- 1Immunization Center, Ningbo Municipal Center for Disease Control and Prevention, Ningbo, China
- 2Immunization Center, Ninghai County Center for Disease Control and Prevention, Ningbo, China
Background: Concern about the risk of peripheral facial palsy (PFP) following vaccination is one reason for hesitancy in influenza vaccination. However, the association between the flu vaccine and PFP is still controversial, and further evidence is urgently needed.
Methods: This self-controlled case series study evaluated PFP risk following inactivated influenza vaccine in the elderly using a large linked database in Ningbo, China. Relative incidence ratios (RIRs) and 95% confidence intervals (CIs) estimated using conditional Poisson regression were utilized to determine whether the risk of PFP was increased after vaccination.
Results: This study included 467 episodes, which occurred in 244 females and 220 males. One hundred twenty-four episodes happened within 1–91 days after vaccination, accounting for 26.7%. The adjusted RIRs within 1–30 days, 31–60 days, 61–91 days, and 1–91 days after influenza vaccination were 0.95 (95% CI 0.69–1.30), 1.08 (95% CI 0.78–1.49), 1.01 (95% CI 0.70–1.45), and 1.00 (95% CI 0.81–1.24), respectively. Similar results were found in subgroup analyses and sensitivity analyses.
Conclusions: Influenza vaccination does not increase PFP risk in the elderly population. This finding provides evidence to overcome concerns about facial paralysis after influenza vaccination.
1. Introduction
Peripheral facial palsy (PFP) is the partial or total loss of function of some or all of the tissues innervated by the facial nerve, of which Bell's palsy is a type of acute peripheral facial paralysis. The pathogenesis of the disease is still unclear, and it may be related to viral infection, autoimmune diseases, and neurological ischemia (1).
In 2004, a Swiss study reported an increased risk of Bell's palsy with inactivated intranasal influenza vaccine, directly triggering public concerns about the safety of influenza vaccines at the time (2). Since then, studies have suggested a possible link between influenza vaccination and facial paralysis (3–5). However, some studies have given conflicting results that influenza vaccines do not increase the risk of PFP (6–9). In the past 2 years, the case reports of facial paralysis after the 2019-nCoV vaccination have aroused the issue of vaccination and facial paralysis again (10–14). To our knowledge, so far, the association between influenza vaccination and PFP remains inconclusive, and further evidence is urgently needed (15). In addition, little is known about the risk of PFP following influenza vaccination in elderly Chinese.
According to the World Health Organization, the elderly are the highest-risk priority group for seasonal influenza vaccination. However, worries about vaccine-induced immune-mediated adverse reactions directly influence vaccination willingness (16). In reality, the coverage rate of influenza vaccine among the elderly population in most countries in the world is not high, far below the 75% vaccination coverage recommended by the World Health Organization (17).
During the 2020/2021 flu season, Ningbo, China implemented a free influenza vaccination campaign for the elderly. From September 2020 to February 2021, 278,959 people over the age of 70 were vaccinated against influenza, with a coverage rate of nearly 46%. In the current study, we used a self-controlled case series (SCCS) design for the first time to assess the risk of PFP after influenza vaccination in the elderly Chinese population through an electronic database linking vaccination records with regional diagnosis and treatment information.
2. Materials and methods
2.1. Data sources
Ningbo is a port city on the southeast coast of China and is geographically adjacent to Shanghai. In 2020, the population was over 9.4 million. The Ningbo Regional Health Care Database (NRHCD), a standardized medical information network in Ningbo, China, collects and integrates electronic health records from hospitals and community clinics in the jurisdiction. In 2016, the platform reached the top level in the regional health information interconnection standardization and maturity measurement of the National Health Commission (18–20). By 2019, the platform covered all 65 public hospitals and 154 primary medical institutions. Vaccination data from all clinics in the region are recorded electronically and transmitted in real time to the Ningbo Vaccination Registration Database (NVRD).
In this study, the NRHCD provided information on outpatient cases of PFP diagnosed between June 2020 and April 2021. The NVRD provided influenza vaccination information for the 2020/2021 influenza season (the last vaccination date was February 27, 2021). Both medical visits and vaccinations in the study area require registration of citizens' unique personal identification numbers, so two databases can be linked at an individual level through identification numbers. The linked records were deidentified before they were further processed and analyzed. This study was approved by the Ethical Review Committee of the Center for Disease Control and Prevention of Ningbo (IRB. No: Y2021005). The requirement for informed consent was waived.
