- 1Department of Neonatology, Guangzhou Women and Children's Medical Centre, Guangzhou Medical University, Guangzhou, China
- 2Department of Neonatology, The Fifth Affiliated Hospital of Southern Medical University, Guangzhou, China
- 3Medical Department, The First People's Hospital of Chenzhou, Chenzhou, China
- 4Rehabilitation Medicine Center, The First Dongguan Affiliated Hospital, Guangdong Medical University, Dongguan, China
- 5Advanced Institute of Natural Sciences, Beijing Normal University at Zhuhai, Zhuhai, China
Background: To investigate the association between insufficient maternal gestational weight gain (GWG) during dietary treatment, and neonatal complications of small-for-gestational-age (SGA) infants born to mothers with Gestational diabetes mellitus (GDM).
Methods: A retrospective case-control study was conducted, involving 1,651 infants born to mothers with GDM. The prevalence of a perinatal outcome and maternal GWG were compared among SGA, adequate- (AGA), and large-for-gestational-age (LGA); association with birth weight and GWG was identified using Pearson's correlation analysis; binary logistic regression was performed to determine the odds ratio (OR) associated with SGA.
Results: In total, 343 SGA, 1025 AGA, and 283 LGA infants met inclusion criteria. The frequency of SGA infants who were siblings (41.7 vs. 4.3 vs. 1.9%) and composite of complications (19.2 vs. 12.0 vs. 11.7%) were higher in SGA infants than in those in AGA or LGA infants group (both P < 0.01). GWG and pre-partum BMI were lower among the SGA mothers with GDM group (11.7 ± 4.5 kg, 25.2 ± 3.1 kg/m2) than AGA (12.3 ± 4.6 kg, 26.3 ± 3.4 kg/m2) or LGA (14.0 ± 5.1 kg, 28.7 ± 3.9 kg/m2) mothers with GDM group. Binary logistic regression showed that siblings who were SGA (AOR 18.06, 95% CI [10.83–30.13]) and preeclampsia (AOR 3.12, 95% CI [1.34–7.30]) were associated with SGA, but not GWG below guidelines (P > 0.05). The risk of SGA (25.7 vs. 19.1 vs. 14.2%) and FGR (15.3 vs. 10.9 vs. 7.8%) was higher in GWG below guidelines group than those in GWG above and within guidelines group, the risk of low Apgar score (6.4 vs. 3.0 vs. 2.8%) was higher in GWG above guidelines group than that in GWG below and within guidelines group (P < 0.05).
Conclusion: Our findings demonstrated that GWG above and below guidelines, compared with GWG within guidelines, had a higher risk of adverse infant outcomes. Our findings also suggested that GWG below guidelines did not increase the risk for SGA, though SGA infants had more adverse outcomes among neonates born to mothers with GDM.
Introduction
Gestational diabetes mellitus (GDM), a common pregnancy complication, affects 1–28% of all pregnancies. Maternal GDM contributes to short- and long-term health risks both for mothers (including polycystic ovarian syndrome, obesity, and type 2 diabetes) and children (including respiratory distress syndrome, hypoglycemia, hyperbilirubinemia, obesity, and metabolic syndrome) (1). The majority of newborns born to women with GDM are large-for-gestational-age (LGA) infants.
At present, the treatment of GDM includes dietary interventions and drugs. The current first-line therapy for GDM is dietary interventions, including lifestyle changes, weight management, physical activity, and medical nutrition therapy; 70–80% of GDM women were given dietary interventions to control blood glucose (2–4). Dietary interventions can reduce gestational weight gain (GWG). The targets of GWG during dietary interventions in many countries refer to the recommendations of the US Institute of Medicine (IOM), which were updated in 2009 based on pre-pregnancy body mass index (BMI) (5). GWG is an important antenatal factor, a few studies demonstrated that insufficient GWG (GWG below the IOM guidelines) during pregnancy was associated with a higher incidence of small-for-gestational-age (SGA) (6, 7). Studies demonstrated that SGA infants born to mothers with GDM had a higher risk of hypoglycemia and hyperbilirubinemia in infants and long-term cardiovascular hospitalizations in adulthood than LGA or appropriate-for-gestational-age (AGA) (8–10). However, it is not known if SGA associated with GDM is a risk factor for other perinatal complications. Moreover, the frequency of SGA born to mothers with GDM who were given dietary treatment has been reported to be as high as 11%, and this rate is increasing worldwide; the prevalence of insufficient GWG is also increasing (11, 12). Additionally, there is limited research on the association of insufficient GWG with SGA infants born to mothers with GDM. To better understand the relationship between insufficient GWG during the dietary intervention and perinatal outcomes, the aim of this study was to examine whether abnormal GWG will increase the frequency of SGA and the risk of adverse outcomes.
Materials and methods
Study design and participants
This study was a population-based, retrospective case-control study and approved by the research ethical committee in Guangzhou Women and Children's Medical Centre of Guangzhou Medical University, Number 2014121402, date of approval 12 December 2014. The selection of participants is shown in Figure 1. All parents provided written informed consent, and the ethics committee approved this consent procedure. This study was conducted from December 2014 to March 2022.
