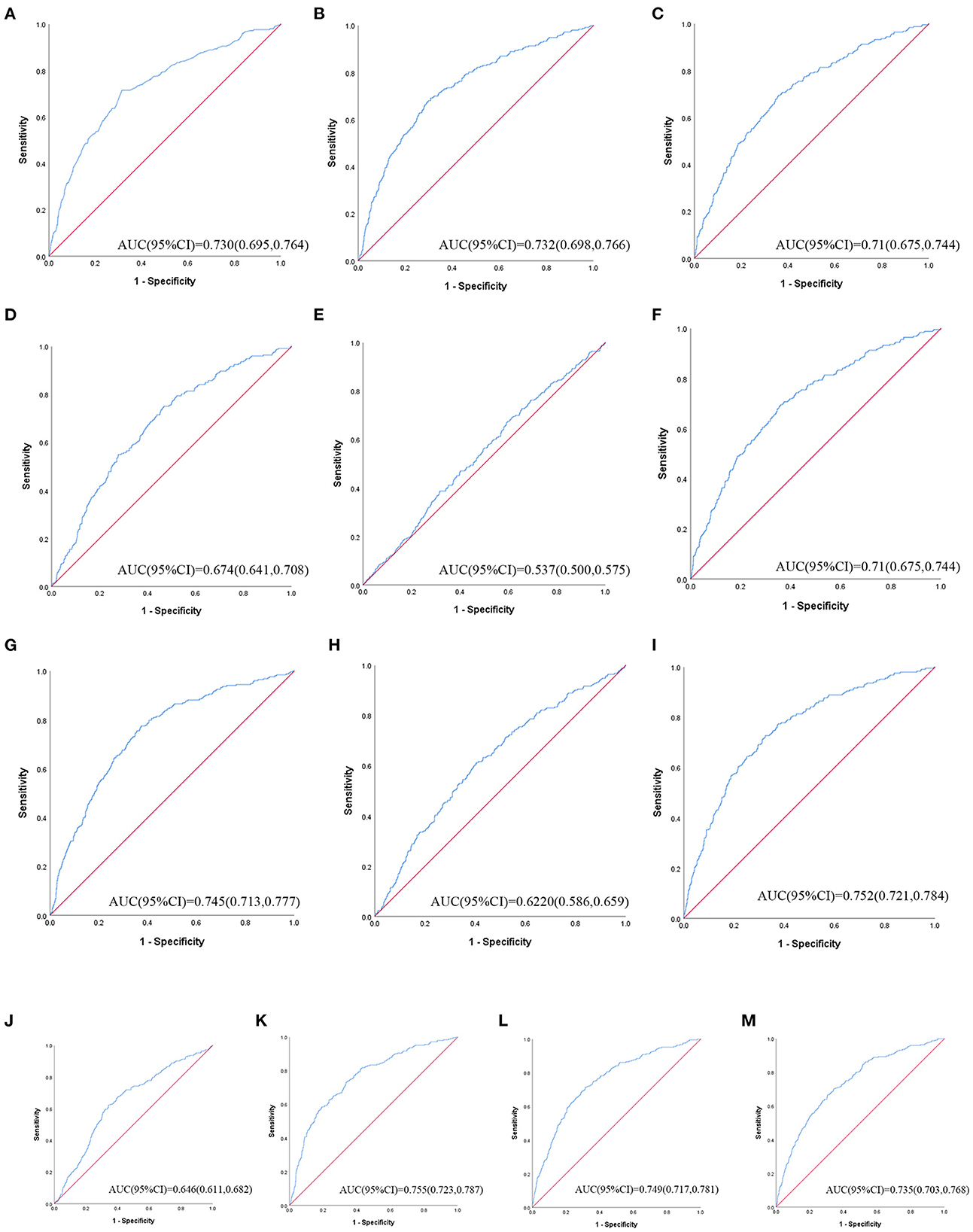
Figure 3. The ROC curves of each indicator in the prediction of MetS risk in male. (A) = WC, (B) = BMI, (C) = WHtR, (D) = VAI, (E) = ABSI, (F) = BRI, (G) = LAP, (H) = CI, (I) = CVAI, (J) = TyG-index, (K) = TyG-BMI, (L) = TyG-WC, (M) = TyG-WHtR.
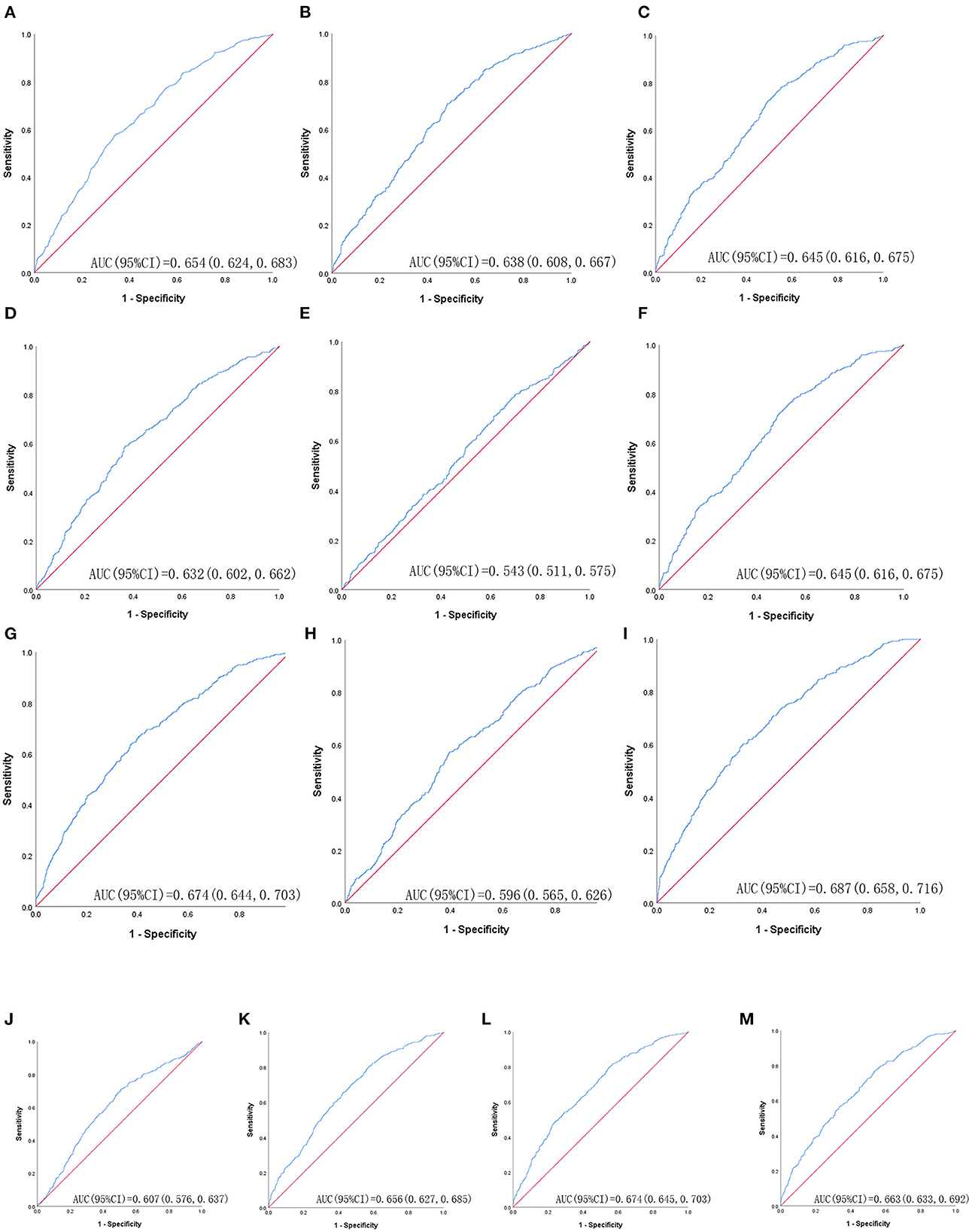
Figure 4. The ROC curves of each indicator in the prediction of MetS risk in female. (A) = WC, (B) = BMI, (C) = WHtR, (D) = VAI, (E) = ABSI, (F) = BRI, (G) = LAP, (H) = CI, (I) = CVAI, (J) = TyG-index, (K) = TyG-BMI, (L) = TyG-WC, (M) = TyG-WHtR.
Table 4 shows that MetS and its components increase gradually with the increase of obesity- and lipid-related indices in both sexes. For instance, in men, a unit increase in WC was associated with a 5.574-fold increased odds of metabolic syndrome (aOR: 