2.2. Study population
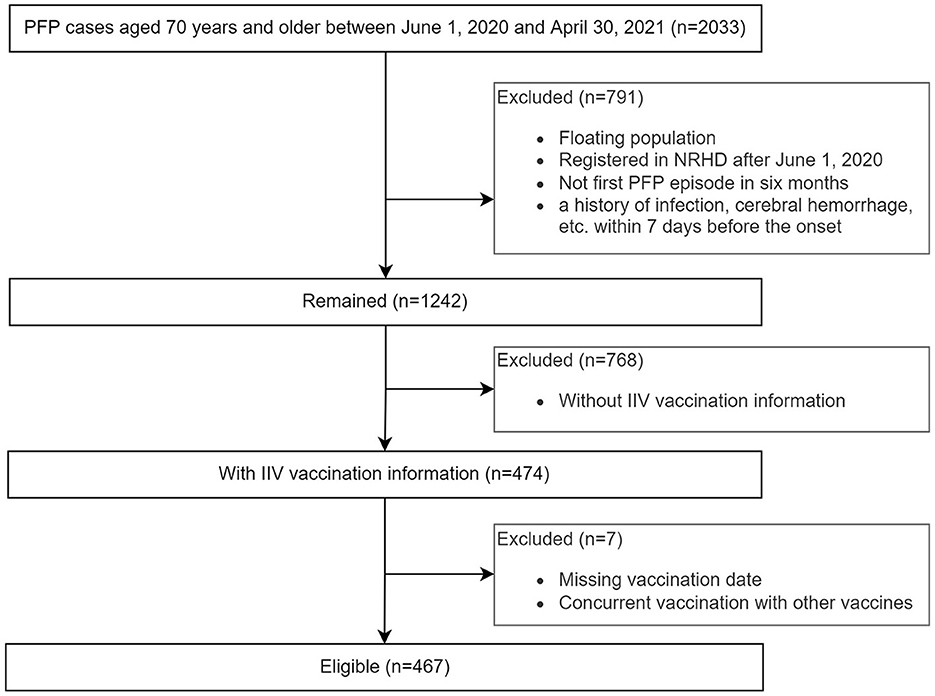
This study was restricted to outpatients aged 70 years and older who had at least one episode of PFP between June 1, 2020, and April 30, 2021. Analysis was further limited to patients who received parenteral trivalent inactivated influenza vaccine (IIV3, containing subtypes A/H1N1, A/H3N2, and B/Victoria) during the 2020/2021 influenza season. The date of onset of the first neurological symptom reported in the outpatient medical record was considered the case index date. If individuals had multiple episodes during the observation period, any recurrence within 6 months of the previous seizure was considered part of the same event. The study exclusion criteria were as follows: floating population, registered with NRHCD after June 1, 2020, missing vaccination date or vaccinated with other vaccines than influenza vaccine during the observation period.
PFP cases were identified by using the International Classification of Diseases version 10 (ICD-10) code G51.0 (Bell's palsy) and G51.9 (Disorder of facial nerve, unspecified). For these cases, we retrospectively reviewed the medical records 7 days before the onset of PFP and confirmed that there was no herpes virus infection, influenza, other acute infectious diseases, cerebral hemorrhage, etc.
2.3. Statistical analysis
We compared the risk of PFP after IIV3 vaccination in a risk period with the risk in a control period using a SCCS method. Referring to previous studies (7), the risk period in the primary analysis was defined as 1–91 days following vaccination, and the remaining observation period was defined as a control period. To avoid the impact of pre-vaccination health effects and day-of-administration chance records on background risk estimates (7, 21), we considered the 14 days before vaccination and the day of vaccination as separate risk periods. The seasonal effect was adjusted for winter-spring (from December to April) and summer-autumn (from June to November). Subgroup analyses were performed by age group and PFP type. Moreover, two sensitivity analyses were conducted: (1) using risk intervals of 1–14 days, 1–77 days, and 1–105 days following vaccination. (2) Only selecting the post-vaccination control period.
Relative incidence ratios (RIRs) and 95% confidence intervals (CIs) were estimated using conditional Poisson regression. A two-sided p < 0.05 indicated statistical significance. All analyses were performed by using R software, version 4.2.0.