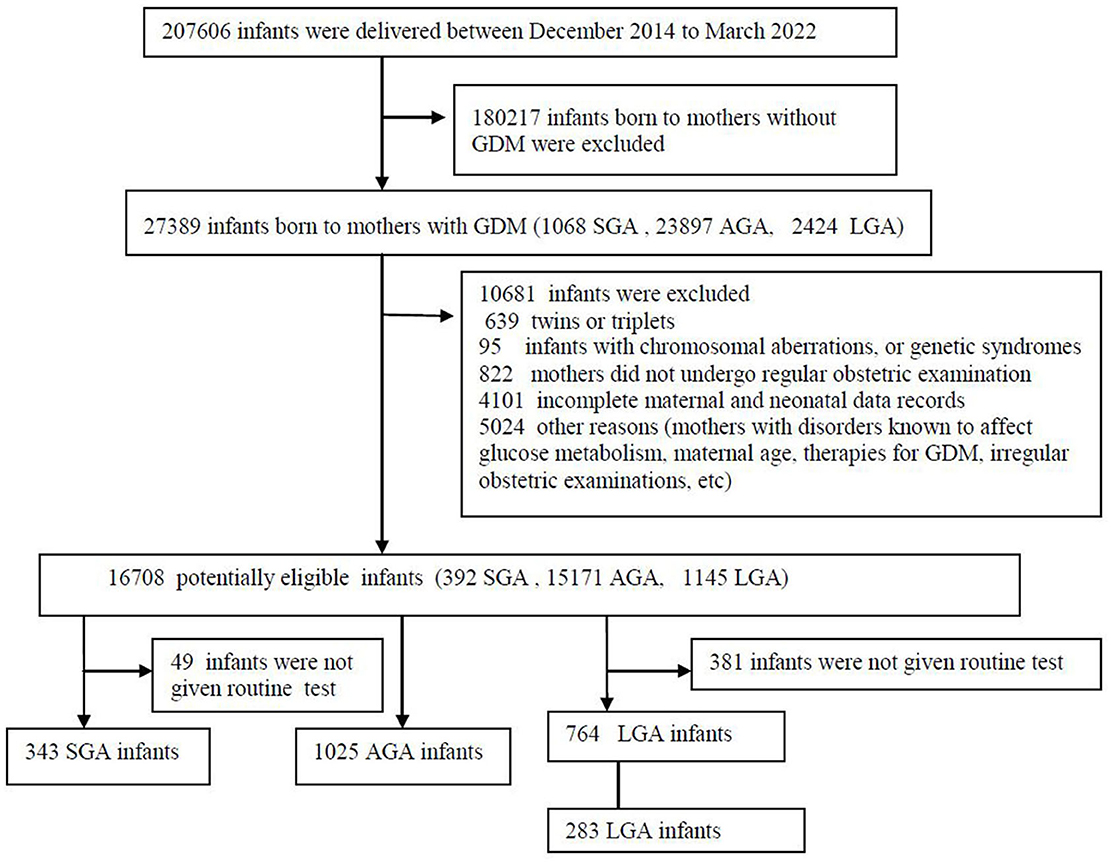
Figure 1. Flow diagram of study population. AGA, appropriate for gestational-age; GDM, gestational diabetes mellitus; LGA, large-for-gestational-age; SGA, small for gestational age.
The inclusion criteria were the followings: (1) mothers were diagnosed with GDM with singleton pregnancy and live birth; (2) mothers were older than 18 years; (3) mothers/infant pairs with complete maternal (delivery data, complication during pregnancy) and neonatal data (birth data, in-hospital outcomes); 4) all newborns were given routine examinations. The exclusion criteria: (1) infants presented with chromosomal aberrations, and genetic syndromes; (2) mothers with concomitant pathology that could affect glucose metabolism (such as diabetes, maturity-onset diabetes in young, polycystic ovarian syndrome, and uncontrolled thyroid during pregnancy); (3) mothers weren't given regular obstetric examinations. Only one birth infant for mother was included in our study. GDM was diagnosed according to guidelines for the diagnosis and treatment of GDM in China (13). The guidelines are similar to the American Diabetes Association and International Association of Diabetes and Pregnancy Study Group (IADPSG) guidelines (14). After the diagnosis of GDM, all GDM women who underwent regular obstetric examinations in hospitals received individualized dietary consultation with a dietitian. Individual recommendations for diet and exercise were based on the guidelines for the treatment of GDM in the China (13), and estimations of daily energy intake and nutrients were computed using a food database. Dietary recommendations were based on the following principles: restricting dietary intake of saturated fat and exchanging carbohydrate-rich foods with a medium-to-high glycaemic index for foods with a lower glycaemic index to reduce the glycaemic load. All women were recommended to participate in the aerobic and strength-conditioning exercises. The women were informed about weekly maternal GWG in late pregnancy, based on their pre-pregnancy BMI, independent of their GWG before GDM diagnosis as follows: gain of 12.5–18 kg for under-weight women (BMI < 18.5 kg/m2), 11.5–16 kg for normal women (BMI 18.5–24.9 kg/m2) and 7–11 kg for overweight women (BMI 25–29.9 kg/m2), 5–9 kg for obese women (BMI ≥30 kg/m2), and physical activity for at least 30 min/day were recommended.
All infants were divided into the following three groups: SGA infants born to mothers with GDM, AGA infants born to mothers with GDM, and LGA infants born to mothers with GDM. The diagnostic criteria of SGA infants: infants whose birth weight is below the 10th percentile at gestational age. The diagnostic criteria of AGA infants: infants whose birth weight is between the 10th and 90th percentile at gestational age. LGA was defined by a birth weight >90th percentile at gestational age. Classification of all newborns was defined according to the Fenton growth chart. GWG was defined as the difference between the final weight before delivery (within the last week before giving birth) and the pre-pregnancy weight (within 3 months before pregnancy). Based on the guidelines for maternal GWG of IOM, GWG was divided into the following three groups for analysis: GWG below guidelines (insufficient GWG), GWG within guidelines (sufficient GWG), and GWG above guidelines (excessive GWG). We performed matching according to neonatal gestational age (difference was ≤ 3 days), sex, and maternal age (difference was ≤ 3 years).
