- Guangdong Provincial Key Laboratory of Tropical Disease Research, Department of Epidemiology, School of Public Health, Southern Medical University, Guangzhou, China
Objectives: SARS-CoV-2 infection and COVID-19 vaccination of homeless people are a serious public health concern during COVID-19 pandemic. We aimed to systematically assess SARS-CoV-2 incidence, seroprevalence, and COVID-19 vaccination coverage in homeless people, which are important to inform resource allocation and policy adjustment for the prevention and control of COVID-19.
Methods: We searched PubMed, Web of Science, and the World Health Organization COVID-19 database for the studies of SARS-CoV-2 incidence, seroprevalence, and COVID-19 vaccination coverage in the homeless population. Subgroup analyses were conducted to pool SARS-CoV-2 incidence and seroprevalence in sheltered homeless, unsheltered homeless, and mixed population, respectively. Potential sources of heterogeneity in the estimates were explored by meta-regression analysis.
Results: Forty-nine eligible studies with a total of 75,402 homeless individuals and 5,000 shelter staff were included in the meta-analysis. The pooled incidence of SARS-CoV-2 infection was 10% (95% CI: 7 to 12%) in the homeless population and 8% (5 to 12%) for shelter staff. In addition, the overall estimated SARS-CoV-2 specific seroprevalence was 19% (8 to 33%) for homeless populations and 22% (3 to 52%) for shelter staff, respectively. Moreover, for the homeless subjects, the pooled incidence was 10% (4 to 23%) for asymptomatic SARS-CoV-2 infections, 6% (1 to 12%) for symptomatic SARS-CoV-2 infections, 3% (1 to 4%) for hospitalization for COVID-19, and 1% (0 to 2%) for severe COVID-19 cases, respectively while no COVID-19-related death was reported. Furthermore, the data derived from 12 included studies involving 225,448 homeless individuals revealed that the pooled proportion of one dose COVID-19 vaccination was 41% (35 to 47%), which was significantly lower than those in the general population.
Conclusion: Our study results indicate that the homeless people remain highly susceptible to SARS-CoV-2 infection, but COVID-19 vaccination coverage was lower than the general population, underscoring the need for prioritizing vaccine deployment and implementing enhanced preventive measures targeting this vulnerable group.
1. Introduction
As of March 10, 2023, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 670 million infections and approximately 6.9 million deaths with a mortality of ~1.0% (1). Within less than 12 months since the initial outbreak of SARS-CoV-2 infection in late December 2019 (2), a large amount of vaccines against the coronavirus disease 2019 (COVID-19) had been developed based on several different technologies and platforms, and authorized for use around the world (3). Till now, 70.3% of the world population have been vaccinated with at least one dose of COVID-19 vaccine (4). However, SARS-CoV-2 variants are continuously emerging and spreading across the world. SARS-CoV-2 variants of concern including Alpha, Beta, Gamma, Delta, and Omicron show specific biological feature, such as enhanced resistance to immunity protection induced by COVID-19 vaccine (5–10). In addition, waning protection over time against the infection of SARS-CoV-2 and COVID-19 has been documented (11–14). Therefore, the ongoing pandemic of COVID-19 has not yet subsided. It is necessary to timely monitor and track SARS-CoV-2 circulation especially in the marginalized population such as homeless people who might move or travel easily from place to place, and make the tracking and prevention of SARS-CoV-2 transmission more difficult (15).
Homelessness is recognized as a serious issue and challenge of global concern due to the possible unprecedented outbreaks of COVID-19 among these people (16). In general, homeless people staying in shelters (sheltered homeless), or on the streets and other similar settings (unsheltered homeless) are denoted as homelessness (17). In 2019, there were about 700,000 homeless people on a single night in the European Union while the number increased by 70% in a decade (18). According to the 2021 annual homeless assessment report released by the U.S. Department of housing and urban development, there were 326,126 sheltered homeless people on any given night in January of 2021 in the United States (19). Homeless people usually possess increased susceptibility to infectious disease and mental disorders (20, 21), and show poor adherence to public health recommendations and limited access to testing, vaccine, or medical service (17, 22–24). Therefore, the containment of SARS-CoV-2 transmission among homeless people may be difficult and complicated. Specht et al. (25) proposes to enhance health communication with homeless people by bridging the “digital gap” and mitigate the structural marginalization of them. In order to interrupt the spread of SARS-CoV-2 among this vulnerable group and further from them into the general population, a comprehensive analysis to clarify SARS-CoV-2 incidence, seroprevalence, and COVID-19 vaccination coverage in homeless people is important for planning and deploying health services tailored to them (20).
One meta-analysis reported the pooled prevalence of 2.3% at baseline and 31.6% in the situation of SARS-CoV-2 outbreak among homeless people between January 2020 and October 2020 (26). In addition, another study identified a prevalence of symptomatic COVID-19 infection of 35% in the homeless and a higher rate of vaccine hesitancy than the general population during the first year of the pandemic (27). However, since 2021, the global COVID-19 pandemic has changed including the emergence of more transmissible SARS-CoV-2 Omicron variant, and worldwide massive vaccination (28). Furthermore, quite different COVID-19 vaccination rates have been reported in the homeless population (24, 29–39). In this study, we conducted an updated meta-analysis and systematic review on SARS-CoV-2 incidence, seroprevalence, and COVID-19 vaccination coverage in homeless individuals.
2. Methods
2.1. Search strategy and selection criteria
We searched PubMed, Web of Science, and the World Health Organization COVID-19 database by using the combinations of terms relating to SARS-CoV-2 infection (2019-nCoV OR SARS-CoV-2 OR COVID-19) and being homelessness (homeless* OR roofless OR shelter*) for studies of SARS-CoV-2 incidence and seroprevalence in the homeless population published from December 1, 2019 to July 31, 2022. We also screened the reference lists of all the eligible primary studies as well as the relevant review articles to identify other related studies. The meta-analysis was conducted following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (40) (Supplementary Checklist S1). Studies on the COVID-19 vaccination coverage in homeless people were identified through searches PubMed, Web of science, the World Health Organization COVID-19 database up to August 10, 2022 using the following search strategy: ((((SARS-CoV-2) OR (Covid-19)) OR (2019-nCoV)) AND (((homeless*) OR (roofless)) OR (shelter*))) AND (vaccine*).
The included studies met the following criteria: (1) study subjects were homeless people; (2) diagnosis of SARS-CoV-2 infection was based on the specific testing assays, such as nucleic acid amplification tests (NAATs), antigen tests, or serological tests (41) (3) anti-SARS-CoV-2 seropositivity was not the immunological response induced by COVID-19 vaccination; (4) the data to determine SARS-CoV-2 incidence or seroprevalence were available. We excluded the studies or papers if: (1) they were reviews, editorial, opinions, case reports or animal studies; (2) the number of homeless individuals was not reported or could not be obtained from the authors.
2.2. Data extraction
Three authors (QS, QL, and YP) independently extracted the following information, i.e., the first author, year of publication, study period, country, study subjects, number of the investigated homeless individuals, gender, age, category of homelessness, diagnostic method/criteria and number of homeless people diagnosed with SARS-CoV-2 infection, number of vaccinated people, number of asymptomatic SARS-CoV-2 infections, number of symptomatic SARS-CoV-2 infections, number of COVID-19-related hospitalization, number of severe COVID-19 cases, and COVID-19-related mortality. The severity of illness was assessed according to the seventh version guideline for the diagnosis and treatment of COVID-19 published by the National Health Commission of China (42) and classified into: (1) a symptomatic carriers present with no clinical symptom but with a positive result of the pathogens tests of SARS-CoV-2 in respiratory tract specimens and so on; (2) mild patients have mild clinical symptoms and no pneumonia on chest imaging; (3) moderate patients have clinical symptoms (i.e., fever and respiratory tract symptoms) and pneumonia on chest imaging. (4) Severe patients who meet any one of the following criteria: respiratory rate ≥30 breaths/min; resting oxygen saturation ≤93%; arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mmHg; disease progression within 24 to 48 h on chest image. Any disagreement between the three authors was resolved by discussing with the corresponding author YL or ST to reach a consensus.
2.3. Quality assessment
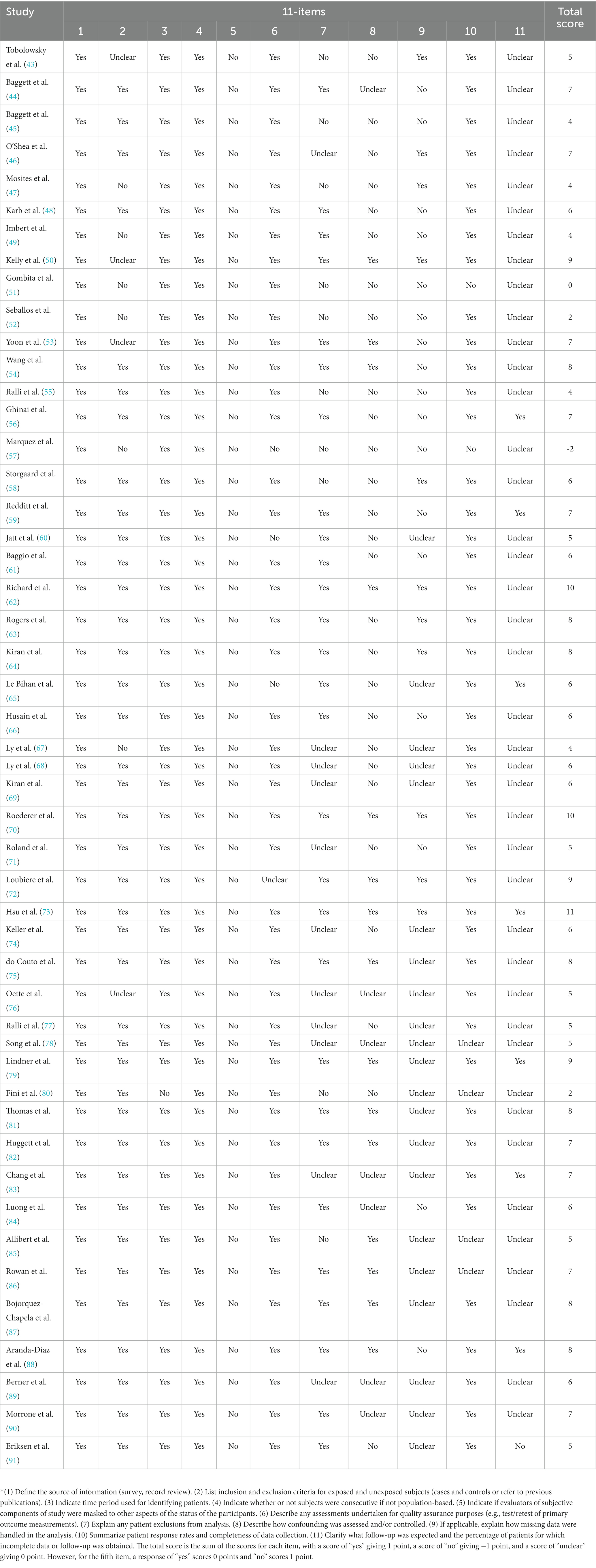
The methodological quality of the included studies was assessed using an 11-item checklist which was recommended by Agency for Healthcare Research and Quality (AHRQ). The total score is the sum of the scores for each item, with a score of “yes” giving 1 point, a score of “no” giving −1 point, and a score of “unclear” giving 0 point (Table 1).