2.4. Sample size
Under the assumption of 11 months of observation duration, 45.6% vaccine coverage, 80% power, and a type 1 alpha level of 0.05, 450 cases were required to identify a RIR of 1.5 or greater during 1–91 days following the IIV3 immunization.
3. Results
3.1. Characteristics of the elderly with PFP
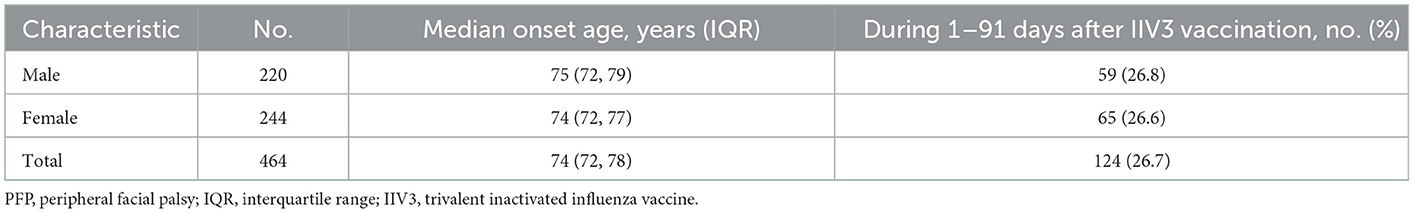
This study included 467 episodes, which occurred in 244 females and 220 males (Figure 1; Table 1). One hundred twenty-four episodes occurred within 1–91 days after IIV3 vaccination, accounting for 26.7%. Three individuals had two episodes. The age of onset ranged from 70 to 95 years, with a median age of 74 years. There were no significant differences in the age of onset and the proportion of PFP episodes within 1–91 days after IIV3 vaccination between males and females.
3.2. Risk of PFP following IIV3 vaccination
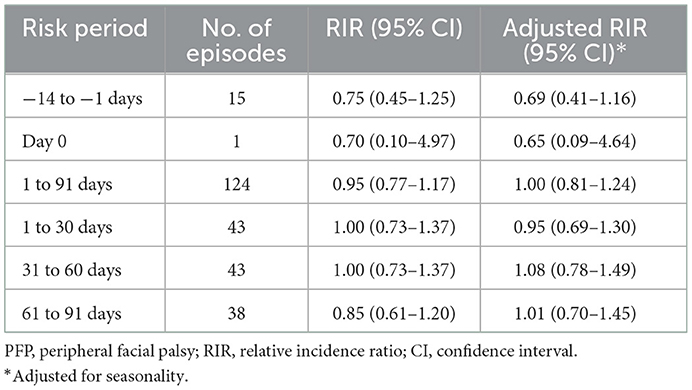
The risk of PFP within 1–91 days after influenza vaccination was not higher than the background risk. We divided the risk period into three 30-day periods and estimated the relative incidence ratio for each period. The results showed no significant risk increase was found in any period (Table 2). In addition, the risk on the day of vaccination and 14 days before vaccination was also comparable to the risk in the background period. Although PFP episodes did not show significant seasonality, we adjusted relative incidence ratio by season in our analysis because influenza vaccination was highly seasonal. Table 2 suggests that there is some confounding by season, but this does not change the interpretation of the results.
Subgroup analysis stratified by age group (Supplementary Table 1) and PFP type (Supplementary Table 2) also showed that the relative incidence ratio of PFP did not increase in each risk period after vaccination.
3.3. Sensitivity analysis
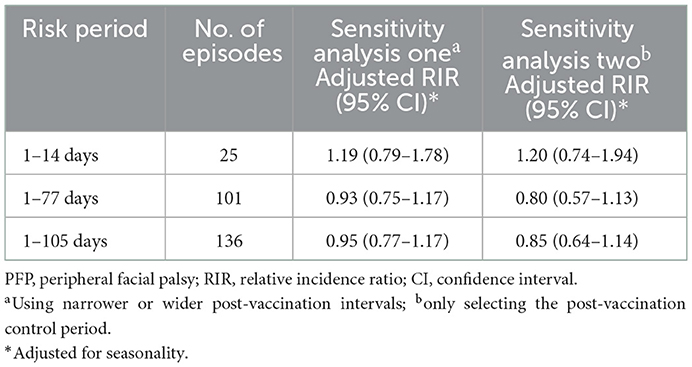
The sensitivity analysis results were consistent with those of the primary analysis regardless of whether a narrower or wider risk period was used, or only the post-vaccination control period was selected (Table 3).