Data collection
Each mother/infant pair's demographic data, intervention condition, and medical information were collected individually using medical records. All mother/infant pairs underwent structured medical examinations and physical examinations. The following data on siblings of included infants also were recorded: maternal blood glucose after a 75-g OGTT, glycated-hemoglobin (Hb) level, gestational age at delivery, sex, birth weight and height of newborn, and mode of delivery. The weight and length/height of mother/infant pairs were measured by trained nurses using standard anthropometric methods, and pre-pregnancy weight was obtained according to maternal self-report. BMI was calculated by dividing weight in kilograms by the square of height in meters. Early thrombocytopenia was defined as a platelet count of < 150 × 10∧9/L in the first 72 h of life. Fetal growth restriction (FGR) was determined by ultrasound, early FGR was defined as the gestational age was < 32 weeks and the following criteria were present: estimated fetal weight (EFW) or abdominal circumference (AC) below the 3rd percentile for the gestational age or absent end-diastolic flow in umbilical artery (UA); late FGR was defined as the gestational age was>32 weeks and the following criteria were present: EFW or AC below the 3rd percentile for the gestational age. Hypoglycemia was defined as blood glucose < 35 mg/dl or plasma glucose < 40 mg/dl within the first 48 h of life. Low Apgar score was defined as a 1-min Apgar score ≤ 7. Necrotizing enterocolitis (NEC) was diagnosed according to Bell criteria. Neonatal respiratory distress syndrome (NRDS) was diagnosed by (1) evidence of respiratory failure, (2) administration of exogenous pulmonary surfactant (3) radiographic evidence. Symptomatic polycythemia was defined as venous hematocrit >65 % or hemoglobin >220 g/L. Prematurity was defined as gestational age at birth of < 37 weeks. The presence of at least one neonatal complication (early thrombocytopenia, hypoglycemia, low Apgar score, NRDS, NEC, or polycythemia) was assessed.
Data entry was performed by a trained clerk and a supervisor. Data were cross-checked by the co-author (VF) for any errors and discrepancies. Data entered incorrectly will be examined and corrected by the supervisor after confirmation with the participants or their obstetric records. Any revision of the original data will be tracked in detail.
Statistical analysis
Sample size calculation was performed using PASS 15. With 90% power and the assumption of relative risk = 2.0, we calculated that 256 SGA infants born to mothers with GDM were needed. Data were analyzed by using SPSS version 22 (SPSS, Chicago, IL, USA). Means (standard deviations) or median (range) was used to describe continuous variables; t-tests, ANOVA or kruskal-wallis test were used to analyze the differences in continuous variables. A two-sided chi-squared or Fisher's exact test was used for categorical variables presented as numbers and percentages. Binary logistic regression was used to determine risk factors for SGA infants born to mothers with GDM after adjusting for potential confounding variables. The association between birth weight and maternal GWG, pre-partum period BMI, and pre-pregnancy BMI was identified using Pearson's correlation analysis. Differences were considered statistically significant at a two-sided P value of < 0.05.
Results
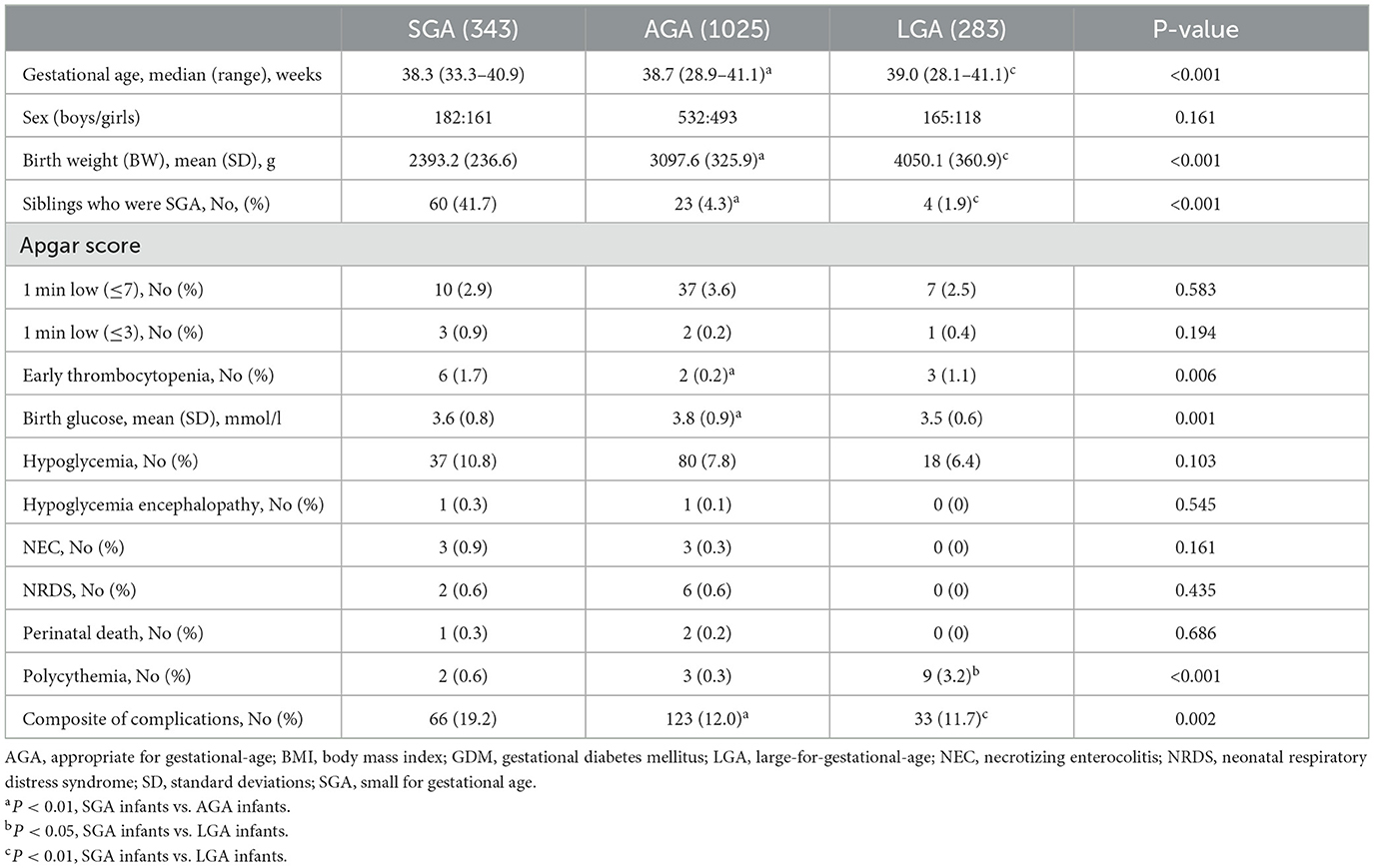
Overall, the final sample consisted of 1,651 mother/infant pairs. Of these, 343 SGA infants born to mothers with GDM, 1,025 AGA infants born to mothers with GDM, and 283 LGA infants born to mothers with GDM. There were 92 (5.6%) premature births, with 8 (2.3%) in the SGA infants born to mothers with GDM.
