- 1School of Medicine, Nankai University, Tianjin, China
- 2Health Management Centre, Tianjin First Central Hospital, Tianjin, China
- 3Tianjin Union Medical Center, Tianjin, China
- 4Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Department of Nephrology, Tianjin Academy of Traditional Medicine Affiliated Hospital, Tianjin, China
Purpose: Research on the relationship between sleep duration and obesity defined using multiple anthropometric and bioelectrical indices in women remains scarce. We aimed to explore the association between sleep duration and body mass index (BMI), waist-hip ratio (WHR), body fat percentage (PBF) and visceral fat area (VFA) among females.
Methods: We recruited women for medical examination using multistage cluster sampling. Sleep was assessed using Pittsburgh Sleep Quality Index (PSQI) and sleep duration was categorized into short (<7 h), optimal (7 <9 h) and long sleep (≥ 9 h). Weight and height were measured using a calibrated stadiometer. Waist circumference was manually measured. PBF, and VFA were estimated by bioelectrical impedance analysis. Data on sociodemographic characteristics and lifestyle factors were also collected and included in the logistic regression models to explore the independent association between sleep duration and obesity defined by different indices.
Results: A total of 7,763 women with a mean age of 42.6 ± 13.5 years were included. The percentage of women reporting short and long sleep was 10.3 and 13.4% respectively. The mean BMI, WHR, PBF and VFA were 23.07 ± 3.30 kg/m2, 0.78 ± 0.06, 32.23 ± 6.08% and 91.64 ± 35.97cm2, respectively. Short sleep was independently associated with 35% (95% CI: 1.05–1.75) increased odds of general obesity (BMI ≥ 28 kg/cm2), and long sleep was associated with 18% (95% CI: 1.01–1.37) increased odds of visceral obesity (VFA > 100 cm2). No association was observed between sleep deprivation or excessive sleep and high WHR or high PBF.
Conclusion: In women, short sleep was associated with an increased odds of general obesity, whereas long sleep was associated with an increased odds of visceral obesity. Longitudinal observations are needed to confirm this cross-sectional relationship.
1. Introduction
The increasing prevalence of obesity has posed significant challenges for public health around the world (1). In parallel, chronic sleep restriction and poor sleep quality raises public health concerns related to both safety and health globally. In China, approximately a quarter of adults sleep <7 h per night (2).
Optimal sleep duration plays an important role in maintaining metabolic homeostasis. Studies suggested that chronic sleep deprivation or excessive sleep was associated with increased risk of common chronic diseases (e.g., hypertension, type 2 diabetes and metabolic syndrome) and all-cause mortality (3–6). The evidence for chronic sleep deprivation as a risk factor for obesity is strong (7, 8). Possible biological mechanisms have been indicated that short sleep duration has adverse impact on metabolism, resulting in damaged metabolic homeostasis and obesity (9, 10).
Though Body mass index (BMI) is the most widely used index to define obesity, it is inefficient in predicting the adverse impact on health of excess adiposity accumulation for it could not distinguish between fat and muscle (7). Research has suggested that not only the mass but also the area of fat accumulation was associated with the unfavorable impact on health (11). Percentage of body fat (PBF), waist-to-hip ratio (WHR) and visceral fat area (VFA) outperform BMI by providing additional information on the body fat content, central obesity, and visceral obesity, respectively (12–14). Therefore, the use of multiple anthropometrics to define obesity has both clinical and practical implications.
Though the evidence for the association of chronic sleep deprivation with obesity is strong, (15) the relationship between sleep duration and obesity among females has not been thoroughly explored and the current evidence is inconsistent (16, 17). In China, the prevalence of obesity for women reached 13.37% with an upward trend (18). Due to physiological cycle changes such as menstruation, pregnancy and menopause, women's sleep was affected by different factors from childhood to menopause. A meta-analysis has shown that women have a 41% higher risk of developing insomnia than men (19). Research indicated that sex modified the association between sleep duration and obesity (20, 21). The circadian system plays a role in the regulation of metabolic homeostasis, circadian desynchrony may be involved in the development of obesity and metabolic disorders. Research has indicated that sex modifies the association between the clock circadian regulator (CLOCK) gene, a core component of the circadian system, and BMI (21).
As numerous studies exploring the relationship between sleep duration and obesity focused on BMI and the results stratified by gender are inconsistent (16, 17) an improved understanding of the association between sleep duration and obesity defined by various indices among women may improve outcomes. The current study therefore aimed to explore the relationship between sleep duration and obesity defined by BMI, PBF, WHR and VFA in non-pregnant and non-breastfeeding women. We used part of the baseline data of the Medical Examination Cohort in Beijing-Tianjin-Hebei Region, China, which was designed to provide reliable data for the health conditions and associated factors among adults.
2. Materials and methods
2.1. Study design and population
This study analyzed part of the baseline data of the Beijing-Tianjin-Hebei Medical Examination Cohort (BTH-MEC), a national key R&D program of China. The study design and population have been reported elsewhere (22). The participants were recruited by multistage stratified cluster sampling among adults for medical examinations from August 2018 to December 2020. Individuals aged 18 years or older who voluntarily participated in the survey were included in the research. Individuals were excluded if they (1) were with cognitive impairment, hearing impairment, articulate problems, or severe mental illness and could not complete the survey; (2) were with heart pacemakers implanted; and (3) could not stand independently. We analyzed the data of female participants in Tianjin. With a response rate of 86.5%, 7,879 women were interviewed and underwent medical examinations and 7,763 were included in the analysis after excluding those who were pregnant (n = 3), lactating (n = 1), taking weight-loss drugs (n = 3) in the prior 3 months or missing one or more variables of interest (n = 109). The research protocol was reviewed and approved by ethical committees from Nankai University (NKUIRB2016063), and written informed consent was got from each participant. The research procedures were carried out strictly following the Declaration of Helsinki. All methods were carried out in accordance with relevant guidelines and regulations.