4. Discussion
During the 2020/2021 influenza season, no increased risk of PFP was observed within 91 days after IIV3 vaccination among Chinese adults aged 70 years and older. Furthermore, subgroup analyses and sensitivity analyses did not reveal an association between influenza vaccine exposure and PFP. To our knowledge, this is the first study of the relationship between influenza vaccination and PFP in an elderly Chinese population using a large linked database. Although controversial in the scientific literature (22), our study provides evidence from China to support that influenza vaccination does not significantly increase the risk of PFP.
The possibility of vaccine-induced immune-mediated adverse effects has raised concerns about a link between vaccines and peripheral facial paralysis (15). However, our data did not identify a significant association between vaccination and PFP, which is consistent with the findings of other studies also designed for SCCS (6–9). Conversely, some studies observed an increased risk of PFP following influenza vaccination (3–5, 23–25). The most influential of these was the intranasal influenza vaccine used in Switzerland in 2000–2001, which was found to significantly increase the risk of PFP in this case-control study (adjusted OR, 84.0; 95% CI 20.1–351.9) (2). However, subsequent experimental studies demonstrated that an E. coli heat-labile toxin as an adjuvant for the intranasal route of administration was responsible for PFP (23). Two disproportionate analyses and one capture-recapture analysis using the Vaccine Adverse Event Reporting System (VAERS) database showed that Bell's palsy reported following seasonal influenza vaccination or influenza A (H1N1) vaccination exceeded the criteria for a potentially relevant signal (3, 4, 24). However, due to the inherent limitations of passive surveillance systems (e.g., underreporting, lack of adequate data quality, etc.) and the exploratory nature of both analyses, the likelihood of risk needs to be further assessed through controlled studies. In addition, a population-based retrospective cohort study in Sweden suggested a slightly increased risk of PFP following H1N1 influenza vaccination (25). However, the excess risk was only found in high-risk groups for influenza (25), and potential causes could be comorbidities, viral infections, and pregnancy (25, 26).
Previous studies have suggested that pre-vaccination healthy effects and opportunistic records on the day of vaccination may lead to bias in baseline risk estimates (7, 21, 27). In this regard, we analyzed the 14 days before vaccination and the day of vaccination as separate risk periods. Unexpectedly, we did not observe a significant reduction in PFP risk before vaccination and an increase in cases due to opportunistic recording on the day of vaccination. It is not inconceivable in clinical practice. After all, a history of PFP does not constitute a contraindication to influenza vaccination.
Our study had several strengths. First, this study was conducted based on comprehensive regional health care data, which allowed us to identify 2,033 cases of PFP, although the disease is uncommon. With 467 cases of vaccinated PFP, our investigation had the power to detect an RIR of 1.5, with an α risk of 5%, >80%. Despite controversial findings from previous studies, our study provides a strong argument to support that seasonal influenza vaccination is not associated with a significant increase in PFP risk. Second, the SCCS design can be used to avoid exposure misclassification bias by including only vaccinated individuals (27). In addition, time-invariant confounders could be also adjusted because vaccines placed themselves in the control group (21, 27). Finally, we conducted several sensitivity analyses to ensure the robustness of our primary results.
However, there were still some limitations. First, we only considered outpatient PFP cases, because NRHIP did not provide information on no-visits and inpatient cases. Due to the impact on appearance and life (28, 29), even mild cases usually need to seek medical advice in time. However, hospitalization is generally not required. Moreover, according to the routine consultation process, the patient will have an outpatient consultation record before being hospitalized. Second, without clinical examination records, we could not verify whether the cases had undergone nerve conduction tests. Third, if the influenza vaccine is given concurrently with other vaccines such as pneumonia or shingles during the observation period, the safety profile may vary. Although the proportion of concurrent vaccinations is <1%, our findings do not apply to this situation. Finally, the findings may not be suitable for extrapolation to all older populations, as only those 70 years of age and older were included in the study.