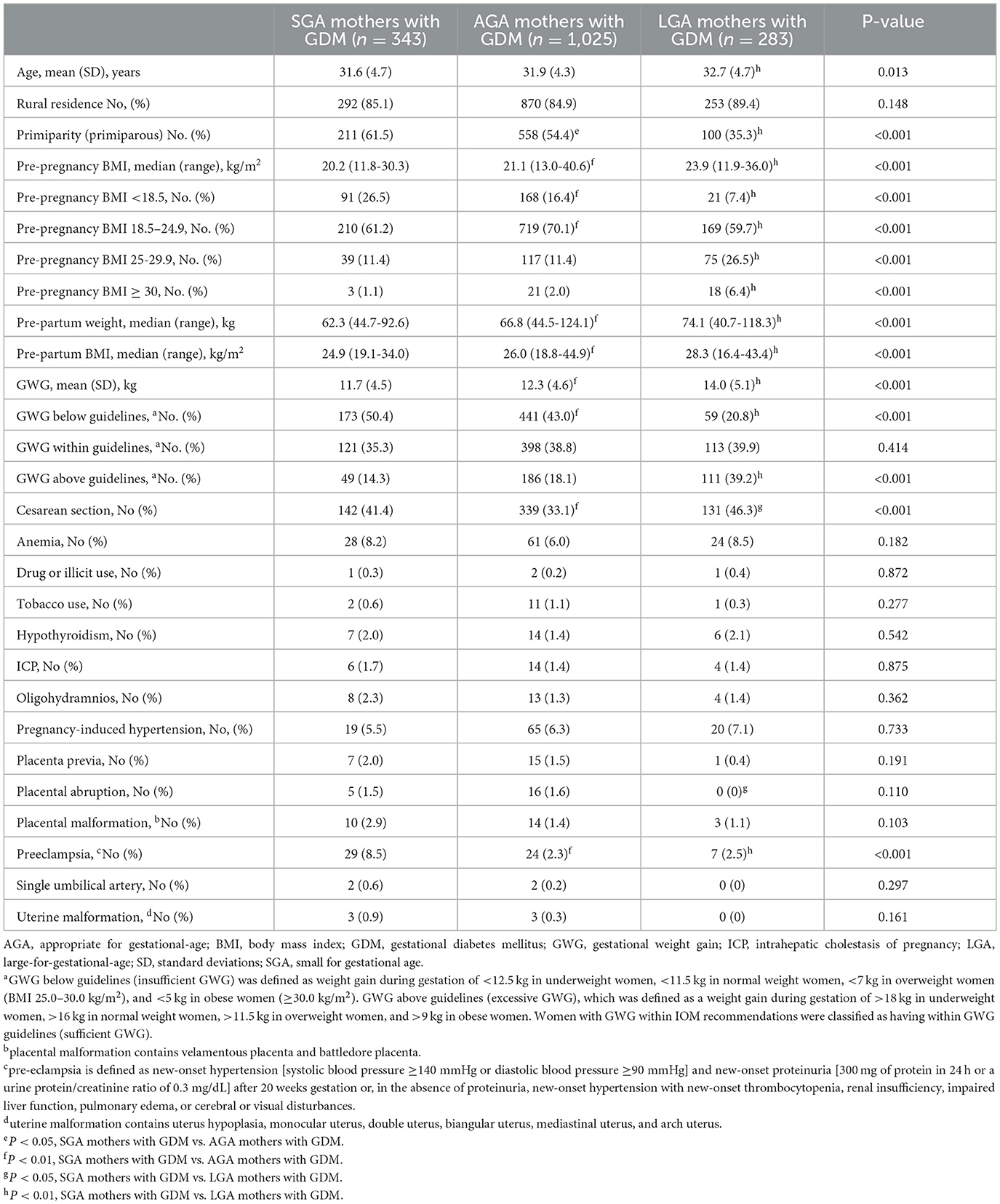
Relevant characteristics of mothers are presented in Table 1. We observed that GWG and Pre-partum BMI were lower in the SGA mothers with GDM group (11.7 ± 4.5 kg, 25.2 ± 3.1 kg/m2) than that in AGA (12.3 ± 4.6 kg, 26.3 ± 3.4 kg/m2) or LGA (14.0 ± 5.1 kg, 28.7 ± 3.9 kg/m2) mothers with GDM group (both P < 0.001). Preeclampsia was higher in SGA mothers with GDM than that in AGA mothers or LGA mothers (8.5 vs. 2.3 vs. 2.5%; P < 0.001). The incidence of primiparity (61.5%) and GWG below guidelines (50.4%) were highest in the SGA mothers group, and LGA mothers had the highest proportion of cesarean section (41.4%) and GWG above guidelines (39.2%).