2.4. Statistical analysis
The SARS-CoV-2 incidence or seroprevalence estimated by individual study was transformed with the Freeman–Tukey double arcsine function before pooling the incidence or seroprevalence to decrease the effect of studies with extremely low frequency on the overall estimate (92). Since the asymptotic method produces intervals that may extend below zero, the 95% confidence intervals (CIs) around these estimates were calculated by the Wilson method (93, 94). Moreover, both Cochran’s Q (reported as χ2 value and p-value) and the I2 statistic were applied to estimate the inter-studies heterogeneity. A p < 0.05 from Cochrane’s chi-square (χ2) test or I2 statistic value >75% indicated substantial heterogeneity (95, 96). A random effect model was used in the situations with substantial inter-studies heterogeneity; otherwise, a fixed effect model was adapted (95). Publication bias was assessed by using Egger and Begg tests (97, 98). Furthermore, subgroup analyses were conducted to explore the SARS-CoV-2 incidence and seroprevalence according to homelessness category (sheltered, unsheltered, and mixed population). If repeat testing was performed in the given shelter for the homeless, the screening with the largest sample size was included in quantitative synthesis. We have also conducted an additional analysis that compared the incidence of SARS-CoV-2 infection in homeless people with the estimated cumulative incidence of SARS-CoV-2 in the total general population during corresponding period to calculate incidence ratios. Information about the cumulative incidence of SARS-CoV-2 in the total general population by country or region was obtained from Our World in Data.1 All the analyses were done by using the Package “meta” in R software (version 4.2.1, R Foundation for Statistical Computing). A two-sided p < 0.05 was considered statistically significant.
3. Results
3.1. Study selection
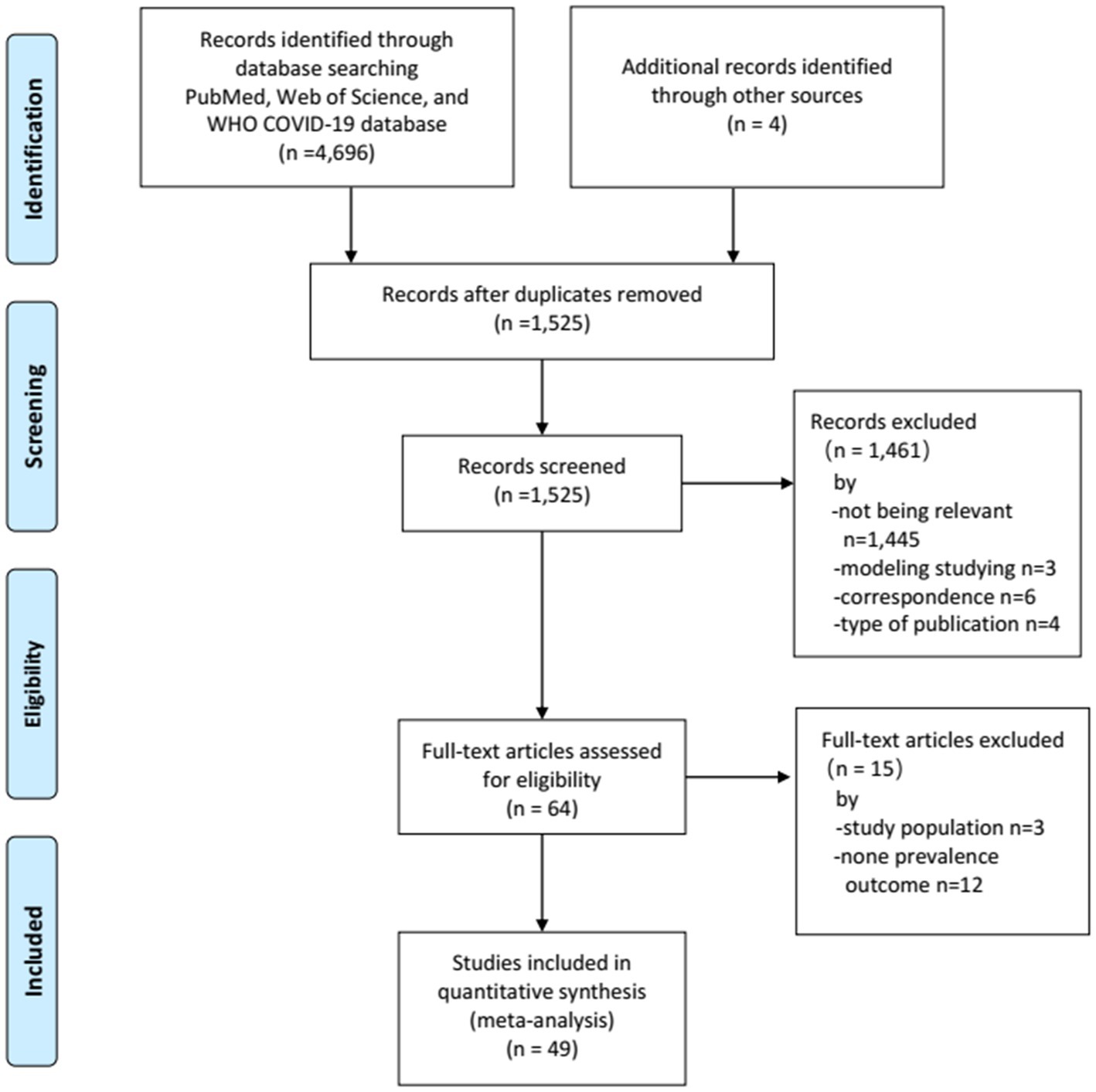
Our literature search yielded a total of 4,696 records, of which 1,230 were retrieved from PubMed, 1,425 from Web of Science, and 2,041 from WHO COVID-19 database. An additional 4 reports were identified from the reference lists of the relevant review articles. After removing the duplicates, 1,525 titles and abstracts were eligible for screening. Of these, 1,461 studies were discarded after reviewing the titles and abstracts. Furthermore, 15 studies were discarded after full-text screening. Finally, 49 studies (43–91) involving 75,402 homeless individuals met the eligibility criteria and were included in the meta-analysis (Figure 1).
3.2. Characteristics of the included studies
Out of the 49 included studies (Supplementary Table S1), 20 eligible studies (N = 29,513) were conducted in the United States (43–45, 47–50, 52, 53, 56, 57, 60, 63, 73, 74, 82, 83, 86, 88, 89), 7 (N = 25,074) in Canada (46, 54, 59, 62, 64, 69, 84), 7 (N = 3,957) in France (65–68, 70, 72, 85), 2 (N = 4,870) in the United Kingdom (78, 81), 2 (N = 757) in Denmark (58, 91), 2 (N = 181) in Germany (76, 79), 2 (N = 6,641) in Italy (55, 90), and one each from Belgium (N = 1,985) (71), Brazil (N = 203) (75), Iran (N = 234) (80), Mexico (N = 481) (87), Slovakia (N = 331) (51), Switzerland (N = 215) (61) and Vatican (N = 960) (77), respectively. The estimated pooled median age of 45.0 (95% CI, 42.9, 47.1) years was reported in 33 articles, and 37 publications reported gender of homeless people whose majority was male.
The majority (83.7%, 41/49) of the included studies was cross-sectional study. Thirty-two studies reported the SARS-CoV-2 incidence or seroprevalence in sheltered homeless and their median sample size was 331 (QTR 51-11,463) (43, 44, 46–51, 53, 54, 56–60, 63, 66–69, 71, 73, 75, 79, 82, 83, 86–89, 91) while 15 studies also simultaneously investigated SARS-CoV-2 incidence or seroprevalence among the shelter staff (N = 5,000) (43, 46, 47, 49, 53, 56, 63, 66, 67, 75, 77, 83, 88, 89, 91) (Supplementary Tables S1, S2). Four studies (N = 1,351) (53, 65, 80, 86) were conducted in the unsheltered homeless people while 15 (N = 31,232) (44, 52, 55, 61, 62, 70, 72, 74, 76–78, 81, 84, 85, 90) in the mixed population comprising sheltered and unsheltered homeless subjects whose SARS-CoV-2 incidence or seroprevalence was not separately reported. For the diagnosis of active SARS-CoV-2 infection in homeless people, 40 studies were based on NAATs alone, 1 study was based on antigen tests alone, and 1 investigation was based on the combination of NAAT and antigen tests. Moreover, the seroprevalence of SARS-CoV-2 was evaluated in the homeless population in 11 surveys (51, 55, 58, 66, 70, 72, 75, 85–87, 91) (Supplementary Table S1).
3.3. SARS-CoV-2 incidence and seroprevalence in the homeless population
SARS-CoV-2 incidence ranged from 0 to 67% with very high heterogeneity among the studies (I2 = 99%, p = 0) (Figure 2). The random-effect pooled incidence of SARS-CoV-2 infection was 10% (95% CI, 7, 12%) whereas 11% (8, 15%) for sheltered homeless, 4% (0, 11%) for unsheltered homeless, and 8% (5, 12%) for the mixed population, respectively (Figure 2). Moreover, the random-effect pooled incidence was 10% (4, 23%) for asymptomatic SARS-CoV-2 infections (Figure 3A), 6% (1, 12%) for symptomatic SARS-CoV-2 infections (Figure 3B), 3% (1, 4%) for the COVID-19-related hospitalization (Figure 4A), 1% (0, 2%) for severe COVID-19 (Figure 4B), respectively although no COVID-19-related death was reported (Figure 4C). Of note, the random-effect pooled incidence of SARS-CoV-2 infection remained 10% (8, 12%) with substantial heterogeneity (I2 = 99%, p = 0) when SARS-CoV-2 infection was diagnosed by NAATs alone in homeless people (Supplementary Figure S1).
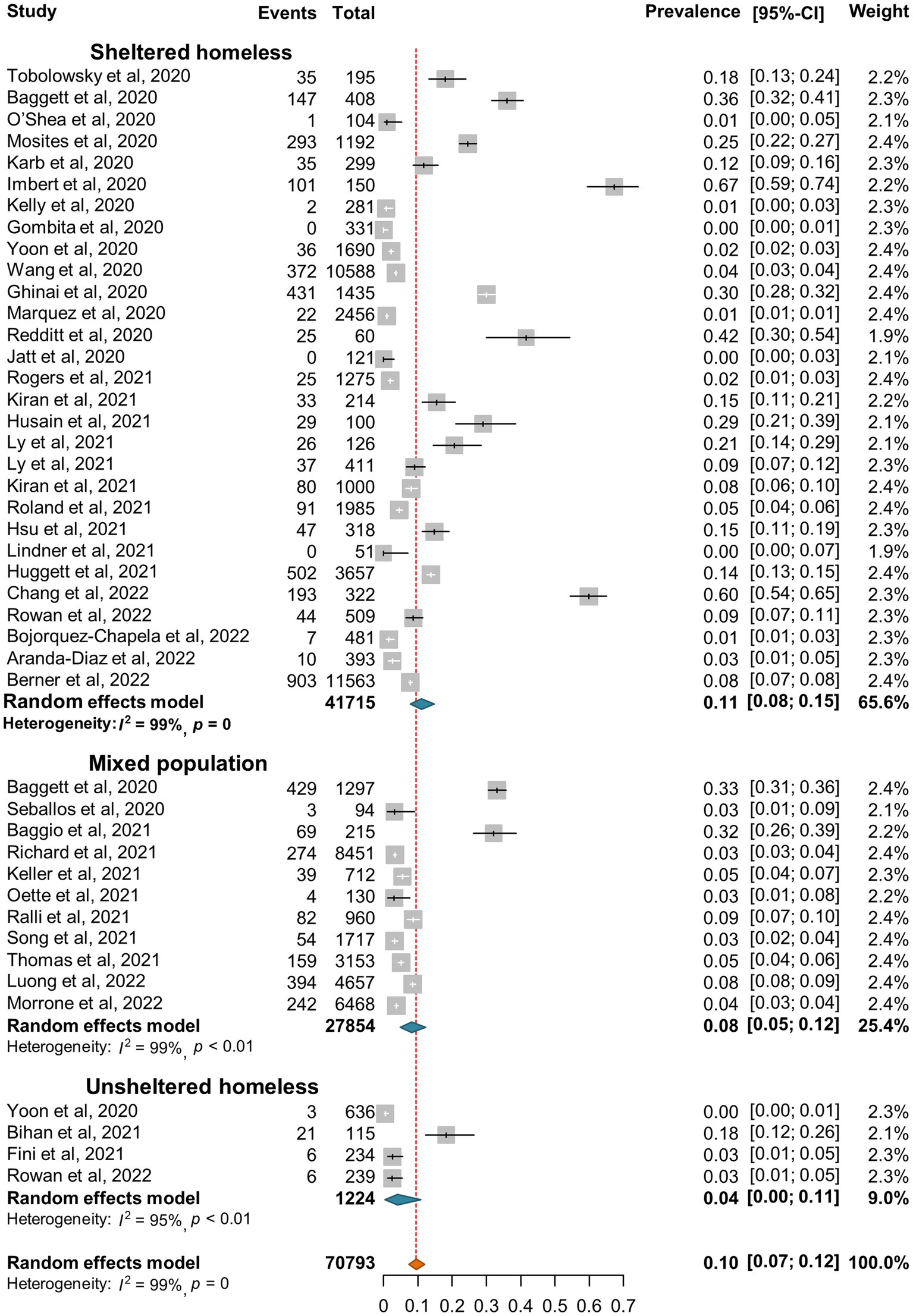
Figure 2. Forest plots of the estimated incidence of SARS-CoV-2 infection in homeless people according to the category of homeless.