In conclusion, our findings are reassuring, and we did not find evidence of an increased risk of PFP within 91 days following IIV3 vaccination. While the antigenic composition of influenza vaccines is adjusted annually to include recently circulating virus strains, it is necessary to continuously monitor the safety of vaccination in future flu seasons, especially when new manufacturing processes or adjuvants are introduced.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ningbo Municipal Center for Disease Control and Prevention. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization: TY, LY, DH, and DZ. Methodology: TY, LY, and YF. Resources: RM, SZ, and XP. Data curation: QM and JW. Writing—original draft preparation: TY. Project administration: TY, DH, and DZ. Writing—review and editing: All authors. All authors have read and agreed to the manuscript.
Funding
This research was funded by the Public Welfare Technology Application Research Project of Zhejiang Province (grant number: GF22H261027), the Public Welfare Science and Technology Project of Ningbo City (grant number: 2022S071), and Ningbo Medical and Health Brand Discipline (grant number: PPXK2018-10). The funders had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication.
Acknowledgments
We thank Hongjun Dong (retired) and Wei Ji (from Big Data Institute, Ningbo Municipal Center for Disease Control and Prevention) for their help in collecting and cleaning the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1047391/full#supplementary-material
References
1. Rath B, Gidudu JF, Anyoti H, Bollweg B, Caubel P, Chen YH, et al. Facial nerve palsy including Bell's palsy: case definitions and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. (2017) 35:1972–83. doi: 10.1016/j.vaccine.2016.05.023
2. Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. (2004) 350:896–903. doi: 10.1056/NEJMoa030595
3. Kamath A, Maity N, Nayak MA. Facial paralysis following influenza vaccination: a disproportionality analysis using the vaccine adverse event reporting system database. Clin Drug Investig. (2020) 40:883–9. doi: 10.1007/s40261-020-00952-0
4. Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT, et al. A potential signal of Bell's palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)-United States, 1991–2001. Pharmacoepidemiol Drug Saf. (2004) 13:505–10. doi: 10.1002/pds.998
5. Gocko X, Poulteau S, Beyens MN, Bertholon P, Pozzetto B. Case report: Recurrent peripheral facial paralysis following two influenza vaccinations in 2009 and 2016. Vaccine. (2019) 37:4864–6. doi: 10.1016/j.vaccine.2019.07.025
6. Jeong N, Kim Y, Kim C, Park S, Lee J, Choi N. Association between influenza vaccination and the risk of Bell's Palsy in the Korean elderly. Vaccines (Basel). (2021) 9:746. doi: 10.3390/vaccines9070746
7. Stowe J, Andrews N, Wise L, Miller E. Bell's palsy and parenteral inactivated influenza vaccine. Hum Vaccin. (2006) 2:110–2. doi: 10.4161/hv.2790
8. Wijnans L, Dodd CN, Weibel D, Sturkenboom M. Bell's palsy and influenza(H1N1)pdm09 containing vaccines: a self-controlled case series. PLoS ONE. (2017) 12:e0175539. doi: 10.1371/journal.pone.0175539
9. Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH, et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. Am J Prev Med. (2011) 41:121–8. doi: 10.1016/j.amepre.2011.04.004
10. Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. (2021) 14:e243829. doi: 10.1136/bcr-2021-243829
11. Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. (2021) 111:310–2. doi: 10.1016/j.ijid.2021.08.071
12. Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. (2021) 268:3589–91. doi: 10.1007/s00415-021-10462-4
13. Gomez de Terreros Caro G, Gil Diaz S, Perez Ale M, Martinez Gimeno ML. Bell's palsy following COVID-19 vaccination: a case report. Neurologia (Engl Ed). (2021) 36:567–8. doi: 10.1016/j.nrleng.2021.04.002
14. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. (2021) 21:450–2. doi: 10.1016/S1473-3099(21)00076-1
15. Ahsanuddin S, Nasser W, Roy SC, Povolotskiy R, Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. (2022) 39:80–4. doi: 10.1093/fampra/cmab068