5.574; 95% CI: 4.133–7.518), and a unit increase in BMI was associated with a 5.137-fold increase in odds of metabolic syndrome (aOR: 5.137; 95% CI: 3.820–6.907). In women, a unit increase in WC was associated with a 2.764-fold increase in odds of metabolic syndrome (aOR: 2.764; 95% CI: 2.195–3.480), and a unit increase in BRI was associated with a 3.045-fold increased odds of metabolic syndrome (aOR: 3.045; 95% CI: 2.363-3.925). The OR and 95% CI of Table 4 are used to draw Figures 5, 6. It is interesting that OR values were generally higher in men than in women for risk factors of MetS. Meanwhile, the OR value of ABSI was much lower than that of the other 13 indices.
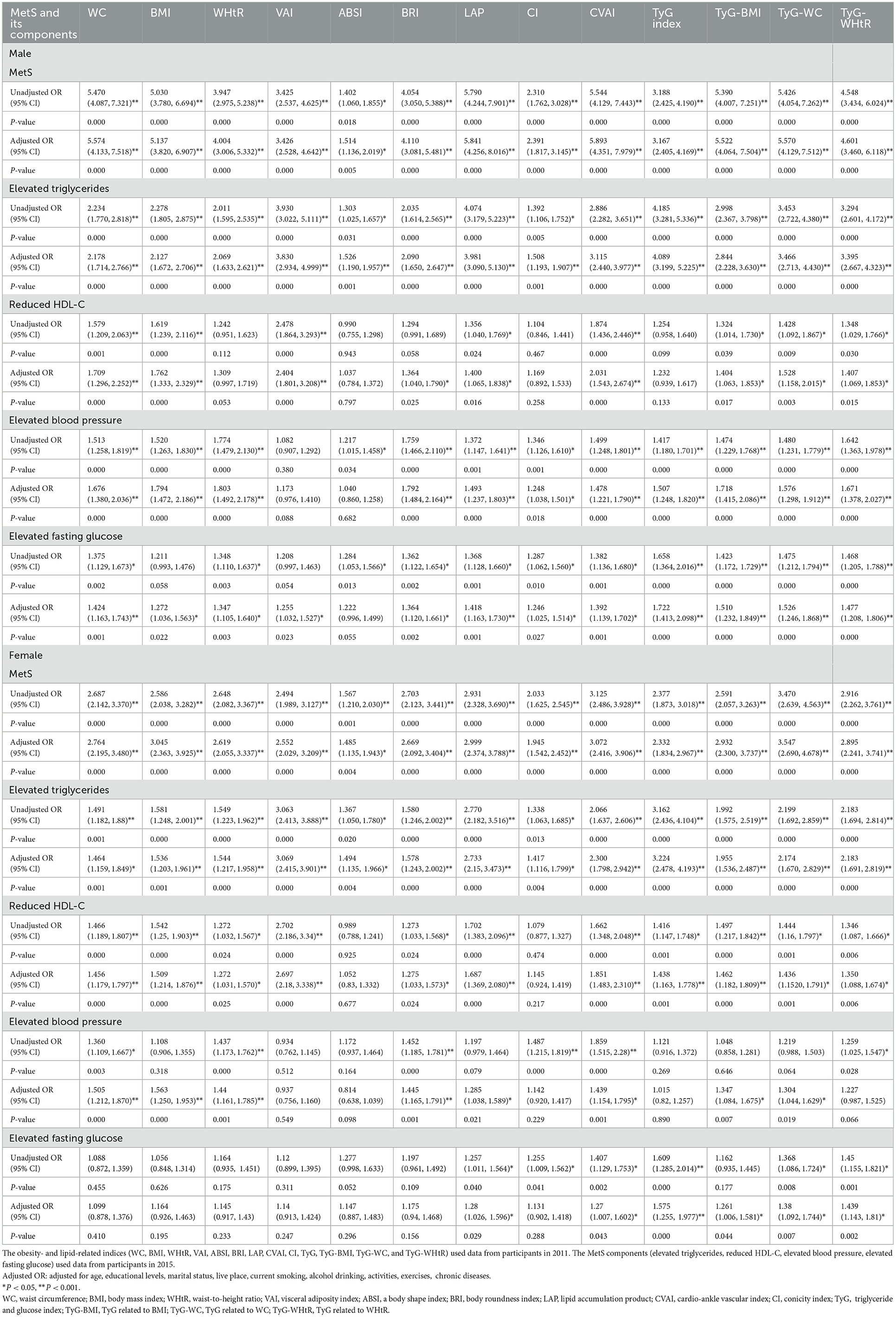
Table 4. Associations of obesity- and lipid-related indices with MetS (CHARLS2011–2015) and its components.