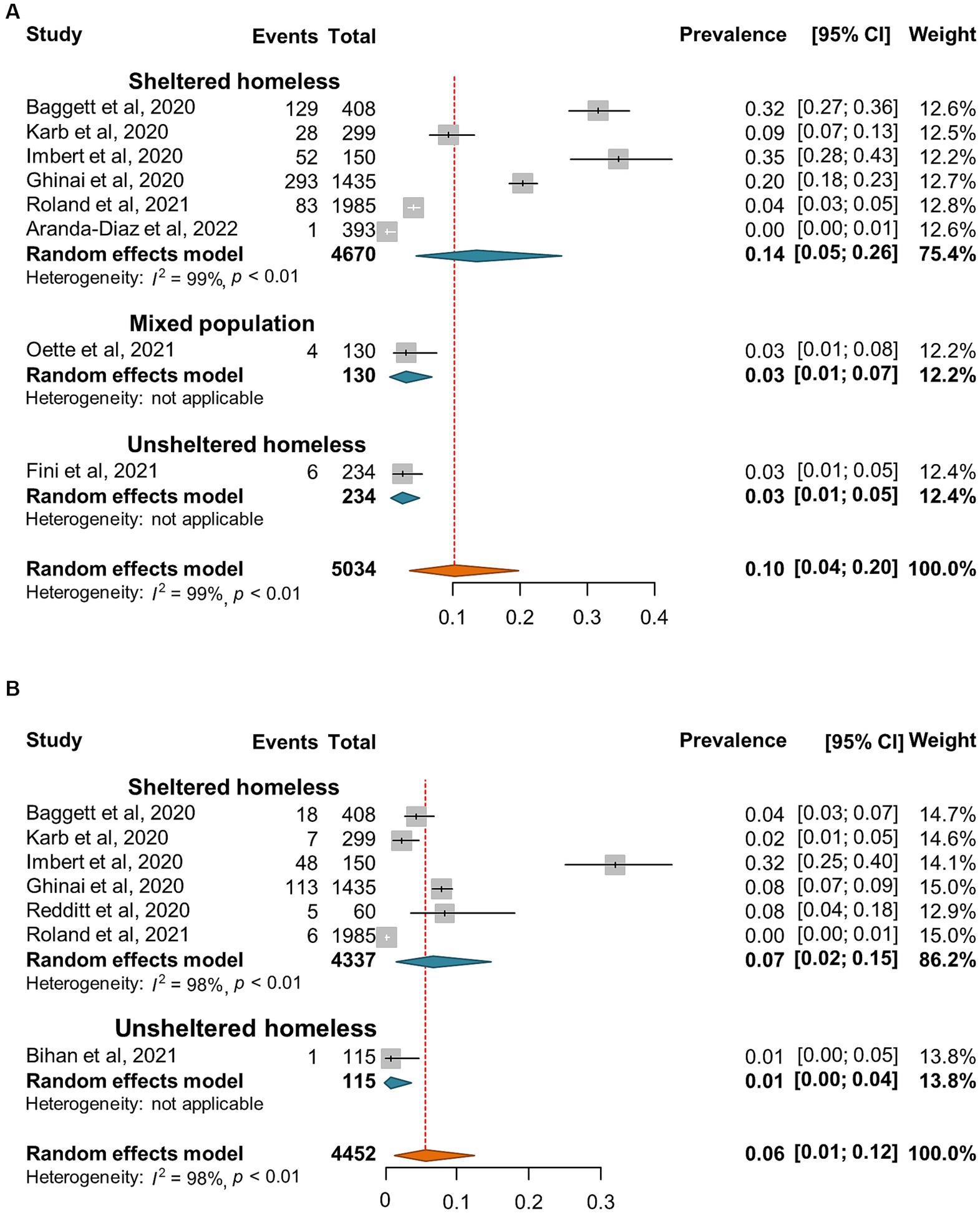
Figure 3. Forest plots of the estimated incidence of asymptomatic SARS-CoV-2 infection (A) and symptomatic infection (B) in homeless people according to the category of homeless.
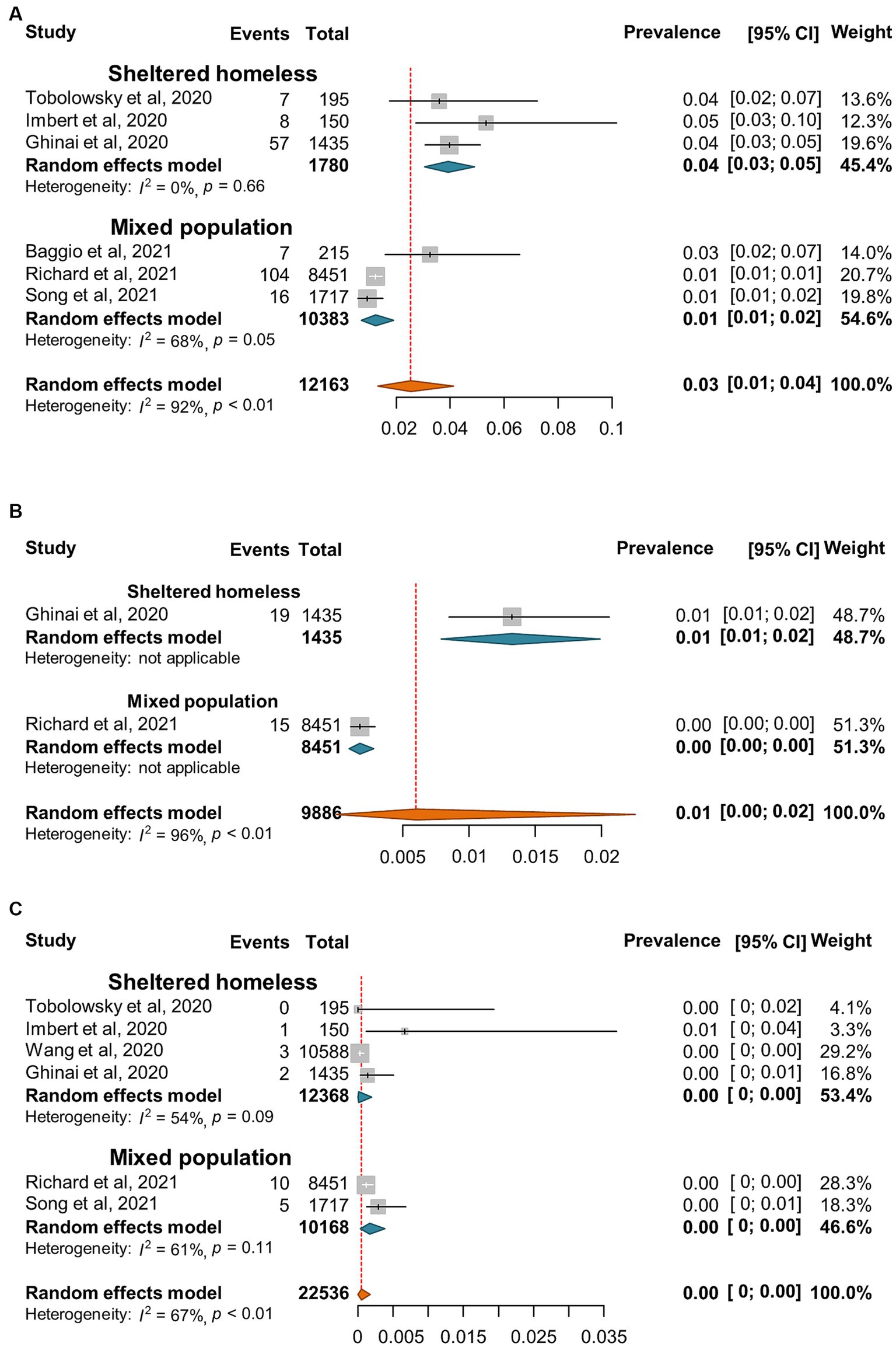
Figure 4. Forest plots of the estimated prevalence of hospitalization (A), sever cases (B), and death (C) caused by SARS-CoV-2 infection in homeless people according to the category of homeless.
Interestingly, in 2020, all the studies showed higher SARS-CoV-2 incidence in homeless people than in the general population and the SARS-CoV-2 incidence ratio between homeless people and general population was 1.8–94.6 (Table 2). However, 4 studies in the United Kingdom, Italy and Mexico showed a reversed SARS-CoV-2 incidence ratio, which ranged from 0.5 to 0.8 between homeless people and general population (Table 2).
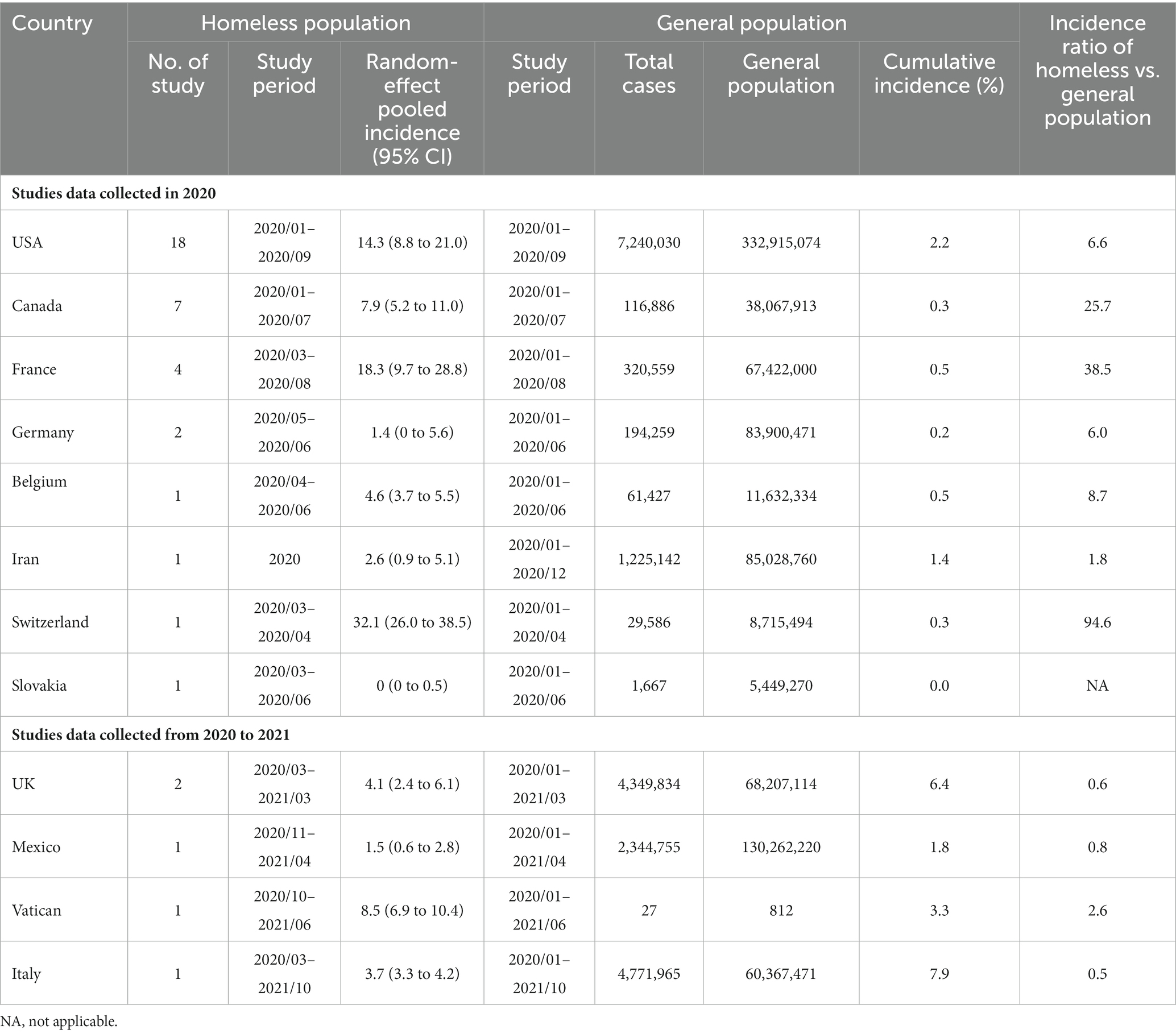
Table 2. Comparison of incidence of SARS-CoV-2 infection between homeless people and general population.