2.2. Outcome variables
Height (0.1 cm precision) and weight (0.1 kg precision) was measured without shoes or heavy clothes using a calibrated stadiometer (GL-310, Seoul, Korea). Waist circumference (WC) and hip circumference was manually measured by medical professionals. PBF, and VFA were estimated by bioelectrical impedance analysis (BIA) using the multifrequency impedance plethysmograph body composition analyzer (Inbody-770, Biospace, Seoul, Korea). The age, sex and height of participants were input into the system prior to the assessment. The participant stood barefoot on the foot electrode of the instrument in a fully vertical position with light clothing, shared the weight evenly on both legs, held the hand electrode with both hands, and was prohibited from speaking during measurement. Measurement was completed after the reading was stable, as reported elsewhere (23).
According to Chinese guidelines, general obesity was defined as BMI ≥ 28 kg/m2 for Chinese women (24, 25). The WHO report suggests high WHR was defined as WHR ≥ 0.80 for Asian females (26). Visceral obesity was defined as VFA ≥ 100 cm2 (27). Due to the lack of criteria on high PBF obesity, we defined high PBF as > 35% in women based on previous literature (28).
2.3. Sleep duration
We used the Pittsburgh Sleep Quality Index (PSQI) (29) to assess the participants' habitual sleep duration and sleep quality. The Chinese version of the PSQI has been shown to have good reliability and validity among Chinese adults (30–32). Higher global PSQI score indicates poor sleep quality, whereas a cutoff of ≤ 7 indicating good sleep quality was suggested to have high diagnostic sensitivity and specificity (98.3 and 90.2%, respectively) in the Chinese population (30). According to the National Sleep Foundation, the optimal sleep duration for adults is 7–9 h per night (33). However, a few participants reported sleeping > 9 h in this study, so we categorized sleep duration into <7 h, 7–9 h (including 7 h but not including 9 h) and ≥ 9 h. Subjective sleep quality (very good, fairly good, fairly bad and very bad) and usage of sleep medicine (not during the past month, less than once a week, once or twice a week, and three or more times a week) was also collected using PSQI.
2.4. Covariates
Trained investigators conducted a face-to-face questionnaire to collect data on health related covariates which were also included in the multivariate logistic regression models including demographic characteristics (age, sex, occupation, education level, and marital status), lifestyle factors (smoking status), alcohol drinking, physical exercise, sedentary (being sitting or reclining) duration, personal history of chronic diseases (hypertension, diabetes, coronary heart disease, dyslipidemia, stroke, chronic kidney disease, chronic obstructive pulmonary disease) and cancer, medication taken in the prior 3 months (such as lowering uric acid agents, anti-arrhythmia agents, hormone agents and sleeping agents).
2.5. Statistical analyses
The normally distributed continuous variables were expressed as mean ± standard deviation (SD). Categorical data were described as rate and proportion. Differences in characteristics of participants across sleep duration categories were compared with Chi-square test and Kruskal–Wallis test. We conducted one-way ANOVA to examine the differences in BMI, WHR, PBF and VFA across sleep duration categories, and Tukey's test was used for pairwise multiple comparison. By including obesity indices as binary outcomes [i.e., general obesity (BMI ≥ 28 kg/m2) or not, elevated WHR (WHR ≥ 80) or not, high PBF (PBF > 35%) or not, and visceral obesity (VFA ≥ 100 cm2) or not], we conducted multivariate logistic regression to explore the associations between sleep duration, sleep quality with obesity defined by different indices. As proposed by previous literature (5, 34, 35) covariates that may act as confounder such as sociodemographic characteristics (age, occupation, marriage status, education level), lifestyles (smoking, alcohol drinking, exercise and sedentary duration), personal history of chronic diseases, regular medication taken in the prior 3 months, and subjective sleep quality and usage of hypnotic were included in the regression models to address the potential confounding. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to measure the risk magnitude of sleep duration, sleep quality for each type of obesity. We constructed Model 1 to examine the associations between sleep duration and obesity defined by different indices, Model 2 to examine the associations between sleep quality and obesity defined by different indices. Model 3 examined the associations of sleep duration and sleep quality with obesity defined by different indices. All analyses were performed using SPSS (version 25.0) software. All tests were 2-sided, with statistical significance set at p < 0.05.