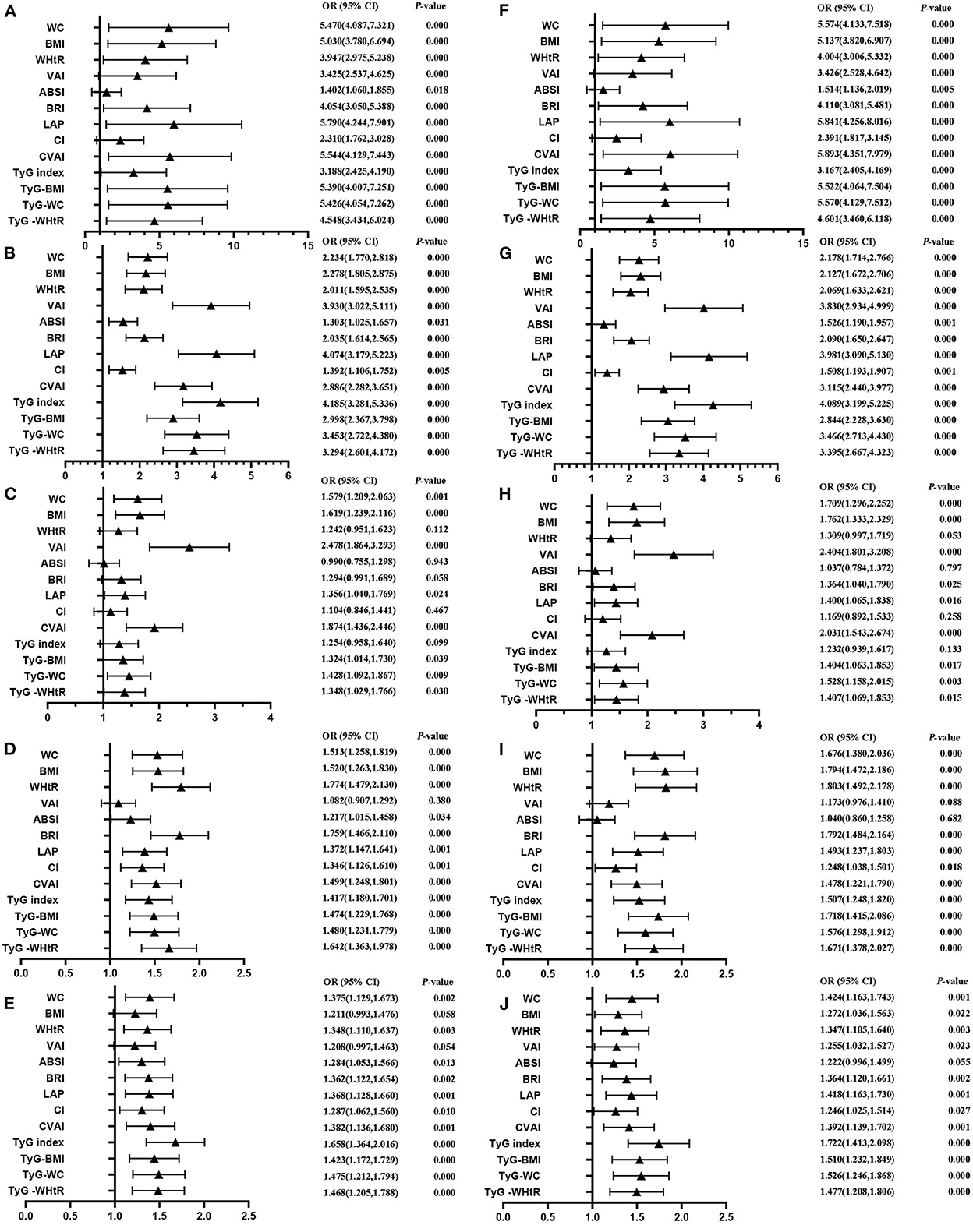
Figure 5. Forest diagram of OR before and after adjustment of confounding factors for male. (A) Mets unadjust, (B) Elevated triglycerides unadjusted, (C) Reduced HDL-C unadjusted, (D) Elevated blood pressure unadjusted, (E) Elevated fasting glucose unadjusted, (F) Mets adjusted, (G) Elevated triglycerides adjusted, (H) Reduced HDL-C adjusted, (I) Elevated blood pressure adjusted, (J) Elevated fasting glucose adjusted. Adjusted OR: Adjusted for age, educational levels, marital status, live place, current smoking, alcohol drinking, activities, exercises, chronic diseases.