Table 2 shows the clinical characteristics of the infants. The frequency of SGA infants who were siblings (41.7 vs. 4.3 vs. 1.9%) and composite of complications (19.2 vs. 12.0 vs. 11.7%) were higher in SGA infants than in those in AGA or LGA infants group (both P < 0.01). The risk of symptomatic polycythemia was more frequent in LGA infants than that in the AGA or SGA infants group (3.2 vs. 0.3 vs. 0.6%, P < 0.001). The risk of early thrombocytopenia was increased in SGA infants compared to that in AGA infants (1.7 vs. 0.2%). However, there were no significant differences in the risk of Apgar score, hypoglycemia, hypoglycemia encephalopathy, NEC, NRDS, or perinatal death among the three groups (all P > 0.05).
Pearson's correlation analysis was used to determine the correlation between maternal factors and birth weight, and the results are shown in Table 3. The results illustrated that GWG, prepregnancy BMI, and prepartum period BMI were significantly weakly correlated with infants' birth weight (Pearson correlation coefficient 0.129, 0.113, and 0.170; all P < 0.01).
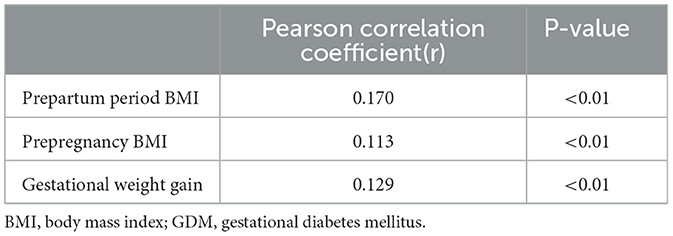
Table 3. Relationships between birth weight and gestational weight gain, prepartum period BMI, pre-pregnancy BMI in infants born with GDM mother (N = 1,651).
Neonates were further grouped by the guidelines for maternal GWG of IOM as follows: GWG below guidelines, GWG within guidelines, and GWG above guidelines. Table 4 shows the influence of GWG on perinatal outcomes. 673 (40.8%) women had GWG below guidelines, 346 (21.0%) had GWG above guidelines, and 632 (38.2%) had GWG within guidelines. We observed that the risk of SGA (25.7 vs. 19.1 vs. 14.2%) and FGR (15.3 vs. 10.9 vs. 7.8%) was higher in GWG below guidelines group than those in GWG above and within guidelines group. However, the risk of low Apgar score (6.4 vs. 3.0 vs. 2.8%) was higher in GWG above guidelines group than that in GWG below or within guidelines group (P < 0.05). There were no significant differences in adverse women outcomes, early thrombocytopenia, NEC, hypoglycemia, or NRDS among these three groups.
Next, multivariate binary logistic regression analysis was performed to identify factors associated with SGA, and the results are shown in Table 5. Adjusting for other confounding variables, the following factors were associated with SGA: preeclampsia (AOR 3.12, 95% CI [1.34–7.30]) and siblings who were SGA (AOR 18.06, 95% CI [10.83–30.13]).
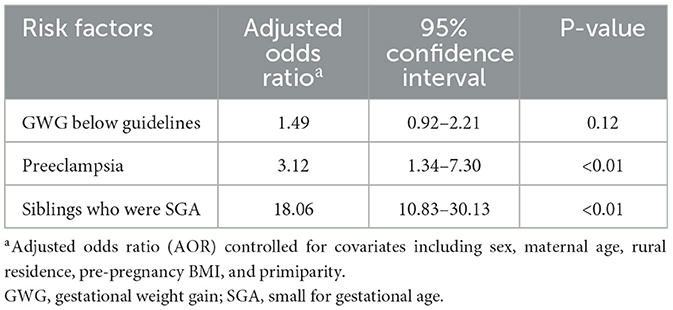
Table 5. Binary logistic analysis for the associations between maternal complications and SGA infants (N = 1,651).
To further evaluate the effect of risk factors for SGA on GWG among women with GDM, mothers were further grouped by risk factors for SGA as follows: GDM mothers with and without preeclampsia, GDM mothers with and without siblings who were SGA. However, groups did not differ significantly in pre-pregnancy BMI, GWG, the incidence of GWG below guidelines, GWG within guidelines, and GWG below guidelines (all P > 0.05) (Table 6).