3. Results
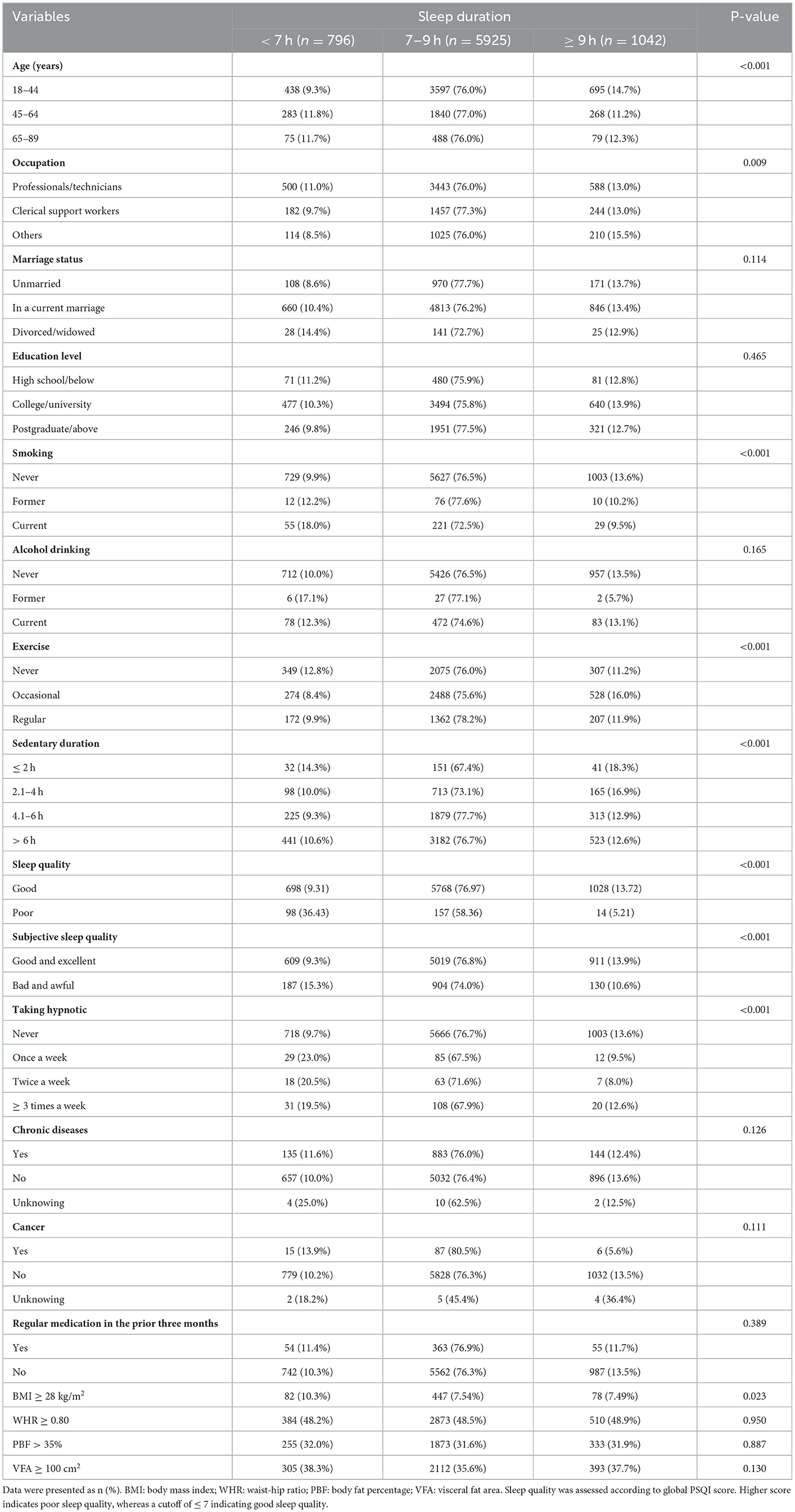
A total of 7,763 non-pregnant and non-breastfeeding women aged 18–89 years with a mean of 42.6 ± 13.5 years were included in the present study. Characteristics of the participants according to the categories of sleep duration were presented in (Table 1). In total, 10.3 and 13.4% reported sleeping <7 h and ≥ 9 h per night respectively. Age, occupation, smoking status, physical exercise, daily sedentary duration, subjective sleep quality and taking hypnotic were all correlated with sleep duration (all p < 0.05), whereas marriage status, education level, alcohol drinking, history of chronic diseases, having regular medication in the prior 3 months were not found to be associated with sleep duration (all p > 0.05).
The prevalence of general obesity (BMI ≥ 28 kg/m2), high WHR and PBF, and visceral obesity were 7.82, 48.53, 31.70, and 36.20%, respectively. The prevalence of high WHR, PBF, and VFA were considerably higher than that of general obesity. As shown in Table 1, the highest prevalence of general obesity, high PBF and visceral obesity was observed in short sleep group, while the highest prevalence of high WHR was observed in long sleep group. Only women reported sleeping <7 h had a higher prevalence of general obesity than that of optimal and long sleep (p < 0.05). The prevalence of high WHR, PBF and VFA were not statistically different across sleep durations (all p > 0.05).
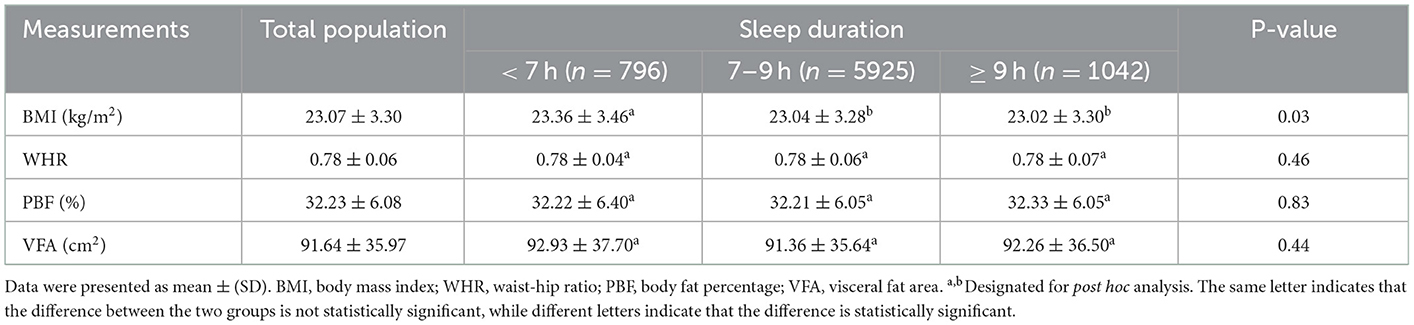
The mean BMI, WHR, PBF and VFA among the total population and women sleeping <7 h, 7–9 h and ≥ 9 h was presented in (Table 2). Women reporting short sleep had higher BMI than that of women reporting 7–9 h of sleep (p = 0.010) and > 9 h of sleep (p = 0.028), whereas WHR, PBF and VFA were not significantly different across sleep duration categories.