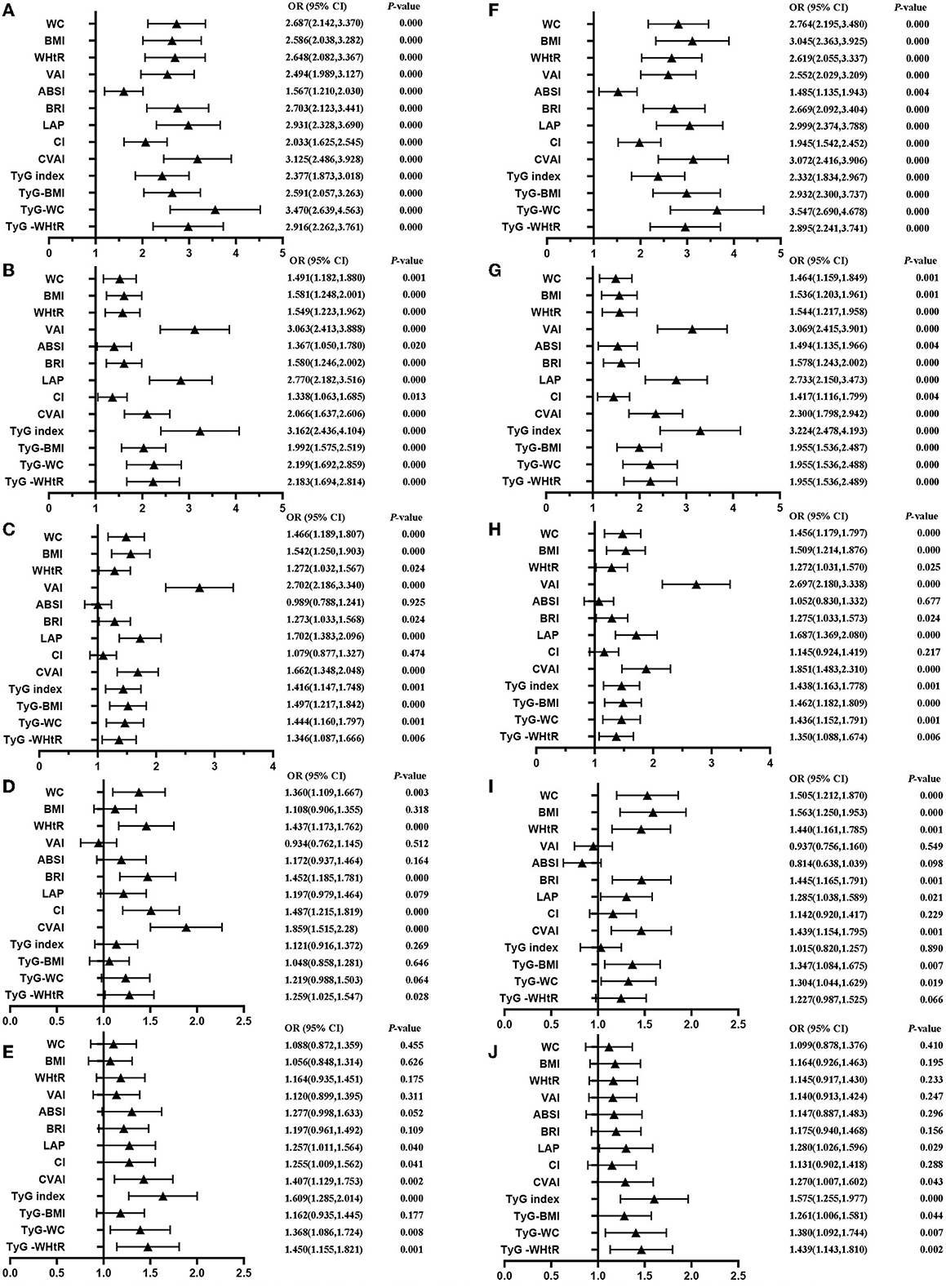
Figure 6. Forest diagram of OR before and after adjustment of confounding factors for female. (A) Mets unadjust, (B) Elevated triglycerides unadjusted, (C) Reduced HDL-C unadjusted, (D) Elevated blood pressure unadjusted, (E) Elevated fasting glucose unadjusted, (F) Mets adjusted, (G) Elevated triglycerides adjusted, (H) Reduced HDL-C adjusted, (I) Elevated blood pressure adjusted, (J) Elevated fasting glucose adjusted. Adjusted OR: Adjusted for age, educational levels, marital status, live place, current smoking, alcohol drinking, activities, exercises, chronic diseases.
According to the diagnostic criteria of MetS issued in 2006, this study evaluated the predictive power of 13 obesity- and lipid-related parameters to identify the risk of MetS in middle-aged and elderly Chinese adults. This article is an original study. To the best of our knowledge, no previous study has compared the ability of these 13 obesity- and lipid-related factors to predict MetS in middle-aged and elderly Chinese populations. There are 3,640 participants in this study. Among 3,640 individuals, the prevalence of MetS was 18.43%. Among 1,968 male participants, 253 (12.86%) were diagnosed with MetS. Among 1,672 female participants, 418 (25.00%) were diagnosed with MetS. According to the meta-analysis report (1), the prevalence of MetS in China was 24.5% in 2016, among which the prevalence of MetS increases with age (15–39 years: 13.9%; 40–59 years: 26.4%; 60 years: 32.4%). Ranasinghe et al.'s (16) research report says that the prevalence of MetS in China is increasing yearly. Based on these studies, further studies are needed to establish a single index for predicting the occurrence of MetS in the middle-aged and elderly population in China.
This national cohort study found that the correlation between ABSI and MetS was far lower than the other 12 obesity- and lipid-related indices. Through ROC analysis of obesity- and blood lipid-related indicators, the results for the ABSI AUC did not reach statistical significance (P = 0.055) in men, similar to the results (P= 0.009) obtained in women. At the same time, some studies (56–58) also show that BRI is better than ABSI in predicting MetS. According to Ji et al. (59), this result can be explained by the following viewpoints: ABSI is highly clustered around the mean value with relatively slight variance, which makes ABSI perform poorly in predicting chronic diseases.
Among the other 12 indices, BMI, WHtR, WC, LAP, and CVAI have received special attention. Ashwell et al. (60) reported that WHtR outperformed WC and BMI in detecting cardiac metabolic risk factors in both men and women. Similarly, in a systematic literature review in 2016, Corrêa et al. (61) also shows that WHtR has better performance than WC and BMI in evaluating MetS. Alves et al.'s (62) study shows that LAP, WC, and WHtR indicators identify MetS in older women and can be used to assess and monitor MetS individually or collectively.
Tyg-related factors such as the TyG index, TYG-BMI, TYG-WC, and TYG-WHTR values may provide a broader basis for assessing the association between MetS and obesity- and lipid-related indicators. Thus, TyG-related factors have also recently been suggested as a method for estimating metabolic disorders. Ahn et al.'s (63) study shows that the TyG index provided a good standard for identifying people with pre-diabetes/diabetes. In the meantime, most of the studies (64–66) also showed a significant correlation between TyG-related factors and pre-diabetes/diabetes. As mentioned earlier, elevated FPG levels were one of the diagnostic criteria for MetS, and pre-diabetes/diabetes leads to elevated FPG levels. Therefore, there must be an association between TyG-related factors and MetS. Ferreira et al. (67) identified the TyG-related factors as an important tool for predicting MetS.