Furthermore, the seroprevalence of SARS-CoV-2 ranged between 0 and 67% with a random-effect pooled estimate of 19% (8, 33%) and substantial heterogeneity (I2 = 99%, p = 0) in the homeless group (Figure 5A). Moreover, there are 4 and 5 articles reported the number of anti-SARS-CoV-2 IgM and IgG positive subjects, respectively. The random effect pooled seropositivity was 2% (1, 3%) for anti-SARS-CoV-2 IgM, and 11% (2, 28%) for anti-SARS-CoV-2 IgG, respectively (Figures 5B,C).
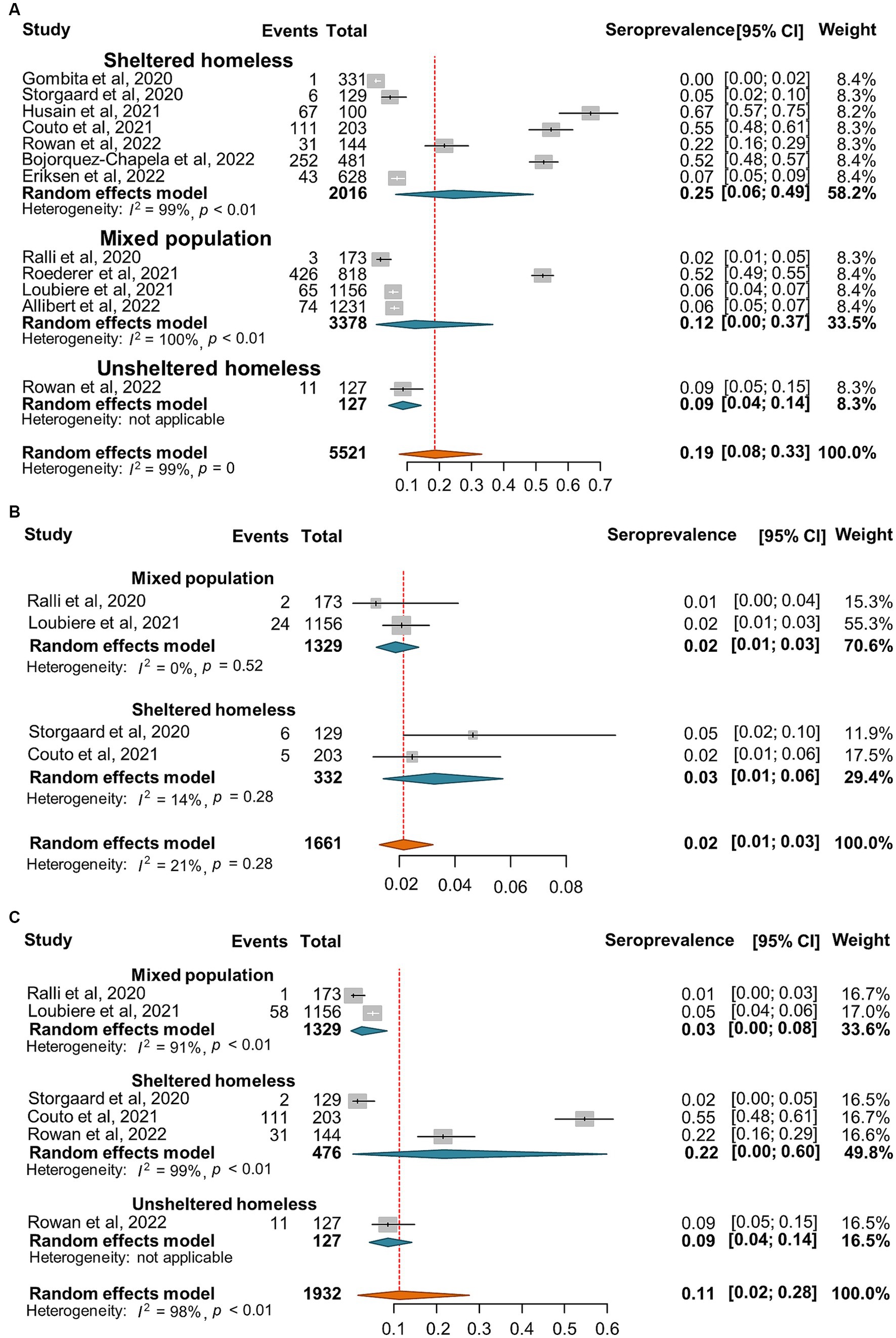
Figure 5. Forest plots of the estimated seroprevalence of anti-SARS-CoV-2 antibodies (A), SARS-CoV-2 specific IgG antibody (B), and SARS-CoV-2 specific IgM antibody (C) in homeless people according to the category of homeless.
3.4. Factors associated with the SARS-CoV-2 incidence or seroprevalence in the homeless population
Substantial heterogeneity was observed between the primary studies; therefore, we explored the potential sources of variations through multivariable meta-regression analysis. Our results indicated that both the incidence and seroprevalence of SARS-CoV-2 infection were not significantly associated with the factors of study period (2021 vs. 2020), study region (Europe vs. America), study design (non-cross-sectional vs. cross-sectional), category of homelessness (unsheltered vs. sheltered; mixed population vs. sheltered), sample size, and mean/median age (Table 3).
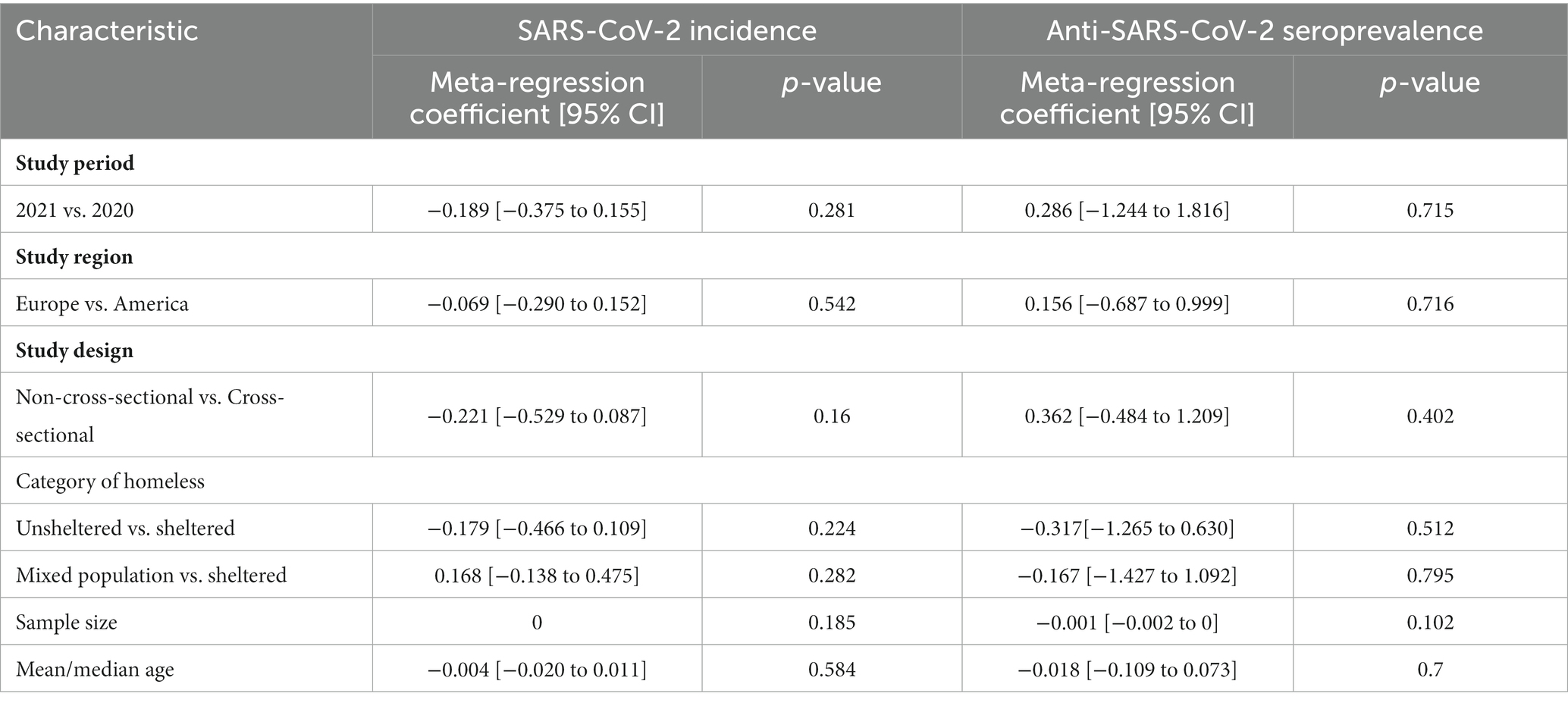
Table 3. Multivariable meta-regression analysis for SARS-CoV-2 incidence and seroprevalence in homeless people.
3.5. SARS-CoV-2 incidence and seroprevalence among shelter staff
Out of the 15 studies that investigated SARS-CoV-2 incidence or seroprevalence among the shelter staff (Supplementary Table S2), there were 12 and 1 investigation diagnosed SARS-CoV-2 infection by NAATs and antigen tests, respectively. The random-effect pooled incidence of SARS-CoV-2 infection was 8% (5, 12%) for diagnosis by NAATs alone and 2% (0, 4%) for antigen tests, respectively (Figure 6). The seroprevalence of SARS-CoV-2 was reported in 3 studies with an estimated pooled seroprevalence of 22% (3, 52%) (Figure 6).