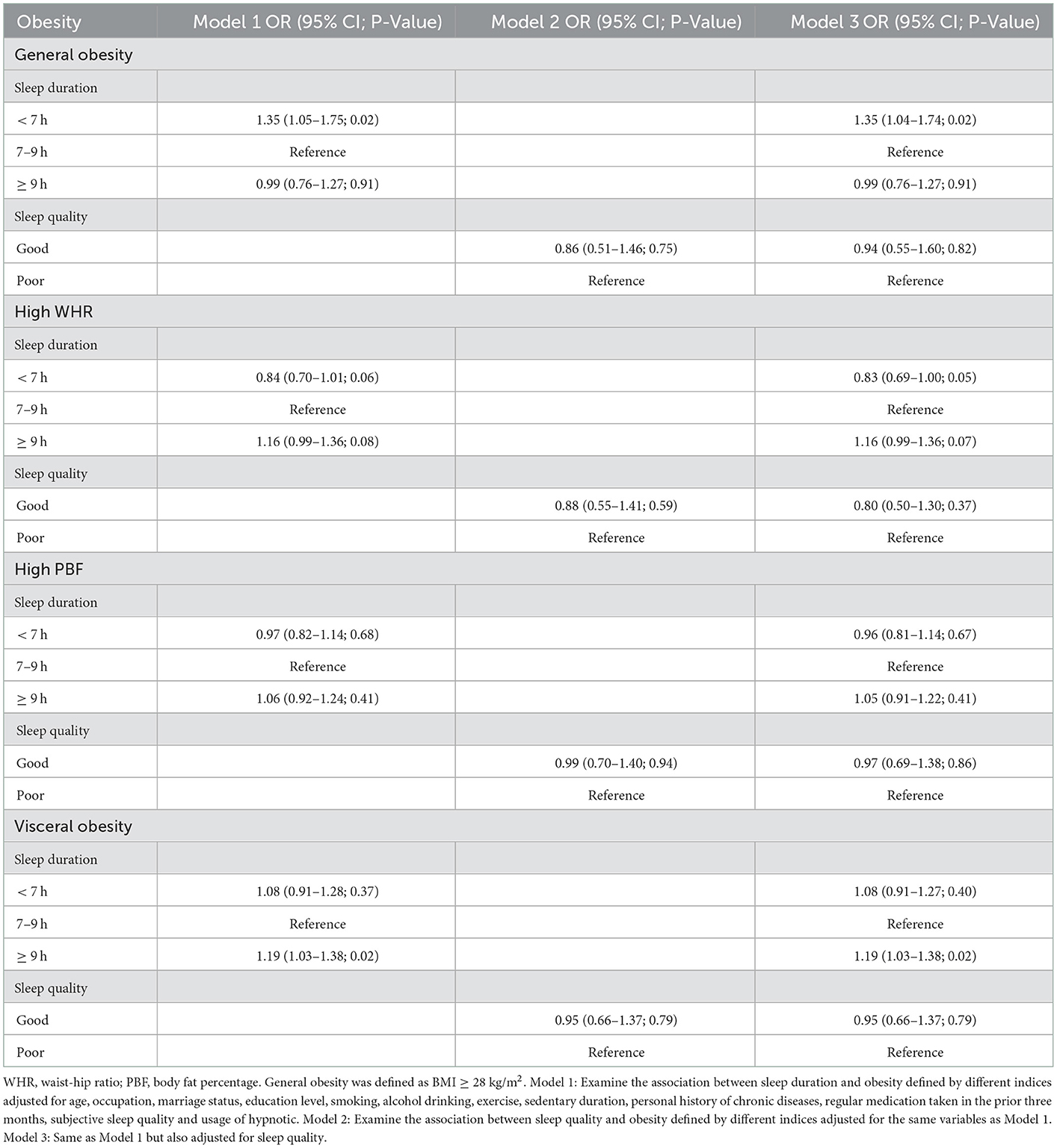
Table 3 showed the ORs and 95% CIs of sleep duration for obesity defined by BMI, WHR, PBF and VFA. After completely adjusting for sleep quality and all selected covariates (age, occupation, marriage status, education level, smoking, alcohol drinking, exercise, sedentary duration, personal history of chronic diseases, regular medication taken in the prior 3 months, subjective sleep quality, usage of hypnotic and sleep quality), sleep <7 h was associated with 35% elevated odds of general obesity (95% CI: 1.04–1.74), whereas sleep ≥ 9 h was associated with 19% elevated odds of visceral obesity (95% CI: 1.03–1.38). In addition, short sleep demonstrated a marginally significant association with high WHR (OR = 0.83, 95% CI: 0.69–1.00). No statistically significant correlations were observed between short sleep and high PBF or visceral obesity, long sleep and general obesity, high PBF or high WHR. We did not observe any significant association between sleep quality and obesity defined by any of the studied adiposity indicators.
4. Discussion
In the present study, we found short sleep was associated with general obesity, and excessive sleep was associated with visceral obesity among females. Women who slept <7 h had 35% increased odds of general obesity, while women who slept ≥ 9 h had 18% increased odds of visceral obesity compared with those sleeping 7–9 h per night. But no statistically significant association was observed between sleep duration and PBF or WHR.