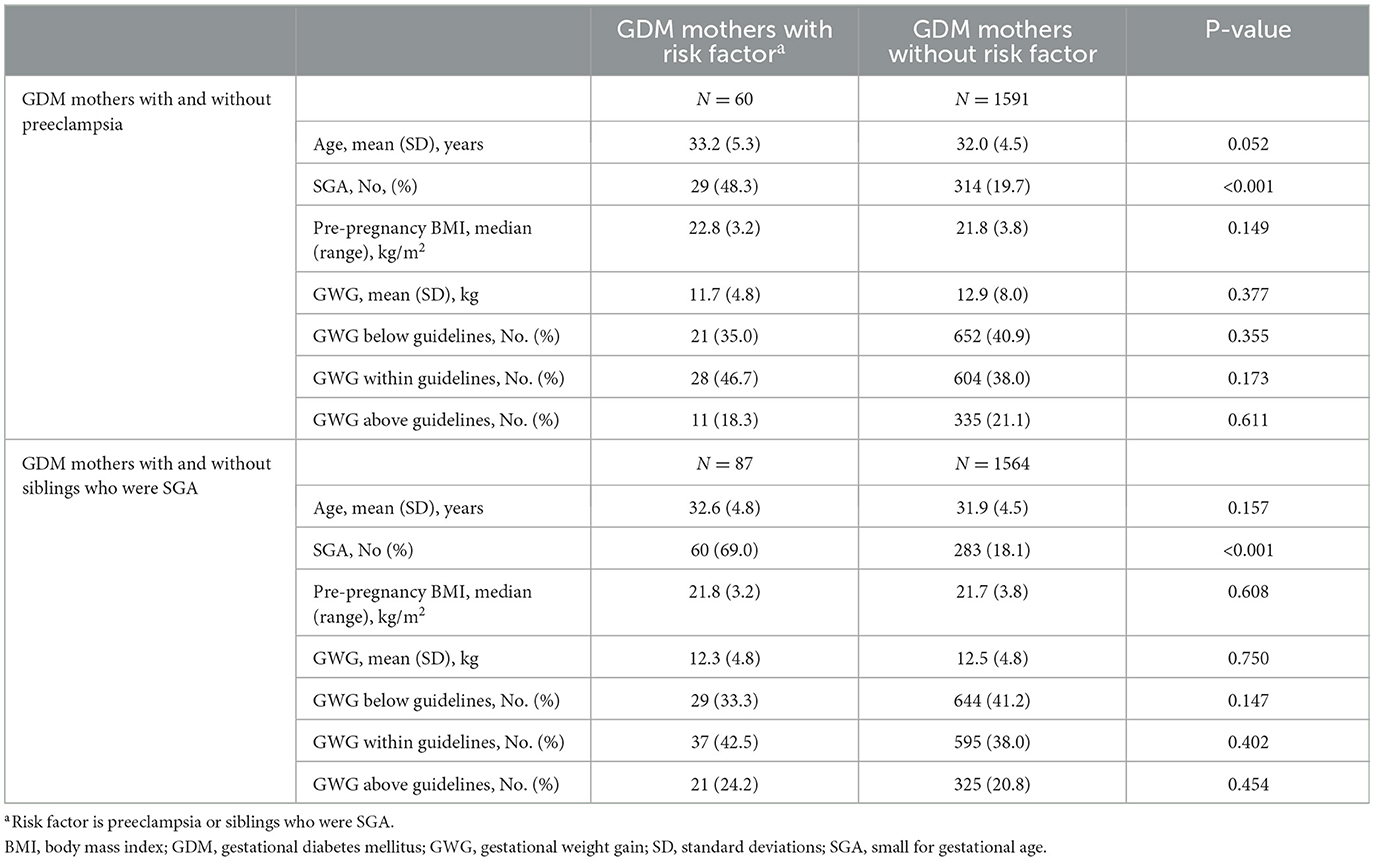
Table 6. The comparison of gestational weight gain and pre-pregnancy BMI between GDM mothers with and without risk factors (N = 1,651).
Discussion
We conducted a population-based, case-control study to explore the association between dietary intervention and the frequency of SGA. The main results of our study suggested the following: (1) siblings who were SGA and pre-eclampsia could be the risk factors for SGA infants born to mothers with GDM, infants' birth weight is also correlated with GWG, prepregnancy BMI, and prepartum period BMI; (2) compared to AGA or LGA, SGA infants born to mothers with GDM have more adverse perinatal outcomes; (3) GWG above and below guidelines increased the risk of adverse infant outcomes.
Dietary advice and exercise interventions are the first-line therapy for pregnant women with GDM. Dietary advice and exercise interventions alone for the prevention of GDM have been widely assessed; dietary and exercise interventions have been proven to reduce GWG in these studies (15, 16). So international guidelines recommend that pregnant women with GDM participated in aerobic and strength-conditioning exercises for 30 min at least 5 days per week along with medical nutritional therapy (17, 18). The main purpose of dietary interventions for pregnant women with GDM is to prevent macrosomia due to its associated risks (19, 20). However, some findings suggested that preventing SGA and related complications should be just as important in the care of pregnant women with GDM (21–23). The birth rate of SGA infants was similar to that of LGA infants in these studies; nevertheless, perinatal complications (20.1%) and mortality (1.6%) were worse in the SGA group than in the AGA or LGA groups (11, 24, 25). Our findings were in agreement with these previous reports. In normal pregnancies, the risk of perinatal asphyxia, hypoglycemia, polycythemia, thrombocytopenia, and other neonatal complications is also higher in SGA neonates than in AGA or LGA neonates (26–28).
Dietary advice and exercise interventions can reduce GWG, Pearson's correlation analysis also showed that GWG was correlated with infants' birth weight in our study; but whether they can increase the incidence of SGA remains controversial (6, 15). IOM recommended different targets for an adequate GWG depending on the pre-pregnancy BMI in 2009. In China, insufficient GWG (GWG below guidelines) occurs in 33.9% of pregnant women with GDM (29). In our study, 40.8% of GDM women presented with a total GWG below the IOM guidelines; this variation may be due to rigorous lifestyle improvements. Recent studies have shown that insufficient GWG results in increased odds of preterm birth and SGA in normal pregnancies and women with GDM (30–33). However, Gou et al. (34) showed that insufficient GWG was not the risk factor for SGA. Adjusting for other confounding variables, the results of logistic regression analysis also showed that there was no positive correlation between SGA and insufficient GWG in our study. The inconsistency may be due to the different study populations, the calculation method of GWG and the adjusted confounding variables. Therefore, more and larger randomized controlled trials are needed to assess the relationship between insufficient GWG and SGA in Chinese.
Moreover, some previous studies reported that excessive GWG (GWG above guidelines) had increased the odds of hypertensive disorders of pregnancy, cesarean delivery, macrosomia, and LGA (35, 36); but the effects of excessive GWG on adverse infant outcomes have not been analyzed in detail in these studies. GDM women with excessive GWG increased the risk for low Apgar score (6.4%) in our study compared to that in GWG below or within guidelines group.