Indeed, in this study, the TyG-related factors were more efficient compared to these other markers. According to the results of this study, obesity- and lipid-related indices are different in predicting MetS because of gender differences. In general, an AUC closer to 1 indicates better predictive power, while an AUC of 0.55 or less indicates no better predictive power than chance. Among men, TyG-BMI performed best, followed by CVAI, TyG-WC, and LAP. TyG-BMI is a surrogate marker used to identify insulin resistance (68). In the ROC analysis of obesity- and blood lipid-related indicators, the largest AUC was observed for the TyG-BMI index (AUC = 0.755, Std. Error = 0.016, 95% CI = 0.723–0.787, and optimal cutoff value = 187.919). Raimi et al. (69) suggested that the product of TyG-related factors and the anthropometric index could improve the identification and prediction of MetS. This view is consistent with this article.
In contrast, according to the report (70), VAI has moderate to high accuracy in screening MetS diagnostic tests. VAI was a reliable index to evaluate the visceral fat function, as proposed by Amato et al. (46). A multi-center cross-sectional study (58) was conducted in Guizhou Province in southwest China, and it was found that LAP and VAI, the visceral obesity markers, were the most effective predictors of MetS, while ABSI and CI were the weakest indicators for screening MetS. Interestingly, many studies (71, 72) have also demonstrated that VAI performs well in predicting MetS.
Considerable evidence suggests that VAI plays a significant role in predicting MetS (73, 74). However, there were significant differences in body fat distribution between ethnic groups. According to the distribution characteristics of Asian body fat, Xia et al. developed CVAI to evaluate the visceral fat area of the Chinese people (51). CVAI is better than VAI in predicting the MetS in middle-aged and older adults in China. Among women, CVAI performed best, followed by LAP, TyG-WC, and TyG-WHtR. In the ROC analysis of obesity- and blood lipid-related indicators, the largest AUC was observed for the CVAI index (AUC = 0.687, Std. Error = 0.015, 95% CI = 0.658–0.716, and optimal cutoff value = 86.785).
Similarly, LAP performed well in predicting metabolic synthesis. LAP was used as an indicator to predict insulin resistance (75, 76), so there is also a significant association between LAP and MetS. In this study, for men, the AUC of LAP (AUC = 0.745, Std. Error = 0.016, 95% CI = 0.713–0.777, and optimal cutoff value = 16.896) was the largest of the other factors in the ROC analysis of obesity- and lipid-related measures, except for the TyG-related factors and CVAI. For women, the AUC of LAP (AUC = 0.674, Std. Error = 0.015, 95% CI = 0.644–0.703, and optimal cutoff value = 23.723) was second only to that of CVAI among the other factors. The rest of the studies (77–79) also showed that LAP works well in predicting MetS.
In this study, 13 obesity- and lipid-related indices were converted into two-category variables according to the values in Table 3. Table 4 is based on the transformed variables. In general, a higher OR indicates a greater risk factor. In Table 4, the OR value of ABSI is much lower than that of the remaining 12 indices in both sexes, which is consistent with the conclusion drawn in Table 3. ABSI is not suitable as a predictor of MetS. Stefanescu A showed in the study that ABSI is much inferior to other indicators for predicting MetS in Peruvian adults (80). Khan et al.'s study showed that WHtR and VAI outperformed ABSI in predicting MetS (22). At the same time, many studies (22, 81, 82) are consistent with the viewpoint of this study. It is interesting that the OR value of men is greater than that of women before and after adjustment. This suggests that an increase in obesity factors may make men more susceptible to MetS. There are many reasons for this. Men are more vulnerable to tobacco damage than women. Many studies (83, 84) have shown that tobacco is more likely to cause sugar and lipid metabolism disorders in the elderly. This may be associated with cigarette smoke impairing the reverse transport of cholesterol (85). At the same time, men are more prone to alcohol abuse than women in daily life. Excessive drinking can significantly increase the risk of MetS (86).
This study has several advantages. This study was based on a nationwide cohort study of middle-aged and older community residents, with participants aged 45 years or older. It compared the effect of different obesity- and lipid-related indices of the MetS and its component symptom. Previous studies have used only a set of single indices to predict the incidence of MetS. It helped us to understand the different obesity- and lipid-related indices on the incidence of MetS. There are several limitations to this study. Many participants were excluded due to missing data, and further studies should gather more complete data.
Among middle-aged and older adults, all obesity- and lipid-related indices, except ABSI, were able to predict MetS after adjustment for age, sex, educational status, history of smoking, taking activities, doing regular exercises, and chronic diseases. In addition, in men, TyG-BMI is the best indicator to predict MetS, and in women, CVAI is considered the best indicator to predict MetS. LAP had performed well in predicting MetS in both men and women.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All data are openly published as microdata at http://charls.pku.edu.cn/ with no direct contact with all participants. Approval for this study was given by the Medical Ethics Committee of Wannan Medical College (approval number 2021–3). The patients/participants provided their written informed consent to participate in this study.
LZ: conceived the research. JG: wrote the paper and analyzed the data. JG, YLi, HL, LG, JinlL, YLe, XL, LS, LY, TY, CW, DZ, HW, JingL, ML, YH, and LZ: revised the paper. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
CHARLS was supported by the NSFC (Grant Nos: 70910107022, 71130002), the National Institute on Aging (R03-TW008358-01; R01-AG037031-03S1), the World Bank (Grant No: 7159234), and the Support Program for Outstanding Young Talents from the Universities and Colleges of Anhui Province for LZ (gxyqZD2021118).
The authors thank the members of the research as well as all participants for their contribution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; WHtR, waist-height ratio; CI, conicity index; VAI, visceral adiposity index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product; ABSI, a body shape index; BRI, body roundness index; TyG, triglyceride glucose; TyG-BMI, triglyceride glucose-body mass index; TyG-WC, triglyceride glucose-waist circumference; TyG-WHtR, triglyceride glucose-waist-height ratio; ROC, receiver operator curve; AUC, area under curve; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose blood glucose; CHARLS, China Health and Retirement Longitudinal Study; CAPI, computer-aided personal interview; IDF, international diabetes federation; OR, odds ratio; ES, effect size; FFA, free fatty acid; IR, insulin resistance; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III.
1. Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. (2016) 16:296. doi: 10.1186/s12889-016-2870-y
2. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
3. Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. (2005) 12:295–300. doi: 10.5551/jat.12.295
4. Park K, Steffes M, Lee DH, Himes JH, Jacobs DR Jr. Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. (2009) 52:2337–44. doi: 10.1007/s00125-009-1486-5
5. DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR. Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr Hypertens Rep. (2010) 12:93–8. doi: 10.1007/s11906-010-0095-5
6. Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. (2018) 40:215–24. doi: 10.1007/s00281-017-0666-5
7. Varghese JF, Patel R, Yadav UCS. Novel insights in the metabolic syndrome-induced oxidative stress and inflammation-mediated atherosclerosis. Curr Cardiol Rev. (2018) 14:4–14. doi: 10.2174/1573403X13666171009112250
8. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. (2019) 129:4050–7. doi: 10.1172/JCI129194
9. Rasaei N, Mirzababaei A, Arghavani H, Tajik S, Keshavarz SA, Yekaninejad MS, et al. A comparison of the sensitivity and specificity of anthropometric measurements to predict unhealthy metabolic phenotype in overweight and obese women. Diabetes Metab Syndr. (2018) 12:1147–53. doi: 10.1016/j.dsx.2018.06.023
10. Ansarimoghaddam A, Adineh HA, Zareban I, Iranpour S, HosseinZadeh A, Kh F. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab Syndr. (2018) 12:195–201. doi: 10.1016/j.dsx.2017.11.004
11. Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link between ‘. Curr Vasc Pharmacol. (2017) 15:30–9. doi: 10.2174/1570161114666161007164510
12. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. (2012) 35:2402–11. doi: 10.2337/dc12-0336
13. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. (2022) 188:109924. doi: 10.1016/j.diabres.2022.109924
14. Gao W. Does the constellation of risk factors with and without abdominal adiposity associate with different cardiovascular mortality risk? Int J Obes. (2008) 32:757–62. doi: 10.1038/sj.ijo.0803797
15. Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll f Cardiol. (2013) 62:697–703. doi: 10.1016/j.jacc.2013.05.064
16. Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. (2017) 17:101. doi: 10.1186/s12889-017-4041-1
17. Low WY, Lee YK, Samy AL. Non-communicable diseases in the Asia-Pacific region: prevalence, risk factors and community-based prevention. Int J Occup Med Environ Health. (2015) 28:20–6. doi: 10.2478/s13382-014-0326-0
18. Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. (2005) 365:1398–405. doi: 10.1016/S0140-6736(05)66375-1
19. Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. (2013) 57:867–71. doi: 10.1016/j.ypmed.2013.09.023
20. Tian T, Zhang J, Zhu Q, Xie W, Wang Y, Dai Y. Predicting value of five anthropometric measures in metabolic syndrome among Jiangsu Province, China. BMC Public Health. (2020) 20:1317. doi: 10.1186/s12889-020-09423-9
21. Ecder SA, Sasak G. Body shape index predicts metabolic syndrome and insulin resistance in renal transplant recipients. Nutrients. (2019) 51:2334–8. doi: 10.1016/j.transproceed.2019.01.172
22. Khan SH, Shahid R, Fazal N, Ijaz A. Comparison of various abdominal obesity measures for predicting metabolic syndrome, diabetes, nephropathy, and dyslipidemia. Obes Surg. (2019) 29:1159–64. doi: 10.29271/jcpsp.2019.12.1159
23. Ching YK, Chin YS, Appukutty M, Gan WY, Chan YM. Comparisons of conventional and novel anthropometric obesity indices to predict metabolic syndrome among vegetarians in Malaysia. Sci Rep. (2020) 10:20861. doi: 10.1038/s41598-020-78035-5
24. Nagayama D, Fujishiro K, Tsuda S, Watanabe Y, Yamaguchi T, Suzuki K, et al. Enhanced prediction of renal function decline by replacing waist circumference with “A Body Shape Index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes. (2022) 46:564–73. doi: 10.1038/s41366-021-01026-7
25. Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, et al. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition. (2019) 57:194–201. doi: 10.1016/j.nut.2018.05.004
26. Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome in urban Asian Indian adults–a population study using modified ATP III criteria. Diabetes Res Clin Pract. (2003) 60:199–204. doi: 10.1016/S0168-8227(03)00060-3
27. De Luis DA, Lopez Mongil R, Gonzalez Sagrado M, Lopez Trigo JA, Mora PF, Castrodeza Sanz J. Prevalence of metabolic syndrome with International Diabetes Federation Criteria and ATP III Program in patients 65 years of age or older. J Nutr Health Aging. (2010) 14:400–4. doi: 10.1007/s12603-010-0087-9
28. Unwin N, Bhopal R, Hayes L, White M, Patel S, Ragoobirsingh D, et al. A comparison of the new international diabetes federation definition of metabolic syndrome to WHO and NCEP definitions in Chinese, European and South Asian origin adults. Ethn Dis. (2007) 17:522–8.