An association between sleep and obesity has been suggested in a substantial body of research, but many previous studies used BMI or other conventional anthropometric indices [e.g., waist circumference (WC), WHR and waist-to-height ration (WHtR)] as adiposity measures (36, 37). It has been suggested that not only the mass but also specific anatomical locations of adipose depots are associated with the adverse impact on health (38). Visceral fat is highly metabolically active and is constantly releasing free fatty acids (FFA) into the portal circulation. As such, visceral fat content contributes to various features of the metabolic syndrome, such as hyperinsulinemia, systemic inflammation, dyslipidemia, and atherosclerosis (39, 40). In addition, visceral fat showed significant positive correlations with cardiovascular risk factor markers (41). In the current study, the prevalence of higher PBF, WHR, and visceral obesity were much higher than that of BMI, suggesting that BMI is not sufficient to identify the excess fat accumulation and the related health risk, a better understanding of the association of sleep duration with obesity defined by various indices may improve outcomes.
At present, several studies have concluded that lack of sleep is related to general obesity in adults, but the results have been inconsistent among women. Being consistent with our findings, studies conducted in Korea and America reported that short sleep was associated with the 18 and 22% increased odds of general obesity in women, respectively (16, 42). But, the study conducted among employees at a Japanese electric power company didn't observe a statistical association between short sleep and general obesity in women (43). Study results on the relationship between sleep duration and PBF had also been mixed. Women who slept for <6 h were found to have an increased risk of high PBF by a study from Urumqi, China (44). However, this study used 6–8 h of sleep as the reference, and high PBF was defined as a PBF higher than 30%, which were different from our study and may account for the inconsistent findings. Being consistent with our research, several studies have not found any relationship between sleep duration and PBF or WHR in women, including research using objectively measured sleep duration (45, 46). In the current study, no statistically significant association was observed between short sleep and VFA, several studies found similar results, whereas others reported positive results (23, 47). Research from China found the adjusted odds ratio for visceral obesity was 1.22 (95% CI: 1.02–1.45) in women sleeping <7 h compared with those sleeping 7–9 h, after adjusting for all confounding factors (23). Inconsistent with the current findings, our previous study linked short sleep with visceral obesity (OR = 1.22 95% CI: 1.02–1.45) (23). The different findings may be partially explained by the fact that the current study included more young women (60.9%) who had longer sleep and less visceral obesity than the previous. Age, independent of other risk factors, plays a fundamental role in both visceral obesity and sleep among females, especially in postmenopausal women. The higher proportion of young long sleepers and the lower proportion of visceral obesity in our study population may have made the association between long sleep and visceral obesity less statistically significant. Though the OR of chronic sleep deprivation for visceral obesity is not statistically significant in the present study, it is greater than the reference, suggesting that short sleep might be a potential risk factor for visceral obesity.
Though research reported a U-shaped relationship between sleep duration and BMI among adults, no association was found between long sleep, BMI, PBF and WHR among women in the present study (9). However, long sleep was found with 18% increased odds of visceral obesity compared with optimal sleep among women. A Swedish study found that long sleep could increase the risk of general obesity in women but decrease the risk of general obesity in men (21). Another study showed that long sleep females (≥ 9 h) demonstrated a higher prevalence of higher PBF (OR = 1.43, 95% CI: 1.04–1.96) compared with those who slept 7–8h (48). However, few studies have examined the relationship between sleep duration and WHR and VFA (46). The current study demonstrated a significant association between long sleep and elevated VFA, and a marginally significant association between long sleep and high WHR. Though research in Japan suggested that sleep duration was not related to visceral fat area and research in Korea demonstrated that the adjusted mean VFA and hepatic fat were highest in the shortest sleep duration group (<5 h) and decreased linearly with increasing sleep duration (49, 50). We found long sleep is associated with elevated odds of visceral obesity, which is similar to our previous study where the OR of long sleep for visceral obesity is marginally significant (OR = 1.10, 95% CI: 0.92–1.32) (23). We speculated the different populations, cutoff values of short or long sleep might contribute to the inconsistent findings. More longitudinal data is needed to further validate this finding.
Although, the pathophysiology of sleep duration and obesity has not yet been fully explored, several mechanisms have been proposed to explain the relationship between short sleep duration and obesity. First, a number of studies have shown that short sleep duration may lead to the decrease of leptin level and the increase of ghrelin level, which leads to the increase of hunger and appetite (9, 51). Chronic sleep restriction may contribute to the long-term hormone alteration (9). Second, chronic sleep deprivation leads to a decrease in melatonin levels, which affects brown adipose tissue replenishment and metabolism, therefore reduces energy expenditure and involves in the development of obesity (52). Third, shorter sleep duration is associated with obesity-related behaviors, changing the quantity, composition and distribution of the daily diet, consuming energy from snacks rather than regular meals, eating fewer vegetables and fruits, and feeling fatigue leading to reduced physical activity (53). At present, there was still few evidence on the potential mechanism of the association between long sleep and visceral obesity. Further studies are needed to investigate the underlying mechanism of this relationship and its implication.
We did not observe any significant relationship between sleep quality and obesity defined by the studied indices. Similar results were found in a study among Turkish women (54). However, studies in Chinese reproductive-aged women, middle-aged Italian adults, and German adults have linked sleep quality with WC, BMI and WC, and BMI and body fat mass, respectively (55–57). Longitudinal observations are needed to further explore the association between sleep quality and obesity defined by various adiposity indices.