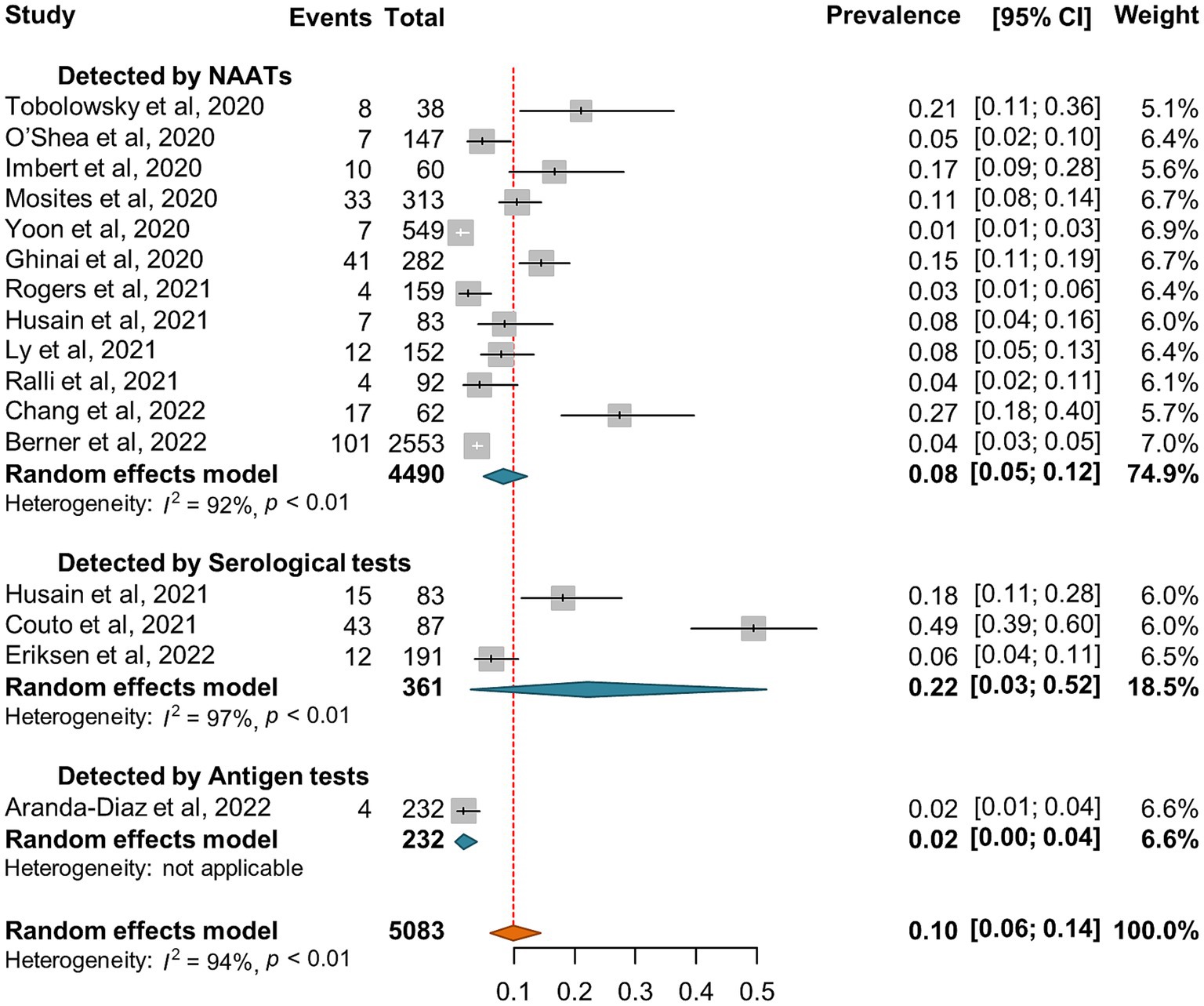
Figure 6. Forest plots of the estimated SARS-CoV-2 incidence and seroprevalence in shelter staff according to the diagnostic method.
3.6. COVID-19 vaccination coverage in the homeless population
A total of 12 reports (24, 29–39) involving 225,448 homeless individuals were selected to assess COVID-19 vaccination and the median sample size of the eligible studies was 2,839 (IQR: 106-83,528) (Supplementary Table S3). All the included studies were conducted in North America (7 in United States and 2 in Canada) and Europe (one each from Denmark, Italy, and United Kingdom, respectively). Out of the 12 studies, 5 reported the proportion of homeless people who had received two doses of COVID-19 vaccine (24, 32, 33, 35, 37). Overall, the pooled proportion of homeless people received at least one dose vaccine was 41% (95% CI: 35, 47%, Figure 7A). The results from 5 studies that reported two doses vaccination coverage showed that 58% (45, 71%) and 43% (32, 54%) of homeless people received one dose and two doses vaccine, respectively (Figures 7B,C). In addition, COVID-19 vaccination coverage in the general population was obtained from 9 studies (24, 29–36) or the global database of COVID-19 vaccinations (4) while one study reported COVID-19 vaccination coverage in the general population aged 18–39 years (99). The proportion ratio between homeless people and the general population was 0.04–2.57 for one dose vaccination and 0.58–1.88 for two doses vaccination, respectively (Table 4).
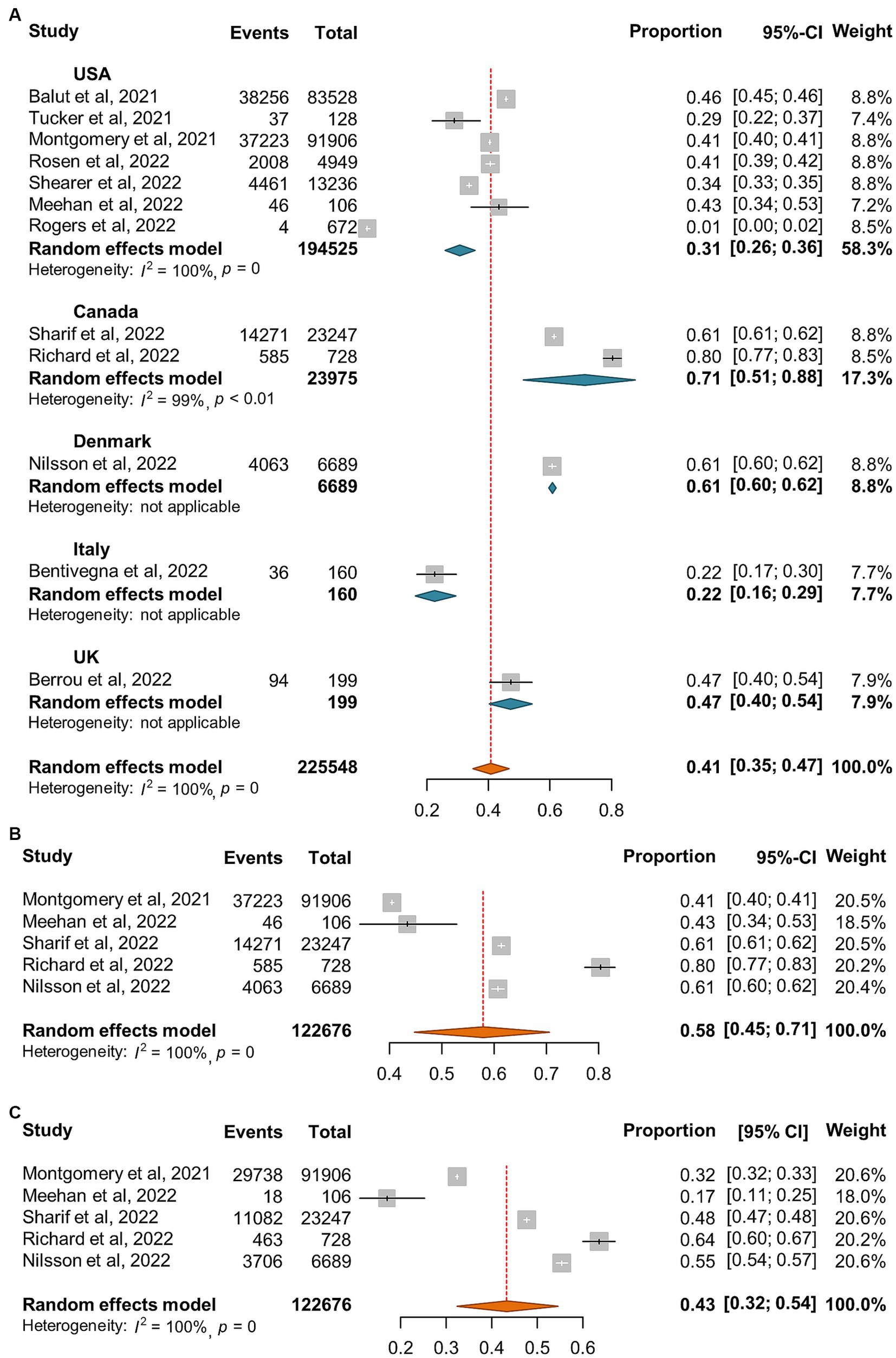
Figure 7. Forest plots of the estimated proportion of vaccinated homeless people. (A) One dose vaccination coverage derived from all studies. (B) One dose vaccination coverage derived from 5 studies that reported both one dose and two dose vaccination coverage. (C) Two dose vaccination coverage derived from 5 studies that reported both one and two dose vaccination coverage.
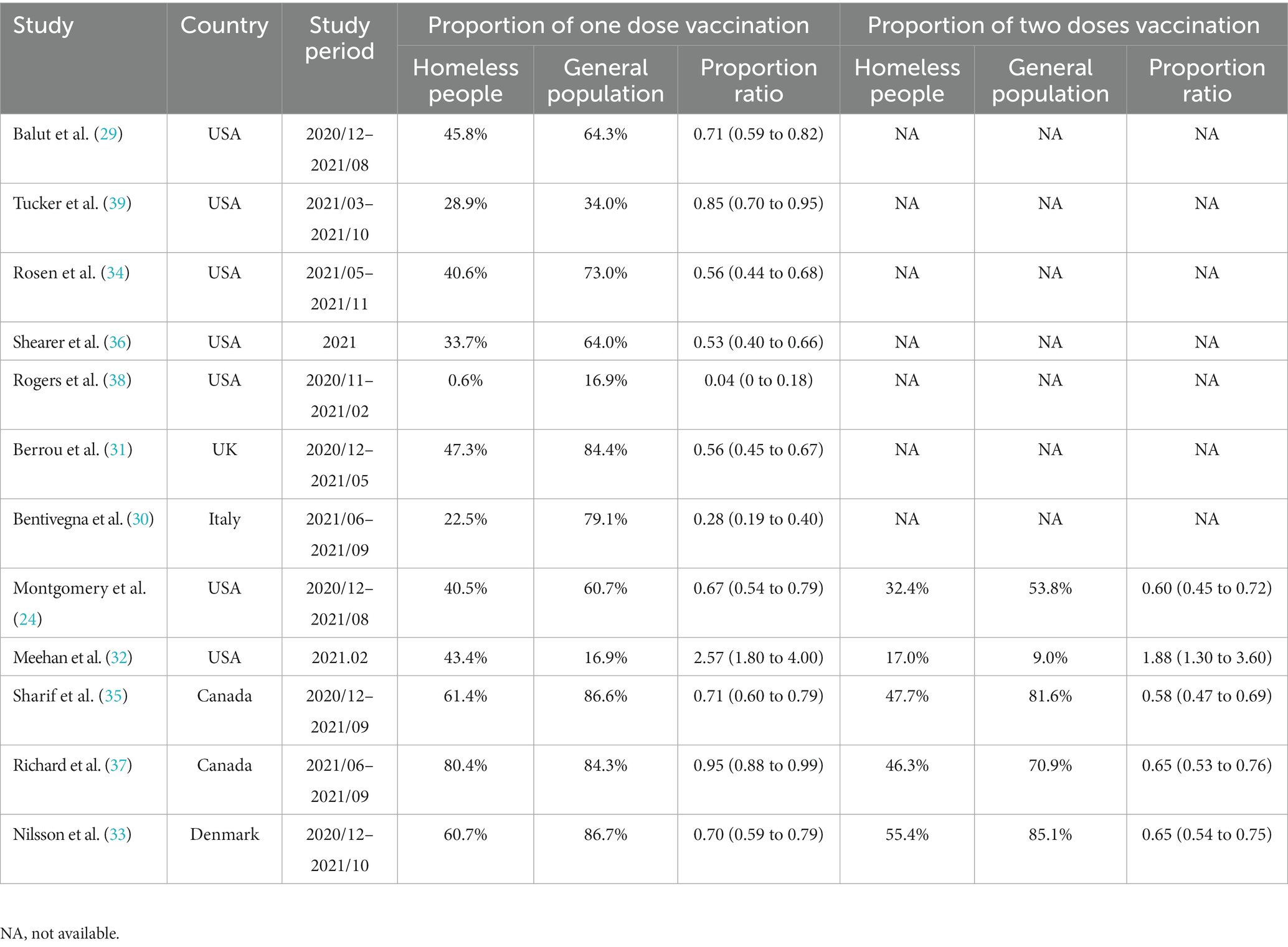
Table 4. Comparison of proportion of COVID-19 vaccination between homeless people and general population during 2020 and 2021.
3.7. Publication bias
Potential publication bias was assessed by Egger and Begg tests. Overall, no evidence of significant publication bias was obtained for the surveys that investigated SARS-CoV-2 incidence (Egger test, p = 0.065; Begg test, p = 0.093) and seroprevalence (Egger test, p = 0.585; Begg test, p = 0.411) among homeless people. In addition, the result of Egger test (p = 0.036) and Begg test (p = 0.131) suggested that the possibility of publication bias was less in the estimated incidence of SARS-CoV-2 infection in shelter staff. Moreover, no significant publication bias was observed for the studies on COVID-19 vaccination coverage of homeless people (Egger test, p = 0.963; Begg test, p = 0.784).