The recurrence risk among siblings is widely used to measure shared genetic contributions. The frequency of SGA siblings in SGA infants born to mothers with GDM group was 10-fold higher than that in the AGA group in our study. Therefore, our findings demonstrated that genetic factors and preeclampsia were risk factors for SGA infants born to mothers with GDM. Recently, Spanish scholars indicated that smoking and neonate prematurity were also risk factors for these infants (11); however, we did not find this result in our study.
Our study presents novel information, not previously reported, as we considered the frequency of siblings who were SGA, neonatal early thrombocytopenia, and abnormal GWG among GDM women. Our findings are also helpful for clinicians to design more accurate care programs for pregnant women with GDM. Our study also has several limitations. First, the number of SGA infants born to mothers with GDM may be small. Second, the other limitation is reliance on self-reported pre-pregnancy weight. Third, the time for the blood routine examination and blood glucose test was varied for each infant, which might affect the results. Fourth, it was a retrospective observational study; therefore, selection and information bias cannot be excluded.
Conclusion
In conclusion, our findings suggested that GWG below guidelines did not increase the risk for SGA, though SGA infants had more adverse outcomes among neonates born to mothers with GDM. However, SGA infants born to mothers with GDM remain a major concern. Moreover, we identified that genetic factors and pre-eclampsia could be the risk factors for SGA infants born to mothers with GDM. Our research also demonstrated that GWG above and below guidelines, compared with GWG within guidelines, had a higher risk of adverse infant outcomes. These findings suggest that it is necessary to maintain a reasonable GWG among pregnant women with GDM to reduce adverse perinatal complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee in Guangzhou Women and Children's Medical Centre of Guangzhou Medical University, Number 2014121402, date of approval 12 December 2014. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
DH, ML, BX, WZ, and JC designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and created the tables and figures. SC, YX, and JC conceptualized and designed the study, coordinated and supervised data collection, and helped draft the initial manuscript. HL, LW, PP, DY, YY, and JY designed the data collection instruments, collected data, and conducted the initial analyses. All authors contributed to the manuscript's critical revision, read, and approved the submitted version.
Funding
This article was supported by Research foundation of Guangzhou Women and Children's Medical Center for Clinical Doctor and Basic research program and applied basic research project of Guangzhou Science and Technology Bureau (2210124-03).
Acknowledgments
The authors thank Huimin Chen (nutrition department, Guangzhou Women and Children's Medical Centre) for data collection. The authors would like to thank the clinical research center staff at the Guangzhou Women and Children's Medical Centre, including study coordinators, doctors, nurses, and supervisors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
2. Reader DM. Medical nutrition therapy and lifestyle interventions. Diabetes Care. (2007) 30:S188–93. doi: 10.2337/dc07-s214
3. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. (2018) 29:743–54. doi: 10.1016/j.tem.2018.09.004
4. Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. (2020) 12:3050. doi: 10.3390/nu12103050
5. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (2009).
6. Allehdan SS, Basha AS, Asali FF, Tayyem RF. Dietary and exercise interventions and glycemic control and maternal and newborn outcomes in women diagnosed with gestational diabetes: systematic review. Diabetes Metab Syndr. (2019) 13:2775–84. doi: 10.1016/j.dsx.2019.07.040
7. Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. (2017) 6:CD012202. doi: 10.1002/14651858.CD012202.pub2
8. Fabricius-Bjerre S, Jensen RB, Færch K, Larsen T, Mølgaard C, Michaelsen KF, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS ONE. (2011) 6:e20595. doi: 10.1371/journal.pone.0020595
9. Barquiel B, Herranz L, Martínez-Sánchez N, Montes C, Hillman N, Bartha JL. Increased risk of neonatal complications or death among neonates born small for gestational age to mothers with gestational diabetes. Diabetes Res Clin Pract. (2020) 159:107971. doi: 10.1016/j.diabres.2019.107971
10. Neimark E, Wainstock T, Sheiner E, Fischer L, Pariente G. Long-term cardiovascular hospitalizations of small for gestational age (SGA) offspring born to women with and without gestational diabetes mellitus (GDM)‡. Gynecol Endocrinol. (2019) 35:518–24. doi: 10.1080/09513590.2018.1541233
11. Wang C, Zhu W, Wei Y, Feng H, Su R, Yang H. Exercise intervention during pregnancy can be used to manage weight gain and improve pregnancy outcomes in women with gestational diabetes mellitus. BMC Pregnancy Childbirth. (2015) 15:255. doi: 10.1186/s12884-015-0682-1
12. Chen J, Xiao H, Yang Y, Tang Y, Yang X, Zhang Z, et al. Demographic and clinical features of small-for-gestational-age infants born to mothers with gestational diabetes mellitus. Front Pediatr. (2021) 9:741793. doi: 10.3389/fped.2021.741793
13. Yang H. Guidelines for diagnosis and treatment of gestational diabetes mellitus (2014 edition). Chin J Obst Gynecol. (2014) 49:561–9. doi: 10.3760/cma.j.issn.0529-567x.2014.08.001
14. Pregnancy Study Groups C. Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
15. Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Sys Rev. (2017) 11:CD010443. doi: 10.1002/14651858
16. Cremona A, O'Gorman C, Cotter A, Saunders J, Donnelly A. Effect of exercise modality on markers of insulin sensitivity and blood glucose control in pregnancies complicated with gestational diabetes mellitus: a systematic review. Obes Sci Pract. (2018) 4:455–67. doi: 10.1002/osp4.283
17. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. (2018) 131:e49–64. doi: 10.1097/AOG.0000000000002501
18. National Institute for Health and Care Excellence. Gestational Diabetes: Risk Assessment, Testing, Diagnosis, and Management. Manchester: NICE (2015).