Cautions should be taken when comparing our findings with other research for different criteria were used for short sleep. Many of the mentioned categorized short sleep as <6 h and even more extreme sleep restriction (≤ 5 h) (16, 43, 45–47). Because very few participants in our study reported <6 h of sleep, we then used 7–9 h as the optimal sleep duration and short sleep was categorized as <7 h. Despite the wide range of short sleep duration, positive results were observed regarding the unfavorable effect of <7 h sleep in the current study. The criteria we use for categorizing sleep duration was supported by the Consensus Statement released by the American Academy of Sleep Medicine and Sleep Research Society. In general, there was consensus that 6 h of sleep or less was associated with unfavorable health outcome, while 7–9 h of sleep were appropriate to support optimal health in adults. Consensus could not be reached on the appropriateness of 6–7 h of sleep, but the median vote suggested this duration was in the inappropriate range and the minimum threshold was set at 7 h as the lowest sleep duration appropriate to support optimal health in adults (58). Nevertheless, a narrower range for the sleep duration category was recommended for use in future studies that include sufficient numbers of people with <6 h of sleep to find a more precise link between sleep duration and obesity.
There were several strengths in the current study. First, the study was the first to investigate the relationship between sleep duration and VFA among Chinese females as far as we know. Second, we adjusted for subjective sleep quality and taking hypnotics as covariates. Prior study had shown that self-reported short sleep duration might be a sign of subjective poor sleep quality (59) and taking hypnotics might affect sleep duration, therefore, including subjective sleep quality and taking hypnotics as a covariate could help to evaluate the relationship between sleep duration and adiposity indices more accurately. Furthermore, short and long sleep was reported to be associated with chronic disease, which may have mixed effects on the relationship between sleep duration and obesity. In the current study, chronic disease status of the participants was also collected and included as covariate in the analysis to improve the accuracy and reliability of the study results.
However, our study also had several limitations. First, the cross-sectional nature of this study prevented a causal interpretation for sleep duration and adiposity indices. As obesity plays an essential role in the pathophysiology of sleep disorders and evidence on the bilateral and mutual interactions linking sleep and metabolic disorders is advanced, the observed correlations between sleep duration and obesity might be supportive for a reverse causation (60–62). Second, sleep duration in this study was self-reported rather than objective measured. However, measurement of objective sleep duration is not applicable for large sample studies for it needs participants to wear wrist activity monitors. Previous research had shown the consistency of sleep parameters derived from subjective questionnaires and those derived from polysomnography (63). Third, visceral fat area was measured by BIA rather than computed tomography and magnetic resonance imaging, However, CT and MRI need expensive and specialized equipment, and require participants to be exposed to radiation, which are not applicable for screening large size of general population. Finally, the participants were recruited at medical examination centers based on voluntary participation. The young age of the study population and the high proportion of desk jobs limits the generalizability of the current findings.
5. Conclusion
In conclusion, our results add to the current evidence to suggest that short sleep was associated with elevated odds of general obesity, whereas long sleep was associated with elevated odds of visceral obesity in women. Most research has suggested an association between sleep duration and obesity as defined by BMI, however, our results suggest that the association also involves obesity as defined by measures other than BMI, demonstrating the importance of using multiple adiposity indices to identify obesity associated with sleep duration. Prospective studies with various measures of adiposity are needed to confirm the observed cross-sectional correlations between sleep duration and obesity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Tianjin First Central Hospital (No. 2017N052KY) and Tianjin Union Medical Center (No. 2018C02). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MinZ has designed the study, supervised and oversaw the study implementation, wrote the manuscript, the guarantors of this work, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. JH conducted the investigation and participated in writing and revising the manuscript. YF conducted the analysis of the data. LZ participated in writing the manuscript. LZ, CL, and MiaZ have organized and managed the investigation. JH, YF, JZ, PG, BZ, CL, JZ, and MiaZ have participated in the investigation and the management of the data. All authors contributed substantially to the study, read, and approved the final manuscript.
Funding
This study was supported by the Chinese Key Research and Development Program (Nos. 2016YFC0900600 and 2016YFC0900604).