4. Discussion
People experiencing homelessness (PEH) are susceptible to infections including SARS-CoV-2 infection because of inadequate access to safe housing, personal protective equipment, vaccine or healthcare and fragile psychiatric conditions due to social marginalization (100). The current meta-analysis confirmed relatively high risk of SARS-CoV-2 infection in homeless people since the pooled incidence and seroprevalence of SARS-CoV-2 infection was 10 and 19% for the homeless population, higher than in the general population (Table 2). Moreover, the global pooled SARS-CoV-2 specific seroprevalence was less than 10% in the general population (101, 102); however, our estimated seroprevalence was 19% for homeless populations and 22% for shelter staff. Therefore, both homeless people and shelter staff are at higher risk of SARS-CoV-2 infection than the general population. Interestingly, our results indicated that the random-effects pooled incidence of SARS-CoV-2 infection was 11% for the sheltered homeless, 4% for the unsheltered homeless, and 8% for the mixed population, respectively (Figure 1), suggesting that sheltered homeless people may be at greater risk of infecting SARS-CoV-2 probably because the sheltered homeless people are often crowded, and difficult to keep social distance. It is worth mentioning that very few of deaths of homeless population caused by COVID-19 were estimated in the current study (Figure 4C). It was hypothesized that implementation of preventive and control interventions for the pandemic, e.g., lockdown and increased infection control, might have reduced large numbers of deaths in homeless people during the pandemic (103).
Of note, no significant difference of SARS-CoV-2 incidence and seroprevalence was observed in our study between shelter staff (Figure 6) and sheltered homeless people (Supplementary Figure S1; Figure 5). Rao et al. (104) reported that 24% of the shelter staff did not use masks all of the time during the interactions with the homeless while 43% of shelter staff had not received training on cleaning surfaces for SARS-CoV-2, which may put shelter staff at increased risk of exposure to SARS-CoV-2 while very limited hygiene resources in the homeless shelter and poor protection awareness for both homeless people and shelter staff may aggravate the mutual transmission of SARS-CoV-2 (104, 105). In addition, some former homeless residents are employed as shelter staff, which may have narrowed the difference between the two groups (104). Furthermore, most of shelter worker have experienced a decline in their mental health and increase of depression, anxiety, stress and fatigue during the COVID-19 pandemic (106). Similarly, homeless people are susceptible to mental disorders which in turn may increase their vulnerability to the infection of SARS-CoV-2 (21).
Incidence ratios suggested that active SARS-CoV-2 infection is at least about 6.6 times more common in homeless people than in total populations in the United States during 2020 (Table 2). However, when the cumulative incidence of general population in the same country during the same study period was used as reference, the incidence ratio might be underestimated. Moreover, during 2020 the SARS-CoV-2 incidence of homeless people is higher than that of general population across various countries or region, whereas the analysis of studies data involving 2021 showed different results (Table 2). The higher SARS-CoV-2 incidence of general population than homeless people in 2021 may be attributed to loosen travel and gathering restriction (107).
Our results confirmed the lower COVID-19 vaccination coverage rate in homeless people than the general population (Table 4) although some contradictive results were reported by Meehan et al. (32) in Detroit (Table 4) (4). However, another study conducted by Rogers and colleagues found that during November 2020 and February 2021, only 0.6% sheltered homeless people in Washington had been vaccinated (38). In addition, 88.3% of the investigated homeless people were Black or African American in Meehan’s report (32) while 37.4% in Rogers’s one (38). However, according to one meta-analysis of COVID-19 vaccine attitudes in the United States, Black American showed the lowest vaccine acceptance (108). Other studies also showed that the proportion of vaccinated Black American was lower than that of White or Hispanic American (36, 109). Therefore, the lower vaccination rate among the homeless may be partially attributed to reduced willingness to be vaccinated (110). Moreover, our results indicated that one dose vaccination was higher than two doses vaccination (58% vs. 43%, Figures 7B,C).
There are some limitations in the current study. First, since only 5 included studies collected data in 2021, and almost none of them involved vaccinated homeless populations; therefore, we were unable to compare the incidence of SARS-COV-2 infection among homeless people between the pre-vaccination period versus post-vaccination period. Moreover, the number of SARS-COV-2 Delta variant-infected cases reached peak in August 2021 (111) and the Omicron variant outcompeted other counterparts and predominantly circulates globally since its emergence around the end of 2021. However, we did not perform a comparison of the SARS-CoV-2 incidence in homelessness between different pandemic periods that experienced the shifting of predominant variants from Delta to Omicron due to the lack of available data. Furthermore, due to the distribution of latent period, i.e., the time interval between infection (dates of exposure) and becoming infectious (dates of first positive PCR test), the SARS-CoV-2 incidence diagnosed by NAATs might be underestimated. Similarly, the incidence of symptomatic infection would also be underestimated because of the existence of incubation period (the time interval from infection through symptom onset). Given that, further research is needed to better understand the incidence and risk factors of SARS-CoV-2 infection in the homeless populations.
Our study has important implications for public health. Firstly, it highlights the need for targeted interventions to address the high incidence and low vaccination rates among homeless individuals. This could involve strategies such as increasing access to testing, vaccines, healthcare services, as well as personal protective equipment to reduce transmission. Secondly, the study underscores the necessity of addressing health disparities in vulnerable populations and promoting health equity and social justice, particularly during public health crises such as the COVID-19 pandemic. Overall, the study provides important information that will be useful in developing effective policies to protect homeless individuals and the broader public from COVID-19.
5. Conclusion
The current study suggests that the homeless people remain highly susceptible to SARS-CoV-2 infection, but their COVID-19 vaccination coverage is lower than general population. These results underscore the need for prioritizing vaccine deployment and implementing enhanced preventive measures targeting this vulnerable group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YL conceived the study, analyzed, interpreted the data, and drafted the manuscript. QS, QL, and YP performed the literature searches, study selection, and data extraction. YL, QS, QL, YP, and ST revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1044788/full#supplementary-material
Footnotes
References
1. Dong, E, Du, H, and Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
2. Zhu, N, Zhang, D, Wang, W, Li, X, Yang, B, Song, J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Tomalka, JA, Suthar, MS, Deeks, SG, and Sekaly, RP. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat Immunol. (2022) 23:360–70. doi: 10.1038/s41590-022-01130-4
4. Mathieu, E, Ritchie, H, Ortiz-Ospina, E, Roser, M, Hasell, J, Appel, C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. (2021) 5:947–53. doi: 10.1038/s41562-021-01122-8
5. Davies, NG, Abbott, S, Barnard, RC, Jarvis, CI, Kucharski, AJ, Munday, JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. (2021) 372:6538. doi: 10.1126/science.abg3055
6. Tegally, H, Wilkinson, E, Giovanetti, M, Iranzadeh, Arash, Fonseca, Vagner, Giandhari, Jennifer, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv (2020): Available at: https://doi.org/10.1101/2020.12.21.20248640. [Epub ahead of preprint]
7. Faria, NR, Mellan, TA, Whittaker, C, Claro, Ingra M., da Candido, Darlan S., Mishra, Swapnil, et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. medRxiv (2021): Available at: https://doi.org/10.1101/2021.02.26.21252554. [Epub ahead of preprint]
8. Melloul, M, Chouati, T, Hemlali, M, Alaoui Amine, S, Touil, N, Elannaz, H, et al. Genome sequences of the Delta variant (B.1.617.2) and the Kappa variant (B.1.617.1) detected in Morocco. Microbiol Resour Announc. (2021) 10:e0072721. doi: 10.1128/MRA.00727-21
9. Garcia-Beltran, WF, Lam, EC, St Denis, K, Nitido, AD, Garcia, ZH, Hauser, BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cells. (2021) 184:2372–83.e9. doi: 10.1016/j.cell.2021.03.013
10. Iketani, S, Liu, L, Guo, Y, Liu, L, Chan, JFW, Huang, Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. (2022) 604:553. doi: 10.1038/s41586-022-04594-4
11. Chemaitelly, H, Tang, P, Hasan, MR, AlMukdad, S, Yassine, HM, Benslimane, FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. (2021) 385:E83. doi: 10.1056/NEJMoa2114114
12. Goldberg, Y, Mandel, M, Bar-On, YM, Bodenheimer, O, Freedman, LS, Ash, N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. (2022) 386:2201–12. doi: 10.1056/NEJMoa2118946
13. Andrews, N, Tessier, E, Stowe, J, Gower, C, Kirsebom, F, Simmons, R, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. (2022) 386:340–50. doi: 10.1056/NEJMoa2115481
14. Feikin, DR, Higdon, MM, Abu-Raddad, LJ, Andrews, N, Araos, R, Goldberg, Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. (2022) 399:924–44. doi: 10.1016/S0140-6736(22)00152-0
15. Tsai, J, and Wilson, M. COVID-19: a potential public health problem for homeless populations. Lancet Public Health. (2020) 5:E186–7. doi: 10.1016/S2468-2667(20)30053-0