19. Kurtzhals LL, Nørgaard SK, Secher AL, Nichum VL, Ronneby H, Tabor A, et al. The impact of restricted gestational weight gain by dietary intervention on fetal growth in women with gestational diabetes mellitus. Diabetologia. (2018) 61:2528–38. doi: 10.1007/s00125-018-4736-6
20. Mustaniemi S, Nikkinen H, Bloigu A, Pouta A, Kaaja R, Eriksson JG, et al. Normal gestational weight gain protects from large-for-gestational-age birth among women with obesity and gestational diabetes. Front Public Health. (2021) 9:550860. doi: 10.3389/fpubh.2021.550860
21. García-Patterson A, Corcoy R, Balsells M, Altirriba O, Adelantado JM, Cabero L, et al. In pregnancies with gestational diabetes mellitus and intensive therapy, perinatal outcome is worse in small-for-gestational-age newborns. Am J Obstet Gynecol. (1998) 179:481–85. doi: 10.1016/s0002-9378(98)70383-7
22. Koren R, Idan C, Elkan M, Koren S, Wiener Y. The risk of small and large for gestational age newborns in women with gestational diabetes according to pre-gestational body mass index and weight gain. J Matern Fetal Neonatal Med. (2021) 20:1–6. doi: 10.1080/14767058.2021.1974390
23. Silva AL, Amaral AR, Oliveira DS, Martins L, Silva MR, Silva JC. Neonatal outcomes according to different therapies for gestational diabetes mellitus. J Pediatr (Rio J). (2017) 93:87–93. doi: 10.1016/j.jped.2016.04.004
24. Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med. (2019) 16:e1002848. doi: 10.1371/journal.pmed.1002848
25. Ludwig DS, Currie J. The association between pregnancy weight gain and birth weight: a within-family comparison. Lancet. (2010) 376:984–90. doi: 10.1016/S0140-6736(10)60751-9
26. Christensen RD, Baer VL, Henry E, Snow GL, Butler A, Sola-Visner MC. Thrombocytopenia in small-for-gestational-age infants. Pediatrics. (2015) 136:e361–70. doi: 10.1542/peds.2014-4182
27. Lee E, Lim Z. Malhotra A.Thrombocytopenia in well small for gestational age neonates. Blood Coagul Fibrinolysis. (2019) 230:104–10. doi: 10.1097/MBC.0000000000000802
28. Finken MJJ, van der Steen M, Smeets CCJ, Walenkamp MJE, de Bruin C, Hokken-Koelega ACS, et al. Children born small for gestational age: differential diagnosis, molecular genetic evaluation, and implications. Endocr Rev. (2018) 39:851–94. doi: 10.1210/er.2018-00083
29. Shi P, Liu A, Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:508. doi: 10.1186/s12884-021-03982-4
30. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. doi: 10.1001/jama.2017.3635
31. Li M, Zhang CY, Yue CY. Effects of pre-pregnancy BMI and gestational weight gain on adverse pregnancy outcomes and complications of GDM. J Obstet Gynaecol. (2022) 42:630–5. doi: 10.1080/01443615.2021.1945009
32. Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new institute of medicine recommendations. Obstetr Gynecol. (2011) 117:1065–70. doi: 10.1097/AOG.0b013e318214f1d1
33. Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. (2018) 16:153. doi: 10.1186/s12916-018-1128-1
34. Gou BH, Guan HM Bi YX, Ding BJ. Gestational diabetes: weight gain during pregnancy and its relationship to pregnancy outcomes. Chin Med J. (2019) 132:154–60. doi: 10.1097/CM9.0000000000000036
35. Ruifrok AE, van Poppel MN, van Wely M, Rogozińska E, Khan KS, de Groot CJ, et al. Association between weight gain during pregnancy and pregnancy outcomes after dietary and lifestyle interventions: a meta-analysis. Am J Perinatol. (2014) 31:353–64. doi: 10.1055/s-0033-1352484
36. Yamamoto JM, Kellett JE, Balsells M, García-Patterson A, Hadar E, Solà I, et al. Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. (2018) 41:1346–61. doi: 10.2337/dc18-0102
Keywords: dietary intervention, gestational diabetes mellitus, gestational weight gain, neonatal complications, risk factors, small-for-gestational age
Citation: Huang D, Liang M, Xu B, Chen S, Xiao Y, Liu H, Yin D, Yang J, Wang L, Pan P, Yang Y, Zhou W and Chen J (2023) The association of insufficient gestational weight gain in women with gestational diabetes mellitus with adverse infant outcomes: A case-control study. Front. Public Health 11:1054626. doi: 10.3389/fpubh.2023.1054626
Received: 27 September 2022; Accepted: 06 February 2023;
Published: 23 February 2023.
Edited by:
Akihito Takeuchi, National Hospital Organization Okayama Medical Center, JapanReviewed by:
Cheng Guoqiang, Fudan University, ChinaZezhong Tang, Department of Pediatrics, First Hospital, Peking University, China
Jian Mao, Sheng Jing Hospital Affiliated to China Medical University, China
Copyright © 2023 Huang, Liang, Xu, Chen, Xiao, Liu, Yin, Yang, Wang, Pan, Yang, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhou,  emhvdXdlaV9wdTAwMkAxMjYuY29t; Juncao Chen,
emhvdXdlaV9wdTAwMkAxMjYuY29t; Juncao Chen,  MzgyNzcwMjY5QHFxLmNvbQ==
MzgyNzcwMjY5QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Dabin Huang
Dabin Huang Mulin Liang
Mulin Liang Bin Xu
Bin Xu Shan Chen
Shan Chen Yan Xiao
Yan Xiao Hui Liu
Hui Liu Dan Yin
Dan Yin Jun Yang
Jun Yang Ling Wang
Ling Wang PianPian Pan
PianPian Pan Yihui Yang
Yihui Yang Wei Zhou
Wei Zhou Juncao Chen
Juncao Chen