Acknowledgments
The survey was performed in Tianjin Union Medical Center and Tianjin First Central Hospital. We sincerely thank all study participants and the staff involved in facilitating and running the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in Body mass Overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/s0140-6736(17)32129-3
2. Sun X, Zheng B, Lv J, Guo Y, Bian Z, Yang L, et al. Sleep behavior and depression: findings from the china kadoorie biobank of 05 million chinese adults. J Affect Disord. (2018) 229:120–4. doi: 10.1016/j.jad.2017.12.058
3. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. (2013) 36:985–95. doi: 10.1038/hr.2013.70
4. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep Duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. (2015) 38:529–37. doi: 10.2337/dc14-2073
5. Smiley A, King D, Bidulescu A. The association between sleep duration and metabolic syndrome: the nhanes 2013/2014. Nutrients. (2019) 11:2582. doi: 10.3390/nu11112582
6. Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. (2017) 32:28–36. doi: 10.1016/j.smrv.2016.02.005
7. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. (2010) 34:791–9. doi: 10.1038/ijo.2010.5
8. Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. (2013) 93:359–404. doi: 10.1152/physrev.00033.2011
9. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. (2004) 1:210–7. doi: 10.1371/journal.pmed.0010062
10. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. (1991) 88:934–42. doi: 10.1172/JCI115396
11. Storz C, Heber SD, Rospleszcz S, Machann J, Sellner S, Nikolaou K, et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br J Radiol. (2018) 91:1089. doi: 10.1259/bjr.20170808
12. Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res. (2012) 45:591–600. doi: 10.1590/S0100-879X2012007500059
13. Mirzaei M, Khajeh M. Comparison of anthropometric indices (body mass index, waist circumference, waist to hip ratio and waist to height ratio) in predicting risk of type ii diabetes in the population of Yazd, Iran. Diabetes Metab Syndr. (2018) 12:677–82. doi: 10.1016/j.dsx.2018.04.026
14. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. (2011) 18:629–39. doi: 10.5551/jat.7922
15. Bacaro V, Ballesio A, Cerolini S, Vacca M, Poggiogalle E, Donini LM, et al. Sleep duration and obesity in adulthood: an updated systematic review and meta-analysis. Obes Res Clin Pract. (2020) 14:301–9. doi: 10.1016/j.orcp.2020.03.004
16. Cho KH, Cho EH, Hur J, Shin D. Association of sleep duration and obesity according to gender and age in korean adults: results from the korea national health and nutrition examination survey 2007-2015. J Korean Med Sci. (2018) 33:e345. doi: 10.3346/jkms.2018.33.e345
17. Li Q. The association between sleep duration and excess body weight of the american adult population: a cross-sectional study of the national health and nutrition examination survey 2015-2016. BMC Public Health. (2021) 21:335. doi: 10.1186/s12889-021-10369-9
18. Jia A, Xu S, Ming J, Zhou J, Guo J, Liu C, et al. Analysis of obesity epidemiology under different diagnostic criteria in China. Chin J Diab. (2017) 9:221–5. doi: 10.3760/cma.j.issn.1674-5809.2017.04.004
19. Zhang B, Wing YK. Sex Differences in insomnia: a meta-analysis. Sleep. (2006) 29:85–93. doi: 10.1093/sleep/29.1.85
20. Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. (2005) 165:25–30. doi: 10.1001/archinte.165.1.25
21. Westerlund A, Bottai M, Adami HO, Bellocco R, Nyren O, Akerstedt T, et al. Habitual sleep patterns and the distribution of body mass index: cross-sectional findings among swedish men and women. Sleep Med. (2014) 15:1196–203. doi: 10.1016/j.sleep.2014.06.012
22. He J, Hu S, Xu X, Guo P, Niu Y, Zhang J, et al. Association of long-term exposure to pm(25) in workplace with fasting plasma glucose among asymptomatic adults: a multicenter study in north China. Environ Int. (2022) 166:107353. doi: 10.1016/j.envint.2022.107353
23. Fan Y, Zhang L, Wang Y, Li C, Zhang B, He J, et al. Gender differences in the association between sleep duration and body mass index, percentage of body fat and visceral fat area among chinese adults: a cross-sectional study. BMC Endocr Disord. (2021) 21:247. doi: 10.1186/s12902-021-00913-4
24. Zhou BF The The Cooperative Meta-analysis Group of Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in chinese adults–study on optimal cut-off points of body mass index and waist circumference in chinese adults. Biomed Environ Sci. (2002) 15:83–96. doi: 10.1046/j.1440-6047.11.s8.9.x
25. WHO. Expert consultation. appropriate body-mass index for asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
26. Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 who expert consultation on waist circumference and waist-hip ratio. Eur J Clin Nutr. (2010) 64:2–5. doi: 10.1038/ejcn.2009.139
27. Yuji M. Examination committee of criteria for 'obesity disease' in Japan, Japan society for the study of obesity. new criteria for 'obesity disease' in Japan. Circ J. (2002) 66:987–92. doi: 10.1253/circj.66.987
28. World Health Organization. Physical status: the use and interpretation of anthropometry. report of a who expert committee. Tech Rep Ser. (1995) 854:1–452.
29. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
30. Liu X, Tang M, Hu L, Wang A, Wu H, Zhao G, et al. Reliability and validity of the pittsburgh sleep quality index. Chin J Psychiatry. (1996) 2:103–7.
31. Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, et al. Psychometric evaluation of the chinese version of the pittsburgh sleep quality index (CPSQI) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
32. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
33. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010
34. Ryu JY, Lee JS, Hong HC, Choi HY, Yoo HJ, Seo JA, et al. association between body size phenotype and sleep duration: korean national health and nutrition examination survey V (Knhanes V). Metabolism. (2015) 64:460–6. doi: 10.1016/j.metabol.2014.12.001