16. Wood, LJ, Davies, AP, and Khan, Z. COVID-19 precautions: easier said than done when patients are homeless. Med J Aust. (2020) 212:384. doi: 10.5694/mja2.50571
17. Fazel, S, Geddes, JR, and Kushel, M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. (2014) 384:1529–40. doi: 10.1016/S0140-6736(14)61132-6
18. Serme-Morin, C, Coupechoux, S, and Aldanas, M-J The sixth overview of housing exclusion in Europe (2021). Available at: https://www.fondation-abbe-pierre.fr/documents/pdf/rapport_europe_2021_gb.pdf. (Accessed July 14, 2022)
19. Henry, M, Sousa, T, and Tano, C The 2021 annual homeless assessment report (AHAR) to congress part 1: estimates of homelessness in the United States. Available at: https://www.huduser.gov/portal/datasets/ahar/2021-ahar-part-1-pit-estimates-of-homelessness-in-the-us.html. (Accessed July, 14 2022)
20. Beijer, U, Wolf, A, and Fazel, S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12:859–70. doi: 10.1016/S1473-3099(12)70177-9
21. Gutwinski, S, Schreiter, S, Deutscher, K, and Fazel, S. The prevalence of mental disorders among homeless people in high-income countries: an updated systematic review and meta-regression analysis. PLoS Med. (2021) 18:e1003750. doi: 10.1371/journal.pmed.1003750
22. Raoult, D, Foucault, C, and Brouqui, P. Infections in the homeless. Lancet Infect Dis. (2001) 1:77–84. doi: 10.1016/S1473-3099(01)00062-7
23. Maxmen, A. Coronavirus spreads under the radar in US homeless shelters. Nature. (2020) 581:129–30. doi: 10.1038/d41586-020-01389-3
24. Montgomery, MP, Meehan, AA, Cooper, A, Toews, KA, Ghinai, I, Schroeter, MK, et al. Notes from the field:COVID-19 vaccination coverage among persons experiencing homelessness—six U.S. jurisdictions, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1676–8. doi: 10.15585/mmwr.mm7048a4
25. Specht, A, Sarma, N, Linzbach, T, Hellmund, T, Hörig, M, Wintel, M, et al. Participatory development and implementation of inclusive digital health communication on COVID-19 with homeless people. Front Public Health. (2022) 10:1042677. doi: 10.3389/fpubh.2022.1042677
26. Mohsenpour, A, Bozorgmehr, K, Rohleder, S, Stratil, J, and Costa, D. SARS-Cov-2 prevalence, transmission, health-related outcomes and control strategies in homeless shelters: systematic review and meta-analysis. EClinicalMedicine. (2021) 38:101032. doi: 10.1016/j.eclinm.2021.101032
27. Ahillan, T, Emmerson, M, Swift, B, Golamgouse, H, Song, K, Roxas, A, et al. COVID-19 in the homeless population: a scoping review and meta-analysis examining differences in prevalence, presentation, vaccine hesitancy and government response in the first year of the pandemic. BMC Infect Dis. (2023) 23:155. doi: 10.1186/s12879-023-08037-x
28. Li, MC, Wang, H, Tian, LL, Pang, Z, Yang, Q, Huang, T, et al. COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther. (2022) 7:146. doi: 10.1038/s41392-022-00996-y
29. Balut, MD, Chu, K, Gin, JL, Dobalian, A, and Der-Martirosian, C. Predictors of COVID-19 vaccination among veterans experiencing homelessness. Vaccines. (2021) 9:1268. doi: 10.3390/vaccines9111268
30. Bentivegna, E, Di Meo, S, Carriero, A, Capriotti, N, Barbieri, A, and Martelletti, P. Access to COVID-19 vaccination during the pandemic in the informal settlements of Rome. Int J Environ Res Public Health. (2022) 19. doi: 10.3390/ijerph19020719
31. Berrou, I, Hamilton, K, Cook, C, Armour, C, Hughes, S, Hancock, J, et al. Leaving no one behind: interventions and outcomes of the COVID-19 vaccine maximising uptake programme. Vaccines. (2022) 10:840. doi: 10.3390/vaccines10060840
32. Meehan, AA, Yeh, M, Gardner, A, DeFoe, TL, Garcia, A, Vander Kelen, P, et al. COVID-19 vaccine acceptability among clients and staff of homeless shelters in Detroit, Michigan, February 2021. Health Promot Pract. (2022) 23:35–41. doi: 10.1177/15248399211049202
33. Nilsson, SF, Laursen, TM, Osler, M, Hjorthøj, C, Benros, ME, Ethelberg, S, et al. Vaccination against SARS-CoV-2 infection among vulnerable and marginalised population groups in Denmark: a nationwide population-based study. Lancet Reg Health Eur. (2022) 16:100355. doi: 10.1016/j.lanepe.2022.100355
34. Rosen, AD, Beltran, J, Thomas, E, Miller, J, Robie, B, Walseth, S, et al. COVID-19 vaccine acceptability and financial incentives among unhoused people in Los Angeles County: a three-stage field survey. J Urban Health. (2022) 99:594–602. doi: 10.1007/s11524-022-00659-x
35. Shariff, SZ, Richard, L, Hwang, SW, Kwong, JC, Forchuk, C, Dosani, N, et al. COVID-19 vaccine coverage and factors associated with vaccine uptake among 23 247 adults with a recent history of homelessness in Ontario, Canada: a population-based cohort study. Lancet Public Health. (2022) 7:E366–e377. doi: 10.1016/S2468-2667(22)00037-8
36. Shearer, RD, Vickery, KD, Bodurtha, P, Drawz, PE, Johnson, S, Jeruzal, J, et al. COVID-19 vaccination of people experiencing homelessness and incarceration in Minnesota. Health Aff. (2022) 41:846–52. doi: 10.1377/hlthaff.2021.02030
37. Richard, L, Liu, M, Jenkinson, JIR, Nisenbaum, R, Brown, M, Pedersen, C, et al. COVID-19 vaccine coverage and sociodemographic, behavioural and housing factors associated with vaccination among people experiencing homelessness in Toronto, Canada: a cross-sectional study. Vaccines. (2022) 10. doi: 10.3390/vaccines10081245
38. Rogers, JH, Cox, SN, Hughes, JP, Link, AC, Chow, EJ, Fosse, I, et al. Trends in COVID-19 vaccination intent and factors associated with deliberation and reluctance among adult homeless shelter residents and staff, 1 November 2020 to 28 February 2021—King County, Washington. Vaccine. (2022) 40:122–32. doi: 10.1016/j.vaccine.2021.11.026
39. Tucker, JS, D’Amico, EJ, Pedersen, ER, Garvey, R, Rodriguez, A, and Klein, DJ. COVID-19 vaccination rates and attitudes among young adults with recent experiences of homelessness. J Adolesc Health. (2022) 70:504–6. doi: 10.1016/j.jadohealth.2021.11.017
40. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
41. Kevadiya, BD, Machhi, J, Herskovitz, J, Oleynikov, MD, Blomberg, WR, Bajwa, N, et al. Diagnostics for SARS-CoV-2 infections. Nat Mater. (2021) 20:593–605. doi: 10.1038/s41563-020-00906-z
42. Xu, Y, Chen, Y, and Tang, X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob Health Med. (2020) 2:66–72. doi: 10.35772/ghm.2020.01015
43. Tobolowsky, FA, Gonzales, E, Self, JL, Rao, CY, Keating, R, Marx, GE, et al. COVID-19 outbreak among three affiliated homeless service sites-King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:523–6. doi: 10.15585/mmwr.mm6917e2
44. Baggett, TP, Keyes, H, Sporn, N, and Gaeta, JM. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. (2020) 323:2191–2. doi: 10.1001/jama.2020.6887
45. Baggett, TP, Racine, MW, Lewis, E, De Las Nueces, D, O’Connell, JJ, Bock, B, et al. Addressing COVID-19 among people experiencing homelessness: description, adaptation, and early findings of a multiagency response in Boston. Public Health Rep. (2020) 135:435–41. doi: 10.1177/0033354920936227
46. O’Shea, T, Bodkin, C, Mokashi, V, Beal, K, Wiwcharuk, J, Lennox, R, et al. Pandemic planning in homeless shelters: a pilot study of a coronavirus disease 2019 (COVID-19) testing and support program to mitigate the risk of COVID-19 outbreaks in congregate settings. Clin Infect Dis. (2021) 72:1639–41. doi: 10.1093/cid/ciaa743
47. Mosites, E, Parker, EM, Clarke, KEN, Gaeta, JM, Baggett, TP, Imbert, E, et al. Assessment of SARS-CoV-2 infection prevalence in homeless shelters—four U.S. cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:521–2. doi: 10.15585/mmwr.mm6917e1
48. Karb, R, Samuels, E, Vanjani, R, Trimbur, C, and Napoli, A. Homeless shelter characteristics and prevalence of SARS-CoV-2. West J Emerg Med. (2020) 21:1048–53. doi: 10.5811/westjem.2020.7.48725
49. Imbert, E, Kinley, PM, Scarborough, A, Cawley, C, Sankaran, M, Cox, SN, et al. Coronavirus disease 2019 outbreak in a San Francisco homeless shelter. Clin Infect Dis. (2021) 73:324–7. doi: 10.1093/cid/ciaa1071
50. Kelly, D, Murphy, H, Vadlamudi, R, Kraut, R, Dalessio, K, Malani, AN, et al. Successful public health measures preventing coronavirus disease 2019 (COVID-19) at a Michigan homeless shelter. Infect Control Hosp Epidemiol. (2021) 42:1155–6. doi: 10.1017/ice.2020.439
51. Gombita, P, Olah, M, Kovac, R, Jurasek, M, Kosticova, M, Taziarova, M, et al. Senior homeless population was COVID-19 free in 3 shelter communities after adapting the Life Island model (note). Clin Soc Work Health Intervention. (2020) 11:78–9. doi: 10.22359/cswhi_11_3_12
52. Seballos, SS, Weleff, J, and Phelan, M. Access to COVID-19 testing by homeless/housing-insecure individuals in Northeast Ohio. Ann Emerg Med. (2020) 76:S91–2. doi: 10.1016/j.annemergmed.2020.09.249
53. Yoon, JC, Montgomery, MP, Buff, AM, Boyd, AT, Jamison, C, Hernandez, A, et al. Coronavirus disease 2019 (COVID-19) prevalences among people experiencing homelessness and homelessness service staff during early community transmission in Atlanta, Georgia, April–May 2020. Clin Infect Dis. (2021) 73:E2978–e2984. doi: 10.1093/cid/ciaa1340
54. Wang, L, Ma, H, Yiu, KCY, Calzavara, A, Landsman, D, Luong, L, et al. Heterogeneity in testing, diagnosis and outcome in SARS-CoV-2 infection across outbreak settings in the greater Toronto area, Canada: an observational study. CMAJ Open. (2020) 8:E627–36. doi: 10.9778/cmajo.20200213
55. Ralli, M, Cedola, C, Urbano, S, Latini, O, Shkodina, N, Morrone, A, et al. Assessment of SARS-CoV-2 infection through rapid serology testing in the homeless population in the City of Rome, Italy. Preliminary results. J Public Health Res. (2020) 9:1986. doi: 10.4081/jphr.2020.1986
56. Ghinai, I, Davis, ES, Mayer, S, Toews, KA, Huggett, TD, Snow-Hill, N, et al. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in homeless shelters in Chicago, Illinois-March–May, 2020. Open Forum Infect Dis. (2020) 7:ofaa477. doi: 10.1093/ofid/ofaa477
57. Marquez, H, Ramers, C, Northrup, A, Tam, A, Liu, J, Rojas, S, et al. Response to the coronavirus disease 2019 pandemic among people experiencing homelessness in congregant living settings in San Diego, California. Rev Infect Dis. (2021) 73:E805–e807. doi: 10.1093/cid/ciaa1668
58. Storgaard, SF, Eiset, AH, Abdullahi, F, and Wejse, C. First wave of COVID-19 did not reach the homeless population in Aarhus. Dan Med J. (2020) 67:A08200594.