29. Song P, Yu J, Chang X, Wang M, An L. Prevalence and correlates of metabolic syndrome in Chinese children: The China Health and Nutrition Survey. Nutrients. (2017) 9:79. doi: 10.3390/nu9010079
30. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
31. Yang BY, Liu KK, Markevych I, Knibbs LD, Bloom MS, Dharmage SC, et al. Association between residential greenness and metabolic syndrome in Chinese adults. Environ Int. (2020) 135:105388. doi: 10.1016/j.envint.2019.105388
32. Balhara YP. Tobacco and metabolic syndrome. Indian J Endocrinol Metab. (2012) 16:81–7. doi: 10.4103/2230-8210.91197
33. Yoon SJ, Kim SK, Lee NY, Choi YR, Kim HS, Gupta H, et al. Effect of Korean Red Ginseng on metabolic syndrome. J Ginseng Res. (2021) 45:380–9. doi: 10.1016/j.jgr.2020.11.002
34. Zhang L, Liu K, Li H, Li D, Chen Z, Zhang LL, et al. Relationship between body mass index and depressive symptoms: the “fat and jolly” hypothesis for the middle-aged and elderly in China. Biomed Res Int. (2016) 16:1201. doi: 10.1186/s12889-016-3864-5
35. Chang HH, Yen ST. Association between obesity and depression: evidence from a longitudinal sample of the elderly in Taiwan. Aging Ment Health. (2012) 16:173–80. doi: 10.1080/13607863.2011.605053
36. Zhang L, Li JL, Zhang LL, Guo LL Li H, Li D. No association between C-reactive protein and depressive symptoms among the middle-aged and elderly in China: Evidence from the China Health and Retirement Longitudinal Study. Medicine. (2018) 97:e12352. doi: 10.1097/MD.0000000000012352
37. Zhang L, Li JL, Zhang LL, Guo LL, Li H, Li D. Association and interaction analysis of body mass index and triglycerides level with blood pressure in elderly individuals in China. BioMed Res Int. (2018) 2018:8934534. doi: 10.1155/2018/8934534
38. Zhang L, Li JL, Zhang LL, Guo LL Li H, Yan W, et al. Relationship between adiposity parameters and cognition: the “fat and jolly” hypothesis in middle-aged and elderly people in China. Medicine. (2019) 98:e14747. doi: 10.1097/MD.0000000000014747
39. Zhang L, Li JL, Guo LL Li H, Li D, Xu G. The interaction between serum uric acid and triglycerides level on blood pressure in middle-aged and elderly individuals in China: result from a large national cohort study. BMC Cardiovasc Disord. (2020) 20:174. doi: 10.1186/s12872-020-01468-3
40. Ding L, Liang Y, Tan ECK, Hu Y, Zhang C, Liu Y, et al. Smoking, heavy drinking, physical inactivity, and obesity among middle-aged and older adults in China: cross-sectional findings from the baseline survey of CHARLS 2011-2012. BMC Public Health. (2020) 20:1062. doi: 10.1186/s12889-020-08625-5
41. Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Combined effect of famine exposure and obesity parameters on hypertension in the midaged and older adult: a population-based cross-sectional study. BioMed Res Int. (2021) 2021:5594718. doi: 10.1155/2021/5594718
42. Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Individual and combined association analysis of famine exposure and serum uric acid with hypertension in the mid-aged and older adult: a population-based cross-sectional study. Biomed Res Int. (2021) 21:420. doi: 10.1186/s12872-021-02230-z
43. Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Mediator or moderator? The role of obesity in the association between age at menarche and blood pressure in middle-aged and elderly Chinese: a population-based cross-sectional study. BMJ Open. (2022) 12:e051486. doi: 10.1136/bmjopen-2021-051486
44. Zhou B. Prospective study for cut-off points of body mass index in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi. (2002) 23:431–4. doi: 10.3760/cma.j.issn.0254-6450.2002.06.107
45. Zhang FL, Ren JX, Zhang P, Jin H, Qu Y, Yu Y, et al. Strong Association of Waist Circumference (WC), Body Mass Index (BMI), Waist-to-Height Ratio (WHtR), and Waist-to-Hip Ratio (WHR) with Diabetes: a population-based cross-sectional study in Jilin Province, China. J Diabetes Res. (2021) 2021:8812431. doi: 10.1155/2021/8812431
46. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. (2010) 33:920–2. doi: 10.2337/dc09-1825
47. Krakauer NY, Krakauer JC, A. new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. (2012) 7:e39504. doi: 10.1371/journal.pone.0039504
48. Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. (2013) 21:2264–71. doi: 10.1002/oby.20408
49. Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. (2005) 5:26. doi: 10.1186/1471-2261-5-26
50. Rato Q. Conicity index: an anthropometric measure to be evaluated. Rev Port Cardiol. (2017) 36:365–6. doi: 10.1016/j.repce.2017.04.006
51. Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. (2016) 6:38214. doi: 10.1038/srep38214
52. Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. (2014) 61:533–40. doi: 10.1016/j.endoen.2014.11.006
53. Lee JJ, Ahn J, Hwang J, Han SW, Lee KN, Kim JB, et al. Relationship between uric acid and blood pressure in different age groups. Clin Hypertens. (2015) 21:14. doi: 10.1186/s40885-015-0022-9
54. Selvi NMK, Nandhini S, Sakthivadivel V, Lokesh S, Srinivasan AR, Sumathi S. Association of triglyceride-glucose index (TyG index) with HbA1c and insulin resistance in type 2 diabetes mellitus. Maedica. (2021) 16:375–81. doi: 10.26574/maedica.2021.16.3.375
55. Jiang C, Yang R, Kuang M, Yu M, Zhong M, Zou Y. Triglyceride glucose-body mass index in identifying high-risk groups of pre-diabetes. Lipids Health Dis. (2021) 20:161. doi: 10.1186/s12944-021-01594-7
56. Calderón-García JF, Roncero-Martín R, Rico-Martín S, De Nicolás-Jiménez JM, López-Espuela F, Santano-Mogena E, et al. Effectiveness of body roundness index (BRI) and a body shape index (ABSI) in predicting hypertension: a systematic review and meta-analysis of observational studies. Int J Environ Res Public Health. (2021) 18:11607. doi: 10.3390/ijerph182111607
57. Rico-Martín S, Calderón-García JF, Sánchez-Rey P, Franco-Antonio C, Martínez Alvarez M, Sánchez Muñoz-Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13023. doi: 10.1111/obr.13023
58. Zhou C, Zhan L, Yuan J, Tong X, Peng Y, Zha Y. Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients. Eat Weight Disord. (2020) 25:727–34. doi: 10.1007/s40519-019-00678-9
59. Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. (2018) 19:737–59. doi: 10.1111/obr.12666
60. Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. (2012) 13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x