35. Wu J, Xu G, Shen L, Zhang Y, Song L, Yang S, et al. Daily sleep duration and risk of metabolic syndrome among middle-aged and older chinese adults: cross-sectional evidence from the dongfeng-tongji cohort study. BMC Pub Health. (2015) 15:178. doi: 10.1186/s12889-015-1521-z
36. Tse LA, Wang C, Rangarajan S, Liu Z, Teo K, Yusufali A, et al. Timing and length of nocturnal sleep and daytime napping and associations with obesity types in high-, middle-, and low-income countries. JAMA Netw Open. (2021) 4:e2113775. doi: 10.1001/jamanetworkopen.2021.13775
37. Al-Rashed F, Sindhu S, Al Madhoun A, Alghaith A, Azim R, Al-Mulla F, et al. Short sleep duration and its association with obesity and other metabolic risk factors in kuwaiti urban adults. Nat Sci Sleep. (2021) 13:1225–41. doi: 10.2147/NSS.S311415
38. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. doi: 10.3389/fcvm.2020.00022
39. Omura-Ohata Y, Son C, Makino H, Koezuka R, Tochiya M, Tamanaha T, et al. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc Diabetol. (2019) 18:137. doi: 10.1186/s12933-019-0941-y
40. Hiuge-Shimizu A, Kishida K, Funahashi T, Okutsu M, Kametani R, Kobayashi H, et al. Coexistence of visceral fat and multiple risk factor accumulations is strongly associated with coronary artery disease in Japanese (the vacation-J study). J Atheroscler Thromb. (2012) 19:657–63. doi: 10.5551/jat.13037
41. Shiina Y, Homma Y. Relationships between the visceral fat area on ct and coronary risk factor markers. Intern Med. (2013) 52:1775–80. doi: 10.2169/internalmedicine.52.9190
42. Mercy UC, Elenwa F, Ogunsola AS, Eniola OA, Yunusa I, Karaye IM. Sex differences in the association between short sleep duration and obesity among US adults: findings from nhanes, 2015-2020. Sleep Med. (2022) 92:59–63. doi: 10.1016/j.sleep.2022.03.004
43. Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. (2010) 33:161–7. doi: 10.1093/sleep/33.2.161
44. Yao XM, Cheng WB, Xiao H. Effects of sleep quality and duration on body composition in adults over 50 years old in Urumqi. Chin General Prace. (2018) 21:4072–6. doi: 10.12114/j.issn.1007-9572.2018.33.008
45. Ouyang Y, Wang Z, Wang H, Zhang B. Relationship between sleep duration and body fat percentage among elderly residents in 15 Provinces of China in 2015. J Environ Occup Med. (2019) 36:1113–8. doi: 10.13213/j.cnki.jeom.2019.19598
46. Larsen SC, Horgan G, Mikkelsen MLK, Palmeira AL, Scott S, Duarte C, et al. Association between objectively measured sleep duration, adiposity and weight loss history. Int J Obes. (2020) 44:1577–85. doi: 10.1038/s41366-020-0537-3
47. Dekker SA, Noordam R, Biermasz NR, de Roos A, Lamb HJ, Rosendaal FR, et al. Habitual sleep measures are associated with overall body fat, and not specifically with visceral fat, in men and women. Obesity. (2018) 26:1651–8. doi: 10.1002/oby.22289
48. Yan LX, Chen XR, Chen B, Bai YM Li JH, Zhang XW, et al. Gender-specific association of sleep duration with body mass index, waist circumference, and body fat in chinese adults. Biomed Environ Sci. (2017) 30:157–69. doi: 10.3967/bes2017.023
49. Kim NH, Lee SK, Eun CR, Seo JA, Kim SG, Choi KM, et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep. (2013) 36:723–9. doi: 10.5665/sleep.2636
50. Arakaki S, Maeshiro T, Hokama A, Hoshino K, Maruwaka S, Higashiarakawa M, et al. Factors associated with visceral fat accumulation in the general population in Okinawa, Japan. World J Gastrointest Pharmacol Ther. (2016) 7:261–7. doi: 10.4292/wjgpt.v7.i2.261
51. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. (2004) 141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008
52. Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. (2011) 12:167–88. doi: 10.1111/j.1467-789X.2010.00756.x
53. Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. (2009) 89:126–33. doi: 10.3945/ajcn.2008.26574
54. Öztürk ME, Yabanci Ayhan N. Associations between poor sleep quality, obesity, and the anthropometric measurements of women In Turkey. Ecol Food Nutr. (2018) 57:3–12. doi: 10.1080/03670244.2017.1406351
55. Li B, Liu N, Guo D, Li B, Liang Y, Huang L, et al. Association between sleep quality and central obesity among southern chinese reproductive-aged women. BMC Womens Health. (2021) 21:280. doi: 10.1186/s12905-021-01407-0
56. Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, et al. Sleep quality in obesity: does adherence to the mediterranean diet matter? Nutrients. (2020) 12:5. doi: 10.3390/nu12051364
57. Rahe C, Czira ME, Teismann H, Berger K. associations between poor sleep quality and different measures of obesity. Sleep Med. (2015) 16:1225–8. doi: 10.1016/j.sleep.2015.05.023
58. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the american academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. (2015) 38:1161–83. doi: 10.5665/sleep.4886
59. Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes. (2008) 32:801–9. doi: 10.1038/ijo.2008.4
60. Martins RC, Andersen ML, Tufik S. The reciprocal interaction between sleep and type 2 diabetes mellitus: facts and perspectives. Braz J Med Biol Res. (2008) 41:180–7. doi: 10.1590/S0100-879X2006005000194
61. Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. (2005) 9:211–24. doi: 10.1016/j.smrv.2005.01.006
62. Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. (2008) 114:211–23. doi: 10.1080/13813450802364627
Keywords: sleep duration, body mass index, percentage of body fat, visceral fat area, women
Citation: He J, Fan Y, Zhang L, Li C, Guo F, Zhu J, Guo P, Zhang B, Zhang M and Zhang M (2023) Habitual night sleep duration is associated with general obesity and visceral obesity among Chinese women, independent of sleep quality. Front. Public Health 11:1053421. doi: 10.3389/fpubh.2023.1053421
Received: 25 September 2022; Accepted: 02 January 2023;
Published: 23 January 2023.
Edited by:
Hidetaka Hamasaki, Hamasaki Clinic, JapanReviewed by:
Jenny Theorell-Haglöw, Uppsala University, SwedenMeram Azzani, MARA University of Technology, Malaysia
Copyright © 2023 He, Fan, Zhang, Li, Guo, Zhu, Guo, Zhang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minying Zhang,  WmhhbmdtaW55aW5nQG5hbmthaS5lZHUuY24=
WmhhbmdtaW55aW5nQG5hbmthaS5lZHUuY24=
 Jiangshan He1
Jiangshan He1 Chunjun Li
Chunjun Li Minying Zhang
Minying Zhang