59. Redditt, V, Wright, V, Rashid, M, Male, R, and Bogoch, I. Outbreak of SARS-CoV-2 infection at a large refugee shelter in Toronto, April 2020: a clinical and epidemiologic descriptive analysis. CMAJ Open. (2020) 8:E819–24. doi: 10.9778/cmajo.20200165
60. Jatt, LP, Winnett, A, Graber, CJ, Vallone, J, Beenhouwer, DO, and Goetz, MB. Widespread severe acute respiratory coronavirus virus 2 (SARS-CoV-2) laboratory surveillance program to minimize asymptomatic transmission in high-risk inpatient and congregate living settings. Infect Control Hosp Epidemiol. (2020) 41:1331–4. doi: 10.1017/ice.2020.301
61. Baggio, S, Jacquerioz, F, Salamun, J, Spechbach, H, and Jackson, Y. Equity in access to COVID-19 testing for undocumented migrants and homeless persons during the initial phase of the pandemic. J Migr Health. (2021) 4:100051. doi: 10.1016/j.jmh.2021.100051
62. Richard, L, Booth, R, Rayner, J, Clemens, KK, Forchuk, C, and Shariff, SZ. Testing, infection and complication rates of COVID-19 among people with a recent history of homelessness in Ontario, Canada: a retrospective cohort study. CMAJ Open. (2021) 9:E1–9. doi: 10.9778/cmajo.20200287
63. Rogers, JH, Link, AC, McCulloch, D, Brandstetter, E, Newman, KL, Jackson, ML, et al. Characteristics of COVID-19 in homeless shelters a community-based surveillance study. Ann Intern Med. (2021) 174:42-+. doi: 10.7326/M20-3799
64. Kiran, T, Craig-Neil, A, Das, P, Lockwood, J, Wang, R, Nathanielsz, N, et al. Association of homelessness with COVID-19 positivity among individuals visiting a testing centre: a cross-sectional study. Healthc Policy. (2022) 17:34–41. doi: 10.12927/hcpol.2022.26730
65. le Bihan, C, Faucherre, V, le Moing, V, Mehenni, A, Nantes, D, da Silva, A, et al. COVID-19: the forgotten cases of hidden exiles. Infect Dis Now. (2021) 51:387–90. doi: 10.1016/j.idnow.2021.01.008
66. Husain, M, Rachline, A, Cousien, A, Rolland, S, Rouzaud, C, Ferre, VM, et al. Impact of the COVID-19 pandemic on the homeless: results from a retrospective closed cohort in France (March–May 2020). Clin Microbiol Infect. (2021) 27:1520.e1–5. doi: 10.1016/j.cmi.2021.05.039
67. TDA, L, Hoang, VT, Goumballa, N, Louni, M, Canard, N, Dao, TL, et al. Variations in respiratory pathogen carriage among a homeless population in a shelter for men in Marseille, France, March–July 2020: cross-sectional 1-day surveys. Eur J Clin Microbiol Infect Dis. (2021) 40:1579–82. doi: 10.1007/s10096-020-04127-9
68. Ly, TDA, Nguyen, NN, Hoang, VT, Goumballa, N, Louni, M, Canard, N, et al. Screening of SARS-CoV-2 among homeless people, asylum-seekers and other people living in precarious conditions in Marseille, France, March–April 2020. Int J Infect Dis. 105:1–6. doi: 10.1016/j.ijid.2021.02.026
69. Kiran, T, Craig-Neil, A, Das, P, Lockwood, J, Wang, R, Nathanielsz, N, et al. Factors associated with SARS-CoV-2 positivity in 20 homeless shelters in Toronto, Canada, from April to July 2020: a repeated cross-sectional study. CMAJ Open. (2021) 9:E302–8. doi: 10.9778/cmajo.20200253
70. Roederer, T, Mollo, B, Vincent, C, Nikolay, B, Llosa, AE, Nesbitt, R, et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health. (2021) 6:E202–e209. doi: 10.1016/S2468-2667(21)00001-3
71. Roland, M, Ben Abdelhafidh, L, Deom, V, Vanbiervliet, F, Coppieters, Y, and Racape, J. SARS-CoV-2 screening among people living in homeless shelters in Brussels, Belgium. PLoS One. (2021) 16:e0252886. doi: 10.1371/journal.pone.0252886
72. Loubiere, S, Monfardini, E, Allaria, C, Mosnier, M, Allibert, A, Ninove, L, et al. Seroprevalence of SARS-CoV-2 antibodies among homeless people living rough, in shelters and squats: a large population-based study in France. PLoS One. (2021) 16:e0255498. doi: 10.1371/journal.pone.0255498
73. Hsu, Y-T, Lan, F-Y, Wei, CF, Suharlim, C, Lowery, N, Ramirez, A, et al. Comparison of COVID-19 mitigation and decompression strategies among homeless shelters: a prospective cohort study. Ann Epidemiol. (2021) 64:96–101. doi: 10.1016/j.annepidem.2021.08.023
74. Keller, M, Shreffler, J, Wilmes, K, Polites, A, and Huecker, M. Equal incidence of COVID-19 among homeless and non-homeless ED patients when controlling for confounders. Am J Emerg Med. (2022) 53:286.e5-.e7. doi: 10.1016/j.ajem.2021.09.057
75. do Couto, AC, Kmetiuk, LB, Delai, RR, Brandão, APD, Monteiro, CO, da Silva, LHA, et al. High SARS-CoV-2 seroprevalence in persons experiencing homelessness and shelter workers from a day-shelter in São Paulo, Brazil. PLoS Negl Trop Dis. (2021) 15:e0009754. doi: 10.1371/journal.pntd.0009754
76. Oette, M, Corpora, S, Baron, M, Laudenberg, M, Kaiser, R, Klein, F, et al. The prevalence of SARS-CoV-2 infection among homeless persons in Cologne, Germany. Dtsch Arztebl Int. (2021) 118:678–9. doi: 10.3238/arztebl.m2021.0327
77. Ralli, M, De-Giorgio, F, Pimpinelli, F, Cedola, C, Shkodina, N, Morrone, A, et al. SARS-CoV-2 infection prevalence in people experiencing homelessness. Eur Rev Med Pharmacol Sci. (2021) 25:6425–30. doi: 10.26355/eurrev_202110_27016
78. Song, J, Moreno-Stokoe, C, Grey, C, and Davies, AR. Individuals with lived experience of homelessness and their COVID-19 outcomes in Wales: a data linkage study. Lancet. (2021) 398:81. doi: 10.1016/S0140-6736(21)02624-6
79. Lindner, AK, Sarma, N, Rust, LM, Hellmund, T, Krasovski-Nikiforovs, S, Wintel, M, et al. Monitoring for COVID-19 by universal testing in a homeless shelter in Germany: a prospective feasibility cohort study. BMC Infect Dis. (2021) 21:1241. doi: 10.1186/s12879-021-06945-4
80. Fini, EA, Asadian, A, Sotoudeh, A, Hadadian, M, Zakeri, A, and Dadras, M. Survey COVID-19 among the homeless residents of Isin camp in Bandar Abbas in South of Iran. J Educ Health Promot. (2021) 10:458. doi: 10.4103/jehp.jehp_1664_20
81. Thomas, I, and Mackie, P. A population level study of SARS-CoV-2 prevalence amongst people experiencing homelessness in Wales, UK. Int J Popul Data Sci. (2020) 5:1695. doi: 10.23889/ijpds.v5i4.1695
82. Huggett, TD, Tung, EL, Cunningham, M, Ghinai, I, Duncan, HL, McCauley, ME, et al. Assessment of a hotel-based protective housing program for incidence of SARS-CoV-2 infection and management of chronic illness among persons experiencing homelessness. JAMA Netw Open. (2021) 4:e2138464. doi: 10.1001/jamanetworkopen.2021.38464
83. Chang, Y-S, Mayer, S, Davis, ES, Figueroa, E, Leo, P, Finn, PW, et al. Transmission dynamics of large coronavirus disease outbreak in homeless shelter, Chicago, Illinois, USA, 2020. Emerg Infect Dis. (2022) 28:77–85. doi: 10.3201/eid2801.210780
84. Luong, L, Beder, M, Nisenbaum, R, Orkin, A, Wong, J, Damba, C, et al. Prevalence of SARS-CoV-2 infection among people experiencing homelessness in Toronto during the first wave of the COVID-19 pandemic. Can J Public Health. (2022) 113:117–25. doi: 10.17269/s41997-021-00591-8
85. Allibert, A, Tinland, A, Landier, J, Loubière, S, Gaudart, J, Mosnier, M, et al. Residential mobility of a cohort of homeless people in times of crisis: COVID-19 pandemic in a European Metropolis. Int J Environ Res Public Health. (2022) 19:3129. doi: 10.3390/ijerph19053129
86. Rowan, SE, McCormick, DW, Wendel, KA, Scott, T, Chavez-van de Hey, J, Wilcox, K, et al. Lower prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among people experiencing homelessness tested in outdoor encampments compared with overnight shelters: Denver, Colorado, June–July 2020. Clin Infect Dis. (2022) 75:e157–64. doi: 10.1093/cid/ciac039
87. Bojorquez-Chapela, I, Strathdee, SA, Garfein, RS, Benson, CA, Chaillon, A, and Ignacio, C, The impact of the COVID-19 pandemic among migrants in shelters in Tijuana, Baja California, Mexico, Sepulveda, J (2022), BMJ Glob Health ; 7,:e007202, doi: 10.1136/bmjgh-2021-007202
88. Aranda-Díaz, A, Imbert, E, Strieff, S, Graham-Squire, D, Evans, JL, Moore, J, et al. Implementation of rapid and frequent SARS-CoV-2 antigen testing and response in congregate homeless shelters. PLoS One. (2022) 17:e0264929-e. doi: 10.1371/journal.pone.0264929
89. Berner, L, Meehan, A, Kenkel, J, Montgomery, M, Fields, V, Henry, A, et al. Clinic-and community-based SARS-CoV-2 testing among people experiencing homelessness in the United States, March–November 2020. Public Health Rep. (2022) 137:764–73. doi: 10.1177/00333549221086514
90. Morrone, A, Buonomini, AR, Sannella, A, Pimpinelli, F, and Rotulo, A. Unequal access to testing and vaccination services for the homeless and undocumented population during COVID-19 pandemic. Int J Public Health. (2022):67. doi: 10.3389/ijph.2022.1604851
91. Eriksen, ARR, Fogh, K, Hasselbalch, RB, Bundgaard, H, Nielsen, SD, Jørgensen, CS, et al. SARS-CoV-2 antibody prevalence among homeless people and shelter workers in Denmark: a nationwide cross-sectional study. BMC Public Health. (2022) 22:1261. doi: 10.1186/s12889-022-13642-7
92. Freeman, MF, and Tukey, JW. Transformations related to the angular and the square root. Ann Math Statist. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
93. Newcombe, RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. (1998) 17:857–72. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E
94. Wilson, EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. (1927) 22:209–12. doi: 10.1080/01621459.1927.10502953
95. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
96. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
97. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
98. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
99. Baack, BN, Abad, N, Yankey, D, Kahn, KE, Razzaghi, H, Brookmeyer, K, et al. COVID-19 vaccination coverage and intent among adults aged 18–39 years—United States, March–May 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:928–33. doi: 10.15585/mmwr.mm7025e2
100. Corey, J, Lyons, J, O’Carroll, A, Stafford, R, and Ivers, J-H. A scoping review of the health impact of the COVID-19 pandemic on persons experiencing homelessness in North America and Europe. Int J Environ Res Public Health. (2022) 19:3219. doi: 10.3390/ijerph19063219
101. Rostami, A, Sepidarkish, M, Fazlzadeh, A, Mokdad, AH, Sattarnezhad, A, Esfandyari, S, et al. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect. (2021) 27:1762–71. doi: 10.1016/j.cmi.2021.09.019
102. Chen, X, Chen, Z, Azman, AS, Deng, X, Sun, R, Zhao, Z, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. (2021) 9:E598–609. doi: 10.1016/S2214-109X(21)00026-7
103. Lewer, D, Braithwaite, I, Bullock, M, Eyre, MT, White, PJ, Aldridge, RW, et al. COVID-19 among people experiencing homelessness in England: a modelling study. Lancet Respir Med. (2020) 8:1181–91. doi: 10.1016/S2213-2600(20)30396-9
104. Rao, CY, Robinson, T, Huster, K, Laws, RL, Keating, R, Tobolowsky, FA, et al. Occupational exposures and mitigation strategies among homeless shelter workers at risk of COVID-19. PLoS One. (2021) 16:e0253108. doi: 10.1371/journal.pone.0253108
105. Casto, AM, Rogers, JH, Link, AC, Boeckh, M, Jackson, ML, Uyeki, TM, et al. Phylogenomics of severe acute respiratory syndrome coronavirus 2 in Emergency shelters for people experiencing homelessness. J Infect Dis. (2022) 226:217–24. doi: 10.1093/infdis/jiac021
106. Kerman, N, Ecker, J, Gaetz, S, Tiderington, E, and Kidd, SA. Mental health and wellness of service providers working with people experiencing homelessness in Canada: a national survey from the second wave of the COVID-19 pandemic. Can J Psychiatry. (2022) 67:371–9. doi: 10.1177/07067437211018782
107. Sehra, ST, Kishfy, LJ, Brodski, A, George, MD, Wiebe, DJ, and Baker, JF. Association of cell phone location data and trends in COVID-19 infections during loosening of stay-at-home restrictions. J Travel Med. (2020) 27:taaa177. doi: 10.1093/jtm/taaa177
108. Dhanani, LY, and Franz, B. A meta-analysis of COVID-19 vaccine attitudes and demographic characteristics in the United States. Public Health. (2022) 207:31–8. doi: 10.1016/j.puhe.2022.03.012
109. Centers for Disease Control and Prevention. Trends in demographic characteristics of people receiving COVID-19 vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker
110. Iacoella, C, Ralli, M, Maggiolini, A, Arcangeli, A, and Ercoli, L. Acceptance of COVID-19 vaccine among persons experiencing homelessness in the City of Rome, Italy. Eur Rev Med Pharmacol Sci. (2021) 25:3132–5. doi: 10.26355/eurrev_202104_25568
Keywords: SARS-CoV-2 infection, seroprevalence, COVID-19 vaccination, homelessness, meta-analysis
Citation: Liang Y, Sun Q, Liu Q, Pang Y and Tang S (2023) SARS-CoV-2 incidence, seroprevalence, and COVID-19 vaccination coverage in the homeless population: a systematic review and meta-analysis. Front. Public Health. 11:1044788. doi: 10.3389/fpubh.2023.1044788
Edited by:
Zhongwei Huang, Lanzhou University, ChinaReviewed by:
Fulvia Pimpinelli, San Gallicano Dermatological Institute IRCCS, ItalyBeatrice Benedetti, Catholic University of the Sacred Heart, Rome, Italy
Max Carlos Ramírez-Soto, University of San Martín de Porres, Peru
Copyright © 2023 Liang, Sun, Liu, Pang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanhao Liang, bHlobHl0bHloQGdtYWlsLmNvbQ==; Shixing Tang, dGFtZ3NoaXhpbmdAc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Yuanhao Liang
Yuanhao Liang Qian Sun†
Qian Sun† Quanxun Liu
Quanxun Liu Yulian Pang
Yulian Pang Shixing Tang
Shixing Tang