61. Corrêa MM, Thumé E, De Oliveira ER, Tomasi E. Performance of the waist-to-height ratio in identifying obesity and predicting non-communicable diseases in the elderly population: a systematic literature review. Arch Gerontol Geriatr. (2016) 65:174–82. doi: 10.1016/j.archger.2016.03.021
62. Alves LF, Cruz JO, da Costa Souza AL, de Oliveira CC. Performance of adiposity indicators in predicting metabolic syndrome in older adults. Arch Endocrinol Metab. (2021) 65:588–95. doi: 10.20945/2359-3997000000372
63. Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. (2019) 9:9693. doi: 10.1038/s41598-019-46187-8
64. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. (2020) 2020:4678526. doi: 10.1155/2020/4678526
65. Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride Glucose (TyG) Index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. (2022) 72:986–8. doi: 10.47391/JPMA.22-63
66. Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, Athanasouli F, Panagiotopoulos I, Kafetzi M, et al. Triglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine. (2020) 70:58–64. doi: 10.1007/s12020-020-02374-6
67. Ferreira JRS, Zandonade E, de Paula Alves Bezerra OM, Salaroli LB. Cutoff point of TyG index for metabolic syndrome in Brazilian farmers. Arch Endocrinol Metab. (2021) 65:704–12. doi: 10.20945/2359-3997000000401
68. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731
69. Raimi TH, Dele-Ojo BF Dada SA, Fadare JO, Ajayi DD, Ajayi EA, et al. Triglyceride-glucose index and related parameters predicted metabolic syndrome in Nigerians. Metab Syndr Relat Disord. (2021) 19:76–82. doi: 10.1089/met.2020.0092
70. Bijari M, Jangjoo S, Emami N, Raji S, Mottaghi M, Moallem R, et al. The accuracy of visceral adiposity index for the screening of metabolic syndrome: a systematic review and meta-analysis. Int J Endocrinol. (2021) 2021:6684627. doi: 10.1155/2021/6684627
71. Durcan E, Sahin S, Dedeoglu SE, Ozkaya HM, Gonen MS. Can “VAI” better indicate metabolic syndrome compared with other metabolic syndrome-related parameters in patients with thyroid nodules? A Study from Turkey. Metab Syndr Relat Disord. (2021) 19:358–66. doi: 10.1089/met.2020.0147
72. Vizzuso S, Del Torto A, Dilillo D, Calcaterra V, Di Profio E, Leone A, et al. Visceral adiposity index (VAI) in children and adolescents with obesity: no association with daily energy intake but promising tool to identify metabolic syndrome (MetS). Nutrients. (2021) 13:413. doi: 10.3390/nu13020413
73. Després JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. (1989) 38:304–9. doi: 10.2337/diab.38.3.304
74. Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes. (2015) 39:1365–70. doi: 10.1038/ijo.2015.75
75. Anoop SS, Dasgupta R, Rebekah G, Jose A, Inbakumari MP, Finney G, et al. Lipid accumulation product (LAP) as a potential index to predict risk of insulin resistance in young, non-obese Asian Indian males from Southern India: observations from hyperinsulinemic-euglycemic clamp studies. BMJ Open Diabetes Res Care. (2021) 9:e002414. doi: 10.1136/bmjdrc-2021-002414
76. Chen J, Sun H, Qiu S, Tao H, Yu J, Sun Z. Lipid accumulation product combined with urine glucose excretion improves the efficiency of diabetes screening in chinese adults. Front Endocrinol. (2021) 12:691849. doi: 10.3389/fendo.2021.691849
77. Ding YS Li Y, Zhang XH, Ma RL, Guo H, Ma L, et al. The improved lipid accumulation product is an accurate index for predicting metabolic syndrome in the Xinjiang Population. Biomed Environ Sci. (2021) 34:503–7. doi: 10.3967/bes2021.070
78. Raposo MA, Guimarães NS, Tupinambás U. Lipid accumulation product index to predict metabolic syndrome in people living with HIV. Clin Med Res. (2020) 18:120–5. doi: 10.3121/cmr.2020.1509
79. Zhang L, Zhang Z, Wang B, Yuan Y, Sun L, Gao H, et al. Relative children's lipid accumulation product is a novel indicator for metabolic syndrome. Front Endocrinol. (2021) 12:645825. doi: 10.3389/fendo.2021.645825
80. Stefanescu A, Revilla L, Lopez T, Sanchez SE, Williams MA, Gelaye B. Using a body shape index (ABSI) and body roundness index (BRI) to predict risk of metabolic syndrome in Peruvian adults. J Int Med Res. (2020) 48:300060519848854. doi: 10.1177/0300060519848854
81. Ismail NA, Ragab SH, El Baky A, Ibrahim MH. Potential role of new anthropometric parameters in childhood obesity with or without metabolic syndrome. Open Access Maced J Med Sci. (2019) 7:3930–6. doi: 10.3889/oamjms.2019.698
82. Guo X, Ding Q, Liang M. Evaluation of eight anthropometric indices for identification of metabolic syndrome in adults with diabetes. Diabetes Metab Syndr Obes. (2021) 14:1431–43. doi: 10.2147/DMSO.S294244
83. Chen HJ, Li GL, Sun A, Peng DS, Zhang WX, Yan YE. Age differences in the relationship between secondhand smoke exposure and risk of metabolic syndrome: a meta-analysis. Int J Environ Res Public Health. (2019) 16:1409. doi: 10.3390/ijerph16081409
84. Choi HI, Lee SJ, Kang JG, Lee SH, Kim BS, Kim BJ. Association of environmental tobacco smoke exposure with metabolic syndrome: a longitudinal cohort study of 71,055 never smokers. Nutr Metab Cardiovasc Dis. (2022) 32:2534–43. doi: 10.1016/j.numecd.2022.07.009
85. Zong C, Song G, Yao S, Guo S, Yu Y, Yang N, et al. Cigarette smoke exposure impairs reverse cholesterol transport which can be minimized by treatment of hydrogen-saturated saline. Lipids Health Dis. (2015) 14:159. doi: 10.1186/s12944-015-0160-9
Keywords: metabolic syndrome, lipids, obesity, national cohort study, middle-aged and elderly Chinese
Citation: Gui J, Li Y, Liu H, Guo L-l, Li J, Lei Y, Li X, Sun L, Yang L, Yuan T, Wang C, Zhang D, Wei H, Li J, Liu M, Hua Y and Zhang L (2023) Obesity- and lipid-related indices as a predictor of obesity metabolic syndrome in a national cohort study. Front. Public Health 11:1073824. doi: 10.3389/fpubh.2023.1073824
Received: 18 October 2022; Accepted: 16 January 2023;
Published: 14 February 2023.
Edited by:
Claudio Davila, Facultad Latinoamericana de Ciencias Sociales Mexico, MexicoReviewed by:
Jingyuan Xiong, Sichuan University, ChinaCopyright © 2023 Gui, Li, Liu, Guo, Li, Lei, Li, Sun, Yang, Yuan, Wang, Zhang, Wei, Li, Liu, Hua and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang,  eWFvcmFuMjAwOEAxNjMuY29t
eWFvcmFuMjAwOEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.