16. Meijome-Blanco S, Gonzalez-Cristobo G, Regueiro-Martinez AA. Analysis of the refusal of the flu vaccination (REGRIVI study). Semergen. (2018) 44:395–9. doi: 10.1016/j.semerg.2017.12.003
17. Ababneh M, Jaber M, Rababa'h A, Ababneh F. Seasonal influenza vaccination among older adults in Jordan: prevalence, knowledge, and attitudes. Hum Vaccin Immunother. (2020) 16:2252–6. doi: 10.1080/21645515.2020.1718438
18. Liu Z, Zhang L, Yang Y, Meng R, Fang T, Dong Y, et al. Active surveillance of adverse events following human papillomavirus vaccination: feasibility pilot study based on the regional health care information platform in the City of Ningbo, China. J Med Internet Res. (2020) 22:e17446. doi: 10.2196/17446
19. Chen Q, Che Y, Xiao Y, Jiang F, Chen Y, Zhou J, et al. Impact of multimorbidity subgroups on the health care use and clinical outcomes of patients with tuberculosis: a population-based cohort analysis. Front Public Health. (2021) 9:756717. doi: 10.3389/fpubh.2021.756717
20. Chen Q, Hu A, Ma A, Jiang F, Xiao Y, Chen Y, et al. Effectiveness of prophylactic use of hepatoprotectants for tuberculosis drug-induced liver injury: a population-based cohort analysis involving 6,743 Chinese patients. Front Pharmacol. (2022) 13:813682. doi: 10.3389/fphar.2022.813682
21. Hawken S, Potter BK, Little J, Benchimol EI, Mahmud S, Ducharme R, et al. The use of relative incidence ratios in self-controlled case series studies: an overview. BMC Med Res Methodol. (2016) 16:126. doi: 10.1186/s12874-016-0225-0
22. Bertin B, Grenet G, Pizzoglio-Billaudaz V, Lepelley M, Atzenhoffer M, Vial T. Vaccines and Bell's palsy: a narrative review. Therapie. (2022). doi: 10.1016/j.therap.2022.07.009
23. Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE. (2009) 4:e6999. doi: 10.1371/journal.pone.0006999
24. Huang WT, Huang WI, Huang YW, Hsu CW, Chuang JH. The reporting completeness of a passive safety surveillance system for pandemic (H1N1) 2009 vaccines: a capture-recapture analysis. Vaccine. (2012) 30:2168–72. doi: 10.1016/j.vaccine.2012.01.013
25. Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. (2011) 343:d5956. doi: 10.1136/bmj.d5956
26. Ortqvist A, Berggren I, Insulander M, de Jong B, Svenungsson B. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pandemic strain of influenza A(H1N1)v, in Stockholm County, Sweden. Clin Infect Dis. (2011) 52:1203–11. doi: 10.1093/cid/cir182
27. Baker MA, Lieu TA Li L, Hua W, Qiang Y, Kawai AT, et al. A vaccine study design selection framework for the postlicensure rapid immunization safety monitoring program. Am J Epidemiol. (2015) 181:608–18. doi: 10.1093/aje/kwu322
28. Moverare T, Lohmander A, Hultcrantz M, Sjogreen L. Peripheral facial palsy: speech, communication and oral motor function. Eur Ann Otorhinolaryngol Head Neck Dis. (2017) 134:27–31. doi: 10.1016/j.anorl.2015.12.002
29. Bruins TE, van Veen MM, Werker PMN, Dijkstra PU, Broekstra DC. Associations between clinician-graded facial function and patient-reported quality of life in adults with peripheral facial palsy: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2021) 147:717–28. doi: 10.1001/jamaoto.2021.1290
Keywords: peripheral facial palsy, inactivated influenza vaccination, self-controlled case series study, elderly, relative incidence ratio
Citation: Yang T, Ma R, Ye L, Mei Q, Wang J, Feng Y, Zhou S, Pan X, Hu D and Zhang D (2023) Risk of peripheral facial palsy following parenteral inactivated influenza vaccination in the elderly Chinese population. Front. Public Health 11:1047391. doi: 10.3389/fpubh.2023.1047391
Received: 18 September 2022; Accepted: 06 January 2023;
Published: 24 January 2023.
Edited by:
Jose Ricardo Testa, Federal University of São Paulo, BrazilReviewed by:
Jifang Zhou, China Pharmaceutical University, ChinaHanqing He, Immunization Program, China
Yan Liu, Hangzhou Center for Disease Control and Prevention (HZCDC), China
Copyright © 2023 Yang, Ma, Ye, Mei, Wang, Feng, Zhou, Pan, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Zhang,  Y24teWFuZ3RjQG91dGxvb2suY29t
Y24teWFuZ3RjQG91dGxvb2suY29t
†These authors have contributed equally to this work
 Tianchi Yang
Tianchi Yang Rui Ma1
Rui Ma1